Abstract
Aims
To develop a clinical cardiac risk algorithm for stable patients with suspected CAD based upon angina typicality and CAD risk factors.
Methods and Results
Between 2004 and 2011, 14,004 adults with suspected CAD referred for cardiac imaging were followed: 1) 9,093 patients for CCTA (CCTA-1) followed for 2.0 years; 2) 2,132 patients for CCTA (CCTA-2) followed for 1·6 years, and 3) 2,779 patients for exercise myocardial perfusion scintigraphy followed for 5.0 years. A best-fit model from CCTA-1 for prediction of death or myocardial infarction (MI) was developed, with integer values proportional to regression coefficients. Discrimination was assessed using C-statistic. The validated model was also tested for estimation of the likelihood of obstructive CAD, defined as ≥50% stenosis, as compared to method of Diamond and Forrester (D-F).
Primary outcomes included all-cause mortality and non-fatal MI. Secondary outcomes included prevalence of angiographically obstructive CAD. In CCTA-1, best-fit model discriminated individuals at risk of death or MI (C-statistic 0·76). The integer model ranged from 3-13, and corresponded to 3-year death risk or MI of 0·25% to 53·8%. When applied to the CCTA-2 and MPS, the model demonstrated C-statistics of 0·71 and 0·77. Both best-fit (C=0·76, 95% CI 0·746-0·771) and integer model (C=0·71, 95% CI 0·693-0·719) performed better than D-F (C=0·64; 95% CI, 0·628-0·659) for estimating obstructive CAD.
Conclusions
For stable symptomatic patients with suspected CAD, we developed a history-based method for prediction of death and obstructive CAD.
Keywords: Coronary artery disease, prognosis, diagnosis
INTRODUCTION
Medical history-based assessment of symptomatic stable patients with suspected coronary artery disease (CAD) has relied upon estimating the likelihood of obstructive CAD rather than the risk of clinical events, such as death or myocardial infarction (MI) (1-5). Recent data have challenged these diagnostic algorithms, and suggest that they overestimate the prevalence of obstructive CAD in patients referred for non-invasive and invasive testing (6-8). The absence of a well-validated pre-test method for determining risk of CAD events, as well as an accurate method for estimating the likelihood of obstructive CAD, may evoke overutilization of testing for individuals at low risk for incident clinical events or obstructive CAD (9). We sought to determine whether information acquired through medical history taking alone could predict risk of myocardial infarction or death and obstructive CAD in symptomatic stable individuals with suspected CAD.
METHODS
Study Participants
This study consisted of 3 distinct non-overlapping cohorts of symptomatic stable individuals with suspected but without prior history of CAD who were referred for non-invasive cardiac imaging between 2004-2011: 1) a development cohort of 9,093 patients from 8 centers and 6 countries referred for coronary CT angiography (CCTA-1) followed for death or MI; 2) a CCTA validation cohort of 2,132 patients from 4 centers and 4 countries referred for CCTA (CCTA-2) followed for death or MI; and 3) a validation cohort of 2,779 patients from 1 center referred for exercise stress myocardial perfusion scintigraphy (MPS) followed for death.
The CCTA-1 derivation cohort comprised patients referred for CCTA from Phase I of the CONFIRM registry (Coronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry), which has been previously described (10). Among 12 participating sites, 4 were excluded due to lack of information related to symptoms or follow-up data for non-fatal MI, leaving 17,226 patients from 8 sites in 6 countries that constituted the derivation dataset for the clinical model. Sites included 3 from the United States (N=5727), and 1 each from Canada (N=2171), Germany (N=1785), Italy (N=1895), South Korea (N=4912), and Switzerland (N=736). In sequential order, we excluded patients with prior coronary revascularization or MI (n=1110); who were asymptomatic (n=4389); who did not have complete symptom and/or CAD risk factor collection (n=1959); who were referred for imaging from the emergency department or inpatient setting (n=604); or who were lost to follow-up (n=71). After exclusions, 9,093 patients comprised the final study dataset. The median follow-up time for CCTA-1 was 2.0 years (Interquartile Range [IQR] = 1.4 to 3.1 years).
The CCTA-2 validation cohort consisted of patients from Phase II of CONFIRM, detailing identical elements to Phase I CONFIRM and prospectively designed specifically to serve as a validation dataset to Phase I CONFIRM findings (10). Among the 5 participating sites, 1 was excluded due to lack of follow-up information for MI, resulting in 3,996 patients from the United States (N=1016), Canada (N=636), South Korea (N=1404) and Austria (N=940). In sequential order, we excluded patients with prior coronary revascularization or MI (n=250); who were asymptomatic (n=1072); who did not have complete symptom and/or CAD risk factor collection (n=542); who were referred for imaging from the emergency department or inpatient setting (n=0); or who were lost to follow-up (n=0). 2,132 patients comprised the dataset, and are herein referred to as CCTA-2. The median follow-up time for CCTA-2 was 1·6 years (IQR = 0·8 to 2·8 years).
The MPS validation cohort was referred for exercise myocardial perfusion scintigraphy (MPS) at the Cedars-Sinai Medical Center and followed for all-cause mortality. Excluded patients include those undergoing prior coronary revascularization or MI or who underwent pharmacologic stress testing (n=6405); who were asymptomatic (n=1095); who did not have complete symptom and/or CAD risk factor collection (n=425); who were referred for imaging from the emergency department or inpatient hospital setting (n=1903); or who were lost to follow-up (n=0). 2,779 comprised the final study dataset and are herein referred to as the MPS validation cohort. The median follow-up time for MPS was 5·0 years (IQR 3·5 to 6·4 years).
Coronary Artery Disease Risk Factor and Symptom Type
Prior to testing, we performed medical histories to ascertain the presence of categorical CAD risk factors, which were defined in accordance to accepted guidelines. Hypertension was defined by a history of blood pressure of ≥140/90 mmHg and/or treatment with anti-hypertensive medications (11). Diabetes mellitus was defined by a fasting glucose of 126 mg/dl or greater and/or use of insulin or oral hypoglycemic agents (12). Dyslipidemia was defined in accordance with National Cholesterol Education Program Adult Treatment Panel III guidelines or by treatment with lipid lowering medication (13). Current smoking status was defined by active smoking within 3 months of presentation. Family history of CAD was defined as MI or cardiac death in a first-degree relative (14).
Symptoms were ascertained through interview by a physician or health professional, or by written questionnaire. Chest pain was categorized according to criteria for angina pectoris (3, 15). Patients with typical angina experienced substernal jaw, and/or arm pressure-like pain that occurred with exertion and resolved within 15 minutes of rest and/or use of nitroglycerin. Patients with atypical angina experienced 2 of these characteristics. Patients with non-anginal chest pain experienced 1 or none of these characteristics. Dyspneic patients without chest pain were categorized as having typical angina, in accordance to their prognostic risk (16). From these data, the pre-test likelihood of obstructive CAD was calculated by the method of Diamond and Forrester (3).
Follow-up and Event Ascertainment
All study individuals for CCTA-1 and CCTA-2 were followed for an endpoint of death or non-fatal myocardial infarction, and for an endpoint of death for MPS. Follow-up procedures were approved by all study centers’ institutional review boards. Death status for non-US centers was gathered by clinical visits, telephone contacts and questionnaires sent by mail; with verification of all reported events by hospital records or direct contact with a patient’s attending physician. Death status for US centers was ascertained by query of the Social Security Death Index or by scripted in-person or phone interview by an experienced physician and/or nurse study investigator.
General Statistical Analyses
Categorical variables are displayed as frequencies and percents, while, continuous variables are described as mean ± standard deviations, or medians with interquartile ranges. Variables were compared with chi-squared statistic for categorical variables and by Student’s unpaired t-test or Wilcoxon non-parametric test where appropriate for continuous variables. A two-tailed p value <0.05 was considered statistically significant. Analyses were conducted using SAS version 9.2 (SAS Institute, www.sas.com, Cary, NC).
Development of a Medical History-Based Model for Adverse Clinical Events
Information from the CCTA-1 cohort was used to develop a clinical prediction model for death or MI (CCTA-1) or death (MPS) based on CAD risk factors and symptom type. The best overall clinical prediction algorithm was fit using Cox proportional hazards models. Eight variables were evaluated for risk prediction, and were restricted to those that could be easily obtained from a standard cardiac medical history: age, gender, diabetes, hypertension, dyslipidemia, family history of CAD, current smoking status and symptom type. Symptom type was categorized dichotomously in accordance to prognostic relevance for individuals referred for non-invasive imaging as typical angina or dyspnea versus atypical angina or non-anginal pain (16). The final best-fit Cox model was selected by applying backwards stepwise regression, examining the - 2loglikelihood, and minimizing Bayes’ Information Criterion (BIC) (17). Interactions between age and all risk factors were examined.
Once variables were selected for the final Cox model, an integer-based model was developed to predict the 3-year probability of death or MI. The integer-based model was created by transformation of the Cox regression coefficients from the best-fit model. To maximize ease of use, risk score values were scaled and transposed such that age points related to the decade of a patient’s age (e.g., a 5 for patients aged 50-59) and each CAD risk factor present added an additional point to the total score.
Validation of the Medical History-Based Model
External validation was conducted for the best-fit model using the CCTA-2 and MPS cohorts. For comparison, a best-fit Cox regression model was constructed based on the same candidate variables as in the CCTA-1 model but using data from the validation cohorts. Similar to the CCTA-1 model, backwards stepwise regression was used to create the final best-fit model. For MPS, we adjusted the predicted risk based upon ratio of risk of death to risk of death or MI from the CCTA-1 best-fit model. The accuracy of the CCTA-1 best-fit model was examined using discrimination and calibration. Discrimination was evaluated by C-statistic, while calibration was described by Nam and D’Agostino’s modification of the Hosmer-Lemeshow goodness of fit procedures (18). We further examined discrimination and calibration of the integer-based model (Table 1). Observed versus predicted risk was computed based on categories defined by deciles of predicted risk, and compared by plotting predicted and actual event rates within each decile.
Table 1.
| Risk Factor | Categories | Points |
|---|---|---|
| Age | ||
| 18-39 | 3 | |
| 40-49 | 4 | |
| 50-59 | 5 | |
| 60-69 | 6 | |
| Greater than 70 | 7 | |
| Sex | ||
| Male | 1 | |
| Female | 0 | |
| Symptom | ||
| Non-Typical | 0 | |
| Typical | 1 | |
| Diabetes | ||
| Non-Diabetic | 0 | |
| Diabetic | 1 | |
| Hypertension | ||
| Normotensive | 0 | |
| Hypertensive | 1 | |
| Family History of CAD | ||
| No Family | ||
| History | 0 | |
| Family History | 1 | |
| Current Smoking | ||
| Non-Smoker | 0 | |
| Current Smoker | 1 |
Discrimination of the Medical History-Based Model for Obstructive Coronary Artery Disease
We evaluated the ability of the integer-based risk model to discriminate between individuals with and without obstructive CAD, as defined by a ≥50% luminal diameter stenosis in any coronary artery ≥1·5 mm in diameter. Obstructive CAD was determined from CCTAs from the CCTA-1 and CCTA-2 cohorts, but was not available within the MPS validation cohort. CCTAs were performed and interpreted by Level III experts in an intent-to-diagnose fashion (19, 20).
We applied the integer-based model from CCTA-1 to estimate the probability of obstructive CAD in CCTA-2. For obstructive CAD, the area under the receiver operator characteristics curve of the integer-based model was directly compared to the method of Diamond and Forrester. Further, we used the aggregate data from CCTA-1 and CCTA-2 to determine the rate of obstructive CAD by integer values from the developed model.
RESULTS
Clinical Characteristics of the Study Cohorts
Baseline characteristics of the CCTA-1 development and CCTA-2 and MPS validation cohorts are listed in Table 2. During follow-up, 65 deaths and 155 MIs occurred in the CCTA-1 cohort, while 14 deaths and 90 MIs occurred in the CCTA-2 validation cohort. In the MPS validation cohort, 51 deaths occurred during follow-up.
Table 2. Baseline characteristics of the study groups.
| Characteristic | CCTA-1 | CCTA-2 | P-value (CCTA-1 vs. CCTA-2) | MPS | P-value (CCTA-1 vs. MPS) |
|---|---|---|---|---|---|
|
| |||||
| N | 9093 | 2132 | - | 2779 | |
|
| |||||
| Age, mean 26 [years] | 56·5 (11·9) | 59·3 (12·3) | <0·0001 | 55·9 (11·6) | 0·0098 |
|
| |||||
| Male Sex | 50·0% | 53·9% | 0·0012 | 54·8% | <0·0001 |
|
| |||||
| Classic Symptoms | 41·5% | 52·7% | <0·0001 | 28·5% | <0·0001 |
|
| |||||
| Diabetes | 13·5% | 34·2% | <0·0001 | 10·2% | <0·0001 |
|
| |||||
| Hypertension | 49·3% | 60·5% | <0·0001 | 48·3% | 0·3289 |
|
| |||||
| Dyslipidemia | 55·8% | 41·7% | <0·0001 | 56·2% | 0·7156 |
|
| |||||
| Family History | 31·3% | 30·8% | 0·5994 | 17·7% | <0·0001 |
|
| |||||
| Current Smoker | 16·1% | 21·7% | <0·0001 | 5·6% | <0·0001 |
|
| |||||
| Median follow-up (IQR) | 2·0 (1·4-3·1) | 1·6 (0·8-2·8) | <0·0001 | 5·0 (3·5-6·4) | <0·0001 |
|
| |||||
| D-F Pre-test LK | |||||
| Low (<15) | 22·8% | 12·9% | <0·0001 | 8·9% | <0·0001 |
| Intermediate (15-85) | 66·5% | 60·2% | 85·1% | ||
| High (>85) | 10·7% | 26·9% | 6·0% | ||
Clinical Prediction Model Derivation
In univariate Cox regression applied to CCTA-1, several CAD risk factors and symptom type predicted risk of death or MI (Appendix). Despite its lack of significance in univariate models, gender was forced into multivariate models given its accepted clinical importance. As no significant interactions were identified, the final best-fit Cox model for prediction of death or MI included age, gender, symptom type, diabetes, hypertension, family history of CAD and smoking status. The BIC value for the best-fit Cox model was 3554·4, which represented a substantial improvement over the null model BIC of 3716.6. The β coefficients, standard errors, hazard ratios and p-values for each of the covariates in the best-fit Cox model are shown in Table 3. The C-statistic for this model was 0·76.
Table 3. Multivariable Cox Proportional Hazards Models for Prediction of 3-Year Risk of Death or Myocardial Infarction (3-year survival = 0.9711).
| Multivariate Best-Fit Cox Model | |||||
|---|---|---|---|---|---|
| Parameter | Effect | β | HR (95% CI) | SE | P value |
| Age | Age in Years | 0.06590 | 1.07 (1.05-1.08) | 0.007 | <0.0001 |
| Sex | Male vs. Female | 0.38154 | 1.46 (1.11-1.93) | 0.140 | 0.0065 |
| Symptom | Typical vs. Non-Typical | 0.51894 | 1.68 (1.28-2.21) | 0.141 | 0.0002 |
| Diabetes | Yes vs. No | 0.33708 | 1.40 (1.01-1.94) | 0.165 | 0.0412 |
| Hypertension | Yes vs. No | 0.4861 | 1.63 (1.21-2.18) | 0.150 | 0.0012 |
| Family history of CAD | Yes vs. No | 0.51748 | 1.68 (1.26-2.23) | 0.145 | 0.0004 |
| Smoking status | Current vs. Not Current | 0.56197 | 1.75 (1.25-2.45) | 0.171 | 0.0010 |
Discrimination and Calibration of the Medical History-Based Prediction Model
Table 4 presents the C-statistics for the best-fit and integer-based Cox model for the CCTA-1, CCTA-2 and MPS validation cohorts. For MPS, for which only death was available during follow-up, probabilities were determined by multiplication of the risk of death or MI by 0·405, which represented the ratio of the 3-year risk of death (1·17%) to the 3-year risk of death or MI (2·89%) in CCTA-1. Discrimination of the best-fit model performed well in both CCTA-2 and MPS, achieving similar discrimination to the CCTA-1 best-fit model. Figure 1 presents the decile plots for assessment of calibration of the CCTA-1 model. Integer models were based upon covariates categorized in binary fashion except for age, which was categorized by range as 18-39 years, 40-49 years, 50-59 years, 60-69 years, and ≥70 years (Table 1).
Table 4. C-Statistic of Prediction Models for 3-Year Risk of Death or Myocardial Infarction.
| CCTA-1 Derivation Cohort | CCTA-2 Validation Cohort | MPS Validation Cohort* | |
|---|---|---|---|
| Best-fit Cox Model | 0.76 (0.758, 0.762) | 0.71 (0.707, 0.713) | 0.77 (0.766, 0.774) |
| Integer-Based Model | 0.73 (0.729, 0.733) | 0.66 (0.659, 0.666) | 0.76 (0.756, 0.764) |
Risk of death alone
Figure 1.
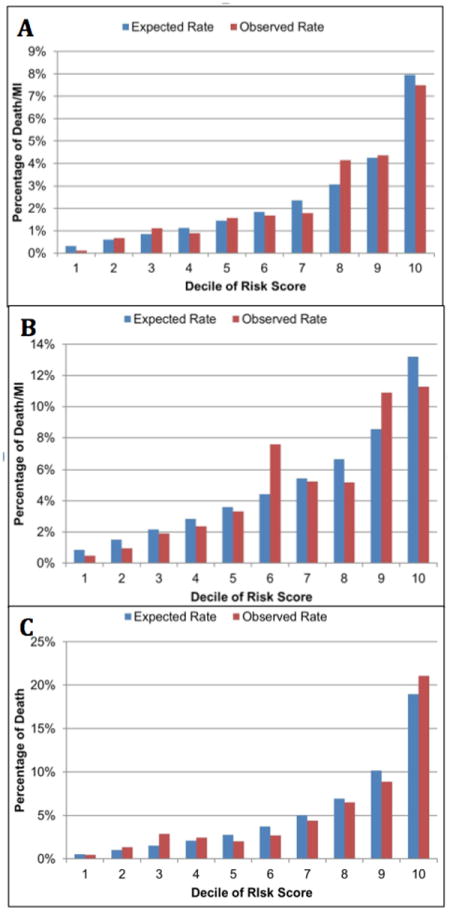
Calibration Plots for (A) CCTA-1 Development, (B) CCTA-2 Validation and (C) MPS Validation Cohort
Integer scores were calculated by the following formula: Total points = [Age 40-49 years = 4; 50-59 years = 5; 60-69 years = 6; ≥70 years = 7) + Gender (1=male, 0=female) + Diabetes (1=diabetic; 0=non-diabetic) + Hypertension (1=hypertensive; 0=normotensive) + Family history of CAD (1=yes, 2=no).
Discrimination of the Medical History-Based Model for Estimating the Likelihood of Obstructive Coronary Artery Disease
The AUC of the best-fit CCTA-1 model (0·76; 95% CI 0·746 to 0·771) was higher than the Diamond-Forrester (0·64; 95% CI 0·628 to 0·659) methods for discriminating individuals with versus without obstructive CAD (p<0·0001). Across the range of integer values, the likelihood of obstructive CAD ranged from 0 to 82·4% (Table 5).
Table 5. 3-year risk of death or MI and likelihood of obstructive CAD (CCTA-1).
| Total Points | Risk of Death or MI over 3 years (%) | Likelihood of obstructive CAD |
|---|---|---|
| 3 | 0.25 | 0 |
| 4 | 0.46 | 1.4 |
| 5 | 0.76 | 3.4 |
| 6 | 1.26 | 5.5 |
| 7 | 2.53 | 13.2 |
| 8 | 4.53 | 21.3 |
| 9 | 8.03 | 31.0 |
| 10 | 15.13 | 43.2 |
| 11 | 23.29 | 52.5 |
| 12 | 34.95 | 82.4 |
| 13 | 53.81 |
Total Points are the range of integer-based model values. Prevalence of obstructive CAD was the observed rates of obstructive CAD for each value of the integer-based model within the CCTA-1 cohort. Risk score values equal the total of each CAD risk factor as a single point plus points related to the decade of a patient’s age (e.g., 5 for patients aged 50-59):fr
Total points = [Age 40-49 years = 4; 50-59 years = 5; 60-69 years = 6; ≥70 years = 7) + Gender (1=male, 0=female) + Diabetes (1=diabetic; 0=non-diabetic) + Hypertension (1=hypertensive; 0=normotensive) + Family history of CAD (1=yes, 2=no).
DISCUSSION
We developed and validated a method for identifying stable individuals with suspected CAD who are at risk of death or myocardial infarction. Our aim was to create a parsimonious model that incorporates CAD factors that can be derived solely from a medical history. We demonstrated the effectiveness of this method for measures of risk stratification, discrimination, and calibration. Further, the model exhibited superior discriminatory performance when tested against the Diamond-Forrester method for determining pre-test likelihood of obstructive CAD.
One potential function of this model may be to serve as an effective “gatekeeper” to identify individuals who are at sufficiently low risk of incident death or MI that the yield from further testing may be low. At present, more than 10 million CAD imaging tests are performed annually in the United States alone at high direct and indirect costs, with rates of test normalcy significantly higher today as compared to only a decade ago (9,21). As an example, in the largest CCTA study to date, more than 42% of individuals were identified as having no CAD (22). Similarly, the rates of test normalcy for MPS have dramatically increased over time, with a prevalence of >90% in a study of 39,515 patients (21).
In this study, those with an integer value ≤7—which represented nearly half of the study population—only 1·0% experienced an adverse clinical event, and only 10% of these individuals possessed obstructive CAD. These findings may convey a greater sense of prognostic and diagnostic certainty towards avoidance of testing for individuals whose risk and prevalence of disease is sufficiently low to preclude the need for further evaluation. Conversely, this model may be used to identify individuals whose risk is high, and who may benefit from further testing. While representing only 3% of the combined cohort, 9% of individuals with an integer score >9 experienced an event and 54% possessed obstructive CAD. The need for testing is apparent in this group, and in accordance with societal guidance documents (23).
To date, no robust medical history-based prediction model has been available for identifying stable symptomatic patients at risk for death or MI. In its absence, clinicians have relied upon estimates of the likelihood of an individual possessing obstructive CAD (3). These methods have served as the cornerstone for assessment of patients with suspected CAD for the last 40 years. Nevertheless, these methods were validated against invasive angiographic and pathologic correlates; and their application demonstrates a 3-fold overestimation of CAD in patients referred for non-invasive imaging that suggests the need for contemporary revision (6). In the present study, the developed medical history-based model was superior to the Diamond-Forrester method for discrimination of individuals with versus without obstructive anatomic CAD. In this regard, this model may serve a dual role not only as a prognostic instrument but also as a diagnostic tool.
This study is not without limitations. We studied patients referred for CCTA and exercise MPS—given their common performance and similarities for indications considered appropriate for use—with our study findings robustly applicable to both modalities (24,25). Yet, whether the model can be employed for patients being considered for exercise treadmill testing, other imaging tests, or in patients not considered for any testing remains unknown. Second, patient-reported histories may have resulted in ascertainment bias, and CAD risk factor profiles may have been more accurate with adjunctive confirmation by vital sign or laboratory values. One advantage of the present study is the applicability to practicing physicians who routinely solicit histories in the office setting. Third, the prediction model included all-cause rather than cardiac-specific death. In doing so, this study is disencumbered from information bias but may result in reduced prognostic specificity. Finally, we examined stable symptomatic individuals referred for outpatient imaging, and caution should be taken to apply the model to patients in the emergency department or inpatient setting.
CONCLUSIONS
We developed and validated a simple medical-history based method that predicts risk of death and myocardial infarction in symptomatic stable patients with suspected CAD. Further, this method exhibits superior performance to traditional methods for identifying individuals with obstructive CAD.
TRANSLATIONAL PERSPECTIVE.
A simple and easy-to-develop model derived solely from a patients’ medical history is useful in identifying stable individuals with suspected CAD who are at risk of death or myocardial infarction. This history-based model may potentially serve a useful purpose as an effective “gatekeeper” towards avoidance of testing for individuals whose risk and prevalence of disease is sufficiently low to preclude the need for further evaluation. Conversely, this model may be used to identify individuals whose risk is high, and who may benefit from further testing.
Acknowledgments
Financial support: Research reported in this publication was supported by the Heart Lung and Blood Institute of the National Institutes of Health under award number 1R01HL115150. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was supported by Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (MSIP) (2012027176). This study was also funded, in part, by a generous gift from the Dalio Institute of Cardiovascular Imaging (New York, NY) and the Michael Wolk Foundation (New York, NY). Dr. James Min discloses receiving research support from GE Healthcare and serving on the Speaker’s Bureau of GE Healthcare.
ABBREVIATIONS
- CAD
coronary artery disease
- MPS
myocardial perfusion scintigraphy
- MI
Myocardial infarction
- CCTA
coronary CT angiography
- D-F
Diamond and Forrester
- CONFIRM
Coronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry
- BIC
Bayes’ Information Criterion
References
- 1.Fraker TD, Fihn SD, Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, Ferguson TB, Jr, Fihn SD, Fraker TD, Jr, Gardin JM, O’Rourke RA, Williams SV, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW American College of Cardiology, American Heart Association, American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group. 2007 chronic angina focused update of the ACC/AHA 2002 Guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 Guidelines for the management of patients with chronic stable angina. Circulation. 2007;116:2762–2772. doi: 10.1161/CIRCULATIONAHA.107.187930. [DOI] [PubMed] [Google Scholar]
- 2.Chaitman BR, Bourassa MG, Davis K, Rogers WJ, Tyras DH, Berger R, Kennedy JW, Fisher L, Judkins MP, Mock MB, Killip T. Angiographic prevalence of high-risk coronary artery disease in patient subsets (CASS) Circulation. 1981;64:360–367. doi: 10.1161/01.cir.64.2.360. [DOI] [PubMed] [Google Scholar]
- 3.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350–1358. doi: 10.1056/NEJM197906143002402. [DOI] [PubMed] [Google Scholar]
- 4.Pryor DB, Harrell FE, Jr, Lee KL, Califf RM, Rosati RA. Estimating the likelihood of significant coronary artery disease. Am J Med. 1983;75:771–780. doi: 10.1016/0002-9343(83)90406-0. [DOI] [PubMed] [Google Scholar]
- 5.Pryor DB, Shaw L, Harrell FE, Jr, Lee KL, Hlatky MA, Mark DB, Muhlbaier LH, Califf RM. Estimating the likelihood of severe coronary artery disease. Am J Med. 1991;90:553–562. [PubMed] [Google Scholar]
- 6.Cheng VY, Berman DS, Rozanski A, Dunning AM, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Chinnaiyan K, Chow BJ, Delago A, Gomez M, Hadamitzky M, Hausleiter J, Karlsberg RP, Kaufmann P, Lin FY, Maffei E, Raff GL, Villines TC, Shaw LJ, Min JK. Performance of the traditional age, sex, and angina typicality-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: results from the multinational coronary CT angiography evaluation for clinical outcomes: an international multicenter registry (CONFIRM) Circulation. 2011;124:2423–2432. 2421–2428. doi: 10.1161/CIRCULATIONAHA.111.039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoilund-Carlsen PF, Johansen A, Vach W, Christensen HW, Moldrup M, Haghfelt T. High probability of disease in angina pectoris patients: is clinical estimation reliable? Can J Cardiol. 2007;23:641–647. doi: 10.1016/s0828-282x(07)70226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genders TS, Steyerberg EW, Alkadhi H, Leschka S, Desbiolles L, Nieman K, Galema TW, Meijboom WB, Mollet NR, de Feyter PJ, Cademartiri F, Maffei E, Dewey M, Zimmermann E, Laule M, Pugliese F, Barbagallo R, Sinitsyn V, Bogaert J, Goetschalckx K, Schoepf UJ, Rowe GW, Schuijf JD, Bax JJ, de Graaf FR, Knuuti J, Kajander S, van Mieghem CA, Meijs MF, Cramer MJ, Gopalan D, Feuchtner G, Friedrich G, Krestin GP, Hunink MG Consortium CAD. A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating, and extension. Eur Heart J. 2011;32:1316–1330. doi: 10.1093/eurheartj/ehr014. [DOI] [PubMed] [Google Scholar]
- 9.Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah MH, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan KM, Chow B, Delago A, Hadamitzky M, Hausleiter J, Karlsberg RP, Kaufmann P, Maffei E, Nasir K, Pencina MJ, Raff GL, Shaw LJ, Villines TC. Rationale and design of the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter) Registry. J Cardiovasc Comput Tomogr. 2011;5:84–92. doi: 10.1016/j.jcct.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ National Heart Lung, Blood Institute Joint National Committee on Prevention Detection, Evaluation, and Treatment of High Blood Pressure, National High Blood Pressure Education Program Coordinating Commitee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd-Jones DM, Nam BH, D’Agostino RB, Sr, Levy D, Murabito JM, Wang TJ, Wilson PW, O’Donnell CJ. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA. 2004;291:2204–2211. doi: 10.1001/jama.291.18.2204. [DOI] [PubMed] [Google Scholar]
- 15.Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, Ferguson TB, Jr, Fihn SD, Fraker TD, Jr, Gardin JM, O’Rourke RA, Pasternak RC, Williams SV, Gibbons RJ, Alpert JS, Antman EM, Hiratzka LF, Fuster V, Faxon DP, Gregoratos G, Jacobs AK, Smith SC, Jr American College of Cardiology, American Heart Association Task Force on Practice Guidelines. Committee on the Management of Patients With Chronic Stable Angina. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina) Circulation. 2003;107:149–158. doi: 10.1161/01.cir.0000047041.66447.29. [DOI] [PubMed] [Google Scholar]
- 16.Abidov A, Rozanski A, Hachamovitch R, Hayes SW, Aboul-Enein F, Cohen I, Friedman JD, Germano G, Berman DS. Prognostic significance of dyspnea in patients referred for cardiac stress testing. N Engl J Med. 2005;353:1889–1898. doi: 10.1056/NEJMoa042741. [DOI] [PubMed] [Google Scholar]
- 17.Harrell FE. Regression modeling strategies : with applications to linear models, logistic regression, and survival analysis. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- 18.D’Agostino RB, Nam B-H. Evaluation of the performance of survival analysis models: discrimination and calibration measures. New York, NY: Elsevier; 2003. [Google Scholar]
- 19.Abbara S, Arbab-Zadeh A, Callister TQ, Desai MY, Mamuya W, Thomson L, Weigold WG. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2009;3:190–204. doi: 10.1016/j.jcct.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Raff GL, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ, Cheng V, DeFrance T, Hellinger JC, Karlsberg RP Society of Cardiovascular Computed Tomography. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3:122–136. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Rozanski A, Gransar H, Hayes SW, Min J, Friedman JD, Thomson LE, Berman DS. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing: 1991 to 2009. J Am Coll Cardiol. 2013;61:1054–1065. doi: 10.1016/j.jacc.2012.11.056. [DOI] [PubMed] [Google Scholar]
- 22.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan K, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Kaufmann P, Maffei E, Raff G, Shaw LJ, Villines T, Berman DS CONFIRM Investigators. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–860. doi: 10.1016/j.jacc.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 23.Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, O’Gara PT, Carabello BA, Russell RO, Jr, Cerqueira MD, St John Sutton MG, DeMaria AN, Udelson JE, Kennedy JW, Verani MS, Williams KA, Antman EM, Smith SC, Jr, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Faxon DP, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO American College of Cardiology, American Heart Association, American Society of Nuclear Cardiology. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging) J Am Coll Cardiol. 2003;42:1318–1333. doi: 10.1016/j.jacc.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O’Gara P, Rubin GD, Kramer CM, Berman D, Brown A, Chaudhry FA, Cury RC, Desai MY, Einstein AJ, Gomes AS, Harrington R, Hoffmann U, Khare R, Lesser J, McGann C, Rosenberg A, Schwartz R, Shelton M, Smetana GW, Smith SC American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society of Cardiovascular Computed Tomography, American College of Radiology, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, North American Society for Cardiovascular Imagin, Society for Cardiovascular Angiography and Interventions, Society for Cardiovascular Magnetic Resonance. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56:1864–1894. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, Pohost GM, Williams KA American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Nuclear Cardiology, American College of Radiology, American Heart Association, American Society of Echocardiography, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, Society of Nuclear Medicine. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol. 2009;53:2201–2229. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Thiele H, Schuler G, Neumann FJ, Hausleiter J, Olbrich HG, Schwarz B, Hennersdorf M, Empen K, Fuernau G, Desch S, de Waha S, Eitel I, Hambrecht R, Bohm M, Kurowski V, Lauer B, Minden HH, Figulla HR, Braun-Dullaeus RC, Strasser RH, Rochor K, Maier SK, Mollmann H, Schneider S, Ebelt H, Werdan K, Zeymer U. Intraaortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock: design and rationale of the Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial. Am Heart J. 2012;163:938–945. doi: 10.1016/j.ahj.2012.03.012. [DOI] [PubMed] [Google Scholar]


