Abstract
Oligodendrocytes, which are the main cell type in cerebral white matter, are generated from their precursor cells (oligodendrocyte precursor cells: OPCs). However, the differentiation from OPCs to oligodendrocytes is disturbed under stressed conditions. Therefore, drugs that can improve oligodendrocyte regeneration may be effective for white matter-related diseases. Here we show that a vasoactive peptide adrenomedullin (AM) promotes the in vitro differentiation of OPCs under pathological conditions. Primary OPCs were prepared from neonatal rat brains, and differentiated into myelin-basic-protein expressing oligodendrocytes over time. This in vitro OPC differentiation was inhibited by prolonged chemical hypoxic stress induced by non-lethal CoCl2 treatment. However, AM promoted the OPC differentiation under the hypoxic stress conditions, and the AM receptor antagonist AM22–52 cancelled the AM-induced OPC differentiation. In addition, AM treatment increased the phosphorylation level of Akt in OPC cultures, and correspondingly, the PI3K/Akt inhibitor LY294002 blocked the AM-induced OPC differentiation. Taken together, AM treatment rescued OPC maturation under pathological conditions via an AM-receptor-PI3K/Akt pathway. Oligodendrocytes play critical roles in white matter by forming myelin sheath. Therefore, AM signaling may be a promising therapeutic target to boost oligodendrocyte regeneration in CNS disorders.
Keywords: Adrenomedullin, oligodendrocyte precursor cell, oligodendrocyte, cell culture, PI3K
Introduction
Oligodendrocytes are one of the major cell types in the cerebral white matters. They produce a lipid-rich membrane called myelin, which enwrap up to 60 axonal segments each (i.e. myelin sheath). Myelin sheaths enable effective nerve impulse conduction and play a supporting role for axons and neuron homeostasis [1]. During development, oligodendrocyte precursor cells (OPCs) are generated from germinal zones, then proliferate and migrate to both grey and white matter areas, where most of them differentiate into mature oligodendrocytes and form myelin sheaths. However, some OPCs may persist in an immature state. These residual OPCs are widely distributed in adult brains, comprising 5–8% of all cells [2]. Although myelinated tracts are formed early in life, renewal of myelin and oligodendrocyte continues throughout most of the adult life [3–5]. In fact, myelin sheaths in the adult CNS exhibit some plasticity in response to changes in neural activity [6] and brain injury [7]. Residual OPCs may play an important part in these endogenous mechanisms of white matter repair and renewal.
In demyelinating diseases, injury of myelin/oligodendrocytes results in a profound loss of myelin sheaths, axonal injury and degeneration, which eventually lead to long-lasting functional disabilities [8, 9]. Mouse models of oligodendrocyte injury, such as proteolipid protein (plp1)-null mice or Cnp mutant mice, show axon loss without considerable demyelination [10, 11], suggesting that oligodendrocytes may also support axon survival through a myelin-independent mechanisms [12]. Endogenous repair attempts by OPC proliferation and differentiation would occur at the early stage of demyelinating disorders but often fail as disease progresses [13]. Although no clinically proven agents currently exist to protect/support OPCs under prolonged or acute pathological conditions, enhancement of oligodendrogenesis (regeneration of mature myelinating oligodendrocytes) should be a promising approach for treatment of demyelinating diseases [8, 14].
One potential target candidate for promoting oligodendrogenesis during pathological conditions may be adrenomedullin (AM). AM was discovered as a vasoactive peptide from human pheochromocytoma in 1993 [15, 16]. AM is widely distributed in tissues, and secreted from various organs such as adrenal medulla, heart, kidney, lung, and vascular wall as well as the brain. AM has diverse biological actions, including cell proliferation and differentiation in a paracrine and autocrine manner [17–20]. AM also have important roles for cellular function of immature cells, such as endothelial progenitor cell (EPC), mesenchymal stem cell, hematopoietic stem cell, adrenocortical stem cell, and neural stem/progenitor cell (NSPC) [18, 21, 22]. Recently, it has been reported that AM may also regulate white matter function in the brain. In a mouse model of white matter injury by prolonged cerebral hypoperfusion, increased levels of circulating AM preserved oligodendrocyte/myelin integrity along with restoring cerebral hemodynamic, promoting arteriogenesis/angiogenesis, and alleviating oxidative damage in cerebral microvessels [23, 24]. In addition, AM deficiency exacerbated white matter injury during prolonged cerebral hypoperfusion conditions [25]. However, mechanisms that underlie the ability of AM to protect myelin and oligodendrocytes in damaged white matter remain unclear. In this proof-of concept study, we tested the hypothesis that AM can promote OPC differentiation under pathological conditions.
Materials and Methods
All experiments were performed following protocols approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Reagents
AM and AM receptor antagonist AM22–52 were purchased from Peptide Institute Inc., and dissolved in distilled water. CoCl2 and LY294002 were purchased from Sigma, and dissolved in dimethysulphoxide. The final concentration of dimethysulphoxide in the culture medium was less than 0.1%, which had no effect on OPC survival and function.
Cell culture
OPCs were prepared as previously described [26, 27]. Briefly, cerebral cortices from 1–2 day old Sprague Dawley rats were dissected, minced, and digested. Dissociated cells were plated in poly-D-lysine-coated 75 cm2 flasks, and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 20% heat-inactivated fetal bovine serum and 1% penicillin/streptomycin. After the cells were confluent (~10 d), the flasks were shaken for 1 h on an orbital shaker (218 rpm) at 37°C to remove microglia. They are then changed to new medium and shaken overnight (~20 h). The medium was collected and plated on non-coated tissue culture dishes for 1 h at 37°C to eliminate contaminating astrocytes and microglia. The non-adherent cells were collected and replated in Neurobasal (NB) medium containing 2 mM glutamine, 1% penicillin/streptomycin, 10 ng/ml PDGF, 10 ng/ml FGF, and 2% B27 supplement onto poly-DL-ornithine-coated plates. Four to 5 days after plating, the OPCs were used for the experiments. To differentiate OPCs to oligodendrocytes, the culture medium was switched to DMEM containing 1% penicillin/streptomycin, 10 ng/ml CNTF, 15 nM T3, and 2% B27 supplement.
CoCl2 treatment
To mimic hypoxic conditions in vitro, cells were incubated with non-lethal concentration of cobalt chloride (CoCl2) according to our previous reports [28, 29]. For experiments in Figures 1 and 3, cells were maintained in the differentiation culture media with or without 1 µM CoCl2 for 7 days. Freshly prepared cultured media containing CoCl2 were applied to OPC cultures on days 0, 3, and 5 (i.e. after starting OPC differentiation, we changed the culture media on days 3 and 5). Then on day 7 after starting OPC differentiation, cells were used for cell proliferation/survival assay, immunocytochemistry, and western blot. For experiments in Suppl Figure S1, cells were maintained in the differentiation culture media for 7 days. We prepared four groups: (i) control (no CoCl2 stress), (ii) 1 µM CoCl2 (for 7 days), (iii) 1 µM CoCl2 (for 7 days) plus 10 nM AM (for 7 days), and (iv) 1 µM CoCl2 (for 7 days) plus 10 nM AM (from day 0 to day 1). After starting OPC differentiation, we changed the cultured media on days 1, 3, 5 and then on day 7, cells were used for western blot.
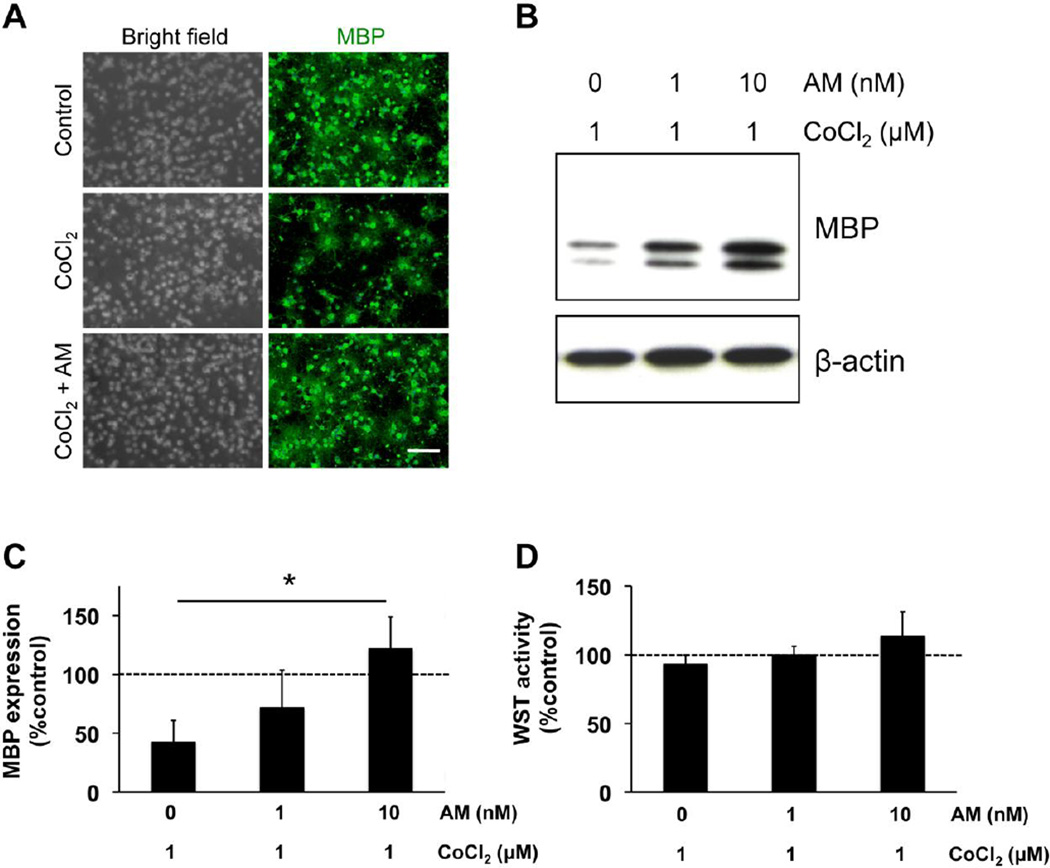
Figure 1. AM promoted OPC differentiation under prolonged hypoxic conditions.
(A) Cultured rat OPCs were maintained in the differentiation media for 7 days. Under normal conditions, the OPCs were successfully maturated into MBP-expressing oligodendrocytes. On the other hand, when OPCs were incubated with non-lethal 1 µM CoCl2 (e.g. chemical hypoxic conditions), they were not differentiated. However, AM treatment (10 nM) promoted the in vitro OPC differentiation under the stressed conditions. MBP is a marker for mature oligodendrocytes. Scale bar = 200 nm. (B-C) Western blot analyses also confirmed that the hypoxic conditions disturbed the OPC differentiation but AM promoted the process. MBP is a marker for mature oligodendrocytes, and β-actin is an internal control. Percentages of MBP expression were calculated based on the values in control group (i.e. no CoCl2 stress). Values are mean ± SD from 4 independent experiments. *P<0.05. (D) The WST assay showed that either AM or CoCl2 did not induce overt cell death in our OPC culture system. Percentages of WST activity were calculated based on the values in control group (i.e. no CoCl2 stress). Values are mean ± SD from 4 independent experiments.
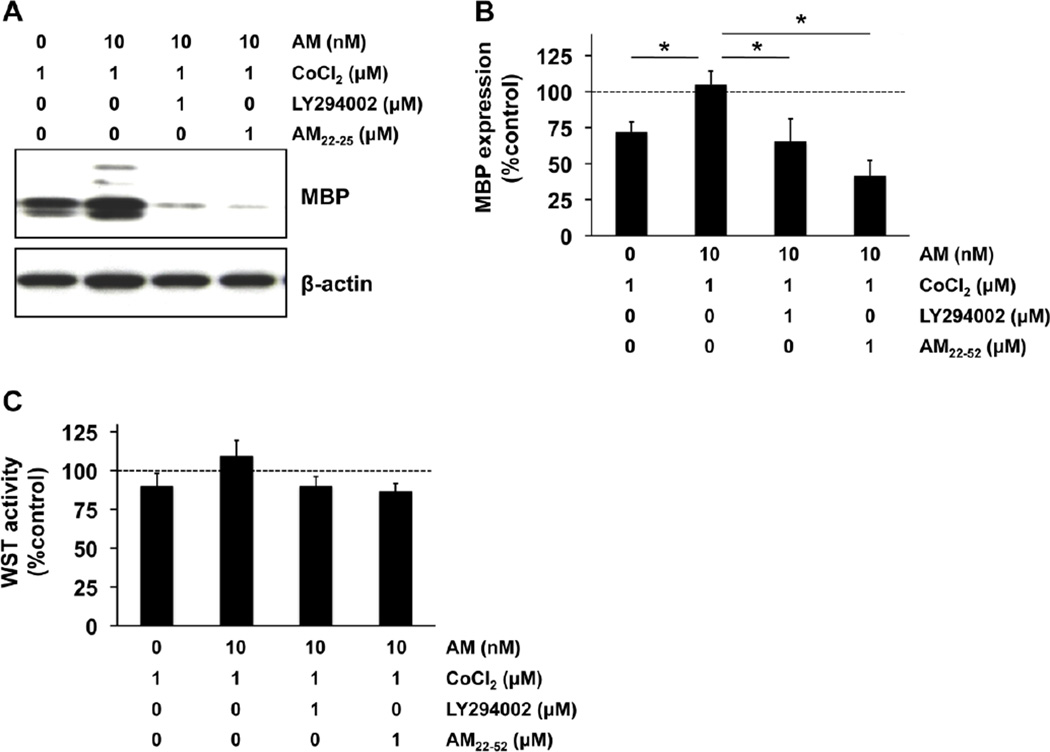
Figure 3. AM-receptor-PI3K/Akt pathway mediated AM-induced OPC maturation.
(A-B) Cultured rat OPCs were maintained in the differentiation media for 7 days. The prolonged chemical hypoxic conditions by non-lethal 1 µM CoCl2 disturbed OPC maturation, but AM treatment promoted the process. This AM-induced OPC maturation was inhibited by either co-treatment with 1 µM LY294002 (PI3K inhibitor) or 1 µM AM22–52 (AM-receptor antagonist). Percentages of MBP expression were calculated based on the values in control group (i.e. no CoCl2 stress). N=3 of independent experiments. *P<0.05. (B) The WST assay showed that in any treatment groups, there was no significant OPC damage. Percentages of WST activity were calculated based on the values in control group (i.e. no CoCl2 stress). Values are mean ± SD from 3 independent experiments.
Cell proliferation/survival assay
Cell proliferation/survival was assessed by WST reduction assay (Cell Counting Kit-8, Dojindo). WST assay is a sensitive colorimetric method to detect cell viability. Highly water-soluble tetrazolium salt WST-8 is reduced by dehydrogenase activities in live cells to give a yellow-color formazan dye, which is soluble in the tissue culture media. Therefore, the intensity of the formazan dye can be an indicator correlating to the number of viable cells. The cells were incubated with 10% WST solution for 1 h at 37°C. Then the absorbance of the culture medium was measured with a microplate reader at a test wavelength of 450 nm and a reference wavelength of 630 nm.
Immunocytochemistry
The cells were washed with ice-cold PBS, pH 7.4, followed by 4% paraformaldehyde for 15 min. After being further washed three times in PBS containing 0.1% Triton X-100, they were incubated with 1% bovine serum albumin in PBS for 1 h. Then cells were incubated with primary antibodies against MBP (1:100, Thermo Scientific) at 4°C overnight. After washing with PBS, they were incubated with secondary antibodies for 1 h at room temperature. Finally, nuclei were counterstained with DAPI.
Western blotting
Cells were rinsed twice with ice-cold PBS, and the cells were collected into cell lysis buffer (Pro-PREP™ Protein Extraction Kit, iNtRON Biotechnology). After the protein concentrations were quantified and adjusted to the same concentrations by adding PBS, samples were mixed with equal volumes of sample buffer containing 91% SDS (Novex) and 9% 2-mercaptoethanol (Sigma). Subsequently, samples were heated at 95°C for 5 min, and each sample was loaded onto 4–20% Tris–glycine gels. After electrophoresis and transferring to nitrocellulose membranes (Novex), the membranes were blocked in Tris buffered saline with 0.1% Tween 20 (TBS-T) containing 5% nonfat dry milk for 60 min at room temperature. Membranes were then incubated overnight at 4°C with myelin basic protein (MBP) antibody (1:500, Thermo Scientific), Akt antibody (1:1000, Cell Signaling), phosphorylated Akt (Ser473) antibody (1:1000, Cell Signaling), or anti-β-actin antibody (1:10000, Sigma Aldrich) followed by incubation with peroxidase-conjugated secondary antibodies and visualization by enhanced chemiluminescence (Amersham).
Statistical analysis
Experiments were performed in duplicate, repeated 3–4 times independently. Quantitative data were analyzed by using ANOVA followed by post hoc Tukey test or Tukey-Kramer test. All values are expressed as means ± SD. A value of P <0.05 was considered statistically significant.
Results
Primary OPCs were prepared from rat neonatal cortex. When OPCs were maintained in differentiation media (e.g. DMEM plus 2% B27 including 10 ng/ml CNTF, 15 nM T3 over 7 days), the cells began to exhibit oligodendrocyte-like phenotypes with myelin-basic-protein (MBP) expression (Figure 1A: control group). As previously reported, OPC differentiation was suppressed by prolonged hypoxia induced by non-lethal CoCl2 treatment for 7 days [28, 29] (Figure 1A: CoCl2-treated group). However, 7-day treatment with AM rescued OPC differentiation under the prolonged chemical hypoxic conditions (Fig 1A: AM + CoCl2-treated group). Western blot analyses confirmed that AM promoted oligodendrocyte maturation under the stressed conditions (Fig 1B-C). However, 1-day AM treatment did not rescue the OPC differentiation under the stressed conditions (Suppl Figure S1). A standard WST assay showed that neither AM nor CoCl2 altered the OPC number or induced overt cell death in our cell culture system (Fig 1D).
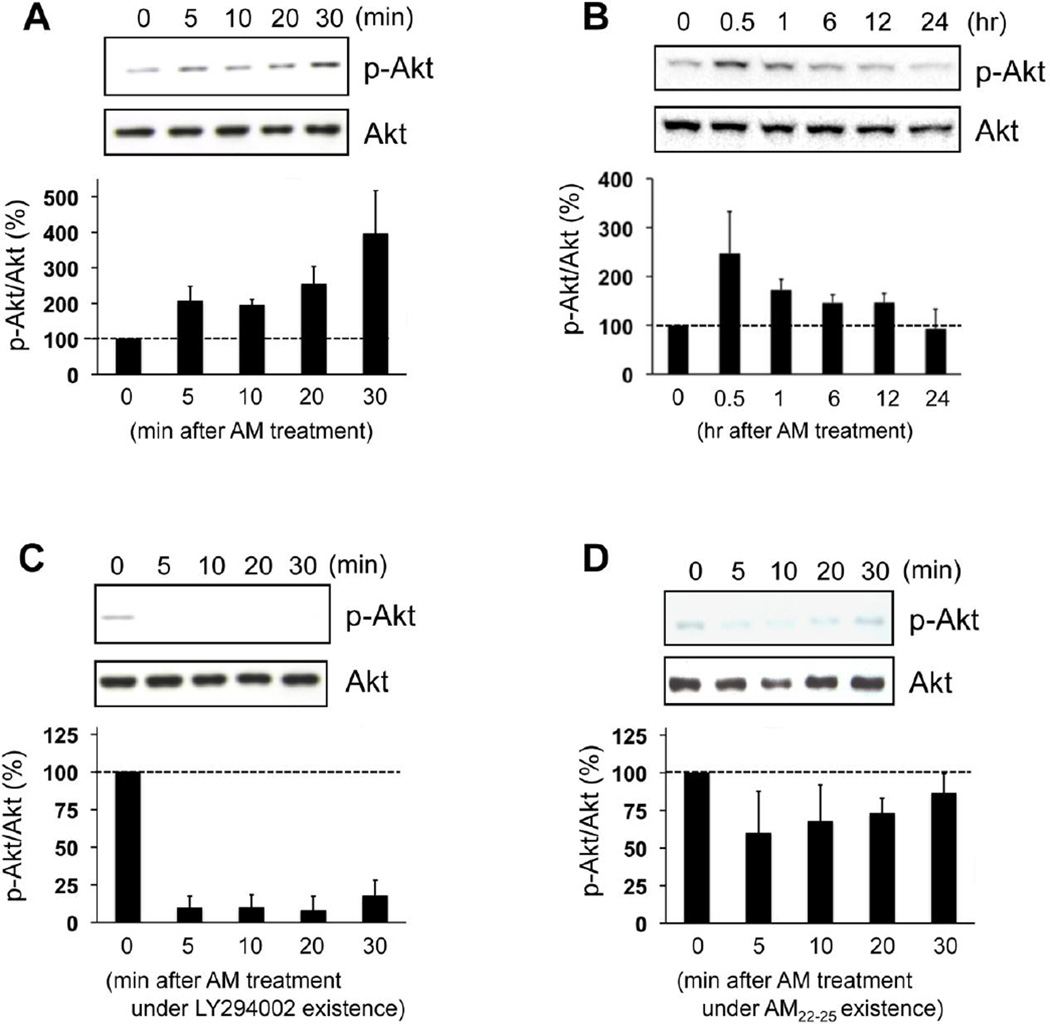
We next examined the mechanisms that may underlie AM-mediated rescue of OPC differentiation. Receptor-mediated Akt signaling may be involved since AM treatment increased the phosphorylation levels of Akt in OPCs within 30 minutes (Fig 2A), and this Akt phosphorylation was back to baseline in 24 hours after AM treatment (Fig 2B). AM-induced Akt phosphorylation was inhibited by the PI3K inhibitor LY294002 (Fig 2C) or blockade of the AM receptor with AM22–52 (Fig 2D). Finally, blockade of PI3K/Akt signaling with LY294002 or AM22–52 negated the ability of AM to rescue OPC differentiation treatment under prolonged hypoxic conditions (Fig 3A-B). These effects were not due to changes in cell survival since the WST assay once again showed that there was no significant cell damage in our experimental conditions (Fig 3C).
Figure 2. AM increased the levels of Akt phosphorylation via AM-receptor-PI3K pathway.
(A-B) Cultured rat OPCs were incubated with 10 nM AM up to 24 hr, and then cell lysates were subjected to western blot analyses with anti-p-Akt or anti-Akt antibodies. AM treatment increased the levels of Akt phosphorylation without changing total Akt levels. Values are mean ± SD from 3 independent experiments. (C) Under the conditions of 1 µM LY294002 (PI3K inhibitor) existence, AM did not increase the phosphorylation levels of Akt. Values are mean ± SD from 3 independent experiments. (D) Similarly, AM did not increase the phosphorylation levels of Akt under the conditions of 1 µM AM22–52 (AM-receptor antagonist) existence. Values are mean ± SD from 3 independent experiments.
Discussion
In this study, we obtained proof-of-concept data to support our hypothesis that AM can rescue OPC differentiation into mature oligodendrocytes under pathological conditions. Our pharmacological approaches also showed that the AM receptor and PI3K/Akt would mediate these AM effects. AM and its receptors are widely expressed in the central nervous system (CNS) [30, 31]. Past studies extensively examined the multiple roles of AM on neuronal and vascular function. AM exerts various actions on the vasculature, such as vasodilation, angiogenesis, and regulation of blood brain barrier. Similarly, AM acts as a neurotransmitter, neuromodulator, or neurohormone [18]. In addition, AM can be considered as a therapeutic target for CNS diseases since several animal studies have demonstrated that AM reduces neuronal injuries [32–35]. Furthermore, compared to wild-type mice, brain-specific conditional AM knockout mice or AM heterozygous KO mice exhibited more neuronal damage after ischemic insults [36, 37]. In vitro cell culture studies also confirmed that AM protected neurons against oxygen glucose deprivation stress in an autocrine and paracrine manner [33, 38]. AM may also be effective in the chronic phase as AM increased mobilization of CD34+ mononuclear cells (so-called EPCs) and subsequent vascular regeneration and neurogenesis after stroke [33]. Our current findings that AM can promote oligodendrogenesis under pathological conditions may support these past studies and confirm that the AM signaling would be the therapeutic target for neurological disorders, especially for white matter-related diseases.
Neurons play the central role in the brain, and therefore, neuroprotection would be the most important approach for CNS diseases. However, oligodendrocytes (and oligodendrocyte-rich white matter) should also be considered when we aim to develop efficient therapies for brain protection. Compared to rodents, primates possess an evolutionally expanded volume of white matter, and white matter damage is a clinically important aspect of several CNS diseases, such as stroke or vascular dementia [39–43]. This may explain the reasons why many neuroprotectants (e.g. glutamate receptor antagonists, antioxidants, etc.) that were proved neuroprotective in rodent CNS disease models have failed to provide efficacy in clinical trials [44]. Even small lesions in the white matter areas (corona radiata or internal capsule) could lead to severe hemiplegia and poor functional prognosis in humans because loss of oligodendroglial supports can cause progressive axon/neuron degeneration and long-term functional disability. Others and we have previously demonstrated that AM might play an important role in the preservation of oligodendrocyte and white matter integrity in mouse models of white matter injury [23–25]. For example, overexpression of circulating AM increased GST-pi-positive oligodendrocytes and preserved myelin integrity accompanied with promotion of neovascularization and vasoprotection after prolonged cerebral hypoperfusion in mice. This "oligo-vascular" protection may lead to the prevention of cognitive decline after demyelination [23, 24]. In addition, a recent report showed that AM knockout mice exhibited decreased OPCs and GST-pi-positive oligodendrocytes and MBP expression in white matter after prolonged cerebral hypoperfusion [25]. Here we show for the first time that AM would directly work on OPCs to promote oligodendrogenesis under pathological conditions in vitro. These findings may explain the mechanisms for beneficial effects of AM on white matter integrity and function. Therefore, the multiple actions of AM on neuro-vascular-oligo protection would have a potential as a promising treatment for cerebrovascular diseases.
Taken together, our findings support the hypothesis that AM can rescue OPC differentiation via receptor-mediated Akt signaling. However, there are some important caveats to keep in mind. First, our current study used only a pure cell culture system. However, to prove clinically-relevant supportive/protective roles of AM on OPCs against stress, we should test the efficacy of AM on in vivo white matter injury animals. Second, our data indicate that short-term AM treatment was not supportive for in vitro OPC differentiation under pathological conditions. A single treatment of AM could activate the downstream pathway (i.e. Akt phosphorylation), but to sufficiently drive the OPC differentiation, multiple rounds of AM treatments would be required. Before testing the efficacy of AM using in vivo animal models, further investigation into the underlying mechanisms of AM/Akt-induced OPC maturation is needed to identify effective treatment schedules of AM. Third, we only examined the PI3K/Akt pathway as an intracellular signaling pathway for in vitro oligodendrogenesis by AM. But the AM receptor would activate other cellular signaling pathways, such as MEK/ERK or cAMP/PKA pathways [45]. Whether these pathways are also involved in the OPC-supportive effects of AM should be carefully examined in future studies. Finally, as OPCs are generated from NSPCs, we may also need to test if AM can enhance the number of newly generated OPCs from NSPCs after white matter injury. A recent study showed that lack of AM results in profound changes in the proliferation and differentiation rates in the progeny of NSPCs isolated from the olfactory bulbs of AM deficient mice. NSPCs derived from the AM deficient mice produced a lower proportion of neuronal-astroglial lineage cells and a higher proportions of oligodendrocyte lineage cells compared to NSPCs from WT mice [22]. Hence, future studies are warranted to examine how AM regulates the cell fates of NSPCs under normal and pathological conditions.
In summary, our data provide proof-of-concept that AM can promote and rescue OPC differentiation into mature oligodendrocytes under pathological conditions in vitro. Preservation and repair of oligodendrocytes should be an important criteria of therapies for CNS disease patients. Therefore, AM signaling may be a novel therapeutic target for accelerating regenerative responses in demyelinating conditions such as stroke, multiple sclerosis or vascular dementia.
Supplementary Material
Highlights.
A vasoactive peptide adrenomedullin (AM) promoted OPC differentiation under pathological conditions in vitro
AM treatment increased phosphorylation level of Akt in OPC cultures
AM receptor antagonist AM22–52 and PI3K inhibitor LY294002 cancelled AM-induced OPC differentiation
Acknowledgments
Funding information: Supported in part by National Institutes of Health (P01 NS055104, R01 NS065089), Research Abroad from the Uehara Memorial Foundation, and the Japan Society for the Promotion of Science. We thank Dr. Noriko Osumi for many helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: none
References
- 1.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiological reviews. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 2.Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends in neurosciences. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- 3.Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 4.Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimou L, Simon C, Kirchhoff F, Takebayashi H, Gotz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nait-Oumesmar B, Picard-Riera N, Kerninon C, Baron-Van Evercooren A. The role of SVZ-derived neural precursors in demyelinating diseases: from animal models to multiple sclerosis. Journal of the neurological sciences. 2008;265:26–31. doi: 10.1016/j.jns.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy, Nature reviews. Neuroscience. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 9.Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330:779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, Schneider A, Zimmermann F, McCulloch M, Nadon N, Nave KA. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- 11.Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- 12.Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maki T, Hayakawa K, Pham LD, Xing C, Lo EH, Arai K. Biphasic mechanisms of neurovascular unit injury and protection in CNS diseases. CNS Neurol Disord Drug Targets. 2013;12:302–315. doi: 10.2174/1871527311312030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotter MR, Stadelmann C, Hartung HP. Enhancing remyelination in disease--can we wrap it up? Brain: a journal of neurology. 2011;134:1882–1900. doi: 10.1093/brain/awr014. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 16.Kato J, Tsuruda T, Kita T, Kitamura K, Eto T. Adrenomedullin: a protective factor for blood vessels. Arterioscler Thromb Vasc Biol. 2005;25:2480–2487. doi: 10.1161/01.ATV.0000184759.91369.f8. [DOI] [PubMed] [Google Scholar]
- 17.Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocrine reviews. 2000;21:138–167. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Herrero S, Larrayoz IM, Ochoa-Callejero L, Garcia-Sanmartin J, Martinez A. Adrenomedullin as a growth and cell fate regulatory factor for adult neural stem cells. Stem cells international. 2012;2012:804717. doi: 10.1155/2012/804717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez J, Martinez A. Cell and molecular biology of the multifunctional peptide, adrenomedullin. International review of cytology. 2002;221:1–92. doi: 10.1016/s0074-7696(02)21010-4. [DOI] [PubMed] [Google Scholar]
- 20.Shindo T, Sakurai T, Kamiyoshi A, Ichikawa-Shindo Y, Shimoyama N, Iinuma N, Arai T, Miyagawa S. Regulation of adrenomedullin and its family peptide by RAMP system--lessons from genetically engineered mice. Current protein & peptide science. 2013;14:347–357. doi: 10.2174/13892037113149990052. [DOI] [PubMed] [Google Scholar]
- 21.Larrayoz IM, Ochoa-Callejero L, Garcia-Sanmartin J, Vicario-Abejon C, Martinez A. Role of adrenomedullin in the growth and differentiation of stem and progenitor cells. International review of cell and molecular biology. 2012;297:175–234. doi: 10.1016/B978-0-12-394308-8.00005-4. [DOI] [PubMed] [Google Scholar]
- 22.Vergano-Vera E, Fernandez AP, Hurtado-Chong A, Vicario-Abejon C, Martinez A. Lack of adrenomedullin affects growth and differentiation of adult neural stem/progenitor cells. Cell and tissue research. 2010;340:1–11. doi: 10.1007/s00441-010-0934-3. [DOI] [PubMed] [Google Scholar]
- 23.Maki T, Ihara M, Fujita Y, Nambu T, Miyashita K, Yamada M, Washida K, Nishio K, Ito H, Harada H, Yokoi H, Arai H, Itoh H, Nakao K, Takahashi R, Tomimoto H. Angiogenic and vasoprotective effects of adrenomedullin on prevention of cognitive decline after chronic cerebral hypoperfusion in mice. Stroke; a journal of cerebral circulation. 2011;42:1122–1128. doi: 10.1161/STROKEAHA.110.603399. [DOI] [PubMed] [Google Scholar]
- 24.Maki T, Ihara M, Fujita Y, Nambu T, Harada H, Ito H, Nakao K, Tomimoto H, Takahashi R. Angiogenic roles of adrenomedullin through vascular endothelial growth factor induction. Neuroreport. 2011;22:442–447. doi: 10.1097/WNR.0b013e32834757e4. [DOI] [PubMed] [Google Scholar]
- 25.Mitome-Mishima Y, Miyamoto N, Tanaka R, Shimosawa T, Oishi H, Arai H, Hattori N, Urabe T. Adrenomedullin deficiency and aging exacerbate ischemic white matter injury after prolonged cerebral hypoperfusion in mice. BioMed research international. 2014;2014:861632. doi: 10.1155/2014/861632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pham LD, Hayakawa K, Seo JH, Nguyen MN, Som AT, Lee BJ, Guo S, Kim KW, Lo EH, Arai K. Crosstalk between oligodendrocytes and cerebral endothelium contributes to vascular remodeling after white matter injury. Glia. 2012;60:875–881. doi: 10.1002/glia.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyamoto N, Maki T, Pham LD, Hayakawa K, Seo JH, Mandeville ET, Mandeville JB, Kim KW, Lo EH, Arai K. Oxidative stress interferes with white matter renewal after prolonged cerebral hypoperfusion in mice. Stroke; a journal of cerebral circulation. 2013;44:3516–3521. doi: 10.1161/STROKEAHA.113.002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyamoto N, Pham LD, Hayakawa K, Matsuzaki T, Seo JH, Magnain C, Ayata C, Kim KW, Boas D, Lo EH, Arai K. Age-related decline in oligodendrogenesis retards white matter repair in mice. Stroke. 2013;44:2573–2578. doi: 10.1161/STROKEAHA.113.001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueta Y, Kitamura K, Isse T, Shibuya I, Kabashima N, Yamamoto S, Kangawa K, Matsuo H, Eto T, Yamashita H. Adrenomedullin-immunoreactive neurons in the paraventricular and supraoptic nuclei of the rat. Neuroscience letters. 1995;202:37–40. doi: 10.1016/0304-3940(95)12204-4. [DOI] [PubMed] [Google Scholar]
- 31.Serrano J, Alonso D, Fernandez AP, Encinas JM, Lopez JC, Castro-Blanco S, Fernandez-Vizarra P, Richart A, Santacana M, Uttenthal LO, Bentura ML, Martinez-Murillo R, Martinez A, Cuttitta F, Rodrigo J. Adrenomedullin in the central nervous system. Microscopy research and technique. 2002;57:76–90. doi: 10.1002/jemt.10053. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe K, Takayasu M, Noda A, Hara M, Takagi T, Suzuki Y, Yoshia J. Adrenomedullin reduces ischemic brain injury after transient middle cerebral artery occlusion in rats. Acta neurochirurgica. 2001;143:1157–1161. doi: 10.1007/s007010100007. [DOI] [PubMed] [Google Scholar]
- 33.Miyashita K, Itoh H, Arai H, Suganami T, Sawada N, Fukunaga Y, Sone M, Yamahara K, Yurugi-Kobayashi T, Park K, Oyamada N, Sawada N, Taura D, Tsujimoto H, Chao TH, Tamura N, Mukoyama M, Nakao K. The neuroprotective and vasculo-neuro-regenerative roles of adrenomedullin in ischemic brain and its therapeutic potential. Endocrinology. 2006;147:1642–1653. doi: 10.1210/en.2005-1038. [DOI] [PubMed] [Google Scholar]
- 34.Xia CF, Yin H, Borlongan CV, Chao J, Chao L. Adrenomedullin gene delivery protects against cerebral ischemic injury by promoting astrocyte migration and survival. Human gene therapy. 2004;15:1243–1254. doi: 10.1089/hum.2004.15.1243. [DOI] [PubMed] [Google Scholar]
- 35.Xia CF, Yin H, Borlongan CV, Chao J, Chao L. Postischemic infusion of adrenomedullin protects against ischemic stroke by inhibiting apoptosis and promoting angiogenesis. Experimental neurology. 2006;197:521–530. doi: 10.1016/j.expneurol.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 36.Hurtado O, Serrano J, Sobrado M, Fernandez AP, Lizasoain I, Martinez-Murillo R, Moro MA, Martinez A. Lack of adrenomedullin, but not complement factor H, results in larger infarct size and more extensive brain damage in a focal ischemia model. Neuroscience. 2010;171:885–892. doi: 10.1016/j.neuroscience.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 37.Miyamoto N, Tanaka R, Shimosawa T, Yatomi Y, Fujita T, Hattori N, Urabe T. Protein kinase A-dependent suppression of reactive oxygen species in transient focal ischemia in adrenomedullin-deficient mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:1769–1779. doi: 10.1038/jcbfm.2009.92. [DOI] [PubMed] [Google Scholar]
- 38.Tixier E, Leconte C, Touzani O, Roussel S, Petit E, Bernaudin M. Adrenomedullin protects neurons against oxygen glucose deprivation stress in an autocrine and paracrine manner. Journal of neurochemistry. 2008;106:1388–1403. doi: 10.1111/j.1471-4159.2008.05494.x. [DOI] [PubMed] [Google Scholar]
- 39.Benowitz LI, Carmichael ST. Promoting axonal rewiring to improve outcome after stroke. Neurobiol Dis. 2010;37:259–266. doi: 10.1016/j.nbd.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Q, Zhang ZG, Chopp M. MRI evaluation of white matter recovery after brain injury. Stroke. 2010;41:S112–S113. doi: 10.1161/STROKEAHA.110.595629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tosto G, Zimmerman ME, Carmichael OT, Brickman AM. Predicting Aggressive Decline in Mild Cognitive Impairment: The Importance of White Matter Hyperintensities. JAMA Neurol. 2014 doi: 10.1001/jamaneurol.2014.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Philips T, Rothstein JD. Glial cells in amyotrophic lateral sclerosis. Exp Neurol. 2014 doi: 10.1016/j.expneurol.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savitz SI, Fisher M. Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. Ann Neurol. 2007;61:396–402. doi: 10.1002/ana.21127. [DOI] [PubMed] [Google Scholar]
- 45.Nagaya N, Mori H, Murakami S, Kangawa K, Kitamura S. Adrenomedullin: angiogenesis and gene therapy, American journal of physiology. Regulatory, integrative and comparative physiology. 2005;288:R1432–R1437. doi: 10.1152/ajpregu.00662.2004. [DOI] [PubMed] [Google Scholar]
- 46.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. The Journal of clinical investigation. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shiojima I, Schiekofer S, Schneider JG, Belisle K, Sato K, Andrassy M, Galasso G, Walsh K. Short-term akt activation in cardiac muscle cells improves contractile function in failing hearts. The American journal of pathology. 2012;181:1969–1976. doi: 10.1016/j.ajpath.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.