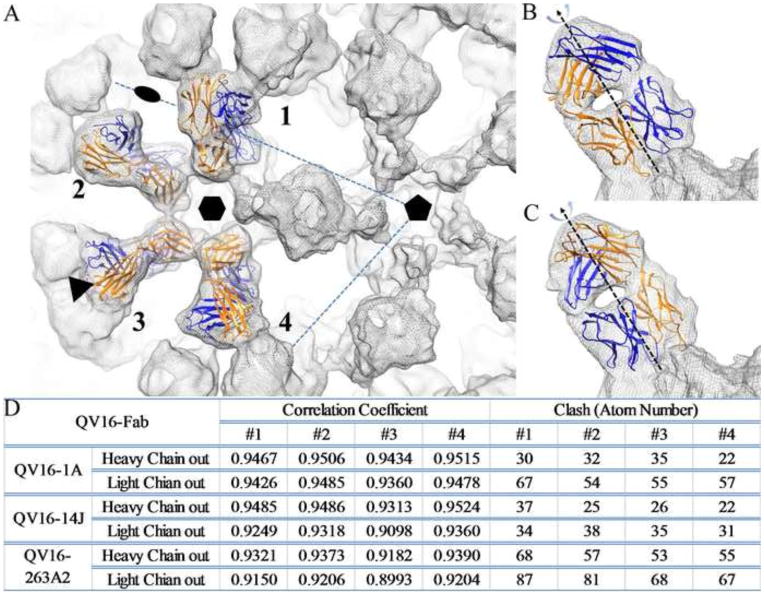
FIGURE 3. Fitting of four Fabs on one asymmetric unit of capsid.
(A) Four Fab densities marked as 1 to 4 on an asymmetric unit of the HPV16 capsid were used for fitting. The icosahedral 2-fold, 3-fold and 5-fold symmetry axes are indicated and the hexavalent capsomer is marked on the virus-Fab complex density map (grey mesh). Due to the pseudo-two-fold symmetry axis between Fab heavy (Blue) and light chains (Orange), two fitting modes were used for refinement of fit: the Fab heavy chain facing outwards from the center of the capsomer (B) and Fab light chain facing outwards (C). Statistics for the fitted Fab structures into corresponding cryo-EM density are reported for both fitting modes (D) according to the correlation coefficient and the number of atomic clashes with the fitted virus structure (crystal structure of the HPV16 L1 pentamer, Protein Data Bank, PDB ID 3OAE) (47)).