Abstract
Objective/Method
Aggrecanase activity, most notably ADAMTS-5, is implicated in pathogenic cartilage degradation. Selective monoclonal antibodies (mAbs) to both ADAMTS-5 and ADAMTS-4 were generated and in vitro, ex vivo and in vivo systems were utilized to assess target engagement, aggrecanase inhibition and modulation of disease-related endpoints with the intent of selecting a candidate for clinical development in osteoarthritis (OA).
Results
Structural mapping predicts the most potent mAbs employ a unique mode of inhibition by cross-linking the catalytic and disintegrin domains. In a surgical mouse model of OA, both ADAMTS-5 and ADAMTS-4-specific mAbs penetrate cartilage following systemic administration, demonstrating access to the anticipated site of action. Structural disease modification and associated alleviation of pain-related behavior were observed with ADAMTS-5 mAb treatment. Treatment of human OA cartilage demonstrated a preferential role for ADAMTS-5 inhibition over ADAMTS-4, as measured by ARGS neoepitope release in explant cultures. ADAMTS-5 mAb activity was most evident in a subset of patient-derived tissues and suppression of ARGS neoepitope release was sustained for weeks after a single treatment in human explants and in cynomolgus monkeys, consistent with high affinity target engagement and slow ADAMTS-5 turnover.
Conclusion
This data supports a hypothesis set forth from knockout mouse studies that ADAMTS-5 is the major aggrecanase involved in cartilage degradation and provides a link between a biological pathway and pharmacology which translates to human tissues, non-human primate models and points to a target OA patient population. Therefore, a humanized ADAMTS-5-selective monoclonal antibody (GSK2394002) was progressed as a potential OA disease modifying therapeutic.
Keywords: ADAMTS-5, ADAMTS-4, Osteoarthritis, Monoclonal Antibody, Cartilage, Disease-Modifying Osteoarthritis Drugs (DMOADs)
Introduction
Translational drug development can be enhanced by addressing a series of guiding principles throughout the pre-clinical stages of development including: strategic drug design for the disease; demonstration of target engagement and pharmacology within the appropriate disease tissue; and co-development of mechanism-related pharmacodynamic markers to assess efficacy, identify likely responders and guide dose selection. Although specific criteria can be tailored for each target and therapeutic modality, these fundamental principles remain consistent. Employing these principles we aimed to produce selective aggrecanase (ADAMTS-4 and ADAMTS-5) inhibitors to guide selection of a disease-modifying osteoarthritis drug (DMOAD).
OA is the most common form of arthritis and recent estimates suggest that half of individuals over age 65 are affected [1]. Between 2002 and 2007, OA moved from the twelfth to the sixth leading cause of years lost to disability or morbidity [2], a trend that is expected to continue as the population ages and obesity rates rise. Yet there are currently no proven disease modifying pharmacologic therapies available. Hallmarks of OA include cartilage destruction with ensuing pain and disability. Pathogenic cartilage degradation processes have been a significant research focus to identify key disease drivers and develop novel treatments. Numerous cartilage degrading proteases have been identified which may contribute to OA pathogenesis [3]. In mouse models of arthritis, ADAMTS-5 is implicated in driving cartilage loss and disease severity [4, 5], whereas, apart from MMP-13 [6], other proteases did not contribute [7]. Aggrecan, a primary aggrecanase substrate, is a key structural component of cartilage. Intact aggrecan consists of N-terminal globular domains, which anchor the glycoprotein to hyaluronic acid, separated by a proteolysis-sensitive interglobular domain (IGD) and a C-terminal region rich in glycosaminoglycan (GAG) side chains important for maintaining cartilage elasticity, water content, and structural integrity. Both aggrecanases and matrix metalloproteases (MMPs) are capable of cleaving within the IGD [8], resulting in release of the GAG-rich regions and contributing to loss of structural integrity and impaired joint function. Notably, mice possessing an aggrecanase-insensitive IGD mutation were protected from experimentally-induced OA and exhibit a pro-regenerative phenotype [9]. In contrast, mice with an MMP-insensitive IGD mutation showed no evidence of cartilage protection and appeared to have exacerbated disease [9]. Studies using human OA tissues suggest that MMP activity is mainly associated with normal turnover rather than pathogenesis [10, 11].
Taken together, these findings highlight a single axis in mice which could be therapeutically targeted in human disease. However, translational uncertainty raises questions about the involvement of ADAMTS-5 in the human condition. Although associations between ADAMTS-5 and human OA have been made [12–15], the link remains controversial as other proteases have been implicated [3, 14–18] and numerous proteases could conceivably combine to drive disease progression. To this point the absence of robust and selective inhibitors and redundancy in signatures of aggrecanase activity have made it difficult to systematically address these questions.
The goal here was to generate selective inhibitors to address fundamental questions about the role of ADAMTS-4 and ADAMTS-5 in human OA and provide a foundation to develop a DMOAD. Fully selective high affinity monoclonal antibodies (mAbs) were assessed for inhibition of recombinant and native aggrecanase activity, target engagement, and modulation of disease endpoints in human ex vivo and preclinical in vivo systems. Based on these studies it appears that, as predicted by the knockout mouse, ADAMTS-5 is a significant protease involved in cartilage degradation in human OA patients and a selected ADAMTS-5 inhibitor mAb (GSK2394002) holds DMOAD potential.
Materials and Methods
Binding Specificity/Affinity
Antibody binding to plate-bound proteins was determined using immunoassays. Immunocytochemical binding of mAbs to BacMam transduced CHO cells [19] was assessed using standard chromogenic and microscopic detection techniques. Antibody/antigen binding kinetics was measured using an OctetQK with streptavidin biosensors (Fortebio) and analyzed using Octet analysis software.
Structural Modeling
Crystal structures were acquired and antigen/antibody binding was computationally modeled as described (supplemental methods).
ARGS Neoepitope Detection
ARGS neoepitope was quantified using sandwich immunoassays in DELFIA (inhibition and explant assay) or electrochemiluminescent (serum) format as described [20] and supplementary methods.
ADAMTS-4 and ADAMTS-5 Inhibition
Aggrecan mAb (Invitrogen, Cat# AHP0022) [100ng] was immobilized on assay plates overnight and bovine aggrecan (Sigma) [10nM] was allowed to bind the immobilized mAb. In a separate polycarbonate plate, various treatment concentrations were pre-incubated with recombinant human ADAMTS-4 [2nM] or ADAMTS-5 [1nM] for 30 minutes, added to the assay plate and incubated for 60 minutes. Plates were washed and detected with biotinylated ARGS neoepitope mAb (OA-1) [21], as described above. Inhibition of proteolysis was quantified and graphed using Prism 5 software (GraphPad).
Human OA Cartilage Explant Efficacy
Tissues from total knee replacement (TKR) surgeries were received within 24 hours (NDRI, Philadelphia), processed and cultured for assessment of treatment efficacy as described in supplemental methods. All human samples were sourced ethically and research use was in accord with the terms of informed consents.
In vivo Antibody Penetration of Cartilage
The destabilization of the medial meniscus (DMM) model of OA [22], was used to assess the ability of mAbs to engage the target in vivo. Male Swiss Webster (SW) mice (Charles River) (12 weeks of age at surgery) with established disease (6 weeks post-surgery) were administered a single dose [16mg/kg, IP] of IR800 dye labelled mAb and periodically imaged using an Odyssey® system (LI-COR) to confirm accurate administration and monitor biodistribution. Mice were sacrificed four days after administration and tissues processed for analysis. Dewaxed and rehydrated 7 μM knee joint sections were imaged (Odyssey® and microscope equipped with an IR800 filter set; Chroma).
In vivo OA Mouse Efficacy
The DMM model [22] was used to assess efficacy in male SW mice (12–16 weeks of age at surgery). Each group included 8–10 mice with joint destabilization performed on each hind leg (n=16–20 knees/group). Age-matched naive and/or sham surgery (open the joint capsule but no DMM) groups were included as negative controls. Treated mice were pre-dosed with mAbs [16mg/kg, IP] three days prior to surgery and once weekly throughout the 8 week study. Efficacy was assessed (readers blinded to treatment identity) using toluidine blue stained 7 μM knee joint sections and a histopathological scoring system, as described [22, 23].
Non-human primate Pharmacokinetic/Pharmacodynamic Model
Healthy cynomolgus monkeys were screened for serum ARGS neoepitope using the electrochemiluminescent immunoassay. Animals with the highest levels received intravenous or subcutaneous GSK2394002 or vehicle control [50 mM sodium acetate buffer, pH 5.5 containing 1% (w/v) L-arginine, 0.05 mM EDTA, 51 mM NaCl and 0.02% (w/v) Tween™ 80 in sterile water for injection, USP]. All animals were subsequently bled and monitored for pharmacokinetic and pharmacodynamic parameters. Serum ARGS neoepitope was quantified and percent change from pre-dose calculated to assess modulation of aggrecanase activity.
Statistical Analysis
Statistical analysis using Mann Whitney test is shown (Fig 3a). The total joint score (Fig 5a) from each knee of the same animal are correlated, so data was analyzed using repeated measure analysis with compound symetry (CS) within animal as covariance matrix. The analysis incorporated correlations for knee observations arising from the same animal. Mechanical allodynia (Fig 5b) was assessed using a one-way ANOVA with Bonferonni post-test. Non-parametric Mann Whitney test was performed for comparison of baseline serum ARGS neoepitope between male and female monkeys (Fig 6a). In addition, a repeated measure analysis was used to analyze the repeated measurement of serum ARGS neoepitope (as meausre of percentage change of predose) (Fig 6b). The repeated measure model included dose, time, and dose by time interaction as fixed effects and subject as a random effect. No multiple comparison adjustment method was used due to the research nature of the study. The repeated measure analysis was performed in SAS 9.3 software. Other analysis was perfromed in GraphPad Prism 5 software.
Results
Monoclonal antibody generation and characterization
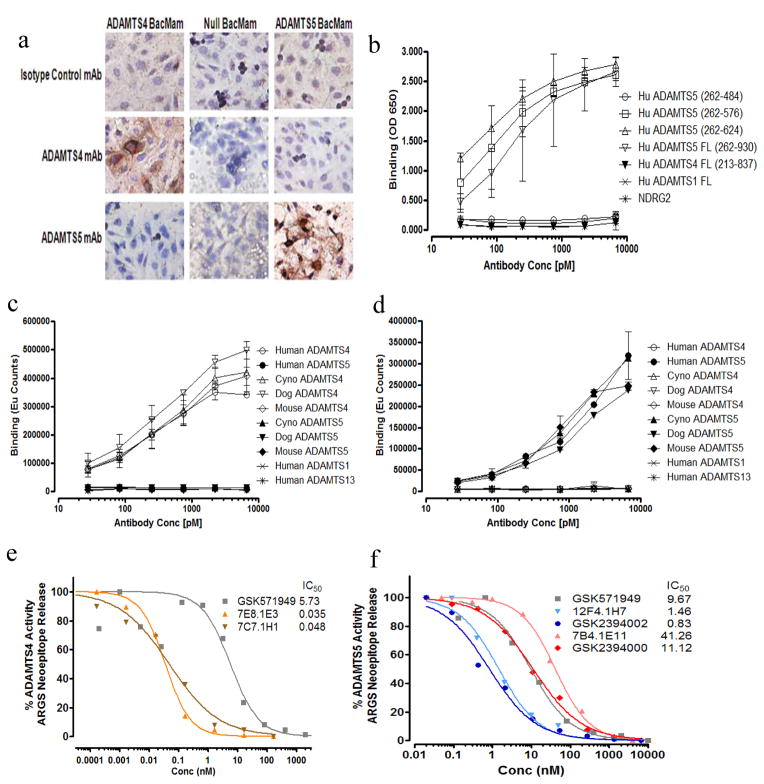
Multiple ADAMTS-4 or ADAMTS-5-selective mAbs were generated and tested for inhibition of aggrecanase activity and only those demonstrating potent inhibition were characterized further. Interestingly, individual mAbs that bound both ADAMTS-4 and 5 lacked functional inhibition. Two ADAMTS-5-specific mAbs, which were subsequently humanized, and two ADAMTS-4-specific mAbs were characterized for binding affinity, selectivity, species cross-reactivity, domain specificity and inhibition of aggrecanase activity (Table 1 and Figure 1). Selectivity profiling demonstrates high target specificity, as no binding was observed for a panel of related proteases (ADAMTS-1 and 13), MMP-1, 3, 9 and 13 (not shown) and unrelated controls (Figure 1a–d). In all species tested, the ability to bind (Figure 1c and d) and inhibit (not shown) the respective protease was retained, suggesting structural conservation across species consistent with the high (≥92%) sequence homology [24].
Table 1.
Characteristics of monoclonal antibody and small molecule aggrecanase inhibitors
| Affinity | |||||||
|---|---|---|---|---|---|---|---|
| Inhibitor | Type | Specificity | Domain Mapping | Ka [1/Ms] | Kd [1/S] | KD | Neutralization IC50 (nM) |
| 12F4.1H7 | Mouse IgG2c | ADAMTS-5 | Cat/Dis | 6.99E+04 | 2.49E-06 | 0.035nM | 1.46 |
| Humanized 12F4 (GSK2394002) | Human IgG1 | ADAMTS-5 | Cat/Dis | 7.54E+04 | 2.88E-06 | 0.038nM | 0.83 |
| 7B4.1E11 | Mouse IgG1 | ADAMTS-5 | Cat/Dis | 9.25E+04 | 2.59E-05 | 0.280nM | 41.26 |
| Humanized 7B4 (GSK2394000) | Human IgG1 | ADAMTS-5 | Cat/Dis | 1.06E+05 | 2.16E-05 | 0.205nM | 11.12 |
| 7E8.1E3 | Mouse IgG1 | ADAMTS-4 | Cat/Dis | 2.81E+05 | 7.08E-05 | 0.252nM | 0.035 |
| 7C7.1H1 | Mouse IgG2c | ADAMTS-4 | C-Terminus | 2.01E+06 | 5.72E-04 | 0.285nM | 0.048 |
| GSK571949 | Small Molecule | Broad Spectrum | Catalytic | 5.73 ADAMTS-4 9.67 ADAMTS-5 |
|||
Catalytic – amino acid sequence 262–484 of human ADAMTS-5 and 213–437 of human ADAMTS-4 Cat/Dis – catalytic + disintegrin domains [262–576 human ADAMTS-5, 213–520 human ADAMTS-4] C-Terminus – amino acid sequence 579–837 of human ADAMTS-4
Figure 1.
ADAMTS-4 and ADAMTS-5 monoclonal antibodies specifically bind to and potently inhibit aggrecanase activity. (a) Selective binding of mAbs for ADAMTS-4 (7E8.1E3) and ADAMTS-5 (7B4.1E11) is demonstrated by immunocytochemistry using ADAMTS-4, ADAMTS-5 and Null BacMam transduced CHO cells. ADAMTS-5 specific domain mapping and selective binding to purified recombinant proteins is profiled by ELISA (b; 12F4.1H7) and DELFIA (c; 7E8.1E3 and d; 12F4.1H7) (Results shown are n=2 independent experiments with 2 dependent replicates for each concentration ± 95% CI). Functional potency of ADAMTS-4 (e) and ADAMTS-5 (f) mAbs and non-selective small molecule (GSK579149) is quantified vs isotype controls (not shown) by measuring percent inhibition of selective aggrecanase-mediated ARGS neoepitope formation from purified bovine aggrecan substrate. IC50 values for each mAb and GSK571949 are shown (inset, e and f) in relation to isotype control mAbs and DMSO, respectively, which did not demonstrate any detectible inhibitory activity. Consistent with binding specificity, no inhibition of aggrecanase activity was observed in this assay for ADAMTS-4 with ADAMTS-5 mAbs and ADAMTS-5 with ADAMTS-4 mAbs (not shown).
Inhibitory potency (IC50) of mAbs against ADAMTS-4 or ADAMTS-5-induced ARGS neoepitope formation was compared to matched isotype control mAbs and a small molecule broad-spectrum metalloprotease inhibitor (GSK571949) [25]. mAb 12F4.1H7 demonstrated a nearly 30-fold lower IC50 value for ADAMTS-5 than the 7B4.1E11 clone (Figure 1f, 1.43 vs. 41.26nM), consistent with the observed binding affinity (Table 1). In both cases, humanization improved potency (Figure 1f, 0.83 vs. 11.12nM), with the relative difference retained. Both ADAMTS-4 mAbs demonstrated comparable IC50 for ADAMTS-4 (Figure 1e, 35 vs 48pM), which was lower than their binding affinity (Table 1) and may relate to the lower specific aggrecanase activity of ADAMTS-4 [26]. As a comparator, GSK571949 demonstrated low nM functional inhibition of both ADAMTS-4 and 5 (Figure 1e and f). ADAMTS-4 and ADAMTS-5 mAb affinities, measured using OctetQK (Table 1) and BiaCore (not shown) systems, ranged from low to mid pM. Consistent with binding, selective inhibition was also maintained (not shown). The differential functional potency between ADAMTS-5 mAbs may be attributed to their respective off-rates (Kd). In addition, the humanized 12F4.1H7 mAb (GSK2394002) dose-dependently suppressed ADAMTS-5-mediated proteolysis of aggrecan and brevican suggesting its inhibition is not substrate-selective (Supplementary Figure 1).
Domain mapping, using truncated forms of each protease (Table 1 and Figure 1b) and competitive mAb binding assays (not shown), suggest that 12F4.1H7 and 7B4.1E11 recognize a similar epitope within the catalytic and disintegrin domains (amino acids 262–576). Both mAbs failed to bind recombinant catalytic domain (amino acids 262–484) in this assay. The ADAMTS-4 mAb domain specificity was distinct, with 7E8.1E3 recognizing the catalytic+disintegrin domain (like the ADAMTS-5 mAbs) and 7C7.1H1 a C-terminal epitope between amino acids 579–837 (Table 1) possibly highlighting a substrate-binding exosite.
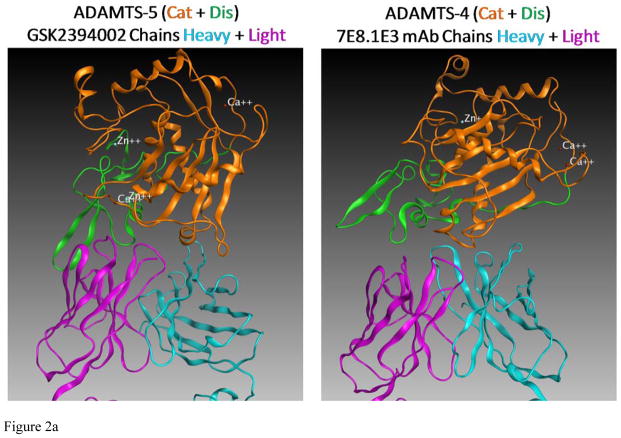
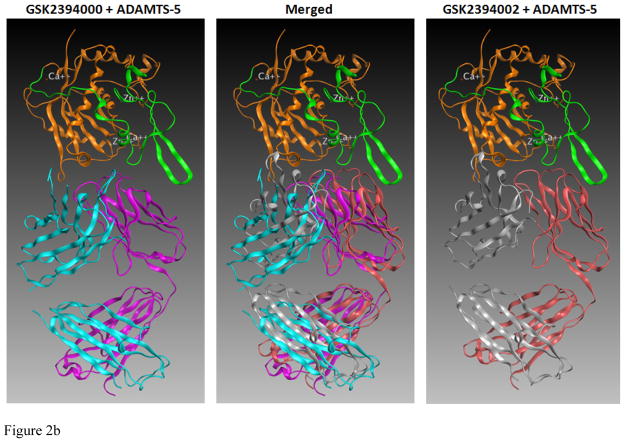
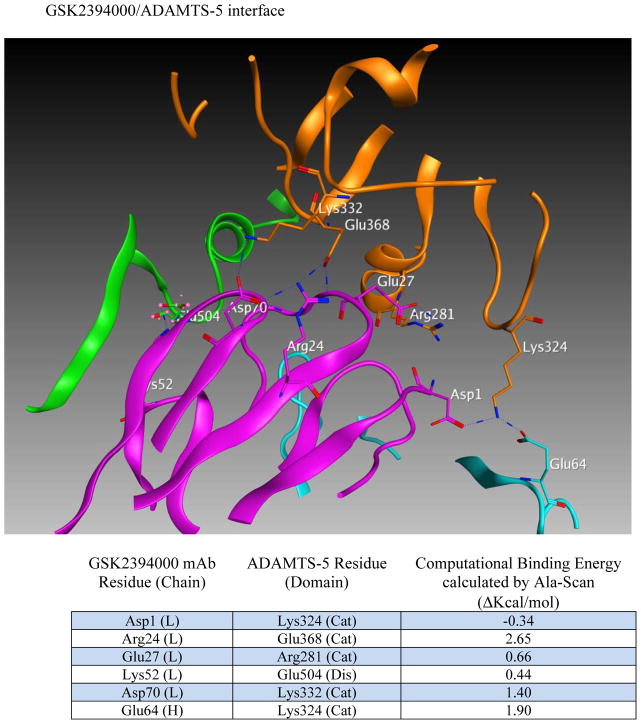
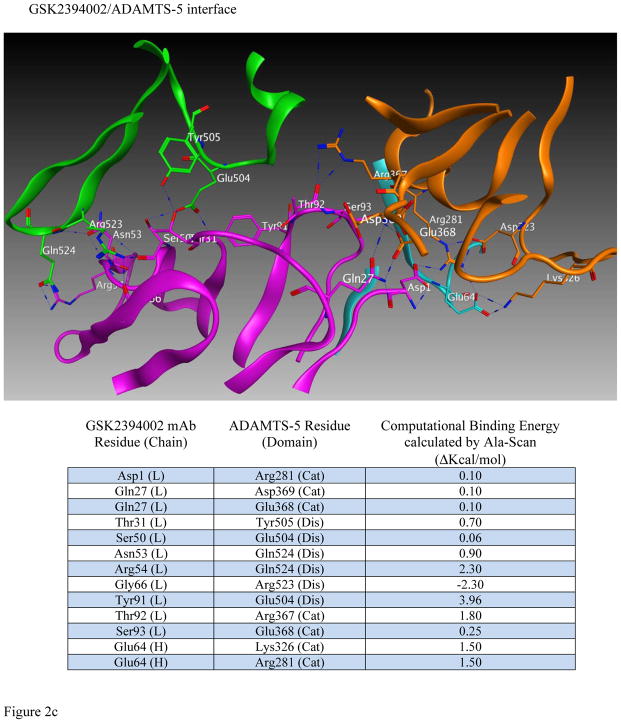
Efforts to further define epitopes employed crystal structures of ADAMTS-4 and ADAMTS-5 with mAb-derived Fab fragments in a computational docking prediction model [27]. This approach was taken because co-crystallization attempts failed to produce crystals suitable for x-ray diffraction. Interestingly, mAbs mapping to the catalytic+disintegrin domains were predicted to recognize a conformational epitope spanning these two domains (Figure 2a and b) and may derive activity from ‘allosterically locking’ the domains together. Comparing ADAMTS-5-specific mAbs (Figure 2b), the number of predicted high-energy (hydrogen bond) interactions was greater for GSK2394002 compared to GSK2394000 (13 vs. 6 sites, Fig 2c), consistent with the differential affinity and inhibition. Furthermore, binding was spread across the heavy and light chain of each mAb with interactions outside the complementarity determining region (CDRH3) that defines classical antibody specificity [28].
Figure 2.
in silico structural modeling of antigen/antibody interactions suggest a conformational binding epitope for select ADAMTS-4 (7E8.1E3) and ADAMTS-5 (GSK2394002) mAbs which spans the catalytic and disintegrin domains of each protease and predicts an ‘allosteric lock’ mechanism of inhibition (a). In all panels the top ranked docking orientation is shown; cyan/light blue is the light chain and magenta/purple the heavy chain of the monoclonal antibodies; orange is the catalytic domain and green the disintegrin domain of ADAMTS-4 or -5. Individual and merged overlay structural models of the inhibitory ADAMTS-5 mAbs (b) and amino acid residues spanning the catalytic and disintegrin domains of ADAMTS-5 predicted to form hydrogen bond interactions (GSK2394000, six bonds and GSK2394002, thirteen bonds) are shown with bond energy predictions from an Alanine substitution scan of the predicted protease/mAb interface (c).
Human OA cartilage explant efficacy
Cartilage explants from human OA patients at TKR were utilized to directly compare the role of ADAMTS-4 and ADAMTS-5 in cartilage degradation. Pharmacology was assessed by measuring inhibition of ARGS release, as a marker of aggrecanase activity, in the presence of ADAMTS-specific and isotype control mAbs (670nM [100ug/mL]) or GSK571949 (2uM). These studies were performed longitudinally with a 3-week unstimulated phase, followed directly by 1-week of cytokine stimulation. A total of 13–28 individual OA donors were assessed for each treatment to compensate for inter-patient variability and each condition was performed on seven replicate cartilage plugs from each donor to reduce intra-patient variability.
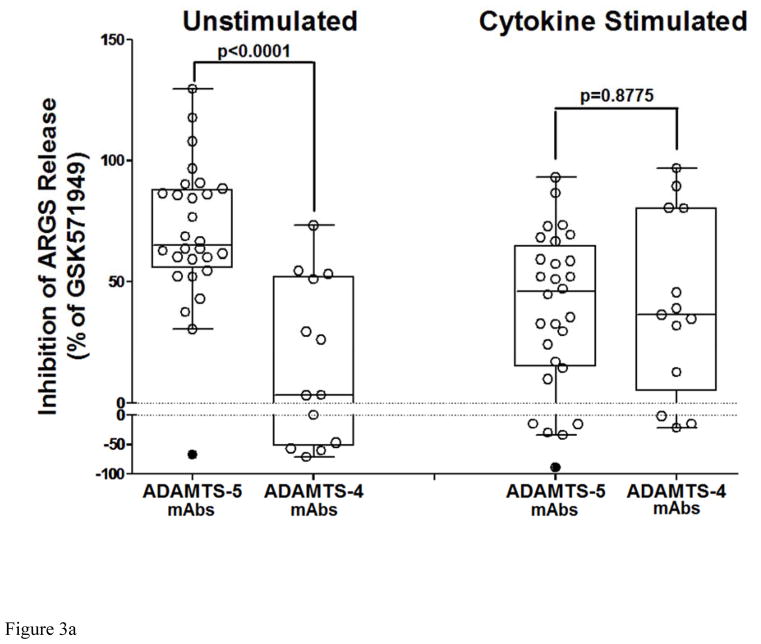
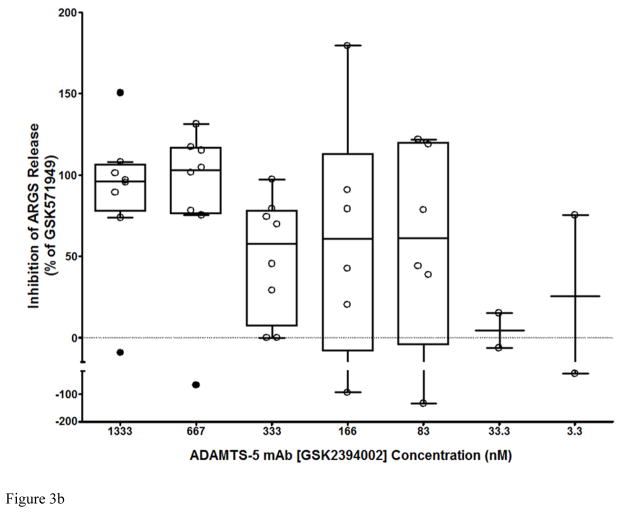
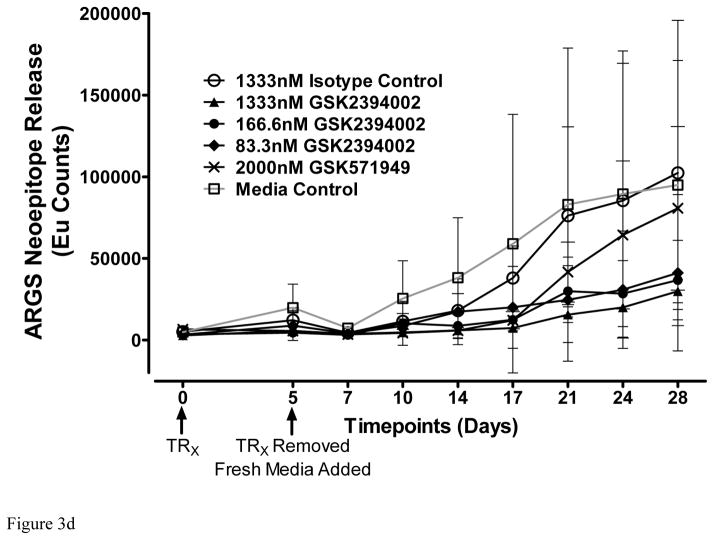
In the unstimulated phase, ADAMTS-5 mAbs potently suppressed ARGS release compared to isotype controls (Figure 3a), while ADAMTS-4 mAbs had only a modest effect, suggesting that ADAMTS-5 is the predominant ARGS-producing aggrecanase in cartilage from these patients. Combination of ADAMTS-4 and ADAMTS-5 mAbs (7E8.1E3 and 7B4.1E11 respectively) at equimolar concentrations (670nM each) showed no benefit over ADAMTS-5 mAbs alone (data not shown). Although ADAMTS-4 mAb inhibition increased following exogenous cytokine stimulation, ADAMTS-5 mAbs retained similar activity suggesting a potential role for both ADAMTS-4 and ADAMTS-5 under this condition. Subsequent studies titrating GSK2394002 in cartilage explants with elevated pre-treatment ARGS levels demonstrated dose-dependent inhibition which was nearly complete at concentrations ≥ 670nM (Figure 3b).
Figure 3.
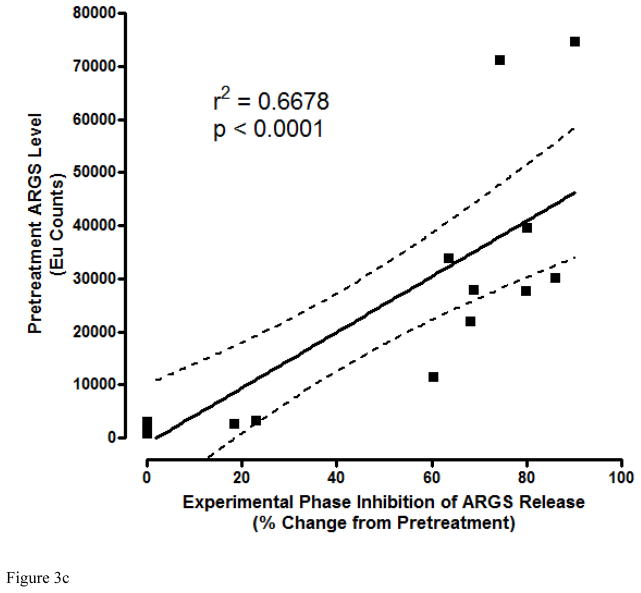
Potent and sustained inhibition of aggrecanolysis from human OA cartilage explants by ADAMTS-5 mAb treatment. ADAMTS-5 mAb treatment inhibits ARGS neoepitope release from sequentially unstimulated and IL-1b/OSM stimulated human OA cartilage explants (a), whereas only modest inhibition is observed with ADAMTS-4 mAb treatment, preferentially under cytokine-stimulated conditions. Mean percent inhibition of mAbs is shown relative to isotype control mAb (0% inhibition, dashed lines in a and b) and GSK571949 (100% inhibition) treatment. Experiments were conducted with 7 replicates for each treatment condition from 8 timepoints across multiple 28-day experiments consisting of a 21 day unstimulated phase followed by a 7-day cytokine-stimulated phase. A total of 13–28 independent OA patient experiments were conducted for each treatment condition. ADAMTS-4 (7E8.1E3 or 7C71.H1) and ADAMTS-5 (12F4.1H7 or 7B4.1E11) mAb treatments are shown as compiled data associated with each mAb specificity. All mAb treatments were held constant at 670nM throughout the experiment and GSK571949 was similarly held at 2 μM. (b) Dose-dependent inhibition of ARGS neoepitope release is evident with GSK2394002 treatment in the absence of exogenous cytokine stimulation using cartilage from individual OA donors with elevated pretreatment ARGS neoepitope levels (n=2–8 independent donor experiments per treatment concentration and each point represents mean % inhibition from 21 day unstimulated phase, as described in (a)). Plots (a and b) are presented in standard box-and-whisker format with Tukey outliers noted (>1.5 times IQR, filled symbols). (c) Linear regression analysis suggests the response to GSK2394002 treatment correlates with pre-treatment ARGS neoepitope levels. Sustained suppression of ARGS neoepitope release is observed with pulse-chase GSK2394002 treatment in relation to small molecule (GSK571949) in individual donor experiments using cultures with elevated pretreatment ARGS neoepitope levels (n=4; each timepoint and ±95% CI error bar represents mean of 4 independent donor experiments where 7 biological replicates for each donor/timepoint was used to calculate a within donor mean for each timepoint) (d).
The premise that endogenous aggrecanase activity could identify patients most likely to respond to ADAMTS-5 mAb treatment framed experiments designed to support a personalized medicine strategy. In these studies, endogenous ARGS levels were quantified in individual patient explant cultures (n=16) prior to treatment with GSK2394002 or an isotype control mAb followed by longitudinal assessment of inhibition. Only half of the individual patient explants demonstrated elevated pre-treatment ARGS levels (Supplementary Figure 2), suggesting distinct phenotypes exist related to endogenous aggrecanase activity in this system, which may correlate to observations made in patients [20]. The response to GSK2394002 treatment correlated with pre-treatment ARGS levels (Figure 3c) while isotype control treatment had little effect (Supplementary Figure 2), suggesting a predominant role for ADAMTS-5-mediated aggrecanolysis at the IGD cleavage site in these patients.
Duration, potency and reversibility of this response were further evaluated using cartilage with elevated pretreatment ARGS levels and a pulse-chase treatment method. GSK2394002 produced sustained ARGS inhibition (Figure 3d) compared to isotype and GSK571949 treatment. These data are consistent with high affinity binding and slow off-rate for GSK2394402 and suggests a modest rate of endogenous ADAMTS-5 turnover in this system. In contrast, GSK571949-treated cultures appeared to steadily lose inhibitory activity as the experiment progressed, demonstrating a transient drug/target interaction.
In vivo target engagement and therapeutic efficacy
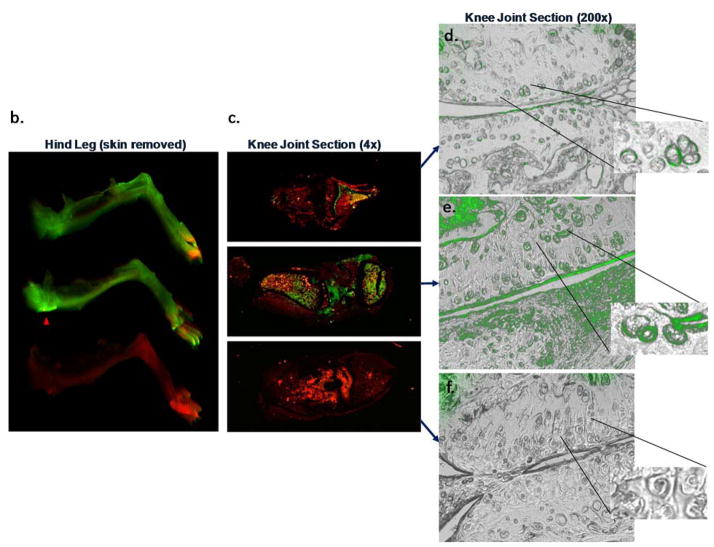
The ability of mAbs to engage their respective targets in vivo was assessed using labeled mAbs in a preclinical OA model. Imaging and microscopic techniques were employed to monitor biodistribution and cartilage penetration. For continuity, the dose [16mg/kg], route (IP), mouse strain (SW) and disease model (DMM) was the same used for subsequent in vivo efficacy assessment. Whole body imaging immediately after dosing and again at day 4 confirmed accurate delivery and systemic biodistribution (Figure 4a). ADAMTS-4 and ADAMTS-5 mAb treated mice showed a systemic signal on day 4, consistent with dermal deposition, a site of reported expression [29]. Consistent with enabling human cartilage experiments (not shown) and literature reports [30] suggesting time-dependent ex vivo mAb penetration of cartilage, in vivo target engagement was observed within 4 days (Figure 4b–e). Characteristic pericellular staining patterns within the cartilage noted for both ADAMTSs [13] were observed (Figure 4d–e, inset) and ADAMTS-5 mAb intensity was qualitatively greater than the ADAMTS-4 mAb and exhibited superficial zone cartilage layer, meniscus and subchondral bone signals (Figure 4d–e).
Figure 4.
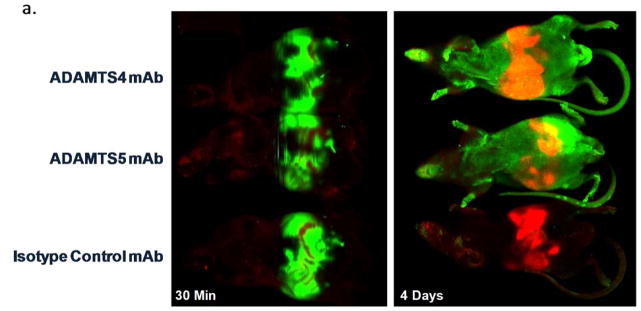
ADAMTS-4 and ADAMTS-5 mAbs engage their targets within the cartilage following systemic administration in a mouse OA model. IR800-labeled ADAMTS-4 (7E8.1E3), ADAMTS-5 (7B4.1E11) or Isotype control mAbs were administered ([16mg/kg] IP) to mice (n=1/group) six weeks after surgical destabilization of the medial meniscus and periodically imaged to monitor systemic biodistribution and tissue penetration. Within 30 minutes of dosing a strong IR800 signal (green) can be observed in the abdomen of all animals (a, left) indicative of accurate administration. The red abdominal signal is related to the inherent infrared signature of the animal diet traversing the digestive system. Systemic IR800 signal is observed in mice receiving ADAMTS-4 or ADAMTS-5 mAb (and to a much lesser extent the isotype control treated animal) 4 days following administration (a, right) when the animals were sacrificed to assess tissue distribution. The right hind leg of each animal is shown (b) with skin removed showing a strong signal intensity in the joint region of the ADAMTS-5 mAb treated mouse (red arrowhead) compared to the other treatments. Similar qualitative differences between treatment groups can be observed in low (c; 4X magnification) and high power (d–f; 200X magnification) images of the knee joint, as well as evidence for cartilage penetration and deposition in the cartilage for the ADAMTS-4 and ADAMTS-5 mAbs in the superficial cartilage zone and pericellular region of the articular chondrocytes (d–e, inset). Merged microscopic bright-field/IR800 images are shown. In the ADAMTS-5 mAb panel (e) staining in the meniscus can be observed (bottom half of image). No signal was observed following administration of a labeled isotype control mAb (f) or following administration of a non-reactive dye control, which was undetectable within 24 hours after dosing (not shown).
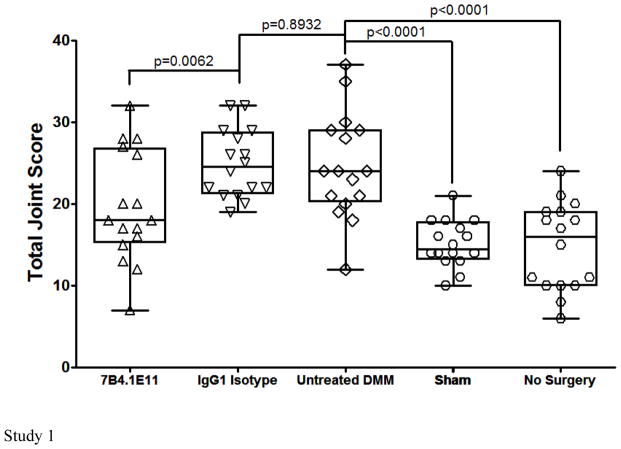
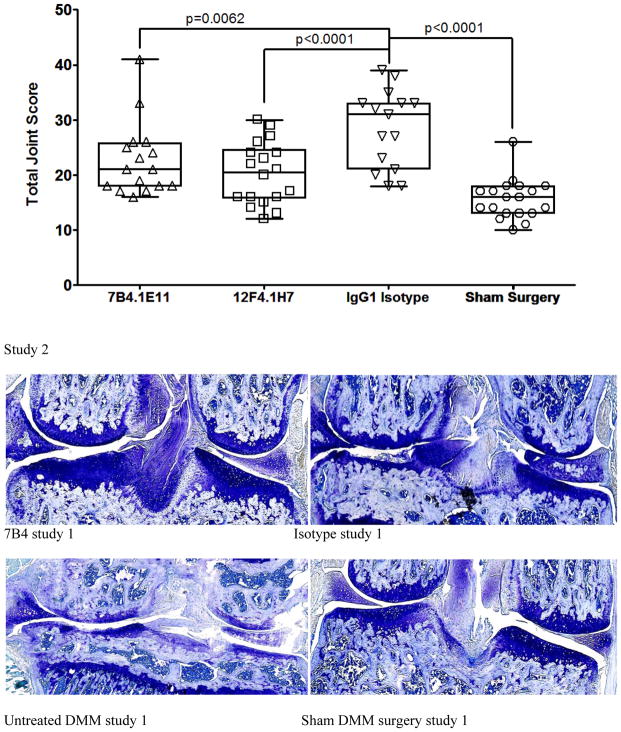
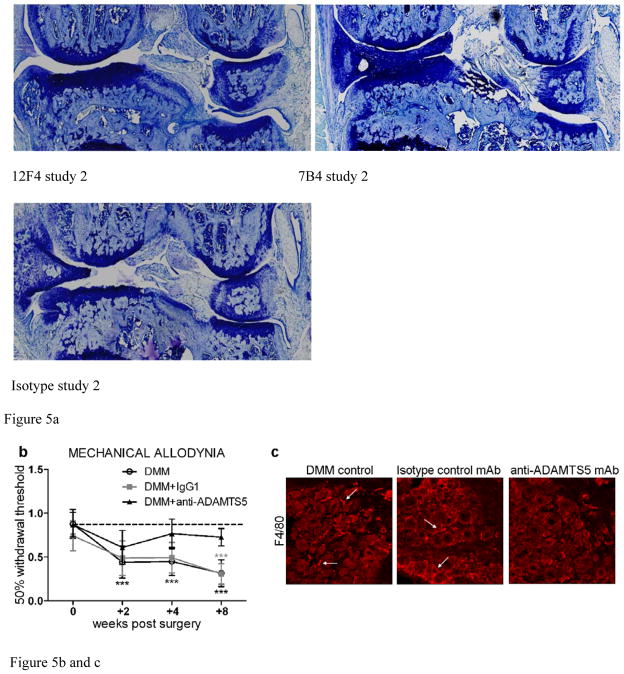
In vivo efficacy was assessed histologically in DMM mice treated with ADAMTS-5 specific mAbs or isotype controls in two independent studies (Figure 5). A prophylactic/weekly treatment regimen was chosen to validate ADAMTS-5 knockout findings [4]. Pharmacokinetic studies using 12F4.1H7 suggests this regimen should maintain circulating concentrations >100μg/mL throughout the study. Both naïve (mean 14.81 ± 5.37) and sham surgery control groups (15.13 ± 2.85 and 15.58 ± 3.63 in each respective study) had comparable joint scores. Whereas isotype control mAb treated (24.88 ± 4.18 and 28.53 ± 7.10) and untreated mice (24.63 ± 6.46) suggest substantial joint damage at 8 weeks after DMM. Alternatively, both ADAMTS-5 mAbs independently imparted statistically significant protection (19.63 ± 6.85 [7B4.1E11, study 1] or 20.28 ± 5.64 and 22.69 ± 6.66 [12F4.1H7 and 7B4.1E11, study 2 respectively]) (Figure 5). Representative images of toluidine blue stained knees, aligned with mean group scores, are shown. In a separate study, ADAMTS-4 mAbs failed to protect from progression of joint damage (not shown).
Figure 5.
ADAMTS-5 mAb treatment suppresses joint disease severity in a mouse model of osteoarthritis. Mice treated prophylactically with anti-ADAMTS-5 (1x/week, i.p. 10–16 mg/kg) were protected from developing (a) histologic cartilage degeneration (two independent studies shown, n=8–10 animals/group each with two DMM knees/animal, although not all knees in study 2 could be scored due to isolated tissue processing errors and in one group loss of a single mouse between surgery and treatment commencement, as demonstrated by individual knee symbols in each group ranging from 15–19/group). Representative toluidine blue stained knee joint sections are shown for each study group. (b) Mice treated prophylactically with anti-ADAMTS5 mAb (1x/week, i.p. 10 mg/kg) were protected from developing mechanical allodynia through 8 weeks post DMM, *** p<0.001 vs time 0, n=12/group. Mean ±95% CI. (c) Prophylactic treatment with anti-ADAMTS5 mAb also protected mice from macrophage infiltration (F4/80 marker) into the DRG at 8 weeks post DMM. Arrows indicate examples of infiltrating macrophages in the DMM control and Isotype control mAb (IgG1) conditions.
In addition to protection from joint damage, an independent study assessed development of mechanical allodynia as a pain-related behavior associated with OA [31] employing the DMM model in C57BL/6 mice. Throughout the course of the 8-week study, mice treated prophylactically with 7B4.1E11 were protected from mechanical allodynia (Figure 5b), consistent with ADAMTS-5 deficient mice [31]. Eight weeks post-surgery, mice showed fewer F4/80 macrophages in innervating dorsal root ganglion [32] compared to untreated and isotype control mAb treated mice (Figure 5c).
Non-human Primate Pharmacokinetic (PK)/Pharmacodynamic (PD) Model
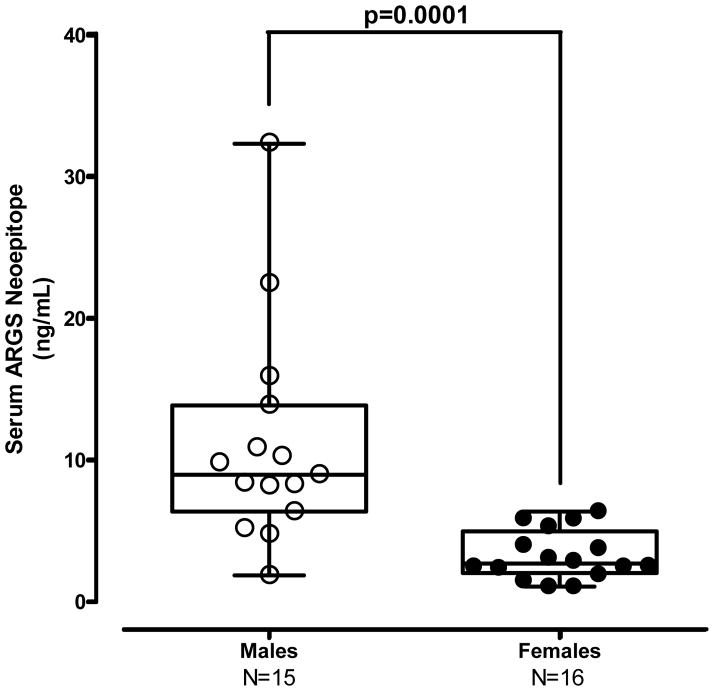
Cynomolgus monkeys were utilized to assess fundamental PK/PD effects of GSK2394002 treatment on circulating ARGS levels as a predictor of target inhibition and translational dose selection. Although not an experimental disease model, cynomolgus monkeys naturally develop a condition consistent with OA [33]. In this study, 31 individual animals were pre-screened for circulating ARGS as an indicator of endogenous aggrecanase activity (Supplementary Table 1). Interestingly, male monkeys typically had higher levels than females (Figure 6a). Significant correlations were also observed between ARGS with both age and weight in males, but not females (Supplementary Table 2).
Figure 6.
Figure 6a – Male cynomolgus monkeys exhibit generally higher endogenous circulating ARGS neoepitope concentrations as compared to females.
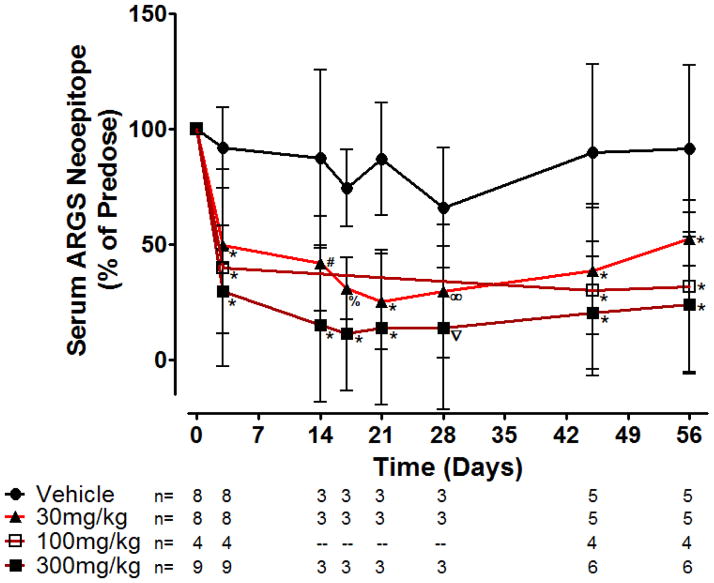
Figure 6b – Serum ARGS neoepitope levels rapidly and dose-dependently decline in response to ADAMTS-5 mAb (GSK2394002) treatment in non-human primates. Monkeys (n/group at each timepoint is shown from two independent studies, both males and females included at each timepoint except days 14–28 which is only females) were dosed every two weeks starting immediately after a pre-dose blood sample at day 0. Serum and plasma was drawn on the 3rd day after dosing, immediately prior to the next dose and periodically throughout the study as shown to monitor circulating PK and PD endpoints. Mid study samples were not taken for the 100mg/kg group and some animals in the other groups, as shown. Data represents mean percent change from pre-dose ± 95% CI with statistical significance (∞p=0.023, %p=0.0046, #p=0.0027, ∇p=0.0001 *p<0.0001) observed for all GSK2394002 dose groupings and at all post-treatment timepoints relative to the vehicle control treated group.
GSK2394002-treated animals exhibited a rapid and sustained reduction in circulating ARGS neoepitope, while levels remained relatively stable following vehicle treatment (Figure 6b). GSK2394002 administration decreased ARGS levels within 3 days relative to pre-dose values (approximately 50–70% dose-dependent reduction) and remained suppressed throughout the study monitoring period with twice-monthly administration (individual animal reduction relative to pre-dose of 50–75% at 30mg/kg, ~60–70% at 100mg/kg and ~70–90% at 300mg/kg). Alternatively, individual vehicle control treated animals remained within ~65–138% of pre-dose levels. Circulating GSK2394002 levels observed following administration were consistent with PK predictions and no immunogenicity was observed (not shown).
Discussion
Strategic drug discovery typically relies on a diverse combination of data to validate a disease target and provide a translational framework for drug development. However, differences in target biology between species, selectivity of tools used to modulate target activity and methods to model a disease state frequently create uncertainty about accurate translation to the human condition. Due to conflicting reports on the role of ADAMTS-4 and ADAMTS-5 in models of murine [4, 5] and human [15, 17] OA, we set out to generate selective monoclonal antibody inhibitors to gain clarity on this discrepancy and guide a human DMOAD strategy.
Here, we demonstrate characterization and validation of selective and potent inhibitory mAbs targeting ADAMTS-4 and ADAMTS-5 in numerous in vitro, ex vivo, in silico and in vivo models of efficacy, target engagement and pharmacology. Although numerous reports of aggrecanase inhibitors exist [25, 34, 35], we believe this is the first example of a direct comparison of fully selective inhibitors with sub-nanomolar potency. The proposed novel ‘allosteric lock’ mechanism of action suggested for some of the mAbs described here (Fig 2) supports the observed selective neutralization of protease activity and appears distinct from other metalloprotease neutralizing mAbs which largely target the catalytic active site directly and frequently lack full selectivity [36, 37]. We propose this selectivity profile can be attributed to these unique epitopes and provides a basis for the ‘allosteric lock’ model where an antibody simultaneously binding the catalytic and disintegrin domans impairs inherent protease flexibility and inhibits its ability to engage and cleave substrate [38]. Although understanding the mechanism of inhibition is not required for therapeutic development, it can provide insight into selecting a clinical candidate and set a strong foundation for securing intellectual property.
The translational role of ADAMTS-4 and ADAMTS-5-selective mAbs was assessed in human OA cartilage explants. Admittedly, this system is cartilage-centric and excludes other tissues of this total joint disease, such as bone, synovium, meniscus and adipose tissue, which may contribute to pathogenesis. However, as endogenous aggrecanase activity was routinely observed, the explant model provides a relevant system to assess the mechanisms in question. Aggrecanase activity leading to ARGS neoepitope generation is important in both mouse [9] and human [8, 14] disease and, although both ADAMTS-4 and ADAMTS-5 are capable of cleaving there (Fig 1 and [39]), ADAMTS-5 has been shown to have significantly greater activity for this site [11]. The human explant model was performed with and without exogenous cytokine stimulation, although our view is that the unstimulated phase, where endogenous factors are allowed to persist in culture, is more representative of native disease. Exogenous cytokine stimulation, which clearly accelerates cartilage degradation, emphasizes specific inflammatory mediators with unclear disease relevance that may bias data interpretation. Our data show markedly greater efficacy with ADAMTS-5 mAb treatment compared to ADAMTS-4 mAbs in the unstimulated phase (Figure 3a) supporting a predominant role for ADAMTS-5 in this system. We cannot rule out a role for ADAMTS-4, as a modest level of inhibition with the ADAMTS-4 mAbs was observed in a subset of patients and suggests an ADAMTS-4 contribution where IL-1 and OSM are involved (Fig 3b). Notably however, combining both ADAMTS-4 and ADAMTS-5 mAbs at fixed equimolar concentrations did not increase inhibition above the ADAMTS-5 mAbs alone (not shown) and higher doses of GSK2394002 were capable of complete inhibition in some patient samples (Figure 3b). This is consistent with single (ADAMTS-5) and dual (ADAMTS-4/5) knockout animal findings [4, 40].
In contrast, efforts using siRNA-mediated inhibition in human OA cartilage suggest both aggrecanses play a role [17]. Potential explanations for this apparent discrepancy may relate to differential mechanistic inhibition between mAbs and siRNA inhibitors, which could be important considering ADAMTS-4 and ADAMTS-5 have distinct transcriptional regulation [41]. The selective mAbs may not inhibit an isoform or other aggrecanase, as numerous variants have been noted for ADAMTS-4 and ADAMTS-5 in human OA cartilage samples [42, 43] and ADAMTS-4/5 dual knockout mice retain residual aggrecanase activity [44]. Finally, target engagement for each mAb in human cartilage explants may differ and was not independently assessed in all cases.
Due to the dense nature of cartilage and large size of antibodies, a widely held perception is that mAbs are unable to penetrate the cartilage matrix. Expression profiles for ADAMTS-4 and ADAMTS-5 localize to the pericellular region of chondrocytes in human OA cartilage [13]. Therefore, in order to be effective, aggrecanase-specific mAbs may need to penetrate cartilage. Prior reports using mAbs and hyaluronan demonstrate in vitro penetration of large proteins in osteoarthritic, and to a lesser extent normal, cartilage [30]. We were able to confirm these findings. However, the process of preparing tissue for culture and disease state may render the cartilage samples more amenable to penetration. Therefore, demonstrating in vivo target engagement within cartilage was important (Fig 4). When viewed alongside the functional modulation of disease endpoints observed in the same model and dose levels (Fig 5), these data strongly suggest effective in vivo target engagement. Interestingly, increased proteoglycan staining seems apparent in ADAMTS-5 mAb treated mice (Fig 5a) compared to controls. Although this may be a result of a chondrogenic activity of these highly potent inhibitors, it may likely be a result of inhibiting normal physiologic turnover of aggrecan leading to accumulation. Therefore, careful consideration of dose and frequency of administration may be warranted.
Building upon these findings, translational PK/PD dose-response relationships in non-human primates may aide human clinical development and are particularly relevant due to similarities in physiology, target homology and lower propensity to develop anti-drug antibodies. These studies clearly demonstrate a potent in vivo modulation of aggrecanase activity (Fig 6b) and provide confidence in the pharmacologic effect of ADAMTS-5 mAb treatment. Moreover, cynomolgus monkeys naturally develop an age-related condition similar to OA [33, 45] and elevated circulating levels of ARGS neoepitope were observed in a subset of animals that correlate with age and weight (Supplementary Table 2) suggesting a level of consistency with human OA [20]. The observed gender differences in circulating ARGS levels are interesting, although they seem to differ from the human condition where females have an increased incidence of OA. It is conceivable that hormones could contribute to this apparent discrepancy especially when considering female OA patients are typically post-menopausal.
In summary, clear preclinical links to the therapeutic mechanism are demonstrated through applying fundamental translational principles for therapeutic development in ex vivo human and in vivo models with a sensitive measure of aggrecanase activity. Ultimately, ARGS may be used in conjunction with GSK2394002 or other drugs to assess clinical parameters of efficacy and patient stratification to prospectively identify those most likely to respond to treatment. Numerous reports describe similar assays and comparisons to small molecule efficacy in rodent systems with similar results [46, 47]. However, this is the first report utilizing fully selective pharmacologic ADAMTS-4 and ADAMTS-5 inhibitors and non-human primates to assess the link.
Together, the current findings are consistent with foundational knockout mouse studies suggesting a predominant role of ADAMTS-5 in osteoarthritic cartilage degradation and allodynia further providing a justification for clinical development of GSK2394002. However, prior to initiating clinical trials, additional studies are necessary to predict safety and tolerability of GSK2394002 and whether, as some recent reports suggest [29, 48–50], inhibition of ADAMTS-5 may lead to undesired effects in other tissues.
Supplementary Material
Acknowledgments
The authors would like to thank: John White and Fred Kull for valuable guidance and support. Jon Dermott and Alastair Morrison for intellectual property and contract support. Neil Burden and Paul Hamblin for expertise of mAb humanization techniques. Jane Zhao, Ed Dul, Linda Myers and Maggie Grimes for molecular cloning and recombinant protein expression. Baoguang Zhao, Paris Ward and Nestor Concha for expertise in crystal structure generation and PDB submission.
Role of funding source
Predominant funding for this work was made by GlaxoSmithKline (JL, TAL, LE, JS, JLS, RM, YX, FL, CG, CCM and CJM). A.M. Malfait acknowledges funding from the US National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR060364 and R01AR064251). Rachel Miller is supported by a Ruth L. Kirschstein National Research Service Award (Individual NIH Post-doctoral fellowship) and by the Arthritis Foundation.
Footnotes
Contributions
| Conception and design | JL, TAL, LE, JS, JLS, REM, AMM, CCM, CJM |
| Analysis and interpretation of data | JL, TAL, LE, JS, REM, PBT, AMM, YX, FL, CCM, CJM |
| Drafting of article | JL |
| Critical Revision of the article | AMM, CJM |
| Final Approval of the article | All |
| Provision of Study Materials | RM |
| Statistical Expertise | FL |
| Administrative, Technical or Logistical Support | JS, CJM |
| Collection and Assembly of data | JL, TAL, LE, RM, REM, JLS, YX, CG |
Competing interests
JL, TAL, LE, JS, JLS, RM, YX, FL, CG, CCM and CJM are current or former employees of, and are shareholders in, GlaxoSmithKline. REM, PBT and AMM declare no competing interests.
References
- 1.Loeser RF. Age-Related Changes in the Musculoskeletal System and the Development of Osteoarthritis. Clin Geriatr Med. 2010;26:371–386. doi: 10.1016/j.cger.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woolfe AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–656. [PMC free article] [PubMed] [Google Scholar]
- 3.Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 2012;1824:133–145. doi: 10.1016/j.bbapap.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 5.Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 6.Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glasson SS. In vivo osteoarthritis target validation utilizing genetically-modified mice. Curr Drug Targets. 2007;8:367–376. doi: 10.2174/138945007779940061. [DOI] [PubMed] [Google Scholar]
- 8.Lark MW, Bayne EK, Flanagan J, Harper CF, Hoerrner LA, Hutchinson NI, et al. Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J Clin Invest. 1997;100:93–106. doi: 10.1172/JCI119526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Little CB, Meeker CT, Golub SB, Lawlor KE, Farmer PJ, Smith SM, et al. Blocking aggrecanase cleavage in the aggrecan interglobular domain abrogates cartilage erosion and promotes cartilage repair. J Clin Invest. 2007;117:1627–1636. doi: 10.1172/JCI30765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Struglics A, Hansson M. MMP proteolysis of the human extracellular matrix protein aggrecan is mainly a process of normal turnover. Biochem J. 2012;446:213–223. doi: 10.1042/BJ20120274. [DOI] [PubMed] [Google Scholar]
- 11.Durigova M, Nagase H, Mort JS, Roughley PJ. MMPs are less efficient than ADAMTS5 in cleaving aggrecan core protein. Matrix Biol. 2011;30:145–153. doi: 10.1016/j.matbio.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu J, Rong J, Guan F, Jiang L, Zhang T, Tao S, et al. Association of ADAMTS5 gene polymorphisms with osteoarthritis in Chinese Han population: a community-based case-control study. Rheumatol Int. 2012 doi: 10.1007/s00296-012-2506-1. [DOI] [PubMed] [Google Scholar]
- 13.Plaas A, Osborn B, Yoshihara Y, Bai Y, Bloom T, Nelson F, et al. Aggrecanolysis in human osteoarthritis: confocal localization and biochemical characterization of ADAMTS5-hyaluronan complexes in articular cartilages. Osteoarthritis Cartilage. 2007;15:719–734. doi: 10.1016/j.joca.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Struglics A, Larsson S, Pratta MA, Kumar S, Lark MW, Lohmander LS. Human osteoarthritis synovial fluid and joint cartilage contain both aggrecanase- and matrix metalloproteinase-generated aggrecan fragments. Osteoarthritis Cartilage. 2006;14:101–113. doi: 10.1016/j.joca.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Verma P, Dalal K. ADAMTS-4 and ADAMTS-5: key enzymes in osteoarthritis. J Cell Biochem. 2011;112:3507–3514. doi: 10.1002/jcb.23298. [DOI] [PubMed] [Google Scholar]
- 16.Malfait AM, Liu RQ, Ijiri K, Komiya S, Tortorella MD. Inhibition of ADAM-TS4 and ADAM-TS5 prevents aggrecan degradation in osteoarthritic cartilage. J Biol Chem. 2002;277:22201–22208. doi: 10.1074/jbc.M200431200. [DOI] [PubMed] [Google Scholar]
- 17.Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, et al. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- 18.Naito S, Shiomi T, Okada A, Kimura T, Chijiiwa M, Fujita Y, et al. Expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic cartilage. Pathol Int. 2007;57:703–711. doi: 10.1111/j.1440-1827.2007.02167.x. [DOI] [PubMed] [Google Scholar]
- 19.Kost TA, Condreay JP, Jarvis DL. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol. 2005;23:567–575. doi: 10.1038/nbt1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Germaschewski FM, Matheny CJ, Larkin J, Liu F, Thomas LR, Saunders JS, et al. Quantitation OF ARGS aggrecan fragments in synovial fluid, serum and urine from osteoarthritis patients. Osteoarthritis Cartilage. 2014;22:690–697. doi: 10.1016/j.joca.2014.02.930. [DOI] [PubMed] [Google Scholar]
- 21.Pratta MA, Su JL, Leesnitzer MA, Struglics A, Larsson S, Lohmander LS, et al. Development and characterization of a highly specific and sensitive sandwich ELISA for detection of aggrecanase-generated aggrecan fragments. Osteoarthritis Cartilage. 2006;14:702–713. doi: 10.1016/j.joca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18 (Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Some homology data for this paper were retrieved from the NCBI Homologene Database. NAtional Center for Biotechnology Information, U.S. National Library of Medicine; Bethesda, Maryland: Mar, 2014. World Wide Web URL: http://www.ncbi.nlm.nih.gov/homologene/5109. [Google Scholar]
- 25.Deng H, O’Keefe H, Davie CP, Lind KE, Acharya RA, Franklin GJ, et al. Discovery of highly potent and selective small molecule ADAMTS-5 inhibitors that inhibit human cartilage degradation via encoded library technology (ELT) J Med Chem. 2012;55:7061–7079. doi: 10.1021/jm300449x. [DOI] [PubMed] [Google Scholar]
- 26.Gendron C, Kashiwagi M, Lim NH, Enghild JJ, Thogersen IB, Hughes C, et al. Proteolytic activities of human ADAMTS-5: comparative studies with ADAMTS-4. J Biol Chem. 2007;282:18294–18306. doi: 10.1074/jbc.M701523200. [DOI] [PubMed] [Google Scholar]
- 27.Lyskov S, Gray JJ. The RosettaDock server for local protein-protein docking. Nucleic Acids Res. 2008;36:W233–238. doi: 10.1093/nar/gkn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu TT. From esoteric theory to therapeutic antibodies. Appl Biochem Biotechnol. 1994;47:107–117. doi: 10.1007/BF02787928. discussion 117–108. [DOI] [PubMed] [Google Scholar]
- 29.Velasco J, Li J, DiPietro L, Stepp MA, Sandy JD, Plaas A. Adamts5 deletion blocks murine dermal repair through CD44-mediated aggrecan accumulation and modulation of transforming growth factor beta1 (TGFbeta1) signaling. J Biol Chem. 2011;286:26016–26027. doi: 10.1074/jbc.M110.208694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Julovi SM, Yasuda T, Shimizu M, Hiramitsu T, Nakamura T. Inhibition of interleukin-1beta-stimulated production of matrix metalloproteinases by hyaluronan via CD44 in human articular cartilage. Arthritis Rheum. 2004;50:516–525. doi: 10.1002/art.20004. [DOI] [PubMed] [Google Scholar]
- 31.Malfait AM, Ritchie J, Gil AS, Austin JS, Hartke J, Qin W, et al. ADAMTS-5 deficient mice do not develop mechanical allodynia associated with osteoarthritis following medial meniscal destabilization. Osteoarthritis Cartilage. 2010;18:572–580. doi: 10.1016/j.joca.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, et al. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci U S A. 109:20602–20607. doi: 10.1073/pnas.1209294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlson CS, Loeser RF, Jayo MJ, Weaver DS, Adams MR, Jerome CP. Osteoarthritis in cynomolgus macaques: a primate model of naturally occurring disease. J Orthop Res. 1994;12:331–339. doi: 10.1002/jor.1100120305. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert AM, Bikker JA, O’Neil SV. Advances in the development of novel aggrecanase inhibitors. Expert Opin Ther Pat. 2011;21:1–12. doi: 10.1517/13543776.2011.539204. [DOI] [PubMed] [Google Scholar]
- 35.Chiusaroli R, Visentini M, Galimberti C, Casseler C, Mennuni L, Covaceuszach S, et al. Targeting of ADAMTS5’s ancillary domain with the recombinant mAb CRB0017 ameliorates disease progression in a spontaneous murine model of osteoarthritis. Osteoarthritis Cartilage. 2013;21:1807–1810. doi: 10.1016/j.joca.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Devy L, Huang L, Naa L, Yanamandra N, Pieters H, Frans N, et al. Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res. 2009;69:1517–1526. doi: 10.1158/0008-5472.CAN-08-3255. [DOI] [PubMed] [Google Scholar]
- 37.Sela-Passwell N, Kikkeri R, Dym O, Rozenberg H, Margalit R, Arad-Yellin R, et al. Antibodies targeting the catalytic zinc complex of activated matrix metalloproteinases show therapeutic potential. Nat Med. 2011;18:143–147. doi: 10.1038/nm.2582. [DOI] [PubMed] [Google Scholar]
- 38.Manka SW, Carafoli F, Visse R, Bihan D, Raynal N, Farndale RW, et al. Structural insights into triple-helical collagen cleavage by matrix metalloproteinase 1. Proc Natl Acad Sci U S A. 2012;109:12461–12466. doi: 10.1073/pnas.1204991109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arner EC. Aggrecanase-mediated cartilage degradation. Curr Opin Pharmacol. 2002;2:322–329. doi: 10.1016/s1471-4892(02)00148-0. [DOI] [PubMed] [Google Scholar]
- 40.Majumdar MK, Askew R, Schelling S, Stedman N, Blanchet T, Hopkins B, et al. Double-knockout of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis. Arthritis Rheum. 2007;56:3670–3674. doi: 10.1002/art.23027. [DOI] [PubMed] [Google Scholar]
- 41.Fosang AJ, Rogerson FM. Identifying the human aggrecanase. Osteoarthritis Cartilage. 2010;18:1109–1116. doi: 10.1016/j.joca.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Gao G, Westling J, Thompson VP, Howell TD, Gottschall PE, Sandy JD. Activation of the proteolytic activity of ADAMTS4 (aggrecanase-1) by C-terminal truncation. J Biol Chem. 2002;277:11034–11041. doi: 10.1074/jbc.M107443200. [DOI] [PubMed] [Google Scholar]
- 43.Zeng W, Corcoran C, Collins-Racie LA, Lavallie ER, Morris EA, Flannery CR. Glycosaminoglycan-binding properties and aggrecanase activities of truncated ADAMTSs: comparative analyses with ADAMTS-5, -9, -16 and -18. Biochim Biophys Acta. 2006;1760:517–524. doi: 10.1016/j.bbagen.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 44.Rogerson FM, Stanton H, East CJ, Golub SB, Tutolo L, Farmer PJ, et al. Evidence of a novel aggrecan-degrading activity in cartilage: Studies of mice deficient in both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2008;58:1664–1673. doi: 10.1002/art.23458. [DOI] [PubMed] [Google Scholar]
- 45.Carlson CS, Loeser RF, Purser CB, Gardin JF, Jerome CP. Osteoarthritis in cynomolgus macaques. III: Effects of age, gender, and subchondral bone thickness on the severity of disease. J Bone Miner Res. 1996;11:1209–1217. doi: 10.1002/jbmr.5650110904. [DOI] [PubMed] [Google Scholar]
- 46.Chockalingam PS, Sun W, Rivera-Bermudez MA, Zeng W, Dufield DR, Larsson S, et al. Elevated aggrecanase activity in a rat model of joint injury is attenuated by an aggrecanase specific inhibitor. Osteoarthritis Cartilage. 2011;19:315–323. doi: 10.1016/j.joca.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Swearingen CA, Chambers MG, Lin C, Marimuthu J, Rito CJ, Carter QL, et al. A short-term pharmacodynamic model for monitoring aggrecanase activity: injection of monosodium iodoacetate (MIA) in rats and assessment of aggrecan neoepitope release in synovial fluid using novel ELISAs. Osteoarthritis Cartilage. 2010;18:1159–1166. doi: 10.1016/j.joca.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 48.Dupuis LE, McCulloch DR, McGarity JD, Bahan A, Wessels A, Weber D, et al. Altered versican cleavage in ADAMTS5 deficient mice; a novel etiology of myxomatous valve disease. Dev Biol. 2011;357:152–164. doi: 10.1016/j.ydbio.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Didangelos A, Mayr U, Monaco C, Mayr M. Novel role of ADAMTS-5 protein in proteoglycan turnover and lipoprotein retention in atherosclerosis. J Biol Chem. 2012;287:19341–19345. doi: 10.1074/jbc.C112.350785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang VM, Bell RM, Thakore R, Eyre DR, Galante JO, Li J, et al. Murine tendon function is adversely affected by aggrecan accumulation due to the knockout of ADAMTS5. J Orthop Res. 2012;30:620–626. doi: 10.1002/jor.21558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.