Abstract
Manganese (Mn) is an essential heavy metal that is naturally found in the environment. Daily intake through dietary sources provides the necessary amount required for several key physiological processes, including antioxidant defense, energy metabolism, immune function and others. However, overexposure from environmental sources can result in a condition known as manganism that features symptomatology similar to Parkinson's disease (PD). This disorder presents with debilitating motor and cognitive deficits that arise from a neurodegenerative process. In order to maintain a balance between its essentiality and neurotoxicity, several mechanisms exist to properly buffer cellular Mn levels. These include transporters involved in Mn uptake, and newly discovered Mn efflux mechanisms. This review will focus on current studies related to mechanisms underlying Mn import and export, primarily the Mn transporters, and their function and roles in Mn-induced neurotoxicity.
Introduction
Manganese (Mn) is a heavy metal found naturally in the earth's crust. This essential metal is the 12th most abundant element and typically exists as oxides, carbonates and silicates. Earth erosion results in the pervasive presence of Mn in air, soil and waterways. Moreover, the natural properties of Mn have resulted in its extensive use in several industrial settings. Mn is used in the manufacturing of batteries, ceramics, steel, cosmetics, leather, fireworks, glass and other textiles. Mn is also a component of an antiknock gasoline additive, known as methylcyclopentadienyl Mn tricarbonyl (MMT), and combustion results in release of Mn phosphates into the ambient air. Additionally, Mn can be found in pesticides and fungicides, smoke inhibitors, and as a contrast reagent for medical magnetic resonance imaging (MRI) purposes (ATSDR 2008). Furthermore, in neonates receiving total parenteral nutrition, the addition of a Mn-containing trace element solution causes a 100-fold increase in the Mn burden compared to those human milk (Aschner & Aschner 2005). Excess Mn exposure is also a concern in drug addicts who illicitly abuse methcathinone, a substance produced from the oxidation of ephedrine and pseudoephedrine via potassium permanganate. Intravenous usage of methcathinone can expose individuals to high levels of Mn derived from the potassium permanganate, which is used as an oxidant in the synthesis of methcathonine (Sikk et al. 2013).
Despite the abundance of Mn in the environment, the primary route of typical human Mn intake is through dietary sources. Mn is found in several foods that compose daily human diets. Legumes, nuts, rice and whole grains contain the highest levels of Mn, while leafy green vegetables, tea, chocolate and some fruits contain moderate levels. Mn is found as a component of some daily multivitamins. The plentiful dietary sources of Mn help ensure adequate levels are reached in humans, with 2.3 mg/day required for men and 1.8 mg/day for women (Aschner & Aschner 2005). The requirement of daily Mn uptake is reflected in its role as a necessary cofactor for several important enzymes, including glutamine synthetase, arginase, pyruvate carboxylase and Mn superoxide dismutase (MnSOD). These metalloproteins are crucial for several enzymatic processes that help regulate development, energy metabolism, digestion, immune function, reproduction and antioxidant defenses (Kanyo et al. 1996, Jitrapakdee et al. 2008, Reddi et al. 2009, Wedler et al. 1982).
The long list of Mn-containing foods, as well as its presence in multivitamins, makes Mn deficiency a rare problem. Moreover, only 3-5% of ingested Mn is absorbed through the gastrointestinal tract (Finley et al. 1994, Davis et al. 1993). However, certain groups can be susceptible to excess Mn from nutritional sources. These include unhealthy neonates receiving total parenteral nutrition (TPN), which is typically supplemented with a trace element solution containing Mn. Importantly, intravenous TPN administration bypasses the gastrointestinal control of Mn absorption, resulting in 100% Mn retention (Aschner & Aschner 2005). Another population at risk of nutritional exposure to excess Mn includes patients suffering from hepatic encephalopathy and/or liver failure, as Mn is excreted from the body predominantly through the biliary system (Zeron et al. 2011, Klos et al. 2005). Finally, individuals with iron (Fe) deficiency (e.g. iron deficiency anemia), a highly prevalent nutritional condition, are at risk for increased Mn body burden, because Mn and Fe use common transporters for uptake, and Fe deficiency increases the expression of these transport systems (Smith et al. 2013).
In addition to nutritional toxicity, excess Mn exposure occurs in occupational settings. Mn-containing fumes, especially in poorly ventilated spaces, can directly affect welders, smelters and other industrial workers (Park 2013). Studies have also found cognitive deficits in populations living close to factories and refineries with high Mn levels (Guarneros et al. 2013, Lucchini et al. 2012). Regardless of the source, excess Mn exposure can lead to a state of Mn poisoning known as “manganism,” an irreversible, progressive condition that resembles Parkinson's disease (PD). PD is a neurodegenerative, motor disorder that results from selective loss of the dopaminergic (DAergic) neurons in the substantia nigra pars compacta (SNpc) (Lees et al. 2009). In contrast, manganism initially targets a different brain region, as Mn shows preferential accumulation in the globus pallidus, but also affects the SNpc to a lesser extent. Early stages of both conditions are characterized by cognitive and emotional problems, including intellectual deficits, mood changes, irritability, restlessness and sleep disturbances (Guilarte 2010). However, likely due to the difference in primary target sites, the motor symptoms are distinctive between the two conditions. The hallmark symptoms of PD include bradykinesia, tremor, rigidity and postural instability (Lees et al. 2009). On the other hand, while still showing signs of rigidity, Mn toxicity typically presents with dystonia, a more upright stance, milder tremors at rest and a signature “cock-like” walk (Guilarte 2010). Another distinguishing feature is the lack of the efficacy that levodopa has in treating patients suffering from manganism (Koller et al. 2004). Current treatment options utilize a combination of levodopa and chelation therapy with either edetate calcium disodium (EDTA) (Herrero Hernandez et al. 2006) or the tuberculosis antibiotic para-aminosalicylic acid (PAS) (Jiang et al. 2006). However, beneficial effects are transient in nature and thereby require prolonged use and high dosage if the source of exposure is not removed.
In order to maintain balance between the essentiality of Mn and its neurotoxicity, several transport mechanisms exist to buffer Mn levels for proper homeostasis. In this review, we will highlight the major uptake mechanisms followed by the more recently discovered efflux mechanisms.
Manganese Import
Mn enters cells through multiple transporters, including the divalent metal transporter 1 (DMT1), the zinc transporters ZIP8 and ZIP14, the citrate transporter, the choline transporter, the dopamine transporter (DAT), the transferrin receptor (TfR) and calcium (Ca) channels (Chen et al. 2014). Among these importers, DMT1 is the primary transporter for divalent Mn and TfR is the primary transporter for trivalent Mn. Notably, in addition to Mn, these transporters also transport other metals, such as Fe, copper (Cu), zinc (Zn), and calcium (Ca), to name a few. Given that excessive cytosolic Mn is toxic to cells, mechanisms regulating the activity and expression of these transporters are noteworthy to investigate within the context of Mn-induced cytotoxicity.
DMT1
DMT1 is also known as natural resistance-associated macrophage protein 2 (NRAMP 2) or divalent cation transporter 1 (DCT1), and is encoded by the SLC11A2 (solute carrier family 11, member 2) gene in humans (Vidal et al. 1995). DMT1 was first identified and characterized in rats and shown to mediate transport of a wide range of substrates, including divalent Fe, Zn, Mn, cobalt (Co), cadmium (Cd), Cu, nickel (Ni) and lead (Pb) (Salazar et al. 2008, Garrick et al. 2006). DMT1 is highly expressed in the basal ganglia of the brain, including SN, GP, hypothalamic nucleus and striatum (Huang et al. 2004, Williams et al. 2000, Burdo et al. 2001), where high levels of Mn accumulation are noted. In addition, its expression level may increase with age (Ke et al. 2005), which may explain the adult-onset (∼ 46 years) of occupational manganism in welders (Racette et al. 2001). DMT1 has a higher transport affinity for Mn than Fe. Indeed, its transport affinities for metals are as follows: Mn>Cd>Fe>Pb∼Co∼Ni>Zn (Garrick et al. 2006). DMT1 preferentially transports Mn into the brain through the blood-brain barrier (BBB), especially under low Fe conditions [due to iron-responsive element (IRE)-mediated regulation], which increases DMT1 expression (Erikson et al. 2005b, Crossgrove & Yokel 2004). In addition, DMT1 also transports Mn from endosomes into cytosol in a TfR dependent manner, which is discussed below.
Changes in DMT1 are associated with several diseases. In the Han Chinese population, the CC haplotype in the DMT1 gene is associated with increased susceptibility for PD (He et al. 2011b). In PD patients, increased expression of DMT1 has been found in the SNpc of brains (Kong et al. 2014, Tsunemi & Krainc 2014, Peng et al. 2009, Peng et al. 2007). In the MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model of PD, it is reported that DMT1 expression is upregulated, associated with Fe accumulation, as well as increased oxidative stress and DAergic cell death (Salazar et al. 2008). In addition to PD, alterations in DMT1 are associated with spinal onset amyotrophic lateral sclerosis (ALS) (Blasco et al. 2011), Alzheimer's disease (AD) onset in males (Jamieson et al. 2005), iron anemia and restless legs syndrome (RLS) (Xiong et al. 2007). Although Fe has been implicated to play an important role in these diseases, data on Fe accumulation in the brain in these diseases is lacking. Whether DMT1-induced changes in Mn contribute to the onset of any of these diseases remains to be investigated.
Transferrin/Trasnferrin Receptor
While DMT1 transports primarily divalent Mn, the transferrin (Tf)/transferrin receptor (TfR) system is responsible for transporting trivalent Mn, which consists of about 20% of total blood Mn. Tf is synthesized in the liver and secreted into the blood plasma (Aisen et al. 1978), where it binds Mn3+. The TfR is expressed in most cells, including neurons, microglia, astrocytes and the endothelial cells of the BBB (Moos & Morgan 2000). Moreover, it has five IREs that sense cytosolic Fe/Mn levels and accordingly regulate the expression of TfR on the plasma membrane (Subramaniam et al. 2002). Tf-bound Mn in the blood is transported by TfR into cells through the ligand-receptor endocytosis mechanism. Using a fluorescent label bound to the Mn3+Tf complex, Gunter and colleagues observed the endocytic transport of Mn3+/Tf through TfR into mouse hippocampal and striatal neuronal cells and into a region adjacent to the mitochondrial network, presumably endosomes (Gunter et al. 2013). Further, available results suggest that, after endocytosis, as endosomes undergo acidification, Mn3+ is released from the Tf/TfR complex (Roth et al. 2002, Tuschl et al. 2013, Gunter et al. 2013). Endosomal Mn3+ is then reduced to Mn2+ by ferrireductase (probably to avoid Mn3+ transport to the cytosol, which can cause oxidative stress), and the Mn2+ is transported to the cytosol by endosomal DMT1 (Tuschl et al. 2013, Au et al. 2008).
Other Manganese Importers
Zinc transporters ZIP8 and ZIP14
ZIP8 (SLC39A8) and ZIP14 (SLC39A14) were first identified as Zn transporters, as cells transfected with these two genes accumulated more Zn than controls (Begum et al. 2002, Liuzzi et al. 2006). ZIP8 is expressed most abundantly in lung, testis and kidney, while ZIP14 expression is highest in the liver, but is also detected in the pancreas and heart; the expression level of both proteins in the brain is relatively low (Jenkitkasemwong et al. 2012). ZIP8 was later found to import Cd and Mn as well, as ZIP8 overexpression stimulated intracellular accumulation of Cd and Mn (He et al. 2006, Dalton et al. 2005). Subsequent studies showed that ZIP14 was able to transport Fe, Mn and Cd as well (Girijashanker et al. 2008, Liuzzi et al. 2006). Both ZIP8 and ZIP14 have high affinity for Mn (He et al. 2006, Girijashanker et al. 2008), e.g., a metal cation competition assay for ZIP8 revealed the following order of affinity: Mn2+ >Hg2+ ≫ Pb2+ =Cu2+ = Zn2+ = Cs2+ (He et al. 2006). Though these transporters may not regulate Mn transport in the brain to a great degree, they may regulate Mn absorption through the liver and lung to help regulate body Mn levels. Notably, ZIP8 and ZIP14 are expressed in the nasal respiratory epithelium and olfactory receptor neuron dendrites, where Mn from inhaled dust can be directly absorbed into the blood or into the brain (Genter et al. 2009). This may facilitate Mn toxicity in industrial workers suffering from manganism who have inhaled fumes containing high levels of Mn.
Dopamine transporter (DAT)
DAT is a membrane-spanning protein that acts to induce reuptake of dopamine into presynaptic vesicles. In rat brain, this protein is highly expressed in the axons, dendrites and cell bodies of neurons in the substantia nigra pars compacta, globus pallidus and striatum (Ciliax et al. 1995). In 1999, Ingersoll and colleagues found that the DAT inhibitor cocaine induced a 10-fold inhibition of Mn uptake in rat brain (particularly in the ventral mesencephalon) (Ingersoll et al. 1999). Subsequently, a study found decreased DAT density and activity in patients chronically exposed to Mn (Kim et al. 2002, Huang et al. 2003). Direct evidence of DAT as a Mn transporter comes from studies using DAT knockout mice, which accumulate significantly less Mn in the striatum compared to wildtype (WT) mice after Mn exposure. Interestingly, the absence of DAT did not affect Mn accumulation in other brain regions not expressing DAT (Erikson et al. 2005a). Indeed, Mn accumulation was selectively decreased by DAT inhibition, but not by inhibition of the serotonin transporter (SERT) or norepinephrine transporter (NET); this decrease was only seen in the globus pallidus of rats upon chronic Mn exposure (Anderson et al. 2007). Surprisingly, DAT levels were significantly increased in the striatum of baboon and rats after acute Mn administration (Chen et al. 2006).
Ca channels
The role of Ca in Mn homeostasis was first reported by Mason et al., noting that Mn uptake was decreased by Ca inhibitors in thymic lymphocytes (Mason et al. 1993). In a human hepato-carcinoma cell line, Mn uptake was shown to be dependent upon Ca concentrations (Finley 1998). Moreover, in human erythrocytes, Mn uptake was shown to be inhibited by nifedipine, a Ca channel blocker (Lucaciu et al. 1997). Later, Mn was found to enter cell membranes through store-operated Ca channels (SOCCs) in rat mast cells, osteoblast-like cells, human platelets and cultured bovine brain microvascular endothelial cells (Baldi et al. 2002, Dobrydneva & Blackmore 2001, Fasolato et al. 1993, Crossgrove & Yokel 2005). SOCCs are expressed in brain endothelial cells (Kim et al. 2004), and therefore, may play a role in Mn accumulation in the brain.
Choline transporters
There are a few different types of choline transporters. In adult rat tissues, a specific choline transporter is expressed in the nervous system, whereas three other forms are primarily distributed in peripheral tissues (Traiffort et al. 2005). Using an in situ brain perfusion technique in rats choline uptake was found to be significantly inhibited in the presence of Cd and Mn, but not Cu or aluminum (Al) (Lockman et al. 2001). Further studies are needed to determine if choline transporters play a direct role in Mn uptake.
Citrate transporters
Citrate transporters have been found in rat liver, testis and brain (Inoue et al. 2002). Similar to the discovery of the choline transporters in regulating Mn uptake using in situ brain perfusion technique, Crogressgrove et al. found that Mn citrate was able to cross the BBB and entered the brain (Crossgrove et al. 2003), indicating the citrate transporters as another putative Mn transporter.
Manganese Export
Efflux is a fundamental process that plays a crucial role in regulating cellular levels of essential metals such as Cu, Zn and Fe, and genetic defects in efflux transporters cause hereditary disorders of metal metabolism (e.g. Wilson's disease and Menke's disease due to mutations in the Cu-transporting pumps, ATP7B and ATP7A, respectively) (Brissot et al. 2011, Donovan et al. 2000, Kitzberger et al. 2005, La Fontaine & Mercer 2007, Liuzzi & Cousins 2004, McKie et al. 2000, Nemeth et al. 2004, Troadec et al. 2010). However, it is only over the last few years that the role of efflux in regulating Mn homeostasis in mammalian systems has begun to be appreciated. Thus far, four transporters have demonstrated Mn efflux activity in various experimental systems: ATPase 13A2 (ATP13A2), SLC30A10, ferroportin and secretory pathway Ca2+-ATPase 1 (SPCA1) (Leyva-Illades et al. 2014, Madejczyk & Ballatori 2012a, Mukhopadhyay et al. 2010, Mukhopadhyay & Linstedt 2011, Tan et al. 2011, Yin et al. 2010). Importantly, the first hereditary or familial form of Mn-induced parkinsonism was recently discovered and genetic studies revealed that there was an association between homozygous loss-of-function mutations in SLC30A10 and the development of this disease (Quadri et al. 2012, Tuschl et al. 2012). The fact that loss-of-function of an efflux transporter is the only genetic factor associated with hereditary Mn-induced parkinsonism underlines the importance of efflux in the regulation of Mn homeostasis and detoxification. The role of ferroportin and SPCA1 in mediating Mn efflux in neuronal systems and in the pathobiology of Mn-induced neurotoxicity are unclear; therefore, these transporters will not be discussed further in this review.
ATPase 13A2 (ATP13A2 or PARK9)
ATP13A2 is a vacuolar/lysosomal transmembrane cation transporting ATPase, and mutated ATP13A2 has been associated with early-onset parkinsonism and Kufor-Rakeb syndrome (Gitler et al. 2009, Behrens et al. 2010, Di Fonzo et al. 2007). Expression of ATP13A2 protected yeast and mammalian cells, and primary rat neurons against Mn-induced lethality (Tan et al. 2011, Gitler et al. 2009). In addition, expression of ATP13A2 reduced intracellular Mn levels in cells (Tan et al. 2011). These results raise the possibility that ATP13A2 may transport Mn from the cytosol to the lumen of lysosomes, and thus, function as a Mn efflux transporter. However, direct Mn efflux activity for ATP13A2 has not yet been demonstrated. Further, it is not known whether patients who harbor mutations in this gene also exhibit Mn deposition the brain. Therefore, further studies are needed to determine whether ATP13A2 is a bona fide Mn efflux transport.
SLC30A10
To date, loss of function mutations in SLC30A10 are the only known cause of a hereditary or familial Mn-induced parkinsonian syndrome. Genetic studies in human patients who developed familial Mn-induced parkinsonism led to the eventual identification of SLC30A10 as a Mn efflux transporter. The first detailed case report of this form of familial parkinsonism was published by Tuschl and colleagues in 2008 (Tuschl et al. 2008). In this study, the authors reported findings from a 12 y old female patient who presented with parkinsonian symptoms (Tuschl et al. 2008). Clinical analyses revealed that the patient had ∼10-fold increase in blood Mn levels. Plasma copper and zinc levels were within the normal range. Magnetic resonance imaging studies provided evidence for Mn deposition in the basal ganglia (Tuschl et al. 2008). Importantly, the individual had no history of exposure to elevated Mn (Tuschl et al. 2008). These findings suggested that the patient suffered from parkinsonism as a consequence of Mn accumulation, and that the increased retention of Mn occurred due to a defect in Mn metabolism.
A few years later, in 2012, two separate studies, one by Tuschl and colleagues and the other by the group of Vincenzo Bonifati, reported findings from an additional set of patients (Quadri et al. 2012, Tuschl et al. 2012). All patients had 10-20 fold increases in blood Mn with no history of exposure to elevated Mn from environmental or occupational sources (Quadri et al. 2012, Tuschl et al. 2012). Whole genome homozygosity mapping revealed that affected patients had one region of homozygosity that mapped to the region coding for the SLC30A10 gene on chromosome 1. Sanger sequencing then determined that patients carried homozygous mutations in the SLC30A10 gene (Quadri et al. 2012, Tuschl et al. 2012). The disease exhibited autosomal recessive inheritance (Tuschl et al. 2012, Quadri et al. 2012). Affected patients were born to consanguineous parents and unaffected siblings and parents of patients, when studied, were found to be heterozygous for SLC30A10 mutations (Tuschl et al. 2012). These studies suggested that mutations in SLC30A10 affected Mn metabolism in a manner that caused increased Mn retention in the body.
The above findings are similar to a prior case report published by Gospe et al in 2000 (Gospe et al. 2000). These authors followed the patient described in their report from age 14 y till death at age 38 y, and then described autopsy findings in a second manuscript (Lechpammer et al. 2014). The patient had homozygous mutations in SLC30A10 (Lechpammer et al. 2014, Tuschl et al. 2012). Important features observed during autopsy included severe neuronal loss in the basal ganglia, particularly in the globus pallidus, along with a 16-fold increase in basal ganglia and a 9-fold increase in liver Mn levels (Lechpammer et al. 2014).
SLC30 proteins belong to the cation diffusion facilitator superfamily of ion transporters (Huang & Tepaamorndech 2013). There are 10 members in the SLC30 family, SLC30A1-A10 (Huang & Tepaamorndech 2013). SLC30A1-A8 transport Zn from the cytosol to the cell exterior or the lumen of various organelles and the function of SLC30A9 is unclear (Huang & Tepaamorndech 2013). The fact that patients with SLC30A10 mutations exhibit increased Mn retention raised the possibility that SLC30A10-WT mediated efflux of Mn, instead of Zn, while disease-causing SLC30A10 mutants lacked this Mn efflux activity. This idea was supported by a heterologous expression experiment performed by Tuschl et al, in which expression of human SLC30A10-WT, but not two separate disease-causing mutants, rescued the enhanced sensitivity to Mn-induced cell death seen in yeast strains that lack pmr1 (Tuschl et al. 2012).
Mechanistic insights into the cellular function of SLC30A10 and the reasons why mutations in this gene affect Mn metabolism were obtained by a recent collaborative study performed by the groups of Michael Aschner and Somshuvra Mukhopadhyay (Leyva-Illades et al. 2014). Localization studies in cell culture, including in GABAergic AF5 cells and primary midbrain neurons, and in C. elegans revealed that SLC30A10-WT trafficked to the cell surface. In contrast, disease-causing mutants tested (L89P, Δ98-134, Δ105-107, T196P and Q308Stop) exhibited a trafficking defect and were trapped in the endoplasmic reticulum (Leyva-Illades et al. 2014). The mutant proteins also underwent increased proteasomal turn-over. Importantly, Mn measurement assays in cell culture revealed that expression of SLC30A10-WT, but not the disease-causing mutant Δ105-107, reduced intracellular Mn levels, and a pulse-chase assay confirmed that this reduction in intracellular Mn was due to an increase in Mn efflux and not a block in Mn influx (Leyva-Illades et al. 2014). Subsequent studies revealed that expression of SLC30A10-WT, but not Δ105-107, protected GABAergic AF5 cells and primary midbrain neurons against Mn toxicity. In contrast, knockdown of SLC30A10 in GABAergic AF5 cells caused Mn accumulation and increased the sensitivity of cells to Mn-induced death (Leyva-Illades et al. 2014). At the organism level, in C. elegans, expression of SLC30A10-WT, but not L89P (another disease-causing mutant), protected dopaminergic neurons against Mn-induced neurodegeneration, rescued a Mn-induced functional defect in locomotion and enhanced the viability of worms exposed to elevated Mn (Leyva-Illades et al. 2014). Put together, the above data strongly suggest that SLC30A10-WT is a cell surface localized Mn efflux transporter and that mutations in the protein that cause familial parkinsonism block the efflux activity of the transporter.
SLC30A10 was initially thought to be a Zn efflux transporter (Bosomworth et al. 2012). A 3-fold increase in liver Zn was reported in one patient of familial Mn-induced parkinsonism (Lechpammer et al. 2014). However, in this patient, there was no change in basal ganglia Zn levels, and, in the liver, there was a 2-fold increase in Cu as well (Lechpammer et al. 2014). A mild increase in liver Cu was also reported in another patient, but the plasma Cu levels in this patient were normal (Tuschl et al. 2008). The above clinical findings suggested that the observed increase in hepatic Zn or Cu levels in a few patients with SLC30A10 mutations was due to compromised liver function, secondary to Mn deposition, and not a primary effect of SLC30A10 on Zn or Cu metabolism. Consistent with this idea, in cell culture assays, expression of SLC30A10-WT did not affect intracellular levels of Zn or Cu or alter viability of cells exposed to elevated Zn or Cu (Leyva-Illades et al. 2014).
How can mutations in SLC30A10 contribute to the development of a familial parkinsonian syndrome? SLC30A10 is expressed in the basal ganglia and the liver (Quadri et al. 2012). Mn is primarily excreted by the liver via transport into bile (Butterworth 2013). Loss-of-function mutations in SLC30A10 will be expected to block the biliary excretion of Mn, and lead to excess Mn retention in the body. The retained Mn will then be expected to cross the blood brain barrier, and eventually accumulate in the basal ganglia. Indeed, increased Mn levels in blood and basal ganglia are seen in patients with SLC30A10 mutations (Quadri et al. 2012, Tuschl et al. 2012). The reasons why basal ganglia neurons, particularly those in the globus pallidus, selectively accumulate Mn and are more sensitive to Mn-induced damage is as yet unclear. However, the fact that SLC30A10 is expressed in basal ganglia neurons, including in the globus pallidus (Quadri et al. 2012), suggests that loss-of-function of SLC30A10 will likely increase the sensitivity of these neurons to Mn accumulation and subsequent damage. This Mn-induced damage likely culminates in the development of parkinsonism.
In sum, among the known Mn efflux transports, SLC30A10 appears to have the most physiological relevance because mutations in this protein cause a hereditary parkinsonian syndrome. The relative contribution of SPCA1 and ferroportin in mediating Mn detoxification at the whole animal level is not yet clear and must be elucidated. Moreover, while mutations in ATP13A2 cause parkinsonism, defects in Mn metabolism are unlikely to be the sole cause of motor defects produced from mutations in this gene. The biology of SLC30A10 has only now begun to be investigated. Fundamental questions, such as what is the mechanism that confers selective Mn transport capability to SLC30A10 and how does Mn control SLC30A10 activity, are as yet unknown. Addressing these issues is an essential step in understanding how SLC30A10 regulates Mn homeostasis and detoxification. It will also be important to determine whether polymorphisisms in SLC30A10 exist in the population and whether these polymorphisms alter the risk of developing Mn-induced parkinsonism. Search for SLC30A10 polymorphisms is supported by the observation that polymorphisms in DMT1 increase the risk of developing Parkinson's disease (He et al. 2011a). Finally, the finding that over-expression of SLC30A10 enhanced Mn efflux and protected against Mn-induced toxicity, (Leyva-Illades et al. 2014), is important from a clinical perspective. Currently, there is no definitive treatment for Mn-induced parkinsonism. Indeed, the only treatment available for patients with SLC30A10 mutations is chelation therapy, which provided partial symptomatic relief (Quadri et al. 2012, Tuschl et al. 2012). The protective effect of enhanced efflux on Mn toxicity raises the possibility that increasing Mn efflux may be a useful strategy for the management of familial and non-familial forms of Mn-induced parkinsonism. It will be important to determine whether increasing Mn efflux can protect against Mn toxicity in a vertebrate animal model without causing side-effects, such as Mn deficiency. Demonstration of a protective effect will justify screening for or generating efflux enhancing small molecules or drugs. Such efflux enhancing drugs, as and when identified, may work by increasing SLC30A10 activity or levels, or by an independent mechanism (e.g. by increasing the efflux activity of SPCA1 or ferroportin). Drugs that require SLC30A10 for activity will likely not be beneficial for patients of familial Mn-induced parkinsonism, where SLC30A10 is mutated. However, such drugs may prove to be useful for non-familial forms of Mn-induced parkinsonism, including forms of the disease that occur due to occupational or environmental over-exposure to Mn. Drugs that work in a SLC30A10-independent manner may help patients with familial and non-familial forms of Mn-induced parkinsonism. Discovery of a viable means to increase Mn efflux combined with advances being made in the identification of non-invasive biomarkers of Mn toxicity [such as hair Mn levels (Eastman et al. 2013)] may eventually come together to create a feasible therapeutic strategy for managing Mn-induced parkinsonism.
Other Manganese Exporters
Ferroportin and SPCA1
Ferroportin is a transmembrane protein expressed in the duodenum, liver, spleen and intestine (Donovan et al. 2005), as well as in the endothelial cells of the BBB, neurons, oligodendrocytes, astrocytes, the choroid plexus and ependymal cells (Wu et al. 2004). SPCA1 is widely expressed in human tissues, with the highest expression in keratinocytes, liver and brain (Hu et al. 2000). It is primarily localized to the Golgi apparatus (Ton et al. 2002), although an additional endosomal/vesicular pool was observed (Leitch et al. 2011). Both ferroportin and SPCA1 are able to mediate Mn efflux (Madejczyk & Ballatori 2012b, Ton et al. 2002). As the role of ferroportin and SPCA1 in the nervous system has yet to be established, it will not be further discussed herein.
Conclusions
The maintenance of Mn homeostasis involves a complex network of proteins that mediate Mn import or export. The major uptake mechanisms identified include DMT1 and Tf-TfR-mediated endocytosis. Other proteins that may play a role in uptake are Ca2+ channels; choline transporters; citrate transporters; and the ZIP family of Zn transporters. Notably, none of these transporters is specific for Mn as they transport other metals/substrates as well. Further work is needed to determine whether changes in cellular Mn controls expression and activity of these transporters in a manner that impacts Mn homeostasis. Compared to the influx transporters, discovery of Mn efflux transporters is relatively recent. SLC30A10 appears to be the most relevant Mn efflux transporter. The roles of ATP13A2, SPCA1 and ferroportin in mediating Mn efflux in neuronal systems and in protecting against Mn-induced neurotoxicity need additional work. Mechanistic insights of Mn transport mechanisms in relevant neurons will likely provide a better understanding of the biology of Mn-induced parkinsonism and contribute to the development of new therapeutic strategies for this devastating disease.
Figure.
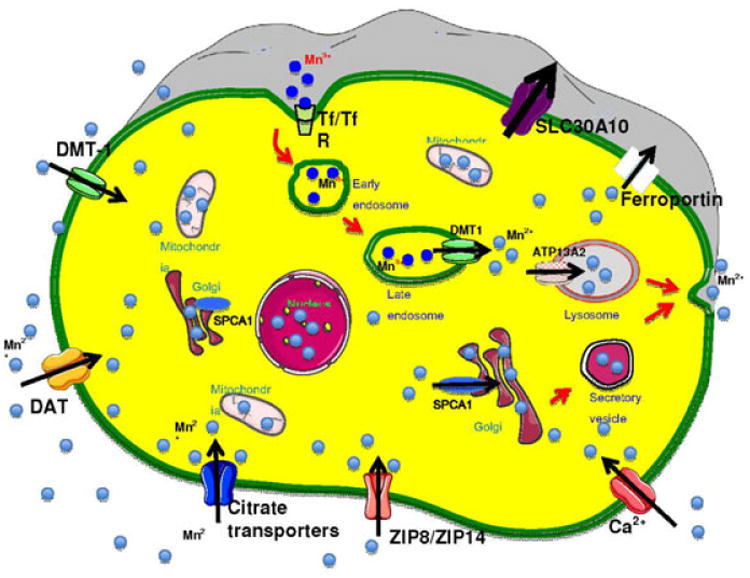
In cells, the intracellular Mn level is tightly regulated by transporters on the cell surface and the intracellular transporters localized on the membrane of internal organelles. On the cell surface, DMT-1, DAT, ZIP8/ZIP14, citrate transporters and Ca channels facilitate Mn2+ influx in to the cytosol, while SLC30A10 and ferroportin mediate efflux of Mn2+. Mn3+ can be directly transported in cells through Tf/TfR. In addition, Mn3+ also enters cells through Tf/TfR mediated endocytosis and finally released into cytoplasm as Mn2+ by DMT1. When the cytosolic Mn reaches a threshold, SPCA1 on the Golgi membrane and ATP13A2 on the lysosome membrane will transport Mn into the Golgi and lysosomes, respectively, which facilitate Mn2+ efflux into the extracellular matrix.
Acknowledgments
The present study was supported in part by NIH grants, NIGMS SC1089630 (EL), R00ES020844 (SM), and R01ES10563 and R01ES10563S1 (MA and ABB).
Footnotes
The authors have no conflicts of interest to declare.
References
- Aisen P, Leibman A, Zweier J. Stoichiometric and site characteristics of the binding of iron to human transferrin. Journal of Biological Chemistry. 1978;253:1930–1937. [PubMed] [Google Scholar]
- Anderson JG, Cooney PT, Erikson KM. Inhibition of DAT function attenuates manganese accumulation in the globus pallidus. Environmental toxicology and pharmacology. 2007;23:179–184. doi: 10.1016/j.etap.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Molecular aspects of medicine. 2005;26:353–362. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR. Toxicological profile for manganese (Draft for Public Comment) US Department of Health and Human Services, Public Service 2008 [Google Scholar]
- Au C, Benedetto A, Aschner M. Manganese transport in eukaryotes: The role of DMT1. NeuroToxicology. 2008;29:569–576. doi: 10.1016/j.neuro.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi C, Vazquez G, Boland R. Characterization of a 1, 25 (OH) 2 - vitamin D3 - responsive capacitative Ca2+ entry pathway in rat osteoblast - like cells. Journal of cellular biochemistry. 2002;86:678–687. doi: 10.1002/jcb.10255. [DOI] [PubMed] [Google Scholar]
- Begum NA, Kobayashi M, Moriwaki Y, Matsumoto M, Toyoshima K, Seya T. Mycobacterium bovis BCG Cell Wall and Lipopolysaccharide Induce a Novel Gene, BIGM103, Encoding a 7-TM Protein: Identification of a New Protein Family Having Zn-Transporter and Zn-Metalloprotease Signatures. Genomics. 2002;80:630–645. doi: 10.1006/geno.2002.7000. [DOI] [PubMed] [Google Scholar]
- Behrens MI, Brüggemann N, Chana P, et al. Clinical spectrum of Kufor-Rakeb syndrome in the Chilean kindred with ATP13A2 mutations. Movement Disorders. 2010;25:1929–1937. doi: 10.1002/mds.22996. [DOI] [PubMed] [Google Scholar]
- Blasco H, Vourc'h P, Nadjar Y, et al. Association between divalent metal transport 1 encoding gene (SLC11A2) and disease duration in amyotrophic lateral sclerosis. Journal of the Neurological Sciences. 2011;303:124–127. doi: 10.1016/j.jns.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Bosomworth HJ, Thornton JK, Coneyworth LJ, Ford D, Valentine RA. Efflux function, tissue-specific expression and intracellular trafficking of the Zn transporter ZnT10 indicate roles in adult Zn homeostasis. Metallomics. 2012;4:771–779. doi: 10.1039/c2mt20088k. [DOI] [PubMed] [Google Scholar]
- Brissot P, Bardou-Jacquet E, Jouanolle AM, Loreal O. Iron disorders of genetic origin: a changing world. Trends Mol Med. 2011;17:707–713. doi: 10.1016/j.molmed.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Burdo JR, Menzies SL, Simpson IA, Garrick LM, Garrick MD, Dolan KG, Haile DJ, Beard JL, Connor JR. Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. J Neurosci Res. 2001;66:1198–1207. doi: 10.1002/jnr.1256. [DOI] [PubMed] [Google Scholar]
- Butterworth RF. Parkinsonism in cirrhosis: pathogenesis and current therapeutic options. Metab Brain Dis. 2013;28:261–267. doi: 10.1007/s11011-012-9341-7. [DOI] [PubMed] [Google Scholar]
- Chen MK, Lee JS, McGlothan JL, Furukawa E, Adams RJ, Alexander M, Wong DF, Guilarte TR. Acute manganese administration alters dopamine transporter levels in the non-human primate striatum. NeuroToxicology. 2006;27:229–236. doi: 10.1016/j.neuro.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Chen P, Parmalee N, Aschner M. Genetic Factors and Manganese-Induced Neurotoxicity. Frontiers in Genetics. 2014;5 doi: 10.3389/fgene.2014.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB, Levey AI. The dopamine transporter: immunochemical characterization and localization in brain. The Journal of neuroscience. 1995;15:1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossgrove JS, Allen DD, Bukaveckas BL, Rhineheimer SS, Yokel RA. Manganese distribution across the blood-brain barrier. I. Evidence for carrier-mediated influx of managanese citrate as well as manganese and manganese transferrin. Neurotoxicology. 2003;24:3–13. doi: 10.1016/s0161-813x(02)00089-x. [DOI] [PubMed] [Google Scholar]
- Crossgrove JS, Yokel RA. Manganese Distribution Across the Blood–Brain Barrier III: The Divalent Metal Transporter-1 is not the Major Mechanism Mediating Brain Manganese Uptake. NeuroToxicology. 2004;25:451–460. doi: 10.1016/j.neuro.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Crossgrove JS, Yokel RA. Manganese distribution across the blood–brain barrier: IV. Evidence for brain influx through store-operated calcium channels. NeuroToxicology. 2005;26:297–307. doi: 10.1016/j.neuro.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Dalton TP, He L, Wang B, Miller ML, Jin L, Stringer KF, Chang X, Baxter CS, Nebert DW. Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3401–3406. doi: 10.1073/pnas.0406085102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CD, Zech L, Greger JL. Manganese metabolism in rats: an improved methodology for assessing gut endogenous losses. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. 1993;202:103–108. doi: 10.3181/00379727-202-43518. [DOI] [PubMed] [Google Scholar]
- Di Fonzo A, Chien HF, Socal M, et al. ATP13A2 missense mutations in juvenile parkinsonism and young onset Parkinson disease. Neurology. 2007;68:1557–1562. doi: 10.1212/01.wnl.0000260963.08711.08. [DOI] [PubMed] [Google Scholar]
- Dobrydneva Y, Blackmore P. 2-Aminoethoxydiphenyl borate directly inhibits store-operated calcium entry channels in human platelets. Molecular pharmacology. 2001;60:541–552. [PubMed] [Google Scholar]
- Donovan A, Brownlie A, Zhou Y, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metabolism. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Eastman RR, Jursa TP, Benedetti C, Lucchini RG, Smith DR. Hair as a biomarker of environmental manganese exposure. Environ Sci Technol. 2013;47:1629–1637. doi: 10.1021/es3035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson KM, John CE, Jones SR, Aschner M. Manganese accumulation in striatum of mice exposed to toxic doses is dependent upon a functional dopamine transporter. Environmental toxicology and pharmacology. 2005a;20:390–394. doi: 10.1016/j.etap.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Syversen T, Aschner JL, Aschner M. Interactions between excessive manganese exposures and dietary iron-deficiency in neurodegeneration. Environmental toxicology and pharmacology. 2005b;19:415–421. doi: 10.1016/j.etap.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Fasolato C, Hoth M, Penner R. Multiple mechanisms of manganese-induced quenching of fura-2 fluorescence in rat mast cells. Pflügers Archiv. 1993;423:225–231. doi: 10.1007/BF00374399. [DOI] [PubMed] [Google Scholar]
- Finley JW. Manganese uptake and release by cultured human hepato-carcinoma (Hep-G2) cells. Biol Trace Elem Res. 1998;64:101–118. [PubMed] [Google Scholar]
- Finley JW, Johnson PE, Johnson LK. Sex affects manganese absorption and retention by humans from a diet adequate in manganese. The American journal of clinical nutrition. 1994;60:949–955. doi: 10.1093/ajcn/60.6.949. [DOI] [PubMed] [Google Scholar]
- Garrick MD, Singleton ST, Vargas F, et al. DMT1: which metals does it transport? Biological research. 2006;39:79–85. doi: 10.4067/s0716-97602006000100009. [DOI] [PubMed] [Google Scholar]
- Genter MB, Kendig EL, Knutson MD. Uptake of Materials from the Nasal Cavity into the Blood and Brain. Annals of the New York Academy of Sciences. 2009;1170:623–628. doi: 10.1111/j.1749-6632.2009.03877.x. [DOI] [PubMed] [Google Scholar]
- Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, Wang B, Dalton TP, Nebert DW. Slc39a14 Gene Encodes ZIP14, A Metal/Bicarbonate Symporter: Similarities to the ZIP8 Transporter. Molecular Pharmacology. 2008;73:1413–1423. doi: 10.1124/mol.107.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Chesi A, Geddie ML, et al. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet. 2009;41:308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospe SM, Jr, Caruso RD, Clegg MS, Keen CL, Pimstone NR, Ducore JM, Gettner SS, Kreutzer RA. Paraparesis, hypermanganesaemia, and polycythaemia: a novel presentation of cirrhosis. Arch Dis Child. 2000;83:439–442. doi: 10.1136/adc.83.5.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneros M, Ortiz-Romo N, Alcaraz-Zubeldia M, Drucker-Colin R, Hudson R. Nonoccupational environmental exposure to manganese is linked to deficits in peripheral and central olfactory function. Chemical senses. 2013;38:783–791. doi: 10.1093/chemse/bjt045. [DOI] [PubMed] [Google Scholar]
- Guilarte TR. Manganese and Parkinson's disease: a critical review and new findings. Environmental health perspectives. 2010;118:1071–1080. doi: 10.1289/ehp.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter TE, Gerstner B, Gunter KK, Malecki J, Gelein R, Valentine WM, Aschner M, Yule DI. Manganese transport via the transferrin mechanism. NeuroToxicology. 2013;34:118–127. doi: 10.1016/j.neuro.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW. ZIP8, Member of the Solute-Carrier-39 (SLC39) Metal-Transporter Family: Characterization of Transporter Properties. Molecular Pharmacology. 2006;70:171–180. doi: 10.1124/mol.106.024521. [DOI] [PubMed] [Google Scholar]
- He Q, Du T, Yu X, et al. DMT1 polymorphism and risk of Parkinson's disease. Neurosci Lett. 2011a;501:128–131. doi: 10.1016/j.neulet.2011.07.001. [DOI] [PubMed] [Google Scholar]
- He Q, Du T, Yu X, et al. DMT1 polymorphism and risk of Parkinson's disease. Neuroscience Letters. 2011b;501:128–131. doi: 10.1016/j.neulet.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Herrero Hernandez E, Discalzi G, Valentini C, Venturi F, Chio A, Carmellino C, Rossi L, Sacchetti A, Pira E. Follow-up of patients affected by manganese-induced Parkinsonism after treatment with CaNa2EDTA. Neurotoxicology. 2006;27:333–339. doi: 10.1016/j.neuro.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Hu Z, Bonifas JM, Beech J, Bench G, Shigihara T, Ogawa H, Ikeda S, Mauro T, Epstein EH., Jr Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nature genetics. 2000;24:61–65. doi: 10.1038/71701. [DOI] [PubMed] [Google Scholar]
- Huang CC, Weng YH, Lu CS, Chu NS, Yen TC. Dopamine transporter binding in chronic manganese intoxication. Journal of neurology. 2003;250:1335–1339. doi: 10.1007/s00415-003-0214-1. [DOI] [PubMed] [Google Scholar]
- Huang E, Ong WY, Connor JR. Distribution of divalent metal transporter-1 in the monkey basal ganglia. Neuroscience. 2004;128:487–496. doi: 10.1016/j.neuroscience.2004.06.055. [DOI] [PubMed] [Google Scholar]
- Huang L, Tepaamorndech S. The SLC30 family of zinc transporters - a review of current understanding of their biological and pathophysiological roles. Mol Aspects Med. 2013;34:548–560. doi: 10.1016/j.mam.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Ingersoll RT, Montgomery EB, Aposhian HV. Central nervous system toxicity of manganese. II: Cocaine or reserpine inhibit manganese concentration in the rat brain. Neurotoxicology. 1999;20:467–476. [PubMed] [Google Scholar]
- Inoue K, Zhuang L, Maddox DM, Smith SB, Ganapathy V. Structure, Function, and Expression Pattern of a Novel Sodium-coupled Citrate Transporter (NaCT) Cloned from Mammalian Brain. Journal of Biological Chemistry. 2002;277:39469–39476. doi: 10.1074/jbc.M207072200. [DOI] [PubMed] [Google Scholar]
- Jamieson SE, White JK, Howson JMM, et al. Candidate gene association study of solute carrier family 11a members 1 (SLC11A1) and 2 (SLC11A2) genes in Alzheimer's disease. Neuroscience Letters. 2005;374:124–128. doi: 10.1016/j.neulet.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Jenkitkasemwong S, Wang CY, Mackenzie B, Knutson M. Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2012;25:643–655. doi: 10.1007/s10534-012-9526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YM, Mo XA, Du FQ, et al. Effective treatment of manganese-induced occupational Parkinsonism with p-aminosalicylic acid: a case of 17-year follow-up study. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2006;48:644–649. doi: 10.1097/01.jom.0000204114.01893.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitrapakdee S, St Maurice M, Rayment I, Cleland WW, Wallace JC, Attwood PV. Structure, mechanism and regulation of pyruvate carboxylase. Biochem J. 2008;413:369–387. doi: 10.1042/BJ20080709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanyo ZF, Scolnick LR, Ash DE, Christianson DW. Structure of a unique binuclear manganese cluster in arginase. Nature. 1996;383:554–557. doi: 10.1038/383554a0. [DOI] [PubMed] [Google Scholar]
- Ke Y, Chang YZ, Duan XL, Du JR, Zhu L, Wang K, Yang XD, Ho KP, Qian Zm. Age-dependent and iron-independent expression of two mRNA isoforms of divalent metal transporter 1 in rat brain. Neurobiology of Aging. 2005;26:739–748. doi: 10.1016/j.neurobiolaging.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim JM, Kim JW, et al. Dopamine transporter density is decreased in parkinsonian patients with a history of manganese exposure: What does it mean? Movement Disorders. 2002;17:568–575. doi: 10.1002/mds.10089. [DOI] [PubMed] [Google Scholar]
- Kim YV, Di Cello F, Hillaire CS, Kim KS. Differential Ca2+ signaling by thrombin and protease-activated receptor-1-activating peptide in human brain microvascular endothelial cells. American Journal of Physiology-Cell Physiology. 2004;286:C31–C42. doi: 10.1152/ajpcell.00157.2003. [DOI] [PubMed] [Google Scholar]
- Kitzberger R, Madl C, Ferenci P. Wilson disease. Metab Brain Dis. 2005;20:295–302. doi: 10.1007/s11011-005-7910-8. [DOI] [PubMed] [Google Scholar]
- Klos KJ, Ahlskog JE, Josephs KA, Fealey RD, Cowl CT, Kumar N. Neurologic spectrum of chronic liver failure and basal ganglia T1 hyperintensity on magnetic resonance imaging: probable manganese neurotoxicity. Archives of neurology. 2005;62:1385–1390. doi: 10.1001/archneur.62.9.1385. [DOI] [PubMed] [Google Scholar]
- Koller WC, Lyons KE, Truly W. Effect of levodopa treatment for parkinsonism in welders: A double-blind study. Neurology. 2004;62:730–733. doi: 10.1212/01.wnl.0000113726.34734.15. [DOI] [PubMed] [Google Scholar]
- Kong SMY, Chan BKK, Park JS, et al. Parkinson's disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes α-Synuclein externalization via exosomes. Human Molecular Genetics. 2014;23:2816–2833. doi: 10.1093/hmg/ddu099. [DOI] [PubMed] [Google Scholar]
- La Fontaine S, Mercer JF. Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis. Arch Biochem Biophys. 2007;463:149–167. doi: 10.1016/j.abb.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Lechpammer M, Clegg MS, Muzar Z, Huebner PA, Jin LW, Gospe SM., Jr Pathology of inherited manganese transporter deficiency. Ann Neurol. 2014;75:608–612. doi: 10.1002/ana.24131. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- Leitch S, Feng M, Muend S, Braiterman LT, Hubbard AL, Rao R. Vesicular distribution of Secretory Pathway Ca(2)+-ATPase isoform 1 and a role in manganese detoxification in liver-derived polarized cells. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2011;24:159–170. doi: 10.1007/s10534-010-9384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva-Illades D, Chen P, Zogzas CE, et al. SLC30A10 Is a Cell Surface-Localized Manganese Efflux Transporter, and Parkinsonism-Causing Mutations Block Its Intracellular Trafficking and Efflux Activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:14079–14095. doi: 10.1523/JNEUROSCI.2329-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proceedings of the National Academy of Sciences. 2006;103:13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–172. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- Lockman PR, Roder KE, Allen DD. Inhibition of the rat blood–brain barrier choline transporter by manganese chloride. Journal of Neurochemistry. 2001;79:588–594. doi: 10.1046/j.1471-4159.2001.00589.x. [DOI] [PubMed] [Google Scholar]
- Lucaciu CM, Dragu C, Copaescu L, Morariu VV. Manganese transport through human erythrocyte membranes. An EPR study. Biochim Biophys Acta. 1997;1328:90–98. doi: 10.1016/s0005-2736(97)00039-4. [DOI] [PubMed] [Google Scholar]
- Lucchini RG, Guazzetti S, Zoni S, et al. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology. 2012;33:687–696. doi: 10.1016/j.neuro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madejczyk MS, Ballatori N. The iron transporter ferroportin can also function as a manganese exporter. Biochim Biophys Acta. 2012a;1818:651–657. doi: 10.1016/j.bbamem.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madejczyk MS, Ballatori N. The iron transporter ferroportin can also function as a manganese exporter. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2012b;1818:651–657. doi: 10.1016/j.bbamem.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MJ, Mayer B, Hymel L. Inhibition of Ca2+ transport pathways in thymic lymphocytes by econazole, miconazole, and SKF 96365. American Journal of Physiology-Cell Physiology. 1993;264:C654–C662. doi: 10.1152/ajpcell.1993.264.3.C654. [DOI] [PubMed] [Google Scholar]
- McKie AT, Marciani P, Rolfs A, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- Moos T, Morgan E. Transferrin and Transferrin Receptor Function in Brain Barrier Systems. Cellular and Molecular Neurobiology. 2000;20:77–95. doi: 10.1023/A:1006948027674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Bachert C, Smith DR, Linstedt AD. Manganese-induced trafficking and turnover of the cis-Golgi glycoprotein GPP130. Molecular biology of the cell. 2010;21:1282–1292. doi: 10.1091/mbc.E09-11-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Linstedt AD. Identification of a gain-of-function mutation in a Golgi P-type ATPase that enhances Mn2+ efflux and protects against toxicity. Proc Natl Acad Sci U S A. 2011;108:858–863. doi: 10.1073/pnas.1013642108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- Park RM. Neurobehavioral deficits and parkinsonism in occupations with manganese exposure: a review of methodological issues in the epidemiological literature. Safety and health at work. 2013;4:123–135. doi: 10.1016/j.shaw.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Peng L, Stevenson FF, Doctrow SR, Andersen JK. Iron and Paraquat as Synergistic Environmental Risk Factors in Sporadic Parkinson's Disease Accelerate Age-Related Neurodegeneration. The Journal of Neuroscience. 2007;27:6914–6922. doi: 10.1523/JNEUROSCI.1569-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Stevenson FF, Oo ML, Andersen JK. Iron-enhanced paraquat-mediated dopaminergic cell death due to increased oxidative stress as a consequence of microglial activation. Free Radical Biology and Medicine. 2009;46:312–320. doi: 10.1016/j.freeradbiomed.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M, Federico A, Zhao T, et al. Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. American journal of human genetics. 2012;90:467–477. doi: 10.1016/j.ajhg.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS. Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology. 2001;56:8–13. doi: 10.1212/wnl.56.1.8. [DOI] [PubMed] [Google Scholar]
- Reddi AR, Jensen LT, Naranuntarat A, Rosenfeld L, Leung E, Shah R, Culotta VC. The overlapping roles of manganese and Cu/Zn SOD in oxidative stress protection. Free Radic Biol Med. 2009;46:154–162. doi: 10.1016/j.freeradbiomed.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JA, Horbinski C, Higgins D, Lein P, Garrick MD. Mechanisms of Manganese-Induced Rat Pheochromocytoma (PC12) Cell Death and Cell Differentiation. NeuroToxicology. 2002;23:147–157. doi: 10.1016/s0161-813x(01)00077-8. [DOI] [PubMed] [Google Scholar]
- Salazar J, Mena N, Hunot S, et al. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson's disease. Proceedings of the National Academy of Sciences. 2008;105:18578–18583. doi: 10.1073/pnas.0804373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikk K, Haldre S, Aquilonius SM, et al. Manganese-induced parkinsonism in methcathinone abusers: bio-markers of exposure and follow-up. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2013;20:915–920. doi: 10.1111/ene.12088. [DOI] [PubMed] [Google Scholar]
- Smith EA, Newland P, Bestwick KG, Ahmed N. Increased whole blood manganese concentrations observed in children with iron deficiency anaemia. Journal of trace elements in medicine and biology : organ of the Society for Minerals and Trace Elements. 2013;27:65–69. doi: 10.1016/j.jtemb.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Sriram K, Lin G, Jefferson A, Roberts J, Chapman R, Chen B, Soukup J, Ghio A, Antonini J. Dopaminergic neurotoxicity following pulmonary exposure to manganese-containing welding fumes. Arch Toxicol. 2010;84:521–540. doi: 10.1007/s00204-010-0525-9. [DOI] [PubMed] [Google Scholar]
- Subramaniam VN, Summerville L, Wallace D. Molecular and cellular characterization of transferrin receptor 2. Cell Biochem Biophys. 2002;36:235–239. doi: 10.1385/CBB:36:2-3:235. [DOI] [PubMed] [Google Scholar]
- Tan J, Zhang T, Jiang L, Chi J, Hu D, Pan Q, Wang D, Zhang Z. Regulation of intracellular manganese homeostasis by Kufor-Rakeb syndrome-associated ATP13A2 protein. J Biol Chem. 2011;286:29654–29662. doi: 10.1074/jbc.M111.233874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton VK, Mandal D, Vahadji C, Rao R. Functional expression in yeast of the human secretory pathway Ca(2+), Mn(2+)-ATPase defective in Hailey-Hailey disease. The Journal of biological chemistry. 2002;277:6422–6427. doi: 10.1074/jbc.M110612200. [DOI] [PubMed] [Google Scholar]
- Traiffort E, Ruat M, O'Regan S, Meunier FM. Molecular characterization of the family of choline transporter-like proteins and their splice variants. Journal of Neurochemistry. 2005;92:1116–1125. doi: 10.1111/j.1471-4159.2004.02962.x. [DOI] [PubMed] [Google Scholar]
- Troadec MB, Ward DM, Lo E, Kaplan J, De Domenico I. Induction of FPN1 transcription by MTF-1 reveals a role for ferroportin in transition metal efflux. Blood. 2010;116:4657–4664. doi: 10.1182/blood-2010-04-278614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemi T, Krainc D. Zn2+ dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation. Human Molecular Genetics. 2014;23:2791–2801. doi: 10.1093/hmg/ddt572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuschl K, Clayton PT, Gospe SM, Jr, et al. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. American journal of human genetics. 2012;90:457–466. doi: 10.1016/j.ajhg.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuschl K, Mills PB, Clayton PT. Manganese and the brain. International review of neurobiology. 2013;110:277–312. doi: 10.1016/B978-0-12-410502-7.00013-2. [DOI] [PubMed] [Google Scholar]
- Tuschl K, Mills PB, Parsons H, Malone M, Fowler D, Bitner-Glindzicz M, Clayton PT. Hepatic cirrhosis, dystonia, polycythaemia and hypermanganesaemia--a new metabolic disorder. J Inherit Metab Dis. 2008;31:151–163. doi: 10.1007/s10545-008-0813-1. [DOI] [PubMed] [Google Scholar]
- Vidal S, Belouchi AM, Cellier M, Beatty B, Gros P. Cloning and characterization of a second human NRAMP gene on chromosome 12q13. Mammalian Genome. 1995;6:224–230. doi: 10.1007/BF00352405. [DOI] [PubMed] [Google Scholar]
- Wedler FC, Denman RB, Roby WG. Glutamine synthetase from ovine brain is a manganese(II) enzyme. Biochemistry. 1982;21:6389–6396. doi: 10.1021/bi00268a011. [DOI] [PubMed] [Google Scholar]
- Williams K, Wilson MA, Bressler J. Regulation and developmental expression of the divalent metal-ion transporter in the rat brain. Cellular and molecular biology (Noisy-le-Grand, France) 2000;46:563–571. [PubMed] [Google Scholar]
- Wu LJc, Leenders AGM, Cooperman S, et al. Expression of the iron transporter ferroportin in synaptic vesicles and the blood–brain barrier. Brain Research. 2004;1001:108–117. doi: 10.1016/j.brainres.2003.10.066. [DOI] [PubMed] [Google Scholar]
- Xiong L, Dion P, Montplaisir J, et al. Molecular genetic studies of DMT1 on 12q in French-Canadian restless legs syndrome patients and families. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144B:911–917. doi: 10.1002/ajmg.b.30528. [DOI] [PubMed] [Google Scholar]
- Yin Z, Jiang H, Lee ES, Ni M, Erikson KM, Milatovic D, Bowman AB, Aschner M. Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation. J Neurochem. 2010;112:1190–1198. doi: 10.1111/j.1471-4159.2009.06534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeron HM, Rodriguez MR, Montes S, Castaneda CR. Blood manganese levels in patients with hepatic encephalopathy. Journal of trace elements in medicine and biology : organ of the Society for Minerals and Trace Elements. 2011;25:225–229. doi: 10.1016/j.jtemb.2011.07.003. [DOI] [PubMed] [Google Scholar]


