Abstract
Metabolic and behavioral changes that occur during pregnancy have well-known effects on maternal and fetal health during the immediate pregnancy and now are thought to be a catalyst for future health throughout later life. Recommendations for appropriate gestational weight gain (GWG) and lifestyle modifications during pregnancy have changed throughout history as more is known about this crucial time. Herein we discuss the current GWG recommendations and the impact of pregnancy and excess GWG gain on the current and future health of women and children including risk of obesity, gestational diabetes, type II diabetes, cardiovascular disease, and metabolic syndrome.
Keywords: pregnancy, diabetes, adiposity, visceral fat, food intake
Gestational weight gain
Gestational weight gain (GWG), or the total amount of weight gained in pregnancy (from conception through to the time of delivery), is highly variable among women. The decreased incidence of preeclampsia with declining food supply during World War I supported the cultural desire to be thin even through pregnancy and there are several reports that women were advised to limit weight gain in pregnancy to less than 15 lbs. This was primarily based on the belief that the growing fetus was able to derive the nutrients needed for adequate growth, and ‘excess’ calories consumed would be stored as maternal fat. 1 The increase incidence stillbirths, low birth weight, and infant mortality during the Dutch famine in 1944 however then lead to a liberalization of GWG recommendations, but never to the current well-accepted social dogma of ‘eating for two’. 1 Globally, it was the increased prevalence of underweight women entering pregnancy together with the issue of maternal under-nutrition that spurred the first international guidelines for weight gain in pregnant women in 1990. 2 With the increased worldwide prevalence of overweight and obesity in reproductive aged women and the observation that many women were exceeding the previous Institute of Medicine (IOM) recommendations, the IOM reconvened and revised the guidelines in 2009. 3 Both the 1990 and 2009 IOM recommendations are specific to the preconception body mass index (BMI) of the woman. Instead of using the Metlife BMI classifications, the 2009 recommendations use the now commonly adopted World Health Organization (WHO) classifications for BMI where underweight is defined as <18.5 kg/m2, normal weight as 18.5 – 24.9 kg/m2, overweight as 25 – 29.9 kg/m2, and obese as ≥30 kg/m2. 4 It is recommended that to prevent adverse maternal as well as infant outcomes, women who are normal weight at the time of conception limit total weight gain in pregnancy to 11.5-16 kg (25-35 lbs), overweight women to 7-11.5 kg (15-25 lbs) and obese women (all classes) to 5-9 kg (11-20 lbs). 3 There is a collection of strong evidence that increased incidences of gestational diabetes mellitus, 5 labor and delivery complications 5 and postpartum weight retention 6,7 are associated with a BMI outside the normal weight range (18.5-24.9 kg/m2) 8 and weight gain above the IOM guidelines, 9 which gives rise to the more stringent weight gain guidelines for women who are overweight or obese at conception. Furthermore, preconception BMI and GWG also affects infant outcomes; infants of overweight/obese mothers are more likely to be preterm, 10 large for gestational age 11 and have an increased risk of developing childhood obesity. 12 The total amount of weight gained during pregnancy, regardless of preconception BMI classification, has dramatically increased over the last 4 decades from 10 to 15 kg (22-33 lbs). 13 The most recent report from the U.S. Centers of Disease Control (2011 Pregnancy Nutrition Surveillance System) shows that more than 48% of all women exceed the 2009 IOM guidelines for appropriate weight gain during pregnancy. 14 Excess GWG in the 2011 CDC report was 38% for normal weight women and was 1.5 times higher in overweight and obese women at 59% and 56%, respectively. While the prevalence of excess GWG within pregravid BMI categories has really not shifted in the past decade, the number of women entering pregnancy as either overweight or obese however, has increased significantly from 30% in 1983 to 54% in 2011, almost 30 years later (Figure 1). Since overweight and obese women are twice as likely to exceed the IOM guidelines, the prevalence of excess GWG can be forecast to continue to rise resulting in only one third of pregnant women achieving the recommended amount of weight gain that is believed to lead to healthy maternal, neonate and infant outcomes. Gestational weight gain therefore presents a major public health concern of the 21st Century and evidenced-based prevention programs are needed for implementation on a wide scale.
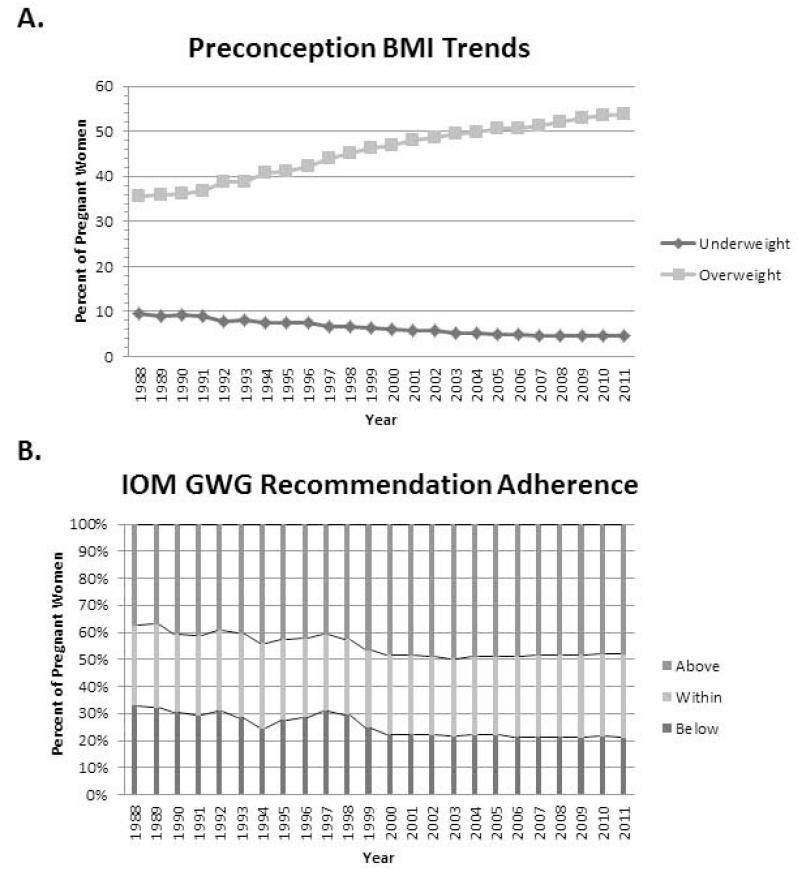
Figure 1.
Preconception BMI has increased steadily since 1988 resulting in more and more women entering pregnancy as overweight or obese (A). With the steady rise in preconception BMI, a larger percent of pregnant women are exceeding the IOM gestational weight gain (GWG) recommendations (B). Light gray squares = % of women entering pregnancy as overweight; Dark gray diamonds = % of women entering pregnancy as underweight (A). Dark gray bar = % of women who gain below the IOM recommendations; Light gray bar = % women who gain within the IOM recommendation; Gray bar = % of women who gain above or exceed the IOM recommendations (B).
Composition of GWG
During pregnancy weight gain is obviously an expected outcome and a minimal amount of weight gain has been determined as essential to provide the nutritional support for fetal growth and the deposition of maternal energy in preparation for lactation postpartum. Therefore total is the sum of both gains in maternal and fetal tissues. GWG is comprised of the accretion of water, protein (fat-free mass, FFM) and fat (fat mass, FM) in the fetus as well as the placenta, uterus and amniotic fluid, expansion of blood maternal volume, mammary gland and maternal adipose tissue. The maternal gains in adipose tissue can be deposited into visceral (central) and subcutaneous (peripheral) adipose tissue depots. The degree to which pregnant women partition excess energy or weight in adipose tissue or other compartments is susceptible to significant inter-individual variation, 15 and understanding the composition of the total weight gained is important as the potential metabolic activity and contribution to chronic disease risk is specific to each fat depot. For example, fat accumulation in visceral depots and around central organs such as the liver positively correlates with insulin resistance, cardiovascular disease risk, and metabolic syndrome. 16
There are few studies that have carefully quantified the composition of weight gain in pregnancy. Much of this is due to the complexity of this work since routine measures for body composition assessment in research such as dual-energy x-ray absorptiometry (DXA) are contraindicated in pregnant women. While magnetic resonance imaging is safe in pregnancy and can provide important information regarding the composition of GWG, particularly abdominal fat, groups are still perfecting these measures 17 and studies are currently collecting this data (NCT01616147). Up until now, the four-compartment model has been used to estimate the composition of weight gain in pregnant women. The four-compartment model as the name suggests requires measurement of body components in four compartments, namely: 1) total body water (TBW), 2) body mass and volume (body density), and 3) bone mineral content (BMC). Total body water is measured using deuterium or 18O labeled water dilution. Body density and volume is measured with a hydrostatic underwater weighing system correcting for residual lung volume. Bone mineral content which is assumed to remain relatively constant throughout pregnancy, is derived from a DXA measurement that is done prior to conception or during the postpartum period. With measures of each of the three compartments, an equation is applied to estimate fat mass 18,19:
| Equation 1 |
A whole-body counter with NaI scintillation detectors can be safely used to measure naturally occurring 40K to estimate total body potassium. Total body potassium measures can be used to estimate FFM 20-22:
| Equation 2 |
Or FFM can more generally be assumed to equal weight minus FM.
These studies clearly show that throughout pregnancy total body water increases to support increased maternal body size and fetal growth mostly as extracellular and amniotic fluids. Maternal FM and FFM are expected to expand throughout pregnancy even when the IOM recommendations are met to support breastfeeding during the postpartum period. 23 With weight gain, more is generally not better, especially for women with higher preconception BMI as exceeding IOM guidelines results in a disproportionate increased maternal FM with little to no improvements in other maternal and fetal components. 24 Data using the 4-compartment model of body composition estimation showed that majority of the variance in GWG is accounted for by increases in FM. The amount of FM gained is associated with total GWG and majority of the weight gained in excess of the IOM guidelines is deposited in maternal adipose tissue. 19 With the increased allowance for GWG with lower BMI, normal weight women accrue more FM than overweight or obese women, however, studies show in women with a normal pregravid BMI, majority of the adipose tissue is deposited in the subcutaneous depots of the hips and thighs, 25,26 but some visceral fat is accumulated in late pregnancy. 27 Data suggests that obese women, who have more subcutaneous fat prior to conception, tend to accumulate adipose during pregnancy in visceral depots. 26 While there are seldom reports of body fat distribution in pregnancy there is one study to suggest that those women who gain more visceral fat during the first trimester are more likely to develop impaired glucose tolerance later in pregnancy, 28 and visceral adipose tissue has been positively correlated with infant birth weight. 29 The number of women with central obesity has increased from 1997 to 2007, especially in reproductive age women, and central obesity increases with increased parity. These data support the idea that not all fat gain in pregnancy can be treated equally and visceral fat accumulation which occurs in pregnancy, particularly obese pregnancies is a cause for postpartum health concerns. 30 Therefore, understanding how fat is deposited in maternal tissues during pregnancy is important not only because of the metabolic and inflammatory characteristics of fat stored in different depots giving rise to undesirable metabolic outcomes in pregnancy (e.g., GDM, dyslipidemia, hypertension, sleep disordered breathing), but also increasing risk for more rapid onset of chronic disease such as type 2 diabetes, postpartum.
Timing of GWG
The IOM guidelines assume a gain of 0.5-2 kg in the first trimester of pregnancy. 3 Much of the weight gained during the first trimester is due to early placental development and expansion of maternal blood volume. As expected, embryonic development and fetal growth are likely to have an insignificant contribution to weight gain in the first trimester. 23 Weight gained that is not attributed to products of conception in the early stages of pregnancy, contributes to an increase in maternal energy stores and weight gain. 19 Excess weight gain experienced in the first trimester has been shown to be predictive of excessive GWG for the entire pregnancy. Women with a normal weight preconception BMI have a 70% probably of excess total GWG when excess weight gain is experienced in the first trimester, while overweight and obese women 90% probability of excess total GWG. 31 This early excess weight gain, independent of total GWG, is associated with impaired maternal glucose tolerance later in pregnancy 32 and greater infant adiposity at birth. 31 These data point to the importance of having conversations about GWG and appropriate amounts of weight gain with patients very early in pregnancy and preferably during an office visit for preconception counselling.
Determinants of GWG
In non-pregnant humans energy intake and energy expenditure are the two primary determinants to energy balance. To support products of conception, fetal growth, expansion of maternal energy stores and tissues, and to sustain increased metabolic size, energy requirements during pregnancy are increased above preconception levels by approximately 200, 300, and 400 kcal/d in the first, second and third trimesters respectively. 18,33 Increased energy intake appears to be the greatest contributor to weight gain in most non-pregnant individuals, 34,35 and one could hypothesize therefore that excess GWG is due in large part to hyperphagia. However, hypothesis this is not supported by self-reported energy intake but before dismissing the hyperphagia hypothesis, objective assessments of energy intake are imperative to enhance our understanding of the role of diet in pregnancy as most individuals grossly underestimate energy intake by 15 – 20% making this an extremely unreliable measure or even an estimate. 36,37 Due to an increase in metabolic size, basal metabolic rate, the largest component of total daily energy expenditure, increases as pregnancy progresses. While total daily energy expenditure increases, activity related energy expenditure and physical activity level decreases, but not at the same magnitude of increased basal metabolic rate resulting in an overall increase in energy expenditure. 18,38 Since total daily energy expenditure increases as pregnancy progresses, an adaptation in energy expenditure or metabolic slowing is not the likely cause of excess GWG.
A key question for physicians in trying to counsel on GWG is how many calories are needed per day to support fetal growth and promote adherence to the IOM guidelines. The few studies that have been conducted to determine the energy requirements of gestation have recently been combined and developed into a complex differential equation. 33 The result is an easy-to-use calculator for estimating pregnancy energy needs during each trimester to achieve the recommended weight gain (http://www.pbrc.edu/research-and-faculty/calculators/gestational-weight-gain/). Using preconception inputs including maternal age, height and weight at conception, this online calculator creates a patient-specific IOM weight graph showing the target weight gain throughout pregnancy defined by IOM guidelines and provides the physician and patient with a calorie goal for each trimester.
EFFECT OF GESTATIONAL WEIGHT GAIN ON FUTURE WEIGHT STATUS
Pregnancy and the postpartum periods are not independent, but highly interrelated and influence one another. As discussed above, many of the physiological and anatomical changes during gestation particularly those to support the expansion of fat mass occur to prepare the mother for the energy costly process of lactation. However, this appears now to be a double-edged sword as the weight gain and metabolic changes that occur during pregnancy, if not controlled, can also have negative consequences for the postpartum woman and increase risk for developing chronic disease later in life.
Many reproductive age women attribute their increasing BMI to pregnancy. Over the course of 5 years postpartum, 89% of women with a normal weight BMI prior to pregnancy became overweight or obese. 39 The postpartum period of the first pregnancy becomes the preconception period of next pregnancy in which the mother begins at a higher BMI and thereby places herself at higher risk for excessive GWG, and increased postpartum BMI. This viscous cycle of repeated weight gain leads to increased prevalence of overweight and obesity in reproductive age and postmenopausal women, 8,40-42 and suggests that the endocrine, metabolic and/or behavioral changes which occur during the pregnancy are likely still playing a role in the weight status of mothers postpartum.
Gestational weight gain, the first step in this vicious cycle, is arguably the most deleterious consequence of pregnancy impacting the future health of the mother. Gestational weight gain in excess of the recommended IOM guidelines is now understood to be particularly important for setting the stage on subsequent health postpartum. 9,43 There are several reports including an extensive meta-analysis in over 69,000 women that indicate the excess weight gained in pregnancy is still retained some 20 years later. 44 Not surprisingly the degree of the weight retained postpartum is highly variable between women (Figure 2), and often dependent on the amount of GWG. Women with excess GWG retain the most weight postpartum, 44,45 and women with elevated preconception BMIs are more likely to experience postpartum weight retention. 46-48 It has been shown that only 11% of overweight and obese pregnant women return to their preconception weight within 5 years postpartum. 39
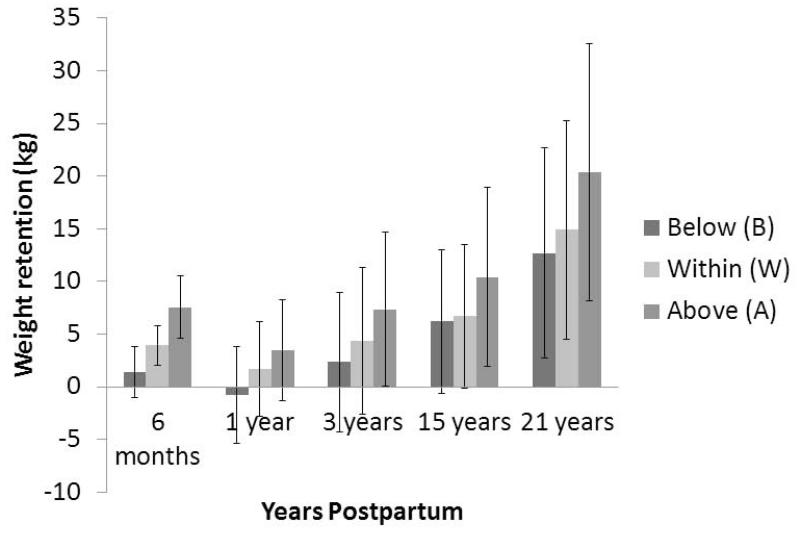
Figure 2.
Average weight gain in kilograms (kg) at different time points postpartum for women gaining below, within, and above IOM guidelines. Data is adapted from Nehring et al 44. Postpartum weight retention (PPWR) from six studies is averaged for the six month time point, PPWR from two studies is averaged for the one year time point, PPWR from two studies is averaged for the three year time point, and PPWR from one study was conducted for both 15 and 21 years postpartum. Weight retention was greatest for women gaining above IOM guidelines at every time point postpartum. Dark gray bars = women gaining below IOM recommendations; Gray bars = women gaining above IOM recommendations; Light gray = women gaining within IOM recommendations.
Postpartum weight retention and body composition
Though total postpartum weight retention is the most reported variable and fewer data on the composition of retained weight is available, it should be noted that the distribution of the postpartum weight being FFM or FM is particularly important. Body composition changes in the postpartum period, particularly in the early postpartum years, may be a tell-tale sign of future metabolic health problems. During the early postpartum period, the demand for expanded extracellular and intracellular fluid, mammary tissue (if not breastfeeding), and uterine tissue is no longer present, so weight attributed to these areas naturally decreases and will return to preconception status usually by 6-12 weeks. Despite the weight loss seen from those tissues, total fat mass is increased by 4% and visceral fat by 33% above preconception values postpartum. 49 Women with excessive GWG, have 3 times greater incidence of abdominal obesity at 8 years postpartum compared with women who gained weight in pregnancy as recommended. 50 In addition to the contribution of excess GWG to increased postpartum abdominal adiposity, parity also correlates positively with increased adnominal adiposity and visceral fat accumulation indicating that the vicious cycle of weight gain in pregnancy and postpartum may be worse than at first glance since weight is being retained as visceral adipose tissue, a potent precursor of metabolic disease. 51,52 Primiparas women gained 13 cm2 of total abdominal adipose tissue more than women with no children, with total 5 year mean increase in visceral adipose tissue of 15.1 cm2 with effects persisting to 15 years postpartum. 51 This observation provides evidence for the perpetuating cycle of weight gain in pregnancy and postpartum and amplification of both outcomes with subsequent pregnancies and points to the detrimental impact of pregnancy and excess GWG on visceral fat accumulation. It is interesting to note that the ability of breastfeeding to reduce total postpartum weight retention has mixed results due to varying levels of compensatory energy intake 53 by lactating women. However, when breastfeeding is sustained over a substantial period of time it leads to a reduction of maternal adipose tissue especially visceral fat. 54 Perhaps another reason why postpartum risk factors for metabolic disease are amplified by parity might be explained by the low levels of breastfeeding in women, especially in the United States. Women choosing to not breastfeed are therefore not having the natural metabolic drive postpartum to utilize the recently accumulated visceral fat. Just as the accumulation of visceral fat is understood to increase risk for pregnancy-related adverse outcomes such as gestational diabetes, 55 increased abdominal adiposity postpartum contributes to the progression of impaired glucose tolerance in pregnancy to insulin resistance and then type 2 diabetes postpartum and other co-morbidities such as cardiovascular disease and metabolic syndrome as the woman ages. 16
IMPACT OF PREGNANCY AND GESTATIONAL WEIGHT GAIN ON MATERNAL AND INFANT METABOLIC PHENOTYPING
Weight and body composition changes seen during pregnancy carry over to the postpartum period suggesting that the metabolic phenotype established during pregnancy remains throughout postpartum and the life span. A clear example of metabolic phenotypes established during pregnancy affecting the mother later in life is gestational diabetes (GDM) in pregnancy as a predictor for developing type II diabetes. 56,57 Gestational diabetes involves the inability of the body to make and use all the insulin it needs for pregnancy, leading to hyperglycemia which is harmful to fetal development. 58 While many women exceed the recommended guidelines for GWG, many more are also subject to developing gestational diabetes (GDM) in combination with pregnancy weight gain. GDM is a threat to long-term health for mother and baby alike, and typically diagnosed around the 24th week of pregnancy. Though not diagnosed until the 24th week of pregnancy, the metabolic environment resulting in GDM was established prior to conception or early in pregnancy through the contributions of elevated preconception BMI, poor dietary habits, visceral fat accumulation, and excess first trimester GWG. 32,59,60 It has been well-documented that the incidence of GDM has skyrocketed by 35-100% 61 between 1991 and 2000, and currently affects close to 7% of all pregnancies in the United States. 62 The most recent statement from the Centers of Disease Control lists the prevalence of GDM being as high as 9.2% 63. In a study of over 5,000 women, Lee et al., 57 demonstrated that the risk of developing diabetes increased with time both GDM and control groups, but was 9.6 times greater for patients with GDM. In the same study, the cumulative risk of developing type II diabetes for these GDM patients was over 25% at 15 years postpartum. 57
Gestational diabetes not only increases maternal risk for developing type II diabetes later in life, it is also a predictor of metabolic syndrome development by 10 years postpartum. 64,65 Metabolic syndrome is characterized by abdominal obesity, abnormal circulating lipid parameters, elevated blood pressure and serum plasma glucose, and insulin resistance, 66 which are symptoms that can easily develop in the pregnant state. 67The gestational period is a common time for exacerbation of these symptoms, and often develop concurrently with excess GWG and GDM alike. For example, Harper et al., 68 showed that 368 out of 635 women diagnosed with GDM gained more than IOM recommendations. Consequently, for every 1 pound per week increase in weight gain after diagnosis of GDM, there was a 36 to 83% increase in the risk of pregnancy related hypertension and other complications. Other symptoms linked to metabolic syndrome include elevated triglycerides, which are potentially detrimental lipid biomarkers that can be easily left unregulated throughout pregnancy. Excess triglycerides often go hand in hand with the diagnosis of type II diabetes, since both conditions involve the inability of the body to regulate sources of energy such as glucose and fat. Although the effects of excess gestational weight gain on triglycerides are sparse and inconclusive, a recent study in overweight/obese pregnant women showed that mothers with excess gestational weight gain also had higher baseline triglycerides (81.7±47.2 vs. 69.7±40.3 mg/dl) when compared to normal weight women. 69 Other studies, such as McClure et al., 50 found that although the 47% of women who gained excessively did not alter their triglycerides, they had the highest rate of metabolic syndrome. The effects of excess GWG go well beyond pregnancy as women who experience excess GWG, have higher incidence of metabolic syndrome and decreased HDL cholesterol levels at 8 years postpartum. 50
The consequences of GDM and excess GWG are not only confined to the mother, however. More immediate effects of GDM and excess GWG include high birth weight, large for gestational age babies, increased infant adiposity, cesarean section and preeclampsia. 70-72 While more long-term effects include children born to mothers with GDM are at higher risk for diabetes as adults. 73 Metabolic syndrome is more likely to affect offspring exposed to GDM in utero as pre-pubertal children born to women with GDM have had one, two, or three metabolic markers of IR with hypertriglyceridemia being the most prevalent (21%). 74 Similarly, Boney et al., 75 showed offspring from mothers with GDM were 3.6 times as likely to develop metabolic syndrome by age 11. The combination of GDM with maternal obesity had an even greater influence on pregnancy and neonatal outcomes than either variable alone, 72 and a groundbreaking study in over 8400 children in the United States identified children born to obese mothers were twice as likely to be obese by the age of 2. 12
Conclusion
The preconception, pregnancy, and postpartum periods are interrelated and have a significant impact on each other for both the mother and infant. Metabolic programming by way of GWG that occurs during pregnancy persists postpartum for the majority of women and the mother and child progress on a trajectory of increased metabolic risk factors such as central adiposity, dyslipidemia and glucose intolerance leading to metabolic disease such as obesity, type II diabetes, and metabolic syndrome. Excessive weight gain in pregnancy is a major public health concern with many opportunities for intervention whether it is through preconception, pregnancy, postpartum. Evidence-based trials are needed to attenuate and possibly even reverse the negative lifestyle behaviors and metabolic phenotypes developed during pregnancy that have lasting effects across the lifespan.
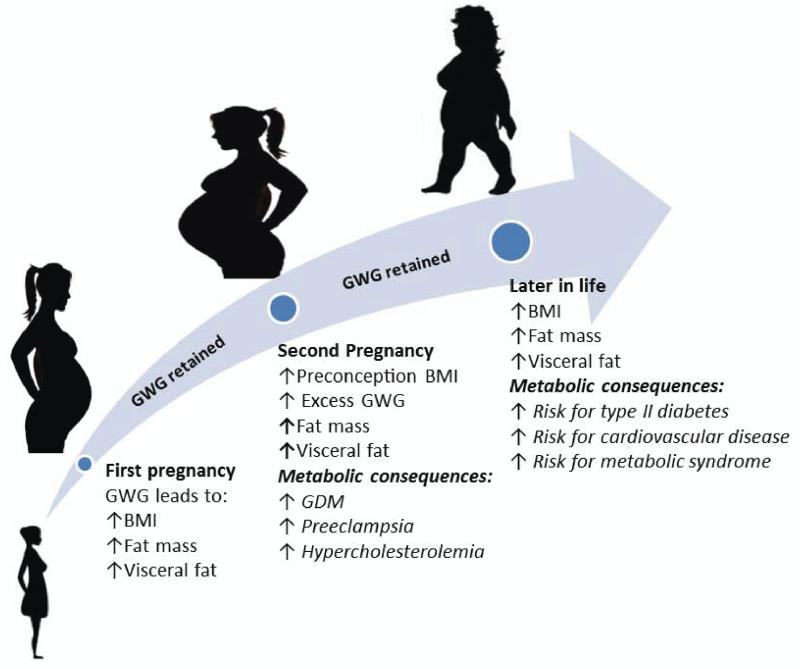
Figure 3.
Long- and short-term metabolic consequences of (excess) GWG and postpartum weight retention in reproductive age women. Excess GWG and postpartum weight retention, especially over the course of multiple pregnancies, results in harmful metabolic consequences due to greater increases in BMI, FM, and visceral fat. BMI = body mass index; GDM = gestational diabetes mellitus; LDL = low-density lipoprotein; HDL = high-density lipoprotein; TG = triglycerides.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health: U01DK094418, R01DK099175, 2T32DK6458411, 2P30DK072476.
Funding: This work is supported by grants from the National Institutes of Health: U01DK099148, 2T32DK645841.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Warsh C, editor. Gender, Health, and Popular Culture. Wilfrid Laurier University Press; Waterloo, Ontario, Canda: 2011. [Google Scholar]
- 2.IOM. Sciences NAo . Nutrition During Pregnancy: Part I: Weight Gain, Part II: Nutrient Supplements. Institute of Medicine (US), National Research Council (US), National Academy of Engineering (US), and National Academy of Sciences (US), Committee on Nutrition Status During Pregnancy and Lactation; 1990. [Google Scholar]
- 3.IOM . Weight Gain During Pregnancy: Reexaming the Guidelines. Institute of Medicine (US), National Research Council (US), Committee to Reexamine IOM Pregnancy Weight Guidelines; 2009. [Google Scholar]
- 4.WHO . Obesity: preventing and managing the global epidemic. Report of a WHO consultation. 2000. 0512-3054 (Print) 0512-3054 (Linking) [PubMed] [Google Scholar]
- 5.Viswanathan M, Siega-Riz AM, Moos MK, et al. Outcomes of maternal weight gain. Evidence report/technology assessment. 2008 May;(168):1–223. [PMC free article] [PubMed] [Google Scholar]
- 6.Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: a review of the evidence. Annals of behavioral medicine: a publication of the Society of Behavioral Medicine. 2003 Oct;26(2):149–159. doi: 10.1207/S15324796ABM2602_07. [DOI] [PubMed] [Google Scholar]
- 7.Rooney BL, Schauberger CW. Excess pregnancy weight gain and long-term obesity: one decade later. Obstetrics and gynecology. 2002 Aug;100(2):245–252. doi: 10.1016/s0029-7844(02)02125-7. [DOI] [PubMed] [Google Scholar]
- 8.Cohen AK, Chaffee BW, Rehkopf DH, Coyle JR, Abrams B. Excessive gestational weight gain over multiple pregnancies and the prevalence of obesity at age 40. International journal of obesity. 2014 May;38(5):714–718. doi: 10.1038/ijo.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keppel KG, Taffel SM. Pregnancy-related weight gain and retention: implications of the 1990 Institute of Medicine guidelines. American journal of public health. 1993 Aug;83(8):1100–1103. doi: 10.2105/ajph.83.8.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyerlein A, Lack N, von Kries R. Within-population average ranges compared with Institute of Medicine recommendations for gestational weight gain. Obstetrics and gynecology. 2010 Nov;116(5):1111–1118. doi: 10.1097/AOG.0b013e3181f1ad8b. [DOI] [PubMed] [Google Scholar]
- 11.Frederick IO, Williams MA, Sales AE, Martin DP, Killien M. Pre-pregnancy body mass index, gestational weight gain, and other maternal characteristics in relation to infant birth weight. Maternal and child health journal. 2008 Sep;12(5):557–567. doi: 10.1007/s10995-007-0276-2. [DOI] [PubMed] [Google Scholar]
- 12.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004 Jul;114(1):e29–36. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- 13.Kinnunen TI, Luoto R, Gissler M, Hemminki E. Pregnancy weight gain from 1960s to 2000 in Finland. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2003 Dec;27(12):1572–1577. doi: 10.1038/sj.ijo.0802471. [DOI] [PubMed] [Google Scholar]
- 14.DHHS. CDC . Pregnancy Nutrition Surveillance 2009 Report. U.S. Department of Health and Human Services and Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 15.Larciprete G, Valensise H, Vasapollo B, et al. Body composition during normal pregnancy: reference ranges. Acta Diabetol. 2003 Oct;40(Suppl 1):S225–232. doi: 10.1007/s00592-003-0072-4. [DOI] [PubMed] [Google Scholar]
- 16.Despres JP. Is visceral obesity the cause of the metabolic syndrome? Annals of medicine. 2006;38(1):52–63. doi: 10.1080/07853890500383895. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Ohkuchi A, Furukawa R, Matsubara S, Suzuki M. Establishing measurements of subcutaneous and visceral fat area ratio in the early second trimester by magnetic resonance imaging in obese pregnant women. The journal of obstetrics and gynaecology research. 2014 May;40(5):1304–1307. doi: 10.1111/jog.12364. [DOI] [PubMed] [Google Scholar]
- 18.Butte NF, Wong WW, Treuth MS, Ellis KJ, O’Brian Smith E. Energy requirements during pregnancy based on total energy expenditure and energy deposition. The American journal of clinical nutrition. 2004 Jun;79(6):1078–1087. doi: 10.1093/ajcn/79.6.1078. [DOI] [PubMed] [Google Scholar]
- 19.Lederman SA, Paxton A, Heymsfield SB, Wang J, Thornton J, Pierson RN., Jr. Body fat and water changes during pregnancy in women with different body weight and weight gain. Obstetrics and gynecology. 1997 Oct;90(4 Pt 1):483–488. doi: 10.1016/s0029-7844(97)00355-4. [DOI] [PubMed] [Google Scholar]
- 20.Ellis KJ, Shypailo RJ. Whole body potassium measurements independent of body size. Basic life sciences. 1993;60:371–375. doi: 10.1007/978-1-4899-1268-8_87. [DOI] [PubMed] [Google Scholar]
- 21.Boddy K, King PC, Hume R, Weyers E. The relation of total body potassium to height, weight, and age in normal adults. Journal of clinical pathology. 1972 Jun;25(6):512–517. doi: 10.1136/jcp.25.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beshyah SA, Freemantle C, Thomas E, Johnston DG. Comparison of measurements of body composition by total body potassium, bioimpedance analysis, and dual-energy X-ray absorptiometry in hypopituitary adults before and during growth hormone treatment. The American journal of clinical nutrition. 1995 Jun;61(6):1186–1194. doi: 10.1093/ajcn/61.6.1186. [DOI] [PubMed] [Google Scholar]
- 23.Hytten FE, Leitch I. The physiology of human pregnancy. 2nd ed. Blackwell Scientific Publications; Oxford, United Kingdom: 1980. [Google Scholar]
- 24.Luke B, Hediger ML, Scholl TO. Point of diminishing returns: when does gestational weight gain cease benefiting birthweight and begin adding to maternal obesity? The Journal of maternal-fetal medicine. 1996 Jul-Aug;5(4):168–173. doi: 10.1002/(SICI)1520-6661(199607/08)5:4<168::AID-MFM2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 25.Sohlstrom A, Wahlund LO, Forsum E. Total body fat and its distribution during human reproduction as assessed by magnetic resonance imaging. Basic life sciences. 1993;60:181–184. doi: 10.1007/978-1-4899-1268-8_41. [DOI] [PubMed] [Google Scholar]
- 26.Ehrenberg HM, Huston-Presley L, Catalano PM. The influence of obesity and gestational diabetes mellitus on accretion and the distribution of adipose tissue in pregnancy. American journal of obstetrics and gynecology. 2003 Oct;189(4):944–948. doi: 10.1067/s0002-9378(03)00761-0. [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita T, Itoh M. Longitudinal variance of fat mass deposition during pregnancy evaluated by ultrasonography: the ratio of visceral fat to subcutaneous fat in the abdomen. Gynecologic and obstetric investigation. 2006;61(2):115–118. doi: 10.1159/000089456. [DOI] [PubMed] [Google Scholar]
- 28.Martin AM, Berger H, Nisenbaum R, et al. Abdominal visceral adiposity in the first trimester predicts glucose intolerance in later pregnancy. Diabetes care. 2009 Jul;32(7):1308–1310. doi: 10.2337/dc09-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cisneiros RM, Dutra LP, Silveira FJ, et al. Visceral adiposity in the first half of pregnancy predicts newborn weight among adolescent mothers. Journal of obstetrics and gynaecology Canada: JOGC = Journal d’obstetrique et gynecologie du Canada: JOGC. 2013 Aug;35(8):704–709. doi: 10.1016/S1701-2163(15)30860-4. [DOI] [PubMed] [Google Scholar]
- 30.Luoto R, Mannisto S, Raitanen J. Ten-year change in the association between obesity and parity: results from the National FINRISK Population Study. Gender medicine. 2011 Dec;8(6):399–406. doi: 10.1016/j.genm.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Knabl J, Riedel C, Gmach J, et al. Prediction of excessive gestational weight gain from week-specific cutoff values: a cohort study. Journal of perinatology: official journal of the California Perinatal Association. 2014 May;34(5):351–356. doi: 10.1038/jp.2014.22. [DOI] [PubMed] [Google Scholar]
- 32.Tomedi LE, Simhan HN, Chang CC, McTigue KM, Bodnar LM. Gestational weight gain, early pregnancy maternal adiposity distribution, and maternal hyperglycemia. Maternal and child health journal. 2014 Jul;18(5):1265–1270. doi: 10.1007/s10995-013-1361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas DM, Navarro-Barrientos JE, Rivera DE, et al. Dynamic energy-balance model predicting gestational weight gain. The American journal of clinical nutrition. 2012 Jan;95(1):115–122. doi: 10.3945/ajcn.111.024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. The American journal of clinical nutrition. 2009 Dec;90(6):1453–1456. doi: 10.3945/ajcn.2009.28595. [DOI] [PubMed] [Google Scholar]
- 35.Tataranni PA, Harper IT, Snitker S, et al. Body weight gain in free-living Pima Indians: effect of energy intake vs expenditure. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2003 Dec;27(12):1578–1583. doi: 10.1038/sj.ijo.0802469. [DOI] [PubMed] [Google Scholar]
- 36.Hill RJ, Davies PS. The validity of self-reported energy intake as determined using the doubly labelled water technique. The British journal of nutrition. 2001 Apr;85(4):415–430. doi: 10.1079/bjn2000281. [DOI] [PubMed] [Google Scholar]
- 37.Trabulsi J, Schoeller DA. Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. American journal of physiology. Endocrinology and metabolism. 2001 Nov;281(5):E891–899. doi: 10.1152/ajpendo.2001.281.5.E891. [DOI] [PubMed] [Google Scholar]
- 38.Melzer K, Schutz Y, Boulvain M, Kayser B. Pregnancy-related changes in activity energy expenditure and resting metabolic rate in Switzerland. European journal of clinical nutrition. 2009 Oct;63(10):1185–1191. doi: 10.1038/ejcn.2009.49. [DOI] [PubMed] [Google Scholar]
- 39.Davis EM, Stange KC, Horwitz RI. Childbearing, stress and obesity disparities in women: a public health perspective. Maternal and child health journal. 2012 Jan;16(1):109–118. doi: 10.1007/s10995-010-0712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mamun AA, Kinarivala M, O’Callaghan MJ, Williams GM, Najman JM, Callaway LK. Associations of excess weight gain during pregnancy with long-term maternal overweight and obesity: evidence from 21 y postpartum follow-up. The American journal of clinical nutrition. 2010 May;91(5):1336–1341. doi: 10.3945/ajcn.2009.28950. [DOI] [PubMed] [Google Scholar]
- 41.Linne Y, Neovius M. Identification of women at risk of adverse weight development following pregnancy. International journal of obesity. 2006 Aug;30(8):1234–1239. doi: 10.1038/sj.ijo.0803258. [DOI] [PubMed] [Google Scholar]
- 42.Amorim AR, Rossner S, Neovius M, Lourenco PM, Linne Y. Does excess pregnancy weight gain constitute a major risk for increasing long-term BMI? Obesity (Silver Spring) 2007 May;15(5):1278–1286. doi: 10.1038/oby.2007.149. [DOI] [PubMed] [Google Scholar]
- 43.Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol Rev. 2000;22(2):261–274. doi: 10.1093/oxfordjournals.epirev.a018038. [DOI] [PubMed] [Google Scholar]
- 44.Nehring I, Schmoll S, Beyerlein A, Hauner H, von Kries R. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. The American journal of clinical nutrition. 2011 Nov;94(5):1225–1231. doi: 10.3945/ajcn.111.015289. [DOI] [PubMed] [Google Scholar]
- 45.Maddah M, Nikooyeh B. Weight retention from early pregnancy to three years postpartum: a study in Iranian women. Midwifery. 2009 Dec;25(6):731–737. doi: 10.1016/j.midw.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Olson CM, Strawderman MS, Hinton PS, Pearson TA. Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 y postpartum. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2003 Jan;27(1):117–127. doi: 10.1038/sj.ijo.0802156. [DOI] [PubMed] [Google Scholar]
- 47.Gunderson EP, Abrams B, Selvin S. Does the pattern of postpartum weight change differ according to pregravid body size? International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2001 Jun;25(6):853–862. doi: 10.1038/sj.ijo.0801631. [DOI] [PubMed] [Google Scholar]
- 48.Mamun AA, O’Callaghan MJ, Williams GM, Najman JM. Change in maternal body mass index is associated with offspring body mass index: a 21-year prospective study. Eur J Nutr. 2013 Sep;52(6):1597–1606. doi: 10.1007/s00394-012-0465-7. [DOI] [PubMed] [Google Scholar]
- 49.Cho GJ, Yoon HJ, Kim EJ, Oh MJ, Seo HS, Kim HJ. Postpartum changes in body composition. Obesity (Silver Spring) 2011 Dec;19(12):2425–2428. doi: 10.1038/oby.2011.163. [DOI] [PubMed] [Google Scholar]
- 50.McClure CK, Catov JM, Ness R, Bodnar LM. Associations between gestational weight gain and BMI, abdominal adiposity, and traditional measures of cardiometabolic risk in mothers 8 y postpartum. The American journal of clinical nutrition. 2013 Nov;98(5):1218–1225. doi: 10.3945/ajcn.112.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gunderson EP, Sternfeld B, Wellons MF, et al. Childbearing may increase visceral adipose tissue independent of overall increase in body fat. Obesity (Silver Spring) 2008 May;16(5):1078–1084. doi: 10.1038/oby.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith DE, Lewis CE, Caveny JL, Perkins LL, Burke GL, Bild DE. Longitudinal changes in adiposity associated with pregnancy. The CARDIA Study. Coronary Artery Risk Development in Young Adults Study. JAMA: the journal of the American Medical Association. 1994 Jun 8;271(22):1747–1751. [PubMed] [Google Scholar]
- 53.Lederman SA. Influence of lactation on body weight regulation. Nutrition reviews. 2004 Jul;62(7 Pt 2):S112–119. doi: 10.1111/j.1753-4887.2004.tb00080.x. [DOI] [PubMed] [Google Scholar]
- 54.McClure CK, Schwarz EB, Conroy MB, Tepper PG, Janssen I, Sutton-Tyrrell KC. Breastfeeding and subsequent maternal visceral adiposity. Obesity (Silver Spring) 2011 Nov;19(11):2205–2213. doi: 10.1038/oby.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gur EB, Genc M, Eskicioglu F, Kurtulmus S, Guclu S. Ultrasonographic visceral fat thickness measurement may be a good scan test for prediction of gestational diabetes mellitus. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2014 Jul 11;:1–2. doi: 10.3109/14767058.2014.936003. [DOI] [PubMed] [Google Scholar]
- 56.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009 May 23;373(9677):1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 57.Lee AJ, Hiscock RJ, Wein P, Walker SP, Permezel M. Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes: a retrospective cohort study using survival analysis. Diabetes care. 2007 Apr;30(4):878–883. doi: 10.2337/dc06-1816. [DOI] [PubMed] [Google Scholar]
- 58.Inturrisi M, Lintner NC, Sorem KA. Diagnosis and treatment of hyperglycemia in pregnancy. Endocrinol Metab Clin North Am. 2011 Dec;40(4):703–726. doi: 10.1016/j.ecl.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Harville EW, Juonala M, Viikari JS, Raitakari OT. Preconception metabolic indicators predict gestational diabetes and offspring birthweight. Gynecological endocrinology: the official journal of the International Society of Gynecological Endocrinology. 2014 Jul 9;:1–5. doi: 10.3109/09513590.2014.937336. [DOI] [PubMed] [Google Scholar]
- 60.Liu Z, Ao D, Yang H, Wang Y. Gestational weight gain and risk of gestational diabetes mellitus among Chinese women. Chinese medical journal. 2014;127(7):1255–1260. [PubMed] [Google Scholar]
- 61.Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991-2000. Obstetrics and gynecology. 2004 Mar;103(3):526–533. doi: 10.1097/01.AOG.0000113623.18286.20. [DOI] [PubMed] [Google Scholar]
- 62.Diagnosis and classification of diabetes mellitus. Diabetes care. 2012 Jan;35(Suppl 1):S64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007-2010. Prev Chronic Dis. 2014;11:E104. doi: 10.5888/pcd11.130415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verma A, Boney CM, Tucker R, Vohr BR. Insulin resistance syndrome in women with prior history of gestational diabetes mellitus. The Journal of clinical endocrinology and metabolism. 2002 Jul;87(7):3227–3235. doi: 10.1210/jcem.87.7.8684. [DOI] [PubMed] [Google Scholar]
- 65.Lauenborg J, Mathiesen E, Hansen T, et al. The prevalence of the metabolic syndrome in a danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. The Journal of clinical endocrinology and metabolism. 2005 Jul;90(7):4004–4010. doi: 10.1210/jc.2004-1713. [DOI] [PubMed] [Google Scholar]
- 66.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Puhkala J, Kinnunen TI, Vasankari T, Kukkonen-Harjula K, Raitanen J, Luoto R. Prevalence of metabolic syndrome one year after delivery in Finnish women at increased risk for gestational diabetes mellitus during pregnancy. J Pregnancy. 2013;2013:139049. doi: 10.1155/2013/139049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harper LM, Tita A, Biggio JR. The Institute of Medicine Guidelines for Gestational Weight Gain after a Diagnosis of Gestational Diabetes and Pregnancy Outcomes. Am J Perinatol. 2014 Jun 27; doi: 10.1055/s-0034-1383846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scifres CM, Catov JM, Simhan HN. The impact of maternal obesity and gestational weight gain on early and mid-pregnancy lipid profiles. Obesity (Silver Spring) 2014 Mar;22(3):932–938. doi: 10.1002/oby.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berggren EK, Stuebe AM, Boggess KA. Excess Maternal Weight Gain and Large for Gestational Age Risk among Women with Gestational Diabetes. Am J Perinatol. 2014 Jun 27; doi: 10.1055/s-0034-1383848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnsson IW, Haglund B, Ahlsson F, Gustafsson J. A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatr Obes. 2014 Jun 11; doi: 10.1111/ijpo.230. [DOI] [PubMed] [Google Scholar]
- 72.Catalano PM, McIntyre HD, Cruickshank JK, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes care. 2012 Apr;35(4):780–786. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. The Journal of maternal-fetal medicine. 2000 Jan-Feb;9(1):83–88. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<83::AID-MFM17>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 74.Keely EJ, Malcolm JC, Hadjiyannakis S, Gaboury I, Lough G, Lawson ML. Prevalence of metabolic markers of insulin resistance in offspring of gestational diabetes pregnancies. Pediatr Diabetes. 2008 Feb;9(1):53–59. doi: 10.1111/j.1399-5448.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- 75.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005 Mar;115(3):e290–296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]