Abstract
Objective
To synthesize the perspectives of a broad range of pediatric palliative care (PPC) clinicians and parents to formulate a consensus on the prioritization of the PPC research agenda.
Study design
A 4-round modified Delphi online survey was administered, to PPC experts and to parents of children who had received PPC. In Round 1, research priorities were spontaneously generated. Rounds 2 and 3 then served as convergence rounds to synthesize priorities. A fourth iteration asked participants to rank the research priorities that had reached at least 80% consensus.
Results
A total of 3093 concepts were spontaneously generated by 170 experts and 72 parents in Round 1 (65.8% response rate [RR]). These concepts were thematically organized into 78 priorities and recirculated for Round 2 ratings (n = 130, 53.7% RR). Round 3 achieved response stability, with 31 consensus priorities oscillating within 10% of the mode (n = 98, 75.4% RR). Round 4 resulted in consensus recognition of 20 research priorities, which were thematically grouped as decision making, care coordination, symptom management, quality improvement, and education.
Conclusions
This modified Delphi survey used professional and parental consensus to identify preeminent PPC research priorities. Attentiveness to these priorities may help direct resources and efforts toward building a formative evidence base. Investigating PPC implementation approaches and outcomes can help improve the quality of care services for children and families.
Palliative care aims at improving the quality of life (QOL) for patients and their families throughout the course of life-threatening conditions, with hospice care being provided at the end of life (EOL). Pediatric palliative care (PPC) is a holistic interdisciplinary care approach with the goal of evaluating and minimizing suffering while promoting personal and spiritual growth. The American Academy of Pediatrics recommends initiation of PPC at diagnosis,1 which could improve QOL for the more than 400,000 pediatric patients living with life-threatening or serious health conditions in the United States2 and for their families. PPC can also reduce suffering and improve satisfaction with care among dying children and their families.3
PPC differs fundamentally from adult palliative carein that it involves parents in decision making and is attentive to the diverse developmental stages represented within service cohorts. However, identifying patients for whom PPC is appropriate may be hindered by definitional and prognostic criteria, as well as by limited access to programs and lack of database registries within those programs.
Ongoing challenges faced by patients, families, and providers include intrinsic difficulty of caring for those with life-threatening conditions, lack of evidence to guide treatment decisions, complex diversity of disease trajectories, and limited financial resources and personnel. In 2003, the Institute of Medicine recommended the development of PPC training programs, guidelines, protocols, and priorities for research.2
In a 2008 Delphi study of Canadian palliative care researchers and clinicians, participants identified research priorities based on patient and family needs assessment standards for symptom management, improvement in EOL care and bereavement.4 However, because of the evolution of PCC and inherent differences between the Canadian and US healthcare systems, these findings may not reflect current research priorities in the US. The present study used Delphi methodology5 to identify and prioritize areas of PPC research through a consensus of PPC providers and parents of patients.
Methods
After obtaining Institutional Review Board approval, we identified potential participants using distribution lists from PPC field conferences. Contacted participants nominated parents whose children had received palliative care or hospice care, thus providing a heterogeneous stakeholder perspective. Participants (n = 368) were informed of the continued commitment involved in the multi-step, iterative Delphi technique (pareonline.net/pdf/v12n10.pdf), with continued eligibility for participation requiring responses in consecutive rounds. Demographic information for participants was collected in Round 1 only.
Solicitation of Opinions
After pilot testing, an anonymous, open-ended questionnaire was administered online via SurveyMonkey® in Round 1. Respondents were asked to name the top 5 research priorities in PPC. A study-team panel comprising 2 physicians, 1 research nurse, and 1 social worker (all trained in qualitative coding) evaluated the responses and used content-analysis techniques to identify and group priorities. Discrepancies were resolved through discussion until consensus was reached.
Synthesis of Perspectives
In Round 2, participants ranked each listed priority as (1) very important: urgent priority; (2) moderately important: intermediate priority; (3) somewhat important: low priority; or (4) not important: not a priority. Consensus on priority was determined from the percentage of respondents who ranked the item as “very important” or “moderately important.” The frequency and mean of each item's rankings were calculated and recirculated to participants to enable further priority convergence in Round 3. The standard of consensus was a greater than 80% frequency of priority selection.6 Individual rankings of priorities from incomplete surveys were still included in data analyses to ensure the broadest representation possible.
Stratification of Priorities
In Round 4, participants received a list of the priority items that had reached greater than 80% consensus and were asked to rank ordinally the top 10 priorities. A total prioritization score was calculated, and priorities constituting more than 10% of the total (the pre-determined standard of consensus6,7) were considered high priorities.
Results
The Figure (available at www.jpeds.com) depicts the multi-step iterative Delphi technique used and the results for each round. A total of 242 individuals, including 72 parents, participated in Round 1 (a 65.8% response rate [RR]). Demographic information and self-reported experience measures of participants are presented in Tables I and II, respectively. In Round 1, 53 parents (72%) identified themselves as bereaved, and 39 parents (54%) also identified themselves as professionals in a pediatric-relevant field. Round 1 yielded 3093 individual responses that led to 1010 free-text priorities after duplicate priorities were removed. These items were organized into 78 priorities by qualitative theme-coding. Although duplicates were removed and responses were thematically consolidated, no response items were omitted. Round 2 included 130 respondents (53.7% RR) with 119 completed surveys (91.5% completion rate). In Round 3, 98 participants responded (75.4% RR) with 83 completed surveys (84.7% completion rate). Round 3 reduced the spread of rankings, as 31 priorities now reached greater than 80% consensus. Fifty-seven participants (58%) elected to create an ordinal top-10 list from 31 circulated priorities. Twenty items reached consensus level7 as research priorities (Table III; available at www.jpeds.com). These 20 items were then thematically grouped by using content analysis into 4 categories: decision making (Priorities 1, 3, 6, 8, 10, 15, and 18); care coordination to include mechanisms of support (Priorities 2, 5, 7, 16, and 20); symptom management (Priorities 9, 12, and 19); and quality improvement (Priorities 4, 11, 13, 14, and 17).
Figure.
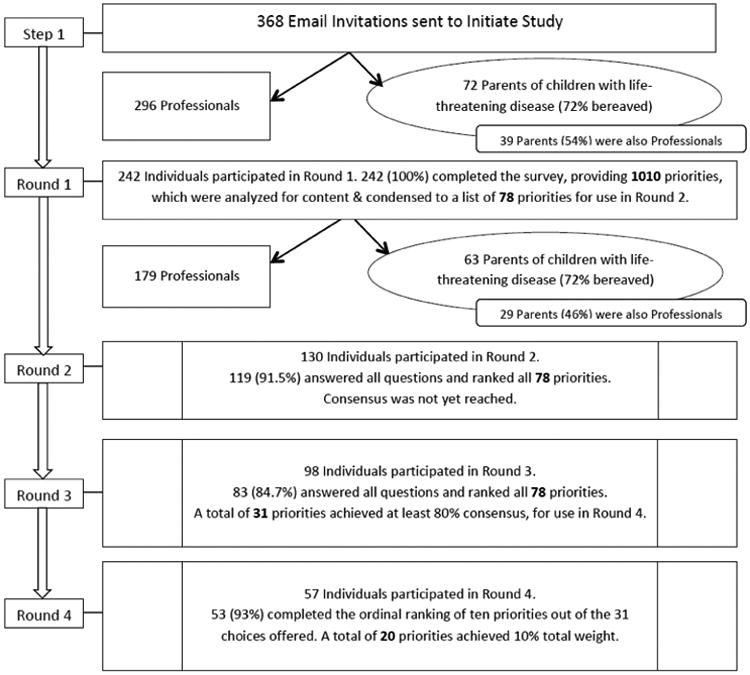
Flow chart of the Delphi technique.
Table I. Demographic information for Round 1 respondents.
| Self-identified role | Responses (n)* | |
|---|---|---|
| Nurse, nurse case manager, or nurse practitioner | 96 (27%) | |
| Certified in hospice/palliative medicine | 23(24%) | |
| Physician | 82 (23%) | |
| Specialization | ||
| Board-certified in hospice/palliative medicine | 35 (43%) | |
| Other | 22 (29%) | |
| Pediatrics | 16 (20%) | |
| Critical care | 15 (18%) | |
| Hematology/oncology | 14 (17%) | |
| Neonatology | 8 (15%) | |
| Parent | 72 | |
| Diagnosis of child | ||
| Neurologic diagnosis | 24 (34%) | |
| Oncologic diagnosis | 14 (20%) | |
| Multi-organ diagnosis | 5 (7%) | |
| Neonatal condition | 4 (<1) | |
| Other diagnosis | 24 (34%) | |
| Parent self-identified as bereaved | 53 (73%) | |
| Parent self-identified as healthcare professional | 39 (54%) | |
| Social worker | 51 | |
| Chaplain | 18 | |
| Administrator | 13 | |
| Child life specialist | 10 | |
| Psychologist | 7 | |
| Pharmacist | 3 | |
Participants may have selected multiple responses. For example, 52 parents of children who had received palliative-care services also self-identified as health professionals. Pediatric providers may have self-identified pediatrics as their primary field in addition to a subspecialty such as oncology or may have self-identified as administrators if this role was relevant to their work.
Table II. Experiential involvement in pediatric palliative care of Round 1 respondents.
| Experience measure | Participant responses | |||
|---|---|---|---|---|
| Years of experience in role(n= 291 responses*) | None | <5 y | 5–10 y | >10 y |
| n = 2 (0%) | n = 62 (21%) | n = 75 (26%) | n = 152 (52%) | |
| No. of children at EOL in past 12 mos. (n = 242 responses) | None | <10 | 10–20 | >20 |
| n = 33 (11%) | n = 53 (37%) | n = 62 (21%) | n = 94 (33%) | |
| Participation in research (n = 242 responses) | Not involved in research | PI or co-PI on a PC project | First author of a PC manuscript | Senior author of a PC manuscript |
| n = 102 (42%) | n = 93 (38%) | n = 27 (11%) | n = 21 (<1%) | |
| Involvement in PC education (n = 242 responses)† | Lectures on PC topics as part of professional role | Active in PC teaching as member of academic institution | Lectures in organized pediatric PC curriculum | Holds education degree (PhD or master's) and is involved in PC education |
| n = 110 (45%) | n = 93 (38%) | n = 79 (33%) | n = 30 (12%) | |
Forty-nine respondents who were both healthcare providers and parents of children who had received palliative services reported duration in each role.
Respondents could select more than 1 answer.
Abbreviations: EOL: end of life; PC: palliative care; PI: principal investigator.
Table III. Description of consensus level research priorities.
| Round 1 | Round 2 | Round 3 | Round 4 | |||
|---|---|---|---|---|---|---|
| Research priority used in Round 4 | Percentage of original free-text responses (n = 1010) | Percentage of participants rating this priority as “very” or “moderately” Important (n = 119) | Percentage of participants rating this priority as “very” or “moderately” important (n = 83) | Percentage of participants choosing this priority in their ordinal top-10 list (n = 53) | Percentage of total weighted score | |
| 1 | Develop and evaluate strategies to help families make difficult end-of-life care decisions. | 20.60 | 79.80 | 94.10 | 77.40 | 63.00 |
| 2 | Compare outcomes for patients and families who have early access to palliative care programs with outcomes for patients and families referred late in the illness trajectory. | 17.00 | 86.90 | 96.90 | 69.80 | 57.90 |
| 3 | Develop strategies to teach clinicians how to help parents make difficult end-of-life decisions and evaluate the impact of this education on relevant clinical outcomes. | 20.40 | 44.60 | 92.90 | 64.20 | 45.50 |
| 4 | Develop and validate evidence-based practice guidelines in pediatric palliative care. | 1.90 | 78.60 | 92.90 | 58.50 | 43.00 |
| 5 | Study strategies to integrate quality palliative care practices into the ongoing care of seriously ill children in a variety of care settings, and evaluate the impact of these strategies on relevant care processes and outcomes. | 21.50 | 77.20 | 88.00 | 56.60 | 32.10 |
| 6 | Study strategies designed to help parents understand what to expect and to prepare them for the possibility of death. | 12.40 | 65.50 | 89.80 | 39.60 | 23.80 |
| 7 | Study the benefits (costs, satisfaction with care, quality of life, burden of care, etc.) of palliative care programs and services for patients, siblings, and parents in diverse clinical contexts. | 21.40 | 90.30 | 88.00 | 35.90 | 23.60 |
| 8 | Study the role of the child in making treatment decisions about their palliative and end-of-life care. | 3.70 | 73.70 | 89.40 | 49.10 | 22.30 |
| 9 | Test symptom interventions (i.e., pain, dyspnea, fatigue, nausea, constipation, disturbed sleep, anxiety, depression, etc.) for infants, children, and adolescents. | 14.10 | 73.90 | 87.10 | 32.10 | 21.50 |
| 10 | Compare outcomes for families who pursue aggressive treatment with curative intent in the care of children with advancing illness with outcomes for families who pursue supportive, non-cure–directed care. | 9.50 | 81.70 | 84.50 | 32.10 | 16.40 |
| 11 | Study barriers to pediatric hospice and palliative care and strategies to overcome those barriers locally, nationally, and internationally (i.e., access, referral, cultural, religious, communication, implementation). | 17.40 | 78.10 | 85.70 | 35.90 | 16.00 |
| 12 | Study the impact of symptom-control interventions on the child's level of comfort, function, and quality of life. | 8.70 | 80.40 | 85.90 | 28.30 | 14.70 |
| 13 | Develop strategies to teach pediatric hospice and palliative care and evaluate the impact of this education on relevant outcomes. | 12.40 | 56.80 | 90.20 | 32.10 | 13.60 |
| 14 | Establish core quality indicators for pediatric hospice and palliative care and evaluate the effect of these measures on care processes and outcomes. | 5.40 | 93.30 | 82.10 | 30.20 | 13.60 |
| 15 | Develop/evaluate strategies to help parents communicate with their child about the child's life-threatening illness and the possibility of death. | 19.46 | 89.08 | 86.75 | 30.19 | 3.02 |
| 16 | Develop and evaluate strategies to support family members of seriously ill children. | 30.97 | 77.31 | 83.13 | 24.53 | 2.45 |
| 17 | Study the experience of children with incurable illness whose lives are prolonged with invasive artificial life-sustaining therapies (i.e., breathing machine, cardio-pulmonary resuscitation, etc.). | 11.93 | 83.19 | 83.13 | 32.08 | 3.21 |
| 18 | Study how to resolve conflicts (i.e., ethical dilemmas) that arise in the care of seriously ill children. | 16.81 | 79.83 | 85.54 | 32.08 | 3.21 |
| 19 | Develop and implement valid symptom-assessment tools in the care of infants, children, and adolescents. | 26.12 | 84.87 | 86.75 | 22.64 | 2.26 |
| 20 | Study the effect of bereavement-care interventions on relevant outcomes. | 20.66 | 71.43 | 83.13 | 30.19 | 3.02 |
Discussion
The priorities most commonly identified priorities emphasized communication with patients and families and shared medical decision making, particularly at EOL (Priority 1). They included a recommendation that clinicians should be taught EOL communication skills with subsequent evaluation of educational impact on clinical outcomes (Priority 3). Historically, such skills have been acquired by trial and error.8 A comprehensive ethics curriculum could provide a valuable educational approach by emphasizing shared values with training in conflict resolution when differences arise (Priority 18). The recent development of PPC workshops and communication training conferences invites comparative longitudinal analyses of their impact.9
As PPC professionals strive to balance hope10 with the reality of disease progression, clear and compassionate communication is recognized as essential. Studies involving bereaved families may indicate how best to prepare patients and their families (Priority 6). Families tend to understand later than clinicians that cure is not possible3; facilitating earlier prognostic realization for families and patients may reduce the burden of further disease-directed therapy and promote earlier hospice referral. Few studies have analyzed the effect of treatment choices and discontinuation of life-sustaining treatment on outcomes such as family decisional regret (Priority 10).
The scope of palliative care extends throughout the trajectory of life-threatening conditions. Recent studies demonstrated the importance of early palliative care and established the concept of palliative care concurrent with disease-directed or life-prolonging treatment.1,11-13 Further research is needed to compare the outcomes of early access to palliative care versus referral late in the illness trajectory (Priority 2). Strategies to integrate high-quality palliative care into ongoing care in supporting families (Priority 16) and to address the practicalities of palliative care delivery in varied settings and transitions warrant evaluation (Priority 5). The benefits of palliative care and bereavement programs in diverse clinical contexts should be studied using measurable outcomes, including biomedical and psychosocial health (Priorities 7 and 20).
Many physicians acquire palliative care competencies experientially through trial and error.14 Evidence-based studies are needed to identify the optimal symptom management strategies for pediatric patients (Priority 9), whose physiology and pharmacokinetics can differ from those of adults. Studies should also assess how specific interventions affect comfort, function, and QOL (Priority 12) and ensure that drugs are both tolerable and adequate to control symptoms. Not only are more effective palliation strategies long overdue, but developmentally appropriate symptom-assessment tools are needed (Priority 19) to ensure that symptoms are recognized and treated.
Evidence-based practice guidelines for PPC were among the most important priorities reported (Priority 4), to ensure consistency of PPC best practice across resource settings. 15 Practices validated in adult palliative care rarely translate to pediatrics, thereby warranting pediatric-specific quality indicators (Priority 14). It is essential to assess the actual experience of children whose lives are prolonged by invasive life-sustaining therapies (Priority 17). The conflict between the immediate need for effective care and the requirement for “proof” of its efficacy can be resolved by consistently evaluating standard practices through process and outcome measures with subsequent guideline distribution.
A key priority was to identify and overcome barriers to PPC and pediatric hospice care (Priority 11). One recent study identified lack of resources, prognostic uncertainty, and different treatment goals as obstacles to PPC consultation.16 Another study reported family reluctance and the perception of palliative care as “giving up.” 17 Studies using objective methods across broader settings are needed to develop strategies to overcome implementation barriers.
Another important priority is greater educational exposure to PC, with measurement of growth in trainee confidence and competence (Priority 13). Many medical students still lack exposure to hospice and palliative medicine.18 Surveyed pediatric residents and fellows reported mainly “none” to “moderate” training, experience, knowledge, competence, and comfort in providing palliative care while anticipating benefit from further palliative training. Most fellowship programs lack formal palliative care curricula.14 There are now fellowships in hospice and palliative medicine, but few are specific to pediatrics. In a survey of general palliative care providers, most described their pediatric training as inadequate, but they nonetheless provided palliative care for dying children.19 Our study identified the theme of education across the priorities of decision making (Priority 3), care coordination (Priority 13), and symptom control (Priority 19). Quality palliative care training includes competencies in symptom assessment, pharmacologic and non-pharmacologic interventions, multidisciplinary team dynamics, decisional ethics, and communication skills.20
One strength of our methodology was the ability to achieve consensus in an area with limited empirical evidence but an increasingly recognized need. However, self-selection bias may have been introduced by the requirement for participation in multiple sequential surveys.5 By including the parental perspective, our design sought to increase heterogeneity and broaden the stakeholder perspective. Anonymity after Round 1 prevented comparison of demographics and of parent, professional, and dual-role respondent ratings. The absence of face-to-face interaction may represent a strength, as it minimized rank-based agreement bias in a field of interdisciplinary roles. Assessment of interventions, best practices for implementation, and outcome metrics can help expand services and improve the quality of palliative care for children with life-threatening conditions.
Acknowledgments
Supported by ALSAC.
Abbreviations
- EOL
end of life
- PPC
pediatric palliative care
- QOL
quality of life
- RR
response rate
Footnotes
The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Academy of Pediatrics. Section on Hospice and Palliative Medicine and Committee on Hospital Care. Pediatric palliative care and hospice care commitments, guidelines, and recommendations. Pediatrics. 2013;132:966–72. doi: 10.1542/peds.2013-2731. [DOI] [PubMed] [Google Scholar]
- 2.Field JF, Behrman RE. When children die: Improving palliative and end-of-life care for children and their families. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 3.Wolfe J, Hammel JF, Edwards KE, Duncan J, Comeau M, Breyer J, et al. Easing of suffering in children with cancer at the end of life: is care changing? J Clin Oncol. 2008;26:1717–23. doi: 10.1200/JCO.2007.14.0277. [DOI] [PubMed] [Google Scholar]
- 4.Steele R, Bosma H, Johnston MF, Cadell S, Davies B, Siden H, et al. Research priorities in pediatric palliative care: a Delphi study. J Palliat Care. 2008;24:229–39. [PubMed] [Google Scholar]
- 5.Powell C. The Delphi technique: myths and realities. J Adv Nurs. 2003;41:376–82. doi: 10.1046/j.1365-2648.2003.02537.x. [DOI] [PubMed] [Google Scholar]
- 6.Vernon W. The Delphi technique: a review. Int J Ther Rehabil. 2009;16:69–76. [Google Scholar]
- 7.Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32:1008–15. [PubMed] [Google Scholar]
- 8.Hilden JM, Emanuel EJ, Fairclough DL, Link MP, Foley KM, Clarridge BC, et al. Attitudes and practices among pediatric oncologists regarding end-of-life care: results of the 1998 American Society of Clinical Oncology Survey. J Clin Oncol. 2001;19:205–12. doi: 10.1200/JCO.2001.19.1.205. [DOI] [PubMed] [Google Scholar]
- 9.Baughcum AE, Gerhardt CA, Young-Saleme T, Stefanik R, Klopfenstein KJ. Evaluation of a pediatric palliative care educational workshop for oncology fellows. Pediatr Blood Cancer. 2007;49:154–9. doi: 10.1002/pbc.21034. [DOI] [PubMed] [Google Scholar]
- 10.Mack JW, Wolfe J, Cook EF, Grier HE, Cleary PD, Weeks JC. Hope and prognostic disclosure. J Clin Oncol. 2007;25:5636–42. doi: 10.1200/JCO.2007.12.6110. [DOI] [PubMed] [Google Scholar]
- 11.Levine D, Lam CG, Cunningham MJ, Remke S, Chrastek J, Klick J, et al. Best practices for pediatric palliative cancer care: a primer for clinical providers. J Support Oncol. 2013;11:114–25. doi: 10.12788/j.suponc.0012. [DOI] [PubMed] [Google Scholar]
- 12.Mack JW, Wolfe J. Early integration of pediatric palliative care: for some children, palliative care starts at diagnosis. Curr Opin Pediatr. 2006;18:10–4. doi: 10.1097/01.mop.0000193266.86129.47. [DOI] [PubMed] [Google Scholar]
- 13.Harris MB. Palliative care in children with cancer: which child and when? J Natl Cancer Inst Monogr. 2004;(32):144–9. doi: 10.1093/jncimonographs/lgh007. [DOI] [PubMed] [Google Scholar]
- 14.Roth M, Wang D, Kim M, Moody K. An assessment of the current state of palliative care education in pediatric hematology/oncology fellowship training. Pediatr Blood Cancer. 2009;53:647–51. doi: 10.1002/pbc.22110. [DOI] [PubMed] [Google Scholar]
- 15.Stevenson M, Achille M, Lugasi T. Pediatric palliative care in Canada and the United States: A qualitative metasummary of the needs of patients and familes. J Palliat Med. 2013;16:566–77. doi: 10.1089/jpm.2011.0076. [DOI] [PubMed] [Google Scholar]
- 16.Davies B, Sehring SA, Partridge JC, Cooper BA, Hughes A, Philp JC, et al. Barriers to palliative care for children: perceptions of pediatric health care providers. Pediatrics. 2008;121:282–8. doi: 10.1542/peds.2006-3153. [DOI] [PubMed] [Google Scholar]
- 17.Knapp C, Thompson L. Factors associated with perceived barriers to pediatric palliative care: a survey of pediatricians in Florida and California. Palliat Med. 2012;26:268–74. doi: 10.1177/0269216311409085. [DOI] [PubMed] [Google Scholar]
- 18.Gibbins J, McCoubrie R, Forbes K. Why are newly qualified doctors unprepared to care for patients at the end of life? Med Educ. 2011;45:389–99. doi: 10.1111/j.1365-2923.2010.03873.x. [DOI] [PubMed] [Google Scholar]
- 19.Rapoport A, Obwanga C, Sirianni G, Librach SL, Husain A. Not just little adults: palliative care physician attitudes toward pediatric patients. J Palliat Med. 2013;16:675–9. doi: 10.1089/jpm.2012.0393. [DOI] [PubMed] [Google Scholar]
- 20.Klick JC, Friebert S, Hutton N, Osenga K, Pituch KJ, Vesel T, et al. Developing competencies for pediatric hospice and palliative medicine. Pediatrics. 2014;134:e1670–7. doi: 10.1542/peds.2014-0748. [DOI] [PubMed] [Google Scholar]


