Abstract
Cisplatin is one of the most widely-used drugs to treat cancers. However, its nephrotoxic and ototoxic side-effects remain major clinical limitations. Recent studies have improved our understanding of the molecular mechanisms of cisplatin-induced nephrotoxicity and ototoxicity. While cisplatin binding to DNA is the major cytotoxic mechanism in proliferating (cancer) cells, nephrotoxicity and ototoxicity appear to result from toxic levels of reactive oxygen species and protein dysregulation within various cellular compartments. In this review, we discuss molecular mechanisms of cisplatin-induced nephrotoxicity and ototoxicity. We also discuss potential clinical strategies to prevent nephrotoxicity and ototoxicity and their current limitations.
Keywords: Cisplatin, Ototoxicity, Nephrotoxicity, Intracellular mechanisms
1. Introduction
Cisplatin (cis-diamminedichloroplatinum [II]) is widely used for chemotherapy to efficaciously treat various cancers, with remission rates >90% in testicular cancers (Einhorn, 2002). However, cisplatin therapy is limited by cellular resistance and severe side-effects in normal tissues (Brock et al., 2012; McWhinney et al., 2009; Shen et al., 2012; Yao et al., 2007). These side-effects include nephrotoxicity, neurotoxicity, and ototoxicity. Cisplatin-induced nephrotoxicity primarily occurs in kidney proximal tubule epithelial cells, neurotoxicity in the upper and lower extremities, and ototoxicity within the mechanosensory outer hair cells of the cochlea.
More than 70% of pediatric patients receiving cisplatin experience renal dysfunction (Skinner et al., 1998). Renal dysfunction typically begins several days after standard doses of cisplatin treatment (50–120 mg/m2) and is revealed by an increase in serum creatinine and blood urea nitrogen levels (Akcay et al., 2010). This renal insufficiency can result in cation wasting, glucose and proteins in the urine (glucosuria and proteinuria). Hypomagnesemia is among the most common manifestations of cisplatin treatment (Schilsky and Anderson, 1979). Progressive and permanent kidney failure can happen with successive cisplatin treatment, in spite of preventive measures (Jakob et al., 1996). While saline hydration and mannitol diuresis can prevent nephrotoxicity (Cornelison and Reed, 1993), few methods to directly prevent ototoxicity have been implemented in clinical practice. One strategy, sodium thiosulfate therapy, has shown efficacy during clinical trials (Brock et al., 2012). Over 60% of cisplatin-treated pediatric patients develop irreversible hearing loss (Knight et al., 2005; Knight et al., 2007). Pediatric hearing loss delays educational attainment and psychosocial development (Bess et al., 1998; Knight et al., 2005). In adults, acquired deafness has an economic cost of ~$300,000 over the lifetime, and due to rehabilitation costs, lifetime costs for those with prelingual onset exceed $1 million, in year 2000 (Mohr et al., 2000). In this review, we discuss molecular mechanisms of cisplatin nephrotoxicity and ototoxicity. Although several reviews discuss cisplatin nephrotoxicity or ototoxicity alone, there are few that discuss both together. Since many studies for each toxicity now exist, it is possible to provide a more integrated view of cisplatin nephrotoxicity and ototoxicity. We also discuss the potential clinical strategies to prevent nephrotoxicity and ototoxicity and their current limitations.
2. Cisplatin and other platinum compounds
The discovery of cisplatin’s cytotoxic effect was observed serendipitously in 1960s. The biophysicist Barnett Rosenberg observed that an electrical field generated via platinum electrodes changed the normal short rod morphology of Escherichia coli into long filaments. After excluding an electric field effect, analysis of electrolytic products revealed that several platinum compounds, including cisplatin, could induce changes in bacterial morphology, and inhibited bacterial cell division (Rosenberg et al., 1965). When mice with tumors were treated with platinum compounds, potent antitumor effects were observed (Rosenberg et al., 1969). These antineoplastic properties of cisplatin rapidly led to clinical testing and FDA approval in 1970s.
Although recent reports suggest that cisplatin induces mitochondrial DNA damage, contributing to cytotoxicity in normal cells (Marullo et al., 2013; Wisnovsky et al., 2013), the predominant antineoplastic action is mediated through the binding of cisplatin to nuclear DNA. As cytoplasmic concentrations of chloride are very low, either or both chlorides attached to platinum become displaced by water (aqua ligands), a process termed aquation. This generates a positively charged electrophile that is highly reactive with nucleophilic sites. As platinum releases water, it covalently binds to DNA at the N7 positions of purine bases, resulting in a DNA-adduct (Jamieson and Lippard, 1999). The formation of DNA-adducts activates several signaling mechanisms including DNA repair, cell cycle arrest, and apoptosis. DNA repair is induced through the nucleotide excision repair (NER) system, where excision repair cross-complementation group 1 (ERCC1) and xeroderma pigmentosum (XP) group proteins work together to remove damaged DNA and allow DNA polymerases and ligases fill the gap with normal strand DNA (Rosell et al., 2003). Cell cycle arrest at G1 phase can also occur via up-regulation of tumor suppressor protein p53 and cyclin-dependent kinase inhibitor p21 (Zamble et al., 1998). Apoptosis is dependent on p53, which activates apoptotic proteins such as bax (Boersma et al., 1997).
Since the 1970s, thousands of platinum analogues (congeners) have been synthesized and tested to identify other antineoplastic compounds with reduced nephrotoxicity and ototoxicity while retaining tumoritoxicity. One of the most successful compounds to meet these criteria was carboplatin (Fig. 1A) (Harrap, 1985). Carboplatin is significantly less nephrotoxic but its antineoplastic efficacy is also lower compared to cisplatin (Knox et al., 1986). Carboplatin has a six membered ring as a leaving group, and is more stable than the labile chlorides. Despite its lower anticancer activity, clinical trials have demonstrated that subjects with ovarian cancer treated with carboplatin had similar survival rates to those treated with cisplatin (Aabo et al., 1998).
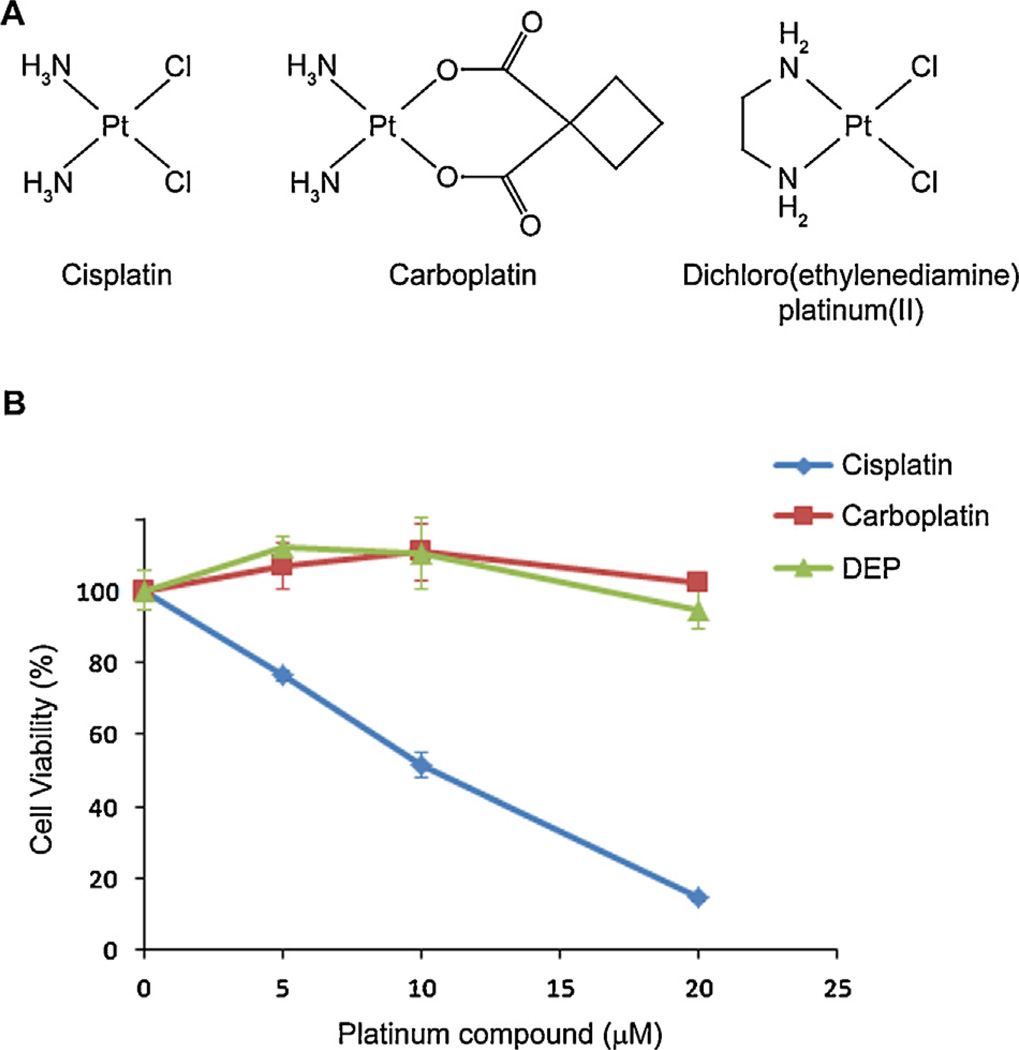
Fig. 1.
(A) Platinum compounds. (B) In vitro cisplatin nephrotoxicity assay results. Mouse kidney proximal tubule KPT11 cells were treated with each platinum compound (all purchased from Sigma) for 2 days before cellular viability was measured by MTT assay.
Our in vitro experiments with a kidney proximal tubule cell line revealed that 20 µM cisplatin killed most cells, while carboplatin, at the same concentration, induced negligible cytotoxicity, likely due to slower rates of aquation compared to cisplatin (Fig. 1B), as described previously (Knox et al., 1986). Interestingly, dichloro (ethylenediamine)platinum(II) (DEP), similar to cisplatin but with the two amino groups replaced by ethylenediamine, also displayed negligible cytotoxicity at this concentration. Although DEP has been used to study the difference between cisplatin-sensitive and -resistant carcinoma cells (Jekunen et al., 1994), our data raises the possibility that amino groups of cisplatin are also important for inducing toxicity.
Acquired and intrinsic resistance of some common cancers to cisplatin has also been a major problem, and other platinum compounds have been developed to overcome cisplatin resistance. For example, oxaliplatin, a platinum compound discovered in 1976, has been used in combination with paclitaxel, a mitotic inhibitor, to effectively kill tumors resistant to cisplatin (Faivre et al., 1999).
2.1. Reactive oxygen species
Cisplatin’s tumoritoxic efficacy is principally due to DNA damage in proliferating cells that leads to apoptosis. However, kidney proximal tubule cells and cochlear cells have low rates of cell proliferation (or none in the case of post-natal cochlear hair cells), yet these cells are especially prone to cisplatin-induced cytotoxicity. Cisplatin has been shown to break double-stranded DNA in strial marginal cells and cochlear hair cells that are transcriptionally active, disrupting cellular physiology and inducing cytotoxicity (Rybak et al., 2007; Slattery et al., 2014). Nonetheless, cisplatin-induced nephrotoxicity and ototoxicity can also occur via the toxic generation of reactive oxygen species (ROS) and binding to various cytoplasmic molecules, including the anti-oxidant glutathione (Fuertes et al., 2003; Karasawa et al., 2013). Carboplatin is less toxic to kidney and cochlea compared to cisplatin, likely due to slower rates of aquation and reduced generation of ROS (Hannemann and Baumann, 1990; Hannemann et al., 1991; Knox et al., 1986).
The aquated forms of cisplatin are highly reactive, particularly with thiol-containing molecules including glutathione (Fuertes et al., 2003). Cisplatin also inhibits antioxidant enzymes including glutathione S-transferase, glutathione peroxidase, and superoxide dismutase (Khynriam and Prasad, 2002; Sadzuka et al., 1992), shifting the cellular redox status, leading to toxic levels of ROS within the cell. Cisplatin conjugation with glutathione can also lead to mitochondrial oxidative stress, mitochondrial lysis, and dysfunction through lipid peroxidation (Kruidering et al., 1997). Cisplatin-dependent increases in ROS levels may also be due to direct drug binding to cytochrome P450 in microsomes where cytochrome P450 is an important source of catalytic iron for ROS generation (Liu and Baliga, 2003). Additional evidence implicating toxic levels of ROS as a major cause of cytotoxicity originate from studies where anti-oxidants, like d-methionine, N-acetylcysteine, sodium thiosulphate or dimethylthiourea, can prevent platinum-induced nephrotoxicity and ototoxicity (Campbell et al., 1996; Dickey et al., 2005; Ramesh and Reeves, 2005).
Recent reports suggest that, in addition to ROS, nitrosative stress plays a significant role in cisplatin-induced nephrotoxicity and ototoxicity. For example, cisplatin increases renal inducible nitric oxide synthase (iNOS) protein expression and nitrotyrosine formation (Mukhopadhyay et al., 2010). iNOS induces reactive nitric species (RNS) formation, which in turn induces nitrotyrosine formation. Like ROS, RNS acts on various targets in the cell, causing cytotoxicity, although its mechanisms are less clear than ROS. In the cochlea, it has been reported that cisplatin nitrates LIM domain only 4 (Lmo4), a transcriptional regulator controlling cell survival and cell death (Jamesdaniel et al., 2012).
Although ROS generation induces signal transduction cascades leading to apoptosis or necrosis, how ROS induces these signaling pathways remains unclear. One mechanism involves lipid peroxidation in the lipid bilayer of the cell membrane to generate highly toxic aldehyde 4-hydroxynonenal (4-HNE). Increasing levels of 4-HNE induces increased Ca2+ influx, which causes apoptosis in the outer hair cells of the cochlea (Clerici et al., 1995; Ikeda et al., 1993). Most of the cisplatin nephrotoxicity and ototoxicity mechanisms that we discuss below involve ROS.
2.2. Mechanisms of cisplatin nephrotoxicity
A major consideration for cisplatin nephrotoxicity is its uptake into kidney cells. The kidney takes up cisplatin at higher concentrations compared to other tissues, particularly in the kidney proximal tubule. This is primarily due to membrane transporters such as the high affinity copper transporter 1 (CTR1) and the organic cation transporter (OCT2) localized to the basolater al membrane of proximal tubule cells (Filipski et al., 2009; Pabla et al., 2009).
There is an extrinsic pathway for cisplatin-induced apoptosis activated via death receptors, like those for tumor necrosis factor (TNF) or Fas, in the plasma membrane (Fig. 2). Kidney epithelial cells deficient for TNFR1 or Fas receptors are resistant to cisplatin treatment (Tsuruya et al., 2003). Ligand binding of TNF-α to TNF receptors activates caspase 8, which in turn triggers caspase 3, leading to apoptosis (Juo et al., 1998; Srinivasula et al., 1996). TNF-α also stimulates an inflammatory response which plays a critical role in cisplatin-induced nephrotoxicity (Zhang et al., 2007). Cisplatin treatment increases expression of inflammatory cytokines and chemokines in the kidney, including interleukin-1β (IL-1β), IL-18, CX3CL1 and IL3 in mice treated with cisplatin (Faubel et al., 2007; Lu et al., 2008). Activation of caspase 1 also increases levels of IL-1β and IL-18, and deletion of caspase 1 gene protects mice against cisplatin-induced apoptosis and acute kidney injury (Faubel et al., 2004). Cisplatin-induced apoptosis can also be triggered by endoplasmic reticulum (ER) stress following cisplatin activation of caspase 12 localized at the cytosolic membrane of ER (Liu and Baliga, 2005). ER stress activates a Ca2+-independent phospholipase A2 and inhibition of this enzyme ameliorates cisplatin-induced apoptosis (Cummings et al., 2004).
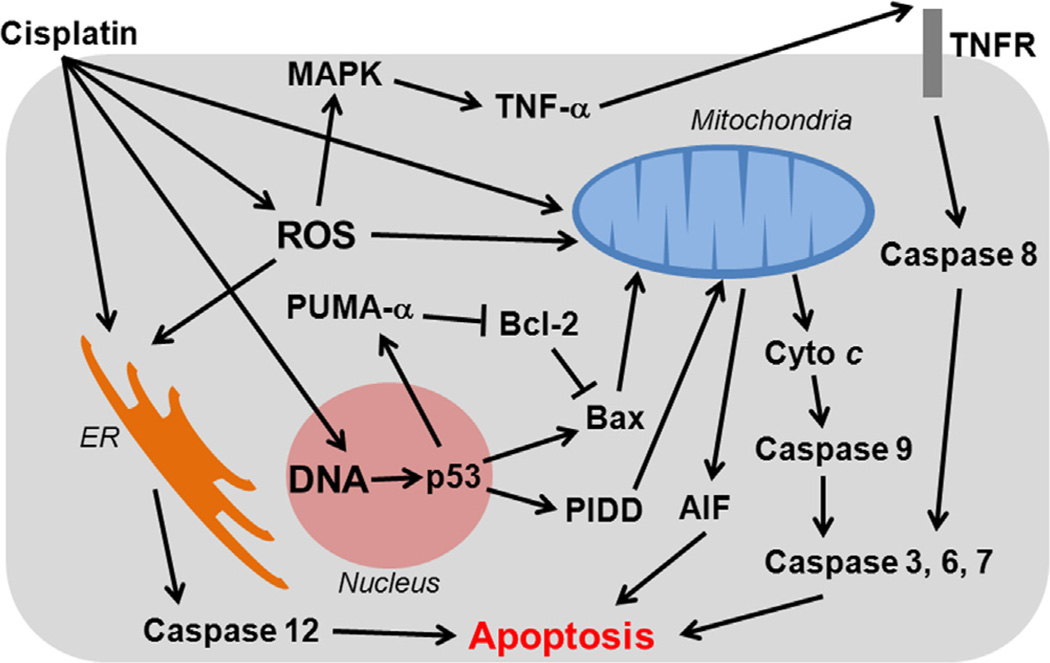
Fig. 2.
Apoptosis signaling pathways in cisplatin nephrotoxicity. In the extrinsic pathway, binding of TNF-α to TNF receptors activates caspase 8, which in turn triggers caspase 3, leading to apoptosis. ER stress induced by cisplatin activates caspase 12 also leads to apoptosis. DNA damage triggers p53, which in turn activates PUMA-α and PIDD. PUMA-α is a pro-apoptotic Bcl-2 family protein which inhibits anti-apoptotic Bcl-2. PIDD activation leads to release of apoptosis-inducing factor (AIF) from mitochondria. This intrinsic pathway leads to release of mitochondrial cytochrome c that sequentially activates caspase 9, 3, 6 and 7, resulting in apoptosis.
Although the tumor suppressor gene p53 does not appear to play a major role, pharmacologic inhibition by pifithrin-α, or genetic inhibition of p53 in dominant-negative mutants, suppressed cisplatin-induced apoptosis in kidney in vitro (Cummings and Schnellmann, 2002; Jiang et al., 2004). The p53-dependency of cisplatin-induced nephrotoxicity has been confirmed using the same pharmacologic and genetic strategies in vivo (Molitoris et al., 2009; Wei et al., 2007). More recent studies identified two transcription factors, p53 upregulated modulator of apoptosis-α (PUMA-α) and p53-induced protein with a death domain (PIDD), as major downstream targets (Jiang et al., 2006; Seth et al., 2005). PUMA-α is a proapoptotic Bcl-2 family protein which can inhibit antiapoptotic Bcl-2 and Bcl-xL (Jiang et al., 2006). PIDD activates caspase 2, inducing release of apoptosis inducing factor (AIF) from mitochondria (Seth et al., 2005). This intrinsic pathway leads to release of mitochondrial cytochrome c that sequentially activates caspase 9, 3, 6 and 7, triggering apoptosis (Lee et al., 2001; Park et al., 2002; Yang et al., 2008).
Cell cycle proteins such as cyclin-dependent kinase (CDK) family proteins and E2F1 are also implicated in cisplatin-induced nephrotoxicity. CDK2 is a cell cycle protein that is required for cycle progression during late G1 through S phases. Expression of a CDK2 inhibitor, p21, is cytoprotective against nephrotoxicity (Price et al., 2004; Yu et al., 2005). However, pharmacological inhibitors of the cell cycle itself are insufficient to protect cells from cisplatin-induced nephrotoxicity (Price et al., 2004).
Cisplatin also activates mitogen-activated protein kinases (MAPKs) including extracellular signal-regulated kinase (ERK), p38 and c-Jun N-terminal protein kinase (JNK). How cisplatin activates MAPKs is unclear, yet inhibition of MAPKs ameliorates cisplatin-induced nephrotoxicity. ERK is activated by epidermal growth factor receptor (EGFR/Src) signaling (Henson and Gibson, 2006). Cisplatin also activates EGFR/Src triggering ERK and caspase-3 leading to apoptosis and cisplatin-induced nephrotoxicity (Arany et al., 2004). p38 and JNK are activated by a variety of cellular stress pathways including oxidative stress and inflammation. Activation of p38 leads to transcriptional induction of TNF-α, contributing to nephrotoxicity (Mishima et al., 2006; Ramesh and Reeves, 2005). It is not clear how JNK plays a role, but a recent report suggests that it acts on both apoptosis and inflammatory responses (Francescato et al., 2007).
2.3. Mechanisms of cisplatin ototoxicity
Many studies report that cisplatin-induced ototoxicity is predominantly sensory hair cell death within the cochlea, or inner ear (Fig. 3A), of the peripheral auditory sensory system (Rybak et al., 2007). However, cisplatin-induced auditory dysfunction is composed of multi-level effects, with damage to strial intermediate and marginal cells in the cochlear lateral wall causing loss of the endolymphatic potential, or breakdown of the blood-labyrinth barrier (Laurell et al., 2007; Laurell and Engstrom, 1989). Multiple low-dose administration (up to 2 mg/kg) in guinea pigs and rats also causes outer hair cell loss and a decrease in auditory sensitivity without loss of the endolymphatic potential or blood-labyrinth barrier integrity (Cardinaal et al., 2000; Laurell and Engstrom, 1989; Laurell et al., 2000).
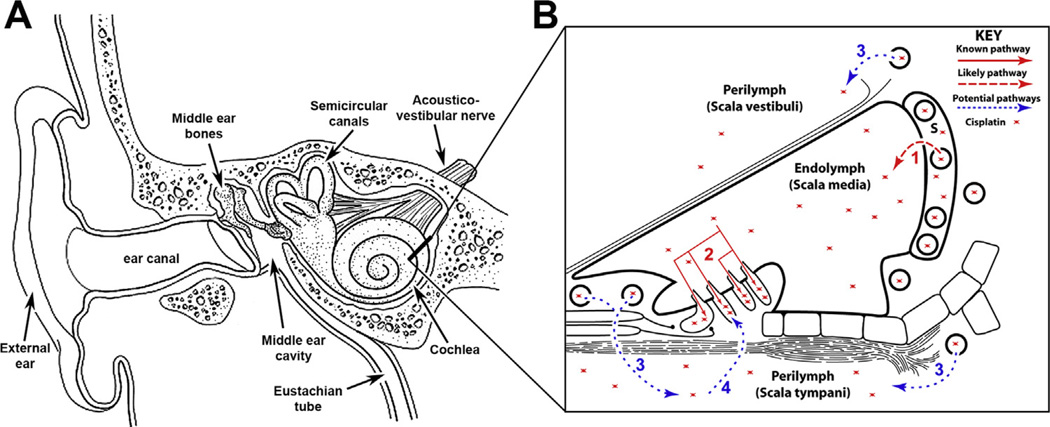
Fig. 3.
(A) A cross-section of the right-side of the human head, showing the peripheral auditory (external and middle ear, as well as the cochlea [inner ear]) system, and the semicircular canals of the peripheral vestibular system (adapted from an image by British Columbia Children’s Hospital, image is not to scale). (B) A cross-section of the cochlear duct shown in (A) as a thick line. The cochlear duct is divided into three compartments, two of which contain perilymph, and an inner compartment, the scala media, containing endolymph. Systemic cisplatin could cross the BLB via a trans-strial trafficking route from strial capillaries, across the stria vascularis in endolymph (1) prior to entering hair cells across their apical membranes (2). Alternatively, cisplatin could traverse the BLB into perilymph and pass through the basilar membrane into extracellular fluids within the organ of Corti and enter hair cells across their basolateral membranes. S, stria vascularis; adapted from Brock et al. (2012), image is not to scale.
Following systemic injection, cisplatin is trafficked into the inner ear fluids (Fig. 3B) and taken up by a variety of cochlear cells. Although it is unclear how transporters like OCT2 and CTR1 facilitate cisplatin trafficking through the cochlea, competitive inhibition of CTR1 and genetic loss of function in the OCT2 gene offer protection from cisplatin-induced ototoxicity (Ciarimboli et al., 2010; Hellberg et al., 2015; Lanvers-Kaminsky et al., 2015; More et al., 2010). Unlike most cells, sensory hair cells have mechano-electrical transduction (MET) channels that are uniquely gated by tip links between individual stereocilia in their apically-located hair bundle (Assad et al., 1991; Hudspeth, 1986; Hudspeth and Corey, 1977; Pickles et al., 1984). Data from electrophysiological and time-lapse imaging studies, as well as drug uptake studies in hair cells with dysfunctional MET channels strongly suggest that the ototoxic aminoglycosides (molecular diameter ~0.81 nm; molecular mass ~445–477 for gentamicin) can permeate through the MET channel pore of at least 1.25 nm (Alharazneh et al., 2011; Farris et al., 2004; Marcotti et al., 2005; Vu et al., 2013). A recent study demonstrated that functional MET channels are required for cytosolic uptake of fluorescently-tagged cisplatin, and for cisplatin-induced hair cell death (Thomas et al., 2013). This raises the possibility that cisplatin, with a lower molecular mass (300.05) and narrower molecular diameter (~0.48 nm) than aminoglycosides, can also permeate hair cell MET channels, although non-MET channel entry routes may also be involved (Hilder and Hill, 2007; Thomas et al., 2013).
The unique structure of the cochlea makes it difficult to remove cisplatin from the cochlea, which is surrounded by the blood-labyrinth barrier (BLB; Fig. 3B), blocking the drug from rapidly clearing the cochlea (Jacobs et al., 2005; van Ruijven et al., 2005). Platinated DNA-adducts have been observed in marginal cells, organ of Corti and the spiral ligament (van Ruijven et al., 2005). The rapid appearance of DNA-adducts in marginal cells is suggestive of a trans-strial trafficking pathway into endolymph and thereby hair cells (Fig. 3B), as demonstrated for aminoglycosides (Li and Steyger, 2011).
Despite recent advances in understanding cisplatin-induced ototoxicity, many gaps remain to understand the mechanisms involved, due largely to limited tissue availability. For example, in the avian inner ear, cisplatin treatment causes a dose-dependent reduction in the proliferation of supporting cells, which severely impairs the ability to regenerate hair cells after ototoxic injury (Slattery and Warchol, 2010). In mammals, cochlear hair cells do not spontaneously regenerate following hair cell death, unlike kidney proximal tubular cells which can regenerate (Berger and Moeller, 2014). These observations also show that cisplatin-induced cytotoxicity occurs in cells that are not actively proliferating. Thus, cisplatin-induced cytotoxicity may be due to cisplatin-induced dysregulation of active DNA transcription error, as well as or non-DNA damage mechanisms. Nonetheless, non-DNA damage mechanisms described for cisplatin-induced nephrotoxicity likely also occur during ototoxicity. Several extrinsic and intrinsic mechanisms described for nephrotoxicity have been confirmed in cochlear cells (Devarajan et al., 2002). Below, we discuss mechanisms identified using cochlear cells.
3. Hair cells
There is growing evidence that ROS production plays an important role in cisplatin-induced ototoxicity (Mukherjea et al., 2011b; Rybak et al., 2009; Rybak and Ramkumar, 2007). In cochlear cells, NADPH oxidase 3 (NOX3), an isoform of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, is a major source of ROS (Fig. 4). NOX3 is highly expressed in the organ of Corti and spiral ganglion (Banfi et al., 2004; Mukherjea et al., 2006). Cisplatin activates NOX3, although the mode of activation remains unclear. Later studies confirmed that NOX1 and NOX4 also generate ROS (Kim et al., 2010). Most interestingly, ROS generation induced by cisplatin in cochlear hair cells is dependent on activation of the transient receptor potential vanilloid 1 channel (TRPV1) (Mukherjea et al., 2008), suggesting that Ca2+ influx is a key factor in generating ROS (Caterina et al., 1997). This was confirmed by siRNA knock down of TRPV1 expression that suppressed NOX3 expression, TRPV1 activation and Ca2+ influx (Mukherjea et al., 2008). The sub-cellular localization of these TRPV1 channels remains to be determined.
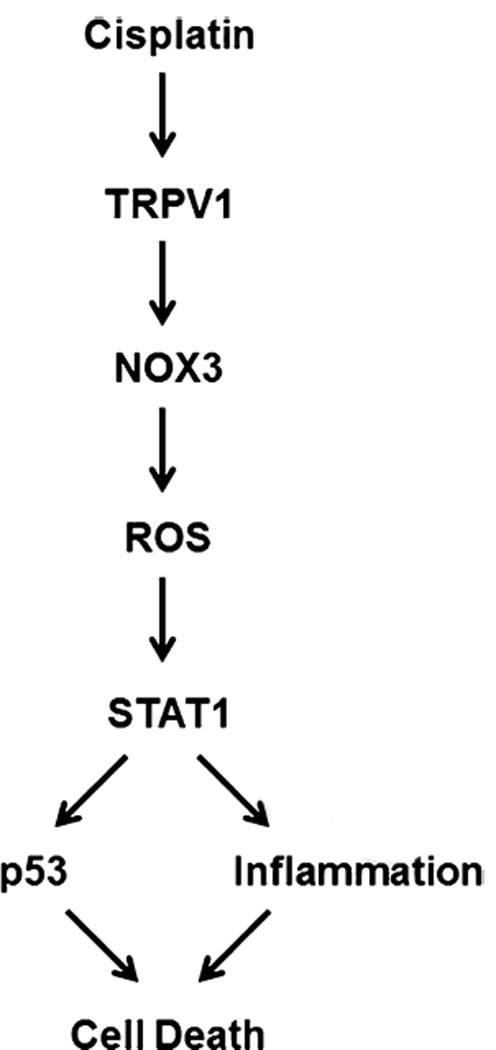
Fig. 4.
A schematic pathway for one mechanisms of cisplatin-induced ototoxicity involves TRPV1 activation by cisplatin leads to NOX3-dependent generation of ROS to trigger STAT1 activation. STAT1 promotes an intracellular inflammatory response and p53 activation, resulting in cell death, adapted from Mukherjea et al. (2011a, 2008).
The well-recognized tumor suppressor, p53, is involved in cisplatin ototoxicity, which is likely triggered by DNA damage (Devarajan et al., 2002 Zhang et al., 2003). The transcription factor, signal transducer and activator of transcription 1 (STAT1) can also mediate cisplatin-induced hair cell death (Schmitt et al., 2009). Recent evidence suggests that STAT1 siRNA abolished cisplatin-induced p53 activation (Kaur et al., 2011). Finally, the TRPV1 and NOX3 signaling pathway has been connected to STAT1, leading to inflammation and cisplatin-induced hair cell death, resulting in hearing loss (Mukherjea et al., 2011a).
4. Stria vascularis and spiral ganglia
Cisplatin also has effects on other cochlear structures, including the stria vascularis and spiral ganglion neurons, inducing independent manifestations of ototoxicity. Cisplatin-treated mice showed increased expression of NF-κB and inducible nitric oxide synthase (iNOS) in stria vascularis and spiral ligament (Watanabe et al., 2002). Since NF-κB is essential for survival of various cell types, including auditory hair cells, it is expected that this protein expression is induced in response to cisplatin treatment to protect cochlear cells. Degeneration of stria vascularis appears to be triggered by iNOS activity, which generates nitric oxide (NO) (Watanabe et al., 2002).
In spiral ganglia, an increase in high mobility group (HMG1) protein was observed following cisplatin treatment (Li et al., 2006). Since HMG1 binding to DNA is associated with anti-cancer actions of cisplatin, and increased HMG1 expression sensitizes target cells to cisplatin cytotoxicity, it is likely that the increase of the protein in spiral ganglion cells by cisplatin treatment leads to DNA damage-dependent apoptosis in these non-proliferating cells. Additional confirmation that cisplatin induces DNA damage-dependent apoptosis in spiral ganglion cells comes from a study on NER factors, in which cytoplasmic to nuclear translocation of NER proteins was detected in spiral ganglia from rats treated with cisplatin (Guthrie et al., 2008).
4. 1. Cisplatin-binding proteins
To better understand how cisplatin induces DNA-independent cytotoxicity, we hypothesized that cisplatin also binds to cytoplasmic proteins, disrupting their normal physiological function, leading to cytotoxicity. To identify such proteins, we generated platinum compounds that could be conjugated to agarose beads, and performed pull-down assays (Karasawa et al., 2013). By mass spectrometric analysis, we identified several proteins that bind to cisplatin at the amino groups with high affinities. Interestingly, many of them are known to be over-expressed in cancer, suggestive of a role in sensitivity or resistance to cisplatin. Of particular interest was heat shock protein 90 (HSP90) because of its low protein expression in kidney proximal tubule cells. Thus, we predicted HSP90 to be cytoprotective against cisplatin and that kidney proximal tubule susceptibility to cisplatin-induced toxicity was due to low HSP90 expression. However, proximal tubule-derived cells engineered to express higher levels of HSP90 were more sensitive to cisplatin treatment than cells with low HSP90 expression. However, cells with high HSP90 expression had higher proliferation rates, suggesting that accelerated cell division conferred greater susceptibility to cisplatin that could not be attenuated by HSP90 (Karasawa et al., 2013).
Physiologically, HSP90 functions to maintain cellular homeostasis, by facilitating protein folding and degradation of misfolded proteins (Taipale et al., 2010). Cancer cells exploit the HSP90 chaperone machinery to protect mutated and over-expressed oncoproteins from degradation (Trepel et al., 2010). Therefore, a number of inhibitory drugs for HSP90 have been developed and tested (Lu et al., 2012). Although the significance of cisplatin interaction with HSP90 in nephrotoxicity or ototoxicity remains unclear, cisplatin appears to block HSP90 interactions with its binding targets, including inositol hexakisphosphate kinase-2 (IP6K2), an enzyme that mediates apoptosis through inositol pyrophosphate generation, and this disruption is likely one of cytotoxic mechanism of cisplatin (Chakraborty et al., 2008).
5. Risk factors that enhance cisplatin toxicity
Clinical data suggests that a poor performance status and the regular use of non-steroidal anti-inflammatory drugs are associated with cisplatin nephrotoxicity (Kidera et al., 2014). For ototoxicity, high cumulative doses of cisplatin and history of noise exposure have been established as significant risk factors (Bokemeyer et al., 1998). A study of pediatric oncology patients have found that, other than high cumulative doses of cisplatin, males are at significantly greater risk to develop hearing loss compared to females, and age at cancer diagnosis is inversely related to severity of ototoxicity (Yancey et al., 2012). Interestingly, another study suggests that females are at greater risk for developing nephrotoxicity as well as increasing age (de Jongh et al., 2003). However, patient group differences need to be considered because the latter study was conducted on patients of median age 54 years. Therefore, risk factors on gender and age could be the same between ototoxicity and nephrotoxicity, if similar patient groups were studied.
Another risk factor that predispose individuals to cisplatin-induced toxicity includes polymorphisms in genes for DNA adduct repair enzymes like ERCC2 and XPC (Caronia et al., 2009; Suk et al., 2005) and in membrane pumps, drug efflux or detoxifying genes (ACYP2, ABCC3, COMT, TPMT) that are also associated with hearing loss (Carleton et al., 2009; Ross et al., 2009; Xu et al., 2015). Systemic inflammation can exacerbate cochlear uptake of ototoxic drugs and potentiate cisplatin-induced ototoxicity (Koo et al., 2015; Oh et al., 2011). Thus, radiation therapy that kills commensal bacteria could inadvertently induce systemic inflammation and potentiate cisplatin-induced ototoxicity during combination anti-neoplastic therapies.
5.1. Preventing cisplatin nephrotoxicity and ototoxicity
While some approaches are specific to prevent nephrotoxicity such as pharmacological inhibition of the transporter OCT2 (Sprowl et al., 2013), there are also some common approaches to protect the cochlea and kidney from cisplatin toxicity (Ciarimboli et al., 2010). One critical consideration for these approaches is prevention of cisplatin toxicity without compromising its antineoplastic efficacy.
In preclinical studies, antioxidants have been shown to reduce cisplatin nephrotoxicity and ototoxicity. For example, several thiol antioxidants like amifostine, N-acetylcysteine and superoxide dismutase are electrophilic and act as free radical scavengers. Amifostine has been approved by FDA for use in patients with advanced ovarian cancer who undergo repeated cisplatin treatment (Hensley et al., 2008). However, certain thiols like sodium thiosulfate and mesna form a complex with cisplatin which lead to drug inactivation and excretion from the kidney, reducing antineoplastic efficacy (Boven et al., 2002). This can be overcome by pretreatment with a drug like procainamide that also forms a complex with cisplatin to maintain antitumor activity, while reducing renal excretion and nephrotoxicity (Viale et al., 2000). For these thiol compounds, drug application through round window, one of the two openings into the cochlea, is possible, but for clinical consideration, this could be invasive and difficult (Li et al., 2001). Another approach to avoid protective agents interfering with the tumoricidal effect of cisplatin is delayed administration. For example, administration of sodium thiosulfate 6 h after cisplatin did not compromise antineuroblastoma activity of cisplatin (Harned et al., 2008).
Inhibition of cell death pathways also reduces nephrotoxicity and ototoxicity in vitro and in vivo. For this method, local administration to the cochlea is required to avoid conflicts with chemotherapy, because the antineoplastic action of cisplatin uses the same cell death pathways. As a method of such administration, intracochlear perfusion of caspase 3 and caspase 9 inhibitors reduced apoptosis of hair cells in guinea pigs treated with cisplatin (Wang et al., 2004). A gene therapy approach using X-linked inhibitor of apoptosis (XIAP) has also been tested using adeno-associated virus (AAV), and cisplatin-induced auditory threshold shifts and hair cell loss were reduced by the AAV encoding XIAP (Cooper et al., 2006). However, these methods are currently too invasive to use in the clinical setting, and alternative approaches for local application are needed.
A third approach to prevent or reduce cisplatin nephrotoxicity and ototoxicity is inhibition of inflammation. For example, inhibitors of TNF-α ameliorated cisplatin-induced renal dysfunction in mice and apoptosis of cochlear cells (Ramesh and Reeves, 2002; So et al., 2007). The effects of TNF-α inhibitors on cancer cells are unclear. Since TNF-α acts as tumor promoter in some cancer cells, it is likely that inhibitors of TNF-α can enhance cancer remission by cisplatin (Popivanova et al., 2008). Anti-inflammatory cytokines, like IL-10, also inhibit cisplatin-induced acute kidney injury (Deng et al., 2001).
More recently, a strategy targeted for NOX3 has emerged as a method to reduce ototoxicity. In mice treated with siRNA for NOX3 as trans-tympanic administration preserved hearing thresholds and inner ear hair cells, preventing cisplatin ototoxicity (Mukherjea et al., 2010). Although it is unclear if delivering siRNA into the cochlea is feasible in the clinical setting, this could lead to a new direction in preventive clinical strategy against cisplatin.
A new approach called sound preconditioning has been developed to inhibit cisplatin-induced hearing loss (Roy et al., 20 13). Cochlear expression of heat shock proteins (HSPs) can be induced by sound stimuli and are protective against cisplatin ototoxicity in mouse models of cisplatin-induced hearing loss. Since this method also protects against aminoglycoside ototoxicity, sound preconditioning is a promising strategy to prevent hearing loss in patients receiving these drugs.
6. Conclusions
Although more studies are needed to understand molecular mechanisms of cisplatin ototoxicity, both nephrotoxicity and ototoxicity induced by cisplatin likely use similar intracellular mechanisms. On the other hand, clinical strategies to prevent nephrotoxicity and ototoxicity will likely be quite different because cellular uptake mechanisms into kidney and cochlear sensory hair cells may be quite different. Although OCT2 and CTR1 transporters are likely involved in cisplatin uptake in both kidney and cochlear non-sensory cells, cytosolic uptake of cisplatin by sensory hair cells is also facilitated by functional mechanotransduction channels. Additionally, local administration of preventive drug or other agents is possible for the cochlea, but such approaches are difficult for the kidney. Improved understanding of the molecular mechanisms involved in cisplatin nephrotoxicity and ototoxicity will provide more insight to open new clinical strategies to prevent nephrotoxicity and ototoxicity.
HIGHLIGHTS.
Differing mechanisms of platinum-induced cytotoxicity are introduced.
Known pathways of cisplatin-induced nephrotoxicity are discussed.
Known mechanisms and cochlear sites of cisplatin-induced ototoxicity are reviewed.
Examples of risk factors enhancing cisplatin-induced ototoxicity are highlighted.
Potential strategies to reduce cisplatin-induced ototoxicity are introduced.
Acknowledgements
This work was supported by National Institute on Deafness and Other Communication Disorders at the National Institutes of Health (R21 DC010231; R01 DC012588) and Medical Research Foundation of Oregon grants to P.S.S. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Aabo K, Adams M, Adnitt P, Alberts DS, Athanazziou A, Barley V, Bell DR, Bianchi U, Bolis G, Brady MF, Brodovsky HS, Bruckner H, Buyse M, Canetta R, Chylak V, Cohen CJ, Colombo N, Conte PF, Crowther D, Edmonson JH, Gennatas C, Gilbey E, Gore M, Guthrie D, Yeap BY, et al. Chemotherapy in advanced ovarian cancer: four systematic meta-analyses of individual patient data from 37 randomized trials. Advanced Ovarian Cancer Trialists’ Group. Br. J. Cancer. 1998;78:1479–1487. doi: 10.1038/bjc.1998.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akcay A, Turkmen K, Lee D, Edelstein CL. Update on the diagnosis and management of acute kidney injury. Int. J. Nephrol. Renovasc. Dis. 2010;3:129–140. doi: 10.2147/IJNRD.S8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharazneh A, Luk L, Huth M, Monfared A, Steyger PS, Cheng AG, Ricci AJ. Functional hair cell mechanotransducer channels are required for aminoglycoside ototoxicity. PLoS One. 2011;6:e22347. doi: 10.1371/journal.pone.0022347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany I, Megyesi JK, Kaneto H, Price PM, Safirstein RL. Cisplatin-induced cell death is EGFR/src/ERK signaling dependent in mouse proximal tubule cells. Am. J. Physiol. Renal Physiol. 2004;287:F543–F549. doi: 10.1152/ajprenal.00112.2004. [DOI] [PubMed] [Google Scholar]
- Assad JA, Shepherd GM, Corey DP. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron. 1991;7:985–994. doi: 10.1016/0896-6273(91)90343-x. [DOI] [PubMed] [Google Scholar]
- Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- Berger K, Moeller MJ. Mechanisms of epithelial repair and regeneration after acute kidney injury. Semin. Nephrol. 2014;34:394–403. doi: 10.1016/j.semnephrol.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Bess FH, Dodd-Murphy J, Parker RA. Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear Hear. 1998;19:339–354. doi: 10.1097/00003446-199810000-00001. [DOI] [PubMed] [Google Scholar]
- Boersma AW, Nooter K, Burger H, Kortland CJ, Stoter G. Bax upregulation is an early event in cisplatin-induced apoptosis in human testicular germ-cell tumor cell line NT2, as quantitated by flow cytometry. Cytometry. 1997;27:275–282. doi: 10.1002/(sici)1097-0320(19970301)27:3<275::aid-cyto10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Berger CC, Hartmann JT, Kollmannsberger C, Schmoll HJ, Kuczyk MA, Kanz L. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br. J. Cancer. 1998;77:1355–1362. doi: 10.1038/bjc.1998.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boven E, Verschraagen M, Hulscher TM, Erkelens CA, Hausheer FH, Pinedo HM, van der Vijgh WJ. BNP7787, a novel protector against platinum-related toxicities, does not affect the efficacy of cisplatin or carboplatin in human tumour xenografts. Eur. J. Cancer. 2002;38:1148–1156. doi: 10.1016/s0959-8049(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Brock PR, Knight KR, Freyer DR, Campbell KC, Steyger PS, Blakley BW, Rassekh SR, Chang KW, Fligor BJ, Rajput K, Sullivan M, Neuwelt EA. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J. Clin. Oncol. 2012;30:2408–2417. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KC, Rybak LP, Meech RP, Hughes L. D-methionine provides excellent protection from cisplatin ototoxicity in the rat. Hear Res. 1996;102:90–98. doi: 10.1016/s0378-5955(96)00152-9. [DOI] [PubMed] [Google Scholar]
- Cardinaal RM, de Groot JC, Huizing EH, Veldman JE, Smoorenburg GF. Histological effects of co-administration of an ACTH((4–9)) analogue, ORG 2766, on cisplatin ototoxicity in the albino guinea pig. Hear Res. 2000;144:157–167. doi: 10.1016/s0378-5955(00)00061-7. [DOI] [PubMed] [Google Scholar]
- Carleton B, Poole R, Smith M, Leeder J, Ghannadan R, Ross C, Phillips M, Hayden M. Adverse drug reaction active surveillance: developing a national network in Canada’s children’s hospitals. Pharmacoepidemiol. Drug Saf. 2009;18:713–721. doi: 10.1002/pds.1772. [DOI] [PubMed] [Google Scholar]
- Caronia D, Patino-Garcia A, Milne RL, Zalacain-Diez M, Pita G, Alonso MR, Moreno LT, Sierrasesumaga-Ariznabarreta L, Benitez J, Gonzalez-Neira A. Common variations in ERCC2 are associated with response to cisplatin chemotherapy and clinical outcome in osteosarcoma patients. Pharmacogenomics J. 2009;9:347–353. doi: 10.1038/tpj.2009.19. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chakraborty A, Koldobskiy MA, Sixt KM, Juluri KR, Mustafa AK, Snowman AM, van Rossum DB, Patterson RL, Snyder SH. HSP90 regulates cell survival via inositol hexakisphosphate kinase-2. Proc. Natl. Acad. Sci. U.S.A. 2008;105:1134–1139. doi: 10.1073/pnas.0711168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarimboli G, Deuster D, Knief A, Sperling M, Holtkamp M, Edemir B, Pavenstadt H, Lanvers-Kaminsky C, am Zehnhoff-Dinnesen A, Schinkel AH, Koepsell H, Jurgens H, Schlatter E. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am. J. Pathol. 2010;176:1169–1180. doi: 10.2353/ajpath.2010.090610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici WJ, DiMartino DL, Prasad MR. Direct effects of reactive oxygen species on cochlear outer hair cell shape in vitro. Hear Res. 1995;84:30–40. doi: 10.1016/0378-5955(95)00010-2. [DOI] [PubMed] [Google Scholar]
- Cooper LB, Chan DK, Roediger FC, Shaffer BR, Fraser JF, Musatov S, Selesnick SH, Kaplitt MG. AAV-mediated delivery of the caspase inhibitor XIAP protects against cisplatin ototoxicity. Otol. Neurotol. 2006;27:484–490. doi: 10.1097/01.mao.0000202647.19355.6a. [DOI] [PubMed] [Google Scholar]
- Cornelison TL, Reed E. Nephrotoxicity and hydration management for cisplatin, carboplatin, and ormaplatin. Gynecol. Oncol. 1993;50:147–158. doi: 10.1006/gyno.1993.1184. [DOI] [PubMed] [Google Scholar]
- Cummings BS, Schnellmann RG. Cisplatin-induced renal cell apoptosis: caspase 3-dependent and -independent pathways. J. Pharmacol. Exp. Ther. 2002;302:8–17. doi: 10.1124/jpet.302.1.8. [DOI] [PubMed] [Google Scholar]
- Cummings BS, McHowat J, Schnellmann RG. Role of an endoplasmic reticulum Ca2+-independent phospholipase A2 in cisplatin-induced renal cell apoptosis. J. Pharmacol. Exp. Ther. 2004;308:921–928. doi: 10.1124/jpet.103.060541. [DOI] [PubMed] [Google Scholar]
- Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001;60:2118–2128. doi: 10.1046/j.1523-1755.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- Devarajan P, Savoca M, Castaneda MP, Park MS, Esteban-Cruciani N, Kalinec G, Kalinec F. Cisplatin-induced apoptosis in auditory cells: role of death receptor and mitochondrial pathways. Hear Res. 2002;174:45–54. doi: 10.1016/s0378-5955(02)00634-2. [DOI] [PubMed] [Google Scholar]
- Dickey DT, Wu YJ, Muldoon LL, Neuwelt EA. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J. Pharmacol. Exp. Ther. 2005;314:1052–1058. doi: 10.1124/jpet.105.087601. [DOI] [PubMed] [Google Scholar]
- Einhorn LH. Curing metastatic testicular cancer. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4592–4595. doi: 10.1073/pnas.072067999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre S, Kalla S, Cvitkovic E, Bourdon O, Hauteville D, Dourte LM, Bensmaine MA, Itzhaki M, Marty M, Extra JM. Oxaliplatin and paclitaxel combination in patients with platinum-pretreated ovarian carcinoma: an investigator-originated compassionate-use experience. Ann. Oncol. 1999;10:1125–1128. doi: 10.1023/a:1008334215414. [DOI] [PubMed] [Google Scholar]
- Farris HE, LeBlanc CL, Goswami J, Ricci AJ. Probing the pore of the auditory hair cell mechanotransducer channel in turtle. J. Physiol. 2004;558:769–792. doi: 10.1113/jphysiol.2004.061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubel S, Ljubanovic D, Reznikov L, Somerset H, Dinarello CA, Edelstein CL. Caspase-1-deficient mice are protected against cisplatin-induced apoptosis and acute tubular necrosis. Kidney Int. 2004;66:2202–2213. doi: 10.1111/j.1523-1755.2004.66010.x. [DOI] [PubMed] [Google Scholar]
- Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, Oh DJ, Lu L, Klein CL, Dinarello CA, Edelstein CL. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J. Pharmacol. Exp. Ther. 2007;322:8–15. doi: 10.1124/jpet.107.119792. [DOI] [PubMed] [Google Scholar]
- Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin. Pharmacol. Ther. 2009;86:396–402. doi: 10.1038/clpt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francescato HD, Costa RS, Junior FB, Coimbra TM. Effect of JNK inhibition on cisplatin-induced renal damage. Nephrol. Dial. Transplant. 2007;22:2138–2148. doi: 10.1093/ndt/gfm144. [DOI] [PubMed] [Google Scholar]
- Fuertes MA, Alonso C, Perez JM. Biochemical modulation of cisplatin mechanisms of action: enhancement of antitumor activity and circumvention of drug resistance. Chem. Rev. 2003;103:645–662. doi: 10.1021/cr020010d. [DOI] [PubMed] [Google Scholar]
- Guthrie OW, Li-Korotky HS, Durrant JD, Balaban C. Cisplatin induces cytoplasmic to nuclear translocation of nucleotide excision repair factors among spiral ganglion neurons. Hear Res. 2008;239:79–91. doi: 10.1016/j.heares.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Hannemann J, Baumann K. Nephrotoxicity of cisplatin, carboplatin and transplatin. A comparative in vitro study. Arch. Toxicol. 1990;64:393–400. doi: 10.1007/BF01973462. [DOI] [PubMed] [Google Scholar]
- Hannemann J, Duwe J, Baumann K. Iron- and ascorbic acid-induced lipid peroxidation in renal microsomes isolated from rats treated with platinum compounds. Cancer Chemother. Pharmacol. 1991;28:427–433. doi: 10.1007/BF00685818. [DOI] [PubMed] [Google Scholar]
- Harned TM, Kalous O, Neuwelt A, Loera J, Ji L, Iovine P, Sposto R, Neuwelt EA, Reynolds CP. Sodium thiosulfate administered six hours after cisplatin does not compromise antineuroblastoma activity. Clin. Cancer Res. 2008;14:533–540. doi: 10.1158/1078-0432.CCR-06-2289. [DOI] [PubMed] [Google Scholar]
- Harrap KR. Preclinical studies identifying carboplatin as a viable cisplatin alternative. Cancer Treat. Rev. 1985;12(Suppl A):21–33. doi: 10.1016/0305-7372(85)90015-5. [DOI] [PubMed] [Google Scholar]
- Hellberg V, Gahm C, Liu W, Ehrsson H, Rask-Andersen H, Laurell G. Immunohistochemical localization of OCT2 in the cochlea of various species. Laryngoscope. 2015 doi: 10.1002/lary.25304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley ML, Hagerty KL, Kewalramani T, Green DM, Meropol NJ, Wasserman TH, Cohen GI, Emami B, Gradishar WJ, Mitchell RB, Thigpen JT, Trotti A, 3rd, von Hoff D, Schuchter LM. American society of clinical oncology clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J. Clin. Oncol. 2008;27:127–145. doi: 10.1200/JCO.2008.17.2627. [DOI] [PubMed] [Google Scholar]
- Henson ES, Gibson SB. Surviving cell death through epidermal growth factor (EGF) signal transduction pathways: implications for cancer therapy. Cell. Signal. 2006;18:2089–2097. doi: 10.1016/j.cellsig.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Hilder TA, Hill JM. Modelling the encapsulation of the anticancer drug cisplatin into carbon nanotubes. Nanotechnology. 2007;18:275704. [Google Scholar]
- Hudspeth AJ, Corey DP. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc. Natl. Acad. Sci. U.S.A. 1977;74:2407–2411. doi: 10.1073/pnas.74.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ. The ionic channels of a vertebrate hair cell. Hear Res. 1986;22:21–27. doi: 10.1016/0378-5955(86)90070-5. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Sunose H, Takasaka T. Effects of free radicals on the intracellular calcium concentration in the isolated outer hair cell of the guinea pig cochlea. Acta Otolaryngol. 1993;113:137–141. doi: 10.3109/00016489309135781. [DOI] [PubMed] [Google Scholar]
- Jacobs SS, Fox E, Dennie C, Morgan LB, McCully CL, Balis FM. Plasma and cerebrospinal fluid pharmacokinetics of intravenous oxaliplatin, cisplatin, and carboplatin in nonhuman primates. Clin. Cancer Res. 2005;11:1669–1674. doi: 10.1158/1078-0432.CCR-04-1807. [DOI] [PubMed] [Google Scholar]
- Jakob SM, Arnold W, Marti HP. Progressive renal failure after cisplatin therapy. Nephrol. Dial. Transplant. 1996;11:370–373. doi: 10.1093/oxfordjournals.ndt.a027273. [DOI] [PubMed] [Google Scholar]
- Jamesdaniel S, Coling D, Hinduja S, Ding D, Li J, Cassidy L, Seigel GM, Qu J, Salvi R. Cisplatin-induced ototoxicity is mediated by nitroxidative modification of cochlear proteins characterized by nitration of Lmo4. J. Biol. Chem. 2012;287:18674–18686. doi: 10.1074/jbc.M111.297960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson ER, Lippard SJ. Structure, recognition, and processing of cisplatin-DNA adducts. Chem. Rev. 1999;99:2467–2498. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- Jekunen AP, Hom DK, Alcaraz JE, Eastman A, Howell SB. Cellular pharmacology of dichloro(ethylenediamine)platinum(II) in cisplatin-sensitive and resistant human ovarian carcinoma cells. Cancer Res. 1994;54:2680–2687. [PubMed] [Google Scholar]
- Jiang M, Yi X, Hsu S, Wang CY, Dong Z. Role of p53 in cisplatin-induced tubular cell apoptosis: dependence on p53 transcriptional activity. Am. J. Physiol. 2004;287:F1140–F1147. doi: 10.1152/ajprenal.00262.2004. [DOI] [PubMed] [Google Scholar]
- Jiang M, Wei Q, Wang J, Du Q, Yu J, Zhang L, Dong Z. Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene. 2006;25:4056–4066. doi: 10.1038/sj.onc.1209440. [DOI] [PubMed] [Google Scholar]
- Juo P, Kuo CJ, Yuan J, Blenis J. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr. Biol. 1998;8:1001–1008. doi: 10.1016/s0960-9822(07)00420-4. [DOI] [PubMed] [Google Scholar]
- Karasawa T, Sibrian-Vazquez M, Strongin RM, Steyger PS. Identification of cisplatin-binding proteins using agarose conjugates of platinum compounds. PLoS One. 2013;8:e66220. doi: 10.1371/journal.pone.0066220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T, Mukherjea D, Sheehan K, Jajoo S, Rybak LP, Ramkumar V. Short interfering RNA against STAT1 attenuates cisplatin-induced ototoxicity in the rat by suppressing inflammation. Cell Death Dis. 2011;2:e180. doi: 10.1038/cddis.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khynriam D, Prasad SB. Changes in glutathione-related enzymes in tumor-bearing mice after cisplatin treatment. Cell Biol. Toxicol. 2002;18:349–358. doi: 10.1023/a:1020899221192. [DOI] [PubMed] [Google Scholar]
- Kidera Y, Kawakami H, Sakiyama T, Okamoto K, Tanaka K, Takeda M, Kaneda H, Nishina S, Tsurutani J, Fujiwara K, Nomura M, Yamazoe Y, Chiba Y, Nishida S, Tamura T, Nakagawa K. Risk factors for cisplatin-induced nephrotoxicity and potential of magnesium supplementation for renal protection. PloS One. 2014;9:e101902. doi: 10.1371/journal.pone.0101902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Lee JH, Kim SJ, Oh GS, Moon HD, Kwon KB, Park C, Park BH, Lee HK, Chung SY, Park R, So HS. Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J. Neurosci. 2010;30:3933–3946. doi: 10.1523/JNEUROSCI.6054-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J. Clin. Oncol. 2005;23:8588–8596. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- Knight KR, Kraemer DF, Winter C, Neuwelt EA. Early changes in auditory function as a result of platinum chemotherapy: use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. J. Clin. Oncol. 2007;25:1190–1195. doi: 10.1200/JCO.2006.07.9723. [DOI] [PubMed] [Google Scholar]
- Knox RJ, Friedlos F, Lydall DA, Roberts JJ. Mechanism of cytotoxicity of anticancer platinum drugs: evidence that cis-diamminedichloroplatinum(II) and cis-diammine-(1,1-cyclobutanedicarboxylato)platinum(II) differ only in the kinetics of their interaction with DNA. Cancer Res. 1986;46:1972–1979. [PubMed] [Google Scholar]
- Koo JW, Quintanilla-Dieck L, Jiang M, Liu J, Urdang ZD, Allensworth JJ, Cross CP, Li H, Steyger PS. Endotoxemia-mediated inflammation potentiates aminoglycoside-induced ototoxicity. Sci. Transl. Med. 2015 doi: 10.1126/scitranslmed.aac5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruidering M, Van de Water B, de Heer E, Mulder GJ, Nagelkerke JF. Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: mitochondrial dysfunction by inhibition of complexes I to IV of the respiratory chain. J. Pharmacol. Exp. Ther. 1997;280:638–649. [PubMed] [Google Scholar]
- Lanvers-Kaminsky C, Sprowl JA, Malath I, Deuster D, Eveslage M, Schlatter E, Mathijssen RH, Boos J, Jurgens H, Am Zehnhoff-Dinnesen AG, Sparreboom A, Ciarimboli G. Human OCT2 variant c.808G>T confers protection effect against cisplatin-induced ototoxicity. Pharmacogenomics. 2015;16:323–332. doi: 10.2217/pgs.14.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell G, Engstrom B. The combined effect of cisplatin and furosemide on hearing function in guinea pigs. Hear Res. 1989;38:19–26. doi: 10.1016/0378-5955(89)90124-x. [DOI] [PubMed] [Google Scholar]
- Laurell G, Viberg A, Teixeira M, Sterkers O, Ferrary E. Blood-perilymph barrier and ototoxicity: an in vivo study in the rat. Acta Otolaryngol. 2000;120:796–803. doi: 10.1080/000164800750061624. [DOI] [PubMed] [Google Scholar]
- Laurell G, Ekborn A, Viberg A, Canlon B. Effects of a single high dose of cisplatin on the melanocytes of the stria vascularis in the guinea pig. Audiol. Neurootol. 2007;12:170–178. doi: 10.1159/000099020. [DOI] [PubMed] [Google Scholar]
- Lee RH, Song JM, Park MY, Kang SK, Kim YK, Jung JS. Cisplatin-induced apoptosis by translocation of endogenous Bax in mouse collecting duct cells. Biochem. Pharmacol. 2001;62:1013–1023. doi: 10.1016/s0006-2952(01)00748-1. [DOI] [PubMed] [Google Scholar]
- Li H, Steyger PS. Systemic aminoglycosides are trafficked via endolymph into cochlear hair cells. Sci. Rep. 2011;1:159. doi: 10.1038/srep00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Frenz DA, Brahmblatt S, Feghali JG, Ruben RJ, Berggren D, Arezzo J, Van De Water TR. Round window membrane delivery of -methionine provides protection from cisplatin ototoxicity without compromising chemotherapeutic efficacy. Neurotoxicology. 2001;22:163–176. doi: 10.1016/s0161-813x(00)00010-3. [DOI] [PubMed] [Google Scholar]
- Li G, Liu W, Frenz D. Cisplatin ototoxicity to the rat inner ear: a role for HMG1 and iNOS. Neurotoxicology. 2006;27:22–30. doi: 10.1016/j.neuro.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Liu H, Baliga R. Cytochrome P450 2E1 null mice provide novel protection against cisplatin-induced nephrotoxicity and apoptosis. Kidney Int. 2003;63:1687–1696. doi: 10.1046/j.1523-1755.2003.00908.x. [DOI] [PubMed] [Google Scholar]
- Liu H, Baliga R. Endoplasmic reticulum stress-associated caspase 12 mediates cisplatin-induced LLC-PK1 cell apoptosis. J. Am. Soc. Nephrol. 2005;16:1985–1992. doi: 10.1681/ASN.2004090768. [DOI] [PubMed] [Google Scholar]
- Lu LH, Oh DJ, Dursun B, He Z, Hoke TS, Faubel S, Edelstein CL. Increased macrophage infiltration and fractalkine expression in cisplatin-induced acute renal failure in mice. J. Pharmacol. Exp. Ther. 2008;324:111–117. doi: 10.1124/jpet.107.130161. [DOI] [PubMed] [Google Scholar]
- Lu X, Wang L, Ruden DM. Hsp90 inhibitors and the reduction of anti-cancer drug resistance by non-genetic and genetic mechanisms. Pharmaceuticals. 2012;5:890–898. doi: 10.3390/ph5090890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, van Netten SM, Kros CJ. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J. Physiol. 2005;567:505–521. doi: 10.1113/jphysiol.2005.085951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marullo R, Werner E, Degtyareva N, Moore B, Altavilla G, Ramalingam SS, Doetsch PW. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PloS One. 2013;8:e81162. doi: 10.1371/journal.pone.0081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol. Cancer Ther. 2009;8:10–16. doi: 10.1158/1535-7163.MCT-08-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima K, Baba A, Matsuo M, Itoh Y, Oishi R. Protective effect of cyclic AMP against cisplatin-induced nephrotoxicity. Free Radic. Biol. Med. 2006;40:1564–1577. doi: 10.1016/j.freeradbiomed.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Mohr PE, Feldman JJ, Dunbar JL, McConkey-Robbins A, Niparko JK, Rittenhouse RK, Skinner MW. The societal costs of severe to profound hearing loss in the United States. Int. J. Technol. Assess. Health Care. 2000;16:1120–1135. doi: 10.1017/s0266462300103162. [DOI] [PubMed] [Google Scholar]
- Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E, Brafman A, Faerman A, Atkinson SJ, Thompson JD, Kalinski H, Skaliter R, Erlich S, Feinstein E. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J. Am. Soc. Nephrol. 2009;20:1754–1764. doi: 10.1681/ASN.2008111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More SS, Akil O, Ianculescu AG, Geier EG, Lustig LR, Giacomini KM. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J. Neurosci. 2010;30:9500–9509. doi: 10.1523/JNEUROSCI.1544-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjea D, Whitworth CA, Nandish S, Dunaway GA, Rybak LP, Ramkumar V. Expression of the kidney injury molecule 1 in the rat cochlea and induction by cisplatin. Neuroscience. 2006;139:733–740. doi: 10.1016/j.neuroscience.2005.12.044. [DOI] [PubMed] [Google Scholar]
- Mukherjea D, Jajoo S, Whitworth C, Bunch JR, Turner JG, Rybak LP, Ramkumar V. Short interfering RNA against transient receptor potential vanilloid 1 attenuates cisplatin-induced hearing loss in the rat. J. Neurosci. 2008;28:13056–13065. doi: 10.1523/JNEUROSCI.1307-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjea D, Jajoo S, Kaur T, Sheehan KE, Ramkumar V, Rybak LP. Transtympanic administration of short interfering (si)RNA for the NOX3 isoform of NADPH oxidase protects against cisplatin-induced hearing loss in the rat. Antioxid. Redox Signal. 2010;13:589–598. doi: 10.1089/ars.2010.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjea D, Jajoo S, Sheehan K, Kaur T, Sheth S, Bunch J, Perro C, Rybak LP, Ramkumar V. NOX3 NADPH oxidase couples transient receptor potential vanilloid 1 to signal transducer and activator of transcription 1-mediated inflammation and hearing loss. Antioxid. Redox Signal. 2011a;14:999–1010. doi: 10.1089/ars.2010.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjea D, Rybak LP, Sheehan KE, Kaur T, Ramkumar V, Jajoo S, Sheth S. The design and screening of drugs to prevent acquired sensorineural hearing loss. Expert Opin. Drug Deliv. 2011b;6:491–505. doi: 10.1517/17460441.2011.562887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P, Rajesh M, Pan H, Patel V, Mukhopadhyay B, Batkai S, Gao B, Hasko G, Pacher P. Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radic. Biol. Med. 2010;48:457–467. doi: 10.1016/j.freeradbiomed.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh GS, Kim HJ, Choi JH, Shen A, Kim CH, Kim SJ, Shin SR, Hong SH, Kim Y, Park C, Lee SJ, Akira S, Park R, So HS. Activation of lipopoly saccharide-TLR4 signaling accelerates the ototoxic potential of cisplatin in mice. J. Immunol. 2011;186:1140–1150. doi: 10.4049/jimmunol.1002183. [DOI] [PubMed] [Google Scholar]
- Pabla N, Murphy RF, Liu K, Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am. J. Physiol. Renal Physiol. 2009;296:F505–F511. doi: 10.1152/ajprenal.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MS, De Leon M, Devarajan P. Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J. Am. Soc. Nephrol. 2002;13:858–865. doi: 10.1681/ASN.V134858. [DOI] [PubMed] [Google Scholar]
- Pickles JO, Comis SD, Osborne MP. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res. 1984;15:103–112. doi: 10.1016/0378-5955(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price PM, Safirstein RL, Megyesi J. Protection of renal cells from cisplatin toxicity by cell cycle inhibitors. Am. J. Physiol. Renal Physiol. 2004;286:378F–3384. F378. doi: 10.1152/ajprenal.00192.2003. [DOI] [PubMed] [Google Scholar]
- Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Invest. 2002;110:835–842. doi: 10.1172/JCI15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh G, Reeves WB. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am. J. Physiol. Renal Physiol. 2005;289:F166–F174. doi: 10.1152/ajprenal.00401.2004. [DOI] [PubMed] [Google Scholar]
- Rosell R, Taron M, Barnadas A, Scagliotti G, Sarries C, Roig B. Nucleotide excision repair pathways involved in cisplatin resistance in non-small-cell lung cancer. Cancer Control. 2003;10:297–305. doi: 10.1177/107327480301000404. [DOI] [PubMed] [Google Scholar]
- Rosenberg B, Vancamp L, Krigas T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature. 1965;205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg B, VanCamp L, Trosko JE, Mansour VH. Platinum compounds: a new class of potent antitumour agents. Nature. 1969;222:385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- Ross CJ, Katzov-Eckert H, Dube MP, Brooks B, Rassekh SR, Barhdadi A, Feroz-Zada Y, Visscher H, Brown AM, Rieder MJ, Rogers PC, Phillips MS, Carleton BC, Hayden MR. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat. Genet. 2009;41:1345–1349. doi: 10.1038/ng.478. [DOI] [PubMed] [Google Scholar]
- Roy S, Ryals MM, Van den Bruele AB, Fitzgerald TS, Cunningham LL. Sound preconditioning therapy inhibits ototoxic hearing loss in mice. J. Clin. Invest. 2013;123:4945–4949. doi: 10.1172/JCI71353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak LP, Ramkumar V. Ototoxicity. Kidney Int. 2007;72:931–935. doi: 10.1038/sj.ki.5002434. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226:157–167. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Mukherjea D, Jajoo S, Ramkumar V. Cisplatin ototoxicity and protection: clinical and experimental studies. Tohoku J. Exp. Med. 2009;219:177–186. doi: 10.1620/tjem.219.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadzuka Y, Shoji T, Takino Y. Mechanism of the increase in lipid peroxide induced by cisplatin in the kidneys of rats. Toxicol. Lett. 1992;62:293–300. doi: 10.1016/0378-4274(92)90033-g. [DOI] [PubMed] [Google Scholar]
- Schilsky RL, Anderson T. Hypomagnesemia and renal magnesium wasting in patients receiving cisplatin. Ann. Intern. Med. 1979;90:929–931. doi: 10.7326/0003-4819-90-6-929. [DOI] [PubMed] [Google Scholar]
- Schmitt NC, Rubel EW, Nathanson NM. Cisplatin-induced hair cell death requires STAT1 and is attenuated by epigallocatechin gallate. J. Neurosci. 2009;29:3843–3851. doi: 10.1523/JNEUROSCI.5842-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth R, Yang C, Kaushal V, Shah SV, Kaushal GP. p53-dependent caspase-2 activation in mitochondrial release of apoptosis-inducing factor and its role in renal tubular epithelial cell injury. J. Biol. Chem. 2005;280:31230–31239. doi: 10.1074/jbc.M503305200. [DOI] [PubMed] [Google Scholar]
- Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol. Rev. 2012;64:706–721. doi: 10.1124/pr.111.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner R, Pearson AD, English MW, Price L, Wyllie RA, Coulthard MG, Craft AW. Cisplatin dose rate as a risk factor for nephrotoxicity in children. Br. J. Cancer. 1998;77:1677–1682. doi: 10.1038/bjc.1998.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery EL, Warchol ME. Cisplatin ototoxicity blocks sensory regeneration in the avian inner ear. J. Neurosci. 2010;30:3473–3481. doi: 10.1523/JNEUROSCI.4316-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery EL, Oshima K, Heller S, Warchol ME. Cisplatin exposure damages resident stem cells of the mammalian inner ear. Dev. Dyn. 2014;243:1328–1337. doi: 10.1002/dvdy.24150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So H, Kim H, Lee JH, Park C, Kim Y, Kim E, Kim JK, Yun KJ, Lee KM, Lee HY, Moon SK, Lim DJ, Park R. Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-kappaB. J. Assoc. Res. Otolaryngol. 2007;8:338–355. doi: 10.1007/s10162-007-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprowl JA, van Doorn L, Hu S, van Gerven L, de Bruijn P, Li L, Gibson AA, Mathijssen RH, Sparreboom A. Conjunctive therapy of cisplatin with the OCT2 inhibitor cimetidine: influence on antitumor efficacy and systemic clearance. Clin. Pharmacol. Ther. 2013;94:585–592. doi: 10.1038/clpt.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri ES. Molecular ordering of the fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14486–14491. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk R, Gurubhagavatula S, Park S, Zhou W, Su L, Lynch TJ, Wain JC, Neuberg D, Liu G, Christiani DC. Polymorphisms in ERCC1 and grade 3 or 4 toxicity in non-small cell lung cancer patients. Clin. Cancer Res. 2005;11:1534–1538. doi: 10.1158/1078-0432.CCR-04-1953. [DOI] [PubMed] [Google Scholar]
- Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Hailey DW, Stawicki TM, Wu P, Coffin AB, Rubel EW, Raible DW, Simon JA, Ou HC. Functional mechanotransduction is required for cisplatin-induced hair cell death in the zebrafish lateral line. J. Neurosci. 2013;33:4405–4414. doi: 10.1523/JNEUROSCI.3940-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruya K, Ninomiya T, Tokumoto M, Hirakawa M, Masutani K, Taniguchi M, Fukuda K, Kanai H, Kishihara K, Hirakata H, Iida M. Direct involvement of the receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Kidney Int. 2003;63:72–82. doi: 10.1046/j.1523-1755.2003.00709.x. [DOI] [PubMed] [Google Scholar]
- Viale M, Vannozzi MO, Pastrone I, Mariggio MA, Zicca A, Cadoni A, Cafaggi S, Tolino G, Lunardi G, Civalleri D, Lindup WE, Esposito M. Reduction of cisplatin nephrotoxicity by procainamide: does the formation of a cisplatin-procainamide complex play a role? J. Pharmacol. Exp. Ther. 2000;293:829–836. [PubMed] [Google Scholar]
- Vu AA, Nadaraja GS, Huth ME, Luk L, Kim J, Chai R, Ricci AJ, Cheng AG. Integrity and regeneration of mechanotransduction machinery regulate aminoglycoside entry and sensory cell death. PLoS One. 2013;8:e54794. doi: 10.1371/journal.pone.0054794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ladrech S, Pujol R, Brabet P, Van De Water TR, Puel JL. Caspase inhibitors, but not c-Jun NH2-terminal kinase inhibitor treatment, prevent cisplatin-induced hearing loss. Cancer Res. 2004;64:9217–9224. doi: 10.1158/0008-5472.CAN-04-1581. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Inai S, Jinnouchi K, Bada S, Hess A, Michel O, Yagi T. Nuclear-factor kappa B (NF-kappa B)-inducible nitric oxide synthase (iNOS/NOS II) pathway damages the stria vascularis in cisplatin-treated mice. Anticancer Res. 2002;22:4081–4085. [PubMed] [Google Scholar]
- Wei Q, Dong G, Yang T, Megyesi J, Price PM, Dong Z. Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am. J. Physiol. Renal Physiol. 2007;293:F1282–F1291. doi: 10.1152/ajprenal.00230.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnovsky SP, Wilson JJ, Radford RJ, Pereira MP, Chan MR, Laposa RR, Lippard SJ, Kelley SO. Targeting mitochondrial DNA with a platinum-based anticancer agent. Chem. Biol. 2013;20:1323–1328. doi: 10.1016/j.chembiol.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Robinson GW, Huang J, Lim JY, Zhang H, Bass JK, Broniscer A, Chintagumpala M, Bartels U, Gururangan S, Hassall T, Fisher M, Cohn R, Yamashita T, Teitz T, Zuo J, Onar-Thomas A, Gajjar A, Stewart CF, Yang JJ. Common variants in ACYP2 influence susceptibility to cisplatin-induced hearing loss. Nat. Genet. 2015;47:263–266. doi: 10.1038/ng.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey A, Harris MS, Egbelakin A, Gilbert J, Pisoni DB, Renbarger J. Risk factors for cisplatin-associated ototoxicity in pediatric oncology patients. Pediatr. Blood Cancer. 2012;59:144–148. doi: 10.1002/pbc.24138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Kaushal V, Shah SV, Kaushal GP. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am. J. Physiol. Renal Physiol. 2008;294:F777–F787. doi: 10.1152/ajprenal.00590.2007. [DOI] [PubMed] [Google Scholar]
- Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am. J. Med. Sci. 2007;334:115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- Yu F, Megyesi J, Safirstein RL, Price PM. Identification of the functional domain of p21(WAF1/CIP1) that protects cells from cisplatin cytotoxicity. Am. J. Physiol. Renal Physiol. 2005;289:F514–F520. doi: 10.1152/ajprenal.00101.2005. [DOI] [PubMed] [Google Scholar]
- Zamble DB, Jacks T, Lippard SJ. p53-Dependent and -independent responses to cisplatin in mouse testicular teratocarcinoma cells. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6163–6168. doi: 10.1073/pnas.95.11.6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Liu W, Ding D, Salvi R. Pifithrin-alpha suppresses p53 and protects cochlear and vestibular hair cells from cisplatin-induced apoptosis. Neuroscience. 2003;120:191–205. doi: 10.1016/s0306-4522(03)00286-0. [DOI] [PubMed] [Google Scholar]
- Zhang B, Ramesh G, Norbury CC, Reeves WB. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-alpha produced by renal parenchymal cells. Kidney Int. 2007;72:37–44. doi: 10.1038/sj.ki.5002242. [DOI] [PubMed] [Google Scholar]
- de Jongh FE, van Veen RN, Veltman SJ, de Wit R, van der Burg ME, van den Bent MJ, Planting AS, Graveland WJ, Stoter G, Verweij J. Weekly high-dose cisplatin is a feasible treatment option: analysis on prognostic factors for toxicity in 400 patients. Br. J. Cancer. 2003;88:1199–1206. doi: 10.1038/sj.bjc.6600884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ruijven MW, de Groot JC, Hendriksen F, Smoorenburg GF. Immunohistochemical detection of platinated DNA in the cochlea of cisplatin-treated guinea pigs. Hear Res. 2005;203:112–121. doi: 10.1016/j.heares.2004.12.007. [DOI] [PubMed] [Google Scholar]