Abstract
The tyrosine kinase Fyn has two regulatory tyrosine residues that when phosphorylated either activate (Tyr420) or inhibit (Tyr531) Fyn activity. Within the central nervous system, two protein tyrosine phosphatases (PTPs) target these regulatory tyrosines in Fyn. PTPα dephosphorylates Tyr531 and activates Fyn, while STEP (STriatal-Enriched protein tyrosine Phosphatase) dephosphorylates Tyr420 and inactivates Fyn. Thus, PTPα and STEP have opposing functions in the regulation of Fyn; however, whether there is cross talk between these two PTPs remains unclear. Here, we used molecular techniques in primary neuronal cultures and in vivo to demonstrate that STEP negatively regulates PTPα by directly dephosphorylating PTPα at its regulatory Tyr789. Dephosphorylation of Tyr789 prevents the translocation of PTPα to synaptic membranes, blocking its ability to interact with and activate Fyn. Genetic or pharmacologic reduction of STEP61 activity increased the phosphorylation of PTPα at Tyr789, as well as increased translocation of PTPα to synaptic membranes. Activation of PTPα and Fyn and trafficking of GluN2B to synaptic membranes are necessary for ethanol intake behaviors in rodents. We tested the functional significance of STEP61 in this signaling pathway by ethanol administration to primary cultures as well as in vivo, and demonstrated that the inactivation of STEP61 by ethanol leads to the activation of PTPα, its translocation to synaptic membranes, and the activation of Fyn. These findings indicate a novel mechanism by which STEP61 regulates PTPα and suggest that STEP and PTPα coordinate the regulation of Fyn.
Keywords: STEP, PTPα, dephosphorylation, ethanol administration, lipid rafts, dorsomedial striatum
Introduction
PTPα is a member of the receptor-type protein tyrosine phosphatases (PTPs) family and is characterized by a transmembrane domain and two intracellular catalytic domains (Sap et al. 1990; Wang and Pallen 1991; Paul and Lombroso 2003; Tonks 2006). It is expressed in many tissues, including the brain (Sap et al. 1990; Sahin et al. 1995). Several reports implicate PTPα in the regulation of integrin signaling, neurite outgrowth, oligodendrocyte differentiation, and myelination through activation of its substrates Fyn and Src and modulation of signaling by NCAM (neural cell adhesion molecule) and CHL1 (close homolog of L1) (Bodrikov et al. 2005; Ye et al. 2008; Chen et al. 2006; Wang et al. 2009; Zeng et al. 2003).
PTPα activates Fyn by dephosphorylating its inhibitory Tyr residue (Y531), allowing full activation of Fyn by auto-phosphorylation at a second regulatory Tyr (Y420) (Engen et al. 2008, Ingley 2008). PTPα knockout (KO) mice have increased phosphorylation of Fyn at its inhibitory site (Y531) and decreased Fyn activity (Ponniah et al. 1999; Su et al. 1999). PTPα KO mice show deficits in LTP as well as in learning and memory, consistent with a role of Fyn in regulating NMDA receptor trafficking to synaptic membranes (Skelton et al. 2003; Petrone et al. 2003).
STriatal-Enriched protein tyrosine Phosphatase (STEP) is widely expressed in multiple brain regions including the striatum, where two major isoforms, STEP61 and STEP46, are expressed (Lombroso et al. 1991; Boulanger et al. 1995). STEP61 is enriched in membrane fractions while STEP46 is enriched in cytosol fractions (Lombroso et al. 1993; Bult et al. 1996). STEP normally opposes the development of synaptic strengthening through dephosphorylation and inactivation of several kinases, including Fyn, as well as endocytosis of both NMDARs and AMPARs (Snyder et al. 2005; Zhang et al. 2008; Zhang et al. 2010; Zhang et al. 2011). Both high and low activities of STEP disrupt synaptic plasticity, and dysregulation of STEP activity is implicated in several neuropsychiatric and neurodegenerative disorders, including Alzheimer’s disease (Zhang et al. 2010), schizophrenia (Carty et al. 2012), Parkinson’s disease (Kurup et al. 2015), Huntington’s disease (Saavedra et al. 2011; Gladding et al. 2012), post-traumatic stress disorder (Yang et al. 2012), and stress-induced anxiety disorders (Dabrowska et al. 2013).
Recent studies have indicated that PTPα is a critical determinant of ethanol consumption in rodent models (Gibb et al. 2011; Ben Hamida et al. 2013). PTPα, Fyn and STEP are all expressed in the striatum, and ethanol administration or binge drinking results in a PTPα-mediated activation of Fyn in the dorsomedial striatum (DMS) but not in the nearby dorsolateral striatum (DLS) or the nucleus accumbens (NAc). Moreover, viral-based knockdown of PTPα or Fyn in the DMS reduces ethanol intake in rats (Wang et al. 2007; Wang et al. 2010; Ben Hamida et al. 2013).
In contrast, recent findings have shown that STEP is phosphorylated and inactivated specifically in the DMS during ethanol administration, and that STEP KO mice or shRNA knockdown of STEP in the DMS increases ethanol consumption (Darcq et al. 2014; Legastelois et al. in press). STEP and PTPα act on one, and not the other, of the two regulatory tyrosines in Fyn (Bhandari et al. 1998; Nguyen et al. 2002). This led to our hypothesis that PTPα may be a novel substrate for STEP to coordinate the bidirectional regulation of Fyn by STEP and PTPα, which we test here using genetic, pharmacological, and molecular techniques. The results suggest that inactivation of STEP is required for activation of PTPα and Fyn both in rat primary corticostriatal cultures and in vivo after ethanol administration to mice.
Materials and reagents
All antibodies used in this study are listed in Table S1. Two dopamine D1 receptor agonists SFK-82958 and SKF-38393, the PKA inhibitor H-89, the PKA activator forskolin, and ethanol (190 proof) were purchased from Sigma-Aldrich (St. Louis, MO), while the selective phosphodiesterase 4 inhibitor rolipram was obtained from Tocris Biosciences (Ellisville, MO). Recombinant glutathione S-transferase (GST)-tagged PTPα protein was purchased from Sino Biological (Beijing, China), while active Fyn kinase was obtained from Millipore (Bedford, MA).
Animals
The wild type (WT) and STEP knockout (KO) male mice used in these experiments were 3–4 months of age, maintained on a C57BL/6 background, and generated at Yale University from heterozygous crosses as described previously (Venkitaramani et al. 2009). Experimental mice were group-housed with a maximum of 5 mice per cage in a climate-controlled facility with 12h light-dark cycle with access to food and water ad libitum. All experiments were carried out during the light phase of the cycle. All procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Yale University.
Tissue processing
Total homogenates and crude synaptic membrane fractions (P2) were obtained from WT and STEP KO mouse striatum following previous protocols (Xu et al. 2012). Briefly, tissues were homogenized in ice-cold TEVP buffer (in mM): 10 Tris, pH 7.4, 5 NaF, 1 Na3VO4, 1 EDTA, 1 EGTA and 320 sucrose with protease inhibitor cocktail (Roche, Indianapolis, IN). Aliquots of samples were saved as total homogenates. The remaining homogenates were centrifuged at 1000 g for 10 min and the supernatants were further spun at 12,000 g for 20 min to isolate crude synaptic membrane fractions (P2). The pellets were resuspended and briefly sonicated in RIPA buffer (Pierce Biotechnology, Rockford, IL) with protease and phosphatase inhibitors (Roche). Total protein concentrations were determined using a BCA protein assay kit (Pierce Biotechnology).
Immunoblotting
All procedures were previously described (Xu et al. 2012). Briefly, 30 μg of samples were resolved on 8% SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad Laboratories, Richmond, CA). Blots were blocked, incubated with primary and secondary antibodies (Table S1) and developed using a Chemiluminescent Substrate kit (Pierce Biotechnology). Densitometry was performed and analyzed using Genetools program (Syngene, Cambridge, UK). All phospho-protein levels were normalized to total protein levels and then to the loading control β-actin.
GST fusion proteins and pull-down assays
PCR-amplified open reading frames of WT STEP61, the substrate trapping STEP61 C472S, or the substrate trapping STEP46 C300S were inserted into pGEX4T1 vectors (GE Lifesciences, Piscataway, NJ). The substrate-trapping STEP protein has a point mutation at its critical cysteine within the phosphatase domain, rendering STEP inactive. This variant of STEP still binds to its substrates, but does not release them, as dephosphorylation is required for release; these constructs have been used to identify STEP substrates in the past (Nguyen et al. 2002; Paul et al. 2003; Xu et al. 2012). Glutathione S-transferase (GST) fusion proteins were expressed in E. coli BL21 (DE3) and purified on glutathione sepharose (GE Lifesciences) as described (Xu et al. 2012). For pull-down assays, GST fusion proteins were conjugated to glutathione sepharose beads and incubated with mouse striatal lysates overnight at 4 °C. STEP interacting proteins were probed with specific antibodies.
Immunoprecipitation
WT and STEP KO mouse striatum or rat primary corticostriatal neurons were lysed in immunoprecipitation (IP) buffer (in mM): 10 Tris-HCl pH 7.4, 150 NaCl, 1% Triton X-100, 1 EDTA and 1 EGTA with protease and phosphatase inhibitor cocktail (Roche). Lysates were incubated with anti-STEP or anti-PTPα antibodies overnight at 4 °C. On the second day, Protein A/G plus agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) were added for 4 h. Beads were washed 3 times and resuspended in 2×Laemmli buffer (Bio-Rad Laboratories) and subjected to SDS-PAGE and western blotting.
Dephosphorylation of PTPα by STEP in vitro
Recombinant GST-PTPα was phosphorylated at pY789 by Fyn kinase in vitro in kinase assay buffer (in mM): 50 Tris-HCl, pH 7.5, 0.1 EGTA, 10 MgCl2, 500 μM ATP for 30 min at 30 °C. Total reaction volume of kinase assay was 30 μl. Adding EDTA/EGTA mix to a final concentration of 5 mM stopped the phosphorylation reaction. GST-pY789 PTPα was then incubated with active WT GST-STEP61 or inactive GST-STEP61 C472S (0–200 nM) in phosphatase assay buffer (in mM): 25 HEPES pH 7.3, 5 EDTA, 10 DTT) for 30 min at 30 °C. The amount of pY789 PTPα remaining was visualized using the phospho-specific antibody to this site.
Primary neuronal cultures and treatments
Primary corticostriatal cultures were derived from rat Sprague-Dawley E18 embryos (Jackson Laboratory, Bar Harbor, Maine) or from mouse STEP KO E18 embryos as described (Xu et al. 2012). Both male and female embryos were used in this study. Cultures were treated with SFK-82958 (10 μM, 30 min), SFK-38393 (10 μM, 30 min), forskolin (100 μM, 10 min) or rolipram (1 μM, 30 min). In some cases, cultures were pre-treated with the PKA inhibitor H-89 (10 μM, 30 min) followed by SKF-82958 or SKF-38393 stimulations. After treatments, neurons were lysed in RIPA buffer (Pierce Biotechnology) with protease and phosphatase inhibitors (Roche). Lysates were spun at 1000 g for 10 min and supernatants were saved for further analyses.
Viral infection
A recombinant adeno-associated virus of mixed serotype 1/2 (AAV1/2) was custom made (GeneDetect LTD, Auckland, New Zealand). Viruses contained either HA-tagged STEP61 or a HA-tagged empty vector, both with a hybrid chicken β-actin/CMV enhancer (CAG) promoter, rAAV2 inverted terminal repeat, a cis-acting woodchuck post-transcriptional regulatory element, and a bovine growth hormone polyadenylation signal sequence. The titers of the viral preparations were >1×1012 genomic particles/ml. At DIV (days in vitro) 5, STEP KO mouse cultures were infected with AAV1/2-STEP61 or AAV1/2-control vector for 10 days.
Lentivirus-based STEP shRNA (LV-STEP; a gift from Thomas Lanz, Pfizer Research & Development, Cambridge, MA) was made as described (Reinhart et al. 2014). The target sequence for STEP shRNA was: 5′-GCATGACTCTTTGGCAACATG-3′ using a loop sequence of 5′-TTCAAGAGA-3′. Control shRNA (5′-AATTCAGCGGGAGCCACCTGA-3′) was designed to target firefly luciferase, which is not homologous to any endogenous rat transcripts and therefore should work as a scrambled sequence. Validation of STEP shRNA and control shRNA was described (Reinhart et al. 2014; Lanz et al. 2013). Rat corticostriatal cultures were infected with LV-STEP or a luciferase control at DIV 7 for 7 days.
Ethanol administration
Male C57BL/6 mice (2–3 months, Jackson Laboratory) were injected intraperitoneally with saline or 20% ethanol (190 proof diluted in saline, 2 g/kg) and sacrificed 15 min post injection. For repeated treatment, mice were administrated with saline or 20% ethanol (2 g/kg, i.p.) once daily for 7 days and sacrificed 16 h after the last injection. In some experiments, STEP KO mice (C57BL/6 background) were administrated with saline or 20% ethanol (190 proof diluted in saline, 2 g/kg, i.p.) for 15 min. The DMS, DLS and NAc were microdissected and kept at −80 °C until use.
Lipid rafts isolation
A detergent-free protocol was used to isolate lipid rafts from mouse brain as described (Persaud-Sawin et al. 2009). Briefly, WT or STEP KO mouse DMS were collected after ethanol administration. Tissues were homogenized in lysis buffer, as described above. Homogenates were centrifuged at 1000 g for 10 min and supernatants were saved. The pellets were resuspended in lysis buffer and passed through a 23-gauge needle, followed by 1000 g spin for 10 min. The second supernatant was pooled with the first one and 250 μl of pooled supernatant was mixed gently with equal amount of 80% sucrose in lysis buffer. Five hundred μl of 30% sucrose was layered on top, followed by a third layer of 5% sucrose. Gradients were centrifuged at 200,000 g in a Beckman Coulter TLA120.1 rotor for 18 h at 4 °C. Twelve sequential fractions (120 μl) were collected and assayed using immunoblotting.
Statistical analysis
All experiments were repeated at least three times. Data were expressed as means ± SEM. Statistical significance was determined by Student’s t-test or one-way ANOVA with post hoc Tukey’s test. For co-immunoprecipitation and rafts isolation experiments in WT and STEP KO mice, a two-way ANOVA with genotype and treatment as main factors followed by post hoc Tukey’s test was used to determine statistical significance, with p values < 0.05 considered significant.
Results
Increased phosphorylation of PTPα at Tyr789 in STEP KO mouse brains
Previous studies have established that loss of STEP leads to elevated basal tyrosine phosphorylation of all STEP substrates identified to date (Venkitaramani et al. 2009; Nguyen et al. 2002; Xu et al. 2012). We reasoned that if PTPα is a novel substrate for STEP, there would be an increase in the tyrosine phosphorylation of PTPα at Y789 in STEP KO mouse brains. There was a significant increase in the phosphorylation of this site (1.47 ± 0.12 of WT, p < 0.05), with no change in total PTPα level (p > 0.05, Fig. 1a). We then examined the phosphorylation of the two regulatory tyrosine sites in Fyn. STEP KO brains showed elevated tyrosine phosphorylation of Fyn at pY420 (1.55 ± 0.13 of WT, p < 0.01), consistent with previous findings that this site is directly regulated by STEP (Nguyen et al. 2002). In contrast, there was a significant decrease in Fyn phosphorylation at the PTPα site (pY531: 0.60 ± 0.10 of WT, p < 0.05), consistent with an increase in PTPα activity (Ponniah et al. 1999; Su et al. 1999).
Figure 1.
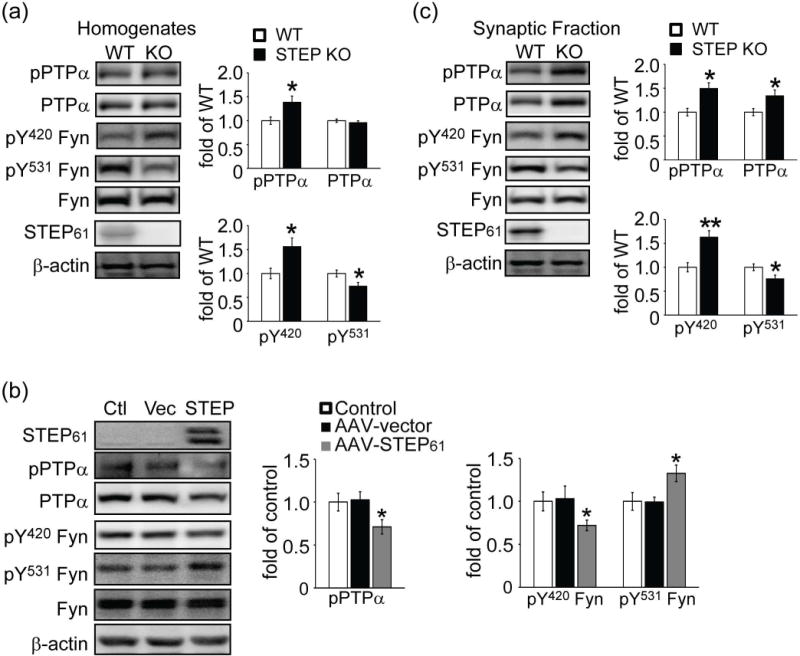
Phosphorylation level of PTPα at Tyr789 is elevated in STEP KO mouse striatum and decreased when STEP61 is restored to STEP KO cultures. Tyrosine phosphorylation levels of PTPα (pY789), Fyn (pY420 and pY531), and total protein levels were determined in total homogenates from wild-type (WT) and STEP KO (KO) mouse striatum (a), corticostriatal neurons from STEP KO mice with restoration of STEP61 expression using AAV1/2-STEP61 (b) or synaptic membrane fractions from wild-type (WT) and STEP KO (KO) mouse striatum (c) Quantification of phosphorylation levels were normalized to total protein levels and then to β-actin as a loading control. All data were expressed as mean ± SEM (*p < 0.05, **p < 0.01, Student’s t-test for (a) and (c), one-way ANOVA with Tukey’s test for (b); n = 6).
These results indicate that PTPα Y789 phosphorylation is increased in STEP KO samples. We next determined whether re-expressing STEP61 into STEP KO cultures was sufficient to reverse the increase in PTPα phosphorylation. Pilot studies using adeno-associated virus1/2 (AAV1/2)-STEP61 confirmed a robust STEP61 expression when corticostriatal cultures were infected with AAV1/2-STEP61 but not with vector alone (Fig. S1). The doublet present is due to differential phosphorylation of STEP61 (Paul et al. 2000). Restoration of STEP61 into STEP KO cultures led to significant decreases in the Tyr phosphorylation of PTPα and a concomitant increase in the phosphorylation of the PTPα site on Fyn (PTPα pY789: 0.71 ± 0.08 of control; Fyn pY531: 1.33 ± 0.10 of control, p values < 0.05, Fig. 1b). In addition, re-expression of STEP61 into these cultures resulted in a decrease in the Tyr phosphorylation of Fyn at the STEP61 site (Fyn pY420: 0.72 ± 0.06 of control, p < 0.05).
Phospho-Fyn antibodies also recognize other Src family kinases, including Src at equivalent phosphorylation sites. To determine whether the changes in Fyn phosphorylation were specific to Fyn, we immunoprecipitated Fyn and Src from STEP KO lysates with specific antibodies, followed by probing with phospho-antibodies. We found alterations in Fyn phosphorylation but not Src (Fig. S2). These results are in agreement with previous findings showing Src is not a direct target of STEP (Nguyen et al. 2002).
PTPα translocation to synaptic membrane fractions is required for full activation of Fyn (Gibb et al. 2011). Thus we next investigated the phosphorylation levels of PTPα and Fyn in synaptic fractions of striatum from WT and STEP KO mice. There was a significantly higher basal level of phospho-PTPα in the striatum of STEP KO mice (1.42 ± 0.15 of WT, p < 0.05), as well as an increase in total PTPα (Fig. 1c). The higher levels of pPTPα in synaptic membranes correlated with a decrease in the Tyr phosphorylation of the PTPα site in Fyn, while the Tyr phosphorylation of the STEP site was increased (PTPα site on Fyn (Y531): 0.75 ± 0.03 of WT; STEP site on Fyn (Y420): 1.44 ± 0.15 of WT, p values < 0.05), with no changes in total Fyn levels in P2 fractions. In addition, there were no changes in the Tyr phosphorylation of PTPα and Fyn in homogenates or P2 fractions from cerebellum (Fig. S3), a brain region in which STEP is not expressed.
STEP61 binds to and dephosphorylates PTPα at Tyr789
We next examined whether there is physical association between STEP61 and PTPα. We took advantage of a substrate-trapping form of STEP61 that contains a mutation of the catalytic site cysteine to a serine residue that inactivates STEP61. This mutated isoform of STEP binds to its substrates but does not release them, as release requires dephosphorylation of substrates (Xu et al. 2012; Nguyen et al. 2002). The N-terminal unique sequence of STEP61 contributes to the binding of some substrates (Fyn and Pyk2) (Nguyen et al. 2002; Xu et al. 2012), while STEP46 preferentially binds to ERK2 and p38. Here we detected binding of endogenous PTPα to recombinant STEP61 C/S but not to STEP46 C/S. Positive controls included Fyn and ERK2 (Fig. 2a). We confirmed that there was no binding of either STEP isoform to Src (Nguyen et al. 2002). These results indicate that STEP61, and not STEP46, interacts with PTPα in vitro.
Figure 2.
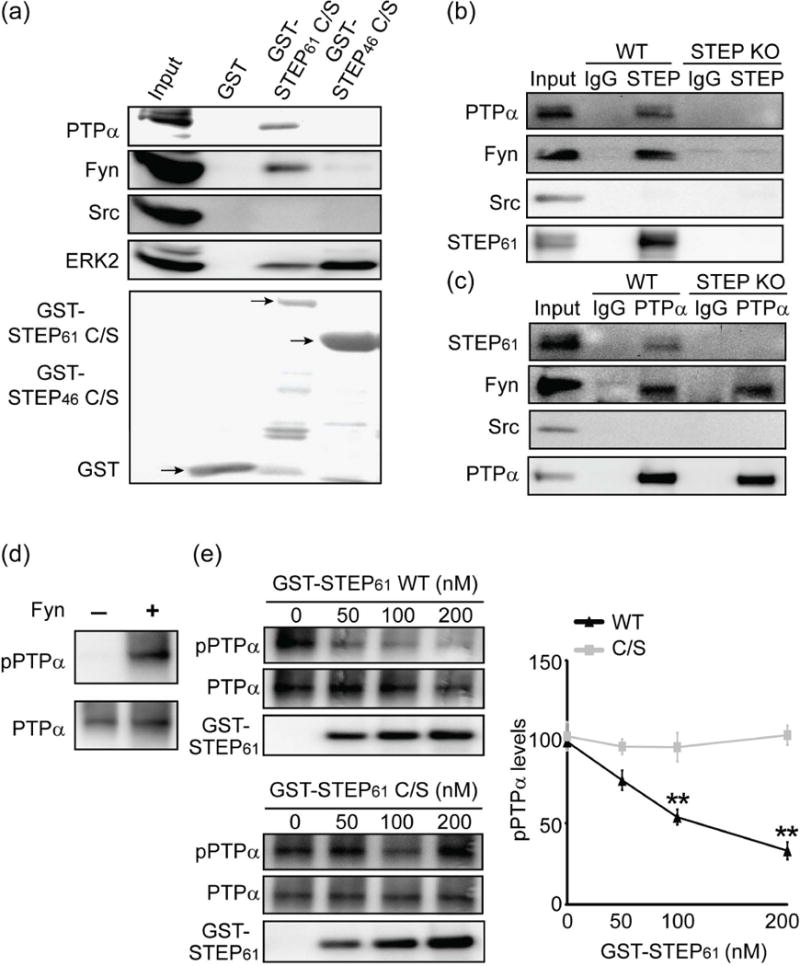
STEP61 binds to and dephosphorylates PTPα at Tyr789. (a) The substrate-trapping (C472S) mutants STEP61 C/S and STEP46 C/S or GST tag alone were adsorbed to glutathione sepharose beads and incubated with mouse striatal homogenates. Bound proteins were visualized with specific antibodies as indicated in the figure. (b, c) STEP61 is associated with PTPα in mouse striatum. WT or STEP KO mouse striatal lysates (300 μg) were incubated with mouse IgG and anti-STEP (23E5) mouse monoclonal antibody (b) or goat IgG and anti-PTPα goat polyclonal antibody (c) overnight at 4 °C. Co-immunoprecipitation of STEP interacting proteins (b) or PTPα interacting proteins (c) was probed with antibodies indicated in the figure. Thirty μg of WT striatal lysates were used as input. Representative blots were shown from three independent experiments (n = 3). (d) Recombinant PTPα was phosphorylated by Fyn in vitro. (e) STEP61 dephosphorylates PTPα at Tyr789. In vitro phosphorylated recombinant PTPα was incubated with active STEP61 (WT) or inactive STEP61 (C/S). The residual phosphorylation of PTPα at Y789 was assessed using a phospho-specific antibody. Data were expressed as mean ± SEM (WT versus C/S at the same dose: **p < 0.01, one-way ANOVA with post hoc Tukey’s test; n = 4).
To investigate possible associations in vivo, we preformed reciprocal immunoprecipitation (IP) of STEP or PTPα from WT and STEP KO mouse striatal lysates. STEP IP co-precipitated PTPα and Fyn from WT lysates but not from STEP KO lysates (Fig. 2b, replicates shown in Fig. S4), while IgG alone and an antibody to Src were used as negative controls. A two-way ANOVA revealed that there was a main effect of treatment (IgG vs anti-STEP: F(1,8) = 44.22, p <0.001) and genotype (WT vs KO: F(1,8) = 40.46, p <0.001) with an interaction between these factors (F(1,8) = 38.77, p <0.001, Fig. S4a). Association was confirmed with the reciprocal IP; STEP61 co-precipitated with PTPα from WT lysates but not from STEP KO lysates (treatment: F(1,8) = 12.00, p <0.01); genotype: F(1,8) = 14.19, p <0.01) and interaction: (F(1,8) = 13.68, p <0.01, Fig. S4b). The absence of STEP did not affect the known interaction between PTPα and Fyn (Bhandari et al. 1998) (Fig. 2c, Fig. S4b). Reciprocal IP was also performed with rat primary corticostriatal cultures to confirm the interaction between STEP61 and PTPα (Fig. S5).
We next examined whether STEP61 could dephosphorylate PTPα. We phosphorylated recombinant PTPα using Fyn and confirmed the phosphorylation using a phospho-specific antibody to Y789 (Fig. 2d). We also assayed PTPα phosphatase activity using para-nitrophenyl phosphate (pNPP) and determined that phosphorylation of PTPα by Fyn did not alter PTPα activity (data not shown). We then incubated phosphorylated PTPα with active WT GST-STEP61 or inactive GST-STEP61 C/S proteins and saw a dose-dependent dephosphorylation of PTPα by active but not inactive STEP61 (Fig. 2e). Together, these results indicate that PTPα is a direct substrate of STEP61.
Regulation of PTPα by STEP in primary cell cultures
If PTPα is a substrate for STEP, we reasoned that the tyrosine phosphorylation level of PTPα would change as we modulated STEP61 activity. It is known that D1 dopamine receptor (D1R) stimulation results in a PKA-mediated phosphorylation of STEP at serine221 (Ser221) within the substrate-binding domain (kinase interacting motif, KIM) (Paul et al. 2000). Phosphorylation at this regulatory serine prevents STEP from interacting with its substrates. We treated corticostriatal cultures with two D1R agonists, SKF-82958 and SKF-38393, and showed the expected increase in STEP61 phosphorylation (SKF-82958: 1.46 ± 0.14 of control; SKF-38393: 1.43 ± 0.15 of control, p values < 0.05) (Fig. 3a). We also found an increase in pY789 PTPα levels when STEP61 was phosphorylated at Ser221 (inactive STEP61) (SKF-82958: 1.53 ± 0.19 of control; SKF-38393: 1.43 ± 0.11 of control, p values < 0.05). Phosphorylation levels of the STEP site in Fyn (Y420) was also increased (SKF-82958: 1.55 ± 0.17 of control; SKF-38393: 1.50 ± 0.06 of control, p values < 0.05). Moreover, there was a decrease in the phosphorylation of the PTPα site on Fyn (pY531) (SKF-82958: 0.72 ± 0.07 of control; SKF-38393: 0.64 ± 0.11 of control, p values < 0.05), presumably due to enhanced dephosphorylation by PTPα. Total protein levels did not alter during drug treatments. As expected, the PKA inhibitor H-89 blocked these effects (Fig. 3a).
Figure 3.
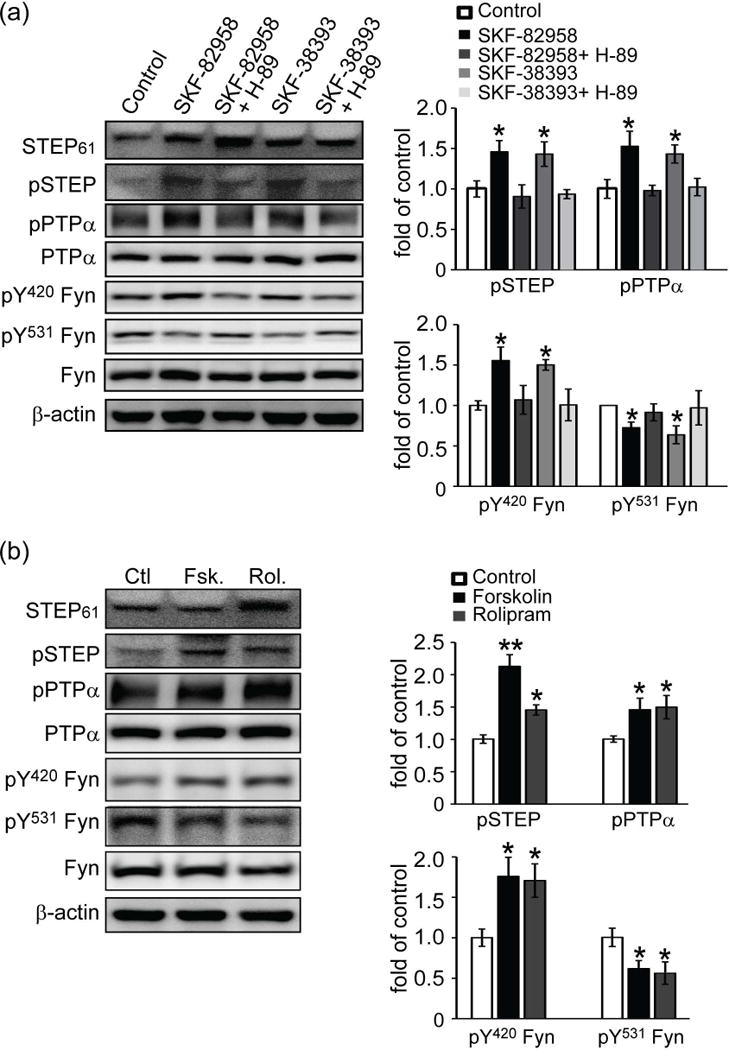
Inactivation of STEP61 results in increased phosphorylation of PTPα. (a) D1 dopamine receptor activation leads to PKA-mediated phosphorylation and inactivation of STEP61. Corticostriatal neurons were treated with SKF-82958 (10 μM) or SKF-38393 (10 μM) for 30 min. Some cultures were preincubated with H-89 (10 μM) for 30 min, followed by D1R agonists stimulations. (b) Inhibition of STEP61 leads to increased phosphorylation of PTPα. Neurons were treated with forskolin (Fsk, 100 μM, 10 min) and rolipram (Rol, 1 μM, 30 min) prior to western blotting. Phospho-protein and total protein levels were assayed with phospho-specific and pan-antibodies as indicated. Phospho-proteins were normalized to total protein levels and then to β-actin as a loading control. All data were compared to controls and expressed as mean ± SEM and statistical significance was determined with one-way ANOVA with Tukey’s test (*p < 0.05, **p < 0.01; n = 4).
To further confirm these results, we activated PKA using forskolin and rolipram in corticostriatal cultures. Both forskolin and rolipram induced robust increases in phosphorylation of STEP61 at Ser221 (Fsk: 2.13 ± 0.18 of control, p < 0.01; Rol: 1.45 ± 0.08 of control, p < 0.05) and subsequent increases in the Tyr phosphorylation of PTPα (Fsk: 1.46 ± 0.18 of control; Rol: 1.50 ± 0.18 of control, p values < 0.05) and Fyn at the STEP site (Y420) (Fsk: 1.76 ± 0.24 of control; Rol: 1.71 ± 0.21 of control, p values < 0.05) (Fig. 3b). In addition, forskolin and rolipram treatments led to a significant decrease in the phosphorylation of the PTPα site on Fyn (Y531), consistent with increased activation of PTPα (Fsk: 0.62 ± 0.09 of control; Rol: 0.59 ± 0.12 of control, p values < 0.05).
Next we used gene-specific knockdown to confirm the regulation of PTPα by STEP61. Lentiviral-STEP shRNA was added to cultures (DIV 5 days) for 7 days as described (Reinhart et al. 2014) and resulted in a significant decrease in STEP61 expression compared to control (0.24 ± 0.03 of control, p < 0.01, Fig. 4). We observed an increase in PTPα phosphorylation and a decrease in the Tyr phosphorylation of the PTPα site on Fyn (PTPα (pY789): 1.37 ± 0.08 of control; Fyn (pY531): 0.77 ± 0.08 of control, p values < 0.05). Moreover, the knockdown of STEP61 expression correlated with an increase in the Tyr phosphorylation of the STEP61 site on Fyn (pY420) (1.36 ± 0.09 of control, p < 0.05). Taken together, these knockdown experiments confirmed the earlier results of acute pharmacological inactivation of STEP.
Figure 4.
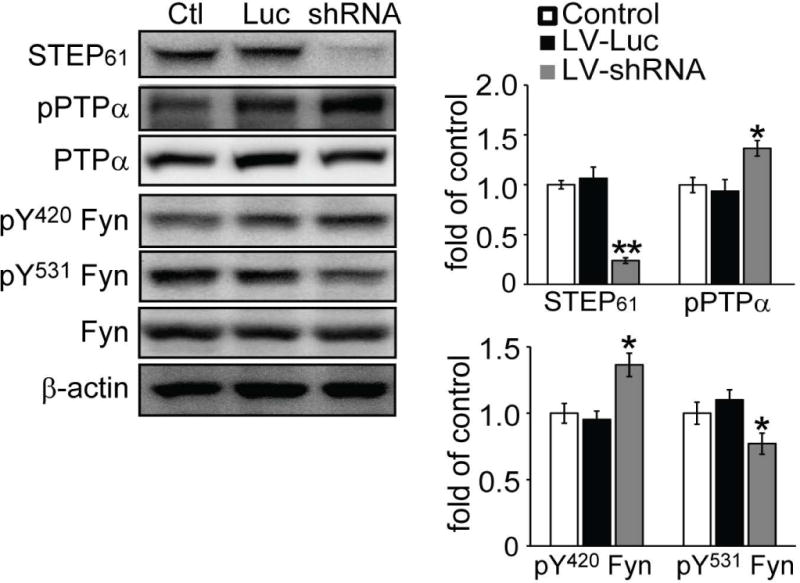
STEP61 knockdown results in increased PTPα phosphorylation at Tyr789 in corticostriatal cultures. Neuronal cultures were treated with lentivirus containing luciferase control (Luc) or STEP shRNA (shRNA) for 7 days. Neurons were lysed in RIPA buffer with protease inhibitors and phosphatase inhibitors and subjected to western blotting. STEP61 and its substrates were probed with phospho-specific or pan-antibodies. Phospho-protein levels were normalized to total protein levels, and then to β-actin as a loading control. All data were compared to controls and expressed as mean ± SEM and statistical significance was determined using one-way ANOVA with Tukey’s test (*p < 0.05, **p < 0.01; n = 6).
Ethanol administration leads to phosphorylation and inactivation of STEP61, and subsequent translocation of PTPα to lipid rafts fraction
We next examined the functional significance of the regulation of PTPα by STEP61. PTPα and Fyn both play a critical role in modulating ethanol (EtOH) intake. EtOH administration in rodents leads to activation of Fyn and an increase in the localization of PTPα within synaptic membranes specifically in the dorsomedial striatum (DMS) (Gibb et al. 2011; Ben Hamida et al. 2013). EtOH treatment also results in the phosphorylation and inactivation of STEP61, as well as activation of Fyn and the phosphorylation of the Fyn target GluN2B, again specifically in the DMS, but not in the adjacent dorsolateral striatum (DLS) or nucleus accumbens (NAc) (Darcq et al. 2014). Given these findings, we asked if STEP61 modulated PTPα phosphorylation and translocation during ethanol administration.
We employed two paradigms for these experiments: acute ethanol injection and repeated ethanol injections in mice followed by a withdrawn period (Gibb et al. 2011; Ben Hamida et al. 2013). C57BL/6 mice were acutely injected with ethanol (2 g/kg, i.p.) and sacrificed 15 min later. Synaptic membrane fractions from DMS, DLS or NAc were processed for levels of STEP61 and its substrates. Consistent with previous findings (Gibb et al. 2011), we observed an increased localization of PTPα in the synaptic membrane fractions in DMS (1.40 ± 0.18 of saline, p < 0.05) but not in DLS or NAc (Fig. 5a). We also confirmed an increase in STEP61 phosphorylation following ethanol injection in DMS (1.58 ± 0.27 of saline, p < 0.05) (Darcq et al. 2014). Both total and phospho-PTPα at Y789 increased in synaptic membrane fractions (Fig. 5a), supporting the observation that phosphorylation at this site is required for PTPα trafficking to membrane fractions (Maksumova et al. 2005; Gibb et al. 2011). Consistent with the enhanced trafficking of PTPα to synaptic fractions, we found a decrease in the phosphorylation of the PTPα site in Fyn (Y531: 0.66 ± 0.10 of saline, p < 0.05), and an increase in the phosphorylation of the STEP61 site in Fyn (Y420: 1.46 ± 0.18 of saline, p < 0.05), possibly due to inactivation of STEP61. We observed none of these changes in the DLS or NAc (Fig. 5b and c). These results suggest a model of synergistic regulation of Fyn by STEP and PTPα in DMS upon acute EtOH administration.
Figure 5.
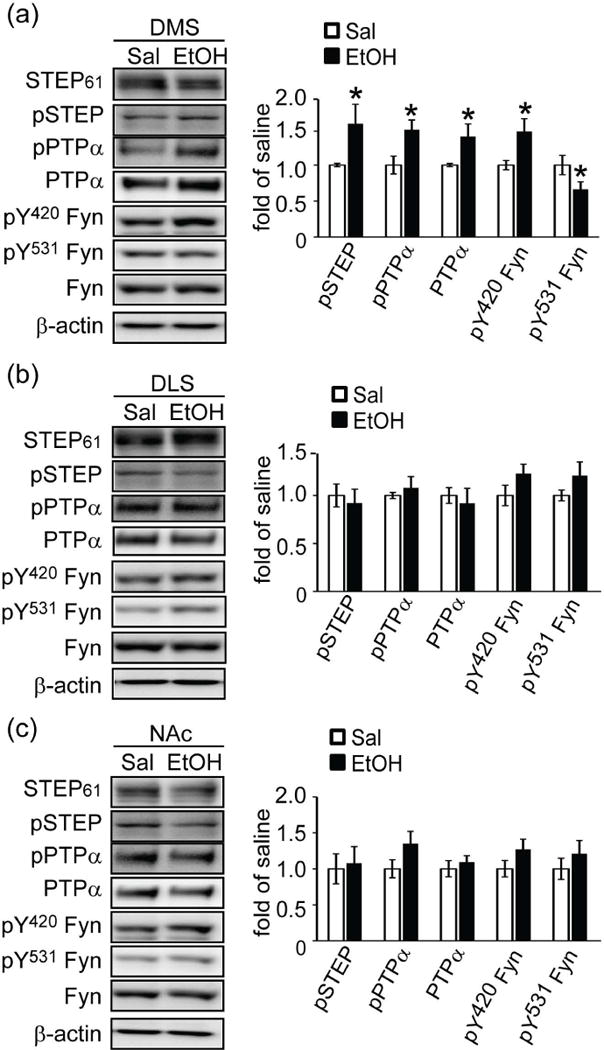
Acute ethanol administration results in phosphorylation and inactivation of STEP61 and increased synaptic localization of PTPα in DMS. C57BL/6 mice (2–3 months) were injected with ethanol (EtOH) acutely (2 g/kg, i.p.) for 15 min. Mice brain regions DMS (a), DLS (b) and NAc (c) were dissected and crude synaptic membrane fractions were isolated and used for western blotting with phospho-specific and pan antibodies. Quantification of phosphorylation levels were normalized to total protein levels and then to β-actin as a loading control. All data were expressed as mean ± SEM (*p < 0.05, Student’s t-test; n = 5).
Previous studies showed that repeated ethanol exposure increased the phosphorylation of Fyn at Y420, but decreased the phosphorylation of Fyn at Y531 (Wang et al. 2010). We followed a similar protocol with repeated ethanol injection (2 g/kg, i.p. daily for 7 days followed by 16 h withdrawn) and examined the effects of ethanol treatment on STEP61, PTPα and Fyn in the DMS, DLS and NAc (Fig. S6). We confirmed the changes in phosphorylation of Fyn, finding increased pY420 Fyn and decreased pY531 Fyn only in the DMS (pY420: 1.46 ± 0.15 of saline; pY531: 0.66 ± 0.08 of saline, p values < 0.05). Moreover, we found increased phosphorylation (inactivation) of STEP61 and a concomitant increased phosphorylation of PTPα at Y789 and translocation of PTPα to synaptic fractions in DMS (pSTEP: 1.57 ± 0.21 of saline; PTPα: 1.39 ± 0.17 of saline; pY789 PTPα: 1.47 ± 0.19 of saline, p values < 0.05). These results suggest that inactivation of STEP61 may be a required for PTPα phosphorylation and translocation to synaptic membrane compartments, resulting in maximal activation of Fyn.
To follow translocation of PTPα into lipid rafts fraction upon EtOH administration, we used a detergent-free protocol (Persaud-Sawin et al. 2009). We first validated our preparation by two criteria commonly used in the field: (1) presence or absence of marker proteins and (2) high cholesterol and low protein content of the rafts fraction (Persaud-Sawin et al. 2009). Flotillin-1, a marker present in lipid rafts, was enriched and correlated with high cholesterol and low protein content in fractions 3 and 4 (Fig. S7). In contrast, the transferrin receptor (TfR), which is excluded from lipid rafts, was recovered mainly in fractions 10–12, and correlated with high protein and low cholesterol content (Fig. S7). Consistent with previous findings (Gibb et al. 2011), we confirmed the expression of Fyn in both lipid rafts and non-rafts fractions (Fig. 6a). In addition, we found that PTPα and STEP61 were primarily in non-rafts fractions at baseline in mouse DMS. We acutely administrated mice with EtOH (2 g/kg, i.p.) or vehicle for 15 min. EtOH administration led to PTPα re-distribution into rafts fractions, without altering the localization of STEP61 or Fyn (Fig. 6a).
Figure 6.
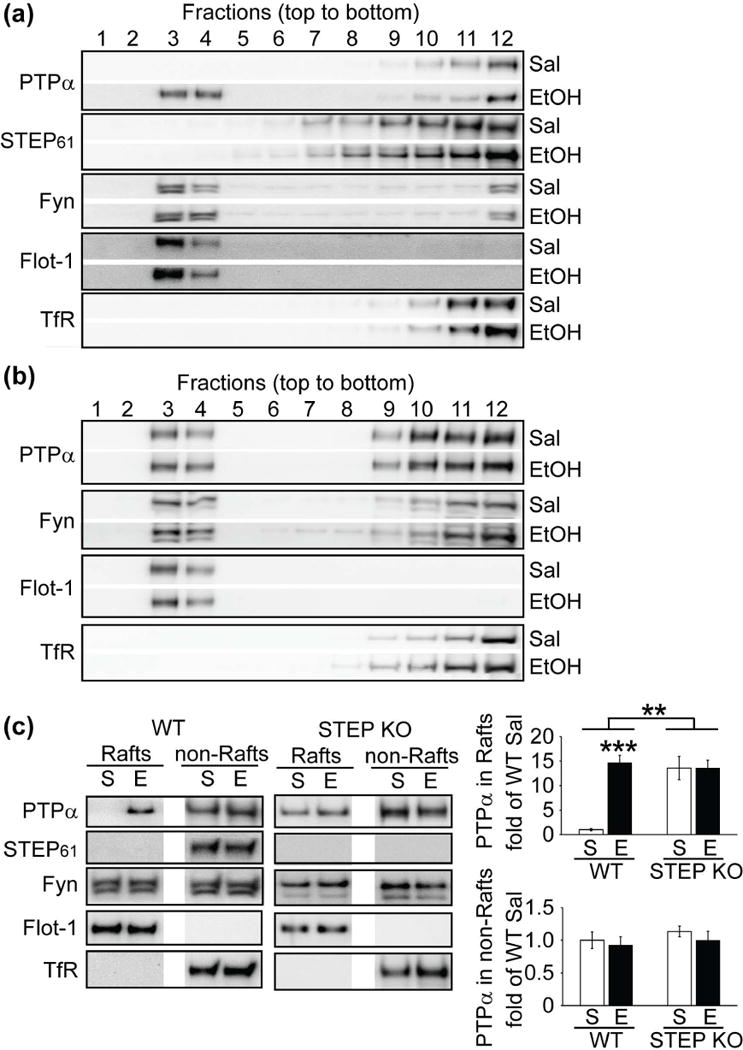
STEP61 regulates translocation of PTPα into lipid rafts fraction in DMS upon acute ethanol administration. (a, b) WT (C57BL/6, 2–3 months) and STEP KO (C57BL/6 background) mice were injected with ethanol (EtOH) acutely (2 g/kg, i.p.) for 15 min. DMS was dissected out and subjected to sucrose gradient fractionation to obtain lipid rafts and non-rafts fraction. Distribution of PTPα, STEP61 and Fyn in each fraction were probed with specific antibodies. Flotillin-1 (Flot-1) and transferrin receptor (TfR) were used as markers. (c) Distribution of PTPα, STEP61 and Fyn in rafts fraction (fractions 3 and 4) and non-rafts fraction (fractions 10–12) were probed with specific antibodies. Representative results were shown from 5 mice each group (n = 5). Data were expressed as mean ± SEM (**p < 0.01, ***p < 0.001, two-way ANOVA with post hoc Tukey’s test; n = 5).
If EtOH-induced phosphorylation and inactivation of STEP61 promoted PTPα translocation to the rafts fractions in WT mice, we reasoned that PTPα might be constitutively localized in the rafts fractions derived from STEP KO mice. We found that PTPα was present in both rafts and non-rafts fractions under baseline conditions (Fig. 6b). A two-way ANOVA analysis of PTPα expression in the rafts showed a main effect of treatment (Sal vs EtOH: F(1,16) = 17.46, p < 0.001) and genotype (WT vs KO: F(1,16) = 12.48, p < 0.01) with an interaction between these factors (F(1,16) = 17.46, p < 0.001, Fig. 6c). However, no changes were observed in PTPα levels in the non-rafts fractions (treatment: F(1,16) = 0.76, p = 0.39; genotype: F(1,16) = 0.74, p = 0.40; interaction: F(1,16) = 0.06, p = 0.80, Fig. 6c). Together, these results suggest STEP61 is involved in PTPα translocation to lipid rafts upon EtOH administration.
Discussion
Here we establish that PTPα is a novel substrate for STEP61 and that STEP61 binds to and dephosphorylates PTPα at Y789. We used STEP KO mice, knockdown of STEP61, and pharmacological interventions to lower STEP activity, and find increased phosphorylation of at Y789 on PTPα, while overexpression of STEP61 results in a decreased phosphorylation of PTPα. The data indicate that inactivation of STEP61 contributes to the increased tyrosine phosphorylation of PTPα and subsequent translocation into lipid raft fractions, leading to the activation of Fyn. We also demonstrated the functional significance of this pathway by showing that it is activated specifically in the DMS during ethanol administration.
PTPα is a receptor-type protein tyrosine phosphatase that is widely expressed in many tissues. Within the CNS, PTPα is implicated in the development of synaptic plasticity and long-term potentiation (LTP) through its ability to activate Fyn and potentiate NMDA receptor signaling. In support of this, PTPα KO mice have decreased Fyn activity and deficits in memory consolidation and LTP (Skelton et al. 2003; Petrone et al. 2003). PTPα is regulated by several mechanisms, including oxidation-induced dimerization and inactivation (Jiang et al. 1999; Yang et al. 2007), phosphorylation (den Hertog et al. 1994; Zheng et al. 2000; Zheng et al. 2002), and translocation between cytoplasm and lipid rafts (Maksumova et al. 2005; Gibb et al. 2011). We find an elevation of PTPα pY789 in dorsal striatum but not in cerebellum of STEP KO mice, consistent with the absence of STEP protein in the cerebellum. It would be interesting to determine whether PTP-SL (Pulido et al. 1998), a closely related PTP to STEP that is present in cerebellum, similarly regulates PTPα in that brain region.
Early reports suggested that pY789 provides a binding site for the SH2 domain of Src/Fyn, thus facilitating the activation of Src/Fyn by removing its intramolecular inhibition (Zheng et al. 2000; Bhandari et al. 1998); however, there are conflicting results, as site-directed mutation did not affect downstream Src signaling, nor was the Y789 site involved in mediating the interaction of PTPα with Src/Fyn (Chen et al. 2006; Lammers et al. 2000; Vacaru and den Hertog 2010). Other studies suggest that the phosphorylation at Tyr789 is needed for Grb2 binding and subsequent activation of MAPK/ERK signaling (den Hertog et al. 1994; den Hertog and Hunter 1996; Su et al. 1996). The direct modulation of intrinsic phosphatase activity by phosphorylation at Y789 is also under debate. Some report that dephosphorylation of this site affects PTPα activity (Maksumova et al. 2007), while others suggest that it does not (den Hertog et al. 1994; Su et al. 1996; Zheng et al. 2000). Our in vitro assay indicate that phosphorylation of PTPα at Y789 does not alter its phosphatase activity using pNPP as substrate.
The possible regulation of PTPα translocation by pY789 is relevant to this study. Previous findings suggested that upon integrin activation, PTPα translocates to focal adhesions in an Y789 dependent-manner (Lammers et al. 2000; Sun et al. 2012), and translocation is critical for full activation of Fyn (Maksumova et al. 2005; Vacaresse et al. 2008). Ethanol administration was shown to induce translocation of PTPα to synaptic membrane fractions (Gibb et al. 2011). Consistent with these reports, we find elevated PTPα levels in synaptic membranes of STEP KO striatum, with a proportional increase in pY789. Acute or repeated ethanol administration also increased the phosphorylation and inactivation of STEP61, increased the phosphorylation of the STEP target PTPα, and increased trafficking of PTPα to synaptic membranes. Moreover, using a lipid rafts extraction protocol, we show that acute EtOH resulted in the re-distribution of PTPα to rafts fractions, where Fyn is enriched, in WT mice. In support of our hypothesis, we find increased localization of PTPα in rafts fractions under baseline conditions in STEP KO mice and EtOH administration did not induce a further translocation of PTPα in STEP KO mice.
These data suggest that decreased STEP61 activity leads to increased phosphorylation of PTPα at Y789 and subsequent translocation of PTPα to lipid rafts, facilitating its interaction with Fyn and NMDA receptors, both critical players in regulating EtOH-related behaviors (Wang et al. 2007; Ben Hamida et al. 2013). Interestingly, a recent report shows that STEP KO mice have more EtOH consumption (Legastelois et al. in press), possibly due to enhanced PTPα, Fyn and NMDAR signaling in rafts fractions in STEP KO mice. The molecular mechanisms that underlie PTPα translocation are not well understood. Several SH2-domain containing adaptor proteins are present at focal adhesions (Boivin et al. 2013; Shen and Guan 2004) or associated with raft targeting proteins (Liu et al. 2002; Kimura et al. 2001; Limpert et al. 2007), thus it is possible that phosphorylated PTPα is recruited by these adaptor proteins to neuronal lipid rafts via pY789-SH2 domain interaction during ethanol administration.
One unanswered question is what determines the brain region specificity of ethanol-induced phosphorylation and inactivation of STEP61 in the DMS, but not in DLS or NAc. STEP61 is phosphorylated by PKA at a regulatory serine residue within its KIM domain upon dopamine D1 receptor (D1R) activation (Paul et al. 2000), while PP2B/PP1 dephosphorylates and activates STEP61 (Snyder et al. 2005; Valjent et al. 2005). Activation of D1R and PKA by ethanol is well documented; however, activation of PKA by ethanol is not restricted to the DMS (Di Chiara and Imperato 1988; Asyyed et al. 2006; Ron and Messing 2013). Studies are needed to clarify the mechanism for the differential regulation of STEP in distinct brain regions, such as DMS, after EtOH administration. Although recent studies have suggested that subregions of the striatum are involved in the regulation of distinct aspects of alcohol abuse (Chen et al. 2011), the mechanisms by which this occurs remain unclear. The differential regulation of STEP within specific brain regions has been previously reported. For example, STEP levels are elevated in striatum in human sporadic Parkinson’s disease and in MPTP-treated mouse models, but not in cortex (Kurup et al. 2015), while STEP levels are elevated in cortex of patients with Alzheimer’s disease and schizophrenia (Zhang et al. 2010; Carty et al. 2012).
A-kinase anchoring proteins (AKAPs) represent a family of adapter proteins that bind to the regulatory subunits of PKA and provide temporal-spatial control by localizing PKA in proximity to substrates and optimal pools of cAMP (Wong and Scott 2004). AKAP proteins show distinct tissue and subcellular distribution, such as the enrichment of AKAP79/150 in neuronal postsynaptic densities. In addition to binding to PKA, it also binds to PP2B and PKC (Dell’Acqua et al. 2002; Klauck et al. 1996), both of which regulate STEP (Snyder et al., 2005 and unpublished data). Meanwhile, distinct combination of regulatory and catalytic subunits of PKA may also contribute to the region-specific regulation of STEP proteins (Gamm et al. 1996). It has been shown that different regulatory subunits of PKA display different sensitivity to cAMP levels. Moreover, RIIβ KO but not RIβ KO mice show increased EtOH consumption (Thiele et al. 2000). Thus it would be important to determine whether AKAP79/150 provides a platform to facilitate the convergent regulation of STEP by these signaling pathways and distinct combination of PKA holoenzyme in a region or compartmental-specific manner.
The synergistic regulation of Fyn by STEP and PTPα is of interest. STEP dephosphorylates and inactivates Fyn directly (Nguyen et al. 2002) and we demonstrate a parallel pathway by which STEP dephosphorylates and suppresses PTPα function also leading to Fyn inactivation (Engen et al. 2008; Ingley 2008). On the other hand, Fyn may provide a positive feedback by phosphorylating PTPα at Y789 and enhancing its signaling, which is inhibited by STEP61 at baseline and disinhibited upon ethanol exposure. Ethanol administration leads to activation of PKA (Ron and Messing 2013; Ortiz et al. 1995), and PKA phosphorylation of STEP isoforms results in the inability of STEP to interact with its substrates (Paul et al. 2000; Paul et al. 2003). At the same time, PKA phosphorylation of DARPP-32 results in inhibition of PP1, which is the phosphatase that dephosphorylates the PKA site in STEP isoforms (Paul et al. 2000). The inactivation of STEP61 results in an increase in the Tyr phosphorylation of PTPα (Y789) and a decrease in the Tyr phosphorylation of Fyn at its inhibitory site (Y531) by PTPα. In this example, the initial activation of PKA results in inactivation of STEP61 and a subsequent translocation of PTPα to synaptic membranes and the full activation of Fyn.
In addition to ethanol-related disorders, the cross-talk between STEP and PTPα has been implicated in several neuropsychiatric disorders including schizophrenia (SZ). Previous findings indicate an elevation of STEP61 level and activity in SZ postmortem brains and animal models of SZ (Carty et al. 2012). Interestingly, hypofunction of Fyn and PTPα are associated with neurobehavioral endophenotypes of SZ (Bjarnadottir et al. 2007; Takahashi et al. 2011), and the present results suggest a possible mechanism for these findings.
In summary, we show that PTPα is a novel substrate for STEP. The phosphorylation and inactivation of STEP61 upon ethanol administration facilitates the translocation of PTPα to lipid rafts and subsequent activation of Fyn-NMDA receptor signaling (Fig. 7). Further studies are needed to investigate possible signaling pathways that underlie the specific phosphorylation and inactivation of STEP61 in the DMS.
Figure 7.
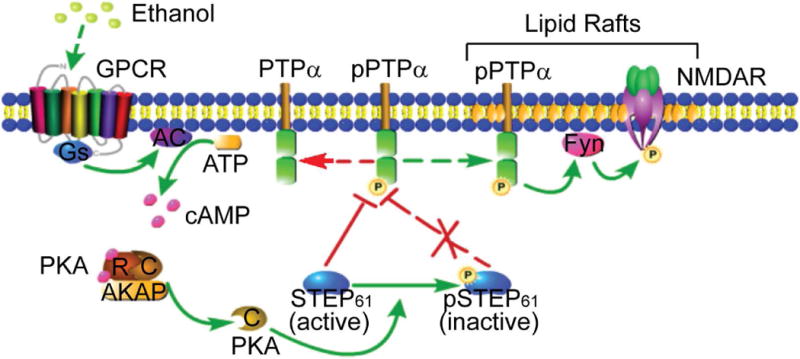
Schematic model of regulation of PTPα by STEP61 during EtOH treatment in DMS. At basal conditions, active STEP61 gates phosphorylation of PTPα and its translocation to the lipid rafts fractions where Fyn and NMDAR reside. Upon EtOH exposure, GPCR (such as D1R and A2AR) is activated, followed by activation of adenylate cyclase (AC) and production of cyclic AMP (cAMP). Binding of cAMP to the regulatory subunits (R) of PKA releases the active catalytic subunits (C) and subsequent phosphorylation of STEP61 at a regulatory site (Ser221), which is known to disrupt the interactions between STEP61 and several of its substrates. Phosphorylation of STEP61 (inactive) blocks its action on dephosphorylation of PTPα at Y789. Phosphorylated PTPα translocates to lipid rafts (maybe through binding to some adaptor proteins, with the mechanisms unclear), activates Fyn by dephosphorylating its inhibitory site (Y531) and enhances NMDAR signaling, which is implicated in EtOH-related behaviors. The mechanisms underlying the region specificity (i.e. DMS versus DLS and NAc) remain unclear. We propose that distinct PKA regulatory and catalytic subunits and region-specific or compartment-specific distribution of AKAPs may be involved. GPCR, G-protein coupled receptor; D1R, dopamine D1 receptor; A2AR, adenosine A2A receptor; NMDAR, N-methyl-D-aspartate receptor; R, regulatory subunit; C, catalytic subunit; AKAP, A-kinase anchor protein; DMS, dorsomedial striatum; DLS, dorsolateral striatum; NAc, nucleus accumbens. This figure was created with the aid of the Pathway Builder Tool 2.0 (www.proteinlounge.com).
Supplementary Material
Acknowledgments
This work was supported by NIH grants P50 AA012870, MH052711 and MH091037 (PJL). The authors thank Drs. Thomas Lanz and Thao Nguyen (Pfizer Research & Development, Cambridge, MA) for providing the lentiviral-STEP shRNA reagents.
Abbreviations
- AAV1/2
adeno-associated virus of mixed serotype ½
- AKAP
A-kinase anchoring protein
- AMPAR
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- CHL1
close homolog of L1
- DIV
days in vitro
- DLS
dorsolateral striatum
- DMS
dorsomedial striatum
- ERK
extracellular-signal regulated kinase
- GST
glutathione S-transferase
- IP
immunoprecipitation
- KIM
kinase interacting motif
- KO
knock out
- MAPK
mitogen-activated protein kinase
- NAc
nucleus accumbens
- NCAM
neural cell adhesion molecule
- NMDAR
N-methyl-D-aspartate receptor
- PAGE
polyacrylamide electrophoresis
- pNPP
para-nitrophenyl phosphate
- PTPα
receptor-type Protein Tyrosine Phosphatase alpha
- pyk2
proline-rich tyrosine kinase 2
- SDS
sodium dodecyl sulfate
- SH2 domain
Src homology 2 domain
- shRNA
short hairpin RNA
- STEP
STriatal-Enriched protein tyrosine Phosphatase
- WT
wild type
Footnotes
The authors declare no conflicts of interest.
References
- Asyyed A, Storm D, Diamond I. Ethanol activates cAMP response element-mediated gene expression in select regions of the mouse brain. Brain Res. 2006;1106:63–71. doi: 10.1016/j.brainres.2006.05.107. [DOI] [PubMed] [Google Scholar]
- Ben Hamida S, Darcq E, Wang J, Wu S, Phamluong K, Kharazia V, Ron D. Protein tyrosine phosphatase alpha in the dorsomedial striatum promotes excessive ethanol-drinking behaviors. J Neurosci. 2013;33:14369–14378. doi: 10.1523/JNEUROSCI.1954-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari V, Lim KL, Pallen CJ. Physical and functional interactions between receptor-like protein-tyrosine phosphatase alpha and p59fyn. J Biol Chem. 1998;273:8691–8698. doi: 10.1074/jbc.273.15.8691. [DOI] [PubMed] [Google Scholar]
- Bjarnadottir M, Misner DL, Haverfield-Gross S, Bruun S, Helgason VG, Stefansson H, Sigmundsson A, Firth DR, Nielsen B, Stefansdottir R, Novak TJ, Stefansson K, Gurney ME, Andresson T. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1+/− knock-outs compared with wild-type mice. J Neurosci. 2007;27:4519–4529. doi: 10.1523/JNEUROSCI.4314-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodrikov V, Leshchyns’ka I, Sytnyk V, Overvoorde J, den Hertog J, Schachner M. RPTPalpha is essential for NCAM-mediated p59fyn activation and neurite elongation. J Cell Biol. 2005;168:127–139. doi: 10.1083/jcb.200405073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin B, Chaudhary F, Dickinson BC, Haque A, Pero SC, Chang CJ, Tonks NK. Receptor protein-tyrosine phosphatase alpha regulates focal adhesion kinase phosphorylation and ErbB2 oncoprotein-mediated mammary epithelial cell motility. J Biol Chem. 2013;288:36926–36935. doi: 10.1074/jbc.M113.527564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger LM, Lombroso PJ, Raghunathan A, During MJ, Wahle P, Naegele JR. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci. 1995;15:1532–1544. doi: 10.1523/JNEUROSCI.15-02-01532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult A, Zhao F, Dirkx R, Jr, Sharma E, Lukacsi E, Solimena M, Naegele JR, Lombroso PJ. STEP61: a member of a family of brain-enriched PTPs is localized to the endoplasmic reticulum. J Neurosci. 1996;16:7821–7831. doi: 10.1523/JNEUROSCI.16-24-07821.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty NC, Xu J, Kurup P, Brouillette J, Goebel-Goody SM, Austin DR, Yuan P, Chen G, Correa PR, Haroutunian V, Pittenger C, Lombroso PJ. The tyrosine phosphatase STEP: implications in schizophrenia and the molecular mechanism underlying antipsychotic medications. Transl Psychiatry. 2012;2:e137. doi: 10.1038/tp.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Cuzon Carlson VC, Wang J, Beck A, Heinz A, Ron D, Lovinger DM, Buck KJ. Striatal involvement in human alcoholism and alcohol consumption, and withdrawal in animal models. Alcohol Clin Exp Res. 2011;35:1739–1748. doi: 10.1111/j.1530-0277.2011.01520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Chen SC, Pallen CJ. Integrin-induced tyrosine phosphorylation of protein-tyrosine phosphatase-alpha is required for cytoskeletal reorganization and cell migration. J Biol Chem. 2006;281:11972–11980. doi: 10.1074/jbc.M600561200. [DOI] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Guo JD, Li C, Dewitt S, Xu J, Lombroso PJ, Rainnie DG. Striatal-enriched protein tyrosine phosphatase-STEPs toward understanding chronic stress-induced activation of corticotrophin releasing factor neurons in the rat bed nucleus of the stria terminalis. Biol Psychiatry. 2013;74:817–826. doi: 10.1016/j.biopsych.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcq E, Hamida SB, Wu S, Phamluong K, Kharazia V, Xu J, Lombroso P, Ron D. Inhibition of striatal-enriched tyrosine phosphatase 61 in the dorsomedial striatum is sufficient to increased ethanol consumption. J Neurochem. 2014;129:1024–1034. doi: 10.1111/jnc.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Acqua ML, Dodge KL, Tavalin SJ, Scott JD. Mapping the protein phosphatase-2B anchoring site on AKAP79. Binding and inhibition of phosphatase activity are mediated by residues 315–360. J Biol Chem. 2002;277:48796–48802. doi: 10.1074/jbc.M207833200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hertog J, Hunter T. Tight association of GRB2 with receptor protein-tyrosine phosphatase alpha is mediated by the SH2 and C-terminal SH3 domains. EMBO J. 1996;15:3016–3027. [PMC free article] [PubMed] [Google Scholar]
- den Hertog J, Tracy S, Hunter T. Phosphorylation of receptor protein-tyrosine phosphatase alpha on Tyr789, a binding site for the SH3-SH2-SH3 adaptor protein GRB-2 in vivo. EMBO J. 1994;13:3020–3032. doi: 10.1002/j.1460-2075.1994.tb06601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engen JR, Wales TE, Hochrein JM, Meyn MA, 3rd, Banu Ozkan S, Bahar I, Smithgall TE. Structure and dynamic regulation of Src-family kinases. Cell Mol Life Sci. 2008;65:3058–3073. doi: 10.1007/s00018-008-8122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamm DM, Baude EJ, Uhler MD. The major catalytic subunit isoforms of cAMP-dependent protein kinase have distinct biochemical properties in vitro and in vivo. J Biol Chem. 1996;271:15736–15742. doi: 10.1074/jbc.271.26.15736. [DOI] [PubMed] [Google Scholar]
- Gibb SL, Hamida SB, Lanfranco MF, Ron D. Ethanol-induced increase in Fyn kinase activity in the dorsomedial striatum is associated with subcellular redistribution of protein tyrosine phosphatase alpha. J Neurochem. 2011;119:879–889. doi: 10.1111/j.1471-4159.2011.07485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladding CM, Sepers MD, Xu J, Zhang LY, Milnerwood AJ, Lombroso PJ, Raymond LA. Calpain and STriatal-Enriched protein tyrosine phosphatase (STEP) activation contribute to extrasynaptic NMDA receptor localization in a Huntington’s disease mouse model. Hum Mol Genet. 2012;21:3739–3752. doi: 10.1093/hmg/dds154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingley E. Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta. 2008;1784:56–65. doi: 10.1016/j.bbapap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Jiang G, den Hertog J, Su J, Noel J, Sap J, Hunter T. Dimerization inhibits the activity of receptor-like protein-tyrosine phosphatase-alpha. Nature. 1999;401:606–610. doi: 10.1038/44170. [DOI] [PubMed] [Google Scholar]
- Kimura A, Baumann CA, Chiang SH, Saltiel AR. The sorbin homology domain: a motif for the targeting of proteins to lipid rafts. Proc Natl Acad Sci U S A. 2001;98:9098–9103. doi: 10.1073/pnas.151252898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- Kurup PK, Xu J, Videira RA, Ononenyi C, Baltazar G, Lombroso PJ, Nairn AC. STEP61 is a substrate of the E3 ligase parkin and is upregulated in Parkinson’s disease. Proc Natl Acad Sci U S A. 2015;12:1202–1207. doi: 10.1073/pnas.1417423112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers R, Lerch MM, Ullrich A. The carboxyl-terminal tyrosine residue of protein-tyrosine phosphatase alpha mediates association with focal adhesion plaques. J Biol Chem. 2000;275:3391–3396. doi: 10.1074/jbc.275.5.3391. [DOI] [PubMed] [Google Scholar]
- Lanz TA, Guilmette E, Gosink MM, Fischer JE, Fitzgerald LW, Stephenson DT, Pletcher MT. Transcriptomic analysis of genetically defined autism candidate genes reveals common mechanisms of action. Mol Autism. 2013;4:45. doi: 10.1186/2040-2392-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpert AS, Karlo JC, Landreth GE. Nerve growth factor stimulates the concentration of TrkA within lipid rafts and extracellular signal-regulated kinase activation through c-Cbl-associated protein. Mol Cell Biol. 2007;27:5686–5698. doi: 10.1128/MCB.01109-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Kimura A, Baumann CA, Saltiel AR. APS facilitates c-Cbl tyrosine phosphorylation and GLUT4 translocation in response to insulin in 3T3-L1 adipocytes. Mol Cell Biol. 2002;22:3599–3609. doi: 10.1128/MCB.22.11.3599-3609.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombroso PJ, Murdoch G, Lerner M. Molecular characterization of a protein-tyrosine-phosphatase enriched in striatum. Proc Natl Acad Sci U S A. 1991;88:7242–7246. doi: 10.1073/pnas.88.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombroso PJ, Naegele JR, Sharma E, Lerner M. A protein tyrosine phosphatase expressed within dopaminoceptive neurons of the basal ganglia and related structures. J Neurosci. 1993;13:3064–3074. doi: 10.1523/JNEUROSCI.13-07-03064.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksumova L, Le HT, Muratkhodjaev F, Davidson D, Veillette A, Pallen CJ. Protein tyrosine phosphatase alpha regulates Fyn activity and Cbp/PAG phosphorylation in thymocyte lipid rafts. J Immunol. 2005;175:7947–7956. doi: 10.4049/jimmunol.175.12.7947. [DOI] [PubMed] [Google Scholar]
- Maksumova L, Wang Y, Wong NK, Le HT, Pallen CJ, Johnson P. Differential function of PTPalpha and PTPalpha Y789F in T cells and regulation of PTPalpha phosphorylation at Tyr-789 by CD45. J Biol Chem. 2007;282:20925–20932. doi: 10.1074/jbc.M703157200. [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Liu J, Lombroso PJ. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem. 2002;277:24274–24279. doi: 10.1074/jbc.M111683200. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, Shoemaker W, Duman RS, Nestler EJ. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse. 1995;21:289–298. doi: 10.1002/syn.890210403. [DOI] [PubMed] [Google Scholar]
- Paul S, Lombroso PJ. Receptor and nonreceptor protein tyrosine phosphatases in the nervous system. Cell Mol Life Sci. 2003;60:2465–2482. doi: 10.1007/s00018-003-3123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- Paul S, Snyder GL, Yokakura H, Picciotto MR, Nairn AC, Lombroso PJ. The Dopamine/D1 receptor mediates the phosphorylation and inactivation of the protein tyrosine phosphatase STEP via a PKA-dependent pathway. J Neurosci. 2000;20:5630–5638. doi: 10.1523/JNEUROSCI.20-15-05630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud-Sawin DA, Lightcap S, Harry GJ. Isolation of rafts from mouse brain tissue by a detergent-free method. J Lipid Res. 2009;50:759–767. doi: 10.1194/jlr.D800037-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone A, Battaglia F, Wang C, Dusa A, Su J, Zagzag D, Bianchi R, Casaccia-Bonnefil P, Arancio O, Sap J. Receptor protein tyrosine phosphatase alpha is essential for hippocampal neuronal migration and long-term potentiation. EMBO J. 2003;22:4121–4131. doi: 10.1093/emboj/cdg399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponniah S, Wang DZ, Lim KL, Pallen CJ. Targeted disruption of the tyrosine phosphatase PTPalpha leads to constitutive downregulation of the kinases Src and Fyn. Curr Biol. 1999;9:535–538. doi: 10.1016/s0960-9822(99)80238-3. [DOI] [PubMed] [Google Scholar]
- Pulido R, Zuniga A, Ullrich A. PTP-SL and STEP protein tyrosine phosphatases regulate the activation of the extracellular signal-regulated kinases ERK1 and ERK2 by association through a kinase interaction motif. EMBO J. 1998;17:7337–7350. doi: 10.1093/emboj/17.24.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart VL, Nguyen T, Gerwien R, Jr, Kuhn M, Yates PD, Lanz TA. Downstream effects of striatal-enriched protein tyrosine phosphatase reduction on RNA expression in vivo and in vitro. Neuroscience. 2014;278:62–69. doi: 10.1016/j.neuroscience.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Ron D, Messing RO. Signaling pathways mediating alcohol effects. Curr Top Behav Neurosci. 2013;13:87–126. doi: 10.1007/7854_2011_161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra A, Giralt A, Rué L, Xifró X, Xu J, Ortega Z, Lucas JJ, Lombroso PJ, Alberch J, Pérez-Navarro E. Striatal-enriched protein tyrosine phosphatase expression and activity in Huntington’s disease: a STEP in the resistance to excitotoxicity. J Neurosci. 2011;31:8150–8162. doi: 10.1523/JNEUROSCI.3446-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin M, Dowling JJ, Hockfield S. Seven protein tyrosine phosphatases are differentially expressed in the developing rat brain. J Comp Neurol. 1995;351:617–631. doi: 10.1002/cne.903510410. [DOI] [PubMed] [Google Scholar]
- Sap J, D’Eustachio P, Givol D, Schlessinger J. Cloning and expression of a widely expressed receptor tyrosine phosphatase. Proc Natl Acad Sci U S A. 1990;87:6112–6116. doi: 10.1073/pnas.87.16.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen TL, Guan JL. Grb7 in intracellular signaling and its role in cell regulation. Front Biosci. 2004;9:192–200. doi: 10.2741/1229. [DOI] [PubMed] [Google Scholar]
- Skelton MR, Ponniah S, Wang DZ, Doetschman T, Vorhees CV, Pallen CJ. Protein tyrosine phosphatase alpha (PTP alpha) knockout mice show deficits in Morris water maze learning, decreased locomotor activity, and decreases in anxiety. Brain Res. 2003;984:1–10. doi: 10.1016/s0006-8993(03)02839-7. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Su J, Muranjan M, Sap J. Receptor protein tyrosine phosphatase alpha activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr Biol. 1999;9:505–511. doi: 10.1016/s0960-9822(99)80234-6. [DOI] [PubMed] [Google Scholar]
- Su J, Yang LT, Sap J. Association between receptor protein-tyrosine phosphatase RPTPalpha and the Grb2 adaptor. Dual Src homology (SH) 2/SH3 domain requirement and functional consequences. J Biol Chem. 1996;271:28086–28096. doi: 10.1074/jbc.271.45.28086. [DOI] [PubMed] [Google Scholar]
- Sun G, Cheng SY, Chen M, Lim CJ, Pallen CJ. Protein tyrosine phosphatase alpha phosphotyrosyl-789 binds BCAR3 to position Cas for activation at integrin-mediated focal adhesions. Mol Cell Biol. 2012;32:3776–3789. doi: 10.1128/MCB.00214-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Nielsen KS, Aleksic B, Petersen S, Ikeda M, Kushima I, Vacaresse N, Ujike H, Iwata N, Dubreuil V, Mirza N, Sakurai T, Ozaki N, Buxbaum JD, Sap J. Loss of function studies in mice and genetic association link receptor protein tyrosine phosphatase alpha to schizophrenia. Biol Psychiatry. 2011;70:626–635. doi: 10.1016/j.biopsych.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, McKnight GS. High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. J Neurosci. 2000;20:Rc75. doi: 10.1523/JNEUROSCI.20-10-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- Vacaresse N, Moller B, Danielsen EM, Okada M, Sap J. Activation of c-Src and Fyn kinases by protein-tyrosine phosphatase RPTPalpha is substrate-specific and compatible with lipid raft localization. J Biol Chem. 2008;283:35815–35824. doi: 10.1074/jbc.M807964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacaru AM, den Hertog J. Serine dephosphorylation of receptor protein tyrosine phosphatase alpha in mitosis induces Src binding and activation. Mol Cell Biol. 2010;30:2850–2861. doi: 10.1128/MCB.01202-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Hervé D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaramani DV, Paul S, Zhang Y, Kurup P, Ding L, Tressler L, Allen M, Sacca R, Picciotto MR, Lombroso PJ. Knockout of striatal enriched protein tyrosine phosphatase in mice results in increased ERK1/2 phosphorylation. Synapse. 2009;63:69–81. doi: 10.1002/syn.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci. 2007;27:3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci. 2010;30:10187–10198. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PS, Wang J, Xiao ZC, Pallen CJ. Protein-tyrosine phosphatase alpha acts as an upstream regulator of Fyn signaling to promote oligodendrocyte differentiation and myelination. J Biol Chem. 2009;284:33692–33702. doi: 10.1074/jbc.M109.061770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Pallen CJ. The receptor-like protein tyrosine phosphatase HPTP alpha has two active catalytic domains with distinct substrate specificities. EMBO J. 1991;10:3231–3237. doi: 10.1002/j.1460-2075.1991.tb04886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- Xu J, Kurup P, Bartos JA, Patriarchi T, Hell JW, Lombroso PJ. Striatal-enriched protein-tyrosine phosphatase (STEP) regulates Pyk2 kinase activity. J Biol Chem. 2012;287:20942–20956. doi: 10.1074/jbc.M112.368654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Huang CC, Hsu KS. A critical role for protein tyrosine phosphatase nonreceptor type 5 in determining individual susceptibility to develop stress-related cognitive and morphological changes. J Neurosci. 2012;32:7550–7562. doi: 10.1523/JNEUROSCI.5902-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Groen A, Lemeer S, Jans A, Slijper M, Roe SM, den Hertog J, Barford D. Reversible oxidation of the membrane distal domain of receptor PTPalpha is mediated by a cyclic sulfenamide. Biochemistry. 2007;46:709–719. doi: 10.1021/bi061546m. [DOI] [PubMed] [Google Scholar]
- Ye H, Tan YL, Ponniah S, Takeda Y, Wang SQ, Schachner M, Watanabe K, Pallen CJ, Xiao ZC. Neural recognition molecules CHL1 and NB-3 regulate apical dendrite orientation in the neocortex via PTP alpha. EMBO J. 2008;27:188–200. doi: 10.1038/sj.emboj.7601939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Si X, Yu WP, Le HT, Ng KP, Teng RM, Ryan K, Wang DZ, Ponniah S, Pallen CJ. PTP alpha regulates integrin-stimulated FAK autophosphorylation and cytoskeletal rearrangement in cell spreading and migration. J Cell Biol. 2003;160:137–146. doi: 10.1083/jcb.200206049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kurup P, Xu J, Anderson GM, Greengard P, Nairn AC, Lombroso PJ. Reduced levels of the tyrosine phosphatase STEP block beta amyloid-mediated GluA1/GluA2 receptor internalization. J Neurochem. 2011;119:664–672. doi: 10.1111/j.1471-4159.2011.07450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kurup P, Xu J, Carty N, Fernandez SM, Nygaard HB, Pittenger C, Greengard P, Strittmatter SM, Nairn AC, Lombroso PJ. Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer’s disease mouse model. Proc Natl Acad Sci U S A. 2010;107:19014–19019. doi: 10.1073/pnas.1013543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Venkitaramani DV, Gladding CM, Kurup P, Molnar E, Collingridge GL, Lombroso PJ. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28:10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XM, Resnick RJ, Shalloway D. A phosphotyrosine displacement mechanism for activation of Src by PTPalpha. EMBO J. 2000;19:964–978. doi: 10.1093/emboj/19.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XM, Resnick RJ, Shalloway D. Mitotic activation of protein-tyrosine phosphatase alpha and regulation of its Src-mediated transforming activity by its sites of protein kinase C phosphorylation. J Biol Chem. 2002;277:21922–21929. doi: 10.1074/jbc.M201394200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


