Abstract
Background
Cardiometabolic disease is a major cause of morbidity and mortality in persons with chronic kidney disease (CKD). Fractalkine (CX3CL1) is a potential mediator of both atherosclerosis and metabolic disease. Studies on the relationship of CX3CL1 with risk of CVD events and metabolic traits are lacking, particularly in the high-risk setting of CKD.
Study Design
Cross-sectional and longitudinal observational analysis.
Setting & Participants
Adults with CKD from 7 US sites participating in the Chronic Renal Insufficiency Cohort (CRIC) Study.
Predictor
Quartiles of plasma CX3CL1 levels at baseline.
Outcomes
Baseline estimated glomerular filtration rate (eGFR) from a creatinine- and cystatin C–based equation, prevalent and incident CVD, diabetes, metabolic syndrome and its criteria, homeostatic model assessment of insulin resistance, hemoglobin A1C, myocardial infarction, all-cause mortality, and the composite outcome of myocardial infarction/all-cause mortality.
Results
Among 3687 participants, baseline CX3CL1 levels were positively associated with several CVD risk factors and metabolic traits, lower eGFR, and higher levels of inflammatory cytokines as well as prevalent CVD (OR, 1.09; 95% CI, 1.01–1.19; p=0.03). Higher CX3CL1 was also associated with prevalent diabetes (OR, 1.26; 95% CI, 1.16–1.38; p<0.001) in adjusted models. During a mean follow up of 6 years, there were 352 deaths, 176 myocardial infarctions, and 484 with composite outcomes. In fully-adjusted models, 1-SD higher CX3CL1 increased the hazard for all-cause mortality (1.11; 95% CI, 1.00–1.22; p=0.02) and the composite outcome (1.09; 95% CI, 1.00–1.19; p=0.04).
Limitations
Study design did not allow evaluation of changes over time, correlation with progression of phenotypes, or determination of causality of effect.
Conclusions
Circulating CX3CL1 may contribute to both atherosclerotic CVD and diabetes in a CKD cohort. Further studies are required to establish mechanisms through which CX3CL1 affects pathogenesis of atherosclerosis and diabetes.
INDEX WORDS: cardiometabolic disease, chronic kidney disease (CKD), cardiovascular disease (CVD), atherosclerosis, metabolic syndrome, diabetes, fractalkine (CX3CL1), Chronic Renal Insufficiency Cohort (CRIC) Study
Chronic kidney disease (CKD), regardless of etiology, confers a markedly elevated risk for morbidity and mortality from cardiometabolic disease1. Elucidating novel pathophysiologic pathways of these diseases may lead to more effective therapies. Fractalkine (CX3CL1) is a possible mediator of atherogenic cardiovascular disease (CVD) and diabetes mellitus (DM)2,3. This chemokine promotes leukocyte adhesion and migration to vascular lesions in animal models of atherosclerosis4; similar effects are hypothesized to occur in obese adipose tissue3 in the pathogenesis of DM.
CX3CL1 is an inflammatory chemokine that promotes monocyte adhesion to endothelial cells5 and adipocytes and is elevated in persons with diabetes3. Humans with coronary artery disease (CAD) have increased CX3CR1-expressing peripheral blood mononuclear cells as well as higher serum CX3CL1 levels6; elevated levels are also found in patients with unstable angina pectoris4. Nonsynonymous variants of CX3CR1 (the CX3CL1 receptor), namely, a valine to isoleucine substitution at amino acid 249 (V249I) and a threonine to methionine substitition at amino acid 280 (T280M), which decrease binding of CX3CL1 to monocytes in functional assays, have been associated with reduced atherosclerosis and cerebrovascular disease7,8. Polymorphisms in the CX3CR1 gene have also been associated with obesity and metabolic traits9,3.
Animal and in vitro models and cross-sectional studies in humans suggest that CX3CL1 is associated with both atherogenesis4,6–8 and diabetes3, however, the relationship between plasma CX3CL1 with incident myocardial infarction (MI) and death as well as diverse metabolic traits have not been described in humans. To address whether CX3CL1 may contribute to the development of atherogenic CVD or associate with insulin resistance and diabetes, we performed a cross-sectional analysis of CX3CL1 with prevalent CVD and metabolic phenotypes in a population with CKD and determined whether CX3CL1 levels predict longitudinal outcomes. We examined in the Chronic Renal Insufficiency Cohort (CRIC) Study whether baseline plasma levels of CX3CL1 were associated with CVD risk factors, diabetes, metabolic traits, and the outcomes of MI and all-cause mortality in adults with CKD.
Methods
Study Population
The CRIC Study is an ongoing prospective, observational study of CKD10. The design of this cohort study and baseline characteristics of the participants have been reported11. Briefly, 3939 individuals were recruited at seven US sites and followed up annually for an average of 6 years. Participants were aged 21–74 years, 46% female, ethnically diverse (45% white, 46% black, 5% Hispanic, 4% Asian/Pacific Islander/Native American), with a broad span of kidney function (estimated glomerular filtration rate [eGFR] range of ~15–90 [mean, 43.4±13.5 (standard deviation)] ml/min/1.73 m2) and ~48% having DM12. We measured plasma CX3CL1 in 3,869 of the 3,939 (98.2%) participants. Among the participants with plasma CX3CL1 values, 182 were excluded from the analysis because of missing values for other variables, resulting in a sample of 3,687. The institutional review boards of all participating institutions approved the study protocol and all participants provided written informed consent.
Exposures
Levels of CX3CL1 were measured in duplicate using a commercial ELISA (Quantikine Immunoassay (R&D Systems Inc, Minneapolis, MN)13,14. Intra-assay and inter-assay coefficients of variation were 9.9% and 12.0%, respectively, for low-concentration and 6.3% and 8.2% for high-concentration controls. High-sensitivity plasma C-reactive protein (hsCRP) was assayed by nephelometry and interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) were measured by high-sensitivity ELISA15 (R&D Systems Inc, Minneapolis, MN).
Cross-sectional Outcomes
Demographic factors and clinical data were obtained at baseline and annually by interview and questionnaire. Blood and urine laboratory tests were measured at a central laboratory using standard assays. Lipids, including total and high- and low-density cholesterol and triglycerides, were measured enzymatically (Hitachi 912, Roche Diagnostic Systems Inc., NJ, USA). An equation derived from CRIC Study data (including serum creatinine, cystatin C, age, sex, and race) was used to determine eGFR16. Diabetes was defined as fasting glucose ≥126 mg/dl, random glucose ≥200 mg/dl, or use of insulin or anti-diabetic medication. Metabolic syndrome was defined by accepted guidelines17, requiring at least three of the following: (1) history of hypertension, systolic blood pressure (SBP) >130 mm Hg, or diastolic blood pressure (DBP) >85 mm Hg, (2) history of diabetes or plasma glucose ≥100 mg/dl, (3) waist circumference >102 cm for men and > 88 cm for women, (4) triglycerides ≥150 mg/dl, and (5) HDL <40 mg/dl for men or <50 mg/dl for women. Homeostatic model assessment of insulin resistance (HOMA-IR) was estimated as follows: [Glucose (mmol/L) × Insulin (mU/L)]/22.518. Hypertension was defined as a SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, or use of antihypertensive medications. Hyperlipidemia was defined as use of cholesterol-lowering medications or total serum cholesterol >200 mg/dL.
Our primary metabolic outcome was diabetes at baseline. The presence of HOMA-IR and other metabolic syndrome components were examined in secondary analyses to provide greater insight into the relationships with metabolic traits.
Longitudinal Outcomes
Our primary longitudinal outcomes were the occurrence of probable or definite MI, all-cause mortality, and a composite of these endpoints during the average six years of follow-up. The MI and mortality events were assessed as previously described19, including review of detailed hospital records by two physician adjucators. Participants were followed annually in person with interim telephone calls until death, withdrawal from study, lost to follow-up, or June 30, 2009, when data were locked for this analysis. More than 90% of the participants were retained during this period. Prevalent CVD at enrollment (defined as self-reported prior diagnosis of CVD, stroke or peripheral vascular disease) was analyzed as a secondary outcome.
Statistical Analysis
One-way analysis of variance (continuous variables) and Pearson’s chi-squared tests (categorical variables) were used to compare characteristics across baseline quartiles of plasma CX3CL1 levels. We used logistic regression to examine unadjusted and multivariable adjusted relationships between plasma CX3CL1 level and DM, and prevalent CVD at enrollment. The CX3CL1 values were standardized by subtracting the sample mean and dividing by the standard deviation (SD). Odds ratios (ORs) are reported per 1 SD increment of standardized CX3CL1.
Statistical models with prevalent CVD as the outcome were adjusted sequentially as follows: Model 1, for demographic factors (age, sex and race); Model 2, for demographic factors and traditional CVD risk factors (body mass index [BMI] and binary indicators for DM, hypertension, hyperlipidemia, and tobacco use); Model 3, for demographic factors, traditional CVD risk factors, and inflammatory biomarkers (log transformed IL-6, TNF-α, and hsCRP levels); Model 4, for demographic factors, traditional CVD risk factors, inflammatory biomarkers, and kidney function measures (eGFR and urinary albumin-creatinine ratio); and Model 5, for demographic factors, traditional CVD risk factors, inflammatory biomarkers, kidney function measures, and the binary indicator for metabolic syndrome.
For longitudinal outcomes there was an additional Model 6, which included Model 5 above plus prevalent CVD at baseline. Cox proportional hazards regression was used to examine unadjusted and multivariable adjusted relationships between CX3CL1, per 1-SD increment of standardized CX3CL1, and each of the following incident outcomes: (1) probable or definite MI, (2) all-cause mortality, and (3) a composite outcome of probable or definite MI and all-cause mortality (MI/all-cause mortality). Adjusted survival curves were generated using quartiles of CX3CL1 to illustrate findings fully adjusted for age, sex, race, traditional CVD risk factors, inflammatory markers, kidney function measures, and metabolic syndrome (Model 5). We explored potential impact of plasma CX3CL1 on the prognostic performance in risk prediction models: adjusted for age, sex, and race (Model 2) or fully adjusted (Model 5) with change in the area under the curve (AUC) for MI, all-cause mortality and combined outcomes using approaches described by Pencina et al20.
Statistical models with diabetes or HOMA-IR as outcomes were identical to those of prevalent CVD except Model 2 contained only BMI. Model 5 was included only for the analysis with diabetes as an outcome. Analyses were performed using the R statistical software, version 2.15.2 (R Foundation for Statistical Computing). All statistical tests were 2-sided, with p values <0.05 considered statistically significant.
Results
Association of Plasma CX3CL1 Levels With Demographic and Clinical Factors
Baseline characteristics of study participants by plasma CX3CL1 quartiles are presented in Table 1. In unadjusted analyses, increasing CX3CL1 quartiles were associated with female and non-White participants. Higher plasma CX3CL1 was found among persons with diabetes, hypertension, and hyperlipidemia; higher BMI; higher plasma levels of the inflammatory markers IL-6, TNF-α, and hsCRP; and history of CVD. Persons with lower eGFR, higher plasma cystatin C and FGF-23, lower calcium, higher phosphorous, and higher urinary albumin-creatinine ratio had higher levels of CX3CL1. Because of the association noted, we looked further at direct correlation between inflammatory markers and CX3CL1 levels and found the following correlations by Spearman’s ρ: TNF-α, 0.24 (p<0.001); hsCRP, −0.04 (p=0.03); and IL-6, 0.68 (p<0.001).
Table 1.
Baseline characteristics by quartiles of plasma CX3CL1 (fractalkine)
| Characteristics | Total (N=3,687) |
Q1: <0.66 ng/ml (n=923) |
Q2: 0.66–0.85 ng/ml (n=923) |
Q3: 0.86–1.08 ng/ml (n=921) |
Q4: >1.08 ng/ml (n=920) |
P † |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (y) | 60 (52, 65) | 61 (54, 67) | 61 (53, 67) | 59 (51, 66) | 0.09 | |
| Female Sex | 1655 (44.9) | 391 (42.4) | 406 (44.0) | 407 (44.2) | 451 (49.0) | 0.03 |
| Race | <0.001 | |||||
| White | 1568 (42.5) | 450 (48.8) | 426 (46.2) | 391 (42.5) | 301 (32.7) | |
| Black | 1518 (41.2) | 329 (35.6) | 357 (38.7) | 388 (42.1) | 444 (48.3) | |
| Hispanic | 454 (12.3) | 88 (9.5) | 105 (11.4) | 115 (12.5) | 146 (15.9) | |
| Other | 147 (4.0) | 56 (6.1) | 35 (3.8) | 27 (2.9) | 29 (3.2) | |
| Traditional CV Risk Factors | ||||||
| Tobacco Use^ | 475 (12.9) | 98 (10.6) | 126 (13.7) | 122 (13.2) | 129 (14.0) | 0.1 |
| Family history CAD | 583 (15.8) | 136 (14.7) | 146 (15.8) | 149 (16.2) | 152 (16.5) | 0.7 |
| Hypertension | 3170 (86.0) | 740 (80.2) | 782 (84.7) | 806 (87.5) | 842 (91.5) | <0.001 |
| Prior CVD | 1227 (33.28) | 242 (26.2) | 309 (33.5) | 311 (33.8) | 365 (39.7) | <0.001 |
| Diabetes | 1778 (48.22) | 323 (35.0) | 412 (44.6) | 484 (52.6) | 559 (60.8) | <0.001 |
| BMI (kg/m2) | 30.3 [26.7–34.8] | 31.2 [27.1–36.4] | 30.8 [26.8–36.1] | 31.2 [26.6–37.3] | 0.002 | |
| High Cholesterol | 3032 (82.2) | 725 (78.6) | 756 (81.9) | 777 (84.4) | 774 (84.1) | 0.003 |
| Kidney function/damage measures |
||||||
| eGFR (ml/min/1.73 m2) | 53.6 (42.8, 65.3) | 45.6 (35.6, 56.2) | 39.9 (31.0, 51.0) | 34.5 (26.1, 44.2) | <0.001 | |
| Urinary ACR (µg/mg) ‡ | 16.5 (5.4, 102) | 41 (7.9, 259) | 100 (11.4, 622) | 265 (22.7, 1245) | <0.001 | |
| Cystatin C (mg/L) | 1.2 (1, 1.5) | 1.3 (1.1, 1.7) | 1.5 (1.2, 1.9) | 1.7 (1.4, 2.1) | <0.001 | |
| Measures of Mineral Metabolism |
||||||
| FGF-23 (RU/ml) | 107 (75.8, 166) | 138 (94.1, 213) | 158 (107, 252) | 195 (126, 314) | <0.001 | |
| Serum phosphorous (mg/dl) | 3.5 (3.2, 3.9) | 3.6 (3.2, 4.0) | 3.7 (3.3, 4.1) | 3.8 (3.4, 4.3) | <0.001 | |
| Serum calcium (mg/dL) | 9.2 (8.9, 9.5) | 9.2 (8.9, 9.5) | 9.2 (8.9, 9.5) | 9.1 (8.8, 9.4) | <0.001 | |
| Inflammatory biomarkers | ||||||
| hsCRP (mg/l) ‡ | 2.6 (1.1, 6.6) | 2.7 (1.1, 6.7) | 2.6 (1.1, 6.3) | 2.3 (0.9, 5.9) | 0.1 | |
| TNF-α (pg/ml) ‡ | 1.8 (1.2, 2.7) | 2.0 (1.4, 3.1) | 2.3 (1.6, 3.2) | 2.7 (1.9, 3.8) | <0.001 | |
| IL-6 (pg/ml) ‡ | 1.6 (0.9, 2.5) | 1.9 (1.2, 3.1) | 2 (1.2, 3.2) | 2.2 (1.4, 3.5) | <0.001 |
Note: Values for categorical variables are given as number (percentage of column category); values for continuous variables are given as median [interquartile range]. Conversion factors for units: phosphorous in mg/dl to mmol/L, ×0.3229; calcium in mg/dl to mmol/L, ×0.2495.
p-value by ANOVA (continuous variables) and Chi-squared test (categorical variables)
log-transformed to meet normality assumption
Abbreviations and definitions: BMI, body mass index; CAD, coronary artery disease; CV, cardiovascular; CVD, cardiovascular disease (defined as self-reported prior diagnosis of CVD, stroke or peripheral vascular disease); eGFR, estimated glomerular filtration rate (using equation from Chronic Renal Insufficiency Cohort Study data)16; FGF, fibroblast growth factor; hsCRP, high-sensitivity C-reactive protein; IL, interleukin; TNF, tumor necrosis factor; ACR, albumin-creatinine ratio; Q, quartile
tobacco use defined as self-reported current smoker
Plasma CX3CL1 and Self-reported Prevalent CVD at Baseline
Increased baseline levels of CX3CL1 were associated with a higher prevalence of self-reported CVD at enrollment (overall prevalence in study population, 33%) in models adjusting for demographic factors, known CVD risk factors (including DM) and inflammatory biomarkers (IL6, TNF-α and hsCRP): the OR per 1-SD increase in standardized CX3CL1 was 1.17 (p<0.001). This effect was attenuated but remained statistically significant after adjusting further for baseline eGFR and urinary albumin-creatinine ratio (OR, 1.09; p=0.03) (Table 2) as well as presence of the metabolic syndrome (OR, 1.09; p=0.03).
Table 2.
Association of plasma CX3CL1 (fractalkine) with self-reported CVD at baseline
| OR (95% CI)† | P value | |
|---|---|---|
| Model 1 | 1.29 (1.20–1.38) | <0.001 |
| Model 2 | 1.21 (1.13–1.31) | <0.001 |
| Model 3 | 1.17 (1.08–1.26) | <0.001 |
| Model 4 | 1.09 (1.01–1.19) | 0.03 |
| Model 5 | 1.09 (1.01–1.19) | 0.03 |
Note: Prevalent CVD is defined as prior myocardial infarction or coronary revascularization at baseline (n with any CVD=1227). Model 1: CX3CL1 + Demographic factors (age, sex, race); Model 2: CX3CL1 + Demographic factors + Traditional risk factors (diabetes, hypertension, hyperlipidemia, tobacco use, body mass index); Model 3: CX3CL1 + Demographic factors + Traditional risk factors + plasma inflammatory biomarkers (log transformed IL6, tumor necrosis factor α and high-sensitivity C-reactive protein); Model 4: CX3CL1 + Demographic factors + Traditional risk factors + plasma inflammatory biomarkers + kidney function measures (estimated glomerular filtration rate using equation from Chronic Renal Insufficiency Cohort Study data16 and log transformed urinary albumin-creatinine ratio); Model 5: CX3CL1 + Demographic factors + Traditional risk factors + plasma inflammatory biomarkers + kidney function measures + metabolic syndrome
CI, confidence interval; CVD, cardiovascular disease; OR, odds ratio
for one standard deviation increase in standardized CX3CL1
Plasma CX3CL1 and Occurrence of MI and All-Cause Mortality
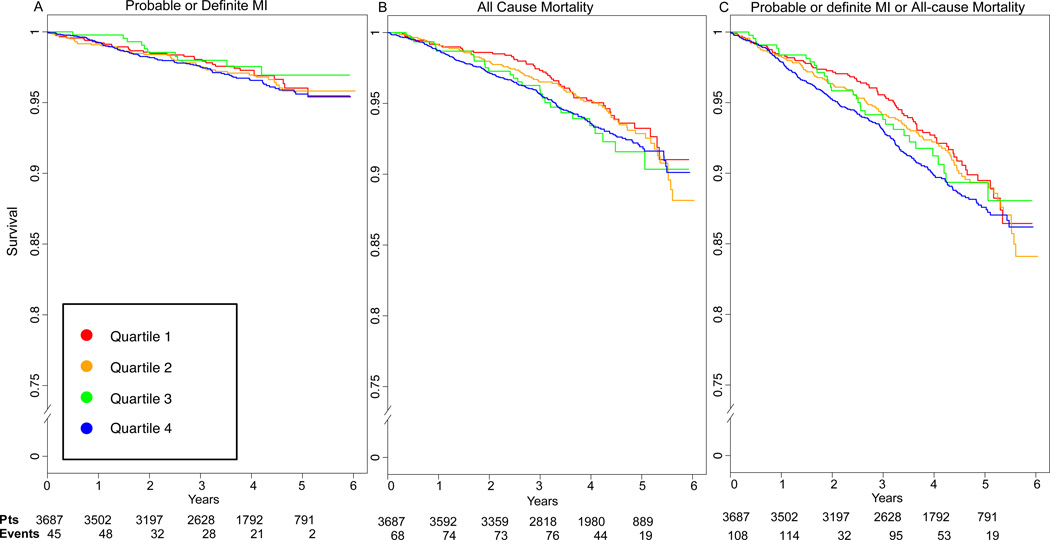
During a median 6 years’ follow-up, a total of 176 participants were identified as having a probable MI (127 definite MI), 352 died, and 484 had either an MI or died, leading to a cumulative incidence of these outcomes of 4.7% for probable or definite MI, 9.6% for death, and 13.2% for the composite outcome of MI and death. In Table 3, multivariable-adjusted hazard ratios (HRs) are presented for probable MI, all-cause mortality, or the composite outcome for a 1-SD difference in the standardized CX3CL1 concentration. Higher CX3CL1 levels were associated with all-cause mortality (HR, 1.23; p<0.001) and the MI/all-cause mortality composite outcome (HR, 1.22; p<0.001) in models adjusted for demographic factors, known risk factors (including diabetes) and inflammatory biomarkers (IL-6, TNF-α, and hsCRP) and remained significant for all-cause mortality (HR, 1.13; p=0.02) and the composite outcome (HR, 1.11; p=0.01) once additional adjustment for study entry eGFR and albumin-creatinine ratio had been done. For MI alone, trends were similar but were not statistically significant in fully adjusted models. Further adjustment for prevalent CVD somewhat attenuated the estimates (HRs for all-cause mortality and for the MI/all-cause mortality composite outcome of 1.11 and 1.09 [P=0.02 and p=0.04], respectively). Adjusted survival curves with fully adjusted associations between quartiles of plasma CX3CL1 and MI, all-cause mortality, or the composite outcome are shown in Figure 1. Similar to the results shown in Table 3, increasing quartiles of CX3CL1 were associated with decreased event-free survival curves for all-cause mortality as well as the composite outcome.
Table 3.
Multivariable association of plasma CX3CL1 (fractalkine) with incident MI and all-cause mortality
| Failure event | HR*(95% CI) | P value |
|---|---|---|
| Probable or definite MI (n=176) | ||
| Model 1 | 1.21 (1.06, 1.38) | 0.01 |
| Model 2 | 1.17 (1.02, 1.34) | 0.03 |
| Model 3 | 1.14 (1.00, 1.31) | 0.06 |
| Model 4 | 1.05 (0.90, 1.22) | 0.6 |
| Model 5 | 1.05 (0.90, 1.22) | 0.5 |
| Model 6 | 1.03 (0.89, 1.20) | 0.7 |
| All-cause mortality (n=352) | ||
| Model 1 | 1.32 (1.21, 1.45) | <0.001 |
| Model 2 | 1.29 (1.17, 1.41) | <0.001 |
| Model 3 | 1.23 (1.13, 1.35) | <0.001 |
| Model 4 | 1.13 (1.02, 1.24) | 0.02 |
| Model 5 | 1.13 (1.02, 1.24) | 0.02 |
| Model 6 | 1.11 (1.01, 1.22) | 0.04 |
| Any MI or all-cause mortality (n=484) | ||
| Model 1 | 1.30 (1.21, 1.40) | <0.001 |
| Model 2 | 1.26 (1.17, 1.36) | <0.001 |
| Model 3 | 1.22 (1.13, 1.32) | <0.001 |
| Model 4 | 1.11 (1.02, 1.21) | 0.01 |
| Model 5 | 1.11 (1.02, 1.21) | 0.01 |
| Model 6 | 1.09 (1.00, 1.19) | 0.04 |
for one standard deviation increase in standardized CX3CL1
Note: N=3,687. Please see Table 2 for definitions of models 1–5; Model 6: CX3CL1 + Demographic factors + Traditional risk factors + plasma inflammatory biomarkers + kidney function measures + metabolic syndrome + prevalent cardiovascular disease at baseline.
CI, confidence interval; HR, hazard ratio; MI, myocardial infarction;
Figure 1.
Adjusted survival curves for effect of plasma CX3CL1 (fractalkine) quartiles on incident events. Fully adjusted for A) probable and definite MI, B) all-cause mortality, and C) composite probable and definite MI / all-cause mortality.
We assessed the AUC to explore the predictive performance of plasma CX3CL1 when added to traditional and novel risk factors. The change in AUC for MI, all-cause mortality and the composite outcome was evaluated in incremental models (Models 2 and 5) (Table S1, available as online supplementary material). Plasma CX3CL1 enhanced the AUC modestly when added to demographic characteristics and traditional risk factors for all three outcomes (e.g., 0.68 to 0.70 for all-cause mortality [P<0.001]) and modestly increased the AUC for all-cause mortality (0.74 to 0.76 [p<0.001]) and the composite outcome in the fully adjusted models.
Plasma CX3CL1, Metabolic Syndrome, and DM
Diabetes and metabolic syndrome at baseline were each strongly associated with increasing CX3CL1 quartiles (Table S2). Blood glucose and blood pressure were positively associated with increasing levels of CX3CL1. However, waist circumference, triglycerides, high-density lipoprotein cholesterol components and HOMA-IR levels were not.
In multivariable models, higher CX3CL1 levels at study entry were significantly associated with higher baseline prevalence of diabetes (overall prevalence in study population of 48%) after adjusting for demographic factors, BMI, and inflammatory biomarkers (IL-6, TNF-α and hsCRP): OR of 1.36 per 1-SD increase in standardized CX3CL1 (p<0.001). This association persisted after further adjustment for measures of kidney disease (OR, 1.22; p<0.001) and metabolic syndrome (OR, 1.26: p<0.001) (Table 4). Analysis of HOMA-IR with multivariable models showed no significant association between baseline CX3CL1 levels and this surrogate marker of insulin resistance (Table S3).
Table 4.
Association of plasma CX3CL1 (fractalkine) with diabetes mellitus at baseline
| OR (95% CI)* | |
|---|---|
| Model 1 | 1.45 (1.35–1.56) |
| Model 2 | 1.46 (1.36–1.58) |
| Model 3 | 1.36 (1.26–1.47) |
| Model 4 | 1.22 (1.16–1.38) |
| Model 5 | 1.26 (1.16–1.38) |
for one standard deviation increase in standardized CX3CL1
Note: N=3,687. P<0.001 for all rows. Please Model 1: CX3CL1 + Demographic factors (age, sex, race); Model 2: CX3CL1 + Demographic factors + body mass index (BMI); Model 3: CX3CL1 + Demographic factors + BMI + plasma inflammatory biomarkers (log transformed IL6, TNFα and hsCRP); Model 4: CX3CL1 + Demographic factors + BMI + plasma inflammatory biomarkers + kidney function measures (CRIC-defined estimated glomerular filtration rate16 and log transformed urinary albumin: creatinine ratio); Model 5: CX3CL1 + Demographic factors + BMI + plasma inflammatory biomarkers + kidney function measures (CRIC-defined estimated glomerular filtration rate16 and log transformed urinary albumin: creatinine ratio) +Metabolic syndrome.
CI, confidence interval; OR, odds ratio
Discussion
Among a large cohort of subjects with CKD and diverse clinical and demographic characteristics, baseline levels of CX3CL1 were associated not only with CVD at enrollment but also with incident events including death and the composite of MI and death during approximately six years of follow-up. Levels of CX3CL1 also were independently associated with prevalent diabetes but not with measures of insulin resistance (HOMA-IR). These associations remained after adjustment for indices of kidney function and damage in addition to multiple demographic and CVD risk factors, suggesting complex cardiometabolic associations of the CX3CL1-CX3CR1 system in persons with CKD.
To our knowledge, this is the first large study of the association of circulating CX3CL1 with CVD in CKD and the largest prospective study of incident events to date. In a study of 46 patients with unstable angina pectoris, increased plasma CX3CL1 and CX3CR1-expressing monocytes were found in subjects with plaque rupture vs. those without21. Multiple studies in rodent models suggest causality for CX3CL1-CX3CR1 signaling in promoting monocyte recruitment and progression of atherosclerosis22–24,25. Circulating CX3CL1 levels have also been reported to predict all-cause and cardiovascular mortality in a small study of advanced heart failure patients26, although the mechanism underlying this relationship in heart failure remains uncertain.
Our analysis of the association between CX3CL1 and CVD outcomes and all-cause mortality provide several novel insights. First, higher CX3CL1 levels were directly related to increased prevalent CVD as well as the composite outcome of incident MI and all-cause mortality in fully adjusted analyses. It is not surprising that the strength of prediction for incident MI and all-cause mortality was weaker in adjusted models since other inflammatory and metabolic risk factors may be acting upon the same pathway as CX3CL1. It appears unlikely, though, that CX3CL1’s association with diabetes explains the prediction of longitudinal outcomes, as adjustment for metabolic factors and diabetes status had minimal impact on the risk estimates for mortality and MI. In our analysis, plasma CX3CL1 was a stronger predictor of all-cause mortality than MI. Although trends were in the same direction for prediction of MI, the association did not meet significance in adjusted analysis, raising the question of whether CX3CL1 relates to death via a mechanism distinct from the modulation of atherosclerosis, as might also be the case in heart failure patients26.
Prior studies have suggested that CX3CL1 may modulate glucose homeostasis and insulin resistance. We have reported that CX3CL1 is induced in inflamed human adipose and that human adipocytes support monocyte adhesion via CX3CL13. Sirois-Gagnon et al. reported an association of genetic variants in CX3CR1 with obesity in humans; we also found modest association of CX3CR1 genetic variation with obesity and DM type 23,9. Findings from rodent studies are conflicting. In obese mice fed a high-fat diet, knockout of the CX3CR1 gene does not attenuate adipose inflammation or the development of peripheral insulin resistance27; these observations are consistent with the lack of an association of CX3CL1 with metabolic syndrome and HOMA-IR among CRIC Study participants. Recently, Lee et al. reported another strain of CX3CR1 knockout mice had a defect in pancreatic β-cell insulin secretion28 and developed glucose intolerance with overt hyperglycemia on high-fat diet, suggesting a protective role for CX3CL1-CX3CR1 in β-cell function. In contrast, we found an apparent opposite pattern in humans with higher levels of plasma CX3CL1 associating with higher prevalence of DM. Further validation of mice model findings and characterization of human CX3CL1 effects on pancreatic β-cell function are warranted29.
We evaluated association of CX3CL1 with metabolic traits, CVD, and all-cause mortality in the CRIC Study, a large, prospective, well-phenotyped cohort of subjects with CKD and a high prevalence of diabetes and risk for CVD. The study was limited by CX3CL1 levels having been assessed only at baseline, as we were thus unable to evaluate change in levels over time, correlate with progression of CVD or metabolic disease, or assess levels near the time of incident events. While we were unable to observe an association of CX3CL1 with HOMA-IR, we cannot definitively exclude an association with insulin resistance given the lack of gold-standard measures of hepatic and peripheral insulin sensitivity. We acknowledge that cross sectional analysis does not allow determination of directionality and we cannot exclude the possibility that DM itself leads to elevation of CX3CL1, rather than CX3CL1 contributing to development of DM. Further, the components of all-cause mortality were not available, thus the association of CX3CL1 to specific causes of death could not be evaluated.
We also recognize the possibility that plasma CX3CL1 accumulates with kidney failure as a function of reduced GFR. Our analysis did show positive association of plasma CX3CL1 with reduced GFR, although association with death and myocardial infarction persisted after correction for kidney function measures. While several studies report CX3CL1 expression in the kidney and suggest a role in kidney inflammation and disease30–32, none directly address the question of whether plasma CX3CL1 levels increase with increasing GFR. One group described positive association of plasma CX3CL1- and CX3CR1-positive CD4 T cells with carotid intima-media thickness in CKD patients33, a finding that supports a functional role of CX3CL1 in CVD, rather than simple accumulation due to reduced filtration.
In conclusion, higher levels of plasma CX3CL1 were associated with traditional cardiovascular risk factors, prevalent CVD, and diabetes in adults with CKD. Levels of CX3CL1 also predicted all-cause mortality after adjustment for traditional risk. These findings, in the context of our current mechanistic knowledge of CX3CL1-CX3CR1, suggest that CX3CL1 may contribute to both atherosclerotic CVD and diabetes. This is consistent with cell-specific, context- and time-dependent roles for other chemokine systems, e.g., CXCL1219, in cardiometabolic biology and disease. Mechanistic and human genomic studies are needed to establish the precise role of CX3CL1-CX3CR1 in diverse metabolic and cardiovascular disorders, determine whether mechanisms in atherosclerosis and diabetes are discrete or overlapping, and establish if these are amenable to therapeutic targeting.
Supplementary Material
Acknowledgements
The CRIC Principal Investigators are Lawrence J. Appel, MD, MPH, Harold I. Feldman, MD, MSCE, Alan S. Go, MD, Jiang He, MD, PhD, John W. Kusek, PhD, James P. Lash, MD, Akinlolu Ojo, MD, PhD, Mahboob Rahman, MD, and Raymond R. Townsend, MD.
The authors thank the participants, investigators, and staff of the CRIC Study for their time and commitment.
Support: This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) at the National Institute of Health (NIH; R01-DK071224 and R01-DK090505 to M.P.R, K23-DK095913 to R.S, K24-DK002651 to H.F). The National Heart, Lung and Blood Institute of the NIH also provided support (R01-HL107196 to A.F. and K24-HL107643, U01-HL108636, and R01-HL113147 to M.P.R.). The US Department of Veterans Affairs Health Services Research and Development Service Award supports M.J.F. N.N.M. is supported by NIH Intramural Award [HL-Z0000]. Funding for the CRIC Study was obtained under a cooperative agreement from the NIDDK (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by the following: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award (CTSA) NIH/National Center for Advancing Translational Sciences (NCATS) UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the NCATS component of the NIH and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, and Kaiser Permanente NIH/National Center for Research Resources UCSF-CTSI UL1 RR-024131.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: MPR, RS; data acquisition: BC, DX; data analysis/interpretation: RS, CM, MPR, RYS, SRM, JC, MW, DJR, DSR, NNM, MJF, MB, ASG, RRT, JH, JWK, HIF; statistical analysis: GJM, ASF; supervision or mentorship: MPR. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. RS takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
References
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003 Oct 28;108(17):2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 2.Apostolakis S, Spandidos D. Chemokines and atherosclerosis: focus on the CX3CL1/CX3CR1 pathway. Acta pharmacologica Sinica. 2013 Oct;34(10):1251–1256. doi: 10.1038/aps.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah R, Hinkle CC, Ferguson JF, et al. Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes. 2011 May;60(5):1512–1518. doi: 10.2337/db10-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White GE, Greaves DR. Fractalkine: a survivor's guide: chemokines as antiapoptotic mediators. Arteriosclerosis, thrombosis, and vascular biology. 2012 Mar;32(3):589–594. doi: 10.1161/ATVBAHA.111.237412. [DOI] [PubMed] [Google Scholar]
- 5.Chapman GA, Moores KE, Gohil J, et al. The role of fractalkine in the recruitment of monocytes to the endothelium. European journal of pharmacology. 2000 Mar 31;392(3):189–195. doi: 10.1016/s0014-2999(00)00117-5. [DOI] [PubMed] [Google Scholar]
- 6.Schlitt A, Heine GH, Blankenberg S, et al. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thrombosis and haemostasis. 2004 Aug;92(2):419–424. doi: 10.1160/TH04-02-0095. [DOI] [PubMed] [Google Scholar]
- 7.Moatti D, Faure S, Fumeron F, et al. Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood. 2001 Apr 1;97(7):1925–1928. doi: 10.1182/blood.v97.7.1925. [DOI] [PubMed] [Google Scholar]
- 8.Lavergne E, Labreuche J, Daoudi M, et al. Adverse associations between CX3CR1 polymorphisms and risk of cardiovascular or cerebrovascular disease. Arteriosclerosis, thrombosis, and vascular biology. 2005 Apr;25(4):847–853. doi: 10.1161/01.ATV.0000157150.23641.36. [DOI] [PubMed] [Google Scholar]
- 9.Sirois-Gagnon D, Chamberland A, Perron S, Brisson D, Gaudet D, Laprise C. Association of common polymorphisms in the fractalkine receptor (CX3CR1) with obesity. Obesity. 2011 Jan;19(1):222–227. doi: 10.1038/oby.2010.125. [DOI] [PubMed] [Google Scholar]
- 10.Fischer MJ, Go AS, Lora CM, et al. CKD in Hispanics: Baseline Characteristics From the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am J Kidney Dis. 2011;58(2):214–227. doi: 10.1053/j.ajkd.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009 Aug;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003 Jul;14(7 Suppl 2):S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 13.Damas JK, Waehre T, Yndestad A, et al. Stromal cell-derived factor-1alpha in unstable angina: potential antiinflammatory and matrix-stabilizing effects. Circulation. 2002 Jul 2;106(1):36–42. doi: 10.1161/01.cir.0000020001.09990.90. [DOI] [PubMed] [Google Scholar]
- 14.Mehta NN, Li M, William D, et al. The novel atherosclerosis locus at 10q11 regulates plasma CXCL12 levels. Eur Heart J. 2011 Mar 17;2011:963–971. doi: 10.1093/eurheartj/ehr091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta J, Mitra N, Kanetsky PA, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012 Dec;7(12):1938–1946. doi: 10.2215/CJN.03500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson AH, Yang W, Hsu CY, et al. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012 Aug;60(2):250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Townsend RR, Anderson AH, Chen J, et al. Metabolic syndrome, components, and cardiovascular disease prevalence in chronic kidney disease: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. American journal of nephrology. 2011;33(6):477–484. doi: 10.1159/000327618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Mehta NN, Matthews GJ, Krishnamoorthy P, et al. Higher plasma CXCL12 levels predict incident myocardial infarction and death in chronic kidney disease: findings from the Chronic Renal Insufficiency Cohort study. Eur Heart J. 2014 Aug 14;35(31):2115–2122. doi: 10.1093/eurheartj/eht481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008 Jan 30;27(2):157–172. doi: 10.1002/sim.2929. discussion 207-112. [DOI] [PubMed] [Google Scholar]
- 21.Ikejima H, Imanishi T, Tsujioka H, et al. Upregulation of fractalkine and its receptor, CX3CR1, is associated with coronary plaque rupture in patients with unstable angina pectoris. Circulation journal : official journal of the Japanese Circulation Society. 2010 Feb;74(2):337–345. doi: 10.1253/circj.cj-09-0484. [DOI] [PubMed] [Google Scholar]
- 22.Ancuta P, Rao R, Moses A, et al. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 2003 Jun 16;197(12):1701–1707. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211(6–8):609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Landsman L, Bar-On L, Zernecke A, et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009 Jan 22;113(4):963–972. doi: 10.1182/blood-2008-07-170787. [DOI] [PubMed] [Google Scholar]
- 25.Saederup N, Chan L, Lira SA, Charo IF. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2−/− mice: evidence for independent chemokine functions in atherogenesis. Circulation. 2008 Apr 1;117(13):1642–1648. doi: 10.1161/CIRCULATIONAHA.107.743872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richter B, Koller L, Hohensinner PJ, et al. Fractalkine is an independent predictor of mortality in patients with advanced heart failure. Thrombosis and haemostasis. 2012 Dec;108(6):1220–1227. doi: 10.1160/TH12-03-0195. [DOI] [PubMed] [Google Scholar]
- 27.Morris DL, Oatmen KE, Wang T, DelProposto JL, Lumeng CN. CX3CR1 deficiency does not influence trafficking of adipose tissue macrophages in mice with diet-induced obesity. Obesity. 2012 Jun;20(6):1189–1199. doi: 10.1038/oby.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YS, Morinaga H, Kim JJ, et al. The fractalkine/CX3CR1 system regulates beta cell function and insulin secretion. Cell. 2013 Apr 11;153(2):413–425. doi: 10.1016/j.cell.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. Journal of immunology. 2004 Mar 1;172(5):2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimoto S, Nakatani K, Iwano M, et al. Elevated levels of fractalkine expression and accumulation of CD16+ monocytes in glomeruli of active lupus nephritis. Am J Kidney Dis. 2007 Jul;50(1):47–58. doi: 10.1053/j.ajkd.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Katsuyama K, Fujinaka H, Yamamoto K, et al. Expression of the chemokine fractalkine (FKN/CX3CL1) by podocytes in normal and proteinuric rat kidney glomerulus. Nephron. Experimental nephrology. 2009;113(2):e45–e56. doi: 10.1159/000228408. [DOI] [PubMed] [Google Scholar]
- 32.Ito Y, Kawachi H, Morioka Y, et al. Fractalkine expression and the recruitment of CX3CR1+ cells in the prolonged mesangial proliferative glomerulonephritis. Kidney international. 2002 Jun;61(6):2044–2057. doi: 10.1046/j.1523-1755.2002.00369.x. [DOI] [PubMed] [Google Scholar]
- 33.Yadav AK, Lal A, Jha V. Association of circulating fractalkine (CX3CL1) and CX3CR1(+)CD4(+) T cells with common carotid artery intima-media thickness in patients with chronic kidney disease. Journal of atherosclerosis and thrombosis. 2011;18(11):958–965. doi: 10.5551/jat.8722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.