Abstract
Objective
To develop and initially validate a global cognitive performance score for the Pediatric Automated Neuropsychological Assessment Metrics (PedANAM-CPS) to serve as a screening tool of cognition in childhood lupus.
Methods
Patients (n=166) completed the nine subtests of the PedANAM battery, each of which provides three principal performance parameters (accuracy, mean reaction time for correct responses, throughput). Cognitive ability was measured by formal neurocognitive testing or estimated by the Pediatric Perceived Cognitive Function Questionnaire-43 to determine the presence or absence of neurocognitive dysfunction (NCD). A subset of the data was used to develop four candidate PedANAM-CPS indices with supervised or unsupervised statistical approaches: PedANAM-CPSUWA i.e. unweighted averages of the accuracy scores of all PedANAM-subtests; PedANAM-CPSPCA, i.e. accuracy scores of all PedANAM-subtests weighted through principal components analysis; PedANAM-CPSlogit i.e. algorithm derived from logistic models to estimate NCD-status based on the accuracy scores of all of the PedANAM-subtests; and PedANAM-CPSmultiscore i.e. algorithm derived from logistic models to estimate NCD-status based on select PedANAM performance parameters. Using the remaining data PedANAM-CPS candidates were validated.
Results
PedANAM-CPS indices were moderately correlated with each other (|r|>0.65). All of the PedANAM-CPS’s discriminated children by NCD-status across datasets (p<0.036). The PedANAM-CPSmultiscore had the highest area under the receiver operating characteristic curve (AUC) across all datasets for identifying NCD-status (AUC >0.74), followed by the PedANAM-CPSlogit, the PedANAM-CPSPCA and the PedANAM-CPSUWA respectively.
Conclusions
Based on preliminary validation and considering ease of use, the PedANAM-CPSmultiscore and the PedANAM-CPSPCA appear to be best suited as global measures of PedANAM performance.
Keywords: SLE, cognitive dysfunction, children, NPSLE, PedANAM, Screening
Systemic Lupus Erythematosus is a relatively common autoimmune disease which affects an estimated 1 in 10,000 children in the United States (1, 2). Childhood-onset SLE (cSLE) is often associated with major organ involvement, including neuropsychiatric disease (NPSLE) affecting between 43–95% of patients (1, 2). Although it is a risk factor for poor disease outcomes in all age groups (3–8), NPSLE is of special concern in pediatric populations. Children with NPSLE may experience school failure due to attention and learning difficulties (9). Additionally, NPSLE affecting the maturing brain may have significant long-term consequences, as initial evidence demonstrates that NPSLE interferes with developmentally appropriate acquisition of new cognitive skills (10).
Screening has been defined as the systematic application of a test for the early identification of individuals at risk for a specific disorder who will benefit from further investigation (11). Within a general pediatric rheumatology practice context, there is a need for an efficient screening test for monitoring cognition and for early detection of clinically relevant neurocognitive dysfunction (NCD) in cSLE patients (12). Effective screening can support early identification of individuals who require more comprehensive assessment of cognitive ability as well as help guide medical treatment decisions.
Neurocognitive function may be considered a measure of global brain health (13). Traditionally, neurocognitive function is assessed through formal neurocognitive testing (FNCT) using a battery of individually-administered standardized tests that probe various cognitive domains (14). Although FNCT generally is considered the criterion standard for quantifying cognitive ability, it has its shortcomings. FNCT is time-consuming, costly, can be difficult to access, and serial administration of FNCT can result in training effects which complicate its interpretation. Taken together, these issues suggest that FNCT is not the ideal route for surveillance of NPSLE in children.
The Pediatric Automated Neuropsychological Assessment Metrics (PedANAM) is a computerized library of tests designed to measure cognitive ability, mental processing speed, memory, and cognitive efficiency in children age 9 years and older(15). Recent studies suggest that the PedANAM can be used for the screening of NCD in cSLE (16). The PedANAM is time efficient, has minimal practice effects and only requires access to a standard computer without need for other specialized equipment, training, or a psychometric specialist.
One shortcoming of the PedANAM is related to its complexity and its lack of a validated summary or overall performance statistic. It is a data rich instrument that yields multiple scores for each subtest, including scores for response speed, accuracy, variability, and speed/accuracy trade-offs. The large amount of data generated by the PedANAM cannot be easily synthesized in a clinical setting to help determine whether a child’s overall cognitive performance has changed. This differs from the adult version of the ANAM, for which various composite scores have been proposed, including summary statistics derived from regression models or averaged ANAM performance scores (17, 18).
The current study sought to use common statistical methods, such as those proposed for the ANAM, to develop a summary cognitive performance score (PedANAM-CPS). Specifically, the objective of this study was to develop and initially validate the calculation of a single composite index (PedANAM-CPS) to serve as a summary measure of children’s cognitive performance on the PedANAM.
PATIENTS AND METHODS
This study was approved by the institutional review boards at each participating center with written consent and assent obtained as appropriate. Participants were 9 to 17 years old at the time of study enrollment and carried either a diagnosis of cSLE (n=108) (19) or juvenile idiopathic arthritis (JIA) (n=18) (20), or were healthy controls (n=40). JIA patients were used as controls as it was felt to be a population easy to access at a rheumatology clinic and not to have abnormal cognitive functioning related to the primary rheumatologic diagnosis. Healthy controls were cSLE patients’ friends within one year if their age, of the same gender, and in the same school grade. English was the primary language of participants and their caregivers. Routine sociodemographic and clinical data were collected. For participants with cSLE disease activity (SLEDAI) (3), laboratory data, and medications were recorded throughout the study course.
Definition of Datasets
FNCT and PedANAM were completed by 40 cSLE patients and 40 age and sex-matched healthy controls at baseline and 18 months later. Data collected from these 80 participants at baseline served as the development-dataset and were used to derive weightings for all of the PedANAM-CPS indices.Validation-dataset-1 was comprised of data from 61 of the 80 participants mentioned above for which 18-month follow-up data (FNCT, PedANAM) were available.
Validation-dataset-2 was collected from a separate sample, with cross-sectional assessment of cSLE patients (n=68; all different from those described above) and 18 JIA patients followed at seven pediatric rheumatology centers. These participants completed the PedANAM but were not administer FNCT. Instead, cognitive ability was estimated via caretaker proxy-report using the Pediatric Perceived Cognitive Function Questionnaire-43 (PedsPCF-43, see details below).
Measurements
Pediatric Automated Neuropsychological Assessment Metrics
The PedANAM is a battery of computer administered and scored tests of cognitive processing efficiency designed for repeated administrations to individuals ages 9 years and older. PedANAM subtests were adapted from the adult version of ANAM to display age-appropriate stimuli and instructions and to allow sufficient time for responses in a pediatric population(15).The PedANAM subtests included in this study were: Code Substitution Learning, Spatial Processing, Mathematical Processing, Matching to Sample Test, Sternberg Memory Search, Code Substitution Delayed Memory Test, Logical Relations Symbolic Test, Matching Grids Test, and Continuous Performance Test.
Each subtest produces at least three performance parameter scores: accuracy (percent correct responses), mean reaction time (for correct responses), and throughput (correct responses/minute) as a measurement of efficiency. Of all of the PedANAM performance parameters, the accuracy score has shown the highest consistency and reliability (16). Additionally, the coefficient of variation of time required for a correct response (CVc) has been proposed as a measure of consistency on the performance of a given PedANAM-subtest (21).
Measurement of Cognitive Ability
Various approaches for the assessment of cognitive functioning have been proposed in the medical literature and for cSLE (22). At present, there is no consensus about the most appropriate definition of a clinically relevant deficit or impairment in cognitive performance in cSLE (23). Therefore, we assessed cognitive ability in two ways: via FNCT and the PedsPCF-43. Given the limited sample size, we restricted analysis to a single definition of NCD for the FNCT and the PedsPCF-43, respectively.
Formal Neuropsychological Testing
FNCT was administered to the development group and validation group-1 by a trained psychometrician, using a recommended standardized neuropsychological battery for cSLE (14). The tests can be clustered into four cognitive domains as previously reported: Working Memory, Psychomotor Speed, Visuoconstructional Ability, and Attention/Executive Functioning. Using published norms, participants’ performance on each of the neuropsychological tests was expressed as a Z-score with a mean of 0 and standard deviation of 1. Following the definition used in previous studies (16, 21, 23), NCD was operationally defined as having at least one domain Z-score below −2 standard deviation or at least two domain Z-scores below −1 standard deviation on FNCT.
Pediatric perceived cognitive function questionnaire-43
The PedPCF-43 measures the caregiver’s perceptions of a child’s cognitive functioning as observed in everyday life (24). This questionnaire was completed by the caregivers of validation group-2. PedsPCF-43 items were determined via Item Response Theory (IRT) analyses with input from parents, teachers, and clinicians experienced at working with pediatric cancer survivors. The focus of the PedsPCF-43 is on fluid cognitive abilities sensitive to changes in mental status secondary to neurologic and systemic medical events. It measures aspects of cognitive functioning such as attention, memory retrieval, and working memory. PedsPCF-43 correlates with computerized neuropsychological testing and neuroimaging findings (25–27). Its reliability, validity, and clinical utility have been investigated in the U.S. general population and pediatric cancer survivors (28). However, the PedsPCF-43 has not been validated for use in cSLE. Ratings on the PedsPCF-43 are expressed in gender and age-standardized scores (T-scores), with lower scores indicating greater cognitive dysfunction. In this study, participants with a T-score <50 on PedsPCF-43 completion were classified as having NCD.
Statistical Analysis
Information collected in all groups included baseline disease characteristics, ongoing disease activity, physical examination, standard clinical and laboratory information, and PedANAM performance. Numeric variables were summarized by means and standard deviations (SD), and categorical variables by percentage values.
General Plan
As cognition is closely related to brain development, and given the influence of age on PedANAM performance (29), our analyses were statistically adjusted for age. However, analysis of unadjusted data did not differ significantly from analysis of adjusted data; therefore, only adjusted analyses are presented. It has been suggested that PedANAM accuracy scores are especially suited to capture the presence of NCD (16). Hence, accuracy scores were considered for the derivation of all candidate PedANAM-CPS indices with the exception of the PedANAM-CPSmultiscore. We had previously derived the PedANAM-CPSmultiscore by logistic regression considering various PedANAM performance parameters as predictors. Performance parameters from each PedANAM-subtest were normalized using Z-scores, i.e. Z-score= [(raw test score-mean)/test standard deviation of the mean] prior to consideration in statistical analyses. Candidate PedANAM-CPS indices were then developed by applying the different statistical methods described below with data from the development group. For validation purposes, the performances of the PedANAM-CPS indices were evaluated using data from validation datasets 1 and 2.
Composition of four candidate PedANAM-CPS indices
We used statistical modeling methods previously proposed for the development of summary scores of the instrument with details provided below.
PedANAM-CPSmultiscore
Based on earlier analyses using the development dataset, this score has a reported sensitivity and specificity to detect NCD of 100% and 86%, respectively (21). Detailed description of the methods used for derivation of the PedANAM-CPSmultiscore can be found elsewhere (21). In brief, the PedANAM-CPSmultiscore includes the accuracy score of the PedANAM Spatial Processing test, the CVc of reaction time for correct responses on the Continuous Performance and Matching-to-Sample tests, and the mean reaction time for correct responses on the Code Substitution Delayed test.
PedANAM-CPSUWA
This PedANAM-CPS index constitutes an unweighted average of each of the nine subtests’ (normalized) accuracy scores.
PedANAM-CPSlogit
This PedANAM-CPS index is based on an algorithm derived from a logistic regression model to predict the dependent variable NCD (yes/no as based on FNCT), using all (normalized) subtests’ accuracy scores as independent (i.e. predictor) variables. The intercept and slope coefficients from this model were used to combine accuracy scores from the individual Ped-ANAM-subtests into a single predicted Logit score for each participant. Since PedANAM-CPSlogit utilizes information from both the criterion standard (FNCT result) as the dependent variable and from the PedANAM subtests as independent variables during the modeling process, it can be considered a supervised statistical method for summary score derivation.
PedANAM-CPSPCA
In Principal Components Analysis (PCA) the variance-covariance matrix of the (normalized) accuracy scores of PedANAM subtests was decomposed into a series of eigenvectors with corresponding eigenvalues. Each eigenvalue constitutes the variance of the linear combination of all test accuracy scores weighted by values contained in the corresponding eigenvector. In order to preserve the largest portion of the total variance of the variance-covariance matrix, the first eigenvector was used in the derivation of the PedANAM-CPSPCA. Since a criterion standard (FNCT) is not required for PCA, it is considered a non-supervised statistical method for summary score derivation.
PedANAM-CPS interpretation
Due to their methods of development, the higher the PedANAM-CPSUWA or the PedANAM-CPSPCA index the higher the performance accuracy on the PedANAM subtests and the lower the probability of NCD. In contrast, higher PedANAM-CPSlogit or PedANAM-CPSmultiscore values indicate higher probability of NCD.
Validation of candidate PedANAM-CPS
Following their initial development, the PedANAM-CPS indices were assessed for differences in mean values for groups of participants with different NCD-status using a Student’s t-test. Pearson’s correlation coefficients were calculated to assess relationships among the candidate PedANAM-CPS indices.
To further assess the measurement properties of the candidate PedANAM-CPS indices, a receiver operating characteristics (ROC) curve analysis was done for identifying NCD-status (yes/no). Overall precision for correctly identifying NCD-status was determined by calculating the area under the ROC curve (AUC). In addition, the sensitivity and specificity of each index were calculated under a preferred threshold approach at 80% or higher.
The ability of the candidate PedANAM-CPS indices to identify NCD (from FNCT) was first evaluated in the development group and then re-tested using validation datasets-1 and −2, defining NCD-status based on FNCT and Ped-PCF43, respectively. Values of the AUC can be interpreted as outstanding (1.0–0.91), excellent (0.81–0.90), good (0.71–0.8), fair (0.61–0.7), and poor (<0.60) performance in predicting NCD (30).
RESULTS
Study participants
Demographics of the participants and disease information are presented in Table 1. cSLE patients and controls enrolled in the development and validation cohorts did not differ on sociodemographic variables (maternal education level, family income). Over 80% of the participants were female and at least a third of those with cSLE were African American. The age (mean±SD) of participants with cSLE in the development group and the validation groups 1 and 2 was comparable, as was their disease activity. Based on FNCT, NCD was present in 22.5% of the cSLE participants of the development group.
Table 1.
Demographics of Development-group and Validation-groups at enrollment *
| Variable | Category | Development Group | Validation Group-1 | Validation Group-2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cSLE (n=40) | Control (n=40) | p-value | cSLE (n=34) | Control (n=27) | p-value | cSLE (n=68) | Control (n=18) | p-value | ||
| Age (years) | 14.8 ± 2.3 | 13.9 ± 3.2 | 0.21 | 16.17 ± 2.3 | 15.6 ± 2.3 | 0.34 | 15.3 ± 3.3 | 14.0 ± 2.5 | 0.131 | |
| Female (%) | Yes | 85 | 85 | 1.0 | 82.4 | 85.2 | 0.77 | 91.2 | 72.2 | 0.032 |
| Ethnicity (%) | ||||||||||
| White | 30 | 32.5 | 0.98 | 29.4 | 33.3 | 0.96 | 30.9 | 94.4 | <0.001 | |
| African American | 45 | 47.5 | 47.1 | 40.7 | 30.9 | 5.6 | ||||
| Hispanic | 17.5 | 15 | 14.7 | 14.8 | 11.8 | 0 | ||||
| Asian and other | 7.5 | 5 | 8.8 | 11.1 | 26.5 | 0 | ||||
| Grade Level (%) | ||||||||||
| Elementary School (4–6) | 20 | 20 | 1.0 | 13.3 | 16.7 | 0.91 | 18.6 | 22.2 | 0.321 | |
| Middle School (7–8) | 17.5 | 17.5 | 6.7 | 8.3 | 15.3 | 33.3 | ||||
| High School (9–12) | 62.5 | 62.5 | 80 | 75 | 55.9 | 38.9 | ||||
| College | 0 | 0 | 0 | 0 | 10.2 | 5.6 | ||||
| Maternal education level (%) | ||||||||||
| No High School Diploma | 7.5 | 10 | 0.7 | 3.1 | 3.9 | 0.72 | 4.5 | 5.6 | 0.523 | |
| Complete High School Diploma | 30 | 37.5 | 21.9 | 30.1 | 16.7 | 11.1 | ||||
| Education Beyond High School | 62.5 | 52.2 | 75 | 65.4 | 78.8 | 83.3 | ||||
| Annual Family Income | ||||||||||
| < $ 25K | 20 | 15.8 | 0.81 | 24.2 | 7.4 | 0.24 | 14.3 | 5.6 | 0.057 | |
| $26K-$50K | 35 | 34.2 | 27.3 | 33.3 | 31.7 | 5.6 | ||||
| $51K-$75K | 20 | 28.9 | 18.2 | 33.3 | 12.7 | 16.7 | ||||
| >$75K | 25 | 21.1 | 30.3 | 25.9 | 41.3 | 72.1 | ||||
| Treated with oral steroids (%) | 77.5 | 50 | 76.5 | |||||||
| Prednisone dose (mg/day) | 19.8 ± 17.4 | 10.15 ±10.2 | 17.1 ± 16.1 | |||||||
| Disease activity, SLEDAI‡ | 4.9 ± 4.4 | 4.2 ± 3.6 | 4.3 ± 4.7 | |||||||
| PCF-43 (T-score)† | - | - | - | - | 60.5 ± 7.9 | 63.2 ± 5.8 | 0.167 | |||
| Neurocogn. dysfunction (%)¥ | 22.5 | 7.5 | 0.06 | 18.2 | 14.3 | 0.682 | 8.8 | 11.1 | 0.766 | |
Values are means± standard deviations unless stated otherwise
cSLE = childhood-onset systemic lupus erythematosus
Systemic Lupus Disease Activity Index 2k version; range 0 – 104; 0 = inactive SLE
PCF-43 questionnaire: Perceived Cognition Functioning −43 questionnaire
Neurocognitive dysfunction categories are defined based on z-scores of the standardized tests completed for the formal neurocognitive testing (FNCT) on the research cohort, and on T-scores of the pediatric perceived cognitive function questionnaire-43 (PedsPCF-43) on the clinical cohort.
Development Dataset Analyses of PedANAM-CPS candidates
Associations between the four candidate PedANAM-CPS indices and details about the algorithms used to calculate them are presented in Table 2. The PedANAM-CPSPCA and the PedANAM-CPSUWA were strongly correlated with each other (r=0.99; p<0.001), while the PedANAM-CPSlogit was moderately correlated with the PedANAM-CPSPCA, and the PedANAM-CPSUWA (r=−0.60 and −0.57 respectively; p<0.001). The PedANAM-CPSmultiscore was moderately correlated with the other three PedANAM-CPS indices (p<0.001).
Table 2.
Pearson correlation coefficients of the candidate PedANAM-CPS in the development dataset of 80 participants
| PedANAM-CPSUWA | PedANAM-CPSPCA | PedANAM-CPSlogit | |
|---|---|---|---|
| PedANAM-CPS UWA | 1.000 | ||
| PedANAM-CPSPCA | 0.990** | 1.000 | |
| PedANAM-CPSlogit | −0.579** | −0.601** | 1.000 |
| PedANAM-CPSmultiscore | −0.670** | −0.675** | 0.650** |
p-values<0.001
PedANAM-CPSUWA is calculated by taking the average of 9 battery accuracy scores; if PedANAM-CPSUWA < 0.25, further evaluation is recommended.
PedANAM-CPSPCA is calculated by taking weights of 0.29, 0.13, 0.45, 0.42, 0.26, 0.35, 0.33, 0.27 and 0.39 on battery accuracy scores of CDD, CPT, CS, LRS, M2S, MG, MATH, ST6 AND SPD respectively; if PedANAM-CPSPCA < 0.25, further evaluation is recommended.
PedANAM-CPSlogit is calculated using the following formula: PedANAM-CPSlogit = −1.99 – 0.24*CDD - 0.21*CPT - 0.07*CS - 0.35*LRS + 0.19*M2S + 0.85*MG + 0.02*Math - 0.37*ST6 - 1.00*SPD; if PedANAM-CPSlogit > −2.7, further evaluation is recommended.
PedANAM-CPSmultiscore is calculated using the following formula: PedANAM-CPSmultiscore = 15.42 – 0.19*AC.SPD + 9.75*CVc.CPT + 7.29*CVc.M2S – 0.01*MNc.CDD; if PedANAM-CPSmultiscore > 0.09, further evaluation is recommended.
Where CDD = Code Substitution Delayed accuracy score; CPT = Running Memory accuracy score; CS = Code Substitution accuracy score; LRS = Logical Relations Symbolic accuracy score; M2S = Matching to Sample accuracy score; MG = Matching Grids accuracy score; Math = Math Processing accuracy score; ST6 = Memory Search accuracy score (6); SPD = Spatial Processing (simultaneous) accuracy score; AC.SPD = Percent of Accuracy in SPD, CVc; CPT=Coefficient of Variation in CPT; CVc.M2S=Coefficient of Variance in M2S; MNc.CDD=Mean Reaction Time in CDD.
PedANAM-CPS indices differ between patients with and without NCD across all datasets
NCD status was based on FNCT (development-dataset and validation-dataset 1) or the PedsPCF-43 (validation-dataset 2). The ability of the PedANAM-CPSUWA, PedANAM-CPSPCA, PedANAM-CPSlogit and PedANAM-CPSmultiscore to discriminate participants as per their NCD-status (FNCT) after adjusting for age differences between groups was assessed. In contrast to individual PedANAM subtests, the PedANAM-CPS indices differ in groups of patients with and without NCD (Table 3). All candidate PedANAM-CPS indices discriminated between participants with different NCD-status (p’s< 0.036).
Table 3.
Performance of the candidate PedANAM-CPS for identifying neurocognitive dysfunction ^
| Non-NCD (n=68)* | NCD (n=12)* | p-value† | |
|---|---|---|---|
| PedANAM-CPSUWA | 0.08 ± 0.07 | −0.39 ± 0.18 | 0.036 |
| PedANAM-CPSPCA | 0.09 ± 0.08 | −0.42 ± 0.19 | 0.027 |
| PedANAM-CPSlogit | −2.26 ± 0.16 | −0.60 ± 0.37 | 0.001 |
| PedANAM-CPSmultiscore | 4.01±0.31 | 7.97±0.74 | <0.001 |
PedANAM-CPS: Pediatric Automated Neuropsychological Assessment Metrics-Cognitive Performance Score; UWA: unweighted average method; PCA: principal component analysis method; Logit: logistic regression method of all the PedANAM subtests accuracy scores; Multiscore: logistic regression method of selected PedANAM subtests accuracy, coefficient of variation and mean reaction time to correct responses scores.
NCD: Neurocognitive dysfunction as determined by Formal Neurocognitive Testing
Values are mean ± Standard error
From fixed effect models adjusted for age
Discrimination by NCD-status
The overall precision of PedANAM-CPSUWA, PedANAM-CPSPCA, PedANAM-CPSlogit, and PedANAM-CPSmultiscore at identifying NCD in the development group using ROC curve analysis is summarized in Table 4. The PedANAM-CPSmultiscore and the PedANAM-CPSlogit indices showed good to excellent ability to identify NCD in the development group (both AUC>0.77). For each candidate PedANAM-CPS index, specificities were determined for sensitivity values of 80% or higher as well as the statistically preferred threshold values that yielded the overall best combination of sensitivity and specificity.
Table 4.
Accuracy and point sensitivity and specificity of the candidates PedANAM-CPS in the Development-group
| Candidate PedANAM-CPS | AUC ¥ | Cut-off* | Sensitivity& | Specificity |
|---|---|---|---|---|
| PedANAM-CPSUWA | 0.60 | 0.25 | 83.3 % | 37.3 % |
| PedANAM-CPSPCA | 0.60 | 0.25 | 83.3 % | 41.8 % |
| PedANAM-CPSlogit | 0.77 | −2.70 | 91.7% | 31.3 % |
| PedANAM-CPSmultiscore | 0.85 | 0.09 | 91.7% | 66.2% |
Only sensitivities of > 80% were considered
Values are measures of overall PedANAM-CPS performance to identify neurocognitive dysfunction
(NCD) as determined by formal neurocognitive testing. AUC values range from 0 – 1; values of 1.0–0.91: outstanding, 0.81–0.90: excellent, 0.71–0.8: good, 0.61–0.7: fair, and <0.6: poor accuracy to detect NCD
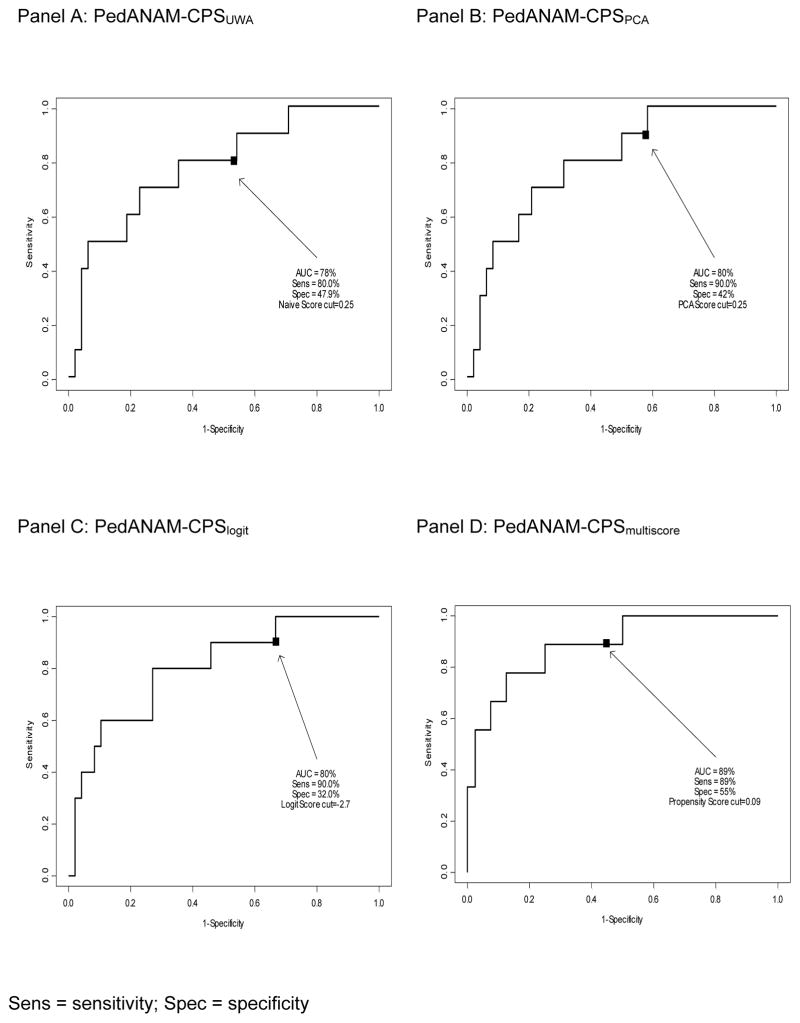
Overall accuracy and sensitivity for identifying NCD (as defined by FNCT) was near 90% in the validation-group-1 (Figure 1). Analyses considering the validation-dataset-2 (Table 5) yielded similar sensitivities to detect NCD (as determined by PedsPCF-43 scores) for all of the PedANAM-CPS indices, except for the PedANAM-CPSmultiscore. The latter, however, showed overall good to excellent ability to detect NCD when applied to validation groups 1 and 2 (AUC= 0.89 and 0.74, respectively).
Figure 1.
Area under (AUC) the receiver operative characteristic curve (ROC) calculated on validation group1
Sens = sensitivity; Spec = specificity
Table 5.
Accuracy and point sensitivity and specificity of the candidates PedANAM-CPS in the validation Groups summary
| Candidate PedANAM-CPS | Cut-off* | Validation Group-1 (n=61) | Validation Group-2 (n=86) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| AUC¥ | Sensitivity | Specificity | AUC¥ | Sensitivity | Specificity | ||
| PedANAM-CPSUWA | 0.25 | 0.78 | 80 % | 47.9 % | 0.68 | 80 % | 39.3 % |
| PedANAM-CPSPCA | 0.25 | 0.80 | 90 % | 42.0 % | 0.67 | 90 % | 39.3 % |
| PedANAM-CPSlogit | −2.70 | 0.80 | 90 % | 32.0 % | 0.62 | 80 % | 31.5 % |
| PedANAM-CPSmultiscore | 0.09 | 0.89 | 89 % | 55.0 % | 0.74 | 60 % | 75.3 % |
Interpretation of AUC values: 1.0–0.91: outstanding, 0.81–0.90: excellent, 0.71–0.8: good, 0.61–0.7: fair, and <0.6: poor
PedANAM-CPSUWA or PedANAM-CPSPCA <0.25 indicate higher likelihood of NCD. PedANAM-CPSlogit > −2.70 indicate higher
DISCUSSION
Based on recent research, the PedANAM software may offer a cost effective approach to screening for cognitive dysfunction associated with NPSLE (21). The development of a summary statistic is complementary to previous research that found the PedANAM to have construct validity and responsiveness to change in cognitive performance in cSLE (16, 21). We developed and initially validated four candidate PedANAM-CPS indices to further increase the clinical usefulness of the PedANAM using commonly accepted statistical approaches. We found that the proposed indices were able to differentiate individuals’ cognitive status. Compared to the PedANAM-CPSlogit, the PedANAM-CPSPCA and the PedANAM-CPSUWA showed similar or even higher accuracy (AUC) and higher specificity at the chosen sensitivity threshold of 80%. The PedANAM-CPSmultiscore featured the highest specificities among the four PedANAM-CPS indices at the chosen sensitivity threshold of 80%. According with our findings we recommend further neurocognitive assessment in subjects with a PedANAM-CPSPCA score <0.25 or a PedANAM-CPSmultiscore >0.09.
Upon completion of the PedANAM a large number of performance variables are provided, making it difficult for clinicians to easily determine whether a patient’s cognitive function has changed or not. We, therefore, propose that the PedANAM-CPS may be used in several ways. Firstly, as the PedANAM is sensitive to cognitive dysfunction when present but not necessarily specific to a particular disease process, the PedANAM-CPS may be used to track cognitive ability over time in children with and without cSLE (31). Secondly, comparing a patient’s score to the threshold values proposed may support the decision of whether or not to pursue FNCT. This is supported by the initial threshold values for each of the PedANAM-CPS indices that are highly sensitive to the presence of NCD.
When developing the PedANAM-CPS we focused on the PedANAM accuracy performance parameter (of each subtest) as this score has demonstrated the highest consistency and reliability when compared to the other PedANAM derived scores (16). This strategy was taken given the known diversity of cognitive deficits observed in cSLE. Conversely, the PedANAM-CPSmultiscore suggests that a shorter PedANAM battery of subtests may be used for NCD-screening. However, this finding needs to be replicated in other prospective studies with larger samples before consideration of a reduced battery.
We identified threshold values for each of the four PedANAM-CPS indices that were able to recognize NCD in the participants with acceptable sensitivities and specificities. Nonetheless, these threshold levels must be considered preliminary and require confirmation in larger cohorts. However, as the performance of a screening device is related to the prevalence of the disease that it is intended to identify, a different population can produce different findings. Based upon the estimated prevalence of cSLE in the US of 9.73 per 100.000 children (32), a sample of more than 100 cSLE patients is actually quite large.
A limitation of our study may be that the distribution of PedsPCF-43 T-score in our study differed from the distribution in pediatric cancer survivors. In the current study, participants’ mean PedsPCF-43 T-score was near 60, and therefore participants with a T-score <50 on PedsPCF-43 were classified as having NCD. Although this is in line with reports from children with attention deficit disorder, epilepsy, and cerebral palsy (24), it differed from what has been suggested in the past for pediatric cancer survivors, in whom the tool was originally developed and where a score of 40 is considered an indication for referral to FNCT. Thus, the differences in performance of the PedsPCF-43 for cSLE and as compared to oncology populations will need further investigation.
Another limitation of this study perhaps will be the significant racial difference observed within cSLE patients and controls in the validation group-2. Female predominance in the cSLE was expected as JIA controls were not recruited based on sex or ethnicity. However, based on previous research it is unlikely that the results are affected by racial differences (33, 34). Rather, social advantage factors such as maternal education (34) and poverty have a stronger association with lower levels of school achievement and IQ later in childhood. Overall in this study the maternal education level was similar among groups.
To ensure the high sensitivity required for neurocognitive screening (11), the threshold levels presented provide greater than 80% sensitivity in detecting NCD. Arguably, a different sensitivity threshold could have been chosen, as we recognize that suggested PedANAM-CPS thresholds have low specificity for identifying NCD. However, we feel it better to have a screening tool that favors false positives as this may only lead to undue referrals for FNCT of children who have normal cognitive function, and will minimize failures to identify children who truly have cognitive dysfunction. Moreover, given the lack of a systematic screening options (i.e. physician perception and parent global impressions are insufficient to detect early cSLE associated cognitive changes) (16, 22) we consider the PedANAM-CPS indices to be an important tool for effective systematic screening for NCD in pediatric rheumatology clinics.
Further study is required to determine whether the PedANAM-CPSPCA and PedANAM-CPSmultiscore indices would be complementary in the detection of NPSLE. In particular, research on the sensitivity of the PedANAM-CPS to clinically meaningful change over time is needed. Additionally, information about the minimal clinically important differences in PedANAM-CPS is needed.
Early detection and screening of cognitive decline and NCD in cSLE is a critical first step to improving prognosis and functioning of affected cSLE patients. The PedANAM-CPS provides a summary measure of cognitive performance that may be simple enough to implement as a clinically relevant NPSLE screening tool. Based on findings from this initial validation study, we recommend the PedANAM-CPSPCA and PedANAM-CPSmultiscore as summary statistics to screen the cognitive performance of children with cSLE. However, larger studies in diverse patient populations are needed to examine the measurement properties of the PedANAM-CPS in more detail.
SIGNIFICANCE & INNOVATION.
Innovation
This study proposes a Pediatric Automated Neuropsychological Assessments Metrics cognitive performance score (PedANAM-CPS) as an overall measure of cognitive ability.
Initial threshold values can assist in the identification of patients with a high likelihood of clinically relevant impairment of cognitive function
Significance
The PedANAM-CPS enhances the suitability of the PedANAM as a screening tool for cognitive dysfunction in clinical settings.
Acknowledgments
This study is supported an NIAMS Center of Clinical Research Award, NIAMS P60 AR47784, a NIAMS Multidisciplinary Clinical Research Award 1P60AR062755 and an Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number 5UL1RR026314-03. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
This work was done in partial completion of a Master of Science in Clinical and Translational Research – University of Cincinnati
Kasha Wiley, Jessica Hummel, Shannen Nelson – Cincinnati Children’s Hospital Medical Center
Paul Nietert - Medical University of South Carolina
Lina Qi and Lawrence Ng – Hospital for Sick Children
Footnotes
The authors deny any conflicts of interest, real or perceived.
References
- 1.Tucker LB, Menon S, Schaller JG, Isenberg DA. Adult- and childhood-onset systemic lupus erythematosus: a comparison of onset, clinical features, serology, and outcome. Br J Rheumatol. 1995 Sep;34(9):866–72. doi: 10.1093/rheumatology/34.9.866. Epub 1995/09/01. eng. [DOI] [PubMed] [Google Scholar]
- 2.Janwityanujit S, Totemchokchyakarn K, Verasertniyom O, Vanichapuntu M, Vatanasuk M. Age-related differences on clinical and immunological manifestations of SLE. Asian Pac J Allergy Immunol. 1995 Dec;13(2):145–9. Epub 1995/12/01. eng. [PubMed] [Google Scholar]
- 3.Brunner HI, Silverman ED, To T, Bombardier C, Feldman BM. Risk factors for damage in childhood-onset systemic lupus erythematosus: cumulative disease activity and medication use predict disease damage. Arthritis Rheum. 2002 Feb;46(2):436–44. doi: 10.1002/art.10072. Epub 2002/02/13. eng. [DOI] [PubMed] [Google Scholar]
- 4.Tokano Y, Morimoto S, Amano H, Kawanishi T, Yano T, Tomyo M, et al. The relationship between initial clinical manifestation and long-term prognosis of patients with systemic lupus erythematosus. Mod Rheumatol. 2005;15(4):275–82. doi: 10.1007/s10165-005-0411-0. Epub 2006/10/10. eng. [DOI] [PubMed] [Google Scholar]
- 5.Moroni G, Quaglini S, Maccario M, Banfi G, Ponticelli C. “Nephritic flares” are predictors of bad long-term renal outcome in lupus nephritis. Kidney Int. 1996 Dec;50(6):2047–53. doi: 10.1038/ki.1996.528. Epub 1996/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 6.Bertoli AM, Alarcon GS, Calvo-Alen J, Fernandez M, Vila LM, Reveille JD. Systemic lupus erythematosus in a multiethnic US cohort XXXIII. Clinical [corrected] features, course, and outcome in patients with late-onset disease. Arthritis Rheum. 2006 May;54(5):1580–7. doi: 10.1002/art.21765. Epub 2006/04/29. eng. [DOI] [PubMed] [Google Scholar]
- 7.Brunner HI, Bishnoi A, Barron AC, Houk LJ, Ware A, Farhey Y, et al. Disease outcomes and ovarian function of childhood-onset systemic lupus erythematosus. Lupus. 2006;15(4):198–206. doi: 10.1191/0961203306lu2291oa. [DOI] [PubMed] [Google Scholar]
- 8.Fragoso-Loyo HE, Sanchez-Guerrero J. Effect of severe neuropsychiatric manifestations on short-term damage in systemic lupus erythematosus. J Rheumatol. 2007 Jan;34(1):76–80. Epub 2006/12/05. eng. [PubMed] [Google Scholar]
- 9.Zelko F, Beebe D, Baker A, Nelson SM, Ali A, Cedeno A, et al. Academic outcomes in childhood-onset systemic lupus erythematosus. Arthritis care & research. 2012 Aug;64(8):1167–74. doi: 10.1002/acr.21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein-Gitelman M, Brunner HI. The impact and implications of neuropsychiatric systemic lupus erythematosus in adolescents. Curr Rheumatol Rep. 2009 Jul;11(3):212–7. doi: 10.1007/s11926-009-0029-x. Epub 2009/07/17. eng. [DOI] [PubMed] [Google Scholar]
- 11.Peckham CS, Dezateux C. Issues underlying the evaluation of screening programmes. Br Med Bull. 1998;54(4):767–78. doi: 10.1093/oxfordjournals.bmb.a011728. Epub 1999/06/15. eng. [DOI] [PubMed] [Google Scholar]
- 12.Kozora E, Hanly JG, Lapteva L, Filley CM. Cognitive dysfunction in systemic lupus erythematosus: past, present, and future. Arthritis Rheum. 2008 Nov;58(11):3286–98. doi: 10.1002/art.23991. Epub 2008/11/01. eng. [DOI] [PubMed] [Google Scholar]
- 13.National Institutes of Health CaEHP. Executive Summary. 2014 Available from: http://trans.nih.gov/CEHP/HBPes.htm.
- 14.Ross GS, Zelko F, Klein-Gitelman M, Levy DM, Muscal E, Schanberg LE, et al. A proposed framework to standardize the neurocognitive assessment of patients with pediatric systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2010 Jul;62(7):1029–33. doi: 10.1002/acr.20152. Epub 2010/07/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cognitive Science Research Center (CSRC) Administration Manual. Vol. 2014 Norman, OK: University of Oklahoma; 2014. The Pediatric Automated Neuropsychological Assessment Metrics (Ped-ANAM) [Google Scholar]
- 16.Brunner HI, Ruth NM, German A, Nelson S, Passo MH, Roebuck-Spencer T, et al. Initial validation of the Pediatric Automated Neuropsychological Assessment Metrics for childhood-onset systemic lupus erythematosus. Arthritis and rheumatism. 2007 Oct 15;57(7):1174–82. doi: 10.1002/art.23005. [DOI] [PubMed] [Google Scholar]
- 17.Roebuck-Spencer TM, Yarboro C, Nowak M, Takada K, Jacobs G, Lapteva L, et al. Use of computerized assessment to predict neuropsychological functioning and emotional distress in patients with systemic lupus erythematosus. Arthritis Rheum. 2006 Jun 15;55(3):434–41. doi: 10.1002/art.21992. Epub 2006/06/02. eng. [DOI] [PubMed] [Google Scholar]
- 18.Vincent AS, Roebuck-Spencer T, Lopez MS, Twillie DA, Logan BW, Grate SJ, et al. Effects of military deployment on cognitive functioning. Military medicine. 2012 Mar;177(3):248–55. doi: 10.7205/milmed-d-11-00156. Epub 2012/04/07. eng. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism. 1997 Sep;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 20.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004 Feb;31(2):390–2. Epub 2004/02/05. eng. [PubMed] [Google Scholar]
- 21.Brunner HI, Klein-Gitelman MS, Zelko F, Thomas EC, Hummel J, Nelson SM, et al. Validation of the Pediatric Automated Neuropsychological Assessment Metrics in childhood-onset systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2013 Mar;65(3):372–81. doi: 10.1002/acr.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vega-Fernandez P, Zelko FA, Klein-Gitelman M, Lee J, Hummel J, Nelson S, et al. Value of questionnaire-based screening as a proxy for neurocognitive testing in childhood-onset systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2014 Jun;66(6):943–8. doi: 10.1002/acr.22247. Epub 2013/12/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams TS, Aranow C, Ross GS, Barsdorf A, Imundo LF, Eichenfield AH, et al. Neurocognitive impairment in childhood-onset systemic lupus erythematosus: measurement issues in diagnosis. Arthritis Care Res (Hoboken) 2011 Aug;63(8):1178–87. doi: 10.1002/acr.20489. Epub 2011/05/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai JS, Butt Z, Zelko F, Cella D, Krull KR, Kieran MW, et al. Development of a parent-report cognitive function item bank using item response theory and exploration of its clinical utility in computerized adaptive testing. J Pediatr Psychol. 2011 Aug;36(7):766–79. doi: 10.1093/jpepsy/jsr005. Epub 2011/03/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007 Sep 1;25(25):3866–70. doi: 10.1200/JCO.2007.10.8639. Epub 2007/09/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahone EM, Zabel TA, Levey E, Verda M, Kinsman S. Parent and self-report ratings of executive function in adolescents with myelomeningocele and hydrocephalus. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence. 2002 Dec;8(4):258–70. doi: 10.1076/chin.8.4.258.13510. Epub 2003/05/22. eng. [DOI] [PubMed] [Google Scholar]
- 27.Lai JS, Zelko F, Krull KR, Cella D, Nowinski C, Manley PE, et al. Parent-reported cognition of children with cancer and its potential clinical usefulness. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2014 May;23(4):1049–58. doi: 10.1007/s11136-013-0548-9. Epub 2013/11/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai JS, Zelko F, Butt Z, Cella D, Kieran MW, Krull KR, et al. Parent-perceived child cognitive function: results from a sample drawn from the US general population. Childs Nerv Syst. 2011 Feb;27(2):285–93. doi: 10.1007/s00381-010-1230-y. Epub 2010/07/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roebuck-Spencer TMBJ, Cernich AN, Ivins B, Schwab K, Sun W, et al. Influence of age, sex, and education on the Automated Neuropsychological Assessment Metrics (ANAM). International Neuropsychological Society Meeting; Baltimore (MD). 2004.2004. [Google Scholar]
- 30.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982 Apr;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. Epub 1982/04/01. eng. [DOI] [PubMed] [Google Scholar]
- 31.Daniel JC, Olesniewicz MH, Reeves DL, Tam D, Bleiberg J, Thatcher R, et al. Repeated measures of cognitive processing efficiency in adolescent athletes: implications for monitoring recovery from concussion. Neuropsychiatry Neuropsychol Behav Neurol. 1999 Jul;12(3):167–9. Epub 1999/08/24. eng. [PubMed] [Google Scholar]
- 32.Mina R, Brunner HI. Update on differences between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis research & therapy. 2013 Aug 21;15(4):218. doi: 10.1186/ar4256. Epub 2013/09/04. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brooks-Gunn J, Duncan GJ. The effects of poverty on children. The Future of children / Center for the Future of Children, the David and Lucile Packard Foundation. 1997 Summer-Fall;7(2):55–71. [PubMed] [Google Scholar]
- 34.Duncan GJ, Brooks-Gunn J, Klebanov PK. Economic deprivation and early childhood development. Child development. 1994 Apr;65(2 Spec):296–318. [PubMed] [Google Scholar]