Abstract
Background
In the general population, circulating adiponectin is associated with a favorable cardiovascular risk profile (e.g., lower triglycerides and body fat) and decreased mortality. Hemodialysis (HD) patients have comparatively higher adiponectin concentrations, but prior studies examining the adiponectin-mortality association in this population have not accounted for body composition nor shown a consistent relationship.
Study Design
Prospective cohort study.
Settings and Participants
We examined baseline serum adiponectin concentrations in 501 HD patients across 13 dialysis centers from the prospective MADRAD (Malnutrition, Diet, and Racial Disparities in Chronic Kidney Disease) cohort (entry period 10/2011-2/2013, follow-up through 8/2013).
Predictor
Serum adiponectin concentration in tertiles (Tertiles 1, 2, and 3 defined as <=16.1, >16.1–30.1, >30.1–100.0 ug/ml, respectively). Adjustment variables included case-mix and laboratory tests (age, sex, race, ethnicity, vintage, diabetes, serum albumin, total iron binding capacity, serum creatinine, white blood cell count, phosphate, hemoglobin, normalized protein catabolic rate), body composition surrogates (subcutaneous, visceral, and total body fat; lean body mass), and serum lipid levels (cholesterol, HDL, triglycerides).
Outcomes
All-cause mortality using survival (Cox) models incrementally adjusted for case-mix and laboratory tests.
Results
Among 501 HD patients, 50 deaths were observed during 631.1 person-years of follow-up time. In case-mix– and laboratory-adjusted Cox analyses, the highest adiponectin tertile was associated with increased mortality vs. the lowest tertile (HR, 3.35; 95% CI, 1.50–7.47). These associations were robust in analyses that additionally accounted for body composition (HR, 3.18; 95% CI, 1.61–8.24) and lipids (HR, 3.64; 95% CI, 1.34–7.58).
Limitations
Residual confounding cannot be excluded.
Conclusions
In conclusion, higher adiponectin is associated with a 3-fold higher death risk in HD patients independent of body composition and lipids. Future studies are needed to elucidate underlying mechanisms, and to determine therapeutic targets associated with improved outcomes in HD patients.
Keywords: Adiponectin; mortality; hemodialysis; body composition; anthropometry; body fat; body mass index (BMI); lipids; cardiovascular disease (CVD); renal replacement therapy (RRT); end-stage renal disease; MADRAD (Malnutrition, Diet, and Racial Disparities in Chronic Kidney Disease) study
Adipose tissue has gained recognition as an important source of biologically-active proteins with metabolic effects, known as adipokines.1 Among the most abundant circulating adipokine is adiponectin, a hormone of 30 kDa produced in inverse proportion to fat mass,1,2 and which circulates in the plasma as a low, middle, and high molecular weight trimer, hexamer, and multimer, respectively.1,3
In the general population, adiponectin has anti-inflammatory, insulin-sensitizing, and anti-atherogenic properties,4 and has been associated with favorable body anthropometry characteristics (e.g., decreased fat mass2) and lipid profiles (e.g., lower triglycerides [TG] and higher HDL cholesterol5). Epidemiologic data have shown that there is an inverse association between adiponectin levels and cardiovascular (CV) morbidity in populations with high underlying CV risk.6–8 However, in hemodialysis (HD) patients, who bear an exceedingly high CV mortality9, two-and-a-half to three-fold higher adiponectin concentrations have been observed.1,10,11
Despite intensive past study, the impact of adiponectin on the CV health and survival of HD patients remains unclear. Whereas early studies suggested that higher adiponectin levels are associated with decreased CV and all-cause mortality risk in the HD population,10,12 recent data indicate that elevated circulating adiponectin is associated with adverse outcomes in non–dialysis-dependent chronic kidney disease and HD patients.13–15 Heterogeneous findings across studies may be due to residual confounding relating to inconsistent covariate adjustment.16,17 Previous studies of the adiponectin–mortality association in this population have not comprehensively considered differences in multiple individual body composition compartments (e.g., visceral and subcutaneous fat mass or lean body mass [LBM]) among patients with varying adiponectin levels. Furthermore, only two studies have accounted for serum lipid components in non–dialysis-dependent chronic kidney disease and HD patients to date.13,14
Accounting for differences in body composition and lipoprotein fractions in the examination of adiponectin and HD patient outcomes bears particular relevance. For example, while body mass index (BMI) has been deemed to be a potent and paradoxical predictor of mortality in HD patients (i.e., higher BMI is associated with decreased mortality),18 some studies suggest that individual body composition components (e.g., waist circumference and triceps skinfold [SF] as surrogates of visceral and subcutaneous fat) have a similar or even stronger association with survival than BMI.19–21 Indeed, recent data suggest that the association between adiponectin and mortality may be dependent upon BMI or waist circumference.16,17 To date, to our knowledge, no studies of adiponectin and mortality have comprehensively accounted for differences in other body composition components such as subcutaneous fat and LBM. Furthermore, some,22–25 but not all,26 studies have shown that total serum cholesterol and its individual components such as LDL and HDL have paradoxical associations with mortality in HD patients (i.e., lower total and LDL cholesterol and higher HDL cholesterol associated with increased death risk). Thus to better inform the field, we sought to examine the association between serum adiponectin levels and mortality in a large prospective cohort of maintenance HD patients undergoing rigorous, protocoled measurement of clinical, laboratory, and individual body anthropometry and serum lipid characteristics.
METHODS
Study Population
The study population comprised a cohort of maintenance HD patients enrolled in the initial phase of the Malnutrition, Diet, and Racial Disparities in Chronic Kidney Disease (MADRAD) study (trial registration: ClinicalTrials.gov; study number NCT01415570), a prospective cohort study examining the differential association between dietary factors and nutritional status across racial and ethnic HD subgroups. In this substudy, patients were recruited from 13 DaVita Healthcare Partners Inc. dialysis facilities in the South Bay–Los Angeles area from October 2011 through February 2013. Patients were included provided that they were aged 18–85 years, received thrice-weekly in-center HD treatment for at least four consecutive weeks, signed a local institutional review board-approved consent form, and had serum adiponectin measurement at study entry. Patients were excluded if they were actively receiving peritoneal dialysis (PD), had a life expectancy of less than six months (e.g., stage IV cancer), or were unable to provide consent without a proxy (e.g., dementia). The study was approved by the Institutional Review Committees of the Los Angeles Biomedical Research Institute at Harbor-UCLA, Torrance, CA, and the University of California Irvine Medical Center, Orange, CA.
Exposure Ascertainment
Baseline adiponectin levels were measured from frozen serum samples that were obtained pre-dialysis during weekday HD treatments at the time of study entry, and that chronologically coincided with routine blood tests conducted at DaVita facilities. Serum adiponectin levels were measured using immunoassay kits based on solid-phase sandwich ELISA (EMD Millipore Corporation, St. Charles, MO) in the General Clinical Research Center Laboratories of Harbor-UCLA Medical Center with a lower limit of detection of 0.0002 µg/ml for adiponectin. The coefficients of variation for intra- and interassay precision were 0.9% and 2.4%, respectively.
In primary analyses, we examined the association between serum adiponectin concentrations, categorized into tertiles, and all-cause mortality. In secondary analyses, adiponectin was considered as a continuous variable and scaled to a 10-µg/ml change. To flexibly model the association between continuous adiponectin concentrations and mortality, we also conducted analyses in which adiponectin was examined as a restricted cubic spline with knots corresponding to the 25th (13.8 µg/ml), 50th (22.6 µg/ml), and 75th (36.3 µg/ml) percentiles of observed values.
Sociodemographic, Comorbidity, and Laboratory Test Measures
At study entry, baseline information on sociodemographics, comorbid conditions, and dialysis treatment characteristics (e.g., vascular access type) were collected. Dialysis vintage was defined as the time period between the date of study entry and the date of HD initiation. Routine dialysis laboratory measurements were performed by DaVita Healthcare Inc. laboratories (Deland, FL) on a monthly or quarterly basis by using automated methods. In this study, baseline values of routine laboratory tests, including serum lipids (total cholesterol, TG, LDL, HDL) were used.
Body Anthropometry Test Measures
At study entry, dialysis center dietitians conducted measurements of body composition surrogates while patients underwent routine HD treatments, which included the following: BMI, subcutaneous fat (determined from biceps and triceps SF), visceral fat (determined from waist circumference), lean muscle mass (determined from mid-arm circumference [MAC] and mid-arm muscle circumference [MAMC]), and body fat percentage (measured by near-infrared [NIR] interactance). The BMI was defined as the post-dialysis weight (kg) divided by height-squared (m2). Biceps and triceps SF thicknesses (mm) were measured in the non-dialysis vascular access arm with a conventional skinfold caliper using standard techniques, and the average values of triplicate measurements were utilized. Waist circumference (cm) was measured in a horizontal plane at the midpoint between the inferior margin of the last rib and crest of the ilium, and MAC (cm) was measured from the lateral tip of the acromion process of the scapula to the tip of the olecranon process with non-stretchable plastic tape. The MAMC (cm) was estimated using the following formula: MAMC = MAC – 3.142 * triceps SF.27,28 To estimate the percentage of body fat and fat-free body mass, NIR interactance body fat (%) was measured by placing a Futrex NIR interactance sensor (portable 6100; Futrex Inc, Gaithersburg, MD; www.futrex.com) on the non-vascular access upper arm for several seconds, after inputting the required data (date of birth, sex, weight, and height) for each patient. As has been shown, NIR interactance body fat is highly correlated with other body fat and nutritional metrics in HD patients.29–31
Outcome Ascertainment
The primary outcome of interest was all-cause mortality. At-risk time began the day after serum adiponectin measurement, and patients were censored for kidney transplantation, transfer to a non-DaVita dialysis unit or PD, or at the end of the study (August 23, 2013). Each semester, information regarding mortality, censoring events, and associated dates from the preceding six months were collected from event forms completed by the MADRAD research assistants and were reviewed by two MADRAD study nephrologists (C.M.R. and K.K.-Z.).
Statistical Methods
Baseline characteristics between exposure groups were compared using chi-squared, ANOVA, and Kruskal-Wallis tests as dictated by data type. We first examined the relationship of relevant clinical, laboratory, and body composition surrogates with (1) serum adiponectin concentrations using Pearson correlation coefficients and (2) high adiponectin (defined as an adiponectin concentration >50th percentile) using logistic regression. For both Pearson correlation and logistic regression analyses, p-values were adjusted using the false discovery rate method to account for multiplicity of comparisons by reducing the proportion of false discoveries.32
We then estimated the association between serum adiponectin and all-cause mortality using Kaplan-Meier plots, log-rank testing, and Cox proportional hazard models with three incremental levels of covariate adjustment:
-
(1)
Unadjusted analyses (Model 1): No adjustment for covariates;
-
(2)
Case-mix–adjusted analyses (Model 2): Adjusted for age, sex, race (black vs. non-black), ethnicity (Hispanic vs. non-Hispanic), and dialysis vintage;
-
(3)
Case-mix– and laboratory-adjusted analyses (Model 3): Adjusted for covariates in Model 2, as well as diabetes, serum albumin, total iron-binding capacity, serum creatinine, white blood cell (WBC) count, phosphate, hemoglobin, and normalized protein catabolic rate.
To test the hypothesis that discordant associations between adiponectin and mortality may be observed with inconsistent adjustment for lipids and body anthropometry surrogates, we incrementally adjusted for lipid and body composition components as follows:
-
(4)
Lipid-adjusted analyses (Model 4): Adjusted for covariates in Model 3, as well as serum total cholesterol, HDL cholesterol, and TG;
-
(5)
Body anthropometry–adjusted analyses (Model 5): Adjusted for covariates in Model 3, as well as waist circumference, biceps SF, MAC, and NIR interactance body fat.
The proportional hazards assumption was confirmed graphically and by Schoenfeld residual function testing. Effect modification of adiponectin–mortality associations on the basis of age (≥65 vs. <65 years), sex, race (black vs. non-black), ethnicity (Hispanic vs. non-Hispanic), diabetes, waist circumference (≥95 vs. <95cm), BMI (≥25 vs. <25kg/m2), and serum albumin (≥4 vs. <4 mg/dl) were explored through the addition of two-way interaction terms with adiponectin (separately) using likelihood ratio testing. Missing covariate data were imputed using the means or medians of observed values. Analyses were carried out using the statistical software Stata version 12.0 (StataCorp LP, College Station, TX) and SAS version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Cohort Description
Among 501 patients meeting eligibility criteria, the mean age of the cohort was 55.2 ± 14.9 (standard deviation) years, of whom 44% were female, 40% were African-American, and 47% had diabetes. The mean, median, and range of serum adiponectin concentrations in the cohort were 26.9 ± 17.6, 22.6 (interquartile range, 13.8–36.3), and 2.83–100 µg/ml, respectively. Compared to patients in the lowest adiponectin tertile, patients in the highest tertile were more likely to be female, Hispanic, of older dialysis vintage; were less likely to be African-American; had lower platelet counts, WBC counts, TG concentrations, BMI, waist circumference, biceps SF, triceps SF, NIR interactance body fat percentage, MAC, and MAMC values; and had higher HDL cholesterol concentrations (Table 1). When examining clinically and statistically significant differences in baseline characteristics among patients with vs. without missing covariate data, we found that those with missing data with respect to Model 3 were more likely to be younger and Hispanic; less likely to be black; and had a lower waist circumference, NIR interactance body fat, and MAMC (Table S1, available as online supplementary material).
Table 1.
Baseline Characteristics According to Adiponectin Tertiles in Maintenance Hemodialysis Patients.
| Tertile 1 | Tertile 2 | Tertile 3 | p-value1 | |
|---|---|---|---|---|
| No. of patients | 32.9% (165) | 33.1% (166) | 33.9% (170) | N/A |
| Adiponectin range (µg/ml) | 0–16.1 | >16.1–<30.1 | >30.1–100 | N/A |
| Age (y) | 52.8 ± 14.5 | 56.6 ± 15.6 | 56.0 ± 14.6 | 0.6 |
| Dialysis vintage (y) | 2.9 (1.2, 5.3) | 3.6 (1.5, 7.3) | 3.9 (1.9, 7.1) | 0.007 |
| Female sex | 34.6% (57) | 48.2% (80) | 47.7% (81) | 0.02 |
| Black race | 45.5% (75) | 36.1% (60) | 38.2% (65) | 0.2 |
| Hispanic ethnicity | 38.2% (63) | 48.8% (81) | 48.8% (83) | 0.08 |
| Diabetes | 46.1% (76) | 48.8% (81) | 46.5% (79) | 0.9 |
| AVF/AVG | 50.9% (84) | 57.2% (95) | 46.5% (79) | 0.1 |
| Smoking | 35.2% (58) | 36.8% (61) | 36.5% (62) | >0.9 |
| Laboratory Tests | ||||
| Serum albumin (g/dl) | 4.1 (3.9, 4.3) | 4.0 (3.7, 4.2) | 4.0 (3.7, 4.2) | 0.06 |
| Creatinine (mg/dl) | 9.9 (8.0, 12.3) | 8.9 (7.2, 11.5) | 9.3 (7.6, 11.0) | 0.05 |
| Calcium2 (mg/dl) | 9.1 (8.7, 9.7) | 9.1 (8.7, 9.5) | 9.2 (8.8, 9.6) | 0.5 |
| Phosphorus (mg/dl) | 4.8 (4.2, 6.1) | 4.9 (4.0, 5.7) | 4.9 (4.1, 6.1) | 0.7 |
| PTH (pg/ml) | 369 (203, 557) | 346 (226, 549) | 352 (250, 575) | 0.8 |
| Hemoglobin (g/dl) | 10.0 (10.6, 11.2) | 10.8 (10.2, 11.3) | 10.7 (10.1, 11.3) | 0.4 |
| Platelet count (×109/L) | 229 (191, 271) | 226 (172, 275) | 198 (156, 234) | <0.001 |
| MPV (fL) | 9.5 (9.0, 10.3) | 9.8 (9.1, 10.6) | 9.9 (9.1, 10.8) | 0.2 |
| WBC count (×109/L) | 7.0 (5.8, 8.4) | 6.6 (5.5, 8.3) | 5.9 (4.5, 7.2) | <0.001 |
| TIBC (mcg/dl) | 224 (207, 251) | 221 (188, 253) | 213 (193, 242) | 0.08 |
| Ferritin (ng/ml) | 676 (422, 899) | 693 (416, 928) | 684 (463, 907) | 0.8 |
| nPCR (g/kg/day) | 1.0 (0.8, 1.2) | 1.0 (0.9, 1.2) | 1.0 (0.8, 1.2) | 0.4 |
| Glucose (mg/dl) | 135 (97, 199) | 142 (102, 196) | 128 (88, 189) | 0.8 |
| Hemoglobin A1c (%) | 6.2 (5.7, 7.0) | 6.2 (5.5, 7.2) | 6.0 (5.5, 7.3) | 0.8 |
| Total cholesterol (mg/dl) | 146 (126, 166) | 128 (112, 170) | 144 (130, 177) | 0.3 |
| HDL cholesterol (mg/dl) | 34 (30, 42) | 39 (30, 49) | 45 (38, 56) | 0.003 |
| LDL cholesterol (mg/dl) | 76 (59, 91) | 61 (47, 74) | 66 (53, 104) | 0.1 |
| Triglycerides (mg/dl) | 164 (111, 265) | 115 (93, 171) | 101 (78, 141) | <0.001 |
| Body anthropometry | ||||
| BMI (kg/m2) | 29.0 (25.5, 33.7) | 26.1 (23.2, 30.3) | 24.1 (21.9, 27.7) | <0.001 |
| Waist circumference (cm) | 101 (91, 115) | 95 (84, 106) | 88.5 (81.25, 100) | <0.001 |
| Biceps SF (mm) | 12.1 (8.0, 22.3) | 11.1 (6.7, 19.7) | 10.3 (5.7, 17.0) | 0.04 |
| Triceps SF (mm) | 20.3 (12.7, 29.3) | 19.2 (12.7, 27.3) | 16.0 (9.0, 23.3) | 0.007 |
| NIR interactance body fat (%) | 30.3 (23.0, 39.0) | 29.6 (23.3, 38.0) | 23.4 (18.3, 34.4) | <0.001 |
| MAC (mm) | 325 (290, 360) | 302 (275, 335) | 285 (261, 317) | <0.001 |
| MAMC (mm) | 261 (228, 293) | 235 (215, 268) | 236 (212, 257) | <0.001 |
Note: Categorical variables are given as number (percentage); continuous variables, as mean ± standard deviation or median [IQR]. Conversion factors for units: calcium in mg/dL to mmol/L, ×0.2495; cholesterol in mg/dL to mmol/L, ×0.02586; creatinine in mg/dL to umol/L, ×88.4; glucose in mg/dL to mmol/L, ×0.05551; fibrinogen in mg/dL to µmol/L, ×0.0294; phosphorus in mg/dL to mmol/L, ×0.3229; triglycerides in mg/dL to mmol/L, ×0.01129.
P-value calculated by analysis of variance, chi-square, or Kruskal-Wallis tests.
Calcium level corrected for serum albumin.
Abbreviations: AVF, arteriovenous fistula; AVG, arteriovenous graft; BMI, body mass index; PTH, parathyroid hormone; MAC, midarm circumference; MAMC, midarm muscle circumference; MPV, mean platelet volume; WBC, white blood cell; TIBC, total iron-binding capacity; nPCR, normalized protein catabolic rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; triglyceride; SF, skinfold; N/A, not applicable; NIR, near-infrared
Cross-sectional Associations With Clinical, Laboratory, and Body Anthropometry Covariates
In correlation analyses adjusted for case-mix and laboratory covariates, adiponectin had the strongest inverse correlations with WBC count, TG concentrations, BMI, waist circumference, NIR interactance body fat percentage, and MAC, and had the strongest positive correlation with HDL cholesterol (Table S2; scatterplots of select bivariate correlations shown in Figure S1). After accounting for multiplicity of comparisons using the false discovery rate method, correlations remained statistically significant except for the TG–adiponectin association. In case-mix– and laboratory-adjusted logistic regression analyses, dialysis vintage and HDL cholesterol were directly associated with high adiponectin, whereas WBC count, total cholesterol, TG, LDL choelsterol, BMI, waist circumference, biceps SF, triceps SF, NIR interactance body fat, MAC, and MAMC were inversely associated with high adiponectin; these associations remained statistically significant after accounting for multiplicity of comparisons (Table S3).
Adiponectin Concentration and Mortality
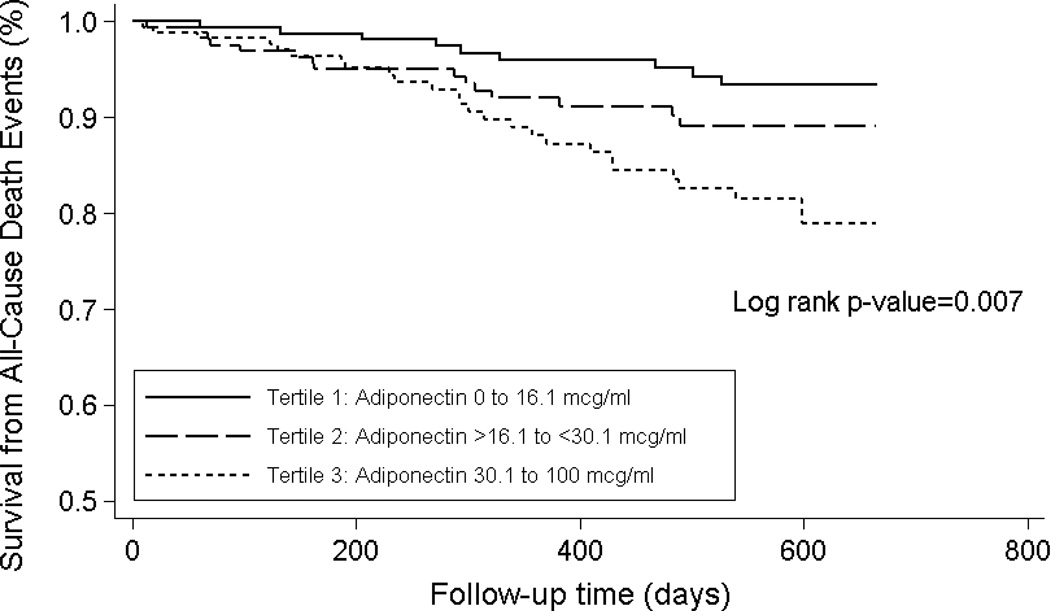
Patients contributed a total of 631.1 person-years of follow-up during which 50 death events were observed. Median at-risk time was 1.47 years. In primary analyses, the highest adiponectin tertile was associated with increased mortality risk in comparison to the lowest tertile in unadjusted Cox regression analyses in Model 1: unadjusted hazard ratio (HR), 3.13; 95% confidence interval (CI), 1.46–6.72; p=0.003 (Table 2; Kaplan Meier curves and log-rank test shown in Figure 1). The magnitude of these estimates became stronger with multivariable adjustment in Models 2 and 3, respectively: adjusted HRs of 3.53 (95% CI, 1.61–7.70; p=0.002) and 3.35 (95% CI, 1.50–7.47; p=0.003), respectively (Table 2). Compared with the lowest adiponectin tertile, the middle tertile was associated with numerically greater risk but did not reach statistical significance in Models 1, 2, and 3. In analyses incrementally adjusted for serum lipids (Model 4) and body composition surrogates (Model 5), the association between higher adiponectin and increased mortality remained robust: adjusted HRs of 3.64 (95% CI, 1.61–8.24; p=0.002) and 3.18 (95% CI, 1.34–7.58; p=0.009), respectively (Table 3). We did not detect effect modification of the adiponectin–mortality association on the basis of age, sex, race, ethnicity, dialysis vintage, presence of diabetes, waist circumference, BMI, or serum albumin: P for interaction of 0.8, 0.5, 0.5, 0.6, >0.9, 0.4, 0.4, >0.9, and 0.6, respectively.
Table 2.
Association Between Adiponectin Level and All-Cause Mortality.
| Model 11 | Model 22 | Model 33 | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) |
p-value4 | HR (95% CI) |
p-value5 | HR (95% CI) |
p-value6 | |
| Categorical* | ||||||
| Adiponectin Tertile 2: >16.1 –<30.1 µg/ml | 1.81 (0.79–4.15) | 0.2 | 1.80 (0.78–4.15) | 0.2 | 1.71 (0.73–4.01) | 0.2 |
| Adiponectin Tertile 3: >30.1 µg/ml | 3.13 (1.46–6.72) | 0.003 | 3.53 (1.61–7.70) | 0.002 | 3.35 (1.50–7.47) | 0.003 |
| Continuous: per each 10.0-µg/ml increase in adiponectin | 1.27 (1.13–1.42) | <0.001 | 1.26 (1.12–1.41) | <0.001 | 1.25 (1.10–1.41) | 0.001 |
CI, confidence interval; HR, hazard ratio
Reference group is adiponectin tertile 1 (0–16.1 ug/mL).
unadjusted for covariates.
adjusted for age, sex, race, ethnicity, and dialysis vintage.
adjusted for age, sex, race, ethnicity, dialysis vintage, diabetes, serum albumin, total iron-binding capacity, serum creatinine, white blood cell count, phosphorus, hemoglobin, and normalized protein catabolic rate.
p for trend for categorical=0.002;
p for trend for categorical=0.001;
p for trend for categorical=0.002
Figure 1. Kaplan Meier Survival Analysis for Adiponectin Tertiles in Maintenance Hemodialysis Patients.
Table 3.
Association Between Adiponectin Level and All-Cause Mortality Incrementally Adjusted for Serum Lipid and Body Anthropometry Covariates.
| Model 41 | Model 52 | |||
|---|---|---|---|---|
| HR (95% CI) | p-value3 | HR (95% CI) | p-value4 | |
| Categorical* | ||||
| Adiponectin Tertile 2: >16.1–<30.1 µg/ml | 1.78 (0.75–4.21) | 0.2 | 1.46 (0.61–3.50) | 0.4 |
| Adiponectin Tertile 3: >30.1 µg/ml | 3.64 (1.61–8.24) | 0.002 | 3.18 (1.34–7.58) | 0.009 |
| Continuous: per each 10.0-µg/ml increase in adiponectin | 1.27 (1.11–1.45) | <0.001 | 1.23 (1.08–1.41) | 0.003 |
CI, confidence interval; HR, hazard ratio
Reference group is adiponectin tertile 1 (0–16.1 µg/mL).
adjusted for covariates in Model 3 (see Table 2) as well as serum total cholesterol, HDL cholesterol, and triglycerides.
adjusted for covariates in Model 3 (see Table 2) as well as waist circumference, biceps skinfold, near-infrared interactance body fat percentage, and mid-arm circumference.
p for trend for categorical<0.001;
p for trend for categorical<0.001
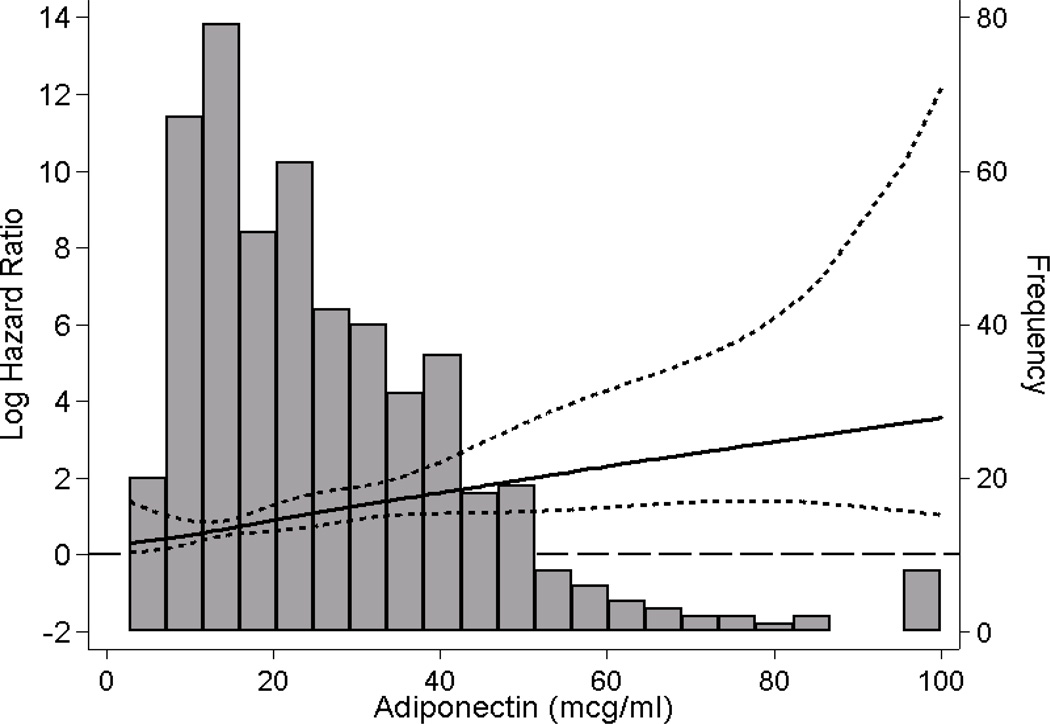
In secondary analyses, we observed that a 10-µg/ml increase in adiponectin concentration was associated with an approximately 25% increased mortality risk across Models 1 through 5 (Tables 2 and 3). In analyses examining the association between continuous adiponectin and all-cause mortality using a cubic spline function adjusted for covariates in Model 3, we observed that there was a monotonic increase in death risk across the entire range of adiponectin concentrations (Figure 2).
Figure 2. Association between Gradations of Adiponectin and All-Cause Mortality in Maintenance Hemodialysis Patients.
Figures present log hazard ratios (short-dashed lines indicate 95% CIs) for adiponectin analyzed as a spline with knots at 14 µg/ml (25th percentile) and 36 µg/ml (75th percentile). A histogram of observed adiponectin values and a log hazard reference ratio of 0 (long-dashed line) is overlaid. Analyses adjusted for age, sex, race, ethnicity, dialysis vintage, diabetes, serum albumin, total iron binding capacity, serum creatinine, white blood cell count, phosphorus, hemoglobin, and normalized protein catabolic rate.
DISCUSSION
In a prospective cohort of maintenance HD patients from the MADRAD study, we observed that HDL cholesterol was directly associated with high adiponectin concentration, whereas visceral, subcutaneous, total body fat, lean body mass, total cholesterol, TG, and LDL cholesterol showed inverse associations. We also found that higher circulating adiponectin concentrations are paradoxically associated with increased mortality risk. These findings were robust across clinically relevant subgroups, as well as analyses that comprehensively adjusted for multiple individual body composition surrogates and serum lipid components.
Adiponectin has known complex associations with multiple biologic pathways, including those that favorably impact atherogenic risk.1,33 In addition to improving the insulin sensitivity of hepatic and skeletal muscle34,35; suppressing and increasing expression of pro-inflammatory and anti-inflammatory cytokines, respectively36,37; maintaining endothelial homeostasis (i.e., augmentation of nitric oxide production, inhibition of neointimal formation)38–41; and attenuating platelet aggregation and thrombus formation1, adiponectin is also associated with a favorable serum lipid profile.5,42,43 Similar to patterns observed in prior studies of the general population and dialysis patients,5,42 we found that adiponectin was inversely associated with TG, LDL cholesterol, and total cholesterol, and was positively associated with HDL cholesterol.
Adiponectin has also been linked with potent predictors of mortality in HD patients, namely unfavorable body anthropometry characteristics such as decreased LBM, subcutaneous fat, and visceral fat.18–21 Indeed, loss of body fat and LBM have been shown to induce adiponectin secretion in both the general and dialysis populations,33,44 and experimental data in animals have shown that adiponectin stimulates energy expenditure and induces weight loss via direct action in the brain.45 Consistent with these data, we found that adiponectin was inversely associated with visceral fat, total body fat, and LBM.33,46–50
There has been inconsistent adjustment for atherogenic risk factors (e.g., serum lipids) and body composition characteristics across prior studies of the adiponectin–mortality association in HD patients, which have shown mixed findings.10,11,13,15 In a seminal study of 227 HD patients by Zoccali et al., a 1-µg/ml higher adiponectin concentration was associated with a 3% lower CV event risk, although an association with mortality was not observed.11 Subsequently, Rao et al. observed that higher baseline and time-dependent adiponectin concentrations were associated with decreased mortality among 182 HD patients from the HEMO (Hemodialysis) Study and two Boston dialysis centers, and these findings have been corroborated in other studies of HD patients.10 In contrast, Menon et al. observed higher adiponectin levels to be associated with increased all-cause and cardiovascular mortality in a secondary analysis of 585 patients with chronic kidney disease stages 3–4 from the Modification of Diet in Renal Disease (MDRD) Study.14 In a cohort of 85 Japanese HD patients, Ohashi et al. showed that a 1-µg/ml higher adiponectin concentration was associated with a 10% increase in all-cause mortality risk, although these estimates were attenuated to the null when patients with serum albumin <3.5 mg/dl (i.e., surrogate of malnutrition) were excluded.15 In the largest study of adiponectin and mortality in dialysis patients to date, Dreschler et al. found that higher adiponectin concentrations were associated with increased risk of sudden cardiac death among 1255 HD patients with diabetes type 2 from 4D (Die Deutsche Diabetes Dialyse), although estimates were no longer significant after adjustment for BMI, serum lipids (TG, HDL) and other covariates.13 Two recent studies by Zoccali et al. and Tsigalou et al. show that the adiponectin–mortality association is dependent upon underlying BMI and waist circumference16,17, but none of the aforementioned studies have concurrently adjusted for individual body composition components (e.g., LBM and visceral, subcutaneous, and total body fat).
To our knowledge, ours is the first study of the adiponectin–mortality association in HD patients to rigorously measure and account for a comprehensive cadre of individual body composition components using reliable metrics (e.g., MAC, MAMC, waist circumference, biceps and triceps SF, NIR interactance body fat). In analyses that incrementally adjusted for these factors, there was a robust association between higher adiponectin concentration and decreased survival. We additionally conducted sensitivity analyses in which we adjusted for serum lipids, and we observed a persistent association between higher adiponectin concentration and death risk in contrast to the aforementioned Dreschler et al. study.
Our findings suggest that past studies’ discordant findings may not in fact be due to variable consideration of body composition characteristics and atherogenic risk factors. Indeed, further studies are needed to determine the mechanisms underlying this robust yet paradoxical association between adiponectin and death, and other mechanisms by which high adiponectin may be linked with heightened mortality including: 1) volume overload (i.e., volume overload stimulates natriuretic peptide release, which in turn increases adiponectin production51), 2) reduced WBC count and impaired immune function resulting from inhibition of bone marrow myelopoiesis15, 3) loss of residual kidney function (i.e., decreased kidney function leads to impaired adiponectin clearance),1 or 4) counter-regulation of inflammation, vascular disease, and atherosclerosis, which are highly prevalent in HD patients.13 Furthermore, additional factors accounting for the discrepant associations across the aforementioned studies including varying durations of follow-up as well as heterogeneous study population characteristics should be explored. We did not detect effect modification of the adiponectin–mortality association by various clinically relevant characteristics including age, sex, race, ethnicity, underlying diabetes, waist circumference, BMI, or serum albumin. Although our study population was of moderately-large size, our analyses may have been underpowered to detect a statistically significant interaction. Future studies are needed to confirm findings, and to account for volume status using sophisticated and validated metrics (i.e., bioelectrical impedance), novel and traditional inflammatory markers, and residual kidney function in order to elucidate the complex association between adiponectin and mortality risk.
The strengths of our study include its 1) examination of a study population with case-mix characteristics similar to that of the HD population in the United States; 2) rigorous, protocoled measurement of sociodemographic, comorbidity, body anthropometry, and laboratory data; and 3) uniform laboratory measurements of adiponectin conducted in one centralized laboratory. However, several limitations bear mention. First, adiponectin concentrations were based on single measurements taken at study entry, and change in adiponectin over time was not considered. Second, we had limited ability to examine markers of insulin resistance and inflammation, and cannot exclude the possibility of residual confounding on this basis. Third, our adiponectin measurements did not differentiate between adiponectin moieties (e.g., high vs. low molecular weight isoforms) which are associated with differential biologic activity.50,52,53 Fourth, the body anthropometry surrogates selected as Cox regression model covariates were moderately correlated (unadjusted Pearson correlation R=0.41–0.52); while these surrogates represent distinct body composition components, and are associated with both adiponectin concentration and mortality based on published evidence, residual confounding by body composition cannot be excluded. Fifth, our analyses did not take into consideration specific causes of death (e.g., cardiovascular). Lastly, as with all observational studies, we cannot exclude the possibility of residual confounding.
In summary, our study supports an association between serum adiponectin and mortality in HD patients independent of body composition. Further studies are needed to confirm findings, explore mechanistic pathways underlying the adiponectin-mortality association, and determine the therapeutic impact of lowering adiponectin concentrations in HD patients.
Supplementary Material
Acknowledgements
Support: This study is supported by research grants from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, including K23-DK102903 (Dr Rhee) and K24-DK091419 and R01-DK078106 (both Dr Kalantar-Zadeh), and philanthropist grants from Mr Harold Simmons and Mr Louis Chang. Dr Nguyen is supported by a National Center for Advancing Translational Sciences grant (UL1 TR000153). The study sponsors did not have a role in the study design; collection, analysis, or interpretation of data; writing the report; or the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of these data have been presented in an abstract published in Nephrology Dialysis Transplantation; as an abstract at the 51st European Renal Association-European Dialysis Transplant Association Congress, May 1-June 30, 2014, Amsterdam, the Netherlands; and as an abstract at the American Society of Nephrology Kidney Week Conference, November 12–16, 2014, Philadelphia, PA.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: CMR, KK-Z; data acquisition: CMR, KK-Z; data analysis/interpretation: CMR, DVN, HM, SMB, RD, JJ, TN, CPK, GAB, KK-Z; statistical analysis: CMR; supervision or mentorship: KK-Z. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. CMR takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Supplementary Material
Table S1: Baseline characteristics in HD patients with vs without missing data.
Table S2: Unadjusted and multivariable-adjusted Pearson correlation coefficients of adiponectin and other relevant covariates.
Table S3: Association of demographic, clinical, and lab measures with high adiponectin using logistic regression.
Figure S1: Bivariate correlations of adiponectin with selected lab test and body composition surrogates.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
REFERENCES
- 1.Adamczak M, Wiecek A. The adipose tissue as an endocrine organ. Seminars in nephrology. 2013 Jan;33(1):2–13. doi: 10.1016/j.semnephrol.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochemical and biophysical research communications. 1999 Apr 2;257(1):79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 3.Fisman EZ, Tenenbaum A. Adiponectin: a manifold therapeutic target for metabolic syndrome, diabetes, and coronary disease? Cardiovascular diabetology. 2014;13:103. doi: 10.1186/1475-2840-13-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes care. 2003 Aug;26(8):2442–2450. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- 5.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003 Apr;46(4):459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 6.Maahs DM, Ogden LG, Kinney GL, et al. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005 Feb 15;111(6):747–753. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 7.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA : the journal of the American Medical Association. 2004 Apr 14;291(14):1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 8.Schulze MB, Shai I, Rimm EB, Li T, Rifai N, Hu FB. Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes. 2005 Feb;54(2):534–539. doi: 10.2337/diabetes.54.2.534. [DOI] [PubMed] [Google Scholar]
- 9.Wheeler DC, Haynes R, Landray MJ, Baigent C. Cardiovascular Aspects of Kidney Disease. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu AS, Brenner BM, editors. Taal: Brenner and Rector’s The Kidney. 9th ed. Philadelphia, PA: Elsevier Saunders; 2012. pp. 2060–2075. [Google Scholar]
- 10.Rao M, Li L, Tighiouart H, Jaber BL, Pereira BJ, Balakrishnan VS. Plasma adiponectin levels and clinical outcomes among haemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2008 Aug;23(8):2619–2628. doi: 10.1093/ndt/gfn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoccali C, Mallamaci F, Tripepi G, et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. Journal of the American Society of Nephrology : JASN. 2002 Jan;13(1):134–141. doi: 10.1681/ASN.V131134. [DOI] [PubMed] [Google Scholar]
- 12.Diez JJ, Estrada P, Bajo MA, et al. High stable serum adiponectin levels are associated with a better outcome in prevalent dialysis patients. American journal of nephrology. 2009;30(3):244–252. doi: 10.1159/000221147. [DOI] [PubMed] [Google Scholar]
- 13.Drechsler C, Krane V, Winkler K, Dekker FW, Wanner C. Changes in adiponectin and the risk of sudden death, stroke, myocardial infarction, and mortality in hemodialysis patients. Kidney international. 2009 Sep;76(5):567–575. doi: 10.1038/ki.2009.200. [DOI] [PubMed] [Google Scholar]
- 14.Menon V, Li L, Wang X, et al. Adiponectin and mortality in patients with chronic kidney disease. Journal of the American Society of Nephrology : JASN. 2006 Sep;17(9):2599–2606. doi: 10.1681/ASN.2006040331. [DOI] [PubMed] [Google Scholar]
- 15.Ohashi N, Kato A, Misaki T, et al. Association of serum adiponectin levels with all-cause mortality in hemodialysis patients. Intern Med. 2008;47(6):485–491. doi: 10.2169/internalmedicine.47.0614. [DOI] [PubMed] [Google Scholar]
- 16.Tsigalou C, Chalikias G, Kantartzi K, et al. Differential effect of baseline adiponectin on all-cause mortality in hemodialysis patients depending on initial body mass index. Long-term follow-up data of 4.5 years. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2013 Jan;23(1):45–56. doi: 10.1053/j.jrn.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Zoccali C, Postorino M, Marino C, Pizzini P, Cutrupi S, Tripepi G. Waist circumference modifies the relationship between the adipose tissue cytokines leptin and adiponectin and all-cause and cardiovascular mortality in haemodialysis patients. Journal of internal medicine. 2011 Feb;269(2):172–181. doi: 10.1111/j.1365-2796.2010.02288.x. [DOI] [PubMed] [Google Scholar]
- 18.Kalantar-Zadeh K. Causes and consequences of the reverse epidemiology of body mass index in dialysis patients. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2005 Jan;15(1):142–147. doi: 10.1053/j.jrn.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Huang CX, Tighiouart H, Beddhu S, et al. Both low muscle mass and low fat are associated with higher all-cause mortality in hemodialysis patients. Kidney international. 2010 Apr;77(7):624–629. doi: 10.1038/ki.2009.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park J, Ahmadi SF, Streja E, et al. Obesity paradox in end-stage kidney disease patients. Progress in cardiovascular diseases. 2014 Jan-Feb;56(4):415–425. doi: 10.1016/j.pcad.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Postorino M, Marino C, Tripepi G, Zoccali C. Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. Journal of the American College of Cardiology. 2009 Apr 14;53(15):1265–1272. doi: 10.1016/j.jacc.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 22.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. Journal of the American College of Cardiology. 2004 Apr 21;43(8):1439–1444. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 23.Kilpatrick RD, McAllister CJ, Kovesdy CP, Derose SF, Kopple JD, Kalantar-Zadeh K. Association between serum lipids and survival in hemodialysis patients and impact of race. Journal of the American Society of Nephrology : JASN. 2007 Jan;18(1):293–303. doi: 10.1681/ASN.2006070795. [DOI] [PubMed] [Google Scholar]
- 24.Moradi H, Streja E, Kashyap ML, Vaziri ND, Fonarow GC, Kalantar-Zadeh K. Elevated high-density lipoprotein cholesterol and cardiovascular mortality in maintenance hemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association -European Renal Association. 2014 Aug;29(8):1554–1562. doi: 10.1093/ndt/gfu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moradi H, Vaziri ND, Kashyap ML, Said HM, Kalantar-Zadeh K. Role of HDL dysfunction in end-stage renal disease: a double-edged sword. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2013 May;23(3):203–206. doi: 10.1053/j.jrn.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sevinc Ok E, Kircelli F, Asci G, et al. Neither oxidized nor anti-oxidized low-density lipoprotein level is associated with atherosclerosis or mortality in hemodialysis patients. Hemodialysis international. International Symposium on Home Hemodialysis. 2012 Jul;16(3):334–341. doi: 10.1111/j.1542-4758.2012.00683.x. [DOI] [PubMed] [Google Scholar]
- 27.Weber J, Kelley J. Assessing nutrition. In: Nieginski E, editor. Health Assessment in Nursing. 3rd Ed. Philadelphia, Lippincott: Williams and Wilkins; 2003. p. 165. [Google Scholar]
- 28.Noori N, Kopple JD, Kovesdy CP, et al. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clinical journal of the American Society of Nephrology : CJASN. 2010 Dec;5(12):2258–2268. doi: 10.2215/CJN.02080310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bross R, Chandramohan G, Kovesdy CP, et al. Comparing body composition assessment tests in long-term hemodialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010 May;55(5):885–896. doi: 10.1053/j.ajkd.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalantar-Zadeh K, Dunne E, Nixon K, et al. Near infra-red interactance for nutritional assessment of dialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association -European Renal Association. 1999 Jan;14(1):169–175. doi: 10.1093/ndt/14.1.169. [DOI] [PubMed] [Google Scholar]
- 31.Kalantar-Zadeh K, Kuwae N, Wu DY, et al. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. The American journal of clinical nutrition. 2006 Feb;83(2):202–210. doi: 10.1093/ajcn/83.2.202. [DOI] [PubMed] [Google Scholar]
- 32.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 33.Park SH, Carrero JJ, Lindholm B, Stenvinkel P. Adiponectin in chronic kidney disease has an opposite impact on protein-energy wasting and cardiovascular risk: two sides of the same coin. Clinical nephrology. 2009 Aug;72(2):87–96. doi: 10.5414/cnp72087. [DOI] [PubMed] [Google Scholar]
- 34.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nature medicine. 2001 Aug;7(8):947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 35.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature medicine. 2001 Aug;7(8):941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 36.Takemura Y, Ouchi N, Shibata R, et al. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. The Journal of clinical investigation. 2007 Feb;117(2):375–386. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochemical and biophysical research communications. 2004 Oct 15;323(2):630–635. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. The Journal of biological chemistry. 2003 Nov 7;278(45):45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi H, Ouchi N, Kihara S, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circulation research. 2004 Mar 5;94(4):e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouchi N, Kihara S, Arita Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001 Feb 27;103(8):1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 41.Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000 Sep 12;102(11):1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 42.Huang JW, Yen CJ, Chiang HW, Hung KY, Tsai TJ, Wu KD. Adiponectin in peritoneal dialysis patients: a comparison with hemodialysis patients and subjects with normal renal function. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2004 Jun;43(6):1047–1055. doi: 10.1053/j.ajkd.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 43.van der Vleuten GM, van Tits LJ, den Heijer M, Lemmers H, Stalenhoef AF, de Graaf J. Decreased adiponectin levels in familial combined hyperlipidemia patients contribute to the atherogenic lipid profile. Journal of lipid research. 2005 Nov;46(11):2398–2404. doi: 10.1194/jlr.M500212-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Yang WS, Lee WJ, Funahashi T, et al. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. The Journal of clinical endocrinology and metabolism. 2001 Aug;86(8):3815–3819. doi: 10.1210/jcem.86.8.7741. [DOI] [PubMed] [Google Scholar]
- 45.Qi Y, Takahashi N, Hileman SM, et al. Adiponectin acts in the brain to decrease body weight. Nature medicine. 2004 May;10(5):524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 46.Jia T, Carrero JJ, Lindholm B, Stenvinkel P. The complex role of adiponectin in chronic kidney disease. Biochimie. 2012 Oct;94(10):2150–2156. doi: 10.1016/j.biochi.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 47.Kaysen GA, Kotanko P, Zhu F, et al. Relationship between adiposity and cardiovascular risk factors in prevalent hemodialysis patients. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2009 Sep;19(5):357–364. doi: 10.1053/j.jrn.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smiechowska J, Utech A, Taffet G, Hayes T, Marcelli M, Garcia JM. Adipokines in patients with cancer anorexia and cachexia. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2010 Mar;58(3):554–559. doi: 10.231/JIM.0b013e3181cf91ca. [DOI] [PubMed] [Google Scholar]
- 49.Stenvinkel P, Marchlewska A, Pecoits-Filho R, et al. Adiponectin in renal disease: relationship to phenotype and genetic variation in the gene encoding adiponectin. Kidney international. 2004 Jan;65(1):274–281. doi: 10.1111/j.1523-1755.2004.00370.x. [DOI] [PubMed] [Google Scholar]
- 50.Tsao TS, Tomas E, Murrey HE, et al. Role of disulfide bonds in Acrp30/adiponectin structure signaling specificity. Different oligomers activate different signal transduction pathways. The Journal of biological chemistry. 2003 Dec 12;278(50):50810–50817. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- 51.Tsukamoto O, Fujita M, Kato M, et al. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. Journal of the American College of Cardiology. 2009 Jun 2;53(22):2070–2077. doi: 10.1016/j.jacc.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 52.Richards AA, Stephens T, Charlton HK, et al. Adiponectin multimerization is dependent on conserved lysines in the collagenous domain: evidence for regulation of multimerization by alterations in posttranslational modifications. Mol Endocrinol. 2006 Jul;20(7):1673–1687. doi: 10.1210/me.2005-0390. [DOI] [PubMed] [Google Scholar]
- 53.Shen YY, Charlesworth JA, Kelly JJ, Peake PW. The effect of renal transplantation on adiponectin and its isoforms and receptors. Metabolism: clinical and experimental. 2007 Sep;56(9):1201–1208. doi: 10.1016/j.metabol.2007.04.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.