Summary
The most commonly used anticoagulants produce therapeutic antithrombotic effects either by inhibiting thrombin or factor Xa, or by lowering the plasma levels of the precursors of these key enzymes, prothrombin and factor X. These drugs do not distinguish between thrombin generation contributing to thrombosis from thrombin generation required for hemostasis. Thus, anticoagulants increase bleeding risk, and many patients who would benefit from therapy go untreated because of comorbidities that place them at unacceptable risk for hemorrhage. Studies in animals demonstrate that components of the plasma contact activation system contribute to experimentally-induced thrombosis, despite playing little or no role in hemostasis. Attention has focused on factor XII, the zymogen of a protease (factor XIIa) that initiates contact activation when blood is exposed to foreign surfaces; and factor XI, the zymogen of the protease factor XIa, which links contact activation to the thrombin generation mechanism. In the case of factor XI, epidemiologic data indicate this protein contributes to stroke and venous thromboembolism, and perhaps myocardial infarction, in humans. A phase 2 trial showing that reduction of factor XI may be more effective than low-molecular-weight heparin at preventing venous thrombosis during knee replacement surgery provides proof of concept for the premise that an antithrombotic effect can be uncoupled from an anticoagulant effect in humans by targeting components of contact activation. Here we review data on the role of factor XI and factor XII in thrombosis, and results of pre-clinical and human trials for therapies targeting these proteins.
Keywords: thrombosis, venous thrombosis, anticoagulant, factor XI, factor XII
Introduction
Exposure of blood to negatively charged substances or artificial surfaces triggers a group of proteolytic reactions in plasma, collectively called contact activation, that lead to thrombin generation and fibrin formation [1–3]. Contact activation involves reciprocal conversion of the zymogens factor XII (FXII) and prekallikrein (PK) to the proteases FXIIa and α-kallikrein, respectively, in the presence of high-molecular-weight kininogen (HK) (Figure 1, right panel). FXIIa promotes thrombin generation by converting factor XI (FXI) to FXIa, while α-kallikrein cleaves HK to liberate the proinflammatory peptide bradykinin (BK). Congenital absence of a contact factor (FXI, FXII, PK or HK) slows the rate of fibrin formation in surface-dependent assays such as the activated partial thromboplastin time (aPTT) [4]. Indeed, FXI and FXII were first identified as missing plasma components in individuals with defects in surface-initiated coagulation [5,6]. However, despite the importance of contact activation to coagulation in the aPTT, absence of FXII, PK or HK is not associated with abnormal hemostasis [1–4]. Therefore, if these proteins contribute to thrombin generation in vivo, it is not required for stemming bleeding at an injury site. Furthermore, as FXI deficiency can cause an injury-related bleeding diathesis [7,8], it must be able to function independently of contact activation in hemostasis.
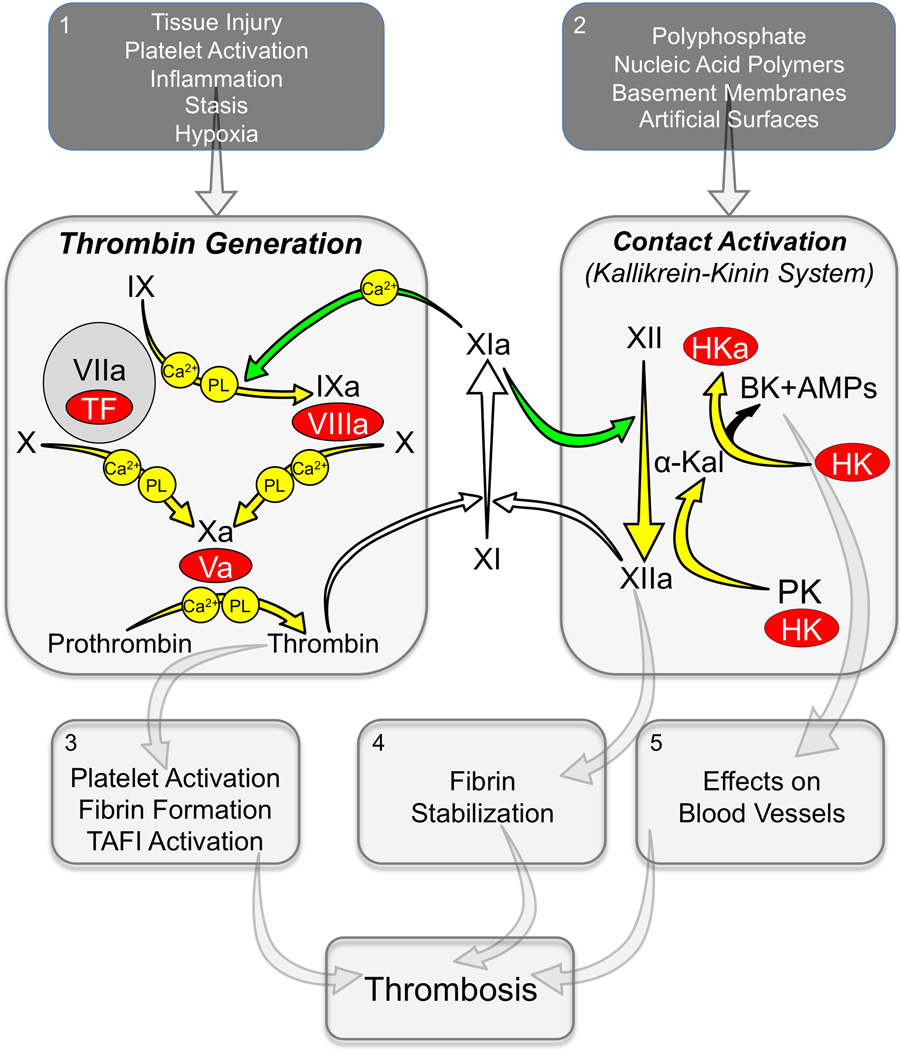
Figure 1. Thrombin Generation and Contact Activation in Thrombosis.
Thrombin Generation (Left Large Panel). Depicted are the calcium and phospholipid (PL) dependent proteolytic reactions that generate thrombin at a site of vascular injury. The process is initiated by activation of factors X and IX by the factor VIIa/tissue factor (TF) complex. Vitamin-K dependent protease zymogens are shown in black type with the active protease forms indicated by a lower case “a”. Cofactors are shown as red ovals. FXI can contribute to hemostasis through its conversion to FXIa by thrombin (white arrows), with subsequent calcium-dependent proteolysis of FIX (green arrow). Contact activation (Right Large Panel). On a surface, FXII undergoes autoactivation to FXIIa. FXIIa converts prekallikrein (PK) to the active protease α-kallikrein, which then activates additional FXII and cleaves the cofactor high-molecular-weight kininogen (HK) to liberate bradykinin (BK). HK cleavage can also produce antimicrobial peptides (AMPs). This system can contribute to thrombin generation through FXIIa-mediated activation of FXI (white arrow). FXIa, in turn can contribute to contact activation through activation of FXII (green arrow). In plasma, PK and FXI circulate as complexes with HK. During contact activation, HK serves as a cofactor by facilitating PK and FXI binding to the surface. Panels 1 and 2 list factors that may contribute to TF-induced thrombin generation or contact activation during thrombosis. Panels 3, 4 and 5 list some consequences of thrombin generation and contact activation that may contribute to thrombus formation and growth.
The work of many investigators over the past twenty-five years has helped to clarify the role of FXI in thrombin generation and its relationship to contact activation [reviewed in 9–13]. While FXI makes a limited contribution to hemostasis (discussed below), epidemiologic data [10], work with animal models [9,13] and recent human trials [14,15] indicate that this protein has important roles in thromboembolic disease. Furthermore, FXIIa activation of FXI may contribute to thrombosis in some settings [1,9,13,16]. These observations are the driving force behind efforts to develop therapies that target the contact system, with the hope and expectation of achieving an antithrombotic effect with minimal or no compromise of hemostasis.
Evolution of the Contact System
Analyses of vertebrate genomes reveal details of the natural history of thrombin generation and contact activation that are illustrative when considering their roles in hemostasis and thrombosis. Teleosts such as zebra fish have a vitamin-K dependent protease-based mechanism for thrombin generation similar to the mammalian system (Figure 1, left panel) [17], but lack FXI, FXII, PK or a kininogen comparable to HK [18,19]. Genes for FXII, PK and HK are present in amphibians, reptiles and mammals, indicating the contact system evolved in early terrestrial vertebrates or their aquatic ancestors [17–19]. However, with the exception of mammals, vertebrates lack FXI and the connection it provides between contact activation and thrombin generation [18]. This explains why plasmas from non-mammalian vertebrates do not clot as quickly as mammalian plasmas in aPTT assays, and supports the premise that thrombin generation was not an original function of contact activation.
FXI arose by a duplication of the PK gene [17,18], and shares features with PK, while having unique functions that allow it to contribute to thrombin generation. Thus, like PK, FXI binds HK [20] and is activated by FXIIa [21,22]. But unlike PK, FXI is activated by thrombin allowing it to participate in tissue factor-initiated coagulation (Figure 1) [22–25]. Similarly, FXIa retains α-kallikrein’s ability to activate FXII [25,26], while having acquired the capacity to activate factor IX [12]. FXI, therefore, should be considered a contact factor and a coagulation factor, and it may function as a bidirectional interface between the more ancient thrombin generation and contact systems.
FXI and Hemostasis
FXI is the only contact factor required for hemostasis, though its role is limited. FXIa’s primary substrate is factor IX (Figure 1), but it may also enhance thrombin generation by activating factors V, VIII and X [27,28] and by proteolysis of tissue factor pathway inhibitor (TFPI) [29]. Humans with severe FXI deficiency may experience excessive injury-induced bleeding, particularly when trauma involves tissues rich in fibrinolytic activity such as the nose, mouth, and genitourinary tract [4,7,8,30]. Hemorrhage at other sites is less common, and tends to occur in individuals with FXI levels <10% of normal [30]. While the correlation between FXI levels and bleeding is weak, it seems reasonable to conclude that FXIa is required in some humans to protect clots from premature degradation. Zucker et al showed that plasma clots from FXI-deficient patients with histories of excessive bleeding are less stable in the presence of tissue plasminogen activator than are clots from FXI-deficient patients without a bleeding history [31]. FXIa-dependent thrombin generation may contribute to clot resistance to fibrinolysis through a number of mechanisms (Figure 1, panel 3), including fibrin formation, platelet activation, and activation of the plasma carboxypeptidase TAFI (Thrombin-Activatable Fibrinolysis Inhibitor) [32,33]. TAFI removes lysine residues on fibrin through which fibrinolytic components bind to clot, inhibiting plasmin generation and plasmin degradation of fibrin. In plasma clot fibrinolysis assays, the anti-fibrinolytic effect of FXI is reduced if TAFI is inhibited [32], consistent with TAFI playing an important role in the FXIa effect on clot integrity.
Contact Activation, Innate Immunity, and Thrombosis
FXII, PK and HK, often referred to as the kallikrein-kinin system (KKS, Figure 1, right panel), may contribute to a variety of host defense and homeostatic processes; although their deficiencies are not easily linked to clear phenotypic abnormalities [1–4]. Here we consider contact activation as part of the innate response to infection, as there may be parallels with its role in thrombosis. Similar to complement proteins, contact factors assemble on surfaces of bacterial pathogens leading to liberation of BK and antimicrobial peptides (AMPs) from HK [34,35]. Rodents treated with FXIIa/kallikrein inhibitors have a defect in bacterial clearance, suggesting such interactions are physiologically relevant [35]. During infection, bacteria and host tissues are sources of polyanions that can promote contact activation. Inorganic phosphate polymers (polyphosphate [polyP]) are ubiquitous in nature [35]. Prokaryotes produce long phosphate polymers (several thousands units) that can induce FXII activation [36,37]. DNA and RNA released from tissue or microorganisms also have phosphate backbones, and promote FXII-dependent processes [38–40]. Activated neutrophils extrude their chromatin, forming neutrophil extracellular traps [NETS] that can induce contact activation [40].
Interactions between the contact system and PolyP, DNA and RNA have also been implicated in thrombotic processes (Figure 1, panel 2). Platelets release polyP of 60–80 phosphate units, inducing a variety of procoagulant effects in human blood [36,37]. Müller et al showed that platelet-sized polyP induces thrombosis in mice in a FXII- and FXI-dependent manner [41]. DNA (including NETS) and RNA promote FXII-dependent thrombosis in mice, and are present in human venous thrombi [42,43]. The contribution of FXII to thrombosis in animal models is probably mediated largely through FXI activation. FXI activation by FXIIa is enhanced by polyP [22,44], DNA [45], and RNA (unpublished observation). FXI binds polyanions through specific anion binding sites, and FXI species lacking these sites support thrombus formation poorly in mice [46]. FXII may also affect clot structure independent of FXI activation and thrombin generation. Konings et al. reported that FXIIa binds with high affinity to fibrin, leading to higher fibrin density and resistance to fibrinolysis (Figure 1, panel 4) [47].
Contact Proteins in Animal Thrombosis Models
Minnema et al reported in 1998 that blocking FXI enhanced lysis of preformed clots introduced into the jugular veins of rabbits [48]. Chan et al noted that FXI deficiency partially ameliorated the thrombo-inflammatory phenotype of protein C-deficient mice [49], while Rosen et al showed that FXI-deficient (FXI−/−) mice are resistant to arterial thrombosis induced by exposing vessels to concentrated FeCl3 [50]. Wang et al expanded on the latter finding, showing that FXI−/− mice are as resistant to FeCl3-induced arterial occlusion as factor IX deficient mice, despite the markedly different propensities to bleed in the two lines [51]. In 2003, Gruber and Hanson reported that FXI inhibition prevented thrombus formation in a primate model, and proposed that targeting FXI could be an effective and safe antithrombotic approach in humans [52]. They observed that a polyclonal anti-FXI antibody attenuated platelet and fibrin deposition within collagen- or tissue factor-coated vascular grafts inserted into femoral arterio-venous (AV) shunts in baboons. The effect of the antibody was comparable to that of heparin, but without a discernable effect on hemostasis.
While mechanisms for FXI activation were not established during these early studies, subsequent work implicated contact activation. Renné et al observed that mice lacking FXII (FXII−/−) are as resistant to thrombosis as FXI−/− mice [53]. Viewed with intravital microscopy, thrombi forming within the lumens of vessels in animals lacking FXII or FXI are unstable and fragment under flow. Mice lacking HK or PK are also resistant to injury-induced thrombosis [54–56]. While these data are consistent with the hypothesis that contact activation-initiated thrombin generation drives thrombosis in the mouse models, this is likely an over-simplification. Stavrou et al reported that the anti-thrombotic phenotype of PK-deficient mice in arterial injury models is partly due to reduced vascular tissue factor and increased plasma prostacyclin, possibly secondary to chronically reduced BK generation (Figure 1, panel 5) [57]. Furthermore, the direct effect of FXIIa on fibrin clot structure (Figure 1, panel 4), as discussed [46], may contribute to thrombus stability in mice independent of FXI. It is important to keep in mind that most experimental thrombosis models involve healthy animals. While suggestive, this type of pre-clinical data does not establish that similar processes occur in diseased vessels in humans. The following sections present data from human populations on contact factors and thrombosis.
FXI and Thrombosis in Humans
Of the four contact factors, the case for a role in thrombosis in humans is strongest for FXI. Salomon et al reported a lower incidence of VTE in patients with severe FXI deficiency compared to the general population [58]. Meijers et al noted that subjects in the Leiden Thrombophilia Study (LETS) with plasma FXI levels in the upper 10% of the population distribution had a two-fold higher risk of venous thromboembolism (VTE) compared with the lower 90%, [59]. Similar results were reported by Cushman et al for the Longitudinal Investigation of Thromboembolism Etiology (LITE) cohort [60]. Severe FXI deficiency appears to confer protection from ischemic stroke [61], and higher FXI levels were associated with increased risk for ischemic stroke in a study by Yang et al [62], and in the Atherosclerosis Risk in Communities (ARIC) study [63]. In young women enrolled in the Risk of Arterial Thrombosis In relation to Oral contraceptives (RATIO) study, levels of FXIa and FXI antigen correlated with stroke risk [64].
A role for FXI in myocardial infarction (MI) is less clear than for VTE or stroke. Salomon et al. reported that the incidence of MI in 96 individuals with severe FXI deficiency was similar to the expected incidence for age-matched controls [65], although FXI-deficient patients had to be alive post-MI to be included in the study, while survival was not evaluated in controls. FXI plasma levels correlated with MI risk in men in the Study of Myocardial Infarction Leiden (SMILE) [66], and patients with acute coronary syndromes have elevated FXIa levels [67]. Berliner et al noted that FXI levels were higher in women with coronary disease than in those without it in a group referred for cardiac catheterization [68]. However, FXI antigen was not a risk factor in ARIC participants after adjustment for other factors [63], and FXI and FXIa levels were not associated with MI in the RATIO study [64]. Similarly, FXIa levels were not associated with coronary disease in men in the second Northwick Park Heart Study (NPHS-II) [69]. Cumulatively, the data for VTE, stroke and MI raise the possibility that the contribution of FXI to thrombosis varies depending on the vascular bed involved.
FXII and Thrombosis in Humans
The argument that FXII, PK or HK contribute to VTE, stroke or MI in humans is less convincing than for FXI. Indeed, patients with hereditary angioedema who have a defect in FXIIa and α-kallikrein regulation do not appear to be thrombosis prone. Famously, John Hageman, the first person identified with FXII deficiency, actually died from a pulmonary embolus [70]. While Mr. Hageman was immobile for nearly two weeks because of a pelvic fracture before his demise, the possibility that FXII deficiency contributes to venous thrombosis was raised in subsequent reports. However, larger studies of FXII-deficient subjects failed to demonstrate a link with VTE [71], and no differences in DVT incidence were noted across a range of FXII levels in LETS [72] or LITE study [60] participants.
Data on FXII in arterial thrombosis paint a complex picture. There was an inverse relationship between FXIIa-C1-INH complex levels and stroke risk in NPHS-II [69], but a direct relationship between the variables in the RATIO study [64]. Despite this, plasma FXII antigen levels in neither study, nor the ARIC study [63], correlated with stroke. Furthermore, FXII deficiency does not provide protection from stroke [71]. Curiously, while elevated FXIIa levels measured by ELISA were associated with increased risk of MI in NPHS-II, elevated FXIIa-C1-INH levels indicated the opposite effect in that trial [69]. In the RATIO study, FXIIa-C1-INH, FXII antigen and PK antigen were not associated with MI [64], and risk of coronary events did not correlate with FXII levels in the ARIC study [63]. Interestingly, data from the SMILE cohort actually showed an inverse relationship between FXII levels and cardiovascular disease [66]. Endler et al also noted an inverse association between FXII and death from ischemic heart disease but, curiously, this relationship did not hold for participants with severe FXII deficiency (<10% of normal), whose risk was comparable to the population mean [73]. It is difficult to draw unifying conclusions from the disparate results, but it could be argued that the contribution of FXII to VTE, stroke, and MI in humans appears to be smaller than for FXI.
The Case for Therapeutic Targeting of Contact Factors
Thrombin is central to formation of hemostatic and thrombotic clots. Two major strategies targeting this protease are currently used to treat or prevent thrombosis. One approach involves inhibiting the activity of thrombin or of factor Xa, the protease that converts prothrombin to thrombin. This is achieved directly by blockade of protease active sites (e.g. argatroban, bivalirudin, dabigatran, rivaroxaban, apixaban, edoxaban), or indirectly by potentiating antithrombin-mediated protease inhibition (e.g. heparin, fondaparinux). Alternatively, synthesis of prothrombin and factor X, the zymogens of thrombin and factor Xa, can be reduced with vitamin K antagonists. These strategies are effective, but carry a risk of major bleeding because they do not distinguish thrombin that drives thrombosis from that required for hemostasis. The use of such agents, therefore, involves striking a somewhat precarious balance between a meaningful antithrombotic effect and an acceptable anticoagulant (antihemostatic) effect. This places limits on the intensity of therapy, the types of patients who can receive therapy, and the clinical situations in which therapy can be safely applied. Therapeutic targeting of contact factors may offer a way to separate antithrombotic and anti-hemostatic effects, predicated, of course, on such treatments demonstrating efficacy.
Given the limited clinical data available, it is difficult at present to draw definitive conclusions as to which protein, factor XI or factor XII, is the better antithrombotic target. Probably the choice will depend on the clinical situation. Table 1 lists some of the potential advantages and disadvantages of targeting FXI or FXII. A number of strategies directed at these zymogens or their active forms have undergone pre-clinical evaluation (Table 2), and one approach (reduction of plasma FXI) has undergone phase 1 and 2 evaluation in humans. The following sections review some of this work.
Table 1.
Possible Advantages and Disadvantages to Targeting Factor XI and Factor XII.
| Target | Advantages | Disadvantages |
|---|---|---|
| Factor XI or Factor XIa |
Increased plasma FXI levels increase risk for VTE, stroke, and possibly MI. |
|
| Lowering plasma FXI level reduced VTE incidence in humans after knee replacement surgery without aggravating bleeding. |
A high level of inhibition could increase bleeding with trauma or surgery, particularly if the nose, mouth or urinary tract is involved. |
|
| Inhibiting FXI would block contribution of contact activation and thrombin-mediated feedback activation on thrombosis. |
Antithrombotic effect may be modest under- conditions where tissue factor is driving thrombosis. |
|
| Severe FXI deficiency is associated with a relatively modest bleeding diathesis. |
A high level of inhibition could cause or aggravate menorrhagia. |
|
| FXI Inhibition causes a potent anti- inflammatory effect and blunts disseminated intravascular coagulation in murine sepsis models. |
||
| Factor XII or Factor XIIa |
FXII deficiency does not affect hemostasis | |
| FXII inhibition may be particularly effective in settings where blood is in contact with artificial surfaces (cardiopulmonary bypass, extracorporeal membrane oxygenation, chronic indwelling vascular catheters, ventricular assist devices, artificial heart valves) |
Plasma FXII levels do not correlate with risk for VTE, stroke, and possibly MI. |
|
| Antithrombotic effect could be countered by a partial defect in fibrinolysis. | ||
| Antithrombotic effect may be modest under- conditions where tissue factor is driving thrombosis. | ||
| Loss of FXIIa effect on fibrin could result in thrombi that fragment and embolize. | ||
| FXII inhibition may produce an anti- inflammatory effect through reduced activation of prekallikrein |
FXII contributes to multiple homeostatic processes. Long-term consequences of inhibition are not known. |
Table 2.
| Compounds Targeting Factor XI and Factor XIa. | ||||
|---|---|---|---|---|
| Inhibitor Type |
Compound | Mechanism of Action | In Vivo Analyses | Reference |
| Monoclonal Antibody |
O1A6 (aXIMAb) | Binds to FXI/XIa Apple 3 domain. Inhibits FXI activation and FXIa activation of factor IX. |
Inhibit thrombus formation in baboon AV shunt model. |
Tucker et al. Blood 2009;113:936 |
| 14E11 | Binds to FXI Apple 2 domain. Inhibits FXI activation by FXIIa, but does not affect FXI activation by thrombin, or FXIa activation of factor IX. |
Inhibits thrombus formation in baboon vascular graft model, and consumptive coagulopathy in a mouse peritonitis model. |
Cheng et al. Blood 2010;116:3981 Tucker et al. Blood 2012;119:4762 |
|
| Antisense Oligo- nucletoide |
FXI ASO (mouse) | Reduces hepatic synthesis of FXI. | Inhibits arterial and venous thrombosis. |
Zhang et al. Blood 2010;116:4684 |
| FXI ASO (rabbit) | Reduces hepatic synthesis of FXI. | Reduces catheter-induced venous thrombosis. |
Yau et al. Blood 2014;123:2102 | |
| FXI ASO (baboon) | Reduces hepatic synthesis of FXI. | Inhibits thrombus formation in vascular graft |
Crosby et al. ATVB 2013;33:1670 | |
| FXI ASO (human) ISIS-FXIRx |
Reduces hepatic synthesis of FXI. | Phase I and II trials in humans. Effective as prophylaxis for VTE in knee replacement surgery. |
Liu et al. Blood 2011;118:Abs209 Büller et al. N Engl J Med 2015;372:232 |
|
| Active site inhibitors |
Aryl boronic acid derivative |
Irreversible inhibitor of FXIa active site. | Not tested in animals. | Lazarova et al. Bioorgan Med Chem Lett 2006;16:5022 |
| Ketoarginine Peptidomimetic |
Irreversible inhibitor of FXIa active site. | Inhibits thrombus formation in a rat venous thrombosis model. |
Deng et al. Bioorgan Med Chem Lett 2006;16:3049 | |
| 4-carboxy-2- azetidinone compound |
Irreversible inhibitor of FXIa active site. | Inhibits thrombus formation in rabbit AV shunt model. |
Wong et al. J Thromb Hameost 2011;32:129 | |
| Tetrahydroquinalone derivative |
Reversible inhibitor of FXIa active site. | Inhibits thrombus formation in rabbit AV shunt model. |
Quan et al. J Med Chem 2014;57;955 | |
| Chloro- and amino- quinalone derivatives |
Inhibitor of FXIa active site. | Not tested in animals. | Fjellström et al. PloS One 2015;10:e0113705 | |
| Phenylimidazoles | Reversible inhibitor of FXIa active site. | Inhibits thrombus formation in rabbit AV shunt model |
Hangeland et al. J Med Chem 2014;57:9915 | |
| Allosteric inhibitors |
Sulfated Pentagalloylglucoside |
Non-competitive inhibitor of FXIa. Binds to charged residues on the FXI/XIa catalytic domain producing allosteric effect on activity. |
Testing underway in rodents | Al-Horani et al. J Med Chem 2013;56:867 Al-Horani and Desai. J med Chem 2013;57:4805 |
| Monosulfated benzofurans |
May bind to the FXIa A3 domain, producing an allosteric effect on activity. |
Not tested in animals. | Argade et al. J Med Chem 2014;57:3559 | |
| Natural Inhibitors |
Protease nexin-2 Kunitz-domain |
Inhibits FXIa active site | Inhibits arterial thrombosis in mice. | Wu et al. Blood 2012;120:671 |
| Clavatadine A | Bromine containing compound from marine sponge S. clavata. FXIa active site inhibitor? |
Not tested in animals. | Buchanan et al. J Med Chem 2008;51:3583 Conn et al. J Nat Prod 2015;78:120 |
|
| Desmolaris | Kunitz-type FXIa active site inhibitor from vampire bat saliva. |
Inhibits arterial thrombosis in mice. | Ma et al. Blood 2013;122:4094 | |
| Compounds Targeting Factor XII and Factor XIIa. | ||||
|---|---|---|---|---|
| Inhibitor Type |
Compound | Mechanism of Action | In Vivo Analyses | Reference |
| Monoclonal Antibody |
15H8 | Binds to the FXII heavy chain and inhibits FXII conversion to FXIIa |
Inhibits thrombus formation in baboon AV shunt model. Inhibits arterial thrombosis in mice. |
Matafonov et al. Blood 2014;123:1739 |
| 3F7 | Binds to the active site of FXIIa. | Inhibit thrombus formation in rabbit ECMO model. |
Larsson et al. Sci Transl Med 2014;6:222ra17 | |
| Antisense Oligo- nucletoide |
FXII ASO (mouse) | Reduces hepatic synthesis of FXII. | Inhibits arterial and venous thrombosis. |
Revenko et al. Blood 2011;118:5302 |
| FXII ASO (rabbit) | Reduces hepatic synthesis of FXII. | Reduces catheter-induced venous thrombosis. |
Yau et al. Blood 2014;123:2102 | |
| Small Molecule |
Bicyclic Peptide | Inhibitor of FXIIa cleavage of a chromogenic substrate – mechanisms not certain. |
Not tested in animals. | Baeriswyl et al. J Med Chem 2013;56:3742 |
| RNA aptamer |
R4cXII-1 | Inhibits FXII autoactivation and FXIIa activation of FXI. |
Not tested in animals. | Woodruff et al. J Thromb Haemost 2013;11:1362 |
| Natural Inhibitors |
Infestin-4 | Inhibitor of FXIIa active site. Inhibitor from the hematophagus insect Triatoma infestan linked to albumin. |
Inhibits arterial thrombosis in mice. Inhibits AV shunt thrombosis in rats and rabbits. |
Hagedorn et al. Circulation 2010;121:1510 Xu et al. Thromb Haemost 2014;111:694 |
FXI and FXII Inhibition in a Primate Thrombosis Model
While FXII and FXI are of comparable importance to thrombus formation in mice, FXI appears to play a greater role than FXII in VTE, stroke and MI in humans. One possible explanation for this discrepancy could relate to differences in mechanisms for FXI activation between species. For example, FXIIa-independent processes such as thrombin-mediated FXI activation (Figure 1) may be more prominent in primates than in mice. Alternatively, the acute injury-induced thromboses in mouse models may not mirror human disease closely. We addressed this recently using a primate model [52]. Anti-FXI monoclonal IgGs that block factor IX activation by FXIa (O1A6) [74,75] or FXI activation by FXIIa (14E11) [76], and an anti-FXII IgG that blocks FXII activation (15H8) [77], were tested for their effects on platelet and fibrin deposition in collagen-coated grafts deployed into temporary femoral AV shunts in baboons. A 2 mg/kg dose of O1A6 lowered plasma FXI activity to undetectable levels for more than a week, and significantly reduced platelet and fibrin accumulation within collagen-coated grafts [74]. As in FXI-deficient mice, thrombi were unstable in O1A6-treated baboons, probably due to reduced thrombin generation, as thrombin-antithrombin complex (TAT) levels downstream from the graft were reduced by >90%. The antithrombotic effect of O1A6 was comparable to full-dose heparin, but without an appreciable effect on hemostasis. In contrast, IgGs 14E11 and 15H8, which inhibit FXIIa-mediated activation of FXI but not FXI activation by thrombin or FXIa activity, did not reduce platelet deposition within the collagen-coated portion of grafts, although they significantly reduced thrombus growth downstream of the graft [76,77]. Therefore, FXI and FXII both contributed to thrombosis in this primate model, but FXIa inhibition was more effective than FXIIa inhibition at reducing thrombus growth. This is reasonably consistent with the human epidemiologic data, and supports the notion that FXI may make a stronger contribution than FXII during thrombotic processes in primates.
Reducing Plasma FXI to Prevent Thrombus Formation in Primates
Antisense-based technologies have been widely used to study the contributions of plasma proteins to thrombus formation. Second-generation DNA antisense oligonucleotides (ASOs) typically have an ~20 nucleotide sequence complementary to the mRNA of a protein of interest, flanked by short sequences of modified base pairs that improve performance properties. ASOs are taken up avidly by hepatocytes, making them ideal for studying coagulation proteins. After entering a cell, ASOs bind target mRNAs through complementary base pairing, leading to selective mRNA degradation and reduced protein synthesis [78]. In our experience, it takes two to three weeks of ASO therapy to achieve the maximum effect in primates (regardless of the protein being targeted), and recovery of plasma proteins levels can take several months after discontinuing treatment. In cynomolgus monkeys, the anti-human FXI ASO ISIS-416858 (4 to 40 mg/kg/wk) produced a dose-dependent reduction in plasma FXI, with ~80% reduction at higher doses [79]. Treated animals had moderately prolonged aPTTs, without evidence of a hemostatic deficit. In the baboon AV shunt model an antithrombotic effect was detected with as little as 50% reduction in plasma FXI, with maximum effect achieved at levels of ≤20% of normal [80].
Results from a phase 1 study of ISIS-416858 were reported in 2011 [14]. Healthy volunteers received three subcutaneous doses (50 to 300 mg) during the first week of therapy, and weekly doses thereafter. A dose response was observed, with higher (200 and 300 mg) ASO doses consistently reducing plasma FXI by ~80%, and >95% in some individuals. Mild irritation at injection sites was the most common side effect. There were no significant hematologic, liver or kidney abnormalities, and no excessive bleeding.
ISIS-416858 was recently studied in a phase 2 multicenter randomized trial for VTE prevention in patients undergoing total knee arthroplasty (TKA) [15]. The ASO was given in 200 or 300 mg subcutaneous doses on study days 1, 3, 5, 8, 15, 22, 29, 36, and 39, with surgery on day 36. The comparator were patients receiving standard enoxaparin prophylaxis. All patients underwent bilateral lower extremity venography 8 to 12 days post-surgery. On day 36, the average plasma FXI levels were 38% and 20% of normal in patients on the 200 or 300 mg ASO doses, respectively, and 93% in patients on enoxaparin. Lower extremity venous thrombi were detected in 30% of enoxaparin-treated patients, 27% of patients on the 200 mg ASO dose, and in only 4% receiving the 300 mg dose. Thrombi detectable by venography are expected in ~45% of TKA patients in the absence of prophylaxis [81]. The 300 mg dose of ISIS-416858 was superior, and the 200 mg dose was similar, to enoxaparin for reducing the primary outcome (VTE). Interestingly, the few thrombi that formed in patients treated with 300 mg ASO were much smaller than with 200 mg ASO or enoxaparin. Thus, there is evidence for a dose effect on thrombus size, as well as on thrombus incidence. In this study, clinically relevant bleeding occurred in 3% of patients on ASOs and 8% on enoxaparin. It is important to note that because ASO therapy was started well before the day of surgery, patients were under the full drug effect during surgery. Despite this, abnormal hemostasis was not observed during surgery, even in patients with FXI levels <5% of normal. Thus, anti-FXI ASO therapy appears to be safe from a bleeding standpoint in TKA.
Targeting FXII to Prevent Thrombus Formation on Artificial Surfaces
While the factor VIIa/TF complex is considered an important trigger of thrombosis in humans [82], there are situations where contact activation might be expected to dominate the process. Contact activation is triggered when blood is exposed to artificial surfaces during cardiopulmonary bypass [83,84] and extracorporeal membrane oxygenation (ECMO) [85], and may be a major driver of thrombosis and inflammation in these settings. In rabbits connected to pediatric ECMO circuits, Larsson et al showed that a monoclonal IgG (3F7) that targets FXIIa was as effective as heparin in preventing thrombotic circuit occlusion [86]. Unlike heparin, 3F7 did not compromise hemostasis. The use of heparin in humans on ECMO, many of whom are infants, can be difficult as patients are often prone to bleeding even while at high risk for thrombosis. Results with 3F7 suggest that it may be possible to replace heparin with a drug targeting contact activation in patients on ECMO. FXIIa converts PK to α-kallikrein, which cleaves HK, liberating BK (Figure 1, right panel). FXIIa inhibition may have an advantage over conventional anticoagulants or a FXI inhibitor with extracorporeal circuits because it may blunt contact activation-driven inflammation more effectively.
Yau et al. studied the effects of ASO-induced depletion (~90% reduction) of factor VII, FXI, FXII or HK on thrombus formation induced by polyurethane catheters inserted into rabbit jugular veins [87]. FXII or FXI reduction prolonged time to thrombus formation more than two-fold, while reducing factor VII or HK had little effect. The negative result with HK knockdown may suggest that a process different from classic contact activation (which is highly dependent on HK) is operating. However, since knockdown was not complete, residual HK may have been sufficient to support contact activation.
Future Considerations
Anticoagulants used in medical practice target the core of the thrombin generation mechanism, in line with the notion that processes that contribute to thrombosis are similar to those required for hemostasis. The counterintuitive discoveries that mice lacking contact proteases [50,51,53–56] and primates treated with anti-FXI antibodies [52,74–77] are at least as resistant to thrombus formation as wild type animals treated with full-dose heparin require that this premise be reconsidered. It could be argued that methods used to induce thrombosis in healthy animals favor contact activation in a manner not always relevant to human disease. However, the demonstration that lowering FXI in patients undergoing TKA produces an excellent therapeutic effect supports the hypothesis that FXI contributes substantively to VTE in some settings. From a practical standpoint, the results of the FXI-ASO in TKA trial confirm that it is possible to dissociate antithrombotic and anticoagulant effects (at least partially) in humans. But the study also raises important questions regarding our understanding of the biology of VTE. The factor VIIa/TF complex is considered a key element in the pathogenesis of thrombosis [82]. While the FXI-ASO study does not exclude a role for factor VIIa/TF in TKA-associated VTE, the strikingly low number of clots in patients treated with the higher FXI-ASO dose, and the small sizes of the few clots that did form [15], indicate factor VIIa/TF does not dominate the process. As a contact factor and a coagulation factor, FXI may be ideally positioned to influence thrombus formation and propagation (Figure 1). Its inhibition would block both contact activation initiated thrombin-generation and the feedback loop that sustains thrombin generation initiated by factor VIIa/TF. Reduced FXI-dependent thrombin generation could impact thrombus formation by reducing fibrin formation and platelet activation, and by increased thrombus instability, rendering clots more susceptible to fibrinolysis. Indeed, the beneficial effect of FXI reduction in the FXI-ASO TKA trial, as demonstrated by venography at a single time point post-surgery, could reflect a defect in thrombus initiation, compromised thrombus growth, enhanced thrombus degradation, or a combination of these factors. In vitro, FXI activation is enhanced by polyanions [37,44–46], and such interactions appear to contribute to thrombus formation in animal models [38–43]. If substances such as polyP, DNA or RNA have similar impacts on thrombotic processes in humans, then they also may be reasonable targets for antithrombotic therapy [88,89]. The contact system has been implicated in the innate response to invading microorganisms, which raises concerns that blocking contact reactions may weaken the host’s ability to ward off infection. However, a critical role for contact factors in fighting infection in humans is not established. Interestingly, while a study in rodents indicated that inhibiting FXII has a detrimental effect on bacterial clearance [34,35], FXI inhibition actually had beneficial effects, including survival advantages, in other infection models [90–92].
Based on the evidence at hand, therapies targeting FXI or FXIa should be tested initially as prophylaxis to prevent VTE and stroke. Data from the FXI-ASO in TKA study raise the possibility that therapy targeting FXI may be superior to standard of care for preventing post-operative DVT, but larger trials would be required to definitively demonstrate that. Inhibition of FXI may have a role in secondary prevention of VTE, and phase 3 trials examining this possibility should be considered. The utility of extending anticoagulation therapy beyond the typical three to six months of treatment for an unprovoked deep vein thrombosis or pulmonary embolus is actively debated [93,94]. While the advantage of preventing relatively rare fatal thrombotic events with extended prophylaxis is partly offset by an increase in major bleeding, lowering the incidence of common complications such as post-thrombotic syndrome and pulmonary hypertension may warrant such treatment. Extrapolating from the TKA data, inhibiting FXI may provide extended protection from recurrence with a lower bleeding risk than with warfarin or a direct oral anticoagulant (DOAC). Inhibiting FXIa may also be useful in primary or secondary prevention in patients with atrial fibrillation or other high-risk conditions who are not good candidates for warfarin or DOAC therapy due to comorbidities, or as short-term prophylaxis after neurosurgery or other procedures where even modest anticoagulant-induced bleeding is unacceptable.
While the efficacy of targeting FXI or contact activation as initial treatment for acute thrombotic events has not been demonstrated, such approaches may be useful in conjunction with current standards of care. A number of anticoagulant and/or antiplatelet agents have been studied in combination, but increased bleeding is often a problem. For example, in the TRACER trial, superimposing the PAR-1 inhibitor vorapaxar onto standard antiplatelet therapy (aspirin and a thienopyridine) in patients with acute coronary syndromes resulted in an increase in intracranial bleeding in patients with a prior history of stroke [95]. Adding the DOAC rivaroxaban to standard anti-platelet therapy in the ATLAS ACS–TIMI 46 trial reduced death from cardiovascular disease, MI and stroke compared to standard therapy alone, but at the cost of a significant increase in major bleeding [96]. Given the anticipated safety profiles of FXIa and FXIIa inhibitors, adding such compounds to standard anti-platelet or anticoagulant therapies may provide additional therapeutic benefit without increased bleeding risk. Indeed, patients with severe FXI deficiency have been treated safely with warfarin or dual anti-platelet therapy for atrial fibrillation and myocardial infarction [97]. It is also possible that the beneficial effects of FXI or FXII inhibitors may allow doses of other antithrombotic drugs to be reduced, lowering the bleeding risks associated with those drugs.
Contact activation is triggered during surgery involving cardiopulmonary bypass and during ECMO, and probably occurs to different degrees with exposure of blood to a variety of extracorporeal circuits and indwelling intravascular devices [83–87]. The recent work showing that FXIIa inhibition prevents thrombus formation in experimental ECMO [86] suggests that FXIIa or FXIa inhibitors may be reasonable replacements or supplements for heparin in patients on extracorporeal circuits. Along similar lines, there is a clear need for alternatives to warfarin for patients with mechanical heart valves. The DOACs are not approved for use in these patients, and the RE-ALIGN trial comparing the thrombin inhibitor dabigatran to warfarin after mechanical valve placement was terminated early because of higher incidences of thrombotic and bleeding episodes in the dabigatran arm [98]. Unlike warfarin, which produces a relatively stable anticoagulant effect over time between doses, there are substantial differences between peak and trough levels with dabigatran. Conceivably, subtherapeutic trough levels of the drug could have contributed to thrombus formation in the RE-ALIGN trial [99]. While the contributions of FXIa and FXIIa to thrombus formation induced by mechanical heart valves have not been evaluated, specific inhibitors targeting these proteases could have long half-lives without compromising safety. In contrast to currently used anticoagulants, it may be possible to maintain near complete inhibition of the target protease with few side effects, as consequences of drug overdose on hemostasis should be small.
We now have clear indications that targeting FXI can produce a useful antithrombotic effect in humans prone to thromboembolism. Future efforts should be directed at establishing the spectrum of clinical scenarios in which FXI and contact activation contribute to thrombosis, and determining which components of the contact system are the best targets for each clinical situation. If drugs targeting contact proteases prove to be effective antithrombotics, their safety profiles could increase the number of patients who are eligible to receive antithrombotic therapy, and widen the spectrum of clinical conditions in which antithrombotic therapy can be safely administered.
ACKNOWLEDGMENTS
The authors wish to acknowledge support from National Institutes of Health Awards (Heart, Lung and Blood Institute) HL81326 and HL58837 (D. Gailini), HL101972 (A. Gruber); (National Institute of Allergy and Infectious Diseases) AI088937 (A. Gruber); and National Institutes of Health awards UL1TR000128 to the Oregon Clinical and Translational Research Institute and RR000163 to the Oregon National Primate Research Center.
D. Gailini reports personal fees from Bayer, Bristol-Myers Squibb, Dyax, Isis, Merck, Ono Pharmaceuticals, Novartis, Diagnostica Stago and Instrument Laboratory, outside the submitted work. A. Gruber is an employee of Aronora and D. Gailini received antibodies for research and is on the Scientific Board of this company. A. Gruber and D. Gailini have a patent Factor XI Antibodies and Uses Thereof with royalties paid, and a patent Factor XII Antibodies and Uses Thereof issued. In addition, A. Gruber and D. Gailini report that Oregon Health and Science University, and Vanderbilt University may have financial interest in some of the work described in this review.
Footnotes
CONFLICT-OF-INTEREST DISCLOSURES
C. E. Bane states that he has no conflict of interest.
REFERENCES
- 1.Renné T. The procoagulant and proinflammatory contact system. Semin Immunopathol. 2012;3:31–41. doi: 10.1007/s00281-011-0288-2. [DOI] [PubMed] [Google Scholar]
- 2.Gailani D, Renné T. J Emsley: Factor XI and the Plasma contact system. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. Metabolic and Molecular Bases of Inherited Disease. NY, NY: McGraw-Hill; 2010. [Google Scholar]
- 3.Schmaier AH. Physiologic activities of the contact activation system. Thromb Res. 2014;133(Suppl 1):S41–S44. doi: 10.1016/j.thromres.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gailani D, Neff AT. Rare coagulation factor deficiencies. In: Hoffman R, Nenz EJ, Silberstein LE, Heslop HE, Weitz JI, Anastasi J, editors. Hematology: basic principles and practice. 6th ed. Saunders-Elsevier; 2010. pp. 1939–1952. [Google Scholar]
- 5.Rosenthal R, Dreskin O, Rosenthal N. Plasma thromboplastin antecedent (PTA) deficiency: Clinical, coagulation, therapeutic and hereditary aspects of a new hemophilia-like disease. Blood. 1955;10:120–131. [PubMed] [Google Scholar]
- 6.Ratnoff O, Colopy J. A familial hemorrhagic trait associated with a deficiency of a clot promoting fraction of plasma. J Clin Invest. 1955;34:602–613. doi: 10.1172/JCI103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James P, Salomon O, Mikovic D, Peyvandi F. Rare bleeding disorders – bleeding assessment tools, laboratory aspects and phenotype and therapy of FXI deficiency. Haemophilia. 2014;20(Suppl 4):71–75. doi: 10.1111/hae.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seligsohn U. Factor XI deficiency in humans. J Thromb Haemost. 2009;7(Suppl 1):84–87. doi: 10.1111/j.1538-7836.2009.03395.x. [DOI] [PubMed] [Google Scholar]
- 9.Van Montfoort ML, Meijers JCM. Recent insights into the role of the contact pathway in thrombo-inflammatory disorders. Hematology Am Soc Hematol Educ Program. 2014;2014:60–65. doi: 10.1182/asheducation-2014.1.60. [DOI] [PubMed] [Google Scholar]
- 10.Key NS. Epidemiologic and clinical data linking factors XI and XII to thrombosis. Hematology Am Soc Hematol Educ Program. 2014;2014:66–70. doi: 10.1182/asheducation-2014.1.66. [DOI] [PubMed] [Google Scholar]
- 11.Woodruff RS, Sullenger B, Becker RC. The many faces of the contact pathway and their role in thrombosis. J Thromb Thrombolysis. 2011;32:9–20. doi: 10.1007/s11239-011-0578-5. [DOI] [PubMed] [Google Scholar]
- 12.Emsley J, McEwan PA, Gailani D. Structure and function of factor XI. Blood. 2010;115:2569–2577. doi: 10.1182/blood-2009-09-199182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renné T, Oschatz C, Seifert S, Müller F, Antovic J, Karlman M, Benz PM. Factor XI deficiency in animal models. J Thromb Haemost. 2009;7(Suppl 1):79–83. doi: 10.1111/j.1538-7836.2009.03393.x. [DOI] [PubMed] [Google Scholar]
- 14.Liu Q, Bethune C, Dessouki E, Grundy J, Monia BP, Bhanot S. ISIS-FXIRx, A Novel and Specific Antisense Inhibitor of Factor XI, Caused Significant Reduction in FXI Antigen and Activity and Increased aPTT without Causing Bleeding in Healthy Volunteers. Blood. 2011;118 Abstract 209. [Google Scholar]
- 15.Büller HR, Bethune C, Bhanot S, Gailani D, Monia BP, Raskob GE, Segers A, Verhamme P, Weitz JI FXI-ASO TKA Investigators. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372:232–240. doi: 10.1056/NEJMoa1405760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gailani D. Future prospects for contact factors as therapeutic targets. Hematology Am Soc Hematol Educ Program. 2014;2014:52–59. doi: 10.1182/asheducation-2014.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doolittle RF. Step-by-step evolution of vertebrate coagulation. Cold Spring Harb Symp Quant Biol. 2009;74:35–40. doi: 10.1101/sqb.2009.74.001. [DOI] [PubMed] [Google Scholar]
- 18.Ponczek MB, Gailani D, Doolittle RF. Evolution of the contact phase of vertebrate blood coagulation. J Thromb Haemost. 2008;6:1876–1883. doi: 10.1111/j.1538-7836.2008.03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou L, Li-Ling J, Huang H, Ma F, Li Q. Phylogenetic analysis of vertebrate kininogen genes. Genomics. 2008;91:129–141. doi: 10.1016/j.ygeno.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Thompson RE, Mandle R, Jr, Kaplan AP. Studies of binding of prekallikrein and Factor XI to high molecular weight kininogen and its light chain. Proc Natl Acad Sci U S A. 1979;76:4862–4866. doi: 10.1073/pnas.76.10.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiggins RC, Bouma BN, Cochrane CG, Griffin JH. Role of high-molecular-weight kininogen in surface-binding and activation of coagulation Factor XI and prekallikrein. Proc Natl Acad Sci U S A. 1977;74:4636–4640. doi: 10.1073/pnas.74.10.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geng Y, Verhamme IM, Smith SB, Sun MF, Matafonov A, Cheng Q, Smith SA, Morrissey JH, Gailani D. The dimeric structure of factor XI and zymogen activation. Blood. 2013;121:3962–3969. doi: 10.1182/blood-2012-12-473629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naito K, Fujikawa K. Activation of human blood coagulation factor XI independent of factor XII. Factor XI is activated by thrombin and factor XIa in the presence of negatively charged surfaces. J Biol Chem. 1991;266:7353–7358. [PubMed] [Google Scholar]
- 24.Gailani D, Broze GJ., Jr Factor XI activation in a revised model of blood coagulation. Science. 1991;253:909–912. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 25.Matafonov A, Sarilla S, Sun MF, Sheehan JP, Serebrov V, Verhamme IM, Gailani D. Activation of factor XI by products of prothrombin activation. Blood. 2011;118:437–445. doi: 10.1182/blood-2010-10-312983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffin JH. Role of surface in surface-dependent activation of Hageman factor (blood coagulation Factor XII) Proc Natl Acad Sci U S A. 1978;75:1998–2002. doi: 10.1073/pnas.75.4.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whelihan MF, Orfeo T, Gissel MT, Mann KG. Coagulation procofactor activation by factor XIa. J Thromb Haemost. 2010;8:1532–1539. doi: 10.1111/j.1538-7836.2010.03899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matafonov A, Cheng Q, Geng Y, Verhamme IM, Umunakwe O, Tucker EI, Sun MF, Serebrov V, Gruber A, Gailani D. Evidence of factor IX-independent roles for factor XIa in blood coagulation. J Thromb Haemost. 2013;11:2118–2127. doi: 10.1111/jth.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puy C, Tucker EI, Matafonov A, Cheng Q, Zientek KD, Gailani D, Gruber A, McCarty OJ. Activated factor XI increases the procoagulant activity of the extrinsic pathway by inactivating tissue factor pathway inhibitor. Blood. doi: 10.1182/blood-2014-10-604587. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salomon O, Steinberg DM, Seligshon U. Variable bleeding manifestations characterize different types of surgery in patients with severe factor XI deficiency enabling parsimonious use of replacement therapy. Haemophilia. 2006;12:490–493. doi: 10.1111/j.1365-2516.2006.01304.x. [DOI] [PubMed] [Google Scholar]
- 31.Zucker M, Seligsohn U, Salomon O, Wolberg AS. Abnormal plasma clot structure and stability distinguish bleeding risk in patients with severe factor XI deficiency. J Thromb Haemost. 2014;12:1121–1130. doi: 10.1111/jth.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Von dem Borne PA, Bajzar L, Meijers JC, Nesheim ME, Bouma BN. Thrombin-mediated activation of factor XI results in thrombin-activatable fibrinolysis inhibitor-dependent inhibition of fibrinolysis. J Clin Invest. 1997;99:2323–2327. doi: 10.1172/JCI119412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marx PF, Verkleij CJ, Valls Seron M, Meijers JC. Recent developments in thrombin-activatable fibrinolysis inhibitor research. Mini Rev Med Chem. 2009;9:1165–1173. doi: 10.2174/138955709789055216. [DOI] [PubMed] [Google Scholar]
- 34.Frick IM, Björck L, Herwald H. The dual role of the contact system in bacterial infectious disease. Thromb Haemost. 2007;98:497–502. [PubMed] [Google Scholar]
- 35.Frick IM, Akesson P, Herwald H, Mörgelin M, Malmsten M, Nägler DK, Björck L. The contact system – a novel branch of innate immunity generating antibacterial peptides. EMBO J. 2006;25:5569–5578. doi: 10.1038/sj.emboj.7601422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith SA, Morrissey JH. Polyphosphate: a new player in the field of hemostasis. Curr Opin Hematol. 2014;21:388–394. doi: 10.1097/MOH.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith SA, Choi SH, Davis-Harrison R, Huyck J, Boettcher J, Rienstra CM, Morrissey JH. Polyphosphate exerts differential effects on blood clotting depending on polymer size. Blood. 2010;116:4353–4359. doi: 10.1182/blood-2010-01-266791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer S, Preissner KT. Extracellular nucleic acids as novel alarm signals in the vascular system. Mediators of defence and disease. Hamostaseologie. 2013;33:37–42. doi: 10.5482/HAMO-13-01-0001. [DOI] [PubMed] [Google Scholar]
- 39.Kannemeier C, Shibamiya A, Nakazawa F, Trusheim H, Ruppert C, Markart P, Song Y, Tzima E, Kennerknecht E, Niepmann M, von Bruehl ML, Sedding D, Massberg S, Günther A, Engelmann B, Preissner KT. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A. 2007;104:6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SH, Weitz JI, Liaw PC. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34:1977–1984. doi: 10.1161/ATVBAHA.114.304114. [DOI] [PubMed] [Google Scholar]
- 41.Müller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renné T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–144. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi SH, Smith SA, Morrissey JH. Polyphosphate as a cofactor for the activation of factor XI by thrombin. Blood. 2011;118:6963–6970. doi: 10.1182/blood-2011-07-368811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matafonov A, Ivanov IS, Sun M-F, Gailani D. A role for coagulation factor XI and factor XII in DNA-induced thrombin generation. Blood. 2013;124 Abstract 581. [Google Scholar]
- 46.Geng Y, Verhamme IM, Smith SA, Cheng Q, Sun M, Sheehan JP, Morrissey JH, C D. Factor XI anion-binding sites are required for productive interactions with polyphosphate. J Thromb Haemost. 2013;11:2020–2028. doi: 10.1111/jth.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konings J, Govers-Riemslag JW, Philippou H, Mutch NJ, Borissoff JI, Allan P, Mohan S, Tans G, Ten Cate H, Ariëns RA. Factor XIIa regulates the structure of the fibrin clot independently of thrombin generation through direct interaction with fibrin. Blood. 2011;118:3942–3951. doi: 10.1182/blood-2011-03-339572. [DOI] [PubMed] [Google Scholar]
- 48.Minnema MC, Friederich PW, Levi M, von dem Borne PA, Mosnier LO, Meijers JC, Biemond BJ, Hack CE, Bouma BN, ten Cate H. Enhancement of rabbit jugular vein thrombolysis by neutralization of factor XI. In vivo evidence for a role of factor XI as an anti-fibrinolytic factor. J Clin Invest. 1998;101:10–14. doi: 10.1172/JCI781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan JC, Ganopolsky JG, Cornelissen I, Suckow MA, Sandoval-Cooper MJ, Brown EC, Noria F, Gailani D, Rosen ED, Ploplis VA, Castellino FJ. The characterization of mice with a targeted combined deficiency of protein c and factor XI. Am J Pathol. 2001;158:469–479. doi: 10.1016/S0002-9440(10)63989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosen ED, Gailani D, Castellino FJ. FXI is essential for thrombus formation following FeCl3-induced injury of the carotid artery in the mouse. Thromb Haemost. 2002;87:774–776. [PubMed] [Google Scholar]
- 51.Wang X, Cheng Q, Xu L, Feuerstein GZ, Hsu MY, Smith PL, Seiffert DA, Schumacher WA, Ogletree ML, Gailani D. Effects of factor IX or factor XI deficiency on ferric chloride-induced carotid artery occlusion in mice. J Thromb Haemost. 2005;3:695–702. doi: 10.1111/j.1538-7836.2005.01236.x. [DOI] [PubMed] [Google Scholar]
- 52.Gruber A, Hanson SR. Factor XI-dependence of surface- and tissue factor-initiated thrombus propagation in primates. Blood. 2003;102:953–955. doi: 10.1182/blood-2003-01-0324. [DOI] [PubMed] [Google Scholar]
- 53.Renné T, Pozgajová M, Grüner S, Schuh K, Pauer HU, Burfeind P, Gailani D, Nieswandt B. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merkulov S, Zhang WM, Komar AA, Schmaier AH, Barnes E, Zhou Y, Lu X, Iwaki T, Castellino FJ, Luo G, McCrae KR. Deletion of murine kininogen gene 1 (mKng1) causes loss of plasma kininogen and delays thrombosis. Blood. 2008;111:1274–1281. doi: 10.1182/blood-2007-06-092338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Revenko AS, Gao D, Crosby JR, Bhattacharjee G, Zhao C, May C, Gailani D, Monia BP, MacLeod AR. Selective depletion of plasma prekallikrein or coagulation factor XII inhibits thrombosis in mice without increased risk of bleeding. Blood. 2011;118:5302–5311. doi: 10.1182/blood-2011-05-355248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bird JE, Smith PL, Wang X, Schumacher WA, Barbera F, Revelli JP, Seiffert D. Effects of plasma kallikrein deficiency on haemostasis and thrombosis in mice: murine ortholog of the Fletcher trait. Thromb Haemost. 2012;107:1141–1150. doi: 10.1160/th-11-10-0682. [DOI] [PubMed] [Google Scholar]
- 57.Stavrou EX, Fang C, Merkulova A, Alhalabi O, Grobe N, Antoniak S, Mackman N, Schmaier AH. Reduced thrombosis in Klkb1-/- mice is mediated by increased Mas receptor, prostacyclin, Sirt1, and KLF4 and decreased tissue factor. Blood. 2015;125:710–719. doi: 10.1182/blood-2014-01-550285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salomon O, Steinberg DM, Zucker M, Varon D, Zivelin A, Seligsohn U. Patients with severe factor XI deficiency have a reduced incidence of deep-vein thrombosis. Thromb Haemost. 2011;105:269–273. doi: 10.1160/TH10-05-0307. [DOI] [PubMed] [Google Scholar]
- 59.Meijers JC, Tekelenburg WL, Bouma BN, Bertina RM, Rosendaal FR. High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med. 2000;342:696–701. doi: 10.1056/NEJM200003093421004. [DOI] [PubMed] [Google Scholar]
- 60.Cushman M, O'Meara ES, Folsom AR, Heckbert SR. Coagulation factors IX through XIII and the risk of future venous thrombosis: the longitudinal investigation of thromboembolism etiology. Blood. 2009;114:2878–2883. doi: 10.1182/blood-2009-05-219915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salomon O, Steinberg DM, Koren-Morag N, Tanne D, Seligsohn U. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood. 2008;111:4113–4117. doi: 10.1182/blood-2007-10-120139. [DOI] [PubMed] [Google Scholar]
- 62.Yang DT, Flanders MM, Kim H, Rodgers GM. Elevated factor XI activity levels are associated with an increased odds ratio for cerebrovascular events. Am J Clin Pathol. 2006;126:411–415. doi: 10.1309/QC259F09UNMKVP0R. [DOI] [PubMed] [Google Scholar]
- 63.Suri MF, Yamagishi K, Aleksic N, Hannan PJ, Folsom AR. Novel hemostatic factor levels and risk of ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Cerebrovasc Dis. 2010;29:497–502. doi: 10.1159/000297966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siegerink B, Govers-Riemslag JW, Rosendaal FR, Ten Cate H, Algra A. Intrinsic coagulation activation and the risk of arterial thrombosis in young women: results from the Risk of Arterial Thrombosis in relation to Oral contraceptives (RATIO) case-control study. Circulation. 2010;122:1854–1861. doi: 10.1161/CIRCULATIONAHA.110.943738. [DOI] [PubMed] [Google Scholar]
- 65.Salomon O, Steinberg DM, Dardik R, Rosenberg N, Zivelin A, Tamarin I, Ravid B, Berliner S, Seligsohn U. Inherited factor XI deficiency confers no protection against acute myocardial infarction. J Thromb Haemost. 2003;1:658–661. doi: 10.1046/j.1538-7836.2003.00195.x. [DOI] [PubMed] [Google Scholar]
- 66.Doggen CJ, Rosendaal FR, Meijers JC. Levels of intrinsic coagulation factors and the risk of myocardial infarction among men: Opposite and synergistic effects of factors XI and XII. Blood. 2006;108:4045–4051. doi: 10.1182/blood-2005-12-023697. [DOI] [PubMed] [Google Scholar]
- 67.Butenas S, Undas A, Gissel MT, Szuldrzynski K, Zmudka K, Mann KG. Factor XIa and tissue factor activity in patients with coronary artery disease. Thromb Haemost. 2008;99:142–149. doi: 10.1160/TH07-08-0499. [DOI] [PubMed] [Google Scholar]
- 68.Berliner JI, Rybicki AC, Kaplan RC, Monrad ES, Freeman R, Billett HH. Elevated levels of Factor XI are associated with cardiovascular disease in women. Thromb Res. 2002;107:55–60. doi: 10.1016/s0049-3848(02)00190-1. [DOI] [PubMed] [Google Scholar]
- 69.Govers-Riemslag JW, Smid M, Cooper JA, Bauer KA, Rosenberg RD, Hack CE, Hamulyak K, Spronk HM, Miller GJ, ten Cate H. The plasma kallikrein-kinin system and risk of cardiovascular disease in men. J Thromb Haemost. 2007;5:1896–1903. doi: 10.1111/j.1538-7836.2007.02687.x. [DOI] [PubMed] [Google Scholar]
- 70.Xu-Cai YO, Shen J, Chen S, Zhou Y, Larusch GA, Stavrou E, Schmaier AH, Wu Q. Factor XII gene mutation in the Hageman family. J Thromb Haemost. 2011;9:2329–2331. doi: 10.1111/j.1538-7836.2011.04508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeerleder S, Schloesser M, Redondo M, Wuillemin WA, Engel W, Furlan M, Lämmle B. Reevaluation of the incidence of thromboembolic complications in congenital factor XII deficiency--a study on 73 subjects from 14 Swiss families. Thromb Haemost. 1999;82:1240–1246. [PubMed] [Google Scholar]
- 72.Koster T, Rosendaal FR, Briet E, Vandenbroucke JP. John Hageman's factor and deep-vein thrombosis: Leiden Thrombophilia Study. Brit J Haematol. 1994;87:422–424. doi: 10.1111/j.1365-2141.1994.tb04937.x. [DOI] [PubMed] [Google Scholar]
- 73.Endler G, Marsik C, Jilma B, Schickbauer T, Quehenberger P, Mannhalter C. Evidence of a U-shaped association between factor XII activity and overall survival. J Thromb Haemost. 2007;5:1143–1148. doi: 10.1111/j.1538-7836.2007.02530.x. [DOI] [PubMed] [Google Scholar]
- 74.Tucker EI, Marzec UM, White TC, Hurst S, Rugonyi S, McCarty OJ, Gailani D, Gruber A, Hanson SR. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood. 2009;113:936–944. doi: 10.1182/blood-2008-06-163675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geng Y, Verhamme IM, Messer A, Sun MF, Smith SB, Bajaj SP, Gailani D. A sequential mechanism for exostie-mediated factor IX activation by factor XIa. J Biol Chem. 2012;287:38200–38209. doi: 10.1074/jbc.M112.376343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheng Q, Tucker EI, Pine MS, Sisler I, Matafonov A, Sun MF, White-Adams TC, Smith SA, Hanson SR, McCarty OJ, Renné T, Gruber A, Gailani D. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood. 2010;116:3981–3989. doi: 10.1182/blood-2010-02-270918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matafonov A, Leung PY, Gailani AE, Grach SL, Puy C, Cheng Q, Sun MF, McCarty OJ, Tucker EI, Kataoka H, Renné T, Morrissey JH, Gruber A, Gailani D. Factor XII inhibition reduces thrombus formation in a primate thrombosis model. Blood. 2014;123:1739–1746. doi: 10.1182/blood-2013-04-499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang H, Löwenberg EC, Crosby JR, MacLeod AR, Zhao C, Gao D, Black C, Revenko AS, Meijers JC, Stroes ES, Levi M, Monia BP. Inhibition of the intrinsic coagulation pathway factor XI by antisense oligonucleotides: a novel antithrombotic strategy with lowered bleeding risk. Blood. 2010;116:4684–4692. doi: 10.1182/blood-2010-04-277798. [DOI] [PubMed] [Google Scholar]
- 79.Younis HS, Crosby J, Huh JI, Lee HS, Rime S, Monia B, Henry SP. Antisense inhibition of coagulation factor XI prolongs APTT without increased bleeding risk in cynomolgus monkeys. Blood. 2012;119:2401–2408. doi: 10.1182/blood-2011-10-387134. [DOI] [PubMed] [Google Scholar]
- 80.Crosby JR, Marzec U, Revenko AS, Zhao C, Gao D, Matafonov A, Gailani D, MacLeod AR, Tucker EI, Gruber A, Hanson SR, Monia BP. Antithrombotic effect of antisense factor XI oligonucleotide treatment in primates. Arterioscler Thromb Vasc Biol. 2013;33:1670–1678. doi: 10.1161/ATVBAHA.113.301282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fuji T, Fujita S, Tachibana S, Kawai Y. A dose-ranging study evaluating the oral factor Xa inhibitor edoxaban for the prevention of venous thromboembolism in pa tients undergoing total knee arthroplasty. J Thromb Haemost. 2010;8:2458–2468. doi: 10.1111/j.1538-7836.2010.04021.x. [DOI] [PubMed] [Google Scholar]
- 82.Owens AP, 3rd, Mackman N. Tissue factor and thrombosis: The clot starts here. Thromb Haemost. 2010;104:432–439. doi: 10.1160/TH09-11-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sonntag J, Dähnert I, Stiller B, Hetzer R, Lange PE. Complement and contact activation during cardiovascular operations in infants. Ann Thorac Surg. 1998;65:525–531. doi: 10.1016/s0003-4975(97)01340-4. [DOI] [PubMed] [Google Scholar]
- 84.Wendel HP, Jones DW, Gallimore MJ. FXII levels, FXIIa-like activities and kallikrein activities in normal subjects and patients undergoing cardiac surgery. Immunopharmacology. 1999;45:141–144. doi: 10.1016/s0162-3109(99)00067-3. [DOI] [PubMed] [Google Scholar]
- 85.Plötz FB, van Oeveren W, Bartlett RH, Wildevuur CR. Blood activation during neonatal extracorporeal life support. J Thorac Cardiovasc Surg. 1993;105:823–832. [PubMed] [Google Scholar]
- 86.Larsson M, Rayzman V, Nolte MW, Nickel KF, Björkqvist J, Jämsä A, Hardy MP, Fries M, Schmidbauer S, Hedenqvist P, Broomé M, Pragst I, Dickneite G, Wilson MJ, Nash AD, Panousis C, Renné T. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med. 2014;6:222ra17. doi: 10.1126/scitranslmed.3006804. [DOI] [PubMed] [Google Scholar]
- 87.Yau JW, Liao P, Fredenburgh JC, Stafford AR, Revenko AS, Monia BP, Weitz JI. Selective depletion of factor XI or factor XII with antisense oligonucleotides attenuates catheter thrombosis in rabbits. Blood. 2014;123:2102–2107. doi: 10.1182/blood-2013-12-540872. [DOI] [PubMed] [Google Scholar]
- 88.Travers RJ, Shenoi RA, Kalathottukaren MT, Kizhakkedathu JN, Morrissey JH. Nontoxic polyphosphate inhibitors reduce thrombosis while sparing hemostasis. Blood. 2014;124:3183–3190. doi: 10.1182/blood-2014-05-577932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith SA, Choi SH, Collins JN, Travers RJ, Cooley BC, Morrissey JH. Inhibition of polyphosphate as a novel strategy for preventing thrombosis and inflammation. Blood. 2012;120:5103–5110. doi: 10.1182/blood-2012-07-444935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tucker EI, Gailani D, Hurst S, Cheng Q, Hanson SR, Gruber A. Survival advantage of coagulation factor XI-deficient mice during peritoneal sepsis. J Infect Dis. 2008;198:271–274. doi: 10.1086/589514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tucker EI, Verbout NG, Leung PY, Hurst S, McCarty OJ, Gailani D, Gruber A. Inhibition of factor XI activation attenuates inflammation and coagulopathy while improving the survival of mouse polymicrobial sepsis. Blood. 2012;119:4762–4768. doi: 10.1182/blood-2011-10-386185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luo D, Szaba FM, Kummer LW, Johnson LL, Tucker EI, Gruber A, Gailani D, Smiley ST. Factor XI-deficient mice display reduced inflammation, coagulopathy, and bacterial growth during listeriosis. Infect Immun. 2012;80:91–99. doi: 10.1128/IAI.05568-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schulman S. Secondary prevention of venous thromboembolism. BMJ. 2013;347:f5440. doi: 10.1136/bmj.f5440. [DOI] [PubMed] [Google Scholar]
- 94.Kearson C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood. 2013;123:1794–1801. doi: 10.1182/blood-2013-12-512681. [DOI] [PubMed] [Google Scholar]
- 95.Tricoci P, Huang Z, Held C, Moliterno DJ, Armstrong PW, Van de Werf F, White HD, Aylward PE, Wallentin L, Chen E, Lokhnygina Y, Pei J, Leonardi S, Rorick TL, Kilian AM, Jennings LHK, Ambrosio G, Bode C, Cequier A, Cornel JH, et al. for the TRACER Investigators. N Engl J Med. 2012;366:20–33. [Google Scholar]
- 96.Mega JL, Braunwald E, Wiviott SD, Bassand J-P, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KAA, Goto, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FWA, Gibson CM for the ATLAS ACS 2–TIMI 51 Investigators. N Engl J Med. 2012;366:9–19. [Google Scholar]
- 97.Salomon O, Seligsohn U. New Observations on factor XI deficiency. Haemophilia. 2004;10(Supple.4):184–187. doi: 10.1111/j.1365-2516.2004.00992.x. [DOI] [PubMed] [Google Scholar]
- 98.Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, Blatchford J, Devenny K, Friedman J, Guiver K, Harper R, Khder Y, Lobmeyer MT, Maas H, Voigt J-W, Simoons ML, Van de Werf F for the RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214. doi: 10.1056/NEJMoa1300615. [DOI] [PubMed] [Google Scholar]
- 99.Hylek EM. Dabigatran and mechanical heart valves – not as easy as we hoped. N Engl J Med. 2013;369:1264–1266. doi: 10.1056/NEJMe1310399. [DOI] [PubMed] [Google Scholar]