Abstract
Background and Aims
After the Diabetes Control and Complications Trial (DCCT), the Epidemiology of Diabetes Interventions and Complications (EDIC) study continued to demonstrate persistent benefit of prior intensive therapy on neuropathy, retinopathy, and nephropathy in type 1 diabetes mellitus (DM)., The relationship between control of glycemia and gastric emptying (GE) is unclear.
Methods
We assessed GE with a 13C-spirulina breath test and symptoms in 78 participants with type 1 diabetes at year 20 of EDIC. The relationship between delayed GE and HbA1c, complications of DM, and gastrointestinal symptoms were evaluated.
Results
GE was normal (37 participants, 50%), delayed (35 participants, 47%), or rapid (2 participants, 3%). The latest mean HbA1c was 7.7%. In univariate analyses, delayed GE was associated with greater DCCT baseline HbA1c and duration of DM prior to DCCT (P ≤ 0.04), greater mean HbA1c over an average of 27 years of follow up (during DCCT-EDIC, P = 0.01), lower R-R variability during deep breathing (P=0.03) and severe nephropathy (P=0.05) and a greater composite upper gastrointestinal symptom score (P<0.05). In multivariate models, retinopathy was the only complication of DM associated with delayed GE. Separately, DCCT baseline HbA1c (OR 1.6, 95% CI 1.1–2.3) and duration of DM (OR 1.2, 95%CI 1.01–1.3) prior to DCCT entry and mean HbA1c during DCCT-EDIC (OR 2.2, 95%CI 1.04–4.5) were independently associated with delayed GE.
Conclusions
In the DCCT/EDIC study, delayed GE was remarkably common and associated with gastrointestinal symptoms and with measures of early and long-term hyperglycemia. ClinicalTrials.gov numbers NCT00360815 and NCT00360893.
Keywords: diabetic gastroparesis, HbA1c, glycemic control, neuropathy
INTRODUCTION
Gastroparesis is a widely recognized complication of diabetes mellitus (DM). The symptoms of diabetic gastroparesis, predominantly early satiety, nausea and vomiting, can be severe, refractory to medical therapy, and may adversely affect blood glucose control. However, gastrointestinal symptoms are often non-specific and may not be associated with delayed gastric emptying (GE); conversely, delayed GE is often asymptomatic.1 Hence, an objective measurement of GE is required to establish gastroparesis.2
Several aspects about the epidemiology and pathophysiology of diabetic gastroparesis are incompletely understood. The prevalence of gastroparesis in type 1 DM (T1DM) has varied widely among studies. In earlier studies from tertiary medical centers, up to 60% of participants with long-standing T1DM and gastrointestinal symptoms had diabetic gastroparesis,3–5 which is associated with cardiovascular autonomic dysfunction and other microvascular complications.2, 6 However, these studies predated the routine use of intensive insulin therapy for T1DM. More recently, (between 1995 and 2006) the community-based cumulative incidence of symptomatic gastroparesis among participants with T1DM was much lower, i.e., only 5%.7 It is unclear whether these different estimates of prevalence between earlier and more recent studies are explained by underlying differences in the definition of gastroparesis, the advent of intensive insulin therapy for T1DM with lower levels of chronic glycemia, and/or differences in blood glucose level during the GE study, which is known to influence GE.2
The Diabetes Control and Complications Trial (DCCT) demonstrated that intensive versus conventional therapy, resulting in mean A1c levels of 7% and 9%, respectively, over an average 6.5 years, reduced the risk of diabetic retinopathy, nephropathy, and peripheral and cardiac autonomic neuropathy by 40–60%.8 The Epidemiology of Diabetes Intensive Complications (EDIC) study is a prospective, longitudinal, observational follow-up study of the long-term effects of improved glycemic control among participants in the DCCT.9 Follow-up in the EDIC study has shown that the differences in retinal, renal, and nerve outcomes observed at the end of the DCCT between the former intensive and conventional treatment groups have persisted for as long as 14 years despite the loss of glycemic separation.9–11 This persistent effect of past glucose control has been called “metabolic memory.12
While acute hyperglycemia delays GE,13 the relationship between long-term control of glycemia and GE is unclear. Increased glycated hemoglobin (HbA1c) levels were associated with a prolonged gastric emptying lag time in Type 1 DM 14 and with gastrointestinal symptoms in people with predominantly Type 2 DM.15 Other reports, however, have reported no differences in HbA1c levels among 3 groups: DM with GI symptoms and delayed GE, DM with GI symptoms and normal GE, and DM without GI symptoms.16 Moreover, in an observational cohort over 25 years, improved glycemic control did not accelerate GE in participants with delayed GE type 1 or 2 DM.17 The relationship between symptoms and delayed GE is weak.18
Our objectives were to evaluate GE and gastrointestinal symptoms in people with long-term Type 1 DM who are being followed in the DCCT-EDIC study and examine the relationship between GE disturbances, control of glycemia and other complications of DM.8 Our hypotheses were that among patients with T1DM in the DCCT-EDIC study (i) delayed gastric emptying is associated with greater glycemic exposure – both early and in the long term, (ii) delayed gastric emptying is associated with long term complications of DM, (iii) the prevalence of delayed gastric emptying is lower in patients who have been treated with “intensive” than “conventional” glycemic therapy in the DCCT, and (iv) delayed gastric emptying is associated with upper gastrointestinal symptoms.
METHODS
Study Design and Participants
The DCCT has been described elsewhere.19 Briefly, 1441 subjects who had T1DM for 1 to 15 years with no (primary prevention cohort) or minimal (secondary intervention cohort) diabetic retinopathy were randomly assigned to either intensive treatment or conventional treatment and were followed for 3 to 9 years (mean, 6.5 years).19 After the DCCT ended in 1993, intensive therapy was recommended for all subjects, and subjects in the conventional treatment group were trained in intensive therapy. All participants returned to their own healthcare providers for diabetes care. Of 1441 people, 1375 (96%) agreed to participate in the annual follow-up evaluations for the EDIC study, which began in 1994. A detailed description of EDIC study procedures and baseline characteristics has been published.20 A standardized history, physical examination, and laboratory testing protocol provided annual clinical and biochemical end points.20 Glycemic control was evaluated by measuring HbA1c quarterly during DCCT and annually during EDIC, using the same methods previously described for the DCCT.19 Nephropathy was evaluated by biennial assessments of urine albumin excretion and annual assessments of serum creatinine. Similar to a previous DCCT-EDIC study, severe nephropathy was defined by an albumin excretion rate of 300 mg/day or a history of renal replacement therapy (dialysis or transplant).21 Cardiac autonomic nervous functions were evaluated by standardized testing (ie, R-R variation during deep breathing, the Valsalva maneuver, and postural testing). Cardiac autonomic neuropathy was defined by one of the following criteria: R-R variation less than 15 beats per minute, R-R variation of less than 20 beats per minute plus a heart rate Valsalva ratio less than or equal to 1.5, or a decrease of 10 mmHg in diastolic blood pressure at any point during 10 min of standing after a period of 30 min of supine rest (postural hypotension). 22 Confirmed clinical peripheral neuropathy, which was the primary outcome measure of peripheral neuropathy during DCCT and EDIC, was defined by the presence of both clinically evident peripheral neuropathy and abnormal nerve conduction studies.11 Retinopathy was graded with the final Early Treatment Diabetic Retinopathy Study (ETDRS) grading scale and DCCT methods23: normal (grade 1), microaneurysms only (grade 2), mild (grade ≤ 3), moderate (grades 4–5), and severe (grade 6 or greater). The GE study was conducted in 2013–2014 (i.e., years 19–20 of EDIC). All variables were collected concurrently except as follows: neuropathy (years 16–17), retinopathy with fundus photography (years 15–18).23
The GE study planned to enroll 80 participants at 7 EDIC centers. The statistician (ARZ) selected 16 participants at each center, i.e., a total of 112 participants, with a random stratification based on 4 criteria (i.e., age, sex, most recent HbA1c, and DCCT treatment group [conventional or intensive therapy]). Age and HbA1c values were each stratified into 2 groups, i.e., less than or greater than median values at each center. Hence, there were 16 strata and 1 subject identified for each of the strata at each center. All subjects on this list who did not have exclusion criteria were approached in the order they appeared on the list. The exclusion criteria were an allergy to eggs, history of or active inflammatory bowel disease, radiation therapy to the abdomen, gastric (including bariatric surgery or gastric banding procedures) or major small bowel (i.e., resection of > 50 cm) or colonic surgery (i.e., hemi or subtotal colectomy), clinically severe cardiopulmonary disease, current dialysis, or use of opioid medications and/or prokinetic agents and inability to discontinue these medications safely for four half-lives prior to the study. All study procedures were approved by the institutional review boards of all participating centers, and all participants provided written informed consent.
Assessment of Gastric Emptying
GE was assessed by a 13C-Spirulina gastric emptying breath test (GEBT, 223 kcal with 19.2 g carbohydrates, 12 g protein, and 10.9 g fat, AB Diagnostics, Brentwood, Tn) under an investigator-initiated IND from the FDA (IND#G130087). The GEBT has been extensively used and validated against scintigraphy in health and disease, including in participants with DM.24, 25 The intra- and inter-individual variability are comparable to that for scintigraphy.25 In addition, this GEBT has been used in over 2000 patients in Phase I and Phase II pharmaceutical investigations of pro-kinetic drugs under sponsors’ IND exemptions (personal communication, AB Diagnostics), some of which have been published.26, 27
After an overnight (minimum 8 hour) fast, participants consumed the test meal containing 13C-Spirulina and either saltines or gluten-free crackers. End tidal breath samples were obtained before and at 15, 30, 45, 60, 90, 120, 150, 180, and 240 minutes after the meal in glass tubes using a straw to blow into the bottom of the tube to displace contained air. The [13C] content of breath samples was determined by Gas Isotope Ratio Mass Spectrometry at AB Diagnostics. The 13C enrichment was expressed as the delta per mL difference between the 13CO2/12CO2 ratio of the sample and the standard. To calculate the quantity of 13C appearing in breath per unit of time, the change over baseline was used where: 0.0112372 is the isotopic abundance of the limestone standard, Pee Dee Belemnite, and CO2 production was corrected for age, sex, height and weight using the algorithms of Schofield et al., as described by Klein.28 The 13C excretion was used to estimate GE T ½ using validated models derived from studies in which GE was simultaneously assessed by the 13C-Spirulina platensis breath test and scintigraphy.24, 25 For the meal employed, the 10th–90th percentile range for GE T ½ in healthy subjects is 50–92 minutes.25 Hence, values less than 50 and greater than 92 minutes reflect rapid and delayed GE, respectively.
To reduce the impact of acute hypoglycemia or hyperglycemia on GE, self-monitored blood glucose (SMBG) assessments were obtained before and at 60 minute intervals for 4 hours after the GEBT meal in order to target a SMBG value between 80 and 200 mg/dL for the duration of the 4-hour test. The clinic coordinators called participants the evening before the test to discuss insulin dosing or food intake. When participants had a hypoglycemic event, defined as a blood glucose < 80 mg/dL 4 hours or more before the start of the test, glucose tablets or, if necessary, a liquid source of carbohydrate was permitted. For hypoglycemic events that required self-treatment within 4 hours of the test start, the GE test was delayed until at least 4 hours had elapsed from the treatment for hypoglycemia. For hypoglycemic events after the start of the breath test, glucose tablets were administered as needed and the test was completed.
When the blood glucose concentration was greater than 200 mg/dL prior to starting the GE test, insulin was administered with the dose individualized according to the patient’s established regimen. SMBG was performed subsequently and additional insulin administered if necessary to try and ensure a value less than 200 mg/dL before starting the test.
Before the test meal and at each breath sampling, participants rated the intensity (0–3; 0=absent; 1=mild, present in a non-bothersome intensity; 2=relevant, clearly present and bothersome but not of such intensity that it would interfere with normal daily activities; and 3=severe, clearly present and of such intensity that it would interfere with normal daily activities) of 6 different symptoms (epigastric pain, fullness, bloating, nausea, belching, and epigastric burning).29, 30
Serological Testing for Celiac Disease
Blood testing for celiac disease was performed using a commercially available kit for tissue transglutaminase IgA antibodies (Inova Diagnostics, San Diego Ca). The assay uses human recombinant tissue transglutaminase as substrate; abnormal results were confirmed by testing for antiendomysial antibodies using indirect immunofluorescence on monkey esophagus.
Assessment of Symptoms
Participants recorded their upper gastrointestinal symptoms in a validated diary (i.e., Gastroparesis Cardinal Symptom Index (GCSI)-Daily Diary) every day for 1 week.31, 32 The GCSI includes 11 of 20 symptoms in the Patient Assessment of Upper Gastrointestinal Disorders-Symptoms (PAGI-SYM) questionnaire, which inquires about upper GI symptoms over the past 2 weeks;33 and is the “recall” version of the daily diary.31, 32 A separate composite subscore consisting of nausea, vomiting, fullness and pain was computed as an exploratory endpoint to summarize symptoms.34 The PAGI-SYM and PAGI-QOL instruments were administered to evaluate symptom severity and the impact of symptoms on quality of life respectively.35, 36 Because the PAGI-SYM instrument does not evaluate bowel symptoms, gastrointestinal symptoms were also evaluated with the Rome III gastrointestinal symptom questionnaire.37, 38 All authors had access to the study data and reviewed and approved the final manuscript.
Statistical Analysis
Univariate associations between various factors and GE, which was characterized as normal or delayed were assessed by Fisher’s exact test for categorical variables and by the Wilcoxon rank sum test for continuous variables. The associations among continuous variables were assessed using Spearman correlation coefficients. Two multiple variable logistic regression models were used to identify factors independently associated with delayed GE. Consistent with our understanding of putative risk factors for delayed GE in DM and the results of the univariate analyses, these models incorporated the other complications of DM (i.e., autonomic neuropathy, retinopathy, and nephropathy, Model 1) and measures of glycemic control at various times (Model 2). Limited by the number of patients with delayed GE, we only incorporated 3 variables in each model. Odds ratios based on the coefficients (and their standard errors) from the logistic models to predict delayed GE (compared to normal GE) are reported. Summary data are reported as mean (±SEM) or median (25th,75th percentiles) for quantitative characteristics and as frequencies (%) for discrete characteristics. All analyses were done using SAS® (Version 9.3, SAS Institute Inc., Cary, NC).
RESULTS
Participation Rates, Demographic Characteristics, and Serological Tests for Celiac Disease
Of 112 eligible participants, 111 were approached regarding participation in the study; 1 was not approached because the clinic had reached its limit of 10 participants. Of the remaining 111 participants, 79 participated in the GE study. The remainder declined to participate (n=22), or were ineligible to participate because of illnesses or surgeries (n=8) or inability to attain a fasting blood glucose concentration between 80–200 mg/dl before the study (n=2). Of the 79 participants, 1 vomited the GEBT meal and 4 participants did not complete breath collections because the blood glucose concentration could not be maintained between 80 and 200 mg/dl. These 74 participants had participated in the DCCT and EDIC studies for an average of 7 and 20 years respectively.
The distributions of age, sex, therapy during DCCT, and most recent HbA1c in EDIC were not significantly different between these 79 participants and the remaining 33 EDIC participants who were identified at the 7 centers but did not participate in the study. Age was not significantly associated with participation (54 ± 1 years for participants vs 52 ± 1 years for non-participants) (Table 1). Fifty percent of participants in each group (i.e., participants and non participants) were women. Fifty percent of participants and 41% of non-participants were randomized to intensive therapy during DCCT. The most recent HbA1c in the EDIC study was higher in nonparticipants (8.2 ± 0.2%) than participants (7.8 ± 0.1%). Tissue transglutaminase antibodies (anti-tTG) were negative (<20 AU/ml) in all patients, indeed less than 1 AU/ml in 78 patients. In 1 patient with an anti-tTG titer of 4.61 AU/ml, testing for antiendomysial antibodies confirmed the absence of celiac disease. A comparison of the 74 participants who completed the study with the entire EDIC cohort of 1275 participants reveals similar distributions of age (55 years in this study versus 53 years in the overall cohort) and sex (50% versus 47% women), and the proportion of participants who received intensive treatment in DCCT (50% versus 51%). However, in the GE subcohort, a greater proportion of participants had severe proliferative retinopathy (38% versus 23%), cardiac autonomic neuropathy (42% versus 32%) and clinically-evident peripheral neuropathy (42% versus 37%).
Table 1.
Demographic, Lifestyle, and Diabetes Characteristics by Gastric Emptying Categories*
| Characteristic | Normal (n = 37)a | Delayed (n = 35) | P Valueb for Association With Group Status |
|---|---|---|---|
| Demographics and Lifestyle | |||
| Women, No (%) | 17 (46%) | 19 (54%) | 0.64 |
| Age, years | 54 ± 1.1 | 56 ± 1.2 | 0.09 |
| BMI, Kg/m2 | 28 ± 0.9 | 29 ± 0.9 | 0.6 |
| Current cigarette smoking, No (%) | 0 | 1 | |
| Diabetes mellitus | |||
| Duration of DM before starting DCCT, months | 69 ± 8.4 | 90 ± 8.4 | 0.04 |
| Total duration of DM | 399 ± 10 | 412 ± 9 | 0.3 |
| DCCT assignment (INT group), No (%) | 18 (49%) | 19 (54%) | 0.65 |
| DCCT - baseline HbA1c, % | 8.3 ± 0.2 | 9.6 ± 0.3 | .002 |
| DCCT – time-weighted average HbA1c, % | 7.8 ± 0.2 | 8.3 ± 0.2 | .17 |
| EDIC - Latest HbA1c, % | 7.6 ± .1 | 7.9 ± .2 | .22 |
| EDIC – time-weighted average HbA1c over 20 years, % | 7.5 ± 0.1 | 8.1 ± 0.2 | .03 |
| DCCT and EDIC – time-weighted average HbA1c | 7.6 ± 0.2 | 8.1 ± 0.2 | 0.01 |
| Current treatment with pump or multiple daily injections, No (%) | 37 (100%) | 33 (94%) | 0.24 |
Values are numbers or mean (SEM) unless stated otherwise. Normal T1/2= 50–92 min.; Delayed T1/2 >92 min.
Abbreviations: DCCT =Diabetes Control and Complications Trial; EDIC =Epidemiology of Diabetes Interventions and Complications Study; INT = intensive diabetes therapy;
Gastric Emptying
Gastric emptying was normal with T1/2 of 50–92 min (37 participants, 50%), delayed with T1/2> 92 min (35 participants, 47%), or rapid with T1/2 <50 min (2 participants, 3%) (Table and Figure 1). The 2 participants with rapid GE, both men, are not included in the remaining analyses. The average GE time was 73 ± 2 minutes in patients with normal and 140 ± 7 minutes in patients with delayed GE. The mean ± SEM (range) fasting SMBG level prior to and during the GEBT study were 151 ± 4 mg/dl (range 80–213 mg/dl) and 159 ± 5 mg/dl (range 82–282 mg/dl) respectively. The gastric emptying T1/2 was not correlated with the following SMBG values during the GEBT: fasting (r = −0.07, P=0.58), average (r = −0.14, P=0.23), maximum (r = −0.15, P=0.20), or the difference (maximum – fasting) SMBG (r = −0.18, P=0.13).
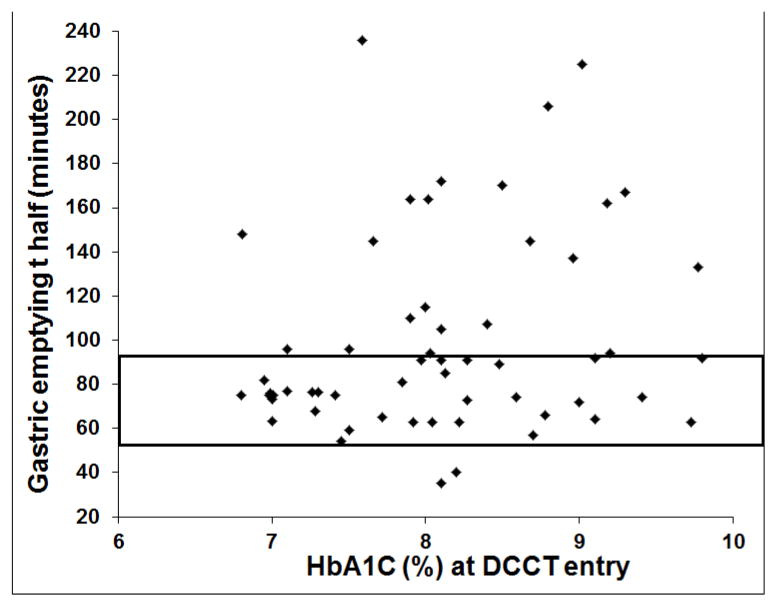
Figure 1.
Relationship between HbA1c at DCCT entry and gastric emptying T ½. The rectangle shows the normal range for GE T½.
Relationship of Demographic Features and Glycemic Control with Gastric Emptying – Univariate Analysis
Gastric emptying was not significantly associated with age, sex or BMI and the proportion of participants who were treated with intensive therapy in DCCT was not significantly associated with GE (Table 1). Participants with delayed GE had DM for a longer duration (90 ± 8 versus 69 ± 8 months P=0.04) and a higher HbA1c (9.6 ± 0.3 vs 8.3 ± 0.2%, P=0.002) before DCCT (Table 1 and Figure 1). The mean HbA1c during EDIC (P=0.03) and the mean HbA1c across DCCT and EDIC (P=0.01) were also associated with GE, being higher in participants with delayed GE (Table 1). However, the mean HbA1c during DCCT, and the most recent HbA1c were not significantly associated with GE.
Relationship between Complications of DM and Gastric Emptying – Univariate Analysis
Table 2 provides the distribution of diabetes complications in participants with normal and delayed GE. Delayed GE was associated with clinically evident (P=0.055) and confirmed peripheral neuropathy (P=0.02) and with lesser mean R-R variation with deep breathing (P=0.03), indicating cardiovagal dysfunction (Table 2). The prevalence of cardiac autonomic neuropathy as defined above, was not significantly greater in delayed (18 participants [51%]) than normal (12 participants [33%]) GE (P=0.15).
Table 2.
Complications of DM by Gastric Emptying Categoriesa
| Characteristic | Normal (n = 37) | Delayed (n = 35) | P Valueb for Association With Group Status |
|---|---|---|---|
| Cardiac autonomic neuropathy | |||
| R-R variation with deep breathing | 27 ± 2.5 | 19 ± 2.5 | 0.03 |
| R-R variation <15 | 10 (27%) | 16 (46%) | 0.14 |
| Valsalva ratio (average of 2 measurements) | 1.8 ± .05 | 1.7 ± .06 | 0.11 |
| Valsalva ratio ≤ 1.5 | 4 (11%) | 12 (35%) | 0.18 |
| Postural change in diastolic blood pressure | 2 (5%) | 3 (9%) | |
| CAN prevalence b | 12 (33%) | 18 (51%) | 0.15 |
| Peripheral neuropathy | |||
| Clinically confirmed | 6 (16%) | 15 (43%) | 0.02 |
| Retinopathy | |||
| No Retinopathy | 3 (8%) | 2 (6%) | NA |
| Microaneurysms only | 4 (11%) | 2 (6%) | NA |
| Mild NPDR | 13 (35%) | 6 (17%) | NA |
| Moderate NPDR | 7 (19%) | 8 (23%) | NA |
| Severe PDR or worse | 10 (27%) | 17 (49%) | 0.02 |
| Nephropathy | |||
| Serum creatinine, mg/dl | 0.9 ± 0.03 | 1 ± 0.06 | NA |
| Estimated GFR, ml/min | 83 ± 3 | 80 ± 4 | NA |
| Sustained estimated GFR < 60 ml/min | 4 (11%) | 7 (20%) | NA |
| Albumin excretion rate c | 11.5 (4.3, 18.7) | 12.2 (6.5, 19.4) | NA |
| Dialysis | 0 | 3 (9%) | NA |
| Renal transplant | 0 | 3 (9%) | NA |
| Severe nephropathy d | 1 (3%) | 6 (17%) | 0.05 |
| Hypoglycemia | |||
| Six or more weekly episodes of mild hypoglycemia | 6 (16%) | 3 (9%) | |
NA – not assessed
Values are numbers (%) or mean (SEM) unless stated otherwise.
CAN prevalence is defined as any one of the following conditions: R-R variation < 15, R-R variation 20 in combination with Valsalva ratio ≤ 1.5, or postural hypotension.22
Data are medians (IQ range)
Severe nephropathy defined as albumin excretion rate > 300 mg/day, renal transplant or dialysis.
Abbreviations: NPDR = non proliferative diabetic retinopathy
A greater proportion of participants with delayed (49%) than with normal (27%) GE had severe proliferative diabetic retinopathy (P=0.02). While the median albumin excretion rate and estimated GFR were not significantly associated with GE status, a greater proportion of participants with delayed (6 participants, 17%) than with normal GE (1 patient, 3%) had a severe nephropathy (P=0.05).
Multiple Variable Models of Risk Factors for Delayed Gastric Emptying
In Model 1, which incorporated complications of DM, retinopathy was significantly associated with an increased risk of delayed versus normal GE (OR 6.5; 95% CI 1.2, 34.1); the odds ratios for autonomic neuropathy and nephropathy were also greater than 1 but 95% CI values crossed 0 (Table 3). In Model 2, which had a higher c-statistic of 0.79, baseline HbA1c before DCCT (OR 1.7; 95% CI 1.2, 2.4), the duration of DM prior to DCCT entry (OR 1.14; 95% CI 1.007, 1.3), and the mean HbA1c during DCCT and EDIC (OR 2.3; 95% CI 1.1, 4.7) were independent predictors of delayed versus normal GE.
Table 3.
Multiple Variable Predictor Models for Delayed Gastric Emptying in Type 1 Diabetes Mellitus
| Parameter | Odds Ratios (95% CI)
|
|
|---|---|---|
| Model 1 | Model 2 | |
| Autonomic neuropathy | 1.2 (0.4, 3.5) | |
| Retinopathy | 6.5 (1.2, 34.1) | |
| Nephropathy | 5.3 (0.53, 52.0) | |
| DCCT – Baseline HbA1c | 1.6 (1.1, 2.3) | |
| Duration of DM prior to DCCT (per 12 months) | 1.2 (1.01, 1.3) | |
| DCCT and EDIC – average HbA1c over 30 years | 2.2 (1.04, 4.5) | |
| C statistic | 0.67 | 0.78 |
Gastrointestinal Symptoms
Gastrointestinal symptoms evaluated by questionnaires were not significantly associated with delayed GE (Table 4). Among patients with delayed GE, functional bowel symptoms included diarrhea or diarrhea-predominant irritable bowel syndrome (n=4), constipation or constipation-predominant irritable bowel syndrome (n=7), and abdominal bloating (n=2). Moreover, neither the total GCSI symptom score (0.11 [0.02, 0.41]; median [IQ range] in delayed vs (0.10 [0.0, 0.40]) in normal GE, P=0.31), nor the subscores for nausea/vomiting, fullness/early satiety, and bloating (data not shown), derived from daily symptom diaries were significantly different between those with delayed compared to those with normal GE. However, the composite score for nausea, fullness, vomiting, and pain was associated with GE (P=0.049) being greater in the delayed group (0.14 [0.0, 0.32]; median [IQ range]) than in the normal group (0.0 [0.0,0.38]). Moreover, this score was correlated (r=0.25, P=0.04) with GE half time.
Table 4.
Gastrointestinal Symptoms by Gastric Emptying Categories
| Characteristic | Normal (n = 37) | Delayed (n = 35) | P Valueb for Association With Group Status |
|---|---|---|---|
| GI symptoms (Rome Criteria) a | |||
| Functional dyspepsia | 3 (8%) | 6 (18%) | 0.29 |
| Functional bowel symptoms | 10 (28%) | 13 (37%) | 0.32 |
| GI Symptom Severity – PAGI scale c | |||
| Heartburn/regurgitation | 0.25 (0.08) | 0.30 (0.07) | 0.60 |
| Nausea, vomiting | 0.06 (0.02) | 0.18 (0.08) | 0.15 |
| Satiety | 0.65 (0.13) | 0.67 (0.15) | 0.92 |
| Abdominal bloating | 0.89 (0.21) | 0.70 (0.17) | 0.50 |
| Upper abdominal pain | 0.28 (0.11) | 0.49 (0.16) | 0.39 |
| Lower abdominal pain | 0.35 (0.13) | 0.42 (0.14) | 0.63 |
| Total PAGI symptom score | 0.41 (0.09) | 0.46 (0.10) | 0.73 |
| Quality of life – PAGI scale c | |||
| Daily activities | 0.018 (0.005) | 0.03 (0.01) | NA |
| Diet | 0.05 (0.017) | 0.07 (0.02) | NA |
| Psychological | 0.04 (0.01) | 0.04 (0.01) | NA |
| Clothing | 0.15 (0.04) | 0.17 (0.09) | NA |
| Relationship | 0.01 (0.006) | 0.05 (0.03) | NA |
| Total | 0.05 (0.02) | 0.08 (0.03) | 0.49 |
NA – not assessed
Values are presented as N (%)
Fisher exact test or Wilcoxon rank sum test.
Symptoms and QOL are ranked on a scale of 0 (none) to 5 (very severe) where 1 represents very mild symptoms
Only 10 patients reported any (of 6) symptoms, mostly of mild severity, before starting the GEBT (Table 5). Forty one participants reported that at least one (of 6) symptoms increased by 1 unit during the GEBT. The GE half time was not significantly correlated with the change (after – before GEBT meal) for any of these 6 symptoms.
Table 5.
Gastrointestinal Symptoms during the Gastric Emptying Breath Test
| Time | Bloating | Burning | Nausea | Abdominal pain | Fullness | Belching |
|---|---|---|---|---|---|---|
| Fasting | 0.12 ±0.04 | 0.01±0.01 | 0.01±0.01 | 0.03±0.02 | 0.13±0.06 | 0.01±0.01 |
| 15 minutes | 0.17±0.06 | 0.00±0.00 | 0.08±0.04 | 0.04±0.02 | 0.41±0.09 | 0.13±0.05 |
| 30 minutes | 0.19±0.07 | 0.05±0.03 | 0.08±0.04 | 0.05±0.03 | 0.38±0.10 | 0.12±0.04 |
| 45 minutes | 0.18±0.07 | 0.04±0.02 | 0.08±0.04 | 0.04±0.02 | 0.37±0.09 | 0.14±0.04 |
| 60 minutes | 0.15±0.06 | 0.08±0.03 | 0.10±0.05 | 0.12±0.06 | 0.42±0.10 | 0.18±0.06 |
| 90 minutes | 0.18±0.06 | 0.08±0.03 | 0.10±0.05 | 0.05±0.03 | 0.32±0.08 | 0.17±0.07 |
| 120 minutes | 0.10±0.05 | 0.06±0.03 | 0.09±0.05 | 0.04±0.02 | 0.29±0.08 | 0.19±0.06 |
| 150 minutes | 0.09±0.04 | 0.03±0.02 | 0.05±0.03 | 0.04±0.02 | 0.27±0.07 | 0.19±0.06 |
| 180 minutes | 0.10±0.04 | 0.08±0.04 | 0.07±0.03 | 0.06±0.03 | 0.25±0.07 | 0.17±0.05 |
| 240 minutes | 0.11±0.05 | 0.06±0.03 | 0.06±0.03 | 0.04±0.02 | 0.13±0.05 | 0.10±0.04 |
| Correlation between change in symptoms (peak after – before GEBT) and gastric emptying T ½ | r=−0.06, p=0.63 | r=0.05, p=0.67 | r=0.21, p=0.07 | r=0.07, p=0.57 | r=−0.17, p=0.13 | r=−0.04, p=0.69 |
Health Care Utilization
Participants with delayed gastric emptying reported more (P=.03) office visits than participants with normal gastric emptying (Table 2). Likewise, the number of emergency room and hospital visits in the past year was also higher; however, associations were not significant. The number of office visits was correlated (r=.27, P=.02) with gastric emptying half time, duration of DM (r=.25, P=.03), BMI (r=.22, P=.06) and the number of hospital visits (r=.30, P=.007), inversely correlated with R-R variability (r= −.30, P<.01), but was not correlated with HbA1c (r= −.03, P=.78) or eGFR (r= −.10, P=.39).
DISCUSSION
By studying a unique cohort with longitudinal and comprehensive assessments of glycemia and other putative risk factors for gastroparesis over an average of 27 years, these findings provide new insights into the prevalence of and risk factors for delayed GE in DM. Among participants with longstanding but generally well-controlled, type 1 DM, 48% had delayed GE, which was associated with generally mild symptoms of dyspepsia. The prevalence of delayed GEis high, indeed comparable to the prevalence among patients with long-standing T1DM and gastrointestinal symptoms who were not treated with intensive insulin therapy.3–5 Furthermore, it is considerably greater than the previously reported cumulative incidence over 11 years of symptomatic gastroparesis among participants with T1DM, which was only 5%, 7 perhaps because the latter estimate was derived from symptomatic patients who presented for care. Three risk factors (i.e., baseline HbA1c and duration of DM at entry into DCCT and mean HbA1c over 30 years during DCCT and EDIC) independently discriminated delayed from normal GE. Other than an association between HbA1c and the GE lag time,14 to our knowledge, this is the first demonstration that long-term glycemic control affects GE in DM. The GE was not correlated with blood glucose values before or during the study, and blood glucose was generally maintained between 80 and 200 mg/dl during the GEBT. However, we cannot exclude the possibility that mild acute hyperglycemia might partly explain delayed GE in this study.
It is useful to consider long-term glycemic exposure during the DCCT-EDIC studies in 3 periods, i.e., before DCCT, during DCCT, and during EDIC. These observations extend findings from the DCCT cohort that “the initial level of HbA1c observed at eligibility screening as an index of pre-DCCT glycemia and the duration of type 1 diabetes on entry were the dominant baseline predictors of the risk of progression” of retinopathy and other complications39. Also, mean HbA1c during EDIC was independently associated with an increased risk of peripheral autonomic neuropathy.40 Similar to delayed GE in the current study, total hyperglycemia exposure, incorporating HbA1c and duration of DM, was also a risk factor for peripheral neuropathy in a population-based study of DM.41, 42 However, in the current study intensive glycemic therapy for an average of 6 years during DCCT did not appear to be associated with a reduced risk of delayed GE measured approximately 21 years later in this study. Because GE was not assessed in the conventional and intensive treated groups during the DCCT, the early effects of intensive therapy on GE in DM are unknown. Taken together, these observations suggest that long-term hyperglycemia is related to the risk of delayed GE in DM and that intensive therapy should reduce delayed GE. While this is sensible, it is unclear if improving glycemic control improves GE in diabetic gastroparesis in this study or elsewhere.43, 44
Compared to participants with normal GE, participants with delayed GE had a lower R-R variation with deep breathing, which suggests a vagal neuropathy, and a higher prevalence of clinically-confirmed peripheral neuropathy at DCCT baseline and closeout and at year 13/14 of EDIC. Other complications (e.g., severe nephropathy) were also nominally more common in participants with delayed GE but differences were not significant. This may be partly attributable to a type II error. Our sample size (i.e., 37 participants with normal and 35 participants with delayed GE) provided 80% power at an α level of 0.05 to identify an absolute difference in the proportions of the prevalence of any risk factor of approximately 0.3 units between the 2 groups. For variables such as cardiac autonomic neuropathy, the actual difference (33% for normal versus 51% for delayed GE) was less than 30%.
The composite score for nausea, fullness, vomiting, and pain derived from daily questionnaires was greater in patients with delayed than normal GE. In addition, the nausea/vomiting subscore of the GCSI was correlated with the GE rate. However, these scores, the symptom scores during the GEBT, and the impact of GI symptoms on QOL were relatively low, even in participants with delayed GE, consistent with mild symptoms and impact on QOL. Indeed, delayed gastric emptying in diabetes is often asymptomatic.2 However, participants with delayed GE reported nausea for an average of 50 minutes daily. In contrast to daily diaries, symptoms recorded by symptom questionnaires were not significantly different between participants with delayed and normal GE, which underscores the importance of assessing symptoms by daily diaries.
Our findings were derived from 74 of approximately 1275 participants in the EDIC cohort. While the distributions of age and sex and the proportion of participants who received intensive treatment in DCCT were similar in this study and the entire cohort, a greater proportion of participants had complications in this cohort than in the overall study. Hence, these findings need to be confirmed in a larger cohort of EDIC participants. While severe hyperglycemia did not occur during the GEBT, even relatively high blood glucose concentrations, albeit in the physiological range, can delay GE.45 Diabetic gastroparesis is associated with small intestinal bacterial overgrowth documented by a lactulose-hydrogen breath test,46 rather than the criterion standard (i.e., culture of small intestinal aspirate). Diabetic diarrhea is very rarely associated with exocrine pancreatic insufficiency or bacterial overgrowth with malabsorption.47 The accuracy of the GEBT has not been formally evaluated in patients with small intestinal bacterial overgrowth or exocrine pancreatic insufficiency. It is theorecticall possible that malabsorption and exocrine pancreatic insufficiency may affect the absorption of 13C, hence the accuracy of the GEBT. Maldigestion or malabsorption severe enough to affect absorption of 13C would likely manifest with symptoms of the same. However, only 6 patients with delayed GE reported any symptoms that might suggest small intestinal bacterial overgrowth (i.e., diarrhea or abdominal bloating); this proportion was not different compared to patients with normal GE. Taken together, these observations suggest that an artifact related to small intestinal bacterial overgrowth or exocrine pancreatic insufficiency is unlikely to explain the observed delayed GE in this study.
In summary, 47% of participants with type 1 DM in the EDIC cohort had delayed GE. Early hyperglycemic exposure, as measured by the duration of DM and HbA1c at DCCT baseline and ongoing hyperglycemia, as quantified by the mean HbA1c during DCCT-EDIC, were associated with delayed GE. Delayed GE was associated with other complications of DM, particularly severe retinopathy, and to a lesser extent with cardiovascular vagal dysfunction, and severe nephropathy.
Acknowledgments
A complete list of participants in the DCCT/EDIC research group can be found in New England Journal of Medicine, 2011;365:2366-2376.
Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Lifescan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Ft. Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), OmniPod® Insulin Management System (Bedford, MA), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN), and Sanofi-Aventis (Bridgewater NJ).
Funding/Support: The DCCT/EDIC has been supported by cooperative agreement grants (1982–1993, 2012–2017), and contracts (1982–2012) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Disease (current grant numbers U01 DK094176 and U01 DK094157), and through support by the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the General Clinical Research Centers Program (1993–2007), and Clinical Translational Science Center Program (2006–present), Bethesda, Maryland, USA.
This ancillary study was supported in part by USPHS NIH Grant P01 DK068055 (Dr. Bharucha). The study was made possible because of the dedication of the DCCT/EDIC researchers and participants. We wish to thank AB Diagnostics for providing the [13C]-Spirulina gastric emptying breath test kits at a discounted rate.
Abbreviations
- DCCT
Diabetes Control and Complications Trial
- DM
diabetes mellitus
- EDIC
Epidemiology of Diabetes Interventions and Complications
- GCSI
Gastrointestinal Cardinal Symptom Index
- GE
gastric emptying
- GEBT
gastric emptying breath tests
- HbA1c
glycated hemoglobin
- SMBG
self-monitored blood glucose
- T1DM
type 1 DM
Footnotes
Disclosures: None.
Trial Registration: clinicaltrials.gov NCT00360815 and NCT00360893.
Author Contributions:
Adil E. Bharucha - Study concept and design; analysis and interpretation of data; drafting of the manuscript
Barbara Batey-Schaefer - Study concept and design; data collection
Joseph A. Murray - Study concept and design; data analysis
Alan R. Zinsmeister – Study design, statistical analysis; drafting of the manuscript
David M. Nathan- writing and editing of manuscript
Gayle Lorenzi, Marsha Driscoll, Judy Harth, Mary Larkin, Margaret Bayless, Nyra
Wimmergren, Kim Jones, Davida Kruger, Marielle Christofi, Cathy Martin, and Georgia Ziegler – data collection
All authors: critical revision of the manuscript for important intellectual content
The authors acknowledge Mary Hawkins of the Biostatistics Center, the George Washington University for technical assistance and editorial compliance with group research specifications and NIH guidelines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kassander P. Asymptomatic gastric retention in diabetics: gastroparesis diabeticorum. Ann Intern Med. 1958;48:797–812. doi: 10.7326/0003-4819-48-4-797. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M, Bharucha AE, Farrugia G. Epidemiology, mechanisms, and management of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2011;9:5–12. doi: 10.1016/j.cgh.2010.09.022. quiz e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldman M, Schiller ER. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med. 1983;98:378–384. doi: 10.7326/0003-4819-98-3-378. [DOI] [PubMed] [Google Scholar]
- 4.Clouse RE, Lustman PJ. Gastrointestinal symptoms in diabetic patients: lack of association with neuropathy. Am J Gastroenterol. 1989;84:868–872. [PubMed] [Google Scholar]
- 5.Jones KL, Russo A, Stevens JE, et al. Predictors of delayed gastric emptying in diabetes. Diabetes Care. 2001;24:1264–1269. doi: 10.2337/diacare.24.7.1264. [DOI] [PubMed] [Google Scholar]
- 6.Bharucha AE, Camilleri M, Low PA, et al. Autonomic dysfunction in gastrointestinal motility disorders. Gut. 1993;34:397–401. doi: 10.1136/gut.34.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choung RS, Locke GR, 3rd, Schleck CD, et al. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol. 2012;107:82–88. doi: 10.1038/ajg.2011.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kilpatrick ES, Rigby AS, Atkin SL. The Diabetes Control and Complications Trial: the gift that keeps giving. Nat Rev Endocrinol. 2009;5:537–545. doi: 10.1038/nrendo.2009.179. [DOI] [PubMed] [Google Scholar]
- 9.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. . Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–2569. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. . Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290:2159–2167. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin CL, Albers JW, Pop-Busui R, et al. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:31–38. doi: 10.2337/dc13-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miao F, Chen Z, Genuth S, et al. Evaluating the role of epigenetic histone modifications in the metabolic memory of type 1 diabetes. Diabetes. 2014;63:1748–1762. doi: 10.2337/db13-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser RJ, Horowitz M, Maddox AF, et al. Hyperglycaemia slows gastric emptying in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990;33:675–680. doi: 10.1007/BF00400569. [DOI] [PubMed] [Google Scholar]
- 14.Horowitz M, Harding PE, Maddox A, et al. Gastric and oesophageal emptying in insulin-dependent diabetes mellitus. J Gastroenterol Hepatol. 1986;1:97–113. [Google Scholar]
- 15.Bytzer P, Talley NJ, Hammer J, et al. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97:604–611. doi: 10.1111/j.1572-0241.2002.05537.x. [DOI] [PubMed] [Google Scholar]
- 16.Hyett B, Martinez FJ, Gill BM, et al. Delayed radionucleotide gastric emptying studies predict morbidity in diabetics with symptoms of gastroparesis. Gastroenterology. 2009;137:445–452. doi: 10.1053/j.gastro.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 17.Chang J, Russo A, Bound M, et al. A 25-year longitudinal evaluation of gastric emptying in diabetes. Diabetes Care. 2012;35:2594–2596. doi: 10.2337/dc12-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacy BE. Functional dyspepsia and gastroparesis: one disease or two? Am J Gastroenterol. 2012;107:1615–1620. doi: 10.1038/ajg.2012.104. [DOI] [PubMed] [Google Scholar]
- 19.The Diabetes Control and Complications Trial Research Group. . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 20.Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. No authors listed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boright AP, Paterson AD, Mirea L, et al. Genetic variation at the ACE gene is associated with persistent microalbuminuria and severe nephropathy in type 1 diabetes: the DCCT/EDIC Genetics Study. Diabetes. 2005;54:1238–1244. doi: 10.2337/diabetes.54.4.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pop-Busui R, Low PA, Waberski BH, et al. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC) Circulation. 2009;119:2886–2893. doi: 10.1161/CIRCULATIONAHA.108.837369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White NH, Sun W, Cleary PA, et al. Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes mellitus: 10 years after the Diabetes Control and Complications Trial. Archives of Ophthalmology. 2008;126:1707–1715. doi: 10.1001/archopht.126.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szarka LA, Camilleri M, Vella A, et al. A stable isotope breath test with a standard meal for abnormal gastric emptying solids in the clinic and in research. Clin Gastroenterol Hepatol. 2008;6:635–643. doi: 10.1016/j.cgh.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bharucha AE, Camilleri M, Veil E, et al. Comprehensive assessment of gastric emptying with a stable isotope breath test. Neurogastroenterol Motil. 2013;25:e60–69. doi: 10.1111/nmo.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez C, Lobo B, Pigrau M, et al. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2013;62:1160–1168. doi: 10.1136/gutjnl-2012-302093. [DOI] [PubMed] [Google Scholar]
- 27.Lembo A, Camilleri M, McCallum RW, et al. A Phase 2, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Safety and Efficacy of RM-131 in Patients with Diabetic Gastroparesis [Abstract 929a] Gastroenterology. 2014;146:S-158–S-159. [Google Scholar]
- 28.Klein PD. Clinical applications of 13CO2 measurements. Fed Proc. 1982;41:2698–2701. [PubMed] [Google Scholar]
- 29.Arts J, van Gool S, Caenepeel P, et al. Influence of intrapyloric botulinum toxin injection on gastric emptying and meal-related symptoms in gastroparesis patients. Aliment Pharmacol Ther. 2006;24:661–667. doi: 10.1111/j.1365-2036.2006.03019.x. [DOI] [PubMed] [Google Scholar]
- 30.Bisschops R, Karamanolis G, Arts J, et al. Relationship between symptoms and ingestion of a meal in functional dyspepsia. Gut. 2008;57:1495–1503. doi: 10.1136/gut.2007.137125. [DOI] [PubMed] [Google Scholar]
- 31.Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670–680. doi: 10.1111/j.1365-2036.2009.04078.x. [DOI] [PubMed] [Google Scholar]
- 32.Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD) Neurogastroenterol Motil. 2012;24:456–463. doi: 10.1111/j.1365-2982.2012.01879.x. [DOI] [PubMed] [Google Scholar]
- 33.Revicki DA, Rentz AM, Dubois D, et al. Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther. 2003;18:141–150. doi: 10.1046/j.1365-2036.2003.01612.x. [DOI] [PubMed] [Google Scholar]
- 34.Shin A, Camilleri M, Busciglio I, et al. The ghrelin agonist RM-131 accelerates gastric emptying of solids and reduces symptoms in patients with type 1 diabetes mellitus. Clin Gastroenterol Hepatol. 2013;11:1453–1459. e1454. doi: 10.1016/j.cgh.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de la Loge C, Trudeau E, Marquis P, et al. Cross-cultural development and validation of a patient self-administered questionnaire to assess quality of life in upper gastrointestinal disorders: the PAGI-QOL. Quality of Life Research. 2004;13:1751–1762. doi: 10.1007/s11136-004-8751-3. [DOI] [PubMed] [Google Scholar]
- 36.Rentz AM, Kahrilas P, Stanghellini V, et al. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual Life Res. 2004;13:1737–1749. doi: 10.1007/s11136-004-9567-x. [DOI] [PubMed] [Google Scholar]
- 37.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 38.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders.[erratum appears in Gastroenterology. 2006 Jul;131(1):336] Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 39.The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44:968–983. No authors listed. [PubMed] [Google Scholar]
- 40.Martin CL, Albers J, Herman WH, et al. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care. 2006;29:340–344. doi: 10.2337/diacare.29.02.06.dc05-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dyck PJ, Davies JL, Wilson DM, et al. Risk factors for severity of diabetic polyneuropathy: intensive longitudinal assessment of the Rochester Diabetic Neuropathy Study cohort. Diabetes Care. 1999;22:1479–1486. doi: 10.2337/diacare.22.9.1479. [DOI] [PubMed] [Google Scholar]
- 42.Dyck PJ, Davies JL, Clark VM, et al. Modeling chronic glycemic exposure variables as correlates and predictors of microvascular complications of diabetes. Diabetes Care. 2006;29:2282–2288. doi: 10.2337/dc06-0525. [DOI] [PubMed] [Google Scholar]
- 43.Bharucha AE, Kudva YC, Basu A, et al. Relationship between glycemic control and gastric emptying in poorly controlled type 2 diabetes. Clin Gastroenterol Hepatol. 2015;13:466–476. doi: 10.1016/j.cgh.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laway BA, Malik TS, Khan SH, et al. Prevalence of abnormal gastric emptying in asymptomatic women with newly detected diabetes and its reversibility after glycemic control-a prospective case control study. J Diabetes Complications. 2013;27:78–81. doi: 10.1016/j.jdiacomp.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Schvarcz E, Palmer M, Aman J, et al. Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulin-dependent diabetes mellitus. Gastroenterology. 1997;113:60–66. doi: 10.1016/s0016-5085(97)70080-5. [DOI] [PubMed] [Google Scholar]
- 46.George NS, Sankineni A, Parkman HP. Small intestinal bacterial overgrowth in gastroparesis. Dig Dis Sci. 2014;59:645–652. doi: 10.1007/s10620-012-2426-7. [DOI] [PubMed] [Google Scholar]
- 47.Valdovinos MA, Camilleri M, Zimmerman BR. Chronic diarrhea in diabetes mellitus: mechanisms and an approach to diagnosis and treatment. Mayo Clin Proc. 1993;68:691–702. doi: 10.1016/s0025-6196(12)60606-5. [DOI] [PubMed] [Google Scholar]