Abstract
OBJECTIVE
To evaluate cross-sectional and longitudinal associations between ankle-brachial index (ABI) and indicators of cognitive function
DESIGN
Randomized clinical trial (Lifestyle Interventions and Independence for Elders Trial)
SETTING
Eight US academic centers
PARTICIPANTS
1,601 adults (ages 70–89 years, sedentary, non-demented, and with functional limitations
MEASUREMENTS
Baseline ABI and interviewer- and computer-administered cognitive function assessments were obtained from which compared a physical activity intervention with a health education control. Cognitive function was re-assessed 24 months later (interviewer-administered) and 18 or 30 months later (computer-administered) and central adjudication was used to classify individuals as having mild cognitive impairment, probable dementia, or neither.
RESULTS
Lower ABI had a modest independent association poorer cognitive functioning at baseline (partial r=0.09; p<0.001). While, lower baseline ABI was not associated with overall changes in cognitive function test scores, it was associated with higher odds for two-year progression to a composite of either mild cognitive impairment or probable dementia (OR=2.60 per unit lower ABI; 95% confidence interval [1.06,6.37]). Across two years, changes in ABI were not associated with changes in cognitive function.
CONCLUSION
In an older cohort of non-demented sedentary individuals with functional limitations, lower baseline ABI was independently correlated with cognitive function and associated with greater 2-year risk for progression to mild cognitive impairment or probable dementia.
Keywords: Cognitive function, ankle-brachial index, peripheral artery disease, dementia
INTRODUCTION
In older adults, lower ankle-brachial index (ABI), a marker of peripheral artery disease, is correlated with poorer cognitive function.1,2 Most,3 but not all,4,5 longitudinal studies have found it to be associated with future cognitive decline and the development of dementia. Because low ABI is a general indicator of systemic atherosclerosis,5 these associations are thought to derive from the shared pathways and risk factors linking peripheral vascular disease with cerebrovascular disease6 and may be strongest among the oldest old due to longer-term exposures to vascular disease.7
Changes in ABI over time in most seniors are gradual, with more rapid declines reflecting the progression of lower extremity artery disease and greater risk factor burden for systemic vascular disease.8 Declines in ABI, therefore, should be associated with declining cognitive function, particularly in older adults who have many of the shared risk factors for vascular disease and cognitive impairment. If so, it may signal that approaches towards slowing or preventing declines in ABI may be promising strategies for preserving cognitive function.
This paper describes results for three specific aims. First, within a randomized trial of a physical activity intervention in individuals with compromised physical function who were aged 70–89 years at enrollment, we report cross-sectional associations between ABI and cognitive function, seeking to confirm that associations seen in other cohorts exist among this ambulatory, though functionally limited and sedentary, cohort of older adults. Second, we describe the extent to which baseline ABI was associated with changes in cognitive function and the incidence of mild cognitive impairment and probable dementia over two years. Finally, we examine whether changes in ABI were associated with changes in cognitive function.
METHODS
The Lifestyle Interventions and Independence for Elders (LIFE) study was an eight-center randomized controlled trial comparing a physical activity intervention with a successful aging health education intervention featuring a series of didactic presentations and related activities.9,10 Inclusion criteria were used to identify a subset of the older population that is non-disabled, at risk for mobility loss, and may benefit from an intervention to prevent disability. Participants were community-dwelling (those currently living in nursing facilities were not eligible for participation), aged 70–89 years old, and met the following inclusion criteria: functional impairment as demonstrated by summary score <10 on the short physical performance battery (SPPB),11,12 a sedentary lifestyle (spending less than 20 minutes per week in regular physical activity), ability to walk 400 meters within 15 minutes without sitting or help from another person or the use of a walker, and scores on the Modified MiniMental State Exam (3MSE) test of global cognitive function13 exceeding cutpoints based on education, language, and race/ethnicity chosen to rule out dementia.
The physical activity intervention focused on walking, strength, flexibility, and balance training through two center-based visits per week and home-based physical activity 3–4 times per week. Center-based sessions were individualized and progressed towards a goal of 30 minutes of walking at moderate intensity, 10 minutes of primarily lower extremity strength training by means of ankle weights, 10 minutes of balance training, and large muscle group flexibility exercises. The successful aging control group attended weekly workshops of health education during the first 26 weeks of the intervention and then monthly sessions thereafter (semi-monthly attendance was optional). The study protocol was approved by the institutional review boards at all participating sites.
Ankle brachial index
The ABI was measured after the participant rested supine for five minutes, using a hand-held Doppler probe to obtain SBP at the right brachial artery, right posterior tibial artery, left posterior tibial artery, and left brachial artery in the order listed.14,15 Measurements were then repeated in reverse order. If absolute differences between replicated measures exceeded 50 mmHg, the blood pressure measurements recorded from that site were excluded from analyses. The ABI was calculated for each leg by averaging the two posterior tibial artery pressures and dividing them by the average of the four brachial artery pressures. However, when one brachial artery pressure was higher than the alternate brachial artery pressure in both measurement sets, and the difference in the right and left brachial artery pressures differed by at least 10 mmHg in both measurement sets, subclavian stenosis was possible and the average brachial artery pressures from the arm with highest pressure were included in the analyses.16
Interviewer-based cognitive testing
The LIFE study included three interviewer-administered cognitive tests that were completed at baseline and 24 months.17 The 3MSE measures global cognitive functioning, with higher scores (range 0 to 100) reflecting better performance. Items include temporal and spatial orientation, immediate and delayed recall, executive function, naming, verbal fluency, abstract reasoning, praxis, writing, and visuo-constructional abilities. The Digit Symbol Coding (DSC) test measures attention and perceptual speed: participants were given a series of numbered symbols and asked to draw the appropriate symbols below a list of random numbers.18 The score is the number of correct matches in 2 minutes. The Hopkins Verbal Learning Test (HVLT) measures episodic memory.19 Participants listened to a list of 12 words and were asked to recall as many as possible. The task was repeated twice, for a total of three trials (immediate recall). Approximately 20 minutes later the participant was asked to recall as many words as possible (delayed recall).
Computer-based cognitive testing
The LIFE computer-based cognitive testing battery, which assessed memory and executive functioning, was administered at baseline and post-randomization (at either 18 or 30 months, depending on randomization timeframe).20 The n-back test measures working memory:22 participants are presented with letters at a 2-second rate on a computer screen and are asked to indicate whether the presented letter is the same as the nth back letter, with n equal to 1 and 2. The Eriksen flanker task measures selective attention and response inhibition:22 participants are presented with an arrow facing either right or left and are asked to press a key to indicate its direction. The target displays are neutral (no flankers), congruent (flanker arrows point in the same direction as the target arrow), or incongruent (the flanker arrows point in the opposite direction). Shorter reaction times under the congruent condition and the incongruent condition (which includes response inhibition) reflect better performance. The Task Switching test measures attentional flexibility:23,24 participants are asked to quickly alternate between performing two different tasks, which requires executive function to reconfigure the cognitive system each time the task demands shift. They are shown single digit numbers and asked to determine if they are odd or even. This is alternated with presentation of single letters, for which participants indicate whether the letter was a consonant or vowel. Shorter reaction times under the no-switch condition and switch condition (which includes switch costs, a measure of executive function) reflect better performance.
Adjudication of mild cognitive impairment and probable dementia
A composite of mild cognitive impairment or probable dementia was an exploratory outcome of the LIFE trial.17 Consensus-based adjudication by experts in the diagnosis of cognitive impairment was used to classify participants at baseline and 24 months as having no impairment, mild cognitive impairment, or probable dementia using standardized protocols based on modified 2011 NIA-Alzheimer's Association clinical consensus criteria.25,26
Cohort characteristics
Demographic data and smoking and diabetes status were collected by self-report. Hypertension was based on self-report or measurement. The SPPB includes timed measures of standing balance, walking speed, and repeated chair stands. A summary score (range 0–12) orders individuals from lowest to highest performance.
Statistical analyses
Cross-sectional associations between baseline ABI and cognitive function scores were assessed with analyses of variance, analyses of covariance, and regression, and those between baseline ABI and subsequent changes in cognitive function with partial correlation coefficients. Initially, quadratic regression was used to introduce curvature in relationships, however, because none of the second degree terms reached p<0.05, linear models were used for inference. Associations with incident mild cognitive impairment or probable dementia were assessed with logistic regression and those between changes in ABI and changes in cognitive function with partial correlation coefficients. The consistency of relationships among subgroups was examined using tests of interactions. We standardized the individual test scores by dividing their difference from the cohort-wide baseline mean by the cohort-wide baseline standard deviation, ordering them so that positive scores reflected better performance. We also developed a composite measure of cognitive function by averaging the scores of each function and renormalizing this average to have a baseline standard deviation of one, computing this from baseline scores and separately from follow-up scores.
RESULTS
Of 1,635 LIFE participants, the 1,602 (98%) for whom baseline ABI measures could be obtained are included in our analysis. Table 1 summarizes the distribution of the cohort at baseline. At this time, only 4 participants reported having stayed in a nursing home in the past six months (but were not current residents); 115 (7.2%) reported being hospitalized during the past 6 months. The average (SD) age, SPPB score, and 400 meter walk times of participants were 78.8 (5.2), 7.4 (1.6), and 8.46 (1.89) minutes. Overall, 211 (13.2%) participants had low (<0.90) ABI and 73 (4.6%) had high (>1.30) ABI. Low ABI was more prevalent among older participants, racial/ethnic minorities, and current smokers, and among those with lower education, diabetes, or hypertension. The overall mean (SD) of ABI was 1.06 (0.20).
Table 1.
Baseline Characteristics of the LIFE Study Population According to Categories of Baseline Ankle Brachial Index Measures
| Baseline Characteristic (N) |
ABI: Mean (SD) |
|||
|---|---|---|---|---|
| ABI Groups |
p-value* | |||
| <0.90 N=211 |
0.90–1.30 N=1318 |
>1.30 N=73 |
||
| Age group | ||||
| 70–79 | 97 (10.6) | 782 (85.1) | 40 (10.6) | 0.002 |
| 80–89 | 113 (16.6) | 536 (78.6) | 33 (16.6) | |
| Gender | ||||
| Female | 143 (13.3) | 911 (84.6) | 23 (2.1) | <0.001 |
| Male | 67 (12.8) | 407 (77.7) | 50 (9.5) | |
| Education group | ||||
| HS or less | 88 (15.3) | 469 (81.4) | 19 (3.3) | 0.04 |
| Post HS | 121 (11.8) | 846 (82.9) | 54 (5.3) | |
| SBPP group | ||||
| < 8 | 96 (13.4) | 580 (81.4) | 37 (5.2) | 0.50 |
| 8–9 | 114 (12.8) | 738 (83.1) | 36 (4.0) | |
| Diabetes | ||||
| No | 143 (12.0) | 1002 (84.1) | 47 (3.9) | 0.006 |
| Yes | 67 (16.4) | 316 (77.3) | 26 (6.4) | |
| Hypertension | ||||
| No | 30 (7.0) | 349 (87.2) | 21 (5.2) | <0.001 |
| Yes | 180 (15.0) | 969 (80.7) | 52 (4.3) | |
| Race/ethnicity | ||||
| African-American | 49 (17.2) | 232 (81.4) | 4 (1.4) | |
| Caucasian | 144 (11.9) | 1002 (82.7) | 65 (5.4) | 0.008 |
| Other | 16 (16.0) | 80 (80.0) | 4 (4.0) | |
| Smoking | ||||
| Never | 89 (10.9) | 692 (84.6) | 37 (4.5) | |
| Former | 94 (13.3) | 580 (81.8) | 35 (4.9) | <0.001 † |
| Current | 22 (45.8) | 25 (52.1) | 1 (2.1) | |
| Not reported | 5 (19.2) | 21 (80.8) | 0 (0.0) | |
Chi-squared tests
Excluding not reported category
Table 2 summarizes the baseline cross-sectional associations between ABI and cognitive function test scores. Listed are mean test scores, after covariate-adjustment for the risk factors in Table 1. Lower ABI was associated with poorer performance on all cognitive functions except the n-back test of working memory.
Table 2.
Mean Scores from Cognitive Function Tests by ABI Group at Baseline, With Covariate Adjustment for All Factors in Table 1: The LIFE Study
Covariate adjustment for risk factors in Table 1
| Cognitive Function† |
ABI |
|||
|---|---|---|---|---|
| ABI Group: Adjusted Mean (SE) |
p-value* | |||
| <0.90 N=210 |
0.90–1.30 N=1318 |
>1.30 N=73 |
||
| 3MSE | 90.8 (0.3) | 91.7 (0.1) | 92.7 (0.6) | <0.0001 |
| DSC | 44.2 (0.8) | 46.5 (0.3) | 47.4 (1.4) | 0.01 |
| HVLT | ||||
| Immediate | 22.98 (0.35) | 23.27 (0.14) | 23.69 (0.59) | 0.03 |
| Delayed | 7.37 (0.19) | 7.75 (0.08) | 8.10 (0.32) | <0.001 |
| N-Back | ||||
| 1-back | 0.81 (0.01) | 0.81 (0.01) | 0.83 (0.02) | 0.27 |
| 2-back | 0.51 (0.02) | 0.50 (0.01) | 0.53 (0.03) | 0.37 |
| Flanker | ||||
| Congruent | 656 (13) | 655 (5) | 614 (22) | 0.01 |
| Incongruent | 724 (16) | 731 (6) | 684 (27) | 0.05 |
| Task Switch | ||||
| No Switch | 1499 (54) | 1453 (21) | 1387 (85) | 0.002 |
| Switch | 2497 (79) | 2409 (30) | 2299 (124) | <0.001 |
| Composite | −0.087 (0.065) | 0.004 (0.253) | 0.179 (0.108) | <0.001 |
For no cognitive function test was there a significant (p<0.05) quadratic relationship, which supports the use of linear models for the following analyses. P-values are based on analyses of covariance assessing linear relationships between ABI and cognitive function test scores.
3MSE (Modified MiniMental State Exam); DSC (Digit Symbol Coding); HVLT (Hopkins Verbal Learning Test)
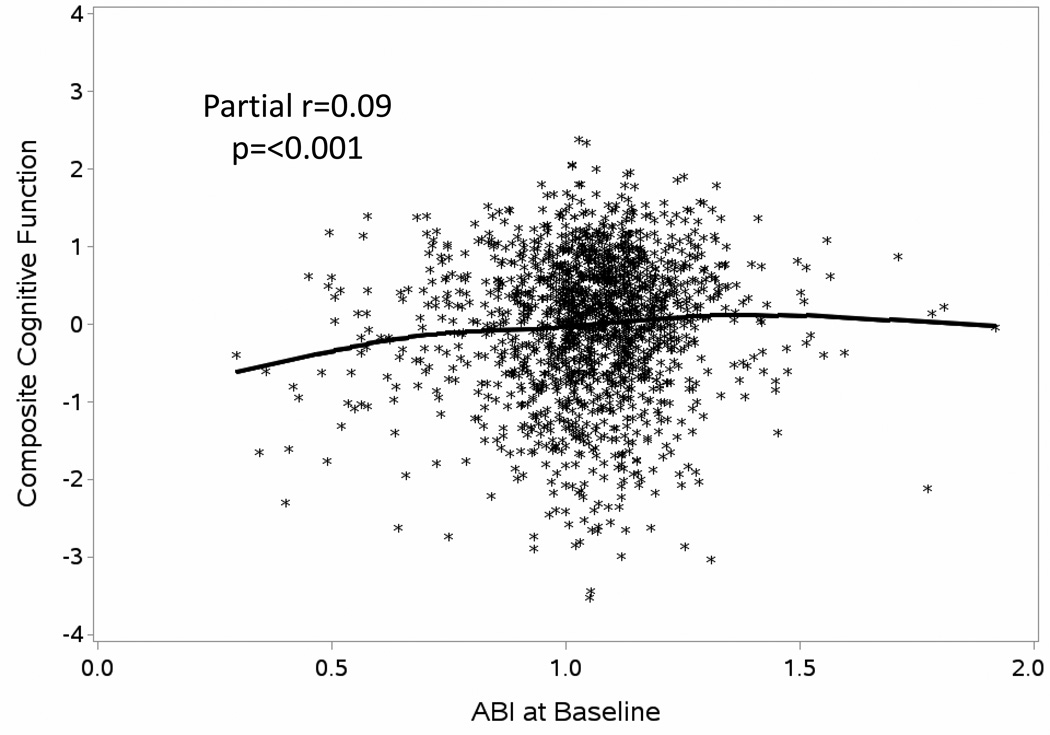
To portray general associations, we plotted the composite measure of cognitive function formed from the separate test scores (after covariate adjustment for the risk factors in Table 1) against ABI (partial r=0.09; p<0.001), overlaying a cubic spline regression curve on the scatterplot (Figure 1).
Figure 1.
Scatterplot of Composite Cognitive Function Scores at Baseline by ABI with Adjustment for Risk Factors in Table 1. Included Is a Cubic Spline Regression Curve.
ABI had a slightly curved association with this composite measure, with lower ABI associated with poorer composite cognitive function and little association across normal and higher ABI: however, the overall curvature was not significant based on quadratic regression (p=0.33). While this independent relationship as modest, the statistical evidence for it was stronger than for several other traditional risk factors for cognitive deficits, such as diabetes (p=0.04), smoking (p=0.07), and hypertension (p=0.25).
We examined whether the relationship between ABI and the composite measure of cognitive function varied among the risk factor subgroups listed in Table 1, using tests of interaction. Only for education was there some evidence for differences (p=0.02), with a steeper slope, indicating a stronger association, among individuals with high school or less education (fitted slope=0.85; standard error=0.21) compared with those with more education (fitted slope=0.26; standard error=0.15).
Table 3 provides results for associations between baseline ABI, as a continuous measure, with changes in standardized measures (i.e. changes in units of standard deviations) of cognitive function after adjustment for the risk factors in Table 1 and intervention assignment. Over the two years of follow-up, mean cognitive function scores changes were within ± 0.14 SDs for all cognitive function tests. After adjustment for the risk factors in Table 1, baseline ABI was not associated with two year changes in cognitive function.
Table 3.
Changes in Cognitive Function Scores over Time and Partial Correlations with Baseline ABI Adjusting For Covariates in Table 1 and Intervention Assignment: The LIFE Study
| Cognitive Function* |
Change Over Time in SD Units Mean (SD) |
Partial Correlation (p-value) With Baseline ABI |
|---|---|---|
| 3MSE | −0.01 (1.06) | 0.01 (p=0.78) |
| DSC | −0.04 (0.61) | 0.03 (p=0.22) |
| HVLT Composite | −0.14 (0.83) | −0.01 (p=0.59) |
| N-Back Composite | 0.01 (0.94) | 0.01 (p=0.62) |
| Flanker Composite | 0.01 (0.75) | 0.00 (p=0.91) |
| Task Switch Composite | −0.10 (0.74) | 0.03 (p=0.31) |
| Executive Function Composite | −0.05 (0.77) | 0.05 (p=0.08) |
| Global Composite | −0.09 (0.67) | 0.00 (P=0.90) |
3MSE (Modified MiniMental State Exam); DSC (Digit Symbol Coding); HVLT (Hopkins Verbal Learning Test)
At 24 months, 55 (3.4%) participants were classified has having probable dementia. Of the 1,459 participants for whom mild cognitive impairment could be ruled out at baseline, 128 (8.8%) were newly classified with mild cognitive impairment at 24 months. Table 4 provides results from logistic regression to assess associations between baseline ABI and progression to the cognitive outcomes. One unit lower ABI at baseline was associated with an increased odds for worsening of cognitive function (either progressing from normal cognition to mild cognitive impairment or from normal cognition or mild cognitive impairment to probable dementia) after covariate adjustment for the risk factors in Table 1 and intervention assignment (OR=2.60; 95% confidence interval [1.06,6.37]). There were non-significant trends for relationships with transitioning from normal to mild cognitive impairment (OR=2.56 [0.89,7.35] and from either normal and mild cognitive function to probable dementia (OR=2.60 [1.06;6.37]). Compared to other participants, those with ABI<0.90 were at increased risk for conversion to mild cognitive impairment (OR=1.72 [1.05,2.82]; p=0.032) but not for progression to probable dementia (OR=0.94 [0.42,2.10)l p=0.889).
Table 4.
Odds Ratios Associated With 1 Unit Lower ABI for 1) Progression from Normal Cognition to Mild Cognitive Impairment, 2) Progression from Normal Cognition or Mild Cognitive Impairment to Dementia, or 3) Progression from Normal Cognitive Function to Mild Cognitive Impairment or Dementia or Progression from Mild Cognitive Impairment to Dementia: Odds Ratios, 95% Confidence Intervention, and P-Value After Covariate Adjustment for All Factors in Table 1.
| Conversion to From Normal Cognition to Mild Cognitive Impairment* |
Conversion From Normal Cognition or Mild Cognitive Impairment to Dementia |
Conversion From Normal Cognition to Mild Cognitive Impairment or from Normal or Mild Cognitive Impairment to Dementia |
|---|---|---|
| OR=2.56 [0.89,7.35] P=0.080 |
OR=2.99 [0.65,13.70] P=0.157 |
OR=2.60 [1.06,6.37] P=0.036 |
Cases at follow-up: Mild cognitive impairment N=128 (8.8%)*; Probable dementia N=55 (3.4%)
MCI could not be ruled out for N=137 at baseline who are not included in this analysis
Assignment to the physical activity intervention was associated with a small but statistically significant relative improvement in ABI measures at 24 months, with a mean (SE) increase among participants assigned to physical activity intervention of 0.011 (0.006) and a mean decrease among participants assigned to successful aging intervention of −0.008 (0.006) units (p=0.036). At follow-up, the prevalence of low ABI was 12.5% in the physical activity intervention participants and 14.4% in the successful aging intervention participants (p=0.84 with covariate adjustment for baseline ABI). Changes in ABI over two years were not associated with changes in any measure of cognitive function, both overall and separately within each intervention group.
Fifteen participants reported having procedures performed “to open up the arteries in either of your legs, such as angioplasty, PTA stent, or lower extremity bypass” sometime during follow-up, 12 of which had ABI<0.90 at baseline. Nine of these occurred among participants assigned to the control group and 6 to participants assigned to the physical activity intervention (p=0.43). Omitting these individuals from the analyses did not alter the associations between intervention assignment and changes in ABI (p-value remained p=0.036) or between changes in ABI and changes in cognitive function scores (all p>0.05).
DISCUSSION
We draw three principal conclusions from this analysis of data from the LIFE study. First, low ABI at baseline was associated with poorer cognitive function in the sedentary and functionally limited LIFE cohort, even after extensive covariate adjustment. Second, over two years of follow-up, baseline ABI was not associated with changes in cognitive function scores. However, lower baseline ABI was associated with increased odds for the outcome of mild cognitive impairment or probable dementia. Third, the LIFE physical activity intervention relative to health education was associated with a small relative increase in ABI over two years, but changes in ABI were not associated with changes in cognitive function.
Cross-sectional associations between ABI and cognitive function
The past two decades have established that low ABI is correlated with poorer cognitive function across many cohorts and settings.2 These associations are evident across many different domains of cognitive function: we found the strongest associations with general measures (e.g. the 3MSE measure of global cognitive function and the composite we formed). However, associations in the LIFE cohort, while statistically significant, were relatively modest, but stronger than for other traditional risk factors for cognitive deficits (i.e. diabetes, smoking, hypertension). LIFE participants had many other conditions and risk factors that may have separately affected these associations and weakened their magnitude, including high rates of diabetes, hypertension, and sedentary lifestyles27 and high rates of hospitalizations during follow-up, and eligibility criteria may have truncated the distribution of lower ABIs.10 Our finding of a fairly linear relationship across the ABI range of the LIFE cohort is consistent with others who have described monotonic relationships.28,29
Artery disease as a risk factor for cognitive decline
There have been several reports that low ABI is associated with greater rates of cognitive decline, although these changes were observed over the course of 7 to 10 years.30,31 Two years is a relatively short time period to observe mean changes in cognitive function, even in a cohort at greater risk for cognitive decline due to older age and sedentary lifestyle. It is possible that learning effects and differential retention may have contributed to the relatively small changes observed in the cohort. It is also possible that due to participant’s advanced age, comorbidities, and compromised physical functioning at baseline, the LIFE cohort may not have been capable of increasing their physical activity to the level required to produce detectable changes in cognitive functioning.
Despite these potential constraints, baseline ABI as a continuous measure was significantly associated with the development of mild cognitive impairment or probable dementia (p=0.037). Our results are consistent with those of several prior studies. Newman, et al., in a cohort of 3,602 with mean age 74 years found, over an average of 5.4 years, that ABI <0.90 was associated with a hazard ratio of 2.4 [95% confidence ratio 1.4,4.0] for dementia.32. Bruce, et al., in a cohort aged 70 years or greater with diabetes, found that ABI ≤0.90 was associated with a 2.03 [1.61,6.26] greater odds of centrally adjudicated composite cognitive impairment and a 4.54 [1.39,14.78] greater odds of dementia over an average of 7.6 years.33 Laurin, et al., in 2,588 participants, aged 71–93 years, of the Honolulu-Asia Aging Study, found that over an average of 5.1 years of follow-up, ABI <0.90 compared with ABI between 0.90 and 1.20 was associated with a hazard ratio of 1.41[1.27,2.52] for centrally adjudicated dementia.28 However, van Oijen, et al., in a population-based study of 6,647 individuals with mean age 69 years found, over an average of 9.0 years, that low ABI was not associated with incident centrally adjudicated dementia: hazard ratio=1.03 [0.85,1.25].3
Peripheral artery disease marks individuals with greater levels of cerebrovascular disease, brain atrophy, and b-amyloid deposition.34–37 Within the LIFE cohort, comprised of older individuals with many risk factors that may accelerate these conditions, two years was sufficient time to observe the link between low ABI and the development of mild cognitive impairment or probable dementia.
Longitudinal association between markers of arterial disease and cognitive function
The LIFE physical activity intervention yielded a small but statistically significant relative increase in mean ABI compared with the successful aging intervention. While this did not translate to a benefit on the prevalence of ABI <0.90, the traditional cutpoint used to define peripheral artery disease, it is encouraging that the progression towards peripheral arterial disease may have been slowed. We saw little evidence that the changes in ABI over two years were associated with changes in cognitive function. Underlying associations may be modest and undetectable over two years. It may also be possible that the time-frames through which changes in arterial disease materially affect changes in cognition may not overlap.
Limitations
While the LIFE Study provided a large, well-characterized cohort, its participants were volunteers in a clinical trial who were selected to have deficits in physical function and reduced physical activity level; thus our findings may not generalize to other cohorts. As noted above, it is also plausible that the LIFE cohort was not capable of performing sufficient doses of physical activity to produce detectable changes in the LIFE cognition battery. Indeed, the dose-response association between physical activity and cognitive functioning may not be well understood.38,39 Two years may have been too short of a follow-up time for detectable longitudinal associations to emerge.
CONCLUSION
In an older cohort of sedentary individuals with compromised physical function, ABI had statistically significant linear relationships with cognitive function, even after covariate adjustment. Furthermore, low baseline ABI predicted of cognitive decline and the incidence of mild cognitive impairment or probable dementia. Longer follow-up beyond two years may be necessary to observe any relationship between changes in ABI and changes in cognitive function.
Appendix: Research Investigators for the LIFE Study
The Lifestyle Interventions and Independence for Elders Study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement #UO1 AG22376 and a supplement from the National Heart, Lung and Blood Institute 3U01AG022376-05A2S, and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH.
The research is partially supported by the Claude D. Pepper Older Americans Independence Centers at the University of Florida (1 P30 AG028740), Wake Forest University (1 P30 AG21332),Tufts University (1P30AG031679), University of Pittsburgh (P30 AG024827), and Yale University (P30AG021342) and the NIH/NCRR CTSA at Stanford University (UL1 RR025744),
Tufts University is also supported by the Boston Rehabilitation Outcomes Center (1R24HD065688-01A1).
LIFE investigators are also partially supported by the following:
Dr. Thomas Gill (Yale University) is the recipient of an Academic Leadership Award (K07AG3587) from the National Institute on Aging.
Dr. Carlos Fragoso (Spirometry Reading Center, Yale University) is the recipient of a Career Development Award from the Department of Veterans Affairs.
Dr. Roger Fielding (Tufts University) is partially supported by the U.S. Department of Agriculture, under agreement No. 58-1950-7-707. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Dept of Agriculture.
Administrative Coordinating Center, University of Florida, Gainesville, FL
Marco Pahor, MD – Principal Investigator of the LIFE Study
Jack M. Guralnik, MD, PhD – Co-Investigator of the LIFE Study (University of Maryland School of Medicine, Baltimore, MD)
Christiaan Leeuwenburgh, PhD
Connie Caudle
Lauren Crump, MPH
Latonia Holmes
Jocelyn Lee, PhD
Ching-ju Lu, MPH
Data Management, Analysis and Quality Control Center, Wake Forest University, Winston-Salem, NC
Michael E. Miller, PhD – DMAQC Principal Investigator
Mark A. Espeland, PhD – DMAQC Co-Investigator
Walter T. Ambrosius, PhD
William Applegate, MD
Daniel P. Beavers, PhD, MS
Robert P. Byington, PhD, MPH, FAHA
Delilah Cook, CCRP
Curt D. Furberg, MD, PhD
Lea N. Harvin, BS
Leora Henkin, MPH, Med
John Hepler, MA
Fang-Chi Hsu, PhD
Laura Lovato, MS
Wesley Roberson, BS,BA
Julia Rushing, BSPH, MStat
Scott Rushing, BS
Cynthia L. Stowe, MPM
Michael P. Walkup, MS
Don Hire, BS
W. Jack Rejeski, PhD
Jeffrey A. Katula, PhD, MA
Peter H. Brubaker, PhD
Shannon L. Mihalko, PhD
Janine M. Jennings, PhD
National Institutes of Health, Bethesda, MD
Evan C. Hadley, MD (National Institute on Aging)
Sergei Romashkan, MD, PhD (National Institute on Aging)
Denise E. Bonds, MD, MPH (National Heart, Lung and Blood Institute)
Kushang V. Patel, PhD (National Institute on Aging)
Field Centers
Northwestern University, Chicago, IL
Mary M. McDermott, MD – Field Center Principal Investigator
Bonnie Spring, PhD – Field Center Co-Investigator
Joshua Hauser, MD – Field Center Co-Investigator
Diana Kerwin, MD – Field Center Co-Investigator
Kathryn Domanchuk, BS
Rex Graff, MS
Alvito Rego, MA
Pennington Biomedical Research Center, Baton Rouge, LA
Timothy S. Church, MD, PhD, MPH – Field Center Principal Investigator
Steven N. Blair, PED (University of South Carolina)
Valerie H. Myers, PhD
Ron Monce, PA-C
Nathan E. Britt, NP
Melissa Nauta Harris, BS
Ami Parks McGucken, MPA, BS
Ruben Rodarte, MBA, MS, BS
Heidi K. Millet, MPA, BS
Catrine Tudor-Locke, PhD, FACSM
Ben P. Butitta, BS
Sheletta G. Donatto, MS, RD, LDN, CDE
Shannon H. Cocreham, BS
Stanford University, Palo Alto, CA
Abby C. King, Ph.D. – Field Center Principal Investigator
Cynthia M. Castro, PhD
William L. Haskell, PhD
Randall S. Stafford, MD, PhD
Leslie A. Pruitt, PhD
Veronica Yank, MD
Kathy Berra, MSN, NP-C, FAAN
Carol Bell, NP
Rosita M. Thiessen
Kate P. Youngman, MA
Selene B. Virgen, BAS
Eric Maldonado, BA
Kristina N. Tarin, MS, CSCS
Heather Klaftenegger, BS
Carolyn A. Prosak, RD
Ines Campero, BA
Dulce M. Garcia, BS
José Soto, BA
Linda Chio, BA
David Hoskins, MS
Tufts University, Boston, MA
Roger A. Fielding, PhD – Field Center Principal Investigator
Miriam E. Nelson, PhD – Field Center Co-Investigator
Sara C. Folta, PhD – Field Center Co-Investigator
Edward M. Phillips, MD
Christine K. Liu, MD
Erica C. McDavitt, MS
Kieran F. Reid, MSc, MPH
Won S. Kim, BS
Vince E. Beard, BS
University of Florida, Gainesville, FL
Todd M. Manini, PhD – Field Center Principal Investigator
Marco Pahor, MD – Field Center Co-Investigator
Stephen D. Anton, PhD
Susan Nayfield, MD
Thomas W. Buford, PhD
Michael Marsiske, PhD
Bhanuprasad D. Sandesara, MD
Jeffrey D. Knaggs, BS
Megan S. Lorow, BS
William C. Marena, MT, CCRC
Irina Korytov, MD
Holly L. Morris, MSN, RN, CCRC (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Margo Fitch, PT (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Floris F. Singletary, MS, CCC-SLP (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Jackie Causer, BSH, RN (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Katie A. Radcliff, MA (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
University of Pittsburgh, Pittsburgh, PA
Anne B. Newman, MD, MPH – Field Center Principal Investigator
Stephanie A. Studenski, MD, MPH – Field Center Co-Investigator
Bret H. Goodpaster, PhD
Nancy W. Glynn, PhD
Oscar Lopez, MD
Neelesh K. Nadkarni, MD, PhD
Kathy Williams, RN, BSEd, MHSA
Mark A. Newman, PhD
George Grove, MS
Janet T. Bonk, MPH, RN
Jennifer Rush, MPH
Piera Kost, BA (deceased)
Diane G. Ives, MPH
Wake Forest University, Winston-Salem, NC
Stephen B. Kritchevsky, Ph.D. – Field Center Principal Investigator
Anthony P. Marsh, PhD – Field Center Co-Investigator
Tina E. Brinkley, PhD
Jamehl S. Demons, MD
Kaycee M. Sink, MD, MAS
Kimberly Kennedy, BA, CCRC
Rachel Shertzer-Skinner, MA, CCRC
Abbie Wrights, MS
Rose Fries, RN, CCRC
Deborah Barr, MA, RHEd, CHES
Yale University, New Haven, CT
Thomas M. Gill, MD – Field Center Principal Investigator
Robert S. Axtell, PhD, FACSM – Field Center Co-Investigator (Southern Connecticut State University, Exercise Science Department)
Susan S. Kashaf, MD, MPH (VA Connecticut Healthcare System)
Nathalie de Rekeneire, MD, MS
Joanne M. McGloin, MDiv, MS, MBA
Karen C. Wu, RN
Denise M. Shepard, RN, MBA
Barbara Fennelly, MA, RN
Lynne P. Iannone, MS, CCRP
Raeleen Mautner, PhD
Theresa Sweeney Barnett, MS, APRN
Sean N. Halpin, MA
Matthew J. Brennan, MA
Julie A. Bugaj, MS
Maria A. Zenoni, MS
Bridget M. Mignosa, AS
Cognition Coordinating Center, Wake Forest University, Winston-Salem, NC
Jeff Williamson, MD, MHS – Center Principal Investigator
Kaycee M Sink, MD, MAS – Center Co-Investigator
Hugh C. Hendrie, MB, ChB, DSc (Indiana University)
Stephen R. Rapp, PhD
Joe Verghese, MB, BS (Albert Einstein College of Medicine of Yeshiva University)
Nancy Woolard
Mark Espeland, PhD
Janine Jennings, PhD
Electrocardiogram Reading Center, University of Florida, Gainesville, FL
Carl J. Pepine MD, MACC
Mario Ariet, PhD
Eileen Handberg, PhD, ARNP
Daniel Deluca, BS
James Hill, MD, MS, FACC
Anita Szady, MD
Spirometry Reading Center, Yale University, New Haven, CT
Geoffrey L. Chupp, MD
Gail M. Flynn, RCP, CRFT
Thomas M. Gill, MD
John L. Hankinson, PhD (Hankinson Consulting, Inc.)
Carlos A. Vaz Fragoso, MD
Cost Effectiveness Analysis Center
Erik J. Groessl, PhD (University of California, San Diego and VA San Diego Healthcare System)
Robert M. Kaplan, PhD (Office of Behavioral and Social Sciences Research, National Institutes of Health)
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov Identifiers: NCT01072500
CONFLICT OF INTEREST STATEMENT
Conflicts of Interest: None.
REFERENCES
- 1.Rafnsson SB, Deary IJ, Fowkes FGR. Peripheral arterial disease and cognitive function. Vasc Med. 2009;14:51–61. doi: 10.1177/1358863X08095027. [DOI] [PubMed] [Google Scholar]
- 2.Guerchet M, Aboyans V, Nubukpo P, et al. Ankle-brachial index as a marker of cognitive impairment and dementia in general population. A systematic review. Atherosclerosis. 2011:251–257. doi: 10.1016/j.atherosclerosis.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 3.van Oijen M, de Jong FJ, Witteman JC, et al. Atherosclerosis and risk for dementia. Ann Neurol. 2007;61:403–410. doi: 10.1002/ana.21073. [DOI] [PubMed] [Google Scholar]
- 4.Johnson W, Price JF, Rafnsson SB, et al. Ankle-brachial index predicts level of, but not change in, cognitive function: The Edinburgh Artery Study at the 15-year follow-up. Vasc Med. 2010;15:91–97. doi: 10.1177/1358863X09356321. [DOI] [PubMed] [Google Scholar]
- 5.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 6.Rafnsson SB, Deary IJ, Smith FB, et al. Cardiovascular diseases and decline in cognitive function in an elderly community population: The Edinburgh Artery Study. Psychosomatic Med. 2007;69:425–434. doi: 10.1097/psy.0b013e318068fce4. [DOI] [PubMed] [Google Scholar]
- 7.Laukka EJ, Starr JM, Deary IJ. Lower ankle-brachial index is related to worse cognitive performance in old age. Neuropyschol. 2014;28:281–289. doi: 10.1037/neu0000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy M, Solomon C, Manolio T, et al. Risk factors for declining brachial-index in men and women 65 years or older. JAMA Intern Med. 2005;165:1896–1902. doi: 10.1001/archinte.165.16.1896. [DOI] [PubMed] [Google Scholar]
- 9.Fielding RA, Rejeski WJ, Church T, et al. The Lifestyle Interventions and Independence for Elders (LIFE) Study: design and methods. J Gerontol Biol Med Sci. 2011;66A:1226–1237. doi: 10.1093/gerona/glr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: The LIFE Study Randomized Clinical Trial. JAMA. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 13.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 14.Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 15.McDermott MM, Applegate WB, Bonds DE, et al. Ankle brachial index values, leg symptoms, and functional performance among community-dwelling older men and women in the Lifestyle Interventions and Independence for Elders Study. J Am Heart Assoc. 2013;2:e000257. doi: 10.1161/JAHA.113.000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: prevlance, risk factors, and association with cardiovascular disease. J Am Coll Cardiol. 2004;44:618–623. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 17.Sink KM, Espeland MA, Rushing J, et al. The LIFE Cognition Study: design and baseline characteristics. Clin Interv Aging. 2014;9:1425–1436. doi: 10.2147/CIA.S65381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wechsler D. WAIS-III manual. New York: Psychological Corporation; 1997. [Google Scholar]
- 19.Brandt J, Benedict RHB. Hopkins Verbal Learning Test-Revised. Professional manual. Lutz, FL: Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- 20.Espeland MA, Katula JA, Rushing J, et al. Performance of a computer-based assessment of cognitive function measures in two cohorts of seniors. Int J Geriatr Psychiatry. 2013;28:1239–1250. doi: 10.1002/gps.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jennings JM, Webster LM, Kleykamp BA, et al. Recollection training and transfer effects in older adults: successful use of a repetition-lag procedure. Aging Neuropsychol C. 2005;12:278–298. doi: 10.1080/138255890968312. [DOI] [PubMed] [Google Scholar]
- 22.Eriksen B, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- 23.Rogers R, Monsell S. Costs of a predictable switch between simple cognitive tasks. J Exp Psychol Gen. 1995;124:207–231. [Google Scholar]
- 24.Kramer AF, Hahn S, McAuley E, et al. Exercise, aging and cognition: healthy body, healthy mind? In: Rogers WA, Fisk AD, editors. Human factors interventions for the health care of older adults. Hillsdale NJ: Erlbaum; 2001. [Google Scholar]
- 25.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer’s Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer’s Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsh AP, Lovato LC, Glynn NW, et al. Lifestyle Interventions and Independence for Elders Study: recruitment and baseline characteristics. J Gerontol A Biol Sci Med Sci. 2013;68:1549–1558. doi: 10.1093/gerona/glt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laurin D, Masaki KH, White LR, Launer LJ. Ankle-to-brachial index and dementia: the Honolulu-Asia Aging Study. Circulation. 2007;116:2269–2274. doi: 10.1161/CIRCULATIONAHA.106.686477. [DOI] [PubMed] [Google Scholar]
- 29.Sugawara N, Yaui-Furukori N, Umeda T, et al. Comparison of ankle-brachial pressure index and pulse wave velocity as markers of cognitive function in a community dwelling population. BMC Psychiatry. 2010;10:46. doi: 10.1186/1471-244X-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haan MN, Shemanski L, Jagust WJ, et al. The role of APOE epislon4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA. 1999;282:40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- 31.Price JF, McDowell S, Whiteman MC, et al. Ankle-brachial index as a predictor of cognitive impairment in the general population: ten-year follow-up of the Edinburgh Artery Study. J Am Geriatr Soc. 2006;54:763–769. doi: 10.1111/j.1532-5415.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- 32.Newman AB, Fitzpatrick Al, Lopez O, et al. Dementia and Alzheimer’s disease incidence in association to cardiovascular disease in the Cardiovascular Heart Study cohort. J Am Geriatr Soc. 2005;53:1101–1107. doi: 10.1111/j.1532-5415.2005.53360.x. [DOI] [PubMed] [Google Scholar]
- 33.Bruce DG, Davis WA, Casey GP, et al. Predictors of cognitive impairment and dementia in older people with diabetes. Diabetologia. 2008;51:241–248. doi: 10.1007/s00125-007-0894-7. [DOI] [PubMed] [Google Scholar]
- 34.Bots ML, van Swieten JC, Breteler MM, et al. Cerebral white matter lesions and atherosclerosis in the Rotterdam study. Lancet. 1993;341:1232–1237. doi: 10.1016/0140-6736(93)91144-b. [DOI] [PubMed] [Google Scholar]
- 35.Geerlings MI, Appleman APA, Vincken KL, et al. Brain volumes and cerebrovascular lesions in MRI patients with atherosclerotic disease, The SMARTA-MR study. Atherosclerosis. 2010;210:130–136. doi: 10.1016/j.atherosclerosis.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 36.Hughes TM, Kuller LH, Barinas-Mitchell EF, et al. Pulse wave velocity is associated with B-amyloid deposition in the brains of very elderly adults. Neurology. 2013;81:1711–1718. doi: 10.1212/01.wnl.0000435301.64776.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hilal S, Saini M, Tan CS, et al. Ankle-brachial index, cognitive impairment, and cerebrovascular disease in a Chinese population. Neuroepidemiology. 2014;42:131–138. doi: 10.1159/000357372. [DOI] [PubMed] [Google Scholar]
- 38.Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. Journal of applied physiology. 2006;101:1237–1242. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- 39.Snowden M, Steinman L, Mochan K, et al. Effect of exercise on cognitive performance in community-dwelling older adults: review of intervention trials and recommendations for public health practice and research. J Am Geriatr Soc. 2011;59:704–716. doi: 10.1111/j.1532-5415.2011.03323.x. [DOI] [PubMed] [Google Scholar]