Abstract
Esophageal adenocarcinoma (EAC) is rapidly increasing in incidence in Western cultures. Barrett’s esophagus (BE) is the presumed precursor lesion for this cancer. Several other risk factors for this cancer have been described, including chronic heartburn, tobacco use, Caucasian race, and obesity. Despite these known associations, most patients with EAC present with symptoms of dysphagia from late-stage tumors—only a small minority of patients are identified in screening and surveillance programs. Diagnostic analysis of EAC usually commences with upper endoscopy, followed by cross-sectional imaging. Endoscopic ultrasound is useful to assess local extent of disease as well as the involvement regional lymph nodes. T1a EAC may be treated endoscopically; some patients with T1b disease might also benefit from endoscopic therapy. Locally advanced disease is generally managed with esophagectomy, often accompanied by neoadjuvant chemoradiotherapy or chemotherapy. The prognosis is based on tumor stage: patients with T1a tumors have an excellent prognoses, whereas few patients with advanced disease have longterm survival.
Keywords: Esophageal neoplasms, adenocarcinoma, risk factors, endoscopic therapy
Esophageal adenocarcinoma (EAC) is among the most lethal conditions gastroenterologists face. Only 16% of patients survive 5 y and median survival time is less than 1 y; relatively little progress has been made in stemming the toll of this condition.1 EAC usually presents at a late stage, with most patients presenting with T3 or T4 disease. This is in part responsible for its high mortality, as few therapies are available to cure patients with advanced-stage disease.
The incidence of EAC has increased greatly in the last 40 y, by approximately 600% from the 1970s. The reasons for this increase are incompletely understood. Many investigators have suggested that the concurrent epidemic of obesity may explain at least part of this increase. Treatment of EAC has also evolved—especially for early stage disease. Although in the past, gastroenterologists only performed the endoscopy and biopsy to diagnose the cancer, they are now integral members of the care team and are involved with many of the patients who are cured. For this reason, it is important for gastroenterologists to understand the risk factors and presentation of EAC, all the options for its treatment, and the expected outcomes based on the treatment provided.
We review the epidemiology, risk factors, staging, and treatment of EAC, focusing on early-stage disease and endoscopic therapy, and prognoses of patients who receive different therapies. We conclude with likely future developments in care of these patients.
Epidemiology
Adenocarcinoma was once an exceedingly rare histological type of esophageal cancer. Beginning as early as the 1960s, the incidence of EAC began to increase in the United States (US).2 By the 1990s, adenocarcinoma was the predominant type of esophageal cancer in the US, surpassing squamous cell carcinoma.3, 4 In 2014, there were approximately 18,170 incident esophageal cancers in the US, 59.9% of which were adenocarcinomas.5, 6 However, worldwide, squamous cell cancer is still the predominant form of esophageal neoplasia. In fact, there were approximately 52,000 incident cases of EAC worldwide in 2012, compared with an estimated 398,000 esophageal squamous cell carcinomas.7
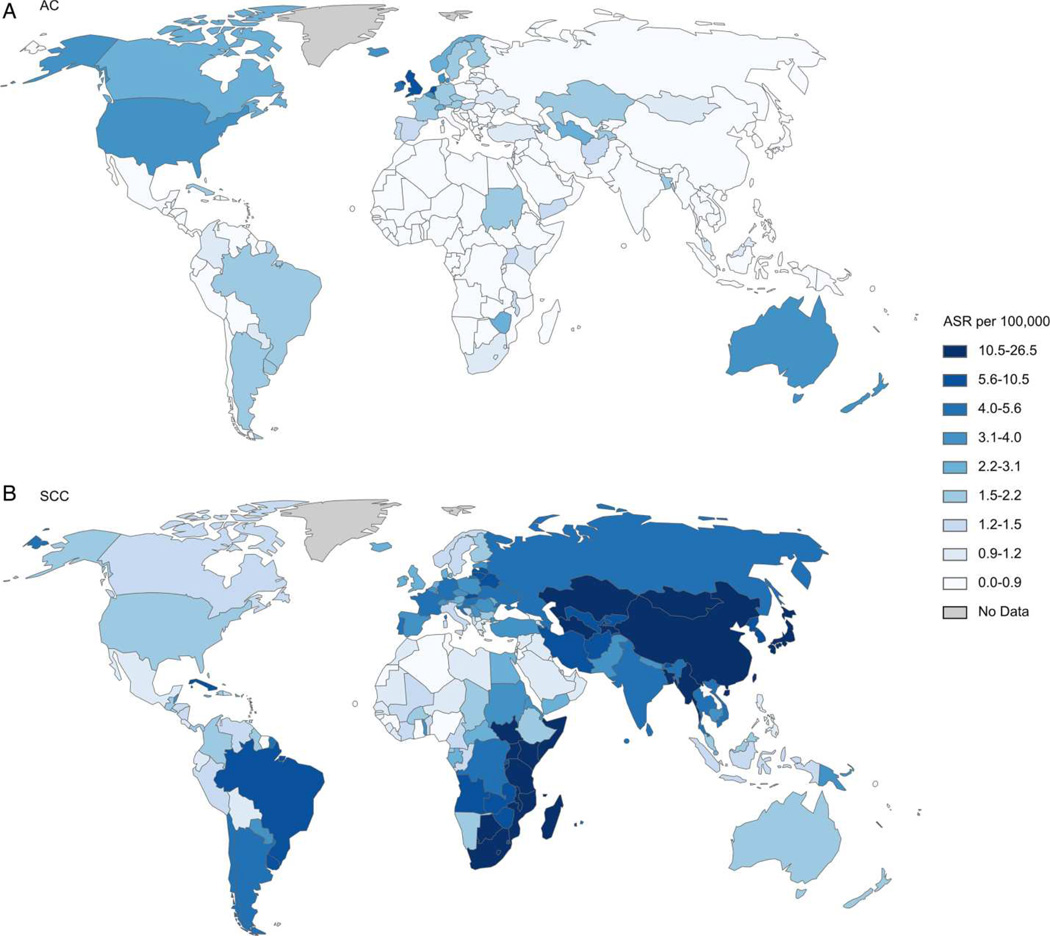
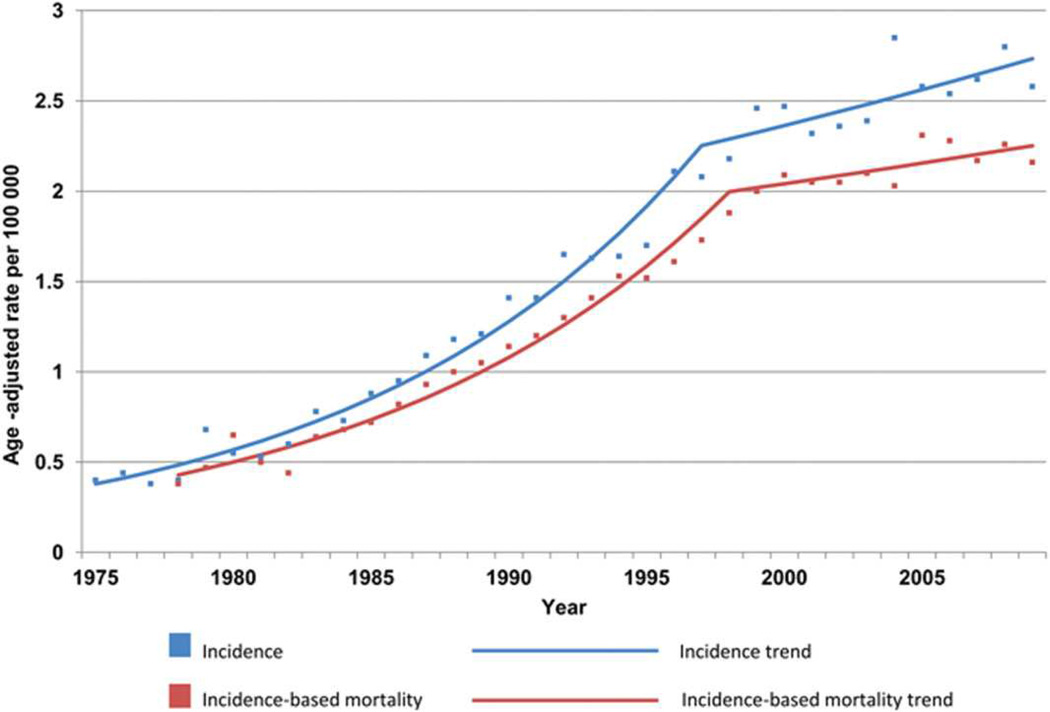
EAC is most common in industrialized countries with populations of predominant European race; nearly 50% of all cases occur in Northwest Europe and North America (Figure 1).7 Incidences are highest in the United Kingdom (UK), Ireland, France, and the Netherlands, indicating a Northern European predilection.8 EAC is rare in Asia and Africa, but China has approximately 18% of all incident cases worldwide, due to its large population.7 The incidence of EAC has continued to increase in the West, but may be reaching a plateau (Figure 2)8–10—the incidence of EAC in the US was 2.5/100,000 individuals/y in 2011.1, 9
Figure 1. Global Differences in Incidence of Histological Subtypes of Esophageal Cancer.
Age-standardized incidence rate (ASR) per 100,000 of (A) esophageal adenocarcinoma and (B) squamous cell carcinoma in men. AC, adenocarcinoma; SCC, squamous cell carcinoma. (Reproduced with permission from: Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut published online 10/15/2014 as doi:10.1136/gutjnl-2014-308124).
Figure 2. Trend in US Incidence of EAC.
Graph shows Surveillance Epidemiology and End Results (SEER) cancer registry 9 esophageal adenocarcinoma incidence and incidence-based mortality, 1975 to 2009. From 1975 to 1997, EAC incidence increased at an annual percentage change (APC) of 8.4 (95% confidence interval [CI] = 7.7–9.1), whereas the APC was 1.6 (95% CI = 0.0–3.3) from 1997 to 2009. For incidence-based mortality, the APC was 8.0 from 1978 to 1998 (95% CI = 7.2–8.8) and 1.1 from 1998 to 2009 (95% CI = 0.7 to 2.9). All rates were age-adjusted to the 2000 Standard population using 19 age groups. (Reproduced with permission from: Hur C, Miller M, Kong CY, et al. Trends in Esophageal Adenocarcinoma Incidence and Mortality. Cancer 2013;119:1149–58).
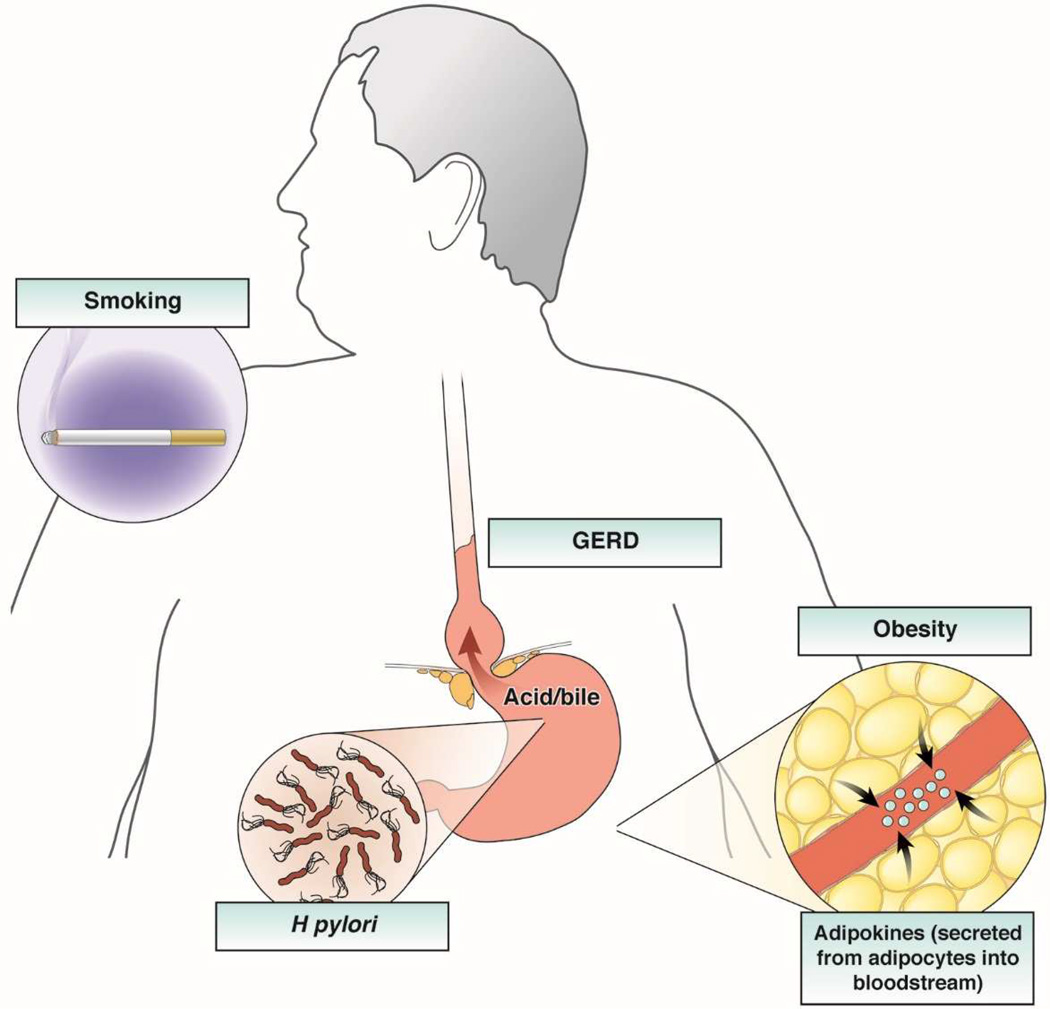
There are several risk factors for EAC (Figure 3). Perhaps the most familiar is gastroesophageal reflux disease (GERD). In 1995, Chow et al associated adenocarcinoma of either the esophagus or gastric cardia with prior documentation of GERD (adjusted odds ratio [OR] = 2.1; 95% confidence interval [CI], 1.2–3.6).11 In 1999, Lagergren et al published a landmark population-based case–control study demonstrating that the risk of GERD was approximately 8-fold greater in patients with recurrent GERD symptoms compared to those without GERD symptoms.12 It is believed that in predisposed individuals, GERD can lead to erosive esophagitis, and after an aberrant healing process a metaplastic, specialized intestinal epithelium (Barrett’s esophagus).13 Barrett’s esophagus progresses to EAC in a small percentage of individuals, at a rate of approximately 0.12%/y to 0.60%/y.14–16 A meta-analysis of population-based studies estimated that weekly GERD symptoms increase the risk of EAC by about 5-fold.17 Patients with long-standing symptoms, nocturnal symptoms or more frequent symptoms are at higher risk. However, the severity of the symptoms is not associated with an increased risk of EAC.18
Figure 3. Risk Factors for EAC.
The primary risk factors for esophageal adenocarcinoma include male sex, advancing age, white race, GERD, obesity, and tobacco use. The effect of obesity is likely mediated both through a mechanical effect promoting GERD and a hormonal effect through alterations in circulating adipokines and other peptides, and appears to be a major risk factor for both BE and EAC. A deranged gastro-esophageal junction, noted above with a large hiatal hernia, allows free reflux of gastric contents. H pylori infection protects against EAC, and Barrett’s esophagus is its only known precursor.
Although GERD is undoubtedly an important risk for EAC, most individuals with GERD never develop EAC.19 A systematic review of population-based studies found that a slight majority of patients with EAC deny any substantial prior symptoms of GERD.20 It is possible that chronic reflux of gastric contents could promote EAC without causing substantial symptoms before patients present with cancer.
Tobacco use is a strong risk factor for esophageal squamous cell carcinoma, but it is also a risk for EAC. Analyses of data from a consortium of researchers found that tobacco use increased the risk for EAC 2.18-fold (95% CI, 1.84–2.58).21 Alcohol use is also a strong risk factor for esophageal squamous cell carcinoma, but has not proven to be a consistent risk factor for EAC. In fact, there appears to be a moderate inverse association between alcohol use and risk of EAC.22 It is not clear whether alcohol use actually protects against EAC; it could be that individuals who develop EAC avoid alcohol use because it worsens the symptoms of GERD.
Obesity is a clear risk factor for EAC. A body mass index (BMI) of 30–34.9 kg/m2 is associated with a 2.39-fold increase in risk EAC, compared to a BMI of less than 25 kg/m2, with stronger effects for those with even greater BMIs.23 Abdominal obesity, in particular, is associated with Barrett’s esophagus, and is also associated with EAC (summary OR 2.51; 95% CI 1.54–4.06).24
How might abdominal obesity contribute to development of EAC? Obesity has the mechanical effect of promoting formation of hiatal hernia, which is associated with an increased risk of GERD.25, 26 But abdominal obesity is associated with Barrett’s esophagus even after adjusting for GERD, and obesity is associated with EAC even in individuals without symptoms of GERD.23 In addition to its mechanical effect, abdominal obesity is associated with alterations in circulating levels of peptides that are associated with Barrett’s esophagus, and may also promote EAC.27 Abdominal obesity is associated with insulin resistance and hyperinsulinemia, which have been associated with multiple epithelial cancers. The metabolic syndrome has been associated with Barrett’s esophagus and EAC.28, 29 However, the evidence for an association between hyperinsulinemia or diabetes mellitus with Barrett’s esophagus or EAC has been inconsistent.27, 30–35
The insulin-like growth factor (IGF) pathway has been more strongly implicated in the development of EAC than insulin itself. Circulating levels of IGF binding protein-3 are inversely associated with the presence of Barrett’s esophagus.31 A polymorphism in the gene encoding IGF1 is associated with Barrett’s esophagus,36 and a polymorphism in the gene encoding the IGF1 receptor modifies the effect of obesity on the risk for Barrett’s esophagus and EAC.37 A polymorphism in the gene encoding IGF2 is also associated with EAC, perhaps more so among smokers.38 The IGF pathway might also be involved in the risk of progression from Barrett’s esophagus to EAC.39
Studies have also found associations between blood levels of leptin and Barrett’s esophagus, and progression of Barrett’s esophagus to EAC.27, 30, 34, 40, 41 Barrett’s esophagus, and progression to EAC, has been associated with decreased levels of adiponectin (particularly the low molecular weight form) in some, but not all studies.27, 34, 40, 42, 43 These metabolic effects of obesity could have synergistic effects with GERD on the risk for Barrett’s esophagus and EAC.30, 44 Although obesity has been the primary focus for studies examining the effect of body habitus on risk of EAC, a recent analysis of data from a consortium of researchers found that short individuals are at greater risk for EAC than taller ones.45 The reason for that association is unclear.
Given the association of obesity with EAC, one might expect that physical activity and dietary habits would also be associated with EAC. Physical activity (either occupational or recreational) is weakly inversely associated with EAC (summary OR of greatest vs lowest category, 0.79; 95% CI, 0.66–0.94), with the strongest effects in people who exercise moderately or vigorously 5 days/week.46 The effect may persist including after adjusting for obesity. Some forms of activity, such as working in a stooped position and weight lifting, have been positively associated with GERD, but their specific effects on the risk of EAC have not been adequately studied.47
The association of dietary habits with the risk of EAC has been examined in many observational studies. Consumption of processed meats is associated with an approximate 23%–37% increase in risk of EAC, when the highest levels of intake are compared with the lowest.48, 49 Dietary fiber intake is inversely associated with EAC (summary OR for greatest vs lowest category of intake, 0.66; 95% CI, 0.44–0.98), but there is substantial heterogeneity in results from individual studies.50 Intake of the anti-oxidant vitamins A, C, and E are inversely associated with EAC.51 Caution is necessary before inferring that any of these dietary habits are causal in the development of EAC, because increased intake of one food type or micronutrient is inextricably linked with increased or decreased intake of other food types and micronutrients. Many observational studies have associated dietary habits with outcomes, but these findings were not supported by subsequent randomized trials of dietary supplementation.52–54
Infection with Helicobacter pylori appears to protect against EAC. Individuals with EAC are approximately half as likely to have H pylori infection as individuals without (OR, 0.56; 95% CI 0.46–0.68).55 In particular, the cytotoxin-associated gene A strain of H pylori appears to reduce risk of EAC. Infection of predominantly the gastric body, or the body and the antrum, reduces gastric acid production, which reduces acidic GERD and risk for EAC.56 However, infection predominantly in the antrum may be associated with increases in gastrin, with subsequent increase in gastric acid production.57 In Western countries, most H pylori infections occur predominantly in the antrum,58 so it is not clear whether its inverse association with EAC is due to a reduced incidence of GERD. H pylori infection is inversely associated with GERD in Asian countries, but does not appear to be so in Western countries.59
Another potential mechanism by which H pylori infection reduces risk for EAC could be that refluxed H pylori DNA reduces the inflammatory response to GERD.60, 61 Additionally, individuals who are genetically predisposed to maintaining persistent infection with H pylori might also be predisposed to an inflammatory response to GERD.62–64 Lack of infection with H pylori might simply be a marker for other alterations in the microbiome of the esophagus and/or stomach that are directly related to the development of EAC.63 Further research is needed to understand the mechanisms of association between H pylori and EAC.
The strongest risk factors for EAC are advancing age and male sex. Men have approximately 6-fold the risk of EAC of women.9 Among men, circulating levels of free testosterone and free dihydrotestosterone are strongly associated with Barrett’s esophagus (adjusted ORs for 4th vs 1st quartile, 5.36; 95% CI, 2.21–13.0 and OR, 4.25; 95% CI, 1.87–9.66, respectively).65 Among women who have had children, breast feeding is inversely associated with the risk of EAC, suggesting hormone effects.66 But no association among women has been found for the number of children, age of menarche or menopause, or use of hormone replacement therapy or oral contraceptives.66
The risk of EAC might be greater in men because of differences between sexes in use of tobacco or types of obesity. The estimated relative effects of tobacco use on EAC risk (ever use, or categorized by pack-y of use) are similar between men and women,21 but men more frequently use tobacco. Similarly, the effect of BMI on risk of EAC is similar between men and women,23 as is the effect of waist circumference on Barrett’s esophagus.67 However, the prevalence of abdominal obesity is greater among men, which could account for some of the increased risk for EAC among men. It is likely that the etiology of the difference in sexes is multifactorial, with differential distribution of some risk factors increasing the risk of EAC in men.
The regional differences observed in the incidence of EAC indicate that race is a strong risk factor for EAC. In the UK, the incidence of EAC is much lower among Asians and Africans than whites.68 Within the US, individuals of Asian descent and African-Americans have greatly decreased risk for EAC compared to non-Hispanic whites, with white Hispanics having an intermediate risk.69 The reasons for the differences across races are not clear. The effect of race might be mediated in part by differences in the prevalence of H pylori infection.70 In addition, although GERD symptoms are equally prevalent among the different races, whites are more likely to have erosive esophagitis, a lesion that is believed to be a necessary step in the development of EAC.71
Three genome-wide association studies have associated loci with Barrett’s esophagus; these are near or within CRTC1, BARX1, FOXF1, FOXP1, GDF7, and TBX5.72–74 CRTC1 encodes a transcription coactivator that regulates the invasiveness and migration of esophageal cancer cells; it is also associated with age at menarche and with obesity.72, 73 BARX1 encodes a homeobox transcription factor involved in esophageal differentiation.72, 73 TBX5, FOXF1, and FOXP1 encode transcription factors that regulate esophageal development.73, 74 GDF7 encodes a protein in the bone morphometric protein pathway.73 Differences in these or other alleles among races might account for the increased risk for EAC among Northern Europeans and their descendants.
The use of certain medications has been associated with an increased or decreased risk of EAC. Observational studies of patients with Barrett’s esophagus demonstrate that that use of proton pump inhibitors reduced the risk of neoplastic progression by 71%, but results from different studies are heterogeneous.75 Conversely, medications that relax the lower esophageal sphincter and could thereby predispose people to GERD have been studied as potential risk factors for EAC.76–79 There appears to be no association between EAC and use of calcium channel blockers. Use of asthma medications such as theophylline or β-agonists have been associated with Barrett’s esophagus and EAC, but these findings could be confounded by the indication for the medications, since asthma is associated with GERD. Use of aspirin and non-steroidal antiinflammatory drugs is associated with a decreased risk of EAC, particularly when used daily and for long duration.80 Finally, use of hydroxymethylglutaryl-CoA reductase inhibitors (statins) are associated with a decreased risk of EAC.81
Clinical Presentation and Diagnosis
Typically, patients with EAC present with dysphagia that progresses rapidly over months. Symptoms begin with subtle difficulty with solids, and progress to dysphagia for liquids as well. Oropharyngeal dysphagia is rare. The point of difficulty may be localized by the patient as cephalad as the sternal notch, even when the mass is in the distal esophagus. Weight loss and fatigue are common. In rare instances, patients present with iron-deficiency anemia. Although patients with GERD frequently undergo upper endoscopy and surveillance for Barrett’s esophagus, fewer than 15% of EAC cases are detected during surveillance endoscopies.82–84 Most patients with EAC present with symptoms and advanced disease. Only approximately 25% of patients with EAC have localized disease at the time of presentation,9 severely limiting effective treatment options.
Cross-sectional imaging analysis, such as with computed tomography (CT), does not accurately identify localized EAC. Barium esophagrams can identify irregular strictures or masses, but upper endoscopy with biopsy collection and histologic analysis is the standard for diagnosis. During endoscopy, EAC can appear as a stricture, mass, raised nodule, ulceration, or a subtle irregularity in the mucosa, including a depression. EAC has been detected in biopsy specimens from regions of Barrett’s esophagus that appear flat during endoscopy. During endoscopy, care should be taken to document the proximal and distal extents of the tumor, and their relation to the gastroesophageal junction, because these have implications for surgical management. For example, if the tumor involves the stomach more distally than the cardia, a gastric pull-up procedure may not be feasible, and colonic interposition might be required. For tumors that involve the entire circumference of the lumen, the normal anatomy of the gastroesophageal junction can be obliterated, making the documentation of its location inaccurate. Occasionally, smaller tumors are encountered that may be amenable to endoscopic therapy. As such, the size, location, and morphology of the tumor, as visualized by endoscopy, should be documented to aid in the planning of such therapy.
Staging
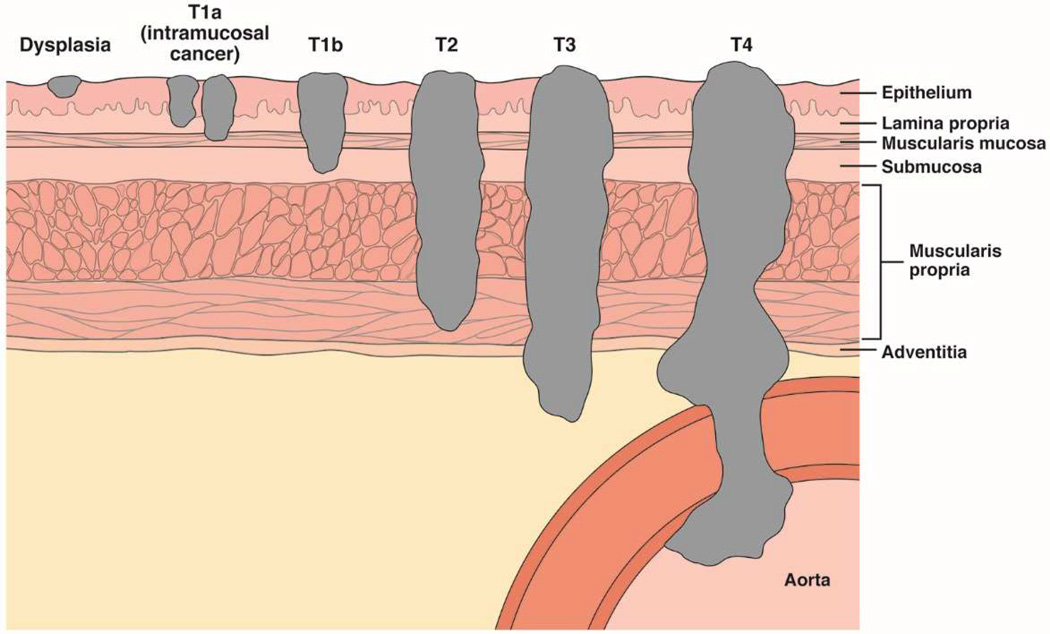
EAC is staged based on the American Joint Committee on Cancer system, last updated in 2010 (Table 1).85 This was the first time staging of EAC was separated from that of squamous cell carcinoma. The esophageal wall has layers of mucosa, submucosa, muscularis propria, and adventitia (Figure 4). The mucosal layer includes the epithelium, lamina propria, and muscularis mucosae, and is separated from the submucosa by a basement membrane. High-grade dysplasia is synonymous with carcinoma in situ (Stage 0), and is confined to the epithelium. If cells with the same appearance have invaded the lamina propria, the lesion is classified as invasive cancer.
Table 1.
Staging of Esophageal and Esophagogastric Junction Adenocarcinoma.
| Primary Tumor (T) | |
| Tis | High-grade dysplasia |
| T1a | Tumor invades lamina propria or muscularis mucosa (intramucosal) |
| T1b | Tumor invades submucosa |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor invades adventitia |
| T4a | Resectable tumor invading pleura, pericardium, or diaphragm |
| T4b | Unresectable tumor invading other adjacent structures, such as aorta, vertebral body, trachea, etc. |
| Regional Lymph Nodes (N) | |
| NX | Regional lymph node(s) cannot be assessed |
| N0 | No regional lymph node metastases |
| N1 | Metastasis in 1–2 regional lymph nodes |
| N2 | Metastasis in 3–6 regional lymph nodes |
| N3 | Metastasis in 7 or more regional lymph nodes |
| Distant Metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| Histologic Grade (G) | |
| GX | Grade cannot be assessed – stage grouping as G1 |
| G1 | Well differentiated |
| G2 | Moderately differentiated |
| G3 | Poorly differentiated |
| G4 | Undifferentiated – stage grouping as G3 squamous |
| Anatomic Stage / Prognostic Groups | ||||
|---|---|---|---|---|
| Stage | T | N | M | G |
| 0 | Tis (HGD) | N0 | M0 | 1,X |
| IA | T1 | N0 | M0 | 1–2,X |
| IB | T1 | N0 | M0 | 3 |
| T2 | N0 | M0 | 1–2,X | |
| IIA | T2 | N0 | M0 | 3 |
| IIB | T3 | N0 | M0 | Any |
| T1-2 | N1 | M0 | Any | |
| IIIA | T1-2 | N2 | M0 | Any |
| T3 | N1 | M0 | Any | |
| T4a | N0 | M0 | Any | |
| IIIB | T3 | N2 | M0 | Any |
| IIIC | T4a | N1-2 | M0 | Any |
| T4b | Any | M0 | Any | |
| Any | N3 | M0 | Any | |
| IV | Any | Any | M1 | Any |
Note: Based on 2010 AJCC TNM
NX: Regional lymph node(s) cannot be assessed; GX: Grade cannot be assessed.
Figure 4. Tumor Depth Staging for EAC.
There are 4 main layers of the esophageal wall: mucosa, submucosa, muscularis propria, and adventitia. The mucosa is further divided into the epithelium, lamina propria, and muscularis mucosae. Dysplasia is confined to the epithelium. Intramucosal tumors (T1a) invade the lamina propria or muscularis mucosae. Tumors that invade the submucosa are classified T1b. T2 tumors invade the muscularis propria, T3 tumors invade the adventitia, and T4 tumors invade adjacent structures.
T1a cancers are confined within the mucosa and are often called intramucosal cancers; they can invade the lamina propria, as deeply as the muscularis mucosae. The intra-observer agreement among pathologists for distinguishing high-grade dysplasia from T1a cancer is poor.16, 17 Therefore, it should not be surprising that the patients high-grade dysplasia vs those with T1a cancers have similar times of survival (Supplemental Figure 1).86 In contrast, patients with tumors that invade even the submucosa (T1b) have considerably worse prognoses, similar to those of patients with T2 tumors (Supplemental Figure 1). T2 tumors invade the muscularis propria, T3 invade the adventitia, and T4 tumors invade adjacent structures (Figure 4). In the 2010 American Joint Committee on Cancer staging system, regional lymph node involvement included periesophageal cervical nodes to the celiac nodes. In the earlier 2002 system, celiac nodes were considered distant metastases. The 2010 system categorizes the number of lymph nodes involved into 0 (N0), 1 or 2 (N1), 3 to 6 (N2), and 7 or more (N3).
A number of modalities are available to stage esophageal cancer. For staging the tumor depth, endoscopic resection distinguishes T1a from those that invade more deeply.87 Cross-sectional imaging techniques such as CT or positron emission tomography (PET) has no practical role in staging depth of early-stage EACs. Although endoscopic ultrasound (EUS) is more accurate than CT or PET at staging tumor depth, none of these accurately stage shallow tumors.88, 89 Furthermore, these techniques can over-stage superficial EAC, which is actually amenable to endoscopic therapy. Therefore, for cases in which EUS analysis indicates T1b disease, endoscopic mucosal resection should still be considered. EUS may be particularly inaccurate if strictures prevent passage of the echoendoscope to the full extent of the tumor. A meta-analysis of studies of regional lymph node staging found EUS to detect T1b disease with the highest level of sensitivity (80%), but slightly less specificity (70%), than PET (57%sensitivity and 85% specificity).90
EUS only rarely identifies distant metastases such as those to the liver or peritoneum. CT with intravenous contrast is the main modality used to identify distant metastases. PET can identify incrementally more metastases than CT alone.90 Increasingly, PET is performed with a hybrid scanner that performs a non-contrast CT in the same setting, to aid in localizing any uptake identified by PET. In practice, once a diagnosis of EAC is made, based on analysis of mucosal biopsies, patients typically undergo PET/CT, or CT with intravenous contrast, to identify distant metastases. PET/CT is the most accurate form of imaging for this purpose, with greater accuracy than either PET or CT alone. If no distant metastases are found, then EUS can be used to stage regional lymph nodes, and to a lesser extent, tumor depth. In cases where the tumor appears to be subtle or small by endoscopy (typically in patients undergoing surveillance for Barrett’s esophagus), endoscopic resection of the lesion is often considered before additional imaging, to determine whether the tumor was invasive and exclude dysplasia confined to the epithelium.
For patients with T1a tumors, the likelihood of metastasis is lower than the false-positive rates of PET/CT or EUS, meaning that most positive results are false.91 Given the extremely low likelihood of lymph node involvement in patients with T1a cancer, the value of PET/CT or EUS is questionable.92–94 Additionally, in patients with more advanced disease, PET/CT is not a good modality for assessing for brain metastases, due to the avid glucose uptake of normal brain.95 Magnetic resonance imaging should be considered for patients with neurologic symptoms, to detect metastases to brain. Locally advanced lesions that extend to the middle third of the esophagus could also involve the posterior membranous trachea. Bronchoscopy should be considered to assess invasion and, in the event of fistula formation, stent the bronchus.96
Treatment
Initial treatment approaches for EAC depend on several factors, including the stage and grade of the tumor, the location of the tumor, the comorbidities and age of the patient, and institutional expertise in providing therapy. Given that systemic therapy for EAC is often not curative, it is imperative to correctly identify patients who may be eligible for curative endoscopic or surgical therapy. Multi-modal therapies are used for patients with late-stage disease, although the value of this approach has not been completely elucidated.
Superficially Invasive Adenocarcinoma (T1a and T1b Disease)
Perhaps no aspect of the treatment of EAC has evolved more in the past 10 y than endoscopic therapy for superficially invasive adenocarcinoma. Although surgical and endoscopic therapies are available for superficial disease, endoscopic therapies have moved to the forefront, due to their high level of efficacy and low morbidity. New endoscopic treatment modalities, and more widespread and aggressive use of endoscopic therapy, have changed outcomes in the small proportion of patients with early-stage EAC.
Endoscopic Therapy
Most endoscopic therapy for EAC begins with endoscopic resection.97 This is performed as a staging and a therapeutic measure, in that complete excision of a mucosal EAC improves a patient’s prognosis. The resection can be performed as an endoscopic mucosal resection, using either band ligation methods or cap-and-snare methods,98 or as an endoscopic submucosal dissection (ESD).99 The chief advantage of ESD is that it allows for complete en bloc resection and assessment of the lateral margins of the lesion.100, 101 However, ESD is technically more challenging, and relatively few US centers have the capability to perform it. The most important piece of prognostic information obtained from endoscopic resection is the depth of invasion; endoscopic mucosal resection and ESD each provide this information and allow for the tumor to be staged. More than 1 endoscopic session may be necessary to achieve complete resection.
Several features define tumors that are amenable to endoscopic therapy.102–105 Moderate- or well-differentiated grade, lack of lymphovascular invasion, lesion size less than 3 cm, and confinement to the mucosa are all features of a good prognosis. Invasion into the submucosa is a marker of T1b disease, and is graded as superficial, moderate, or deep invasion (sm1, sm2, and sm3, respectively). Even superficial (sm1) invasion into the submucosa has been associated with a substantial rate of lymph node involvement, making endoscopic therapy inadequate for patients with any submucosal extension of disease.106–108 However, more recent data indicate that sm1 tumors can be effectively treated by endoscopy.102, 109 Given the inconsistencies in results, such patients require multi-disciplinary treatment teams that consider not only tumor characteristics, but also patient features.
Studies of endoscopic management of superficial EAC at tertiary care centers have reported rates of local control of neoplasia greater than 95%.97 Survival times of these patients may not differ from those of from age- and sex-matched cohorts without neoplasia. These excellent results were achieved by careful selection of patients for the treatment; those with tumor invasion of the submucosa did not receive this conservative therapy, but were instead sent for consultation for surgical therapy.102, 110
After complete endoscopic resection, endoscopic eradication therapy is recommended, to remove any residual Barrett’s esophagus. As many as 30% of patients who undergo endoscopic resection of EAC without adjuvant eradication therapy develop recurrent EAC.97, 111–113 A number of eradication therapies are available, although there is no level 1 evidence for the superiority of one over another after endoscopic resection of superficial EAC.
The most-reported modality is radiofrequency ablation (RFA). Data from prospective cohort studies demonstrate that 80% or more of subjects with T1a EAC can achieve complete eradication of all dysplasia with RFA.114–116 However, in an analysis of a cohort that included patients without cancer at baseline, 33% had recurrence of intestinal metaplasia within 2 y after complete eradication of Barrett’s esophagus.117 Rates of recurrence in patients with T1a tumors may be higher than those of patients who undergo RFA for less-advanced lesions, such as Barrett’s esophagus with no dysplasia (Supplemental Figure 2).118 Additionally, these neoplasias might be of a higher grade in subjects treated endoscopically for EAC than those treated with RFA for BE. For these reasons, aggressive endoscopic surveillance is recommended in patients after they have undergone EMR and RFA for superficial EAC.119
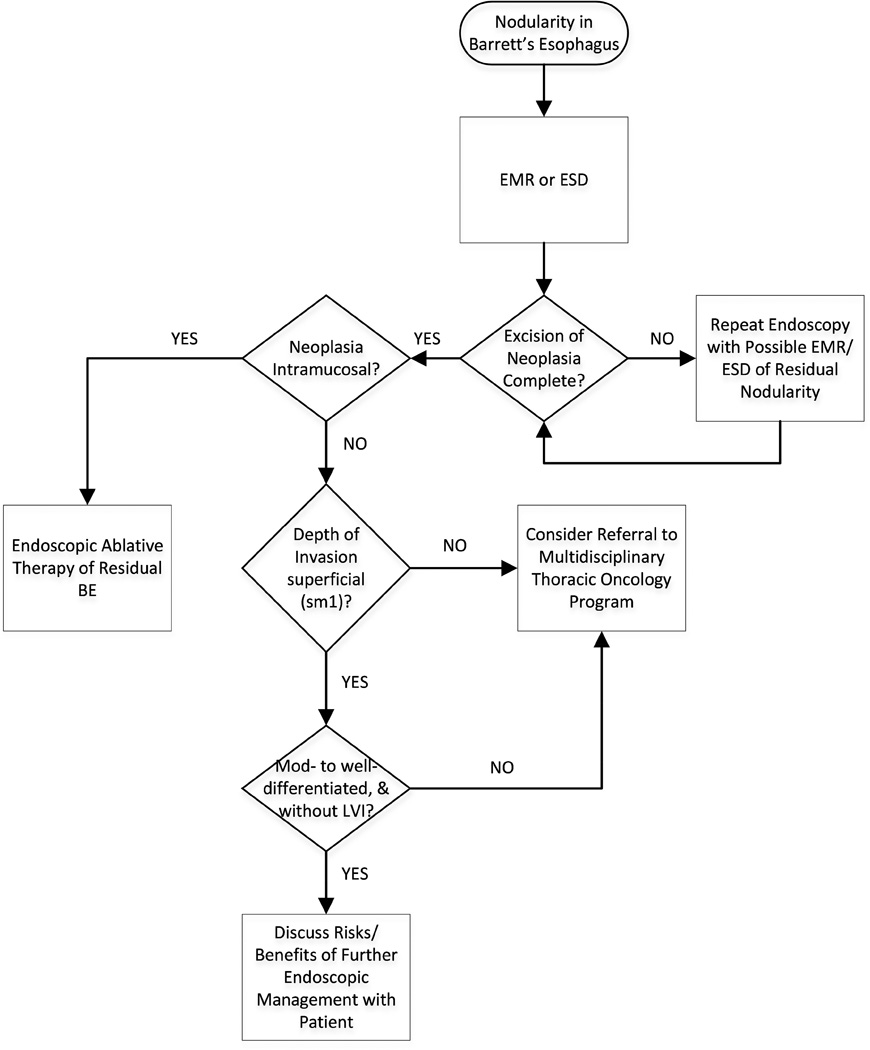
Although evidence-based guidelines are not available, experts recommend surveillance every 3 months for the first year following endoscopic therapy, every 6 months in the second year, and annually thereafter.120 Endoscopic cryotherapy121 and photodynamic therapy122 have also demonstrated efficacy for patients with EAC, although there are fewer data on outcomes. Stepwise radical endoscopic resection of residual BE can be successfully undertaken after EMR of superficial EAC,123, 124 but appears to be associated with an unacceptably high rate of stricture formation, compared to ablative therapy.125 Figure 5 presents a suggested algorithm to manage patients with early-stage EAC.
Figure 5. Endoscopic Management of Early EAC.
Depth of invasion, as assessed by EMR or ESD, is the key to appropriate subsequent therapy. EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; LVI, lymphovascular invasion.
Esophagectomy
Although surgery is less commonly used to treat superficial neoplasia, there are situations in which it is appropriate to consider esophagectomy. For instance, in subjects with poor esophageal transit secondary to stricturing and aperistalsis, preservation of the esophagus with endoscopic therapy can still have poor functional outcome. In these situations, esophagectomy can address the neoplasia as well as the poor esophageal function. In tertiary care centers, esophagectomy for high-grade dysplasia or T1a cancer is associated with less than 3% mortality. However, morbidity, primarily in the form of pulmonary and wound infection, and anastamostic leaks occur in more than 30% of patients undergoing esophagectomy.126, 127
Locally Advanced Adenocarcinoma
Although several high-quality studies have been performed, the optimal approach to locally advanced EAC is unclear. In the US, the most common approach involves neoadjuvant chemotherapy and external beam radiotherapy (CRT). There have been a range of results reported on the benefits of neoadjuvant chemotherapy or CRT before surgery, compared with only surgery. Some studies reported increases in survival and likelihood of pathological R0 resection after combined therapy,128–130 whereas others did not.131, 132 Study findings are difficult to interpret because patients with EAC and those with SCC, as well as patients with different stages of disease have been included in many studies.
Meta-analyses of the value of CRT before resection have found that CRT increases overall survival times and rates of R0 resection, compared to surgery alone, making CRT the most common approach in the US133–135 In most trials, patients were given cisplatin or carboplatin with 5-fluorouracil. Concurrent external beam radiation dosage varied from 35 to 45 Gray. There are data to support the efficacy of an alternative approach, in which only chemotherapy is given before resection; this is commonly used in the UK136, 137 It is unclear how much neoadjuvant CRT improves outcomes of patients with early-invasive EAC (T1b); surgical resection without neoadjuvant therapy can be recommended in this subgroup of patients.
Most patients with locally advanced neoplasia have been treated with surgery, not only with curative intent, but also to relieve dysphagia and improve quality of life for patients who could not be cured. The advent of stenting has decreased the use of surgery for palliation.
Esophagectomy
Esophagectomy is an important component of therapy used to treat patients with locally advanced EAC. Several approaches have been described—each has its merits. Ivor-Lewis esophagectomy involves abdominal and right thoracic incisions. The stomach is mobilized through the abdominal incision, the esophagus removed, and lymph nodes collected through the chest incision; an intra-thoracic esophagogastric anastomosis is then made. This approach has the advantage of providing an excellent yield of lymph nodes for histologic assessment, and good exposure to the lesion, especially for cancers in the distal third of the esophagus.
Transhiatal esophagectomy involves left neck and abdominal incisions.138 The esophagus is mobilized by blunt dissection through the hiatus from the abdominal incision. An anastomosis is made via the neck incision. This approach generally avoids a thoracotomy, but provides less exposure to the tumor site and potentially fewer lymph nodes for examination. Tri-incisional esophagectomy uses a laparotomy, a thoracotomy, and a neck incision, allowing excellent lymphadenectomy of abdominal and thoracic lymph nodes. Neck anastomosis is generally performed on the left side to decrease the likelihood of recurrent laryngeal nerve injury.
Minimally invasive esophagectomy is the most recently described approach, and relies on thoracoscopic access to the chest and laparoscopic access to the abdomen to achieve excision of the esophagus.139 It is associated with the quickest recuperation, and because of the expanded abilities of thoracoscopy, can, in expert hands, provide adequate lymph node yield to assess for metastatic disease.140, 141
Which of these approaches is best is hotly contested issue in the surgical literature.142–144 Single-center studies from groups with expertise in each of the 3 approaches have provided excellent results.139, 145, 146 There is no level-1 evidence that any approach increases survival times, compared with the others. Randomized studies have associated trans-hiatal approaches with less morbidity than transthoracic approaches, but percentages of patients with long-term survival did not differ significantly.147 In general, local expertise is probably a better predictor of outcome than approach.
One recurrent theme in the surgical literature is that center volume of esophagectomy is an important deciding factor of outcome148–150. Thirty-day mortality is associated with volume of esophagectomies performed at the center. In addition to operator technique, aspects of care such as intensive care unit management and early detection of complications likely play a role in these differential outcomes. Despite the availability of neoadjuvant therapy for patients with locally advanced disease, mortality is high. The median survival times reported for patients who received CRT in these trials ranged from 16 to 49 months; 3 y rates of overall survival ranged from 32% to 59%.
These disappointing results led to the development of newer chemotherapeutic agents, designed to target cancer cells. Molecular profiling of individual cancers may allow the use of agents tailored to the susceptibility of the tumor. For example, gastric and gastro-esophageal junction tumors, which overexpress human epidermal growth factor receptor 2, appear to respond to trastuzumab, when combined with cisplatin and a fluoropyrimidine.151 Tumor characterization may also allow clinicians to discern the mechanisms by which the tumor protects itself from the effects of CRT. For instance, because standard CRT induces formation of DNA adducts, therapeutics might be developed to alter production of proteins that repair the adducts.152 Studies are underway to investigate both of these approaches. Ramucirumab, an antibody against vascular endothelial growth factor receptor-2, showed some efficacy as a salvage therapy for gastroesophageal junction and gastric cancers in early-stage trials.153 However, its effect was modest, increasing median survival time by 1.4 months (hazard ratio, 0.77; 95% CI, 0.603−0.998).
Patients with Unresectable or Metastatic Disease, and Patients who Cannot Undergo Surgery
Chemoradiation or chemotherapy is a definitive therapy for patients with advanced disease that cannot be cured by surgery. They are generally treated by chemotherapy or CRT, and their 5 y rate of survival is less than 15%. For these patients, multi-modal therapy with chemoradiation appears superior to radiation alone, increasing median survival by approximately 4 months.154, 155
Endoscopic therapy also has an important role in palliating locally advanced and distant metastatic disease. Dilation using balloons or bougies may provide transient relief of dysphagia, but is unlikely to have long-lasting effect, given the aggressive nature of the lesion. Palliative tumor debulking of more advanced lesions, via resection, thermal therapies, cryotherapy, and/or photodynamic therapy, have all been described, and can improve quality of life, and in some instances, forestall stent placement.121, 156–159 A program of intermittent palliative endoscopic treatments at 2–3 month intervals may attain local control of the disease, allowing adequate oral intake and decreased dysphagia.121
Placement of endoscopic stents has become increasingly less difficult with improved technology. Covered self-expandable metal stents are often used for this indication, and their use is associated with marked improvement in dysphagia symptoms.160, 161 However, this therapy has not been shown to increase survival times, and patients and clinicians must be familiar with the potential side effects of stent placement. One common side effect is stent migration, which occurs in up to 15% of patients and can require surgery for removal.160 Chest pain is also common, and can be difficult to distinguish from pain secondary to the EAC.162, 163 The most catastrophic complication is erosion into a major blood vessel, which is often a terminal event.164, 165 Because of excellent initial rates of response of tumors to CRT, stenting at the time of tumor discovery is generally not recommended, given the additional risk it entails, the chance of migration after tumor debulking from CRT, and the impediment it presents for possible future endoscopic cytoreductive therapy.
Conclusion
EAC is an increasingly common and highly lethal tumor that is associated with Barrett’s esophagus—its precursor lesion. Barrett’s esophagus and EAC are most common in elderly, overweight Caucasian men with symptoms of GERD. Other risk factors include tobacco, lack of H pylori infection, and certain medications; there is an inverse association between EAC and use of NSAIDs. EAC most commonly presents with dysphagia, but patients may also present with weight loss, anemia, or manifestations of distant metastases. Only a small minority of these cancers in most series present as part of an endoscopic surveillance program for BE.
Diagnostic analysis generally begins with an upper endoscopy. The lesion is usually obvious during white-light endoscopy, and biopsies of the lesion confirm the presence of EAC. For tumors that are not bulky, EMR is required to differentiate between T1a and more deeply invasive EAC. PET/CTcan identify distant metastases. In those cases where the disease is confined to the mediastinum, EUS, potentially with sampling of any pathologic-appearing lymph nodes, can help define depth of invasion and nodal status. In some cases, bronchoscopy and/or brain magnetic resonance imaging are necessary to rule out tumor involvement of the trachea or brain.
Multiple disease- and patient-specific characteristics must be considered in selecting the optimal treatment for patients with EAC. The most important of these is the stage of the tumor at diagnosis. T1a tumors can be effectively treated with endoscopic therapy, most commonly by endoscopic resection followed by ablative therapy. Invasion into the submucosa, especially the deep submucosa, is associated with an increased rate of lymph node involvement, making endoscopic therapy a suboptimal approach in the good surgical candidate. For patients with stage T1b tumors, esophagectomy may be employed as a sole therapeutic modality. For patients with locally advanced disease without distant metastases, the most common treatment in the US is neoadjuvant CRT, followed by esophagectomy in patients who are candidates for surgery. For those with distant metastatic disease, definitive CRT is often used. In patients with local advanced disease, as well as those with distant metastases, endoscopic therapy can offer significant palliation of dysphagia. Because of the vital role of endoscopic therapy in the diagnosis and treatment of patients with EAC, endoscopists must be included in the cancer care team and should be engaged in multi-modal planning conferences.
Despite remarkable advances in the care of EAC patients, overall mortality remains high, with 5 y rates of survival less than 20%. This is because most patients present with late-stage disease, and are not eligible for the highly effective, usually curative, endoscopic therapies that have evolved over the last 15 y. Although advances in radiotherapy and surgical technique have reduced the toxicity of these therapies, the likelihood of R0 resection, based on pathology analysis, and long-term survival after these non-endoscopic therapies is unacceptably low. This points to a need for improved screening for this condition, to detect disease at earlier, more curable stages, as well as improved prevention with endoscopic, behavioral, and medical approaches. Also, molecular characterization of these tumors may allow for less toxic, more tumor-specific, therapies.
Supplementary Material
A: Current [2002] classification. Number of patients in each classification and number traced at 5 years, respectively, were as follows: Tis (44 and 21), T1 (98 and 26), T2 (31 and 8), T3 (57 and 12), and T4 (4 and 0).
B: Proposed subclassification of T1 into T1a (intramucosal, n = 66, 18 traced at 5 years) and T1b (submucosal, n = 32, 8 traced at 5 years). Shown for reference are Tis (high grade dysplasia) and T2. Vertical bars represent 68% confidence limits.
(Reproduced with permission from: Rice TW, Blackstone EH, Rybicki LA, et al. Refining esophageal cancer staging. J Thorac Cardiovasc Surg 2003;125: 1103–13)
Durability of complete eradication for intramucosal adenocarcinoma compared to Barrett’s esophagus of varying degrees of dysplasia. Panel A is the overall recurrence rate of the cohort. Panel B is the recurrence rate broken out by baseline histology. IMC (T1a) has a higher recurrence rate than non-dysplastic Barrett’s esophagus. (LGD, low-grade dysplasia; HGD, high-grade dysplasia; IMC, intramucosal adenocarcinoma, aka T1a). (Reproduced with permission from: Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and predictors of successful radiofrequency ablation for Barrett’s esophagus. Clin Gastr Hep. 2014;12:1840–1847.)
Acknowledgments
Conflict of Interest
JHR has served as a consultant to ORC, International and Analogy Growth Partners.
NJS receives research funding from Covidien Medical, CSA Medical, Takeda Pharmaceuticals, CDx Medical, and RedPath.
Financial support
Dr. Shaheen is supported by the National Institutes of Health (DK K24100548).
Dr. Rubenstein is supported by the Department of Veterans Affairs (CSRD I01-CX000899).
Abbreviations
- BMI
body mass index
- CI
confidence interval
- CT
computed tomography
- EAC
esophageal adenocarcinoma
- EUS
endoscopic ultrasound
- GERD
gastroesophageal reflux disease
- IGF
insulin-like growth factor
- OR
odds ratio
- PET
positron emission tomography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions: Both JHR and NJS drafted and edited the manuscript
REFERENCES
- 1.Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2013 Sub (2000–2011) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2012 Counties.: National Cancer Institute, DCCPS, Surveillance Research Program. Cancer Statistics Branch. Released April 2014 (updated 5/7/2014), based on the November 2013 submission.
- 2.Abrams JA, Sharaiha RZ, Gonsalves L, et al. Dating the rise of esophageal adenocarcinoma: analysis of Connecticut Tumor Registry data, 1940–2007. Cancer Epidemiology, Biomarkers & Prevention. 2011;20:183–186. doi: 10.1158/1055-9965.EPI-10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trivers KF, Sabatino SA, Stewart SL, et al. Trends in esophageal cancer incidence by histology, United States, 1998–2003. International Journal of Cancer. 2008;123:1422–1428. doi: 10.1002/ijc.23691. [DOI] [PubMed] [Google Scholar]
- 4.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 5.National Cancer Institute. Surveillance, Epidemiology and End Results (SEER) Program. [accessed January 9, 2015];Esophagus Table 8.22. www.seer.cancer.gov/csr/1975_2010/browse_csr.php.
- 6.National Cancer Institute. Surveillance, Epidemiology and End Results (SEER) Program. [accessed January 9, 2015];SEER Stat Fact Sheets: Esophageal Cancer. http://seer.cancer.gov/statfacts/html/esoph.html.
- 7.Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2014 doi: 10.1136/gutjnl-2014-308124. [DOI] [PubMed] [Google Scholar]
- 8.Bosetti C, Levi F, Ferlay J, et al. Trends in oesophageal cancer incidence and mortality in Europe. International Journal of Cancer. 2008;122:1118–1129. doi: 10.1002/ijc.23232. [DOI] [PubMed] [Google Scholar]
- 9.Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119:1149–1158. doi: 10.1002/cncr.27834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiology, Biomarkers & Prevention. 2010;19:1468–1470. doi: 10.1158/1055-9965.EPI-10-0012. [DOI] [PubMed] [Google Scholar]
- 11.Chow WH, Finkle WD, McLaughlin JK, et al. The relation of gastroesophageal reflux disease and its treatment to adenocarcinomas of the esophagus and gastric cardia. JAMA. 1995;274:474–477. [PubMed] [Google Scholar]
- 12.Lagergren J, Bergstrom R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. New England Journal of Medicine. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 13.Souza RF, Krishnan K, Spechler SJ. Acid, bile, and CDX: the ABCs of making Barrett's metaplasia. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2008;295:G211–G218. doi: 10.1152/ajpgi.90250.2008. [DOI] [PubMed] [Google Scholar]
- 14.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. New England Journal of Medicine. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 15.Desai TK, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett’s oesophagus: a meta-analysis. Gut. 2012;61:970–976. doi: 10.1136/gutjnl-2011-300730. [DOI] [PubMed] [Google Scholar]
- 16.Wani S, Puli SR, Shaheen NJ, et al. Esophageal adenocarcinoma in Barrett's esophagus after endoscopic ablative therapy: a meta-analysis and systematic review. American Journal of Gastroenterology. 2009;104:502–513. doi: 10.1038/ajg.2008.31. [DOI] [PubMed] [Google Scholar]
- 17.Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Alimentary Pharmacology & Therapeutics. 2010;32:1222–1227. doi: 10.1111/j.1365-2036.2010.04471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nason KS, Wichienkuer PP, Awais O, et al. Gastroesophageal reflux disease symptom severity, proton pump inhibitor use, and esophageal carcinogenesis. Archives of Surgery. 2011;146:851–858. doi: 10.1001/archsurg.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubenstein JH, Scheiman JM, Sadeghi S, et al. Esophageal adenocarcinoma incidence in individuals with gastroesophageal reflux: synthesis and estimates from population studies. American Journal of Gastroenterology. 2011;106:254–260. doi: 10.1038/ajg.2010.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubenstein JH. Risk factors for Barrett's esophagus. Current Opinion in Gastroenterology. 2014;30:408–414. doi: 10.1097/MOG.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 21.Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. Journal of the National Cancer Institute. 2010;102:1344–1353. doi: 10.1093/jnci/djq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedman ND, Murray LJ, Kamangar F, et al. Alcohol intake and risk of oesophageal adenocarcinoma: a pooled analysis from the BEACON Consortium. Gut. 2011;60:1029–1037. doi: 10.1136/gut.2010.233866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoyo C, Cook MB, Kamangar F, et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. International Journal of Epidemiology. 2012;41:1706–1718. doi: 10.1093/ije/dys176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh S, Sharma AN, Murad MH, et al. Central Adiposity Is Associated With Increased Risk of Esophageal Inflammation, Metaplasia, and Adenocarcinoma: A Systematic Review and Meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11:1399–1412. e7. doi: 10.1016/j.cgh.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derakhshan MH, Robertson EV, Fletcher J, et al. Mechanism of association between BMI and dysfunction of the gastro-oesophageal barrier in patients with normal endoscopy. Gut. 2012;61:337–343. doi: 10.1136/gutjnl-2011-300633. [DOI] [PubMed] [Google Scholar]
- 26.Pandolfino JE, El-Serag HB, Zhang Q, et al. Obesity: a challenge to esophagogastric junction integrity. Gastroenterology. 2006;130:639–649. doi: 10.1053/j.gastro.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Garcia JM, Splenser AE, Kramer J, et al. Circulating Inflammatory Cytokines and Adipokines Are Associated With Barrett's Esophagus: A Case-Control Study. Clinical gastroenterology and hepatology. 2014;12:229–238. doi: 10.1016/j.cgh.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drahos J, Ricker W, Parsons R, et al. Metabolic Syndrome Increases Risk of Barrett Esophagus in the Absence of Gastroesophageal Reflux: An Analysis of SEER-Medicare Data. J Clin Gastroenterol. 2014 doi: 10.1097/MCG.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindkvist B, Johansen D, Stocks T, et al. Metabolic risk factors for esophageal squamous cell carcinoma and adenocarcinoma: a prospective study of 580,000 subjects within the Me-Can project. BMC Cancer. 2014;14:103. doi: 10.1186/1471-2407-14-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubenstein JH, Morgenstern H, McConell D, et al. Associations of Diabetes Mellitus, Insulin, Leptin, and Ghrelin with Gastroesophageal Reflux and Barrett’s Esophagus. Gastroenterology. 2013;145:1237–1244. doi: 10.1053/j.gastro.2013.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greer KB, Thompson CL, Brenner L, et al. Association of insulin and insulin-like growth factors with Barrett’s oesophagus. Gut. 2012;61:665–672. doi: 10.1136/gutjnl-2011-300641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neale RE, Doecke JD, Pandeya N, et al. Does type 2 diabetes influence the risk of oesophageal adenocarcinoma? British Journal of Cancer. 2009;100:795–798. doi: 10.1038/sj.bjc.6604908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubenstein JH, Davis J, Marrero JA, et al. Relationship between diabetes mellitus and adenocarcinoma of the oesophagus and gastric cardia. Alimentary Pharmacology & Therapeutics. 2005;22:267–271. doi: 10.1111/j.1365-2036.2005.02544.x. [DOI] [PubMed] [Google Scholar]
- 34.Duggan C, Onstad L, Hardikar S, et al. Association Between Markers of Obesity and Progression From Barrett's Esophagus to Esophageal Adenocarcinoma. Clinical Gastroenterology and Hepatology. 2013;11:934–943. doi: 10.1016/j.cgh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iyer PG, Borah BJ, Heien HC, et al. Association of Barrett's esophagus with type II Diabetes Mellitus: results from a large population-based case-control study. Clinical Gastroenterology & Hepatology. 2013;11:1108–1114. e5. doi: 10.1016/j.cgh.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McElholm AR, McKnight A-J, Patterson CC, et al. A population-based study of IGF axis polymorphisms and the esophageal inflammation, metaplasia, adenocarcinoma sequence. Gastroenterology. 2010;139:204–212. e3. doi: 10.1053/j.gastro.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald K, Porter GA, Guernsey DL, et al. A polymorphic variant of the insulin-like growth factor type I receptor gene modifies risk of obesity for esophageal adenocarcinoma. Cancer Epidemiology. 2009;33:37–40. doi: 10.1016/j.canep.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Hoyo C, Schildkraut JM, Murphy SK, et al. IGF2R polymorphisms and risk of esophageal and gastric adenocarcinomas. International Journal of Cancer. 2009;125:2673–2678. doi: 10.1002/ijc.24623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siahpush SH, Vaughan TL, Lampe JN, et al. Longitudinal study of insulin-like growth factor, insulin-like growth factor binding protein-3, and their polymorphisms: risk of neoplastic progression in Barrett's esophagus. Cancer Epidemiology, Biomarkers & Prevention. 2007;16:2387–2395. doi: 10.1158/1055-9965.EPI-06-0986. [DOI] [PubMed] [Google Scholar]
- 40.Thompson OM, Beresford SAA, Kirk EA, et al. Serum leptin and adiponectin levels and risk of Barrett's esophagus and intestinal metaplasia of the gastroesophageal junction. Obesity. 2010;18:2204–2211. doi: 10.1038/oby.2009.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kendall BJ, Macdonald GA, Hayward NK, et al. Leptin and the risk of Barrett's oesophagus. Gut. 2008;57:448–454. doi: 10.1136/gut.2007.131243. [DOI] [PubMed] [Google Scholar]
- 42.Rubenstein JH, Kao JY, Madanick RD, et al. Association of adiponectin multimers with Barrett’s esophagus. Gut. 2009;58:1583–1589. doi: 10.1136/gut.2008.171553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubenstein JH, Dahlkemper A, Kao JY, et al. A pilot study of the association of low plasma adiponectin and Barrett's esophagus. American Journal of Gastroenterology. 2008;103:1358–1364. doi: 10.1111/j.1572-0241.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- 44.Whiteman DC, Sadeghi S, Pandeya N, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57:173–180. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 45.Thrift AP, Risch HA, Onstad L, et al. Risk of esophageal adenocarcinoma decreases with height, based on consortium analysis and confirmed by mendelian randomization. Clin Gastroenterol Hepatol. 2014;12:1667–1676. e1. doi: 10.1016/j.cgh.2014.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Behrens G, Jochem C, Keimling M, et al. The association between physical activity and gastroesophageal cancer: systematic review and meta-analysis. European Journal of Epidemiology. 2014;29:151–170. doi: 10.1007/s10654-014-9895-2. [DOI] [PubMed] [Google Scholar]
- 47.Collings KL, Pierce Pratt F, Rodriguez-Stanley S, et al. Esophageal reflux in conditioned runners, cyclists, and weightlifters. Medicine & Science in Sports & Exercise. 2003;35:730–735. doi: 10.1249/01.MSS.0000064937.99001.56. [DOI] [PubMed] [Google Scholar]
- 48.Salehi M, Moradi-Lakeh M, Salehi MH, et al. Meat, fish, and esophageal cancer risk: a systematic review and dose-response meta-analysis. Nutrition Reviews. 2013;71:257–267. doi: 10.1111/nure.12028. [DOI] [PubMed] [Google Scholar]
- 49.Zhu HC, Yang X, Xu LP, et al. Meat consumption is associated with esophageal cancer risk in a meat- and cancer-histological-type dependent manner. Digestive Diseases & Sciences. 2014;59:664–673. doi: 10.1007/s10620-013-2928-y. [DOI] [PubMed] [Google Scholar]
- 50.Coleman HG, Murray LJ, Hicks B, et al. Dietary fiber and the risk of precancerous lesions and cancer of the esophagus: a systematic review and meta-analysis. Nutrition Reviews. 2013;71:474–482. doi: 10.1111/nure.12032. [DOI] [PubMed] [Google Scholar]
- 51.Kubo A, Corley DA. Meta-analysis of antioxidant intake and the risk of esophageal and gastric cardia adenocarcinoma. American Journal of Gastroenterology. 2007;102:2323–2330. doi: 10.1111/j.1572-0241.2007.01374.x. quiz 2331. [DOI] [PubMed] [Google Scholar]
- 52.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. New England Journal of Medicine. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 53.Klein EA, Thompson IM, Jr, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asano T, McLeod RS. Dietary fibre for the prevention of colorectal adenomas and carcinomas. Cochrane Database Syst Rev. 2002:CD003430. doi: 10.1002/14651858.CD003430. [DOI] [PubMed] [Google Scholar]
- 55.Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res (Phila) 2008;1:329–338. doi: 10.1158/1940-6207.CAPR-08-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graham DY, Opekun AR, Osato MS, et al. Challenge model for Helicobacter pylori infection in human volunteers. Gut. 2004;53:1235–1243. doi: 10.1136/gut.2003.037499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.el-Omar E, Penman I, Ardill J, et al. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology. 1995;109:681–691. doi: 10.1016/0016-5085(95)90374-7. [DOI] [PubMed] [Google Scholar]
- 58.Naylor GM, Gotoda T, Dixon M, et al. Why does Japan have a high incidence of gastric cancer? Comparison of gastritis between UK and Japanese patients. Gut. 2006;55:1545–1552. doi: 10.1136/gut.2005.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raghunath A, Hungin APS, Wooff D, et al. Prevalence of Helicobacter pylori in patients with gastro-oesophageal reflux disease: systematic review. BMJ. 2003;326:737. doi: 10.1136/bmj.326.7392.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luther J, Owyang SY, Takeuchi T, et al. Helicobacter pylori DNA decreases pro-inflammatory cytokine production by dendritic cells and attenuates dextran sodium sulphate-induced colitis. Gut. 2011;60:1479–1486. doi: 10.1136/gut.2010.220087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siersema P, Moons L, Kusters J, et al. A pro-inflammatory IL-10/IL-12 gene profile is associated with an increased risk for developing Barrett’s esophagus. Gastroenterology. 2006;130:A76. [Google Scholar]
- 62.Moons LMG, Kusters JG, van Delft JHM, et al. A pro-inflammatory genotype predisposes to Barrett's esophagus. Carcinogenesis. 2008;29:926–931. doi: 10.1093/carcin/bgm241. [DOI] [PubMed] [Google Scholar]
- 63.Pei Z, Yang L, Peek RM, et al. Bacterial biota in reflux esophagitis and Barrett's esophagus. World Journal of Gastroenterology. 2005;11:7277–7283. doi: 10.3748/wjg.v11.i46.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akhiani AA, Pappo J, Kabok Z, et al. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. Journal of Immunology. 2002;169:6977–6984. doi: 10.4049/jimmunol.169.12.6977. [DOI] [PubMed] [Google Scholar]
- 65.Cook MB, Wood SN, Cash BD, et al. Association Between Circulating Levels of Sex Steroid Hormones and Barrett's Esophagus in Men: A Case-Control Analysis. Clin Gastroenterol Hepatol. 2014 doi: 10.1016/j.cgh.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cronin-Fenton DP, Murray LJ, Whiteman DC, et al. Reproductive and sex hormonal factors and oesophageal and gastric junction adenocarcinoma: a pooled analysis. European Journal of Cancer. 2010;46:2067–2076. doi: 10.1016/j.ejca.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kubo A, Cook MB, Shaheen NJ, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett's oesophagus: a pooled analysis from the international BEACON consortium. Gut. 2013;62:1684–1691. doi: 10.1136/gutjnl-2012-303753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ali R, Barnes I, Cairns BJ, et al. Incidence of gastrointestinal cancers by ethnic group in England, 2001–2007. Gut. 2012 doi: 10.1136/gutjnl-2012-303000. [DOI] [PubMed] [Google Scholar]
- 69.Kubo A, Corley DA. Marked multi-ethnic variation of esophageal and gastric cardia carcinomas within the United States. American Journal of Gastroenterology. 2004;99:582–588. doi: 10.1111/j.1572-0241.2004.04131.x. [DOI] [PubMed] [Google Scholar]
- 70.Everhart JE, Kruszon-Moran D, Perez-Perez GI, et al. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis. 2000;181:1359–1363. doi: 10.1086/315384. [DOI] [PubMed] [Google Scholar]
- 71.El-Serag HB, Petersen NJ, Carter J, et al. Gastroesophageal reflux among different racial groups in the United States. Gastroenterology. 2004;126:1692–1699. doi: 10.1053/j.gastro.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 72.Levine DM, Ek WE, Zhang R, et al. A genome-wide association study identifies new susceptibility loci for esophageal adenocarcinoma and Barrett's esophagus. Nat Genet. 2013;45:1487–1493. doi: 10.1038/ng.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.*Palles C, *Chegwidden L, Li X, et al. Polymorphisms Near TBX5 and GDF7 Are Associated With Increased Risk for Barrett’s Esophagus. Gastroenterology. 2015;148:367–378. doi: 10.1053/j.gastro.2014.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Su Z, Gay L, Strange A, et al. Common variants at the MHC locus and at chromosome 16q24.1 predispose to Barrett's esophagus. Nat Genet. 2012;44:1131–1136. doi: 10.1038/ng.2408. Epub 2012 Sep 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh S, Garg SK, Singh PP, et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett's oesophagus: a systematic review and meta-analysis. Gut. 2014;63:1229–1237. doi: 10.1136/gutjnl-2013-305997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Corley DA, Levin TR, Habel LA, et al. Barrett's esophagus and medications that relax the lower esophageal sphincter. American Journal of Gastroenterology. 2006;101:937–944. doi: 10.1111/j.1572-0241.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- 77.Ladanchuk TC, Johnston BT, Murray LJ, et al. Risk of Barrett's oesophagus, oesophageal adenocarcinoma and reflux oesophagitis and the use of nitrates and asthma medications. Scandinavian Journal of Gastroenterology. 2010;45:1397–1403. doi: 10.3109/00365521.2010.503968. [DOI] [PubMed] [Google Scholar]
- 78.Vaughan TL, Farrow DC, Hansten PD, et al. Risk of esophageal and gastric adenocarcinomas in relation to use of calcium channel blockers, asthma drugs, and other medications that promote gastroesophageal reflux. Cancer Epidemiology, Biomarkers & Prevention. 1998;7:749–756. [PubMed] [Google Scholar]
- 79.Ye W, Chow WH, Lagergren J, et al. Risk of adenocarcinomas of the oesophagus and gastric cardia in patients hospitalized for asthma. British Journal of Cancer. 2001;85:1317–1321. doi: 10.1054/bjoc.2001.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology. 2012;142:442–452. e5. doi: 10.1053/j.gastro.2011.11.019. quiz e22-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh S, Singh AG, Singh PP, et al. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett's esophagus: a systematic review and meta-analysis. Clinical Gastroenterology & Hepatology. 2013;11:620–629. doi: 10.1016/j.cgh.2012.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cooper GS, Kou TD, Chak A. Receipt of Previous Diagnoses and Endoscopy and Outcome From Esophageal Adenocarcinoma: A Population-Based Study With Temporal Trends. Am J Gastroenterol. 2009;104:1356. doi: 10.1038/ajg.2009.159. [DOI] [PubMed] [Google Scholar]
- 83.Dulai GS, Guha S, Kahn KL, et al. Preoperative prevalence of Barrett's esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology. 2002;122:26–33. doi: 10.1053/gast.2002.30297. [DOI] [PubMed] [Google Scholar]
- 84.Verbeek RE, Leenders M, Ten Kate FJ, et al. Surveillance of Barrett's esophagus and mortality from esophageal adenocarcinoma: a population-based cohort study. Am J Gastroenterol. 2014;109:1215–1222. doi: 10.1038/ajg.2014.156. [DOI] [PubMed] [Google Scholar]
- 85.Edge SB American Joint Committee on C. AJCC cancer staging handbook from the AJCC cancer staging manual. New York: Springer; 2010. American Cancer S. [Google Scholar]
- 86.Rice TW, Blackstone EH, Rybicki LA, et al. Refining esophageal cancer staging. Journal of Thoracic & Cardiovascular Surgery. 2003;125:1103–1113. doi: 10.1067/mtc.2003.170. [DOI] [PubMed] [Google Scholar]
- 87.Wani S, Mathur SC, Curvers WL, et al. Greater interobserver agreement by endoscopic mucosal resection than biopsy samples in Barrett's dysplasia. Clinical Gastroenterology & Hepatology. 2010;8:783–788. doi: 10.1016/j.cgh.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 88.Puli SR, Reddy JB, Bechtold ML, et al. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World Journal of Gastroenterology. 2008;14:1479–1490. doi: 10.3748/wjg.14.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Young PE, Gentry AB, Acosta RD, et al. Endoscopic ultrasound does not accurately stage early adenocarcinoma or high-grade dysplasia of the esophagus. Clin Gastroenterol Hepatol. 2010;8:1037–1041. doi: 10.1016/j.cgh.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 90.van Vliet EP, Heijenbrok-Kal MH, Hunink MG, et al. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer. 2008;98:547–557. doi: 10.1038/sj.bjc.6604200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun G, Tian J, Gorospe EC, et al. Utility of baseline positron emission tomography with computed tomography for predicting endoscopic resectability and survival outcomes in patients with early esophageal adenocarcinoma. Journal of gastroenterology and hepatology. 2013;28:975–981. doi: 10.1111/jgh.12148. [DOI] [PubMed] [Google Scholar]
- 92.Pech O, Bollschweiler E, Manner H, et al. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett's esophagus at two high-volume centers. Ann. Surg. 2011;254:67–72. doi: 10.1097/SLA.0b013e31821d4bf6. [DOI] [PubMed] [Google Scholar]
- 93.Sepesi B, Watson TJ, Zhou D, et al. Are endoscopic therapies appropriate for superficial submucosal esophageal adenocarcinoma? An analysis of esophagectomy specimens. J. Am. Coll. Surg. 2010;210:418–427. doi: 10.1016/j.jamcollsurg.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 94.Stein HJ, Feith M, Bruecher BL, et al. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005;242:566–573. doi: 10.1097/01.sla.0000184211.75970.85. discussion 573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Posther KE, McCall LM, Harpole DH, Jr, et al. Yield of brain 18F-FDG PET in evaluating patients with potentially operable non-small cell lung cancer. J Nucl Med. 2006;47:1607–1611. [PubMed] [Google Scholar]
- 96.McGrath EE, Warriner D, Anderson P. The insertion of self expanding metal stents with flexible bronchoscopy under sedation for malignant tracheobronchial stenosis: a single-center retrospective analysis. Arch Bronconeumol. 2012;48:43–48. doi: 10.1016/j.arbres.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 97.Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology. 2014;146:652–660. e1. doi: 10.1053/j.gastro.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 98.Pouw RE, van Vilsteren FG, Peters FP, et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett's neoplasia. Gastrointestinal Endoscopy. 2011;74:35–43. doi: 10.1016/j.gie.2011.03.1243. [DOI] [PubMed] [Google Scholar]
- 99.Sun F, Yuan P, Chen T, et al. Efficacy and complication of endoscopic submucosal dissection for superficial esophageal carcinoma: a systematic review and meta-analysis. J Cardiothorac Surg. 2014;9:78. doi: 10.1186/1749-8090-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901) Gastrointest Endosc. 2013;78:704–710. doi: 10.1016/j.gie.2013.04.182. [DOI] [PubMed] [Google Scholar]
- 101.Neuhaus H, Terheggen G, Rutz EM, et al. Endoscopic submucosal dissection plus radiofrequency ablation of neoplastic Barrett's esophagus. Endoscopy. 2012;44:1105–1113. doi: 10.1055/s-0032-1310155. [DOI] [PubMed] [Google Scholar]
- 102.Manner H, Pech O, Heldmann Y, et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11:630–635. doi: 10.1016/j.cgh.2012.12.040. quiz e45. [DOI] [PubMed] [Google Scholar]
- 103.Dunbar KB, Spechler SJ. The risk of lymph-node metastases in patients with high-grade dysplasia or intramucosal carcinoma in Barrett's esophagus: a systematic review. The American Journal of Gastroenterology. 2012;107:850–862. doi: 10.1038/ajg.2012.78. quiz 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ancona E, Rampado S, Cassaro M, et al. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol. 2008;15:3278–3288. doi: 10.1245/s10434-008-0065-1. [DOI] [PubMed] [Google Scholar]
- 105.Buskens CJ, Westerterp M, Lagarde SM, et al. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc. 2004;60:703–710. doi: 10.1016/s0016-5107(04)02017-6. [DOI] [PubMed] [Google Scholar]
- 106.Badreddine RJ, Prasad GA, Lewis JT, et al. Depth of submucosal invasion does not predict lymph node metastasis and survival of patients with esophageal carcinoma. Clin Gastroenterol Hepatol. 2010;8:248–253. doi: 10.1016/j.cgh.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grotenhuis BA, van Heijl M, Wijnhoven BP, et al. Lymphatic micrometastases in patients with early esophageal adenocarcinoma. Journal of Surgical Oncology. 2010;102:863–867. doi: 10.1002/jso.21719. [DOI] [PubMed] [Google Scholar]
- 108.Westerterp M, Koppert LB, Buskens CJ, et al. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch. 2005;446:497–504. doi: 10.1007/s00428-005-1243-1. [DOI] [PubMed] [Google Scholar]
- 109.Manner H, May A, Pech O, et al. Early Barrett's carcinoma with"low-risk" submucosal invasion: long-term results of endoscopic resection with a curative intent. American Journal of Gastroenterology. 2008;103:2589–2597. doi: 10.1111/j.1572-0241.2008.02083.x. [DOI] [PubMed] [Google Scholar]
- 110.Huntington JT, Walker JP, Meara MP, et al. Endoscopic mucosal resection for staging and treatment of early esophageal carcinoma: a single institution experience. Surg Endosc. 2014 doi: 10.1007/s00464-014-3962-3. [DOI] [PubMed] [Google Scholar]
- 111.Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett's esophagus. Gastroenterology. 2000;118:670–677. doi: 10.1016/s0016-5085(00)70136-3. [DOI] [PubMed] [Google Scholar]
- 112.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut. 2008;57:1200–1206. doi: 10.1136/gut.2007.142539. [DOI] [PubMed] [Google Scholar]
- 113.May A, Gossner L, Pech O, et al. Local endoscopic therapy for intraepithelial high-grade neoplasia and early adenocarcinoma in Barrett's oesophagus: acute-phase and intermediate results of a new treatment approach. European Journal of Gastroenterology & Hepatology. 2002;14:1085–1091. doi: 10.1097/00042737-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 114.Haidry RJ, Dunn JM, Butt MA, et al. Radiofrequency ablation and endoscopic mucosal resection for dysplastic barrett's esophagus and early esophageal adenocarcinoma: outcomes of the UK National Halo RFA Registry. Gastroenterology. 2013;145:87–95. doi: 10.1053/j.gastro.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 115.Bulsiewicz WJ, Kim HP, Dellon ES, et al. Safety and efficacy of endoscopic mucosal therapy with radiofrequency ablation for patients with neoplastic Barrett's esophagus. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11:636–642. doi: 10.1016/j.cgh.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett's esophagus with early neoplasia. Clinical Gastroenterology & Hepatology. 2010;8:23–29. doi: 10.1016/j.cgh.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 117.Gupta M, Iyer PG, Lutzke L, et al. Recurrence of Esophageal Intestinal Metaplasia After Endoscopic Mucosal Resection and Radiofrequency Ablation of Barrett's Esophagus: Results From a US Multicenter Consortium. Gastroenterology. 145:79–86. e1. doi: 10.1053/j.gastro.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and Predictors of Successful Radiofrequency Ablation for Barrett's Esophagus. Clin Gastroenterol Hepatol. 2014;12:1840–1847. e1. doi: 10.1016/j.cgh.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett's Esophagus: systematic review and meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11:1245–1255. doi: 10.1016/j.cgh.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bedi AO, Kwon RS, Rubenstein JH, et al. A survey of expert follow-up practices after successful endoscopic eradication therapy for Barrett's esophagus with high-grade dysplasia and intramucosal adenocarcinoma. Gastrointest Endosc. 2013;78:696–701. doi: 10.1016/j.gie.2013.04.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Greenwald BD, Dumot JA, Abrams JA, et al. Endoscopic spray cryotherapy for esophageal cancer: safety and efficacy. Gastrointest Endosc. 2010;71:686–693. doi: 10.1016/j.gie.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yachimski P, Puricelli WP, Nishioka NS. Patient predictors of histopathologic response after photodynamic therapy of Barrett's esophagus with high-grade dysplasia or intramucosal carcinoma. Gastrointestinal Endoscopy. 2009;69:205–212. doi: 10.1016/j.gie.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 123.Chennat J, Konda VJ, Ross AS, et al. Complete Barrett's eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma--an American single-center experience. American Journal of Gastroenterology. 2009;104:2684–2692. doi: 10.1038/ajg.2009.465. [DOI] [PubMed] [Google Scholar]
- 124.Peters FP, Kara MA, Rosmolen WD, et al. Stepwise radical endoscopic resection is effective for complete removal of Barrett's esophagus with early neoplasia: a prospective study. American Journal of Gastroenterology. 2006;101:1449–1457. doi: 10.1111/j.1572-0241.2006.00635.x. [DOI] [PubMed] [Google Scholar]
- 125.van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett's oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut. 2011;60:765–773. doi: 10.1136/gut.2010.229310. [DOI] [PubMed] [Google Scholar]
- 126.Rice TW, Blackstone EH, Goldblum JR, et al. Superficial adenocarcinoma of the esophagus. J Thorac Cardiovasc Surg. 2001;122:1077–1090. doi: 10.1067/mtc.2001.113749. [DOI] [PubMed] [Google Scholar]
- 127.Pech O, Bollschweiler E, Manner H, et al. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett's esophagus at two high-volume centers. Annals of Surgery. 2011;254:67–72. doi: 10.1097/SLA.0b013e31821d4bf6. [DOI] [PubMed] [Google Scholar]
- 128.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–467. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 129.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. The New England journal of medicine. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 131.Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–313. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 132.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659–668. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 133.Wang DB, Zhang X, Han HL, et al. Neoadjuvant chemoradiotherapy could improve survival outcomes for esophageal carcinoma: a meta-analysis. Dig Dis Sci. 2012;57:3226–3233. doi: 10.1007/s10620-012-2263-8. [DOI] [PubMed] [Google Scholar]
- 134.Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–692. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 135.Ronellenfitsch U, Schwarzbach M, Hofheinz R, et al. Preoperative chemo(radio)therapy versus primary surgery for gastroesophageal adenocarcinoma: systematic review with meta-analysis combining individual patient and aggregate data. Eur J Cancer. 2013;49:3149–3158. doi: 10.1016/j.ejca.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 136.Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727–1733. doi: 10.1016/S0140-6736(02)08651-8. [DOI] [PubMed] [Google Scholar]
- 137.Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 138.Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg. 1999;230:392–400. doi: 10.1097/00000658-199909000-00012. discussion 400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bizekis C, Kent MS, Luketich JD, et al. Initial experience with minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg. 2006;82:402–406. doi: 10.1016/j.athoracsur.2006.02.052. discussion 406-7. [DOI] [PubMed] [Google Scholar]
- 140.Noble F, Kelly JJ, Bailey IS, et al. A prospective comparison of totally minimally invasive versus open Ivor Lewis esophagectomy. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus / I.S.D.E. 2013;26:263–271. doi: 10.1111/j.1442-2050.2012.01356.x. [DOI] [PubMed] [Google Scholar]
- 141.Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg. 2003;238:486–494. doi: 10.1097/01.sla.0000089858.40725.68. discussion 494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Suttie SA, Nanthakumaran S, Mofidi R, et al. The impact of operative approach for oesophageal cancer on outcome: the transhiatal approach may influence circumferential margin involvement. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2012;38:157–165. doi: 10.1016/j.ejso.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 143.Davies AR, Sandhu H, Pillai A, et al. Surgical resection strategy and the influence of radicality on outcomes in oesophageal cancer. The British journal of surgery. 2014;101:511–517. doi: 10.1002/bjs.9456. [DOI] [PubMed] [Google Scholar]
- 144.Ovrebo KK, Lie SA, Laerum OD, et al. Long-term survival from adenocarcinoma of the esophagus after transthoracic and transhiatal esophagectomy. World journal of surgical oncology. 2012;10 doi: 10.1186/1477-7819-10-130. 130-7819-10-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tapias LF, Morse CR. A preliminary experience with minimally invasive Ivor Lewis esophagectomy. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus / I.S.D.E. 2012;25:449–455. doi: 10.1111/j.1442-2050.2011.01269.x. [DOI] [PubMed] [Google Scholar]
- 146.Perry KA, Funk LM, Muscarella P, et al. Perioperative outcomes of laparoscopic transhiatal esophagectomy with antegrade esophageal inversion for high-grade dysplasia and invasive esophageal cancer. Surgery. 2013;154:901–907. doi: 10.1016/j.surg.2013.05.019. discussion 907-8. [DOI] [PubMed] [Google Scholar]
- 147.Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–1669. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- 148.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 149.Brusselaers N, Mattsson F, Lagergren J. Hospital and surgeon volume in relation to long-term survival after oesophagectomy: systematic review and meta-analysis. Gut. 2014;63:1393–1400. doi: 10.1136/gutjnl-2013-306074. [DOI] [PubMed] [Google Scholar]
- 150.Reames BN, Ghaferi AA, Birkmeyer JD, et al. Hospital volume and operative mortality in the modern era. Ann Surg. 2014;260:244–251. doi: 10.1097/SLA.0000000000000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 152.Leichman LP, Goldman BH, Bohanes PO, et al. S0356: a phase II clinical and prospective molecular trial with oxaliplatin, fluorouracil, and external-beam radiation therapy before surgery for patients with esophageal adenocarcinoma. J Clin Oncol. 2011;29:4555–4560. doi: 10.1200/JCO.2011.36.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. The Lancet. 383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 154.Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593–1598. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]
- 155.al-Sarraf M, Martz K, Herskovic A, et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol. 1997;15:277–284. doi: 10.1200/JCO.1997.15.1.277. [DOI] [PubMed] [Google Scholar]
- 156.Manner H, May A, Rabenstein T, et al. Prospective evaluation of a new high-power argon plasma coagulation system (hp-APC) in therapeutic gastrointestinal endoscopy. Scandinavian Journal of Gastroenterology. 2007;42:397–405. doi: 10.1080/00365520600898130. [DOI] [PubMed] [Google Scholar]
- 157.Greenwald BD. Photodynamic therapy for esophageal cancer. Update. Chest Surgery Clinics of North America. 2000;10:625–637. [PubMed] [Google Scholar]
- 158.Freitas D, Gouveia H, Sofia C, et al. Endoscopic Nd-YAG laser therapy as palliative treatment for esophageal and cardial cancer. Hepatogastroenterology. 1995;42:633–637. [PubMed] [Google Scholar]
- 159.Abdel-Wahab M, Gad-Elhak N, Denewer A, et al. Endoscopic laser treatment of progressive dysphagia in patients with advanced esophageal carcinoma. Hepatogastroenterology. 1998;45:1509–1515. [PubMed] [Google Scholar]
- 160.Battersby NJ, Bonney GK, Subar D, et al. Outcomes following oesophageal stent insertion for palliation of malignant strictures: A large single centre series. J Surg Oncol. 2012;105:60–65. doi: 10.1002/jso.22059. [DOI] [PubMed] [Google Scholar]
- 161.Stewart DJ, Balamurugan R, Everitt NJ, et al. Ten-year experience of esophageal self-expanding metal stent insertion at a single institution. Dis Esophagus. 2013;26:276–281. doi: 10.1111/j.1442-2050.2012.01364.x. [DOI] [PubMed] [Google Scholar]
- 162.Pellen MG, Sabri S, Razack A, et al. Safety and efficacy of self-expanding removable metal esophageal stents during neoadjuvant chemotherapy for resectable esophageal cancer. Dis Esophagus. 2012;25:48–53. doi: 10.1111/j.1442-2050.2011.01206.x. [DOI] [PubMed] [Google Scholar]
- 163.Homann N, Noftz MR, Klingenberg-Noftz RD, et al. Delayed complications after placement of self-expanding stents in malignant esophageal obstruction: treatment strategies and survival rate. Dig Dis Sci. 2008;53:334–340. doi: 10.1007/s10620-007-9862-9. [DOI] [PubMed] [Google Scholar]
- 164.Siersema PD, Tan TG, Sutorius FF, et al. Massive hemorrhage caused by a perforating Gianturco-Z stent resulting in an aortoesophageal fistula. Endoscopy. 1997;29:416–420. doi: 10.1055/s-2007-1004227. [DOI] [PubMed] [Google Scholar]
- 165.Burstow M, Kelly T, Panchani S, et al. Outcome of palliative esophageal stenting for malignant dysphagia: a retrospective analysis. Dis Esophagus. 2009;22:519–525. doi: 10.1111/j.1442-2050.2009.00948.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: Current [2002] classification. Number of patients in each classification and number traced at 5 years, respectively, were as follows: Tis (44 and 21), T1 (98 and 26), T2 (31 and 8), T3 (57 and 12), and T4 (4 and 0).
B: Proposed subclassification of T1 into T1a (intramucosal, n = 66, 18 traced at 5 years) and T1b (submucosal, n = 32, 8 traced at 5 years). Shown for reference are Tis (high grade dysplasia) and T2. Vertical bars represent 68% confidence limits.
(Reproduced with permission from: Rice TW, Blackstone EH, Rybicki LA, et al. Refining esophageal cancer staging. J Thorac Cardiovasc Surg 2003;125: 1103–13)
Durability of complete eradication for intramucosal adenocarcinoma compared to Barrett’s esophagus of varying degrees of dysplasia. Panel A is the overall recurrence rate of the cohort. Panel B is the recurrence rate broken out by baseline histology. IMC (T1a) has a higher recurrence rate than non-dysplastic Barrett’s esophagus. (LGD, low-grade dysplasia; HGD, high-grade dysplasia; IMC, intramucosal adenocarcinoma, aka T1a). (Reproduced with permission from: Pasricha S, Bulsiewicz WJ, Hathorn KE, et al. Durability and predictors of successful radiofrequency ablation for Barrett’s esophagus. Clin Gastr Hep. 2014;12:1840–1847.)