Abstract
“Heartwarming” social experiences, when one feels interpersonally connected to others, have recently been linked with physical warmth. According to one theory (Panksepp, 1998), “social warmth” and physical warmth may be closely linked because both experiences are supported by similar neurobiological mechanisms, however, the neurochemical substrates underlying this overlap have not been explored. Here, an opioid antagonist, naltrexone, was administered in order to examine the role of opioids, previously shown to alter temperature and social bonding behavior, on perceived thermal intensity, general positive affect, and feelings of social connection from physical warmth. Thirty-one participants took both naltrexone and placebo and completed a temperature manipulation task (held a warm pack, cold pack, and neutral object) while on each drug. Replicating previous research, holding a warm (vs. a cold or neutral) object increased feelings of social connection. Moreover, blocking opioids reduced this effect. Hence, naltrexone specifically reduced feelings of social connection to holding a warm (vs. neutral) object but not to holding a cold (vs. neutral) object. These results lend further support to the theory that social and physical warmth share neurobiological, opioid receptor dependent mechanisms.
Keywords: opioids, emotion, humans, social warmth, social bonds
“And it’s that reaching, that gesture, that reflex we have to pull what’s warm-whether it’s something or someone-toward us, that feeling we get when we do that, that feeling of being safe in the world…that’s happiness.”
Design Flaws of the Human Condition by Paul Schmidtberger
What does it feel like to connect with someone else? Even though connecting with others is a primary emotional experience in life, there is very little empirical attention given to this question. As a consequence, we know little about the mechanisms underlying the positive affective state associated with experiences of social connection. In many cultures, the language used to describe experiences of social connection, such as ‘heartwarming’ or ‘warm’ moments, reference physical warmth (Alberts & Decsy, 1990). Beyond mere metaphor, a growing body of research now suggests that changes to actual physical warmth can also affect perceptions of “social warmth,” feeling loved by and connected to others. For example, after holding warm objects, participants rated another person as interpersonally warmer (Williams & Bargh, 2008), reported feeling closer to other people (IJzerman & Semin, 2009), increased their trusting behavior (Kang, Williams, Clark, Gray, & Bargh, 2011), and felt more socially connected (Inagaki & Eisenberger, 2013). While these results suggest an overlap between experiences of social and physical warmth, the mechanisms, particularly the neurochemical mechanisms, linking these two concepts are unknown.
From a neurobiological perspective, social and physical warmth may be closely linked because they share some of the same neurocircuitry (Panksepp, 1998; Panksepp, Nelson, & Bekkedal, 1997). Given the importance of social relationships early in life (Bowlby, 1988; Harlow, 1958), social warmth may have piggybacked onto other basic regulatory mechanisms in the body, such as thermoregulatory mechanisms, which then help the individual monitor and reinforce social connections (Panksepp, 1998; Williams, Huang, & Bargh, 2009). That is, mechanisms that regulate our relatively warm internal body temperature (including the motivation to seek out warm stimuli as well as the perceived pleasantness of physical warmth) may also help to regulate feelings of social connection and help maintain our bonds to others. From this perspective, social disconnection elicits feelings of coldness, which then prompts the individual to seek “warmth” in order to alleviate the distress of social isolation. Once the warmth is obtained, one feels the pleasant satisfaction from being reconnected.
As part of the thermoregulatory system, endogenous opioids, known for their role in reward and analgesia, mediate changes in body temperature (Adler, Geller, Roscow, & Cochin, 1988). Thus, administering µ-opioid agonists, such as morphine, to animals leads to increases in body temperature (Clark, Murphy, Lipton, & Clark, 1983) and µ-opioid antagonists, such as naloxone, decrease body temperature (Handler, Geller, & Adler, 1992; Spencer, Hruby, & Burks, 1988). In humans, morphine has also been shown to reduce the unpleasantness of noxious thermal stimuli, but does not alter how objectively warm or cold the same stimuli are rated (Morin, Duncan, Lavigne, Boily, & Bushnell, 1999) suggesting that opioids may be important for the subjective experience of warmth, but not for one’s ability to perceive warmth. In addition, opioid-rich neural regions, such as the insula and ventral striatum (VS), show increased activity to innocuous physical warmth. For instance, participants asked to hold warm (vs. neutral) stimuli show increased activity in the insula (Becerra et al., 1999; Davis, Kwan, Crawley, & Mikulis, 1998; Inagaki & Eisenberger, 2013; Olausson, Charron, Marchand, Villemure, Strigo, & Bushnell, 2005; Rolls, Grabenhorst, & Parris, 2008; Verhagen, Kadohisa, & Rolls, 2004), and increases in self-reported pleasure in response to holding a warm stimulus are associated with increased ventral striatum activity (Rolls et al., 2008).
With regards to social warmth, the opioid system has also long been theorized to be an important component of social bonding (Panksepp, 1998). Thus, administering µ-opioid agonists to animals leads to increases in behavioral displays of social pleasure, while µ-opioid antagonists block this pleasure (Panksepp, Bean, Bishop, Vilberg, & Sahley, 1980). Though not previously published on its own, one study in humans (reviewed in Depue & Morrone-Strupinsky, 2005) reported that naltrexone, a µ-opioid antagonist, (vs. placebo) led to reductions in feelings of affiliation and warmth to an affiliative movie clip for females high in trait levels of affiliation. Similarly, Schweiger and colleagues (2014) have recently shown that a 25mg dose of naltrexone (vs. placebo) reduced affiliative feelings (cozy, comforting, liked, secure) after an economic trust game. Moreover, viewing images of a loved one activates a number of opioid-rich regions, including the VS and mid-insula (Acevedo, Aron, Fisher, & Brown, 2012; Aron, Fisher, Mashek, Strong, Li, & Brown, 2005; Bartels & Zeki, 2000, 2004). Finally, in the first neuroimaging study to examine physical and social warmth together, the VS and mid-insula were the only two regions that showed overlapping neural activity between the two tasks (Inagaki & Eisenberger, 2013). Together, these studies suggest that opioids may be a critical neurochemical substrate underlying physical and social warmth and may contribute to the feelings of connection that come from physical warmth.
To examine the role of µ-opioids in the feelings of connection that come from physical warmth, naltrexone, an opioid receptor antagonist with a high affinity for µ-opioid receptors (Weerts et al., 2008), and placebo were administered prior to completing a temperature manipulation task (holding and evaluating a warm pack, a cold pack, and a neutral object) in the laboratory. Following the theory that social and physical warmth share similar neurocircuitry (Panksepp, 1998; Panksepp, et al., 1997), this study had two predictions. First, holding a warm (vs. neutral or cold) object would lead to increases in feelings of connection and second, that naltrexone (vs. placebo) would reduce these reported feelings of connection. No effects of naltrexone on thermal intensity (i.e. how warm or cold the items felt) were expected. Given the role of opioids in pleasure and reward more generally, changes in positive affect in response to naltrexone were also examined.
Methods
Overview
After extensive telephone and in-person screening, participants were enrolled in a double-blind crossover design such that each participant took both naltrexone and placebo in a randomized order. The results outlined here are from a larger research study on the effects of naltrexone on perceptions of social relationships (reported separately; Inagaki, Ray, Irwin, Way, & Eisenberger, under review). Eligible participants completed a baseline session where they received instructions for taking the study drugs (see Study Drug Schedule below). They then came in for two separate experimental sessions, one on each study drug, separated by a 10-day washout period during which time no study drugs were taken. In addition to the temperature manipulation described below, participants completed a messages task (read messages from close others) and a threat of shock task (viewed images of close others and strangers while anticipating shock) during the laboratory session, and they completed brief daily diary reports on the days when they were taking the study drugs. Results from these additional measures are reported separately. Participants were run between December 2012 and February 2014. The study was registered on the U. S. National Institutes of Health Clinical Trials registry as NCT01672723 and all procedures were run in compliance with UCLA’s Institutional Review Board.
Screening and Study Participants
Interested participants were scheduled for a physical examination at UCLA’s Clinical and Translational Science Institute (CTSI) where a study nurse drew blood to test for liver functioning and pregnancy, if female, and assess vital signs (heart rate, blood pressure, height and weight). The experimenter then measured depression levels by administering the Patient Health Questionnaire (PHQ-9; Spitzer, Kroenke, Williams, 1999) and collected a urine sample to test for drug use (THC, Opiates, Cocaine, AMP, and mAMP).
Inclusion criteria required participants to be in good health, between the ages of 18 and 35, and fluent in English. Participants were excluded if they reported any major physical health or psychiatric disorders (including a PHQ-9 score above a 13), used medication, tested positive on the urine drug test, had a BMI greater than 35, or showed any clinically-relevant abnormalities (e.g., liver function tests) or pregnancy (if female) on the blood test.
After screening 50 potential participants, 37 individuals were enrolled in the study. Out of this sample, 2 participants were removed after being unresponsive to scheduling requests, 1 participant asked the experimenters to be removed from the study prior to the first session, and 3 participants (all females) reported physical symptoms (stomach/abdominal discomfort, nausea) at a severe level after the first day of the study drug and were removed by the study physician. The final sample included 31 participants (21 females, M age = 21.55, SD = 3.34). The sample was ethnically diverse with 38.7% Caucasian, 35.5% Asian, 12.9% Hispanic, 6.5% African American, and 6.5% reporting mixed ethnicity. For completing the entire study, participants were paid up to $160.
Study Drug Schedule
The opioid antagonist used in this study was oral naltrexone, an FDA-approved drug used to help manage alcoholism and opioid addiction. Study drugs were dispensed by UCLA’s Investigational Drug Section. Based on a previously established titration schedule (Bujarski, MacKillop, & Ray, 2012; Ray, Bujarski, Chin, & Miotto, 2011), participants took 4 doses of naltrexone over 4 days (25 mg for days 1 and 2 and 50mg for days 3 and 4) as well as 4 matched placebo pills. The lab session occurred on the fourth day, and thus the fourth pill of each condition was taken in the presence of the experimenter prior to beginning the temperature manipulation (see Temperature Manipulation for more details). To ensure drug compliance, drugs were packed with 50mg of riboflavin. Urine samples were then evaluated at the beginning of each experimental session under a UV light for riboflavin content. All urine samples tested positive for riboflavin indicating 100% compliance with the study drug schedule.
Sample size
Sample size was determined based on 2 factors. First, prior published studies using the same study drug schedule as that used here provided an initial estimation of the appropriate sample size (Bujarski et al., 2012; Ray et al., 2011). Second, based on a power analysis (GPower; Erdfelder, Faul, & Buchner, 1996) with a statistical power of 80% and a medium to large effect size between .50 and .60 (α = 0.05), it was determined that a sample size of 30 would be sufficient to detect naltrexone’s (vs. placebo) effect on warmth-induced feelings of connection. Data collection ended after 31 participants had completed the entire protocol.
Randomization
An individual in the lab, who was not associated with the study, provided the randomization of the study drugs (whether a participant received naltrexone first or second) to the UCLA Pharmacy staff so that the study drugs could be packaged appropriately. The randomization procedure ensured that half of the participants received naltrexone first and the other half received naltrexone second and that both males and females were also assigned to random orders of the drugs. None of the experimenters who screened, enrolled, or ran participants had access to the randomization list until the final participant was run. The individual who managed the randomization list did not interact with or view data from any of the participants.
Physical Symptoms
To assess the success of the study drug blind, participants guessed which drug they were on at the end of each study session. Participants also reported on the following physical symptoms each day they were on a study drug (4 days when on placebo and 4 days when on naltrexone for a total of 8 days): headache, dizziness/faintness, shortness of breath, rapid/irregular heartbeat, stomach/abdominal discomfort, nausea, appetite increase/decrease, difficulty urinating, muscle/bone/joint pain, fever, tiredness/fatigue. Severity of symptoms were reported using a 0 (no symptoms) to 4 (very severe symptoms) scale. In addition, participants were asked how distressing they found the symptoms on a 0 (not at all) to 7 (very) scale in order to better understand the subjective severity of the symptoms as a whole1.
Temperature manipulation
Approximately one hour after taking the study drug, when naltrexone reaches its peak effect (Lee, Wagner, Tanada, Frost, Bice, & Dannals, 1988), participants held and evaluated a warm pack, a cold pack, and a neutral room temperature object (a squeeze ball) under the cover story that the experimenters were interested in their “ratings to some commonly used products.” Participants were cued to pick up the warm pack, the cold pack, and the neutral object and then simply hold each item for 30 seconds. After holding each item, participants made ratings of thermal intensity (how warm and cool the item felt), their feelings of connection (how connected did you feel when holding this item?), and to enhance the cover story, whether they would recommend the product to a friend (How likely are you to recommend this product to a friend?). In addition, general positive affect to holding the items was assessed with the following items: How good did it feel to hold the item?; How much did you enjoy holding this item?; How pleasant was it to hold?; How unpleasant was it to hold? (reverse-coded); How soothing was it to hold this item?; How comforted did you feel when holding this item?; How appreciative or grateful did you feel when holding this item? In scale reliability analyses examining ratings during each drug (placebo, naltrexone) and each type of object (warm pack, neutral object, cold pack) these items showed high convergence (alpha’s range between .86 and .92) and so responses to the seven items were combined into a single measure to look more generally at changes in positive affect.
Statistical Analyses
For ease of interpretation and to be consistent with the way that this task has been analyzed previously (Inagaki & Eisenberger, 2013), difference scores were calculated, subtracting mean ratings in the neutral object control condition from mean ratings in each of the other temperature conditions (warm pack and cold pack). To assess the effect of the pharmacological manipulation on each of the outcomes—thermal intensity, feelings of connection, and positive affect—we conducted 2 (condition: placebo vs. naltrexone) × 2 (type of object: warm pack vs. cold pack) × 2 (order: placebo first vs. naltrexone first) × 2 (gender: male vs. female) repeated measures analyses of variance (ANOVAs) in SPSS. As expected, no interactions with order or gender were found and so both variables were dropped from further analyses.
Results
Validating the reliability of the study drug blind, participants only guessed correctly that they were on placebo 61% of the time and only guessed correctly 55% of the time that they were on naltrexone. These percentages were not significantly different from chance (50%) for either placebo (χ2 (1) = .35, p = .55) or naltrexone (χ2 (1) = .14, p = .71). Physical symptoms were also recorded for each day that participants were on the study drugs (4 days when on naltrexone and 4 days when on placebo). There were significant increases in headaches, dizziness/faintness, nausea, and appetite increase/decrease when on naltrexone compared to placebo (p’s < .05). However, mean ratings for the symptoms were low (M naltrexone = .39, M placebo = .10 on a scale of 0 - 4) suggesting any symptoms experienced due to naltrexone were very mild. Furthermore, there were no differences in how distressing participants found the symptoms when on naltrexone (M = 1.87, SD = 1.07) compared to placebo (M = 1.55, SD = .84, t(20) = .88, p = .39) and overall, levels of distress about the symptoms were also low (ratings between 1 and 2 on a 0 –7 scale) suggesting that the symptoms are unlikely to influence the main study outcomes.
Main effects of temperature manipulation on self-reports
We first examined the effect of the temperature manipulation on ratings of thermal intensity. Confirming that the temperature manipulation worked, there was a significant effect of type of object on ratings of warmth (F(1, 30) = 887.55, p < .001, see Table 1 for raw descriptive values) and coolness (F(1,30) = 727.42, p < .001). Hence, the warm pack was rated as warmer (M = 2.61, SD = 1.28, 95% CI [2.14, 3.08]) than the cold pack (M = −1.94, SD = 1.12, 95% CI [−2.35, −1.52]), and the cold pack was rated as cooler (M = 4.19, SD = 1.60, 95% CI [3.60, 4.78]) than the warm pack (M = −1.23, SD = 1.50, 95% CI [−1.78, −.68]).
Table 1.
Ratings for each condition and each object type.
| Placebo Means (SD) |
Naltrexone Means (SD) |
|
|---|---|---|
| A. Warmth ratings | ||
| Warm Pack | 5.55 (0.85) | 5.65 (0.98) |
| Neutral Object | 2.94 (1.12) | 3.06 (1.34) |
| Cold Pack | 1.00 (0.00) | 1.03 (0.18) |
| B. Coolness ratings | ||
| Warm Pack | 1.35 (0.88) | 1.16 (0.45) |
| Neutral Object | 2.58 (1.54) | 2.42 (1.36) |
| Cold Pack | 6.77 (0.50) | 6.65 (0.55) |
| C. Social connection ratings | ||
| Warm Pack | 3.77 (1.91) | 3.29 (1.94) |
| Neutral Object | 2.68 (1.64) | 2.90 (1.66) |
| Cold Pack | 1.97 (1.47) | 2.16 (1.61) |
| D. Positive affect ratings | ||
| Warm Pack | 5.25 (1.20) | 5.28 (1.15) |
| Neutral Object | 4.42 (1.26) | 4.72 (1.26) |
| Cold Pack | 2.37 (1.23) | 2.83 (1.50) |
We next examined the effect of the temperature manipulation on subjective ratings of social connection and positive affect. Replicating previous work (Inagaki & Eisenberger, 2013) and confirming our first hypothesis, participants reported greater feelings of connection after holding the warm pack (M = 1.10, SD = 1.66, 95% CI [0.49, 1.71]) compared to holding the cold pack (M = −.74, SD = 1.53, 95% CI [−1.30, −0.18]); F(1,30) = 16.53, p < .001, d = .88, see Table 1 for raw descriptive values). In regards to general positive affect, participants reported increased pleasure to holding the warm pack (M = .83, SD = 1.36, 95% CI [.33, 1.33]) compared to holding the cold pack (M = −2.05, SD = 1.48, 95% CI [−2.59, −1.51]; F(1, 30) = 71.75, p < .001, d = 1.53).
Effect of naltrexone on self-reports to temperature manipulation
Thermal intensity
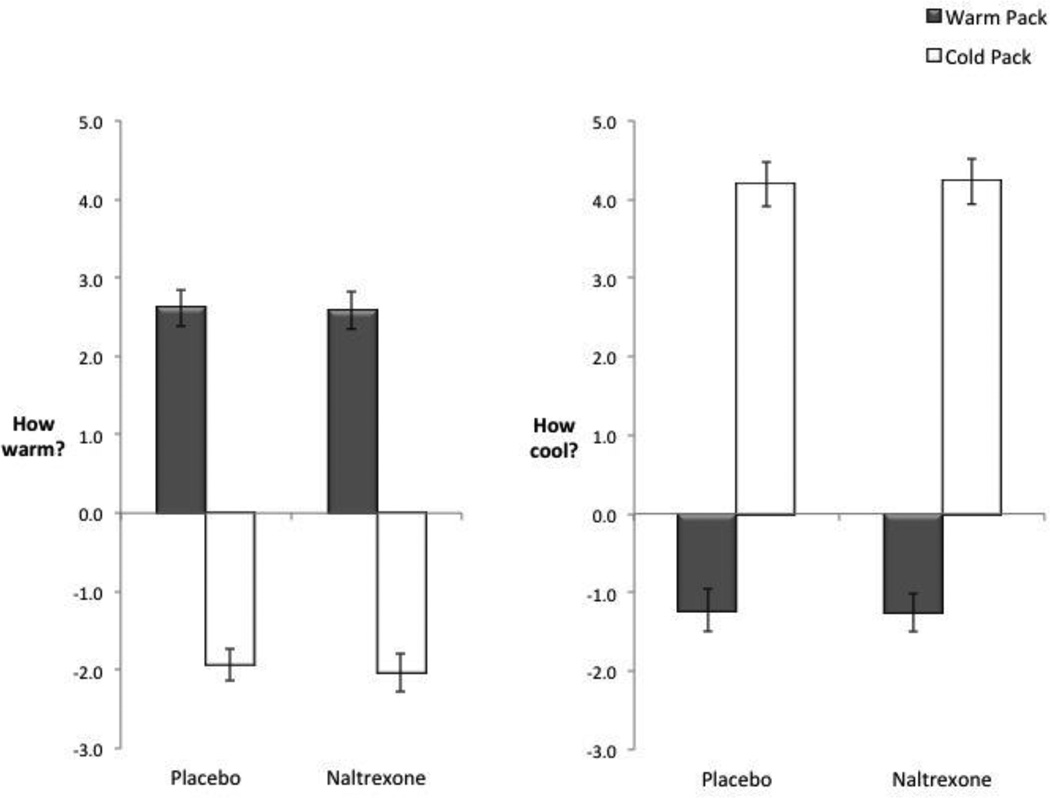
To evaluate the effect of naltrexone on self-reports in response to the temperature manipulation, we ran 2 (condition: placebo vs. naltrexone) × 2 (type of object: warm pack vs. cold pack) repeated measures ANOVA. First, as expected, no interactions between drug condition and type of object were found for ratings of thermal intensity for how warm the objects were rated (F(1, 30) = .13, p = .72, Fig. 1 and also see Table 1 for raw descriptive values) or how cool the objects were rated (F(1, 30) = .22, p = .65). Thus building on prior work showing that naltrexone does not seem to alter objective assessments of sensory intensity (Bertino, Beauchamp, & Engelman, 1991; McCaul, Wand, Eissenberg, Rohde, & Cheskin, 2000; Morin et al., 1999), naltrexone did not alter perceptions of how physically warm or cool the objects were.
Figure 1.
The effect of naltrexone on perceived thermal intensity to holding a warm (vs. neutral) pack and a cold (vs. neutral) pack. Naltrexone (vs. placebo) did not alter perceptions of how warm or cool the objects were rated. Error bars reflect standard errors. Plotted values are difference scores (ratings to the warm pack vs. neutral object and ratings to the cold pack vs. neutral object).
Social connection
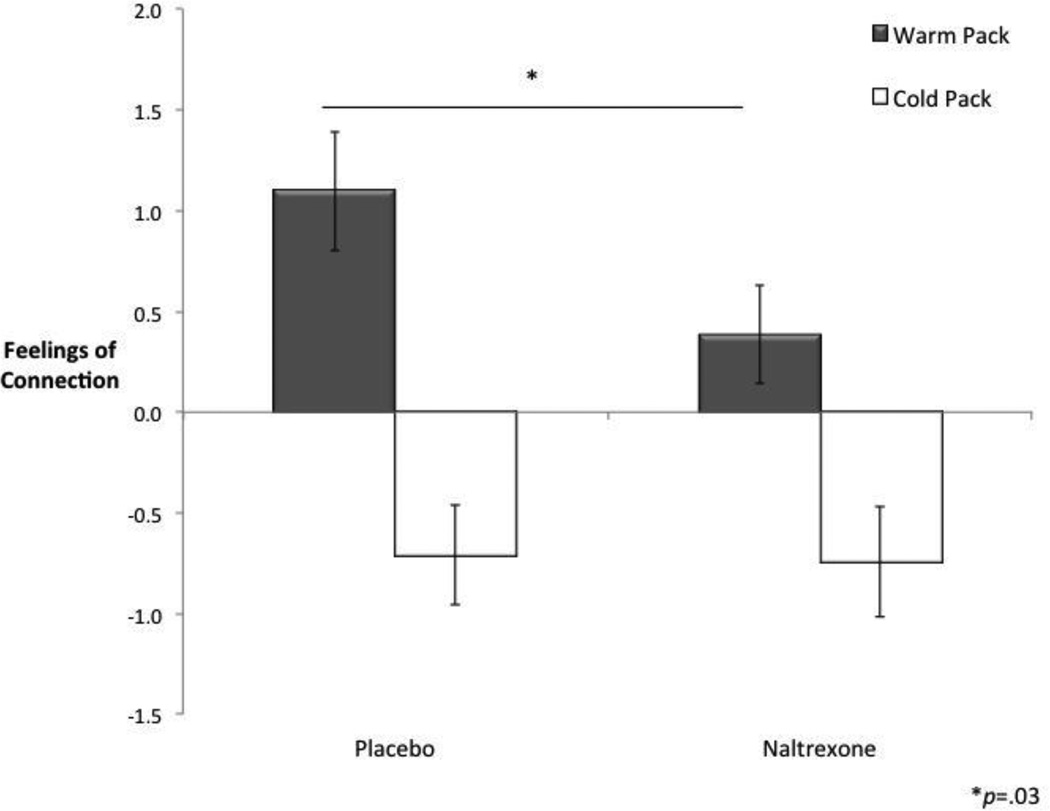
There was, however, a significant interaction between drug condition and type of object on feelings of connection (F(1, 30) = 5.03, p < .05, Fig. 2), such that naltrexone (compared to placebo) significantly reduced feelings of connection to holding the warm pack (F(1,30) = 4.58, p < .05, d = .39, placebo 95% CI [.49, 1.71], naltrexone 95% CI [−.11, .89]) but there was no effect of naltrexone on feelings of connection to holding the cold pack (F(1, 30) = .01, p = .93, d = .02, placebo 95% CI [−1.21, −.21], naltrexone 95% CI [−1.30, −.18]). This finding suggests that the reductions in feelings of connection that occurred following naltrexone administration were specific to physical warmth.
Figure 2.
The effect of naltrexone on feelings of connection to holding a warm (vs. neutral) and cold (vs. neutral) pack. Naltrexone (vs. placebo) led to reduced feelings of connection to holding a warm pack (vs. neutral object). No such effect of naltrexone was found for holding a cold pack. Error bars reflect standard errors. Plotted values are difference scores (ratings to the warm pack vs. neutral object and ratings to the cold pack vs. neutral object).
Positive affect
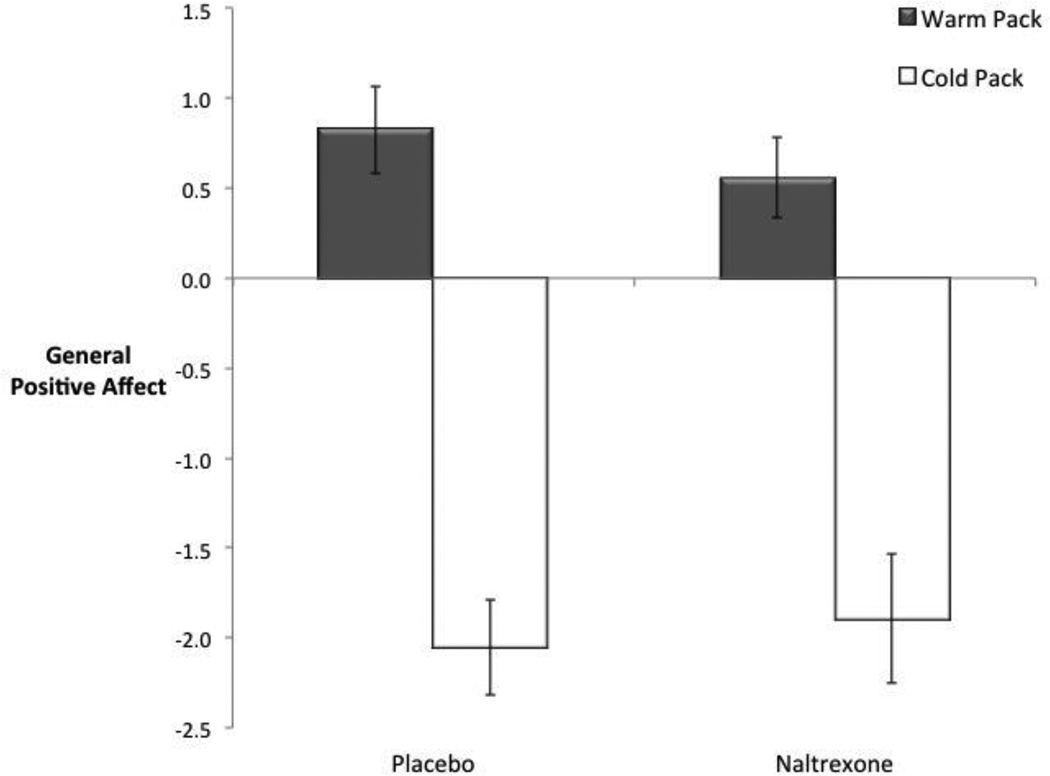
Finally, the effect of naltrexone on more global positive affect was also assessed to see whether opioids were specific to feelings of connection or whether opioid-blockade reduced all positive affect more generally. No interaction between drug condition and type of object was found for positive affect (F(1, 30) = 1.45, p = .24, Fig. 3 and also see Table 1 for raw descriptive values) suggesting that naltrexone did not reduce more general positive affect to holding the objects.
Figure 3.
The effect of naltrexone on general positive affect to holding a warm (vs. neutral) pack and cold (vs. neutral) pack. Naltrexone (vs. placebo) did not alter positive affect ratings to holding the objects. Error bars reflect standard errors. Plotted values are difference scores (ratings to the warm pack vs. neutral object and ratings to the cold pack vs. neutral object).
Together, these findings suggest that naltrexone had specific effects on reducing feelings of social connection after holding something warm, but did not seem to affect ratings of thermal intensity or general positive affect.
Discussion
Social and physical warmth are linked, not just by metaphorical language, but also by neurobiological mechanisms. Here, we show for the first time that an opioid antagonist reduced feelings of social connection in response to holding a warm object (vs. a cold pack), but did not affect ratings of thermal intensity or more general positive affect. We have already shown that experiences of social warmth and physical warmth share overlapping neural circuitry (Inagaki & Eisenberger, 2013). These results further extend an understanding of the basic neurobiological mechanisms that underlie social connection and lend further support to the theory that social and physical warmth share similar neurobiological mechanisms (Panksepp, 1998).
Why might social and physical warmth overlap? An integral part of human experience is connecting with others. Even from birth, we need others as much as we need warmth, food, and water in order to survive. However, due to our altricial nature at birth, the only way to obtain warmth, food, and water after birth is via other human beings, our caregivers. Thus, our basic need for warmth may have been fulfilled concurrently with the presence of a caregiver and so the systems that help regulate temperature may have then been coopted to also help “regulate” social experience with warmth signaling connection and cold signaling the loss of connection (Williams & Bargh, 2008; Williams et al., 2009). While the importance of physical warmth from social interaction has long been suggested by previous work in animals (Blumberg, Efimova, & Alberts, 1992; Harlow, 1958; Stone, Bonnet, & Hofer, 1976), only recently have we begun to understand the extent of the overlap in humans (IJzerman & Semin, 2009; Inagaki & Eisenberger, 2013; Williams & Bargh, 2008).
The current results add to the existing literature that suggests there is a specific effect of naltrexone on affective, but not more “objective” sensory experience. In other words, objective evaluations of a stimulus, such as its intensity, do not appear to be opioid-mediated, whereas affective experience may be. For example, naltrexone (vs. placebo) has no effect on the perceived sweetness of sugary drinks, but does reduce the pleasantness of consuming the drinks (Bertino et al., 1991). Similarly, naltrexone, particularly at high doses (100mg), reduces how much participants report liking an alcoholic beverage, but does not affect more objective indicators of drunkenness such as how “high” or intoxicated they feel (McCaul et al., 2000). In regard to temperature perception, morphine has been shown to reduce how unpleasant noxious thermal stimuli feel, but do not alter how warm or cold the same stimuli are rated (Morin et al., 1999). Collectively, these results suggest that endogenous opioid activity may be more tightly linked with valenced affective experience.
Although opioids are known to be involved more generally in pleasurable affective experiences such as the pleasure from drinking alcohol (McCaul et al., 2000) and the pleasure of consuming sweet (Pecina & Berridge, 2005) and other palatable foods (Yeomans & Wright, 1991), naltrexone specifically reduced feelings of connection to holding a warm pack in the current study, but did not alter other pleasant feelings (e.g. how enjoyable, good, pleasant). This pattern of results is consistent with opioid theories of social attachments (Depue & Morrone-Strupinsky, 2005; Machin & Dunbar, 2011; Panksepp, 1994) which suggest that in the context of social bonding, opioids may be more critical for the pleasure from connection as opposed to other positive feelings. Why might this be the case? If remaining socially bonded and connected to others is critical for survival (e.g. Baumeister & Leary, 1995), then humans should be particularly sensitive to the potential loss of connection because this may have more serious consequences for health and well-being than changes to nonspecific pleasure. Thus, to the extent that physical warmth also signals social connection, any feelings of connection that arise from experiences of physical warmth may also be particularly sensitive to opioid antagonism.
In conclusion, physical warmth has emerged as an important contributor to social warmth—the experience of feeling loved by and connected to others. Furthering the importance of µ-opioids for social bonding more generally, the current study reveals the importance of opioids to feelings of connection that stem from experiences of physical warmth and help uncover another mechanism through which social bonds are regulated.
Acknowledgements
This study was supported by a Dissertation Research Award from UC Berkeley’s Greater Good Science Center awarded to T.K.I. We extend our thanks to UCLA’s Clinical and Translational Science Institute (CTSI: UL1TR000124), Bill Hirokawa, of the UCLA Investigational Drug Section, Keely Muscatell, for assistance with drug randomization and the study blind, Lara Ray for her guidance on the study drug administration, and Cory Higgs and Molly Howland for assistance running the study. We also acknowledge generous support from the National Institutes of Health (R01-AG034588; R01-AG026364; R01 CA160245-01; R01-CA119159; R01 HL095799; R01 DA032922-01; P30-AG028748 to M. R. I.), and the Cousins Center for Psychoneuroimmunology.
Footnotes
How distressing participants found their daily symptoms was added partway into the study and obtained from the final 21 participants.
References
- Acevedo BP, Aron A, Fisher HE, Brown LL. Neural correlates of long-term intense romantic love. Social Cognitive and Affective Neuroscience. 2012;7:145–159. doi: 10.1093/scan/nsq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler MW, Geller EB, Roscow CE, Cochin J. The opioid system and temperature regulation. Annual Review of Pharmacology Toxicology. 1988;28:429–449. doi: 10.1146/annurev.pa.28.040188.002241. [DOI] [PubMed] [Google Scholar]
- Ainsworth MD, Blehar MC, Waters E, Wall S. Patterns of attachment: a psychological study of the strange situation. Hillsdale, NJ: Erlbaum; 1978. [Google Scholar]
- Aron A, Fisher H, Mashek D, Strong G, Li H, Brown L. Reward, motivation and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology. 2005;93:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin. 1995;117:497–529. [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural basis of romantic love. NeuroReport. 2000;11:3829–3834. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Borsook D. Human brain activation under controlled thermal stimulation and habituation to noxious heat: An fMRI study. Magnetic Resonance in Medicine. 1999;41:1044–1057. doi: 10.1002/(sici)1522-2594(199905)41:5<1044::aid-mrm25>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Bertino M, Beauchamp GK, Engelman K. Naltrexone, an opioid blocker, alters taste perception and nutrient intake in humans. American Journal of Physiology. 1991;261:R59–R63. doi: 10.1152/ajpregu.1991.261.1.R59. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Efimova IV, Alberts JR. Ultrasonic vocalizations by rat pups: The primary importance of ambient temperature and the thermal significance of contact comfort. Developmental Psychobiology. 1992;25:229–250. doi: 10.1002/dev.420250402. [DOI] [PubMed] [Google Scholar]
- Bowlby J. A secure base: Parent-child attachment and healthy human development. New York, NY: Basic Books; 1988. [Google Scholar]
- Bujarski S, MacKillop J, Ray LA. Understanding naltrexone mechanism of action and pharmacogenetics in asian americans via behavioral economics: a preliminary study. Experimental and Clinical Psychopharmacology. 2012;20:181–190. doi: 10.1037/a0027379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SM, Murphy MT, Lipton JM, Clark WG. Effects of morphine on body temperature of squirrel monkeys of various ages. Brain Research Bulletin. 1983;10:305–308. doi: 10.1016/0361-9230(83)90095-3. [DOI] [PubMed] [Google Scholar]
- Davis KD, Kwan CL, Crawley AP, Mikulis DJ. Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. Journal of Neurophysiology. 1998;80:1533–1546. doi: 10.1152/jn.1998.80.3.1533. [DOI] [PubMed] [Google Scholar]
- Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. Behavioral and Brain Sciences. 2005;28:350–395. doi: 10.1017/S0140525X05000063. [DOI] [PubMed] [Google Scholar]
- Erdfelder E, Faul F, Buchner A. GPOWER: A general power analysis program. Behavior Research Methods, Instruments, & Computers. 1996;28:1–11. [Google Scholar]
- Handler CM, Geller EB, Adler MW. Effect of µ-, kappa-, and delta-selective opioid agonists on thermoregulation in the rat. Pharmacology Biochemistry & Behavior. 1992;43:1209–1216. doi: 10.1016/0091-3057(92)90504-9. [DOI] [PubMed] [Google Scholar]
- Harlow HF. The nature of love. American Psychologist. 1958;13:673–685. doi: 10.1037/h0029383. [DOI] [PubMed] [Google Scholar]
- IJzerman H, Semin GR. The thermometer of social relations: Mapping social proximity on temperature. Psychological Science. 2009;20:1214–1220. doi: 10.1111/j.1467-9280.2009.02434.x. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Eisenberger NI. Shared neural mechanisms underlying “social warmth” and physical warmth. Psychological Science. 2013;24:2272–2280. doi: 10.1177/0956797613492773. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Ray LA, Irwin MR, Way BM, Eisenberger NI. Opioids and social bonding: Naltrexone reduces feelings of social connection. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Williams LE, Clark M, Gray JR, Bargh JA. Physical temperature effects on trust behavior: The role of the insula. Social Cognitive and Affective Neuroscience. 2011;6:507–515. doi: 10.1093/scan/nsq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Wagner HN, Tanada S, Frost JJ, Bice AN, Dannals RF. Duration of occupancy of opiate receptors by naltrexone. The Journal of Nuclear Medicine. 1988;29:1207–1211. [PubMed] [Google Scholar]
- Machin AJ, Dunbar RIM. The brain opioid theory of social attachment: a review of the evidence. Behaviour. 2011;148:985–1025. [Google Scholar]
- McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ. Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmocology. 2000;22:480–492. doi: 10.1016/S0893-133X(99)00147-5. [DOI] [PubMed] [Google Scholar]
- Morin C, Duncan GH, Lavigne G, Boily J, Bushnell M. Differential effects of morphine on pain and temperature perception in human volunteers. European Journal of Pain. 1999;3:193–204. doi: 10.1053/eujp.1999.0131. [DOI] [PubMed] [Google Scholar]
- Olausson H, Charron J, Marchand S, Villemure C, Strigo IA, Bushnell MC. Feelings of warmth correlate with neural activity in right anterior insular cortex. Neuroscience Letters. 2005;389:1–5. doi: 10.1016/j.neulet.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective neuroscience. New York, NY: Oxford University Press; 1998. [Google Scholar]
- Panksepp J, Bean NJ, Bishop P, Vilberg T, Sahley TL. Opioid blockade and social comfort in chicks. Pharmacology Biochemistry & Behavior. 1980;13:673–683. doi: 10.1016/0091-3057(80)90011-8. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Nelson E, Bekkedal M. Brain systems for the mediation of social separation-distress and social-reward: Evolutionary antecedents and neuropeptide intermediaries. Annals of the New York Academy of Sciences. 1997;807:78–100. doi: 10.1111/j.1749-6632.1997.tb51914.x. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Hedonic hot spots in nucleus accumbens shell: where do µ-opioids cause increased hedonic impact of sweetness? The Journal of Neuroscience. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Chin PF, Miotto K. Pharmacogenetics of naltrexone in asian americans: a randomized placebo-controlled laboratory study. Neuropsychopharmacology. 2011;37:445–455. doi: 10.1038/npp.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F, Parris BA. Warm pleasant feelings in the brain. NeuroImage. 2008;41:1504–1513. doi: 10.1016/j.neuroimage.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Schweiger D, Stemmler G, Burgdorf C, Wacker J. Opioid receptor blockade and warmth-liking: effects on interpersonal trust and frontal asymmetry. Social Cognitive and Affective Neuroscience. 2014;9:1608–1615. doi: 10.1093/scan/nst152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Bonnet KA, Hofer MA. Survival and development of maternally deprived rats: Role of body temperature. Psychosomatic Medicine. 1976;38:242–249. doi: 10.1097/00006842-197607000-00002. [DOI] [PubMed] [Google Scholar]
- Spencer RL, Hruby VJ, Burks TF. Body temperature response profiles for selective mu, delta and kappa opioid agonists in restrained and unrestrained rats. The Journal of Pharmacology and Experimental Therapeutics. 1988;246:92–101. [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JBW. Validation and utility of a self-report version of PRIME-MD: the PHQ Primary Care Study. Journal of the American Medical Association. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Kadohisa M, Rolls ET. Primate insular/opercular taste cortex: Neuronal representations of the viscosity, fat texture, grittiness, temperature, and taste of foods. Journal of Neurophysiology. 2004;92:1685–1699. doi: 10.1152/jn.00321.2004. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Kim YK, Wand GS, Dannals RF, Lee JS, Frost JJ, McCaul ME. Differences in delta- and mu-opioid receptor blockade measured by positron emission tomography in naltrexone-treated recently abstinent alcohol-dependent subjects. Neuropsychopharmacology. 2008;33:653–665. doi: 10.1038/sj.npp.1301440. [DOI] [PubMed] [Google Scholar]
- Williams LE, Bargh JA. Experiencing physical warmth promotes interpersonal warmth. Science. 2008;322:606–607. doi: 10.1126/science.1162548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Huang JY, Bargh JA. The scaffolded mind: higher mental processes are grounded in early experience of the physical world. European Journal of Social Psychology. 2009;39:1257–1267. doi: 10.1002/ejsp.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans MR, Wright P. Lower pleasantness of palatable foods in nalmefene-treated human volunteers. Appetite. 1991;16:249–259. doi: 10.1016/0195-6663(91)90062-w. [DOI] [PubMed] [Google Scholar]