Abstract
In an established rat model of penetrating ballistic-like brain injury (PBBI), arylsulfatase B (ARSB; N-acetylgalactosamine 4-sulfatase) activity was significantly reduced at the ipsilateral site of injury, but unaffected at the contralateral site or in sham controls. In addition, the ARSB substrate chondroitin 4-sulfate (C4S) and total sulfated glycosaminoglycans increased. The mRNA expression of chondroitin 4-sulfotransferase 1 (C4ST1; CHST11) and the sulfotransferase activity rose at the ipsilateral site of injury (PBBI-I), indicating contributions from both increased production and reduced degradation to the accumulation of C4S. In cultured, fetal rat astrocytes, following scratch injury, the ARSB activity declined and the nuclear hypoxia inducible factor (HIF)-1α increased significantly. In contrast, sulfotransferase activity and chondroitin 4-sulfotransferase expression increased following astrocyte exposure to TGF-β1, but not following scratch. These different pathways by which C4S increased in the cell preparations were both evident in the response to injury in the PBBI-I model. Hence, findings support effects of injury due to mechanical disruption inhibiting ARSB and to chemical mediation by TGF-β1 increasing CHST11 expression and sulfotransferase activity. The increase in C4S following TBI is due to contributions from impaired degradation and enhanced synthesis of C4S which combine in the pathogenesis of the glial scar.
Keywords: arylsulfatase B, astrocytes, chondroitin 4-sulfate, CHST11, glycosaminoglycans, neurocan
INTRODUCTION
Recent studies have demonstrated that the enzyme arylsulfatase B (ARSB; Nacetylgalactosamine4-sulfatase), which removes sulfate groups from the non-reducing end of chondroitin 4-sulfate and dermatan sulfate and thereby triggers degradation of the glycosaminoglycan chain, is not just a lysosomal enzyme, but is also present at the cell membrane of human cerebrovascular and epithelial cells and rodent epithelial and endothelial cells [Bhattacharyya et al., 2009a; Bhattacharyya et al., 2009b; Mitsunaga-Nakatsubo et al., 2009; Prabhu et al., 2011; Feferman et al., 2013]. Activity of ARSB is reduced in hypoxic conditions, and molecular oxygen participates in the post-translational modification of ARSB to its active form [Bhattacharyya and Tobacman, 2012; Roeser et al., 2006]. In human bronchial and colonic epithelial cells, silencing ARSB replicated the effects of hypoxia, suggesting that ARSB may mediate intracellular effects of oxygen. In the traumatized brain, oxygenation is impaired [Leung et al., 2013; Murakami et al., 2012] and chondroitin 4-sulfate (C4S) is upregulated [Yi et al., 2012], leading to our consideration that ARSB activity might be reduced in brain injury. The studies in this report were performed to investigate for the first time the effect of brain injury on ARSB, using a well-characterized rat model of penetrating ballistic-like brain injury (PBBI) [Williams et al., 2005] and two in vitro astrocyte injury models, induced by scratch or transforming growth factor (TGF)-β1 [Yu et al., 2012; Yu et al., 1993; Lau and Yu, 2001; DeVellis et al., 2010].
PBBI recapitulates the insult sustained by a penetrating ballistic-like event, and the persistent motor and cognitive deficits associated with PBBI have been well-documented [Shearer et al., 2010]. The temporal modulation of a number of proteins has been previously reported in the PBBI model [Boutté et al., 2012; Yao et al., 2008; Cartagena et al., 2014]. PBBI-induced changes at 24 hours in protein expression have also been reported, with both over- and under-expression of proteins associated with injury and the response to injury, including changes in STAT3, Tau, PKA RII beta, 14-3-3 epsilon, p43/EMAPII, ubiquitin carboxyl-terminal hydrolase isozyme L1, syntaxin-6, hypoxia-inducible factor (HIF)-1α, and aquaporin [Boutté et al., 2012; Yao et al., 2008; Cartagena et al., 2014]. The effect of this model on C4S, sulfated glycosaminoglycans (GAGs), and the chondroitin sulfate proteoglycan neurocan has not previously been evaluated.
In other TBI models and clinical brain injury, increases in chondroitin 4-sulfate (C4S) and chondroitin sulfate proteoglycans (CSPG), including neurocan, have been identified and recognized as major contributors to the scar formation that follows trauma [Yi et al., 2012; Yin et al., 2009; Smith and Strunz, 2005; Siebert et al., 2014; Asher et al., 2001].
In the studies that follow, two well-established models of reactive astrocytes were used to separately analyze mechanical and cytokine-induced effects which were anticipated to be present in combination in the PBBI model. We hypothesized that post-traumatic decline in ARSB would contribute to the accumulation of C4S in TBI, and that an overall increase in C4S would result from both decline in ARSB, leading to inhibition of C4S degradation, and increased CHST11 and sulfotransferase activity, leading to increased synthesis of C4S.
MATERIALS AND METHODS
Rodent model of penetrating ballistic-like brain injury (PBBI)
Male Sprague-Dawley rats (250–300 g; Charles River Labs, Raleigh, VA) were used in all the experiments of traumatic brain injury. All procedures were approved by the Walter Reed Army Institute of Research Animal Care and Use Committee and Material Transfer Agreement was enacted with the University of Illinois at Chicago. All surgical procedures were conducted in compliance with the animal welfare act, the Guide for the Care and Use of Laboratory Animals (National Research Council) and other federal statutes and regulations relating to animals and experiments involving animals. Anesthesia was induced in animals with 5% isoflurane and maintained at 2% isoflurane and was delivered in oxygen. Body temperature was maintained at 37° ± 1°C by means of a homeothermic heating system (Harvard Apparatus, South Natick, MA).
Right frontal ballistic injury was produced by the Dragonfly Variable Pressure Waveform Generator (model no. HPD-1700; Dragonfly Inc., WV) as detailed [Yi et al., 2012]. Injury was induced (10% brain volume) by a high speed, specially designed probe into the hippocampal region of the brain, and rapid inflation of an attached balloon mimicked the temporary cavity induced by a penetrating bullet. For protein assay and RT-PCR experiments, coronal brain tissue slice samples (2-mm thick, from −5-mm bregma to −2 mm bregma) were collected at 24 hours following injury, flash frozen in liquid N2, and stored at −80°C until subsequent testing in the experiments described below. Samples included: ipsilateral peri-traumatic region (PBBI-I), region contralateral to the site of injury in the traumatized brain (PBBI-C), sham-injured ipsilateral site (SHAM-I), and sham-injured contralateral site (SHAM-C). Five brain samples from each condition were tested (total animals=10). For confocal microscopy experiments, PBBI was induced as described above. At 24 hours following injury, animals were transcardially perfused with ice-cold phosphate buffered saline (PBS; pH 7.4). Sham control or injured brains were extracted, immersed in 4% paraformaldehyde for 6 h, and then transferred to 0.1 M phosphate buffer containing 20% sucrose (pH 7.4, 4°C) and stored at −80°C until further processing.
Primary astrocyte cultures and treatments
Fifteen-day time-pregnant Sprague-Dawley rats were purchased from Charles River (Wilmington, MA). Cortical astrocytes were prepared from rat fetuses at gestational day 21 as previously described [Guizzetti, 1996; Zhang, 2014]. Astrocytes were grown in Dulbecco's modified Eagle Medium (DMEM) containing 10% Fetal Bovine Serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin under a humidified atmosphere of 5% CO2-95% air at 37°C. After 9-16 days in culture, astrocytes were plated in 100 mm dishes (2.5 × 106 cells/dish).
After four days in culture, confluent astrocyte monolayers were induced to become reactive by physically scratching the cultures and/or treating them with 10 ng/ml TGF-β1, similarly to what was previously described [Yu et al, 2012; DeVellis, 2010, O’Toole et al, 2007]. The scratch-wound model is a mechanical injury model in which a monolayer of confluent astrocytes is “wounded” by scratching it with the tip of a sterile pipette tip. Using a p100 sterile pipette tip, seven parallel scratches and then seven more scratches at a right angle to the previous seven were made. About 35–40% of the cells were removed. After the injury, the dishes were washed in PBS to remove all the debris and floating cells. Serum-free DMEM medium supplemented with 0.1% Bovine Serum Albumin (BSA) and antibiotics were then added to the culture dishes, which were incubated for 72 h.
Another set of confluent astrocyte dishes was treated with TGF-β1 (10 ng/ml, R&D Systems) in serum-free DMEM medium supplemented with 0.1% BSA and antibiotics for 72 h. A last set of dishes was scratched as described above, washed in PBS, and incubated for 72 h with serum-free DMEM medium supplemented with 0.1% BSA and antibiotics and TGF-β1 (10 ng/ml).
Arylsulfatase B activity assay
ARSB measurements were performed by a fluorimetric assay with the substrate 4- methylumbelliferyl sulfate (4-MUS) [Bhattacharyya et al., 2009b]. Briefly, 20 μl of fetal rat astrocytes or brain tissue homogenate and 80 μl of assay buffer (0.05M Na acetate, pH 5.6) were combined with 100 μl of substrate (5mM 4-MUS in assay buffer) in wells of a microplate. The microplate was incubated for 30 minutes at 37°C. T he reaction was stopped by 150 μl of stop buffer (Glycine-Carbonate buffer, pH 10.7), and fluorescence was measured at 360 nm (excitation) and 465 nm (emission) in a microplate reader (FLUOstar, BMG LABTECH, Inc., Cary, NC). Protein content of the homogenate was measured using BCA™ Protein Assay Kit (Pierce), and enzyme activity was expressed as nmol/mg protein/h.
Sulfated glycosaminoglycans and chondroitin 4-sulfate assay
Total sulfated glycosaminoglycans (GAGs), including chondroitin 4-sulfate (C4S), chondroitin 6-sulfate, dermatan sulfate, keratan sulfate, heparin, and heparan sulfate, in astrocyte or brain tissue lysate, were measured by the sulfated GAG assay (Blyscan, Biocolor Ltd., Newtownabbey, Northern Ireland) [Bhattacharyya et al., 2009b]. The sulfated polysaccharide component of proteoglycans (PG) and protein-free sulfated GAG chains were measured, whereas hyaluronan or degraded disaccharide fragments were not detected. The reaction was performed in the presence of excess unbound 1,9-dimethylmethylene blue dye, and the cationic dye-sulfated GAG at acid pH produced an insoluble complex. The sulfated GAG content was determined by the amount of dye that was recovered from the test sample following exposure to the Blyscan dissociation reagent. Absorbance maximum for measurement of 1,9-dimethylmethylene blue was 656 nm. Concentration was expressed as micrograms/mg protein of cell lysate.
Chondroitin 4-sulfate (C4S) in the samples was determined following immunoprecipitation with C4S antibody (AmsBio, San Diego, CA). Dynabeads (Life Technologies, Carlsbad, CA) were coated with specific C4S antibody, and beads were mixed with samples, incubated, and immunoprecipitated [Bhattacharyya et al., 2009b]. Immunoprecipitated C4S molecules were eluted and subjected to the Blyscan sulfated GAG assay, as above.
Neurocan ELISA
The nervous system proteoglycan neurocan, a chondroitin sulfate proteoglycan (CSPG) with three C4S attachments, has been shown to be increased in neuronal injury [Asher et al., 2000; McKeon et al., 1999]. Neurocan protein levels in the culture medium and cell extracts of fetal rat astrocytes and in the rat brain tissue were measured by a competitive ELISA kit (Cederlane, Burlington, NC), following the recommended procedures. Standards, cell or tissue samples, and neurocan horseradish peroxidase conjugate were added to wells pre-coated with neurocan antibody, incubated for 1 hour at 37°C, and washed three times. Color was developed by adding hydrogen peroxide/tetramethylbenzidine (TMB) substrate, and color development was inversely proportional to the neurocan content in the test samples. The reaction was stopped by 2N sulfuric acid, and the color was read at 450 nm in a plate reader (BMG). The concentration of neurocan in the samples was extrapolated from the standard curve and expressed per mg of total tissue protein, which was measured by protein assay (Pierce). Neurocan protein associated with C4S was determined by ELISA following immunoprecipitation of C4S, as described above. The C4S associated with neurocan was expressed as the ratio of ng C4S / ng neurocan.
Sulfotransferase activity assay
Sulfotransferase activity in the brain tissue and astrocyte samples was measured by a standardized assay that measures all sulfotransferase activity (R&D system, MN) [Bhattacharyya et al., 2014c]. In this assay, sulfotransferases in the samples converted 3–-phosphoadenosine-5–-phosphosulfate (PAPS) to 3–-phosphoadenosine-5–-phosphate (PAP). Subsequently, a coupling 3-phosphatase enzyme released one inorganic phosphate from PAP. The 3-inorganic phosphate was detected by malachite green phosphate detection reagent, and was proportional to the 3’-phosphoadenosine-5’-phosphate generated, thereby indicating the extent of the sulfotransferase reaction which utilized the sulfate group of PAPS.
Measurement of activated HIF-1α
Nuclear extracts of treated and control cell preparations and brain tissue were prepared using a nuclear extract kit (Active Motif, Carlsbad, CA). Activated HIF-1α in the nuclear extract of the control and treated cells was determined by an oligonucleotide-based ELISA (R & D Systems, MN) [Bhattacharyya and Tobacman, 2012]. Activated HIF-1α in the nuclear extract bound to a biotinylated double-stranded oligonucleotide containing a consensus sequence of the HIF-1α binding site, and the HIF-1α-oligonucleotide complexes were captured by an immobilized antibody specific for HIF-1α that was coated onto the wells of a microtiter plate. Captured HIF-1α molecules were subsequently detected by streptavidin-horseradish peroxidase (HRP), and HRP activity was determined by adding hydrogen peroxide-tetramethylbenzidine (TMB) chromogenic substrate. The optical density for the developed color was measured in an ELISA reader (BMG) at 450 nm, after stopping the reaction with 2N sulfuric acid. The intensity of the developed color was proportionate to the quantity of activated HIF-1α in each sample. The sample values were normalized with the total cell protein and expressed as percentage of control.
Quantitative real-time PCR
RNA was isolated from either astrocytes or tissue samples using RNeasy plus Mini Kit Qiagen; Valencia, CA). Total RNA was transcribed into complementary DNA (cDNA) using High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Carlsbad, CA) as described previously [Chen et al., 2013]. ARSB, neurocan, CHST11, transforming growth factor (TGF)-β1 and glial fibrillary-acidic protein (GFAP) mRNA levels in astrocytes and brain samples were quantified by SYBR-Green quantitative real-time polymerase chain reaction (PRT-PCR). The reference gene 60S ribosomal protein L32 (RPL32.2) mRNA was measured in the same samples, and used to normalize the mRNA loading for the genes of interest.
Primers were:
CHST11 (chondroitin 4-sulfotransferase) forward: 5′ - ACT GTG bCGT CCC ATA AAG TC - 3′ and reverse: 5′ - CAA ACC AGA CAT AGC CAG CA – 3’;
NCAN (neurocan) forward: 5′ - CCT TGG AGA GTG ACC AGA GC - 3′ and reverse: 5′ - AGA CTC CTG CTG CCA AGA GA - 3′
TGFB1 (transforming growth factor beta 1) forward: 5′ - CGC CTG AGT GGC TGT CTT TT - 3′ and reverse: 5′ - GCG AAA GCC CTG TAT TCC GT - 3′
GFAP (glial fibrillary-acidic protein) forward: 5′ - CCT TGC GCG GCA CGA ACG AG - 3′ and reverse: 5′ - CCG AGC GAG TGC CTC CTG GT - 3′
ARSB (arylsulfatase B) forward: 5′ - CCT CCT GGA CGA AGC AGT GGG - 3′ and reverse: 5′ - CCC GCC GTT GTC TGT GGA GA - 3′
RPL32.2 (60S ribosomal protein L32) forward: 5′ - AGA TTC AAG GGC CAG ATC CT - 3′ and reverse: 5′ - GTT GCA CAT CAG CAG CAC TT - 3′.
Confocal microscopy
Frozen sections from PBBI-I and PBBI-C tissues were fixed in 4% paraformaldehyde for 90 minutes, washed once in 1×PBS containing 1mM calcium chloride (pH 7.4), and permeabilized with 0.08% saponin. Slides were washed again with PBS, blocked by 5% normal goat serum, and incubated with GFAP or neurocan antibody. After primary antibody incubation, slides were washed and stained with corresponding Alexa Fluor® conjugated secondary antibodies (Life Technologies, Grand Island, NY). Next, the sections were washed thoroughly and coverslipped using DAPI-mounting medium (Vectashield®; Vector Laboratories, Inc., Burlingame, CA) for nuclear staining. Stained tissue sections were observed with a Zeiss LSM 510 laser scanning confocal microscope. Digital images (25x) were taken using the same settings and reproduced in this manuscript without modification.
Statistics
Assays were performed with at least biological triplicates and technical replicates and expressed as mean ± standard deviation (S.D.). Significant differences were determined by InStat software (GraphPad Software, San Diego CA), using one-way ANOVA tests followed by Tukey–Kramer post-test for multiple comparisons. P-values <0.05 are considered statistically significant. In the figures, * represents p<0.05, ** p<0.01, and *** p<0.001.
RESULTS
Decline in arylsulfatase B activity following injury
In the penetrating ballistic-like brain injury (PBBI) model, ARSB activity in the ipsilateral traumatized brain (PBBI-I) declined significantly at 24 hours following injury (p<0.001; one-way ANOVA with Tukey-Kramer post-test), but did not change in the contralateral injured brain or in the sham-injured brain (Fig. 1A). In the scratch model of injury to the fetal rat astrocytes, ARSB activity detected after 72 hours was markedly reduced in the rat brain astrocytes (p<0.001) (Fig. 1B). Exogenous TGF-β1 did not affect the ARSB activity. In contrast to the declines in ARSB activity, ARSB mRNA expression was not significantly reduced in either the PBBI-I ipsilateral brain tissue (Fig. 1C) or in the astrocyte scratch model (Fig. 1D).
Fig. 1. Decline in arylsulfatase B activity following injury.
A. In the PBBI-I tissue, ARSB activity was significantly reduced at 24 h (p<0.001; one-way ANOVA with Tukey-Kramer post-test), in contrast to the contralateral tissue or the sham operated tissues. All statistical analysis was performed with one-way ANOVA and Tukey-Kramer post-test. Graphs show mean values of at least three independent samples, with technical replicates of each measurement, and the standard deviation.
B. In the cultured rat astrocytes, scratch, but not TGF-β1 treatment, produced significant decline in ARSB activity (p<0.001).
C. The mRNA expression of ARSB was not significantly less in the PBBI-I tissue.
D. The mRNA expression of ARSB was not significantly less following scratch than following exposure to TGF-β1.
[Abbreviations in the figures are: ARSB=arylsulfatase B; C4S=chondroitin 4-sulfate; CHST11=carbohydrate (chondroitin-4) sulfotransferase 11=chondroitin 4-sulfotransferase; GAG=glycosaminoglycan; GFAP=glial fibrillary-acidic protein; NCAN=neurocan; N.S.=no significant difference; PBBI-C=penetrating ballistic like brain injury, contralateral; PBBII=penetrating ballistic-like brain injury, ipsilateral; SHAM-C=contralateral sham control; SHAM-1=ipsilateral sham control; TGF=transforming growth factor]
Sulfotransferase activity and CHST11 mRNA expression increased following injury
Sulfotransferase activity was significantly increased in the ipsilateral traumatized rat brains (PBBI-I) (p<0.001) (Fig. 2A) and in the reactive rat astrocytes following TGF-β1 exposure (p<0.001), but not following scratch (Fig. 2B). Consistent with the increase in sulfotransferase activity, the mRNA expression of CHST11 was significantly increased in the ipsilateral, traumatized brain (PBBI-I) at 24 hours (p<0.01) (Fig. 2C) and in the reactive rat astrocytes following exposure to TGF-β1 (p<0.05), but not following scratch (Fig. 2D). The combination of TGF-β and scratch further increased the CHST11 mRNA expression (p<0.01).
Fig. 2. Sulfotransferase activity and CHST11 mRNA expression increased following injury.
A. Sulfotransferase activity was measured in the brain tissue, and was significantly greater in the PBBI-I than in the contralateral tissue or in the sham-operated tissue (p<0.001).
B. In the astrocyte model, sulfotransferase activity increased following TGF-β1 exposure, but not following scratch (p<0.001).
C. CHST11 expression was significantly increased at 24 hours in the PBBI-I tissue, compared with the sham-injured tissue or the PBBI-C (p<0.01, p<0.05, respectively).
D. CHST11 expression was increased in the astrocytes following TGF-β1 (p<0.05), but not scratch. The combination of scratch and TGF-β1 further increased the CHST11 expression from the value following TGF-β1 alone (p<0.01).
Increase in total sulfated glycosaminoglycans and chondroitin 4-sulfate following injury
The content of total sulfated glycosaminoglycans (GAGs) increased significantly in the ipsilateral traumatized rat brains (PBBI-I) (p<0.001) (Fig. 3A). Consistent with the decline in ARSB and the increase in CHST11, the content of C4S increased significantly, from 8.1 ± 0.8 μg/mg protein in the ipsilateral sham control to 12.6 ± 1.2 μg/mg protein in the PBBI-I tissue (p<0.001) (Fig. 3B). In the astrocyte models, the levels of total sulfated GAGs (Fig. 3C) and C4S (Fig. 3D) increased significantly both following scratch (p<0.001) and following exposure to TGF-β1 (p<0.01). The combination of scratch and TGF-β1 produced a larger increase than either stimulus alone.
Fig. 3. Increase in total sulfated glycosaminoglycans and chondroitin 4-sulfate following injury.
A. In the brain injury model, total sulfated glycosaminoglycans (GAGs) were significantly increased at 24 h in the ipsilateral injury (p<0.001), but not in the contralateral brain or in the sham-injury models.
B. In the brain injury model, chondroitin 4-sulfate (C4S) increased at 24 h in the ipsilateral injury to 12.6 ± 1.2 μg/mg protein from a baseline of 8.1 ± 0.8 μg/mg protein in the sham ipsilateral injury (p<0.001).
C. In the cultured rat astrocytes, both scratch (p<0.001) and TGF-β1 (p<0.01) induced increases in total sulfated GAGs. The combination of scratch and TGF-β-1 further increased the level compared to TGF-β1 alone (p<0.01).
D. Corresponding to the increases in total sulfated GAG in the cultured rat astrocytes, both scratch (p<0.001) and TGF-β1 (p<0.01) increased C4S. Following scratch, the C4S increased to 12.2 ± 0.9 μg/mg protein from a baseline of 7.3 ± 0.5 μg/mg protein. TGF-β1 exposure increased the level to 10.7 ± 0.2 μg/mg protein, and the combination produced an increase to 15.1 ± 0.9 μg/mg protein, significantly higher than the levels from either stimulus alone (p<0.01 compared to scratch, and p<0.001 compared to TGF-β1 alone).
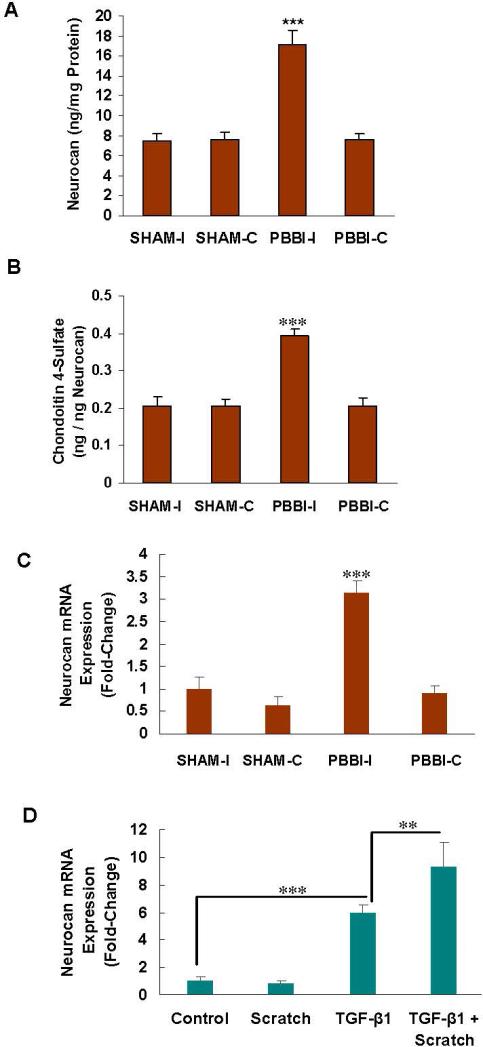
Increase in neurocan and in the C4S that co-immunoprecipitated with neurocan following injury
Following injury, the protein component of the chondroitin sulfate proteoglycan neurocan was significantly increased, as detected by ELISA in the PBBI-I tissue (p<0.001) (Fig. 4A). The amount of C4S that immunoprecipitated with neurocan, expressed as ng C4S per ng neurocan, increased by over 60% from the baseline level following injury. This increase was consistent with the rise in chondroitin 4-sulfation following the decline in ARSB and the increases in CHST11 and sulfotransferase activity (Fig. 4B). These results indicate that the increased C4S following injury is associated with increased neurocan. The mRNA expression of neurocan also increased in the PBBI-I tissue (p<0.001) (Fig. 4C) and in the reactive astrocytes following TGF- β1 (p<0.001), but not scratch (Fig. 4D). The increase in neurocan expression in the astrocytes was greater following the combination of scratch and TGF-β1.
Fig. 4. Increase in neurocan and in C4S immunoprecipitated neurocan following injury.
A. Neurocan, as measured by ELISA, showed significant increase in the ipsilateral traumatized brain tissue (p<0.001).
B. The C4S that immunoprecipitated with neurocan, expressed as ng C4S per ng neurocan, increased following injury, compared to the SHAM controls (p<0.001) and to PBBI-C (p<0.01). This increase is substantial, since the neurocan content was also increased post-trauma. The increase in C4S is attributable to both impaired degradation due to decline in ARSB and increased production due to higher levels of CHST11 expression and sulfotransferase activity.
C. The neurocan mRNA was significantly increased at 24 hours in PBBI-I (p<0.001).
D. In the astrocytes, the neurocan mRNA increased following TGF-β1 (p<0.001), but not scratch. The combination of scratch and TGF-β1 further increased the neurocan expression over TGF-β1 alone (p<0.01).
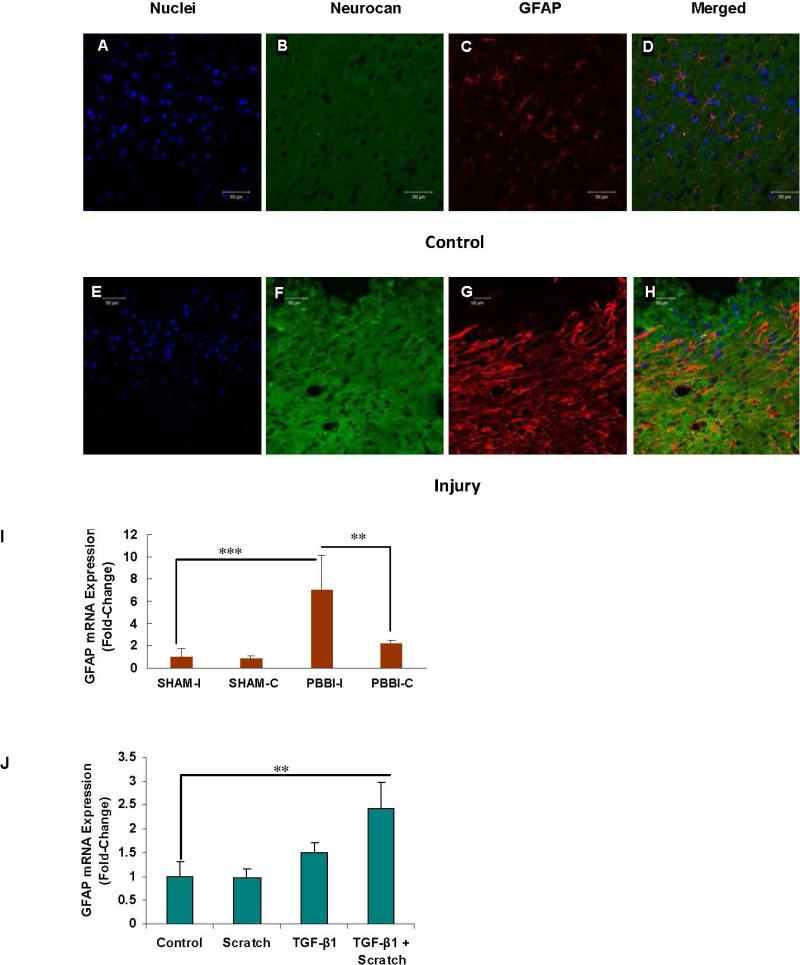
Increases in neurocan and glial fibrillary-acidic protein following TBI
Confocal microscopy demonstrated the increase in neurocan (green) in the ipsilateral injured rat brain (PBBI-I), compared to the contralateral control tissue (PBBI-C) (Fig. 5F vs. Fig. 5B). Glial fibrillary-acidic protein (GFAP; red) was also shown to increase following trauma (Fig. 5G vs. Fig. 5C) and to co-localize with neurocan (Figs. 5D and 5H). QRT-PCR demonstrated increased mRNA expression of GFAP in the ipsilateral peri-traumatic brain tissue at 24 hours (p<0.001) (Fig. 5I). In the neonatal rat astrocytes at 72 h, GFAP mRNA was significantly increased by the combination of scratch and TGF-β1 treatment (p<0.01).
Fig. 5. Increases in Neurocan and Glial Fibrillary-Acidic Protein following TBI.
A-H. Confocal images confirm the increase in neurocan shown by ELISA and mRNA above, comparing the contralateral control tissue (PBBI-C) (A-D) and the PBBI-I tissue (E-H). Both neurocan (green - B, F) and GFAP (red - C, G) increased following injury, as shown by the increases in the PBBI-I images, compared to the contralateral PBBI-C control images. Merged images show colocalization of the neurocan and the GFAP in the injured tissue (H), with little staining in the control (D).
I. mRNA expression of GFAP was markedly increased at 24 hours in the PBBI-I tissue, compared to PBBI-C (p<0.01) and sham controls (p<0.001).
J. In the astrocyte model, mRNA expression of GFAP was significantly increased by the combination of scratch and TGF-β1 exposure (p<0.01).
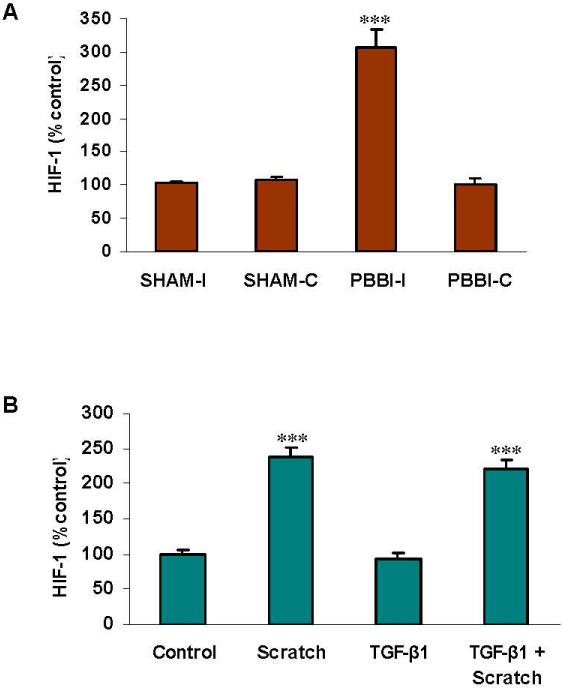
Increased nuclear HIF-1α following injury
Binding of nuclear HIF-1α to a distinct consensus DNA sequence was detected using nuclear extracts of the brain tissues and the cultured astrocytes. The nuclear, activated HIF-1α increased to three times the baseline level in the PBBI-I tissue, in contrast to the contralateral and sham-operated tissues (p<0.001) (Fig. 6A). In the astrocytes, scratch, but not TGF-β1 treatment, enhanced the activation of HIF-1α (p<0.001) (Fig. 6B). Findings are consistent with previously reported increases in rat HIF-1α mRNA at 12 and 24 hours following brain injury [Yao et al., 2008], and the increase in activated HIF-1α that followed silencing of ARSB by siRNA in human bronchial and colonic epithelial cell lines [Bhattacharyya and Tobacman, 2012].
Fig. 6. Increased nuclear HIF-1α following injury.
A. Nuclear HIF-1α increased following injury in the PBBI-I model (p<0.001), compared to the controls.
B. Activated HIF-1α increased following scratch (p<0.001) and the combination of scratch and TGF-β1, but not following TGF-β1 exposure in the cultured rat astrocytes.
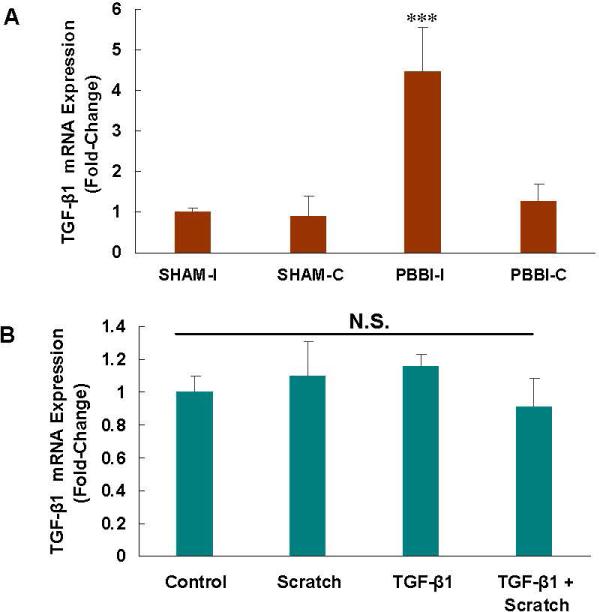
Increased TGF-β1 following injury
In the PBBI-I samples, mRNA expression of TGF-β1 increased significantly at 24 h (p<0.001) (Fig. 7A). However, in the scratch injury model in the astrocytes and following exogenous TGF-β1, the mRNA expression of TGF-β1 did not increase (Fig. 7B).
Fig. 7. Transforming growth factor (TGF)-β1 mRNA increased in PBBI-I, but not following scratch.
A. In the PBBI-I model, mRNA expression of TGF-β1 was increased at 24 h (p<0.001), compared to the controls.
B. In the scratch model of cell injury and following exposure to exogenous TGF-β1, mRNA expression of TGF-β1 did not increase significantly.
DISCUSSION
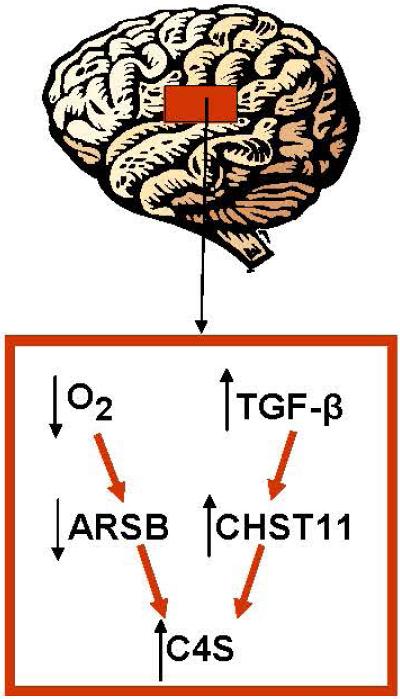
Improvement in the clinical response to traumatic brain injury (TBI) remains a significant challenge, and the long-term and cumulative consequences of TBI can be devastating. Improved understanding of the underlying pathophysiology may lead to better approaches to assessment of injury severity and to effective interventions. In this report, we have presented data which identify two major mechanisms by which injury leads to increased chondroitin 4-sulfate, a major component of the glial scar [Siebert et al., 2014; Asher et al., 2001]. These mechanisms, which were isolated in the reactive astrocyte models, include: 1) direct physical impact leading to decline in ARSB activity inhibiting the degradation of C4S; and 2) TGF-β1-mediated increases in CHST11 expression and sulfotransferase activity. These effects in combination can reduce the degradation and increase the production of C4S, thereby augmenting the accumulation of C4S in the injured brain tissue. The findings in this report are consistent with a two-component reaction to injury in the TBI model, including responses analogous to effects of scratch and to TGF-β1. Scratch is a mechanical injury, comparable to disruption of the blood supply, leading to hypoxia and reduced ARSB activity. TGF-β1 exposure is a cytokine-mediated stimulation, leading to increased CHST11 by transcriptional activation of mRNA expression. Both effects lead to increased C4S, either through inhibition of ARSB or stimulation of CHST11, as summarized in Figure 8.
Fig. 8. Schematic showing two pathways by which chondroitin 4-sulfate increases post-TBI.
C4S increases due to reduced degradation and increased synthesis. Reduced degradation is attributable to hypoxia-induced decline in ARSB activation following injury, with disruption of normal tissue oxygenation and structure, leading to accumulation of more highly sulfated C4S. C4S also accumulates due to increased synthesis from TGF-β1-induced increase in CHST11 expression with increased sulfotransferase activity.
The PBBI model is distinct from the models of hypoxia initiated by arterial occlusion [Yao et al., 2008]. In the PBBI model, brain oxygen tension was stable when the fraction of inspired oxygen was maintained at ~0.26 [Murakami et al., 2012]. However, the brain oxygen tension declined by about 40% in the peri-lesional site immediately following unilateral frontal 10% PBBI, compared to sham controls, and this decline was sustained for at least 150 minutes following trauma [Leung et al, 2013]. Hence, the PBBI produced hypoxia, and hypoxia has been shown to reduce ARSB activity by inhibition of the post-translational modification of ARSB to its active form [Bhattacharyya and Tobacman, 2012]. The measured decline in ARSB activity in the brain tissue following PBBI and in the cultured astrocytes following scratch resemble the effects of hypoxia on ARSB activity. No significant changes in ARSB mRNA expression were observed in PBBI or following scratch. However, decline in ARSB activity is consistent with inhibition of the post-translational modification of ARSB, which requires oxygen and the formylglycine-generating enzyme. The observed increase in activated HIF-1α at the 24 h time point in the PBBI model is attributable to hypoxia from disruption of oxygen delivery following trauma. HIF-1α mRNA expression at 12 and 24 hours in the PBBI model was previously demonstrated to increase [Yao et al., 2008]. In this report, the PBBI studies were performed at 24 hours, consistent with the time interval in the previous studies [Boutté et al., 2012; Yao et al., 2008; Cartagena et al., 2014]. In the cell-based model, the experiments were performed at 72 hours, consistent with previous studies in the cultured astrocyte model [Yu P et al, 2012].
C4S and CSPG represent major components of the glial scar, which prevents the regeneration of axons. Astrocytes respond to a variety of CNS insults, including injury, with morphological and molecular changes. Reactive astrocytes migrate, proliferate, and form the glial scar, a physical and biochemical barrier that limits the extent of injury, but is also responsible for the failure of axonal regeneration. The carbohydrate moiety of CSPG, chondroitin sulfate (CS) glycosaminoglycans (CS-GAGs), and in particular C4S-GAGs, are largely responsible for the axonal inhibitory properties of the glial scar [Silver and Miller, 2004; Laabs et al., 2007]. Since the phenotype of reactive astrocytes is not homogeneous and depends on the type of insult [Zamanian et al., 2012; Sofroniew, 2014; Malhotra et al., 1997], both the scratch wound, mechanical model [Guizzetti et al., 1996] and the transforming growth factor β1 (TGF-β1), chemical astrogliosis model [Yu et al., 2012] were tested for their effects on ARSB, CHST11, and C4S. The scratch model of injury in the astrocyte cell cultures has been regarded as a mechanical insult and distinct from hypoxia. In the scratch studies in this report, nuclear HIF-1α and ARSB were affected, consistent with hypoxic insult, perhaps arising from disruption of cell membranes by the scratch injury. Some reported findings, such as changes in p38 MAPK, indicate overlap between the effects of the two models [Roy Choudhury et al., 2014]. No increase in TGF-β1 expression occurred following scratch or exogenous TGF-β1 in the astrocytes, whereas TGF-β1 mRNA increased in the PBBI injury (Fig. 7). These results indicate that the reactive astrocytes in the cell-based model do not have a significant increase of TGF-β1, and suggest that the source of the increased TGF-β1 in TBI and in PBBI is from infiltrating inflammatory cells or the resident microglia [Weiser-Alves and Milner, 2013; Perry and Gordon, 1988; Streit et al, 1988]. Other activating molecules, which were not present in the in vitro cell model, may be necessary for the upregulation of TGF-β1 in astrocytes. Astrocyte stimulation with TGF-β1 or lipopolysaccharide (LPS) plus interferon (IFN)-γ did not increase TGF-β1 expression; however, when given together, TGF-β1 expression was upregulated [Hamby et al., 2012].
An underlying consideration intrinsic to this report is the extent to which other posttraumatic findings in TBI might be attributable to the decline in the ARSB and the associated increase in chondroitin 4-sulfation. Based on our findings in human epithelial cells [Bhattacharyya and Tobacman, 2012; Bhattacharyya et al., 2014a; Bhattacharyya et al., 2014b], it is likely that transcriptional events will occur when ARSB is reduced, due to decline in binding of galectin-3 to the more highly sulfated C4S and to the role of galectin-3 as a co-transcriptional activator of AP-1 and Sp1. Also, effects on the transcription of CHST11 were identified when ARSB was reduced in human colonic epithelial cells [Bhattacharyya et al., 2014c], attributable to increased binding of bone morphogenetic protein (BMP)-4 to more highly sulfated C4S present when ARSB activity was reduced, leading to decline in the activation of Smad signaling. Effects of TGF-β on CHST11 transcription also proceed through the Smads [Susaria et al., 2011], suggesting that the transcriptional impact of TGF-β on CHST11 may also be regulated by chondroitin 4-sulfation and ARSB. However, like exogenous recombinant BMP-4 in the colonic epithelial cells, TGF-β from inflammatory cells in PBBI or exogenous TGF-β in the astrocyte model may directly interact with cellular receptors and stimulate Smad signaling and CHST11 transcription.
Traditionally, astrogliosis and the formation of the glial scar have been considered an impediment to functional recovery. However, recent studies suggest that astrogliosis represents an adaptive response to a variety of CNS insults and therefore is now viewed as a beneficial process directed at wound repair and tissue protection. Indeed, the prevention of astrogliosis or astrocyte scar formation has been shown to increase inflammation and tissue damage and impair functional recovery in traumatic injury, stroke, infection, inflammation, and neurodegenerative disorders (Sofroniew, 2015). In contrast, under certain circumstances, astrogliosis can engender maladaptive effects leading to harmful effects. The most recognized detrimental effect of astrogliosis and of the glial scar is the inhibition of axon regeneration after brain injury (Silver and Miller 2004). However, because of the beneficial roles of astrogliosis and the glial scar in restricting inflammation and protecting healthy tissue adjacent to lesions, the modulation of glial scar components with the intent of promoting axonal regeneration needs to be carried out with caution and in a targeted manner. Numerous studies investigated the possible use of bacterial chondroitinase ABC (ChABC), which degrades CS-GAGs, to restore axonal outgrowth after CNS injury and in vivo and in vitro studies have shown that this enzyme stimulates axonal regeneration and functional recovery (Zhao and Fawcett, 2013, Crespo et al., 2007, Rhodes and Fawcett, 2004). However, the bacterial origin of ChABC can trigger immune responses and for this reason its use in human therapy has not been successful (Crespo et al, 2007).
The present study identifies ARSB as a novel therapeutic target for the treatment of CNS injury. Prior studies showed that recombinant ARSB restored neurite outgrowth in vitro (Zhang et al, 2014), and recombinant ARSB infusion in the spinal cord after injury decreased CS-GAG levels and increased locomotor function (Yoo et al, 2013). Recombinant human ARSB is clinically effective in treatment of the congenital disorder Mucopolysaccharidosis VI [Harmatz et al, 2004; Harmatz et al, 2008]. Together, these findings suggest that ARSB might be useful as a therapeutic intervention for traumatic brain injury. Future investigations will further assess the interactions between reduced degradation and increased production of C4S on the characteristics of brain injury and the responsiveness to interventions aimed at inhibition of C4S accumulation.
Acknowledgments
This work was supported by the NIH CTSA ULI RR029879 (to JKT), by NIH/NIAAA AA021876 (to MG), and by VHA I01BX001819 (to MG).
Abbreviations
- ARSB
arylsulfatase B = N-acetylgalactosamine 4-sulfatase
- C4S
chondroitin 4-sulfate
- ChABC
chondroitinase ABC
- CS-GAGs
chondroitin sulfate glycosaminoglycans
- CSPG
chondroitin sulfate proteoglycan
- CHST11
carbohydrate (chondroitin-4)sulfotransferase 11
- GAG
glycosaminoglycan
- GFAP
glial fibrillary-acidic protein
- HIF
hypoxia-inducible factor
- NCAN
neurocan
- N.D.
no difference
- PBBI
penetrating ballistic-like brain injury
- PBBI-I
PBBI ipsilateral
- PBBI-c
PBBI contralateral
- SHAM-C
contralateral sham control
- SHAM-1
ipsilateral sham control
- TGF
transforming growth factor
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
REFERENCES
- Asher RA, Morgenstern DA, Moon LD, Fawcett JW. Chondroitin sulfate proteoglycans: Inhibitory components of the glial scar. Prog Brain Res. 2001;132:611–9. doi: 10.1016/S0079-6123(01)32106-4. [DOI] [PubMed] [Google Scholar]
- Asher RA, Morgenstern DA, Fidler PS, Adcock KH, Oohira A, Braistead JE, Levine JM, Margolis RU, Rogers JH, Fawcett JW. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J Neurosci. 2000;20:2427–2438. doi: 10.1523/JNEUROSCI.20-07-02427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Feferman L, Tobacman JK. Arylsulfatase B regulates versican expression by galectin-3 and AP-1 mediated transcriptional effects. Oncogene. 2014a;33(47):5467–76. doi: 10.1038/onc.2013.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Feferman L, Tobacman JK. Increased expression of colonic Wnt9A through Sp1-mediated transcriptional effects involving arylsulfatase B, chondroitin 4-sulfate and galectin-3. J Biol Chem. 2014b;289(25):17564–75. doi: 10.1074/jbc.M114.561589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Feferman L, Tobacman JK. Regulation of chondroitin-4-sulfotransferase (CHST11) by opposing effects of arylsulfatase B on BMP4 and Wnt9A. Biochim Biophys Acta. 2014c Dec. doi: 10.1016/j.bbagrm.2014.12.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Tobacman JK. Hypoxia reduces arylsulfatase B activity and silencing arylsulfatase B replicates and mediates the effects of hypoxia. PLoS One. 2012;7(3):e33250. doi: 10.1371/journal.pone.0033250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Solakyildirim K, Zhang Z, Linhardt RJ, Tobacman JK. Chloroquine reduces Arylsulfatase B activity and increases chondroitin 4-sulfate: Implications for mechanisms of action and resistance. Malaria J. 2009a;8(1):303. doi: 10.1186/1475-2875-8-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Solakyildirim K, Zhang Z, Linhardt RJ, Tobacman JK. Cell-bound IL-8 increases in bronchial epithelial cells following Arylsulfatase B silencing. Am J Respir Cell Mol Biol. 2009b;42(1):51–61. doi: 10.1165/rcmb.2008-0482OC. [DOI] [PubMed] [Google Scholar]
- Boutté AM, Yao C, Kobeissy F, May Lu XC, Zhang Z, Wang KK, Schmid K, Tortella FC. Proteomic analysis and brain-specific systems biology in a rodent model of penetrating ballistic-like brain injury. Electrophoresis. 2012;33(24):3693–704. doi: 10.1002/elps.201200196. [DOI] [PubMed] [Google Scholar]
- Cartagena CM, Phillips KL, Tortella FC, Dave JR, Schmid KE. Temporal alterations in aquaporin and transcription factor HIF1α expression from ballistic-like brain injury. Mol Cell Neurosci. 2014;60:81–7. doi: 10.1016/j.mcn.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang X, Kusumo H, Costa LG, Guizzetti M. Cholesterol efflux is differentially regulated in neurons and astrocytes: Implications for brain cholesterol homeostasis. Biochim Biophys Acta. 2013;1831:263–275. doi: 10.1016/j.bbalip.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo D, Asher RA, Lin R, Rhodes KE, Fawcett JW. How does chondroitinase promote functional recovery in the damaged CNS? Exp Neurol. 2007;206(2):159–71. doi: 10.1016/j.expneurol.2007.05.001. [DOI] [PubMed] [Google Scholar]
- De Vellis J, Ghiani CA, Wanner IB, Cole R. Preparation of normal and reactive astrocytes. Protocols for Neural Cell Culture. 2010:193–215. DOI: 10:1007/978-1-60761-292-6_11. [Google Scholar]
- Feferman L, Bhattacharyya S, Deaton R, Gann P, Guzman G, Kajdacsy-Balla A, Tobacman JK. Arylsulfatase B (N-acetylgalactosamine-4-sulfatase): potential role as a biomarker in prostate cancer. Prostate Cancer Prostatic Dis. 2013;16(3):277–84. doi: 10.1038/pcan.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizzetti M, Costa P, Peters J, Costa LG. Acetylcholine as a mitogen: muscarinic receptor-mediated proliferation of rat astrocytes and human astrocytoma cells. Eur J Pharmacol. 1996;297:265–73. doi: 10.1016/0014-2999(95)00746-6. [DOI] [PubMed] [Google Scholar]
- Harmatz P, Whitley CB, Waber L, Pais R, Steiner R, Plecko B, Kaplan P, Simon J, Butensky E, Hopwood JJ. Enzyme replacement therapy in Mucopolysaccharidosis VI (Maroteaux-Lamy syndrome). J Pediatr. 2004;144(5):574–580. doi: 10.1016/j.jpeds.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Hamby ME, Coppola G, Ao Y, Geschwind DH, Kihakh BS, Sofroniew MV. Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple G-protein- coupled receptors. J Neurosci. 2012;32(42):14489–510. doi: 10.1523/JNEUROSCI.1256-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmatz P, Giugliani R, Schwartz IV, Guffon N, Teles EL, Miranda MC, Wraith JE, Beck M, Arash L, Scarpa M, Ketteridge D, Hopwood JJ, Plecko B, Steiner R, Whitley CB, Kaplan P, Yu ZF, Swiedler SJ, Decker C, MPS VI Study Group Long-term follow-up of endurance and safety outcomes during enzyme replacement therapy for Mucopolysaccharidosis VI: Final results of three clinical studies of recombinant human N-acetylgalactosamine-ASB. Mol Genet Metab. 2008;95(4):469–75. doi: 10.1016/j.ymgme.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Laabs TL, Wang H, Katagiri Y, McCann T, Fawcett JW, Geller HM. Inhibiting glycosaminoglycan chain polymerization decreases the inhibitory activity of astrocyte derived chondroitin sulfate proteoglycans. J Neurosci. 2007;27(52):14494–14501. doi: 10.1523/JNEUROSCI.2807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LT, Yu AC. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor-alpha and interferon-gamma following traumatic and metabolic injury. J Neurotrauma. 2001;18(3):351–9. doi: 10.1089/08977150151071035. [DOI] [PubMed] [Google Scholar]
- Leung LY, Wei G, Shear DA, Tortella FC. The acute effects of hemorrhagic shock on cerebral blood flow, brain tissue oxygen tension, and spreading depolarization following penetrating ballistic-like brain injury. J Neurotrauma. 2013;30(14):1288–1298. doi: 10.1089/neu.2012.2715. [DOI] [PubMed] [Google Scholar]
- Malhotra SK, Luong LT, Bhatnagar R, Shnitka TK. Up-regulation of reactive astrogliosis in the rat glioma 9L cell line by combined mechanical and chemical injuries. Cytobios. 1997;89(357):115–34. [PubMed] [Google Scholar]
- McKeon RJ, Jurynec MJ, Buck CR. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci. 1999;19:10778–10788. doi: 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsunaga-Nakatsubo K, Kusunoki S, Kawakami H, Akasaka K, Akimoto Y. Cell-surface arylsulfatase A and B on sinusoidal endothelial cells, hepatocytes, and Kupffer cells in mammalian livers. Med Mol Morphol. 2009;42(2):63–69. doi: 10.1007/s00795-009-0447-x. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Wei G, Yang X, Lu SC, Leung LY, Shear DA, Tortella FC. Brain oxygen tension monitoring following penetrating ballistic-like brain injury in rats. J Neurosci Methods. 2012;203(1):115–121. doi: 10.1016/j.jneumeth.2011.09.025. [DOI] [PubMed] [Google Scholar]
- O'Toole DA, West AK, Chuah M. Effect of olfactory ensheathing cells on reactive astrocytes in vitro. Cell Mol Life Sci. 2007;64:303–9. doi: 10.1007/s00018-007-7106-y. doi: 10.1007/s00018-007-7106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Gordon S. Macrophages and microglia in the nervous system. Trends Neurosci. 1988;11:273–77. doi: 10.1016/0166-2236(88)90110-5. [DOI] [PubMed] [Google Scholar]
- Prabhu S, Bhattacharyya S, Guzman G, Macias V, Kajdacsy-Balla A, Tobacman JK. Extra-lysosomal localization of arylsulfatase B in human colonic epithelium. J Histochem Cytochem. 2011;59(3):328–335. doi: 10.1369/0022155410395511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KE, Fawcett JW. Chondroitin sulphate proteoglycans: preventing plasticity or protecting the CNS? J Anat. 2004;204(1):33–48. doi: 10.1111/j.1469-7580.2004.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeser D, Preusser-Kunze A, Schmidt B, Gasow K, Wittmann JG, Dierks T, von Figura K, Rudolph MG. A general binding mechanism for all human sulfatases by the formylglycine-generating enzyme. Proc Natl Acad Sci USA. 2006;103(1):81–86. doi: 10.1073/pnas.0507592102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Choudhury G, Ryou MG, Poteet E, Wen Y, He R, Sun F, Yuan F, Jin K, Yang SH. Involvement of p38 MAPK in reactive astrogliosis induced by ischemic stroke. Brain Res. 2014;1551:45–58. doi: 10.1016/j.brainres.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear DA, Lu XC, Bernbard MC, Pedersen R, Chen Z, Davis A, Tortella FC. Longitudinal characterization of motor and cognitive deficits in a model of penetrating ballistic-like brain injury. J Neurotrauma. 2010;27(10):1911–23. doi: 10.1089/neu.2010.1399. [DOI] [PubMed] [Google Scholar]
- Siebert JR, Conta Steencken A, Osterhout DJ. Chondroitin sulfate proteoglycans in the nervous system. Biomed Res Int. 2014;2014:845323. doi: 10.1155/2014/845323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–56. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Smith GM, Strunz C. Growth factor and cytokine regulation of chondroitin sulfate proteoglycans by astrocytes. Glia. 2005;52(3):209–18. doi: 10.1002/glia.20236. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci. 2015;16(5):249–63. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Astrogliosis. Cold Spring Harb Perspect Biol. 2014 doi: 10.1101/cshperspect.a020420. doi:10.1101/cshperspect.a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Graeber MG, Kreutzberg GW. Functional plasticity of microglia: a review. Glia. 1988;1:301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Susaria BT, Laing ED, Yu P, Katagiri Y, Geller HM, Symes AJ. Smad proteins differentially regulate transforming growth factor-β-mediated chondroitin sulfate proteoglycans. J Neurochem. 2011;119(4):868–78. doi: 10.1111/j.1471-4159.2011.07470.x. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser-Alves JV, Milner R. Microglia are the major source of TNF-α and TGF-β1 in postnatal glial cultures; regulation by cytokines, lipopolysaccharide, and vitronectin. Neurochem Int. 2013;63(1):47–53. doi: 10.1016/j.neuint.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AJ, Hartings JA, Lou X-CM, Rolli ML, Dave JR, Tortella FC. Characterization of a new rat model of penetrating ballistic brain injury. J Neurotrauma. 2005;22:313–331. doi: 10.1089/neu.2005.22.313. [DOI] [PubMed] [Google Scholar]
- Yao C, Williams AJ, Ottens AK, May Lu XC, Chen R, Wang KK, Hayes RL, Tortella FC, Dave JR. Detection of protein biomarkers using high-throughput immunoblotting following focal ischemic or penetrating ballistic-like brain injuries in rats. Brain Inj. 2008;22(10):723–32. doi: 10.1080/02699050802304706. [DOI] [PubMed] [Google Scholar]
- Yi JH, Katagiri Y, Susaria B, Figge D, Symes AJ, Geller HM. Alterations in sulfated chondroitin glycosaminoglycans following controlled cortical impact injury in mice. J Comp Neurol. 2012;520(15):3205–313. doi: 10.1002/cne.23156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Sakamoto K, Zhang H, Ito Z, Imagama S, Kishida S, Natori T, Sawada M, Matsuyama Y, Kadomatsu K. Transforming growth factor-beta 1 upregulates keratan sulfate and chondroitin sulfate biosynthesis in microglias after brain injury. Brain Res. 2009;1263:10–22. doi: 10.1016/j.brainres.2009.01.042. [DOI] [PubMed] [Google Scholar]
- Yoo M, Khaled M, Gibbs KM, Kim J, Kowalewski B, Dierks T, Schachner M. Arylsulfatase B improves locomotor function after mouse spinal cord injury. PLoS One. 2013;8:e57415. doi: 10.1371/journal.pone.0057415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AC, Lee YL, Eng LF. Astrogliosis in culture: 1. The model and the effect of antisense oligonucleotides on glial fibrillary acidic protein synthesis. J Neurosci Res. 1993;34(3):295–303. doi: 10.1002/jnr.490340306. [DOI] [PubMed] [Google Scholar]
- Yu P, Wang H, Katagiri Y, Geller HM. An in vitro model of reactive astrogliosis and its effect on neuronal growth. Methods Mol Biol. 2012:327–40. doi: 10.1007/978-1-61779-452-0_21. 10.1007/978-1-61779-452-0_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrocytes. J Neurosci. 2012;32(18):6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DX, Bhattacharyya S, Kusumo H, Goodlett CR, Tobacman JK, Guizzetti M. Arylsulfatase B modulates neurite outgrowth via astrocyte dysregulation by ethanol. Glia. 2014;62(2):259–71. doi: 10.1002/glia.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao RR, Fawcett JW. Combination treatment with chondroitinase ABC in spinal cord injury - breaking the barrier. Neurosci Bull. 2013;29(4):477–83. doi: 10.1007/s12264-013-1359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]