Abstract
Human bone marrow stromal cells (hBMSCs, also known as bone marrow-derived mesenchymal stem cells) are a population of progenitor cells that contain a subset of skeletal stem cells (hSSCs), able to recreate cartilage, bone, stroma that supports hematopoiesis and marrow adipocytes. As such, they have become an important resource in developing strategies for regenerative medicine and tissue engineering due to their self-renewal and differentiation capabilities. The differentiation of SSCs/BMSCs is dependent on exposure to biophysical and biochemical stimuli that favor early and rapid activation of the in vivo tissue repair process. Exposure to exogenous stimuli such as an electromagnetic field (EMF) can promote differentiation of SSCs/BMSCs via ion dynamics and small signaling molecules. The plasma membrane is often considered to be the main target for EMF signals and most results point to an effect on the rate of ion or ligand binding due to a receptor site acting as a modulator of signaling cascades. Ion fluxes are closely involved in differentiation control as stem cells move and grow in specific directions to form tissues and organs. EMF affects numerous biological functions such as gene expression, cell fate, and cell differentiation, but will only induce these effects within a certain range of low frequencies as well as low amplitudes. EMF has been reported to be effective in the enhancement of osteogenesis and chondrogenesis of hSSCs/BMSCs with no documented negative effects. Studies show specific EMF frequencies enhance hSSC/BMSC adherence, proliferation, differentiation, and viability, all of which play a key role in the use of hSSCs/BMSCs for tissue engineering. While many EMF studies report significant enhancement of the differentiation process, results differ depending on the experimental and environmental conditions. Here we review how specific EMF parameters (frequency, intensity, and time of exposure) significantly regulate hSSC/BMSC differentiation in vitro. We discuss optimal conditions and parameters for effective hSSC/BMSC differentiation using EMF treatment in an in vivo setting, and how these can be translated to clinical trials.
Introduction
Human bone marrow stromal cells (hBMSCs, also known as bone marrow-derived mesenchymal stem cells) contain a population of progenitor cells, and a subpopulation of skeletal stem cells (hSSCs) known to be able to recreate cartilage, bone, stroma that supports hematopoiesis and marrow adipocytes. Recently, hSSCs have been found to reside as pericytes on bone marrow sinusoids, and to participate in vascular stability (Sacchetti et al., 2007). As such, human bone marrow stromal stem/ progenitor cells (hSSCs/BMSCs, collectively referred to as hBMSCs below) continue to be a cornerstone in the fields of basic science and medicine due to their regenerative, reparative, and angiogenic properties. These cells are attractive candidates for cell-based tissue regeneration because of their ability to be extensively propagated in culture while retaining their differentiation potential, although overexpansion can lead to senescence and inability to differentiate. Transcription factors [such as RUNX2 and β-CATENIN (CTNNB1) (Ceccarelli et al., 2013; Liu et al., 2009; Takada, et al., 2009)] and signaling molecules [such as WNTs, TGF-β and VEGF (Yang et al., 2012)] work in concert to regulate BMSC differentiation. Studies in developmental biology have revealed that transcription factors are key regulators of embryonic morphogenesis, and play a leading role in the control and regulation of the differentiation pathways of stromal cells. For BMSCs in particular, the main transcription factors that drive differentiation during development are Cbfa-1/Runx2 and Osterix (Sp7) for bone formation (Komori, 2010; Schroeder et al., 2005), while Sox9 and modulation of Wnt/β-catenin signaling pathways drive chondrogenesis (Chen CH et al., 2013; Day et al., 2005; Mayer-Wagner et al., 2011). BMSC differentiation is heavily influenced by molecular and biophysical-regulating factors present within their environment. In culture, these factors include nutrient media, scaffold constructs, and biochemical cues as well as biophysical information exchange. The BMSCs' first line of interaction is with their extracellular matrix (ECM), which serves as an endogenous scaffold. Once proliferation is established in the ECM, differentiation and continued proliferation onto extracellular structures, such as natural or synthetic scaffolds, begin. Sundelacruz et al. reported that manipulation of the membrane potential of cultured BMSCs can influence their fate and differentiation, along the adipogenic and osteogenic lineages (Sundelacruz et al., 2008, 2009). These findings suggest that it may be possible to achieve an unprecedented level of control over BMSC differentiation using exogenous factors such as an electromagnetic field (EMF). In agreement with this assertion are recent studies showing that extremely low frequency (0–100 Hz) electromagnetic fields (ELF-EMF) affect numerous biological functions such as cell differentiation (Funk et al., 2009), gene expression (Mousavi et al., 2014), and cell fate (Kim et al., 2013), and have been reported to promote the release of necessary growth factors and enhance the differentiation process (Funk and Monsees, 2006).
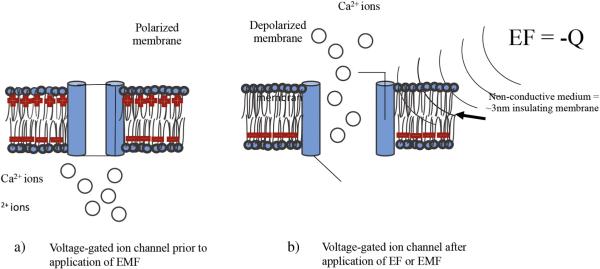
During human development, lineage-committed cells of the three embryonic germ layers migrate and proliferate in appropriate directions to form tissues and organs. Throughout this biological development process, electric fields (EFs) arise in the form of endogenous ionic currents (Levin, 2003; McGaig et al., 2005). While endogenous EFs are present in all developing and regenerating animal tissues, their existence and potential impact on tissue regeneration and repair have been largely ignored. In order to guide cells during migration, endogenous field gradients develop in the embryo by forming voltage gradients between the intracellular and extracellular environment (Levin, 2012a). These voltage gradients are generated by passive sodium (Na+) uptake from the extracellular environment creating potential differences that are time and location specific, and are switched on and off at different developmental stages (Levin, 2003; Levin and Stevenson, 2012b). In most cells, sodium (Na+) and chloride (Cl−) dominate the outside of the plasma membrane and potassium (K+) and organic molecules such as anions (A−) dominate the inside (Sherwood et al., 2005). Na+ and Cl− are the major solutes in the extracellular fluid. These ionic currents are responsible for changes in voltage gradients that correlate with morphogenetic events during growth and patterning (size, shape, and position) of the organism (Hotary and Robinson, 1990; Hotary and Robinson, 1992; Metcalf et al., 1994). The unequal distribution of a few key ions between the intra- and extra-cellular fluid, and their selective movement through the plasma membrane, governs the electrical properties of the membrane. All plasma membranes have a membrane potential, which electrically polarizes them; therefore, the membrane potential (Vmem) refers to a separation of charges across the membrane (Sherwood et al., 2005). Fluctuations in potential serve as electrical signals. These electrical charges are carried by ions. All living cells have a membrane potential, with the cell's interior being slightly more negative than the fluid surrounding the cell when the cell is electrically at rest. Charges are separated across the plasma membrane, and any time the value of the Vmem is anything other than 0 mV, in either the positive or negative direction, the membrane is in a state of polarization. The magnitude of the polarization potential is directly proportional to the number of positive and negative charges separated by the membrane. Changes in Vmem are brought about by changes in ion movement across the membrane. Triggering events such as exposure to EMF can cause changes in membrane permeability. Gated-channels have movable folds in the proteins that can alternately be open, permitting ion passage through the channel, or closed, preventing ion passage through the channel (Fig. 1). Like many proteins, these channels can be inherently flexible molecules whose conformations can be altered in response to external factors (Sherwood et al., 2005). Voltage-gated ion channels, in particular, open or close in response to changes in membrane potential (Panagopoulos et al., 2002; Pall, 2013).
Figure 1.
a) Voltage-gated ion channels control intra- and extra-cellular ion flux due to positive surface charge. b) EF can attenuate the opening and closing of these ion channels to trigger intracellular events due to negative charge (−Q) depolarizing the plasma membrane.
Living systems are constantly in motion, and a changing magnetic field (MF) is associated with a changing electric field (EF). This has been shown via Faraday's Law, which states that a MF will interact with an electric circuit to produce an electromotive force. Endogenous pulsed EMF arises from the movement of muscles, tendons, and the actions of the musculoskeletal system (Hastings and Mahmud, 1988). Mechanical deformation of dry bone ex vivo generates piezoelectricity through bending strains associated with spatial gradients of permanent dipoles in collagen molecules. In living bone however, small piezoelectric potentials are shielded (Otter et al., 1998b). In physiology, mechanical stress-generated potentials are formed by mechanisms such as: 1) the streaming potential, which is the electric potential difference between a liquid and a capillary, diaphragm, or porous solid in which the fluid is forced to flow; or 2) the entrainment of ions caused by fluid motion through the bone (Otter et al., 1998b). The EMF caused by either of these reactions is able to penetrate tissue, and the MF component can induce electric currents in the bone or muscle tissue via Faraday coupling. Faraday coupling is a form of inductance by which the current in one system induces a voltage in another. Vibrations of human muscles induce mechanical strains on bone and currents in the range of 5–30 Hz frequencies during quiet muscle activity (standing), and <10 Hz while walking (Antonsson and Mann, 1985). Bone cells have strong frequency selectivity with EMF effectiveness peaking in the range of 15–30 Hz. In this range, fields as low as 0.01 mV/cm affect remodeling activity (McLeod and Rubin, 1993), and endogenous EMF of 1 Hz, with current densities of 0.1–1.0 mA/cm2 (Lisi et al., 2006) produced during walking.
Research into this phenomenon found that voltage gradients were not just membrane potentials, but specific signals for key metabolic processes in embryonic development and regenerative wound healing (Hotary and Robinson, 1992; Levin, 2007; Nuccitelli, 2003). These signals lead the way for cells to migrate by forming voltage gradients between the intracellular and extracellular environment (Funk and Monsees, 2006). Voltage gradients are localized direct current EFs which are switched on and off at different developmental stages (McGaig et al., 2005). They spread into the extracellular space, as well as into the cytoplasm of one or more cells, coupled by gap junctions (Funk et al., 2009). These gradients can penetrate the cell membrane, into the cytoplasm, and even the nuclear membrane, through signal transduction, whereby the EMF signal is received via receptors on the cell surface, then processed by G-proteins that couple the receptors to effectors, such as ion channels (Ermakov et al., 2012). These signal transduction processes have been reported to show a correlation between the presence of EMF gradients and cellular response in embryogenesis (Funk and Monsees, 2006; Sundelacruz et al., 2013). For hBMSCs to differentiate, there must be effective exogenous stimuli providing direction for their differentiation capabilities. One such stimuli is sinusoidal low-frequency EMF (0.3–100 Hz), which produces fields that are coherent (Adey, 1993), and produce regularly recurring signals — that must be present for a certain minimum duration (Litovitz et al., 1993). This resonant coherence is the key to inducing large effects with low thresholds (Panagopoulos et al., 2002). Conservative estimates show that a 1 μV induced membrane potential can be detected after 10 ms by fewer than 108 ion channels; therefore a strong EMF is not required. According to several different authors (Jacobson, 1994; Jacobson and Yamanashi, 1995; Sandyk, 1996; Persinger, 2006; Persinger and Koren, 2007), picoTesla–nanoTesla intensity EMF is effective with appropriate resonance as a function of the charge and mass of the target molecule (Jacobson, 1994; Jacobson and Yamanashi, 1995; Persinger, 2006; Persinger and Koren, 2007; Sandyk, 1996).
Defining electromagnetic field parameters
When discussing cellular influences by either endogenous or exogenous fields, it is important to define the nomenclature. In this discussion, the term electromagnetic field is used to summarize the whole field, which includes “electric,” “magnetic” and combined “electromagnetic” effects. Electric field (EF) involves a current that can be either direct (DC) or alternating (AC). Electric current units are measured in amperes (A). Electrical potential differences are measured in volts (V). Units of magnetic flux density (intensity) are measured in either Gauss (G), or Tesla (T), which is 10,000 G (see Table 1).
Table 1.
Types of electric, magnetic and electromagnetic fields.
| Field type | Type | Potential difference | Intensity |
|---|---|---|---|
| Electric | Direct (DC) or alternating (AC) | Current | Amperes |
| Magnetic | Static or time-varying | Volt | Gauss or Tesla |
| Electromagnetic | Static or pulsed | Volt | Gauss or Tesla |
Faraday's law of induction and Maxwell's equations explain how an EMF is generated. A static electric field is generated by a static charged particle (q). The electric field (or E component of an EMF) exists whenever charge (Q) is present. Its strength is measured in volts per meter [V/m], and expressed as intensity for field strength. An electric field of 1 V/m is represented by a potential difference of 1 V existing between two points that are 1 m apart. Magnetic field (or M component of an EMF) arises from current flow. The Tesla (or Gauss) is mainly used to express the flux density or field strength produced by the MF. Both EFs and MFs are generated if a charged particle moves at a constant velocity. Combined, they generate an EMF when the charged particle is accelerated. Most often this acceleration takes place in the form of an oscillation, therefore electric and magnetic fields often oscillate. Change in the EF creates an MF, and any change in the MF creates an EF. This interaction suggests the higher the frequency of oscillation, the more the electric and magnetic fields are mutually coupled.
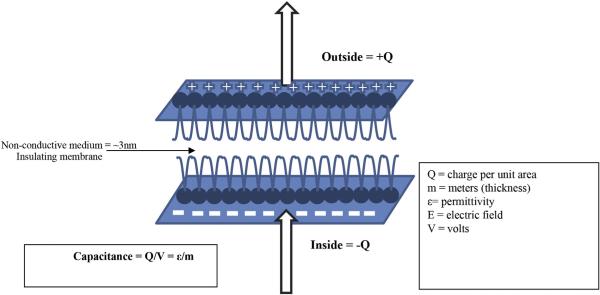
EMF can affect biochemical reactions and the behavior of charged molecules near cell membranes. MF can influence cell behavior by: exerting force on moving charge carriers such as ions; generating electric fields in conductive substances; changing the rate of diffusion across membranes (Ikehara et al., 1998); and distorting bond angles, which affects protein structure binding, and therefore macromolecule synthesis (Barnothy, 1969). Unlike EF, which are shielded by the high dielectric properties of the cell membrane, magnetic gradients penetrate deeper through layers of living tissue (Funk and Monsees, 2006), acting directly on cell organelles. Pulsing the EMF causes a rise and fall in ion fluxes, whereby changes in the membrane potential cause an inward current flow resulting in hyperpolarization of its potential (Alberts et al., 2002). Depending on the parameters involved in the EMF treatment, and the biological process in question, either stimulation or inhibition can occur. In contrast to the membrane, the cytoplasm or fluids in extracellular spaces, contain no free electrons to carry charge, so current is carried by charged ions such as Na+, K+, and Ca2+. The resistivity of the solution can be measured, and is typically ~100 ohms (Ω) (Funk et al., 2009). If there is a voltage difference between any two points in a conductive medium, current will flow. This voltage difference per unit of distance is the EF. Considering the dimension of a cell and the thickness of a cell membrane (~10 nm) with a 0.1 V difference, this corresponds to a field strength of ~70 V/m, meaning that the potential inside the cell is 70 mV less than the potential outside due to a layer of negative charge on the inner surface of the cell membrane and a layer of positive charge on the outer surface. Because a cell's diameter is much larger than the membrane thickness, it is reasonable to ignore the curvature of the cell and think of it as a charged capacitor with a capacitance of approximately 2 μFarad per cm2 of membrane area (Hille, 1992). The differences in various ion concentrations on either side of the membrane can result in a new voltage of between 70 and 80 mV across the membrane. With a sheet of negative charge on the intracellular side of the membrane and a sheet of positive charge on the extracellular side, the cell membrane is best modeled as a parallel-plate capacitor. This voltage difference keeps weaker EF from entering the cell. By adding the magnetic component (MF), the EMF can penetrate the cell membrane (Otter et al., 1998b) (Fig. 2).
Figure 2.
Because a cell's diameter is much larger than its membrane thickness, it is reasonable to ignore the curvature of the cell and think of the plasma membrane as a parallel-plate capacitor, where Q is charge per unit area, and capacitance is equal to charge divided by voltage.
Studies in developmental biology have identified key regulators of morphogenetic properties, and have indicated where endogenous EMF is located in the action potentials of nerves and heart tissue, and in skeletal muscle vibrations, with frequencies elicited by rhythmic activities throughout the human organism (Levin, 2003). Endogenous EMF frequencies act on a cell at the molecular level through extremely low endogenous frequencies (Funk et al., 2009). It is these endogenous frequencies that can be entrained to follow exogenous EMF of the same frequencies. This entrainment (via harmonic resonance) is what influences the differentiation of BMSCs. While there are a multitude of research articles investigating these phenomena, the methods for gathering these data include an overwhelming array of experimental models, EMF devices, waveforms, and clinical applications; therefore, a consensus of standardized methods for experimentation is greatly needed to determine which responses directly result from the EMF exposure. Effective EMF stimuli are coherent, presenting a series of recurring signals that must be present for a minimum amount of time (Adey, 1993). This effect is also tissue specific (Zimmerman et al., 2013; Zimmerman et al., 2012). Therefore, the precise time points during which differentiation occurs under EMF stimuli need to be elucidated. While high frequency (900–1800 MHz) EMF, such as that derived from microwave and mobile phone communication, acts through mixtures of modulated and carrier frequencies, research to-date has focused primarily on the thermal effects of radiation at a tissue-specific absorption rate known as SAR.
EMF and osteogenic differentiation of hBMSCs
Osteogenesis is a complex series of events by which BMSCs differentiate to generate new bone. hBMSCs possess characteristic Ca2+ waves that are involved in intracellular signaling. These waves operate in short and long periods, with the longer periods operating during trans-cellular signaling. In the field of intracellular signaling, the oscillation of cytosolic Ca2+ is perhaps one of the most important discoveries (Parekh, 2011). Research into the molecular information embedded in Ca2+ oscillations is leading to the understanding of dynamic transports of Ca2+ to and from the exterior of the cell, intracellular stores, cytosol, as well as Ca2+ exchanges between cells, diffusion, and buffering due to the binding of Ca2+ to proteins. Ca2+ oscillations vary in amplitude, temporal profile, and spatial properties, and are likely mediated by several influx and efflux pathways depending on different cellular processes (Sun et al., 2007). Ca2+ oscillations have been found to play a key role in EMF-induced cell differentiation (D'Souza et al., 2001; Den Dekker et al., 2001). Sun et al. showed that a direct current (DC) 0.1 V/cm stimulus (30 min/day for 10 days) enhanced expression of osteogenic factors for hBMSC differentiation into the osteogenic cell lineage by reducing the Ca2+ wave frequency typically found in the differentiation process (Sun et al., 2007). For quite some time, mechanical forces have been known to affect molecular signaling and molecules in bone cells via mechanotransduction (Mak and Zhang, 2001). The conversion of mechanical loads to bioelectric signals (i.e., pressure generated potentials also known as piezoelectricity) in bone has been suggested to control repair and remodeling (Yoshida et al., 2009). These signals are attributed to electrically-generated kinetic behavior where mechanical forces generate electrical signals due to the motion of ion-carrying extracellular fluid in the bone matrix. This effect is known as streaming potential (Guzelsu and Walsh, 1990). The use of EMF to stimulate osteogenesis is based on the idea of stimulating the natural endogenous streaming potentials in bone. The same physiological frequencies (8–30 Hz) caused by natural muscle contractions and subsequently induced EF in bony tissue, can be used to regenerate tissue as well as differentiate hBMSCs into osteoblasts. Frequencies in the range of 5–75 Hz have been used to differentiate bone from hBMSCs (Table 2).
Table 2.
Frequency specific effects of EMF on hBMSCs including field strength and time of exposure.
| Authors | EMF type | Freq (Hz) | Intensity | Exposure Time | Outcome |
|---|---|---|---|---|---|
| Fu et al. (2014) | Pulsed | 15 | 2 mT | 30 pulses/d for 21 d | Significantly increased ALP, neovascularization and bone matrix in osteogenic differentiation |
| Ceccarelli et al. (2013) | Pulsed | 75 | 2 mT | 1, 4, 8 h/d | Significantly increased bone matrix deposition in osteoblasts differentiated from hBMSCs |
| Hess et al. (2012) | Pulsed | 3.6 mV/cm | 7 ms pulses | 4h | Synergistic effect of EF and osteogenic media (OM) enhanced proliferation compared with OM only or EF only |
| Luo et al. (2012) | Pulsed | 5, 25, 50, 75, 100, & 150 | 1.1 mT | 30 min/for 21 d | 50 Hz was most effective at differentiation of hBMSCs to osteoblasts via significant increases in ALP, OSTEOCALCIN, COLLAGEN I, and Ca2+ |
| Kaivosoja et al. (2015) | Pulsed | 15 | 1 μT | 24 h/d for 1 d | Increased expression of osteogenic markers ALP, SMAD1, RUNX2, OSTEOPONTIN, and OSTEOCALCIN compared with controls |
| Mayer-Wagner et al. (2011) | Pulsed | 15 | 5 mT | 45 min/(3×/d) every 8 h for 21 d | Increased glycosaminoglycans (GAGs) and COLLAGEN II compared with controls |
| Jansen et al. (2010) | Pulsed | 15 | 1 μT | Continuous for 1, 5, 9 & 14 d | Significant, differentiation stage-dependent, increase in mineralization at days 9 and 14, without altering ALP activity. Increased BMP2, TGF-β, OSTEOPONTIN, MMP1, MMP3, OSTEOCALIN, and BONE SIALOPROTEIN. |
| Sun et al. (2010) | Pulsed | 15 | 1.8 mT | 8 h/d for 2, 4 & 7d | Significantly increased key osteogenic regulatory gene RUNX2 and ALP expression. Substantially enhanced mineralization near midpoint of osteogenesis compared to untreated controls. |
| Sun et al. (2009) | Pulsed | 15 | 1.8 mT | 8 h/d for 3 d | 59% and 40% increased viability in PEMF-exposed cultures at 24 h after plating cell density of 1000 and 3000 cells/cm2, respectively |
| Tsai et al. (2009) | Pulsed | 7.5 | 0.13 mT | 2 h/d for 14 & 28 d | Significantly increased ALP beginning at day 7 and reaching the highest level at day 28; increased early expression of osteogenic marker, RUNX2 |
| Schwartz et al. (2008) | Pulsed | 15 | 0–16 mT | 8 h/d for 20 d | Minor increase ALP with no change in OSTEOCALCIN. Osteogenic media (OM) increased ALP and OSTEOCALCIN by day 6, but not PEMF. BMP2 was stimulatory over OM, and PEMF/BMP2 synergistically increased ALP and OSTEOCALCIN. PEMF also enhanced the effects of BMP2 on PGE2, latent and active TGF-β1, and OSTEOPROTEGERIN. Effects of PEMF on BMP2-treated cells were greatest at days 12 to 20. |
Hz = hertz; T = Tesla; V = volts; min = minutes; ms = milliseconds; μs = microseconds; d = days; h = hours.
Bone remodeling is a highly integrated process of resorption by osteoclasts and formation of bone tissue by osteoblasts, which results in precisely balanced skeletal mass with renewal of the mineralized matrix (Ashton et al., 1980). Hartig et al. reported that a 16 Hz EMF enhances osteoblast activity while reducing osteoclast formation, shifting the balance towards osteogenesis (Hartig et al., 2000). Sun et al. investigated the effect of a 15 Hz, 1.8 mT pulsed EMF (PEMF) on cell proliferation, alkaline phosphatase (ALP) activity, gene expression, and mineralization of the ECM in hBMSCs. Their osteogenic differentiation resulted in a significantly altered temporal expression of osteogenic-related genes, including a 2.7-fold increase in expression of the key osteogenic regulatory gene RUNX2/CBFA1, compared to untreated controls (Sun et al., 2010). In addition, cell exposure to PEMF significantly increased ALP expression during the early stages of osteogenesis and substantially enhanced mineralization near the midpoint of osteogenesis. Increased cell numbers were observed at late stages of osteogenic culture with this same PEMF exposure. The production of ALP, an early marker of osteogenesis, was significantly enhanced at day 7 when exposed to PEMF treatment in both basal and osteogenic cultures as compared to untreated controls. Furthermore, the expression of a key osteogenic regulatory gene RUNX2/CBFA1 and ALP, was also partially modulated by PEMF exposure, indicating that osteo-genesis in hBMSCs was associated with the specific PEMF stimulation (Tsai et al., 2009). Tsai et al. reported similar results when they isolated hBMSCs from adult patients and cultured them in osteogenic medium for up to 28 days. Using a PEMF stimulation of 7.5 Hz, greater cell numbers were observed compared with controls (Tsai et al., 2007). The production of ALP was significantly enhanced at day 7 on both basal and osteogenic cultures as compared to untreated controls. Also the expression of early osteogenic genes RUNX2/CBFA1 and ALP was indicative of PEMF stimulation. ALP accumulation produced by the hBMSCs, along with Ca2+ deposits reached their highest levels at day 28.
EMF alone, and in combination with nanomagnetic particles (MPs), has also been used to promote the differentiation potential of hBMSCs. Kim et al. investigated the effect of both EMF and MPs on hBMSCs by treating them with 50 μg/ml of Fe3O4 MPs and/or an exposure of 45 Hz, 1 mT intensity EMF (Kim et al., 2015). Cells were exposed to EMF twice every 8 h/day for 7 days. Treatment with MP, and/or then exposure to EMF did not cause cytotoxic effects. Strong expression of osteogenic markers OSTEOCALCIN, OSTEOPONTIN, and OSTEONECTIN was observed in the cells treated with MPs, EMF alone, MP alone, or a combination of MP and EMF, as compared with controls. Quantitative RT-PCR revealed that mRNA expression levels of OSTEOCALCIN, OSTEOPONTIN, OSTEONECTIN, COLLAGEN I (COL1A1), COLLAGEN III (COL3A1), BONE MORPHOGENETIC PROTEIN 2 (BMP2), BONE SIALOPROTEIN (IBSP), and RUNX2 were significantly increased in cells treated with MPs, than those exposed to EMF. Furthermore, the mRNA expression of calcium channels, CACNA1C, CACNA1E, CACNA1G and CACNA1l, was activated during osteogenic differentiation. BONE SIALOPROTEIN, BMP2, OSTEOPONTIN and OSTEONECTIN, as well as the phosphorylated extracellular signal-regulated kinase, p-ERK, were all increased in the cells treated with MPs alone, EMF alone, and MP + EMF, compared with the control group. Florescence-activated cell sorting (FACS) analysis of CD73, CD90, and CD105 showed a decrease in these hBMSC cell surface markers in the cells treated with MPs, compared with those exposed to EMF. This was also seen in the cells treated with MPs, then exposed to EMF, as compared with control. Cell mitochondrial activity among the four groups was similar, showing an increase in ALP activity.
Frequencies used thus far for stimulating and enhancing osteogenesis have varied from 7.5 to 75 Hz (De Mattei et al., 1999; Lohmann et al., 2000; Schwartz et al., 2008; Sun et al., 2009; Trock DH et al., 1993; Tsai et al., 2007; Tsai et al., 2009), and have revealed that not only frequency, but also the direction of the EMF makes a difference in the results. For example, hBMSCs exposed to positive (30/45 Hz, 1 mT) and negative (7.5 Hz, 1 mT) EMF for osteogenic differentiation reported increases in ALP mRNA expression. These data indicate that the effect of EMF on osteogenic differentiation is significantly dependent on the direction of the EMF exposure. It is important to point out that the effects occurring at 7.5, 15, 45, and 75 Hz are harmonic waves and these pulsed patterns going from lower to higher order harmonics cause a decrease in relative energy states (Poon et al., 1995).
To date, there have not been a consistent set of EMF stimulus parameters used among research groups reported in the literature; however, results suggest that EMF promotion of bone ECM deposition in vitro is more far more efficient in osteoblasts differentiated from hBMSCs than from cells of other tissues (Bianco et al., 2013). Sun et al. have investigated the effect of PEMF on the proliferation and differentiation potential of human hBMSCs. EMF stimulus was administered to cells for 8 h per day during the culture period. The EMF applied consisted of 4.5 ms bursts repeating at 15 Hz, and each burst contained 20 pulses. Results showed 59% more viable hBMSCs were obtained in the EMF-exposed cultures at 24 h after plating and 20–60% higher cell densities were achieved during the exponentially expanding stage. Many newly divided cells appeared from 12 to 16 h after the EMF treatment; however, cytochemical assays and immunofluorescence analysis showed multilineage differentiation of EMF-exposed hBMSCs to be similar to that of the control group, which used only standard growth media (Sun et al., 2009).
Bone tissue engineering typically uses biomaterial scaffolds, osteoblasts, or cells that can become osteoblasts, and biophysical stimulation to promote cell attachment and differentiation. Saino et al. tested the effects of EMF on hBMSCs seeded on gelatin cryogel disks and compared with control conditions without EMF stimulus. Treatment with EMF (at 2 mT intensity and 75 Hz frequency) increased the cell proliferation and differentiation, as well as enhanced the biomaterial surface coating with bone ECM proteins (Saino et al., 2011). Using this approach, the gelatin biomaterial, coated with differentiated cells and their ECM proteins, has the potential to be used in clinical applications as an implant for bone defect repair. For example, under the appropriate culture conditions, PEMF enhances the osteogenic effects of BMP-2 on hBMSCs. Thus, PEMF could potentially be used clinically to stimulate bone formation from transplanted hBMSCs.
Specific studies investigating whether the effects of PEMF on osteogenic cells were substrate dependent, and could also regulate osteoclastic bone resorption. Schwartz et al. treated hBMSCs and human osteosarcoma cell lines (MG63 cells, SaOS-2 cells) capable of osteoblastic differentiation with BMP-2, then cultured them on calcium phosphate (CaP) or tricalcium phosphate (TCP) to test their response to a 15 Hz PEMF at either 4.5 ms bursts or 20 pulses repeated for 8 h/day. Outcomes were determined to be a function of the decoy receptor, osteoprotegerin (OPG), and RANK ligand (RANKL) production, both of which are associated with the regulation of osteoclast differentiation. Results suggested that when osteogenic cells were cultured on CaP, PEMF decreased cell number and increased production of paracrine factors associated with reduced bone resorption such as OPG (Schwartz et al., 2009). RANKL was unaffected, indicating that the OPG/RANKL ratio was increased, further supporting a surface-dependent osteogenic effect of PEMF. Moreover, effects of estrogen were surface-dependent and enhanced by PEMF, demonstrating that PEMF can modulate osteogenic responses to anabolic regulators of osteoblast function. Therefore, PEMF shows promising results when used in conjunction with complex 3-D cell culture systems as a strategy for tissue engineering approaches.
Influence of EMF on chondrogenic differentiation of hBMSCs
Chondrogenesis is initiated by condensation of embryonic mesenchyme, which induces differentiation of mesenchyme into chondrocytes, and the subsequent secretion of the molecules that form the ECM (Charbord et al., 2011). EMF has been shown to exert beneficial effects on cartilage tissue, and differentiated hBMSCs are being investigated as an alternative approach for cartilage repair. Repair, replacement or regeneration of cartilage tissue is challenging due to the fact that injured articular cartilage is not easily able to repair itself and often the repair of articular cartilage fails because there is a lack of an abundant source of cells to accelerate the healing process and promote host tissue. Research has demonstrated that it is not easy to obtain a sufficient number of hBMSCs for therapeutic use after expansion in vitro, because after thirty population doublings (PDs), hBMSCs exhibit replicative senescence, which blocks their ability to differentiate. However, in vivo studies have shown that PEMF can be used to promote proliferation of endogenous chondroblasts (Fitzsimmons et al., 2008), and suppress inflammatory reactions induced by the repair treatment, thereby enhancing cartilage regeneration (Fini et al., 2013). Successful articular cartilage tissue engineering relies largely on identifying appropriate cell sources, designing the proper formulations of 3D scaffolding matrix, bioactive agents, differentiation stimulants and safe gene delivery (Ahmed and Hincke, 2014).
Osteoarthritis (OA) is a common joint disease associated with articular cartilage degeneration. To improve the therapeutic options of OA, tissue engineering based on the use of hBMSCs has become prominent; however, the presence of inflammatory cytokines, such as interleukin-1β (IL-1β), during the chondrogenic process reduces the efficacy of engineered repair procedures by preventing the differentiation of chondrocytes. Studies show that EMF stimulates anabolic processes in synovial fluid cells in OA cartilage, and limits IL-1β catabolic effects (Ongaro et al., 2012). EMF exposure during chondrogenic differentiation displays the significant role EMF can play in counteracting the IL-1β-induced inhibition of chondrogenesis, suggesting EMF as a therapeutic strategy for improving the clinical outcome of cartilage engineering repair procedures. Mayer-Wagner et al. exposed hBMSC cultures to sinusoidal extremely-low frequency magnetic fields (15 Hz, 5 mT), and reported that chondrogenic differentiation of these cells was improved with regard to collagen type II (COL2A1) expression and glycosaminoglycan (GAG) content (Mayer-Wagner et al., 2011), indicating that EMF has the potential to not only stimulate but also maintain chondrogenesis of hBMSCs.
Discussion
During EMF exposure, intracellular and extracellular mechanisms are activated. The mechanisms through which EMF exchanges information between cells, and how the conversion of this biomechanical signaling is translated have been researched for decades. It has been shown that EMF can permeate both the plasma and nuclear membranes of cells, thereby affecting a variety of cell types and different tissues (Luben et al., 1982; Sun et al., 2012; Volpe, 2003). The concept that the plasma membrane may be sensitive to EMF was first proposed by Adey (1974). Liboff suggested that the transport of Ca2+ through channels of the cell membrane involves a resonance-type response to the applied EMF, which is the mechanism that activates ion flux, receptors, kinases, and even transcription factors (Liboff, 1985). Ca2+ efflux transported from the cytosol to the plasma membrane has been found to be initiated by exposure to EMF, and as reported by McLeod et al., to transport Ca2+ across the membrane (McLeod et al., 1987). This modulation of Ca2+ creates a harmonic resonance pattern in which the innate ions follow the wave function of the exogenously applied EMF. A case in point is the investigation of human neuron-committed teratocarcinoma (NT2) cells that were continuously exposed for up to 5 weeks to both a static MF (10 μT) and an alternating EMF (2.5 μT RMS of intensity) at 7 Hz, matching the cyclotron frequency corresponding to the charge/mass ratio of calcium ion (Ca2+-ICR). Intracellular as well as extracellular mechanisms were activated during this exposure to EMF, showing that EMF can permeate both the plasma and nuclear membranes of cells (Luben et al., 1982; Sun et al., 2012; Volpe, 2003).
Ca2+ plays a pivotal role in signal transduction pathways that include cell growth and division, metabolic function, apoptosis, synaptic transmission and gene expression (Bootman et al., 2001; Mellstrom et al., 2008). The regulation of cytosolic Ca2+ concentrations is mediated by an elaborate system of channels and binding proteins found in both the plasma membrane and on intracellular organelles such as the endoplasmic reticulum (ER) (Harzheim et al., 2010). Ca2+ channels are found in all excitable cells and differ in voltage dependence, inactivation rate, and ionic selectivity. Unlike sodium (Na+) channels, Ca2+ channels do not inactivate quickly, therefore they can supply a maintained inward current for longer depolarizing responses. According to the Lasker Award winning biophysicist, Bertil Hille, they serve as the only link to transduce depolarization into all of the nonelectrical activities that are controlled by excitation, and without Ca++ channels, our nervous system would not have outputs (Hille, 1992). They dominate the electrical response to make a longer depolarization, and they also supply activator Ca2+ as long as the membrane remains depolarized (Petersen, 1980). Ca2+ has been reported to bind to the Ca-binding messenger protein calmodulin (CaM) as the voltage at the binding site increases (Pilla, 2013); however Ca2+ does not immediately dissociate from CaM when the voltage decreases as the waveform decays or the sinusoidal wave changes polarity, because the newly bound Ca2+ is sequestered for almost one second to allow the CaM to activate its target enzyme. This is a very complex process driven by waveform effects. Due to this effect it is not difficult to understand how an exogenous stimulus such as EMF can activate intracellular as well as extracellular mechanisms on cell membranes.
It is well known that specific ion fluxes are necessary for tissue regeneration, and that EMF with frequencies below 100 Hz induce physiological effects as a result of ionic interactions (Funk and Monsees, 2006; Gartzke and Lange, 2002). Adams et al. reported that active up-regulation of a pump mechanism is specifically required during regeneration (Adams et al., 2007), in contrast to passive injury currents that result from trauma to polarized epithelia during limb regeneration in frogs and salamanders (Borgens, 1984). In general, regeneration is accompanied by a stimulation of endogenous currents; and the inhibition of endogenous currents specifically prevents regeneration (Becker, 2002; Levin, 2007). Due to this phenomenon, an exogenous application of fields, such as EMF, can induce a significant degree of regeneration in normally non-regenerating tissues (Becker, 2002; Nuccitelli, 2003). This holds important relevance to the regeneration of tissues in adult organisms. Yamada et al. showed that mild stimulation using EF strongly influences embryonic stem cells (ESCs) to assume a neuronal state (Yamada et al., 2007). They reported that induction of Ca2+ influx is required for the formation of embryoid bodies from ES cells. Because Ca2+ is one of the most important signaling ions, many downstream pathways may be involved, such as Ca2+ involvement in the Wnt signaling pathway. Yamada further suggests that physical alteration of cell surface membranes may initiate signaling, even though innate signaling mechanisms take over later. The ion flux signals differentiation in early development through receptor-ligand signaling systems that have evolved to stabilize and refine environmental cues imposed on cells. Sun et al. reported that a DC 0.1 V/cm stimulus (30 min/day for 10 days) applied with osteogenic induction factors, stimulated hBMSC differentiation into the osteogenic cell lineage by reducing the Ca2+ wave frequency, which is typically found in the differentiation processes (Sun et al., 2007). These naturally occurring fluctuations in Ca2+ or other metabolic or signaling waves, can be accessible to appropriate EMF impulses because cells recognize the Ca2+ oscillations through sophisticated mechanisms that decode the information embedded in the Ca2+ dynamics. For example, where rapid and localized changes of Ca2+ (known as Ca2+ spikes) occur, inter- and intra-cellular propagations known as Ca2+ waves control slower responses (Sun et al., 2007). Here the frequency of the Ca2+ oscillations reflects the extracellular stimulus of the EMF. Examples of this phenomenon are Ca2+-binding proteins such as troponin C in skeletal muscle cells and CaM in eukaryotic cells that serve as transducers of Ca2+ signals by changing their activity as a function of the Ca2+ oscillation frequency (Chawla, 2002). These frequency-modulated responses determine the qualitative and quantitative nature of genomic responses which can be translated into frequency-dependent cell responses such as differentiation (Dolmetsch et al., 1998).
The parameters modulating hBMSC differentiation processes depend on the osteogenic markers of interest. As shown in Table 2, there is a trend for the 15 Hz field to increase osteogenic differentiation in vitro with field strengths at 1 mT. This was measured via increases of early osteogenic markers such as intracellular Ca2+, ALP, RUNX2, GAGs, COL2A1, BMP2, MMP1 and MMP3; however, application times vary greatly, anywhere from 30 min/day for 21 days to continuously for 14 days. An in vitro comparison of 5, 25, 50, 75, 100 and 150 Hz at 1.1 mT for 30 min/day for 21 days, reported that 50 Hz was most effective in differentiation of hBMSCs to osteoblasts via significant increase in ALP, OSTEOCALCIN, COLLAGEN, and Ca2+ (Luo et al., 2012). While fewer investigations have been conducted to study the effect of EMF on chondrogenesis, 15 Hz, 5 mT significantly increased the chondrogenic markers GAGs and COLLAGEN II in a human cell model, compared with controls (Mayer-Wagner et al., 2011).
Electromagnetic field resonance and signal transduction
The application of EMF signals appears to be more than a new tool in biophysics and information medicine. It uses the basic science of physics, which drives the chemistry and the biology, to effect a biological change. Low-frequency EMF is biologically significant in that it is endogenous to cell regulation, and the remarkable effectiveness of EMF resonance treatments reflects a fundamental aspect of biological systems. Although cell signaling is regarded as a fundamental aspect of biology it is usually thought of as a molecular function — for example, the second messenger role of Ca2+. However the volumes of literature published in the past 40 years make it impossible to ignore the underlying electromagnetic nature of cell signaling and signal transduction. Ion cyclotron resonance helps regulate biological information in ways that biochemical remedies and pharmaceuticals cannot (Foletti et al., 2012; Lisi et al., 2008). Experiments in resonance effects involve generating cell communication signals by using ELF-EMF which can trigger specific biological pathways. The resonant frequencies applied to human stem/progenitor cells are able to generate modifications in well-defined cells and strongly affect differentiation processes (Foletti et al., 2012). ELF resonance fields stimulate embryonic stem cell differentiation and demonstrate the synergistic effects of a physical stimulus (EMF) with a biochemical stimulus (differentiation media). The effect of EMF on stem/progenitor cell differentiation depends on specific parameters such as waveform, duration, frequency and field strength, as well as the cell type (Tsai et al., 2009; Schwartz et al., 2008).
Translation from in vitro to in vivo and clinical use
The differentiation of hBMSCs has been extensively studied using in vitro assays with culture-expanded hBMSCs. Results, however, have not always been reliable and fully reproducible because of the vast heterogeneity of in vitro culture conditions and the impact of these conditions on phenotype. hBMSCs are known to undergo phenotypic alternations during ex vivo manipulations, losing expression of some markers while acquiring new ones (Jones et al., 2002). hBMSC phenotype and capabilities vary between in vivo and in vitro settings because of the removal from their natural environment and the use of chemical and physical growth conditions that can alter their characteristics. In vitro data are dependent on culture conditions for differentiation and expansion of hBMSC populations and are unlikely to be extrapolated to the native cells. The idea of monitoring and controlling BMSC differentiation is a crucial regulatory and clinical requirement. hSSCs/BMSCs can be harvested from bone marrow aspirates then isolated, expanded, and characterized (Chim et al., 2008). These stem/progenitor cells for regenerative medical applications should ideally be cultured in large quantities (107–109), and have the ability to be differentiated along multiple cell lineages in a reproducible manner. hBMSCs can express an osteoblastic phenotype when treated with BMP2, which is used clinically to induce bone formation, although high doses are required. PEMF has been reported to promote osteogenesis in vivo, in part through direct action on osteogenic cells (Schwartz et al., 2008). In vivo tissue engineering studies have revealed that the absence of an abundant source of cells accelerating the healing process is a limiting factor in the ability to repair articular cartilage. During cartilage regeneration, proliferation and differentiation of new chondrocytes are required, and in humans, EMF stimulation has been used in order to increase the spontaneous regenerative capacity of bone and cartilage tissue post-op, with no apparent side-effects (Zhong et al., 2012). It is important to note that in vitro assays for osteogenesis, chondrogenesis and adipogenesis have been shown to be unreliable and unable to predict in vivo differentiation. While the cartilage pellet culture is the gold standard by which to assess chondrogenic potential, this assay is prone to misinterpretation based on alcian blue, rather than description of pellets in which chondrocytes can be seen in lacunae, surrounded by matrix that stains purple with toluidine blue (metachromasia). Due to this challenge, certain assay results have not been reproducible (Bianco et al., 2008, 2013). There is also the misconception that clonogenic, adherent fibroblastic cells from any non-skeletal tissue are equivalent to BMSCs.
Consistent protocols for hBMSC differentiation, proliferation, and viability are greatly needed to be able to translate in vitro findings into therapeutic utility in vivo, and ultimately clinical treatments. It would appear that the differentiation capabilities are already put in place during the morphogenetic process, and cells can migrate and differentiate according to preset endogenous conditions; however the signaling information necessary to complete each differentiated cell type remains unknown. The literature shows that an exogenous continuation of this signaling information in the form of an applied EMF can enhance the cells’ encoded ability to differentiate towards certain cell types. Much of this information is transferred through signal transduction pathways that pass signals from outside the cell through the cell surface receptors to the inside of the cell. The Wnt family of proteins is an example of highly conserved secreted signaling molecules that regulate cell-to-cell interactions. One benefit to using EMF after stem cells have begun the differentiation process in vivo, is that an external EMF can be applied to the treated tissue at the appropriate frequency, thereby continuing the differentiation process to the desired cell line after implantation.
Conclusion
Human BMSCs are a promising cell type for regenerative medicine and tissue-engineering applications. They have the capacity for self-renewal and exhibit multipotent differentiation potential through which they can produce lineages such as osteoblasts, chondrocytes, and adipocytes (Charbord et al., 2011). hBMSCs possess characteristic Ca2+ waves that are involved in intracellular signaling, exhibiting both short and long periods — the longer periods also operate during transcellular signaling (Sun et al., 2007). To date, research has focused on exogenous chemical and biological factors without considering physiological factors such as EMF (see https://www.osha.gov/SLTC/elfradiation/healtheffects.html). Perhaps it is due to the lack of knowledge of the difference between ionizing and non-ionizing EMF. In particular, ionizing radiation has been shown to cause harmful effects by breaking the electron bonds that hold molecules like DNA together (Buonanno et al., 2011; Mobbs et al., 2011). EMF capable of generating ionizing fields includes current produced by power lines, electrical wiring, and high-energy electrical equipment. The energy in non-ionizing radiation, however, is not strong enough to break ion bonds in atoms and molecules (Ng, 2003; Tenforde and Kaune, 1987). Another issue in using EMF for differentiation of hBMSCs concerns the fact that investigators rarely discuss why they selected specific parameters for activating cell differentiation in their studies, leaving the reader to assume that their selections were random. In order to truly understand the mechanism of action of EMF on any cell type and its potential utility in developing novel therapies, it is imperative that parameters such as frequency, intensity, and time of exposure be optimized into a single, identified system where the experimental and environmental conditions are fixed, thereby permitting replication and optimization of a treatment shown to have regenerative effects. While much of the EMF research has focused on the differentiation of hBMSCs to bone, it appears that the same 15 Hz frequency stimulates hBMSCs to initiate chondrogenesis, however the field strength is more intense (5 mT versus <2 mT) (Mayer-Wagner et al., 2011). Since cartilage formed by hBMSCs typically undergoes hypertrophy or directly forms bone (Scotti et al., 2010; Serafini et al., 2014), it is not surprising that these two tissues would respond to the same frequency; however it is interesting to note that the field strength is more than double for chondrogenesis than that of osteogenesis.
While it remains difficult to alter the expression of genes to rebuild damaged tissues in humans, especially when considering the use of controversial treatments such as stem cell and gene therapies, a systems-based view of development and regeneration may provide suitable therapeutic alternatives. Complex interactions of multiple genetic substances give rise to physical cues, including mechanical and electrical signals that are relatively easier to control and implement in order to guide repair and regeneration. Treatment using EMF could be an auxiliary approach to enhancing cellular activities for tissue regeneration by stimulating cells with both EMF and the proper chemical signals (differentiation media and growth factors) to promote cellular responses synergistically. Additionally, this inherently noninvasive and noncontact treatment method is easily applied to cells for tissue regeneration using three-dimensional scaffolds (Kim et al., 2011; Konrad et al., 1996; Liu et al., 2012; Trock, 2000; Yun JH et al., 2012). Exposure of the EMF to cells on scaffolds with specific conditions has been reported to accelerate tissue formation (Saino et al., 2011).
Acknowledgments
The authors wish to acknowledge the Guth Family Fund 120-330-740196, NIH grant numbers R01-HL097623 and R21-HL117704, Grant NNX13AB67G from National Aeronautics and Space Administration (NASA), P20-DK097806, DOD W81XWH-14-2-0004, TATRC W81XWHH-12-1-0422, and R01-GM88180.
References
- Adams D, Masi A, Levin M. H+ pump-dependent changes in membrane voltage are in early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007;134:1323–1335. doi: 10.1242/dev.02812. [DOI] [PubMed] [Google Scholar]
- Adey WR. Dynamic patterns of brain cell assemblies. IV. Mixed systems. Influence of exogenous low-level currents. Are weak oscillating fields detected by the brain? Neurosci. Res. Program Bull. 1974;12:140–143. [PubMed] [Google Scholar]
- Adey W. Electromagnetics in biology and medicine. In: Matsumoto H, editor. Modern Radio Science. Oxford University Press; 1993. [Google Scholar]
- Ahmed T, Hincke MT. Mesenchymal stem cell-based tissue engineering strategies for repair of articular cartilage. Histol. Histopathol. 2014;29:669–689. doi: 10.14670/HH-29.669. [DOI] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter K. Molecular Biology of the Cell. 4th edition. Garland Science; New York: 2002. pp. 528–530. [Google Scholar]
- Antonsson E, Mann RW. The frequency content of gait. J. Biomech. Eng. 1985;18:39–47. doi: 10.1016/0021-9290(85)90043-0. [DOI] [PubMed] [Google Scholar]
- Ashton B, Allen TD, Howlett CR, Eaglesom CC, Hattori A, Owen M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin. Orthop. Relat. Res. 1980;151:294–307. [PubMed] [Google Scholar]
- Barnothy ME. Biological Effects of Magnetic Fields. Vol. 2. Plenum Press; NY.: 1969. [Google Scholar]
- Becker R. Induced dedifferentiation: a possible alternative to embryonic stem cell transplants. NeuroRehabilitation. 2002;17:23–31. [PubMed] [Google Scholar]
- Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Cao X, Frenette PS, Mai JJ, Robey PG, Simmons PJ, Wang C-Y. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat. Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman M, Lipp P, Berridge MJ. The organization and functions of local Ca(2+) signals. J. Cell Sci. 2001;114:2213–2222. doi: 10.1242/jcs.114.12.2213. [DOI] [PubMed] [Google Scholar]
- Borgens R. Are limb development and limb regeneration both initiated by an integumentary wounding? A hypothesis. Differentiation. 1984;28:87–93. doi: 10.1111/j.1432-0436.1984.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Buonanno M, Toledo S, Azzam E. Increased frequency of spontaneous neoplastic transformation in progeny of bystander cells from cultures exposed to densely ionizing radiation. PLoS One. 2011;6:e21540. doi: 10.1371/journal.pone.0021540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli G, Bloise N, Mantelli M, Gastalki G, Gassina L, De Aneglis MGC, Ferrar D, Imbriani M, Visai L. A comparative analysis of the in vitro effects of pulsed electromagnetic field treatment on osteogenic differentiation of two different mesenchymal cell lineages. Biores. Open Access. 2013;2:283–294. doi: 10.1089/biores.2013.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbord P, Livne E, Gross G, Häupl T, Neves NM, Marie P, Bianco P, Jorgensen C. Human bone marrow mesenchymal stem cells: a systematic reappraisal via the genostem experience. Stem Cell Rev. 2011;7:32–42. doi: 10.1007/s12015-010-9125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla S. Regulation of gene expression by Ca2+ signals in neuronal cells. Eur. J. Pharmacol. 2002;447:131–140. doi: 10.1016/s0014-2999(02)01837-x. [DOI] [PubMed] [Google Scholar]
- Chen CH, L.Y., Fu YC, Wang CK, Wu SC, Wang GJ, Eswaramoorthy R, Wang YH, Wang CZ, Wang YH, Lin SY, Chang JK, Ho ML. Electromagnetic fields enhance chondrogenesis of human adipose-derived stem cells in a chondrogenic microenvironment in vitro. J. Appl. Physiol. 2013;114:647–655. doi: 10.1152/japplphysiol.01216.2012. [DOI] [PubMed] [Google Scholar]
- Chim H, Schantz JT, Gosain AK. Beyond the vernacular: new sources of cells for bone tissue engineering. Plast. Reconstr. Surg. 2008;122 doi: 10.1097/PRS.0b013e31818236b7. [DOI] [PubMed] [Google Scholar]
- Day T, Guo X, Garrett-Beal L, Yang Y. Wnt/B-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- De Mattei MCA, Traina GC, Pezzetti F, Baroni T, Sollazzo V. Correlation between pulsed electromagnetic fields exposure time and cell proliferation increase in human osteosarcoma cell lines and human normal osteoblast cells in vitro. Bioelectromagnetics. 1999;20:177–182. doi: 10.1002/(sici)1521-186x(1999)20:3<177::aid-bem4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Den Dekker E, Gorter G, van der Vuurst H, Heemskerk JW, Akkerman JW. Biogenesis of G-protein mediated calcium signaling in human megakaryocytes. Thromb. Haemost. 2001;86:1106–1113. [PubMed] [Google Scholar]
- Dolmetsch R, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- D'Souza S, Pajak A, Balazsi K, Dagnino L. Ca2+ and BMP-s signaling regulate E2F during epidermal keratinocyte differentiation. J. Biol. Chem. 276 23531-22358. 2001 doi: 10.1074/jbc.M100780200. [DOI] [PubMed] [Google Scholar]
- Ermakov A, Pells S, Freile P, Ganeva VV, Wildenhain J, Bradley M, Pawson A, Millar R, De Sousa PA. A role for intracellular calcium downstream of G-protein signaling in undifferentiated human embryonic stem cell culture. Stem Cell Res. 2012;9:171–184. doi: 10.1016/j.scr.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Fini M, Pagani S, Giavaresi G, De Mattei M, Ongaro N, Varani K, Vineenzi E, Massari L, Cadossi M. Functional tissue engineering in articular cartilage repair: is there a role for electromagnetic biophysical stimulation? Tissue Eng. B Rev. 2013;19:353–367. doi: 10.1089/ten.TEB.2012.0501. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons R, Gordon SL, Kronberg J, Ganey T, Pilla AA. A pulsing electric field (PEF) increases human chondrocyte proliferation through a transduction pathway involving nitric oxide signaling. J. Orthop. Res. 2008;26:854–859. doi: 10.1002/jor.20590. [DOI] [PubMed] [Google Scholar]
- Foletti A, Grimaldi S, Lisi A, Ledda M, Liboff AR. Bioelectromagnetic medicine: the role of resonance signaling. Electromagn. Biol. Med. 2012;32:484–499. doi: 10.3109/15368378.2012.743908. [DOI] [PubMed] [Google Scholar]
- Fu YC, Lin CC, Chang JK, Chen CH, Tai IC, Wang GJ, HO ML. A novel single pulsed electromagnetic field stimulates osteogenesis of bone marrow mesenchymal stem cells and bone repair. PLoS One. 2014;9:e91581. doi: 10.1371/journal.pone.0091581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk RH, Monsees TK. Effects of electromagnetic fields on cells: physiological and therapeutical approaches and molecular mechanisms of interaction. Cells Tissues Organs. 2006;182:59–78. doi: 10.1159/000093061. [DOI] [PubMed] [Google Scholar]
- Funk RH, Monsees TK, Ozkucur N. Electromagnetic effects — from cell biology to medicine. Prog. Histochem. Cytochem. 2009:177–246. doi: 10.1016/j.proghi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Gartzke J, Lange K. Cellular target of weak magnetic fields: ionic conduction along actin filaments of microvilli. Am. J. Physiol. Cell Physiol. 2002;283:C1333–C1346. doi: 10.1152/ajpcell.00167.2002. [DOI] [PubMed] [Google Scholar]
- Guzelsu N, Walsh WR. Streaming potential of intact wet bone. J. Biomech. 1990;23:7673–7685. doi: 10.1016/0021-9290(90)90167-2. [DOI] [PubMed] [Google Scholar]
- Hartig M, Joos U, Wiesmann HP. Capacitively coupled electric fields accelerate proliferation of osteoblast-like primary cells and increase bone extracellular matric formation in vitro. Eur. Biophys. J. 2000;29:499–506. doi: 10.1007/s002490000100. [DOI] [PubMed] [Google Scholar]
- Harzheim D, Roderick HL, Bootman MD. Intracellular calcium signaling. Handbook of Cell Signaling. 2nd ed. Vol. 2. Academic Press; Amsterdam Netherlands: 2010. pp. 937–942. [Google Scholar]
- Hastings G, Mahmud FA. Electrical effects in bone. J. Biomed. Eng. 1988;10:515–521. doi: 10.1016/0141-5425(88)90109-4. [DOI] [PubMed] [Google Scholar]
- Hess R, Jaeschke A, Neubert H, Hintze V, Moeller S, Schnabeiracuh M, Wiesmann HP, Hart DA, Scharnweber D. Synergistic effect of defined artificial extracellular matrices and pulsed electric fields on osteogenic differentiation of human MSCs. Biomaterials. 2012;33:8975–8985. doi: 10.1016/j.biomaterials.2012.08.056. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Second Edition. Sinauer Associates Inc.; Sunderland, MA: 1992. [Google Scholar]
- Hotary K, Robinson KR. Endogenous electrical currents and the resultant voltage gradients in the chick embryo. Dev. Biol. 1990;140:149–160. doi: 10.1016/0012-1606(90)90062-n. [DOI] [PubMed] [Google Scholar]
- Hotary K, Robinson KR. Evidence of a role for endogenous electrical fields in chick embryo development. Development. 1992;114:985–996. doi: 10.1242/dev.114.4.985. [DOI] [PubMed] [Google Scholar]
- Ikehara T, Yamaguchi H, Miyamoto H. Effects of electromagnetic fields on membrane ion transport of cultured cells. J. Med. Invest. 1998;45:47–56. [PubMed] [Google Scholar]
- Jacobson J. Pineal-hypothalamic tract mediation of picotesla magnetic fields in the treatment of neurological disorders. Panminerva Med. 1994;36:201–205. [PubMed] [Google Scholar]
- Jacobson J, Yamanashi WS. An initial physical mechanism in the treatment of neurological disorders with externally applied pico Tesla magnetic fields. Neurol. Res. 1995;17:144–148. doi: 10.1080/01616412.1995.11740303. [DOI] [PubMed] [Google Scholar]
- Jansen JH, van der Jagt OP, Punt PJ, Verhaar JA, van Leeuwen JP, Weinans H, Jahr H. Stimulation of osteogenic differentiation in human osteoprogenitor cells by pulsed electromagnetic fields: an in vitro study. BMC Musculoskelet. Disord. 2010;11:188. doi: 10.1186/1471-2474-11-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E, Kinsey Se, English A, Jones RA, Straszynski L, Meredith DM, Markham AF, Jack A, Emery P, McGonagle D. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- Kaivosoja SV, Chen Y, Donttinen YT. The effect of pulsed electromagnetic fields and dehydroepiandrosterone on viability and osteo-induction of human mesenchymal stem cells. Tissue Eng. Regen. Med. 2015;9:31–40. doi: 10.1002/term.1612. [DOI] [PubMed] [Google Scholar]
- Kim JLD, Kim YH, Koh YH, Lee MH, Han I. A comparative study of the physical and mechanical properties of porous hydroxyapatite scaffolds fabricated by solid freeform fabrication and polymer replication method. Int. J. Precis. Eng. Manuf. 2011;12:695–701. [Google Scholar]
- Kim H, Jung J, Park JH, Kim JH, Ko KN, Kim CW. Extremely low-frequency electromagnetic fields induce neural differentiation in bone marrow derived mesenchymal stem cells. Exp. Biol. Med. 2013;238:923–931. doi: 10.1177/1535370213497173. [DOI] [PubMed] [Google Scholar]
- Kim M, Jung H, Kim SC, Park JK, Seo YK. Electromagnetic fields and nanomagnetic particles increase the osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Int. J. Mol. Med. 2015;35:153–160. doi: 10.3892/ijmm.2014.1978. [DOI] [PubMed] [Google Scholar]
- Komori T. Regulation of bone development and extra-cellular matrix protein genes by RUNX2. Cell Tissue Res. 2010;339:189–195. doi: 10.1007/s00441-009-0832-8. [DOI] [PubMed] [Google Scholar]
- Konrad K, Sevcic K, Foldes K, Piroska E, Molnar E. Therapy with pulsed electromagnetic fields in aseptic loosening of total hip prostheses: a prospective study. Clin. Rheumatol. 1996;15:325–328. doi: 10.1007/BF02230352. [DOI] [PubMed] [Google Scholar]
- Levin M. Bioelectromagnetics in morphogenesis. Bioelectromagnetics. 2003;24:295–315. doi: 10.1002/bem.10104. [DOI] [PubMed] [Google Scholar]
- Levin M. Large-scale biophysics: ion flows and regeneration. Trends Cell Biol. 2007;17:261–270. doi: 10.1016/j.tcb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Levin M. Molecular bioelectricity in developmental biology: new tools and recent discoveries: control of cell behavior and pattern formation by transmembrane potential gradients. Bioessays. 2012;34:205–217. doi: 10.1002/bies.201100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Stevenson CG. Regulation of cell behavior and tissue patterning by bioelectrical signals: challenges and opportunities for biomedical engineering. Annu. Rev. Biomed. Eng. 2012;14:295–323. doi: 10.1146/annurev-bioeng-071811-150114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liboff A. Geomagnetic cyclotron resonance in living cells. J. Biol. Phys. 1985;13:99–104. [Google Scholar]
- Lisi A, Foletti A, Ledda M, Rosola E, Giuliani L, D'Emilia E, Grimaldi S. Extremely low frequency 7 Hz 100 microT electromagnetic radiation promotes differentiation in the human epithelial cell line HaCaT. Electromagn. Biol. Med. 2006;25:269–280. doi: 10.1080/15368370601044184. [DOI] [PubMed] [Google Scholar]
- Lisi A, Ledda M, de Carlo F, Pozzi D, Messina E, Gaetani R, Chimenti I, Barile L, Giacomello A, D'Emilia E, Giuliani L, Foletti A, Patti A, Vulcano A, Grimaldi S. Ion cyclotron resonance as a tool in regenerative medicine. Electromagn. Biol. Med. 2008;27:27–33. doi: 10.1080/15368370802072117. [DOI] [PubMed] [Google Scholar]
- Litovitz T, Krause D, Penafiel M, Elson EC, Mullins JM. The role of coherence time in the effect of microwaves on ornithine decarboxylase activity. Bioelectromagnetics. 1993;14:395–403. doi: 10.1002/bem.2250140502. [DOI] [PubMed] [Google Scholar]
- Liu G, Vijayakumar S, Grumolato L, Arroyave R, Qiao H, Akiri G, Aaronson SA. Canonical Wnts function as potent regulators of osteogenesis by human mesenchymal stem cells. J. Cell Biol. 2009;185:67–75. doi: 10.1083/jcb.200810137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FH, Shen YK, Lee JL. Selective laser sintering of a hydroxyapatite-silica scaffold on cultured MG63 osteoblasts in vitro. Int. J. Precis. Eng. Manuf. 2012;13:439–444. [Google Scholar]
- Lohmann CH, S.Z., Liu Y, Guerkov H, Dean DD, Simon B, Boyan BD. Pulsed electromagnetic field stimulation of MG63 osteoblast-like cells affects differentiation and local factor production. J. Orthop. Res. 2000;18:637–646. doi: 10.1002/jor.1100180417. [DOI] [PubMed] [Google Scholar]
- Luben R, Cain CD, Chen MC, Rosen DM, Adey WR. Effects of electromagnetic stimuli on bone and bone cells in vitro: inhibition of responses to parathyroid hormone by low-energy low-frequency fields. Proc. Natl. Acad. Sci. U. S. A. 1982;79:4180–4184. doi: 10.1073/pnas.79.13.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Hou T, Zhang Z, Xie Z, Wu X, Xu J. Effects of pulsed electromagnetic field frequencies on the osteogenic differentiation of human mesenchymal stem cells. Orthopedics. 2012;35:e526–e531. doi: 10.3928/01477447-20120327-11. [DOI] [PubMed] [Google Scholar]
- Mak A, Zhang JD. Numerical simulation of streaming potentials due to deformation-induced hierarchical flows in cortical bone. J. Biomech. Eng. 2001;123:66–70. doi: 10.1115/1.1336796. [DOI] [PubMed] [Google Scholar]
- Mayer-Wagner S, Passberger A, Sievers B, Aigner J, Summer B, Schiergens TS, Jansson V, Müller PE. Effects of low frequency electromagnetic fields on the chondrogenic differentiation of human mesenchymal stem cells. Bioelectromagnetics. 2011;32:283–290. doi: 10.1002/bem.20633. [DOI] [PubMed] [Google Scholar]
- McGaig C, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current view and future potential. Physiol. Rev. 2005;85:943–978. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- McLeod K, Rubin CT. Observations from mechanically and electrically induced bone remodeling. In: Blank M, editor. Electricity and Magnetism in Biology and Medicine. San Francisco Press; San Francisco: 1993. pp. 98–700 98–700. [Google Scholar]
- McLeod B, Smith SD, Cooksey KE, Liboff AR. Ion cyclotron resonance frequencies Ca++-dependent motility in diatoms. Bioelectromagnetics. 1987;6:1–12. doi: 10.1002/bem.2250080302. [DOI] [PubMed] [Google Scholar]
- Mellstrom B, Savignac M, Gomez-Villfuertes R, Naranjo JR. Ca2+-operated transcriptional networks: molecular mechanisms and in vivo models. Physiol. Rev. 2008;88:421–449. doi: 10.1152/physrev.00041.2005. [DOI] [PubMed] [Google Scholar]
- Metcalf M, Shi R, Borgens RB. Endogenous ionic currents and voltages in amphibian embryos. J. Exp. Zool. 1994;268:307–322. [Google Scholar]
- Mobbs S, Muirhead C, Harrison J. Risks from ionising radiation: an HPA viewpoint paper for Safegrounds. J. Radiol. Prot. 2011;31:289–307. doi: 10.1088/0952-4746/31/3/R01. [DOI] [PubMed] [Google Scholar]
- Mousavi M, B.J., Shahrokhabadi K. The synergic effects of Crocus sativus L. and low frequency electromagnetic field on VEGFR2 gene expression in human breast cancer cells. Avicenna J. Med. Biotechnol. 2014;6:123–127. [PMC free article] [PubMed] [Google Scholar]
- Ng K-H. Non-ionizing radiations — sources, biological effects, emissions and exposures.. Proceedings of the International Conference on Non-ionizing Radiation at UNITEN ICNIR2003 Electromagnetic Fields and Our Health; October 20–22.2003. [Google Scholar]
- Nuccitelli R. A role for endogenous electric fields in wound healing. Curr. Top. Dev. Biol. 2003;58:1–26. doi: 10.1016/s0070-2153(03)58001-2. [DOI] [PubMed] [Google Scholar]
- Ongaro A, Pellati A, Setti S, Masieri FF, Aquila G, Fini M, Caruso A, De Mattei M. Electromagnetic Fields Counteract IL-1β Activity During Chondrogenesis of Bovine Mesenchymal Stem. 2012 doi: 10.1002/term.1671. [DOI] [PubMed] [Google Scholar]
- Otter M, McLeod KJ, Rubin CT. Effects of electromagnetic fields in experimental fracture repair. Clin. Orthop. Relat. Res. 1998:S90–S104. doi: 10.1097/00003086-199810001-00011. [DOI] [PubMed] [Google Scholar]
- Pall M. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. Journal of cellular and molecular medicine. 2013;17:958. doi: 10.1111/jcmm.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagopoulos D, Karabarbounis A, Margaritis LH. Mechanism for action of electromagnetic fields on cells. Biochem. Biophys. Res. Commun. 2002;298:95–102. doi: 10.1016/s0006-291x(02)02393-8. [DOI] [PubMed] [Google Scholar]
- Parekh A. Decoding cytosolic Ca2+ oscillations. Trends Biochem. Sci. 2011;36:78–87. doi: 10.1016/j.tibs.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Persinger M. A potential multiple resonance mechanism by which weak magnetic fields affect molecules and medical problems: the example of melatonin and experimental “multiple sclerosis”. Med. Hypotheses. 2006;66:811–815. doi: 10.1016/j.mehy.2005.09.044. [DOI] [PubMed] [Google Scholar]
- Persinger M, Koren SA. A theory of neurophysics and quantum neuroscience: implications for brain function and the limits of consciousness. Int. J. Neurosci. 2007;117:157–175. doi: 10.1080/00207450500535784. [DOI] [PubMed] [Google Scholar]
- Petersen O. Electrophysiology of Gland Cells. Vol. 4. Academic Press; London: 1980. [Google Scholar]
- Pilla A. Nonthermal electromagnetic fields: from first messenger to therapeutic applications. Electromagn. Biol. Med. 2013;32:123–136. doi: 10.3109/15368378.2013.776335. [DOI] [PubMed] [Google Scholar]
- Poon P, Koehler RC, Thakor NV. Rapid measurement of somatosensory evoked potential response to cerebral artery occlusion. Med. Biol. Eng. Comput. 1995;33:396–402. doi: 10.1007/BF02510522. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Saino E, Fassina L, Van Vlierberghe S, Avanzini MA, Dubruel P, Magenes G, Visai L, Benazzo F. Effects of electromagnetic stimulation on osteogenic differentiation of human mesenchymal stromal cells seeded onto gelatin cryogel. Int. J. Immunopathol. Pharmacol. 2011;24:1–6. doi: 10.1177/03946320110241S201. [DOI] [PubMed] [Google Scholar]
- Sandyk R. Effects of picotesla flux electromagnetic fields on dopaminergic transmission in Tourette's syndrome. Int. J. Neurosci. 1996;84:187–194. doi: 10.3109/00207459608987264. [DOI] [PubMed] [Google Scholar]
- Schroeder T, Jense ED, Westendorf JJ. Runx2: a master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res. C Embryo Today. 2005;75:213–225. doi: 10.1002/bdrc.20043. [DOI] [PubMed] [Google Scholar]
- Schwartz Z, Simon BJ, Duran MA, Barabino G, Chaudhri R, Boyan BD. Pulsed electromagnetic fields enhance BMP-2 dependent osteoblastic differentiation of human mesenchymal stem cells. J. Orthop. Res. 2008;26:1250–1255. doi: 10.1002/jor.20591. [DOI] [PubMed] [Google Scholar]
- Schwartz Z, Fisher M, Lohmann CH, Simon BJ, Boyan BD. Osteoprotegerin (OPG) production by cells in the osteo-blast lineage is regulated by pulsed electromagnetic fields in cultures grown on calcium phosphate substrates. Ann. Biomed. Eng. 2009;37:437–444. doi: 10.1007/s10439-008-9628-3. [DOI] [PubMed] [Google Scholar]
- Scotti C, Tonnarelli B, Papadimitropoulos A, Scherberich A, Schaeren S, Schauerte A, Lopez-Rios J, Zeller R, Barbero A, Martina I. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc. Natl. Acad. Sci. 2010;107:7251–7256. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini M, Sacchetti B, Pievani A, Redaelli D, Remoli C, Biondi A, Riminucci M, Bianco P. Establishment of bone marrow and hematopoietic niches in vivo by reversion of chondrocyte differentiation of human bone marrow stromal cells. Stem Cell Res. 2014;12:659–672. doi: 10.1016/j.scr.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Sherwood L, Klandorf H, Yancey P. Animal Physiology: From Genes to Organisms. Vol. 3. Thomson, Brooks/Cole Ch; 2005. pp. 95–100. [Google Scholar]
- Sun S, Liu Y, Lipsky S, Cho M. Physical manipulation of calcium oscillation facilitates osteodifferentiation of human mesenchymal stem cells. FASEB J. 2007;21:1472–1480. doi: 10.1096/fj.06-7153com. [DOI] [PubMed] [Google Scholar]
- Sun LY, Hsieh DK, Yu TC, Chiu HT, Lu SF, Luo GH, Kuo TK, Lee OK, Chiou TW. Effect of pulsed electromagnetic field on the proliferation and differentiation potential of human bone marrow mesenchymal stem cells. Bioelectromagnetics. 2009;30:251–260. doi: 10.1002/bem.20472. [DOI] [PubMed] [Google Scholar]
- Sun LY, Hsieh DK, Lin PC, Chiu HT, Chiou TW. Pulsed electromagnetic fields accelerate proliferation and osteogenic gene expression in human bone marrow mesenchymal stem cells during osteogenic differentiation. Bioelectromagnetics. 2010;31:209–219. doi: 10.1002/bem.20550. [DOI] [PubMed] [Google Scholar]
- Sun W, Mogadam MK, Sommarin M, Nittby H, Salford LG, Persson BR, Eberhardt JL. Calcium efflux of plasma membrane vesicles exposed to ELF magnetic fields—test of a nuclear magnetic resonance interaction model. Bioelectromagnetics. 2012;33:535–542. doi: 10.1002/bem.21726. [DOI] [PubMed] [Google Scholar]
- Sundelacruz S, Levin M, Kaplan DL. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS One. 2008;3:e3737. doi: 10.1371/journal.pone.0003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S, Levin M, Kaplan DL. Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev. 2009;5:231–246. doi: 10.1007/s12015-009-9080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S, Li C, Choi YJ, Levin M, Kaplan DL. Bioelectric modulation of wound healing in a 3D in vitro model of tissue-engineered bone. Biomaterials. 2013;34:6695–6705. doi: 10.1016/j.biomaterials.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada i, Kouzmenki AP, Kato S. Wnt and PPAR gamma signaling in osteoblastogenesis and adipogenesis. Nat. Rev. Rheumatol. 2009;5:442–447. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- Tenforde T, Kaune W. Interaction of extremely low-frequency electric and magnetic fields with humans. Health Phys. 1987;53:585–606. doi: 10.1097/00004032-198712000-00002. [DOI] [PubMed] [Google Scholar]
- Trock D. Electromagnetic fields and magnets: Investigational treatment for musculoskeletal disorders. Rheum. Dis. Clin. N. Am. 2000;26:51–62. doi: 10.1016/s0889-857x(05)70119-8. [DOI] [PubMed] [Google Scholar]
- Trock DH, B.A., Dyer RH, Fielding LP, Miner WK, Markoll R. A double-blind trial of the clinical effects of pulsed electromagnetic fields in osteoarthritis. J. Rheumatol. 1993;20:456–460. [PubMed] [Google Scholar]
- Tsai M, Chang WHS, Chang K, Hou RJ, Wu TW. Pulsed electromagnetic fields affect osteoblast proliferation and differentiation in bone tissue engineering. Bioelectromagnetics. 2007;28:519–528. doi: 10.1002/bem.20336. [DOI] [PubMed] [Google Scholar]
- Tsai M, Li WJ, Tuan RS, Chang WH. Modulation of Osteogenesis in Human Mesenchymal Stem. 2009 doi: 10.1002/jor.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe P. Interactions of zero-frequency and oscillating magnetic fields with biostructures and biosystems. Photochem. Photobiol. Sci. Rev. 2003;2:637–648. doi: 10.1039/b212636b. [DOI] [PubMed] [Google Scholar]
- Yamada M, Tanemura K, Okada S, Iwanami A, Nakamura M, Mizuno H, Ozawa M, Ohyama-Goto R, et al. Electrical stimulation modulates fate determination of differentiating embryonic stem cells. Stem Cells. 2007;25:562–570. doi: 10.1634/stemcells.2006-0011. [DOI] [PubMed] [Google Scholar]
- Yang YQ, Tan YY, Wong R, Wenden A, Zhang LK, Rabie AB. The role of vascular endothelial growth factor in ossification. Int. J. Oral Sci. 2012;4:64–68. doi: 10.1038/ijos.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Kim WC, Kubo T. Bone fracture and the healing mechanisms. Fracture treatment using electrical stimulation. Clin. Calcium. 2009;19:709–717. [PubMed] [Google Scholar]
- Yun JH, Y.J., Choi SH, Lee MH, Lee SJ, Song SU. Synergistic effect of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on bone regeneration of calvarial defects in rabbits. Tissue Eng. Regen. Med. 2012;9:17–23. [Google Scholar]
- Zhong C, Zhao TF, Xu ZJ, He RX. Effects of electromagnetic fields on bone regeneration in experimental and clinical studies: a review of the literature. Chin. Med. J. (Engl.) 2012;125:367–372. [PubMed] [Google Scholar]
- Zimmerman JW, Pennison M, Brezovich I, Nengun Y, Yang C, Ramaker R, Absher D, Myers R, Kuster N, Costa F, Barbault A, Pasche B. Cancer cell proliferation is inhibited by specific modulation frequencies. Br. J. Cancer. 2012;106:307–313. doi: 10.1038/bjc.2011.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JW, Jimenez H, Pennison MJ, Brezovich I, Morgan D, Mudry A, Costa FP, Barbault A, Pasche B. Targeted treatment of cancer with radiofrequency electromagnetic fields amplitude-modulated at tumor-specific frequencies. Chin. J. Cancer. 2013;32:573–581. doi: 10.5732/cjc.013.10177. [DOI] [PMC free article] [PubMed] [Google Scholar]