Abstract
Objective
Knowledge of smoking change processes may be enhanced by identifying pathways to stable abstinence. We sought to identify latent classes of smokers based on their day-to-day smoking status in the first weeks of a cessation attempt. We examined treatment effects on class membership and compared classes on baseline individual differences and 6-month abstinence rates.
Method
In this secondary analysis of a double-blind randomized placebo-controlled clinical trial (N=1433) of 5 smoking cessation pharmacotherapies (nicotine patch, nicotine lozenge, bupropion SR, patch and lozenge, or bupropion SR and lozenge), we conducted repeated measures latent class analysis of daily smoking status (any smoking vs. none) for the first 27 days of a quit attempt. Treatment and covariate relations with latent class membership were examined. Distal outcome analysis compared confirmed 6-month abstinence rates among the latent classes.
Results
A 5-class solution was selected. Three-quarters of smokers were in stable smoking or abstinent classes, but 25% were in classes with unstable abstinence probabilities over time. Active treatment (compared to placebo), and particularly the patch and lozenge combination, promoted early quitting. Latent classes differed in 6-month abstinence rates and on several baseline variables, including nicotine dependence, quitting history, self-efficacy, sleep disturbance, and minority status.
Conclusions
Repeated measures latent class analysis identified latent classes of smoking change patterns affected by treatment, related to known risk factors, and predictive of distal outcomes. Tracking behavior early in a change attempt may identify prognostic patterns of change and facilitate adaptive treatment planning.
Keywords: Smoking, Tobacco, Cessation, Repeated Measures Latent Class Analysis
Relapse is the most common outcome of a smoking cessation attempt. As many as 94% of smokers who cease smoking for at least one day resume smoking within months (Centers for Disease Control & Prevention, 2011). Even with the best available treatments, at most, one-third of smokers will achieve 6 or 12 months of continuous abstinence (Fiore et al. 2008; Stead & Lancaster, 2012). Although relapse rates vary in change efforts across drugs of abuse (e.g., Amato, Minozzi, Davoli, Vechhi, 2011; Bonn-Miller & Moos, 2009; Moos & Moos, 2006) and other health-relevant behaviors and conditions (Jeffery et al., 2000; Linke, Gallo, & Norman, 2011), difficulty in initiating and sustaining behavior change are problems common to all of these domains (McLellan, Lewis, O’Brien, & Kleber, 2000).
Although most cessation studies focus on distal outcomes (typically continuous or prolonged abstinence for 6- or 12-months; Fiore et al., 2008; Stead & Lancaster, 2012), survival analyses suggest most returns to smoking begin within the first few days of a change attempt, and this is therefore a critical period in which to intervene (Piasecki, Fiore, McCarthy, & Baker, 2002; Shiffman et al., 2006). Early outcomes are crucial: individuals who smoke at all during the first weeks of their quit attempt are very likely to smoke in the long run (Kenford et al., 1994; Wileyto et al., 2005). Across many other domains (e.g., treatment for depression, anxiety, eating disorders, and alcohol use disorders), early response to treatment is an excellent indicator of later treatment response, in addition to being useful in the development of adaptive treatments (e.g., Ciraulo, Dong, Silverman, Gastfriend, & Pettinati, 2008; Steidmann et al., 2013).
The focus on distal outcomes in smoking cessation research makes sense from public health and treatment efficacy perspectives; lasting abstinence will improve individual and community health (U.S. Department of Health and Human Services, 1990) and this is the outcome we want to influence through treatment. Yet, studies of distal outcomes do not inform our understanding of how smokers achieve distal success or failure or how treatment influences this process (Brandon, Vidrine, & Litvin, 2007).
To advance our understanding of the processes that lead to distal outcomes, we may need to examine the process in new ways. Rather than adopting simple, binary, and ultimately arbitrary, definitions of relapse based on convention (e.g., smoking any amount 7 days in a row, Ossip-Klein et al. 1986; smoking at least 5 cigarettes per day in 3 consecutive days, Shiffman et al., 2006; smoking at least once a month, Herd & Borland, 2009), research might productively focus on objectively characterizing patterns of daily smoking and abstinence. In a similar spirit, Hughes and colleagues have recently tracked day-to-day intentions to smoke and smoking in community smokers hoping to quit, documenting remarkable instability in smoking intentions and behavior across days (Hughes et al., 2013, 2014; Peters & Hughes, 2009). Such natural history studies illustrate the complexity of smoking over time, and the degree to which simple classifications of smokers as smoking/abstinent long-term may mask this complexity, and thus perhaps keep us from understanding the processes we try to affect through treatment.
The current project sought to identify latent classes of smokers based on patterns of smoking status (any smoking vs. no smoking each day) over the first month of a quit attempt. This secondary data analysis aimed to generate new information about the number, nature, and prevalence of smoking or abstinence patterns during the initial phase of an attempt to change. We sought to empirically identify underlying classes of smoking status trajectories, using repeated measures latent class analysis (RMLCA). RMLCA of daily smoking status has the potential to enhance our understanding of the varying paths smokers may take to ultimate abstinence or smoking. Other approaches to modeling daily smoking, such as generalized estimating equations (GEE) or mixed effect logistic regression modeling (cf. Li, Wileyto, & Heitjan, 2011), fit functional forms such as linear or quadratic trends. In contrast, RMLCA can capture and identify arbitrarily complex patterns of smoking (e.g., step patterns) that may be observed early in a quit attempt (Collins & Lanza, 2010). In addition, RMLCA allows investigators to estimate the size of subpopulations of individuals who are expected to follow similar patterns of change over time (i.e., share latent class membership), and to link latent class membership to both predictors and distal outcomes (Bray, Lanza, & Tan, in press; Collins & Lanza, 2010). Class membership may also be linked to treatment, and this may help explain treatment effects on distal outcomes, particularly as treatments often seem to have their greatest effects early in the quit attempt (McCarthy et al., 2008a; Piasecki et al., 2002). Importantly, RMLCA class membership is a categorical latent variable; latent variable approaches help to separate error variance from class-relevant variance. Latent class modeling is a person-centered approach that may be particularly useful in identifying smokers at high-risk of relapse (Collins & Lanza, 2010), which could aid treatment planning. Variable-centered approaches, such as GEE and mixed-effects regression models (e.g., Li et al., 2011), are very useful in testing specific hypotheses about independent variables, including treatment, but do not help identify smokers displaying different patterns of smoking over time.
Although latent class analyses have examined tobacco use before, these analyses have mostly focused on tobacco use and other risk behavior initiation in adolescents and young adults over long time-frames (e.g., Chen et al., 2004; Rose et al., 2007; Sutfin, Reboussin, McCoy, & Wolfson, 2009), rather than on cessation over shorter time-frames. Other latent class analyses have focused on subtypes of tobacco dependence (e.g., Furberg et al., 2005; Storr, Zhou, Liang, & Anthony, 2004; Timberlake, 2008) or stage of change (Guo, Aveyard, Fielding, & Sutton, 2009; Harell, Trenz, Scherer, Martins, & Latimer, 2013). The only latent class analyses related to smoking cessation identified latent classes of first lapse contexts (Deiches, Baker, Lanza, & Piper, 2013) and latent classes of retrospectively assessed withdrawal severity (Xian et al., 2005). To our knowledge, no repeated measures latent class analyses of smoking during the course of a quit attempt have been conducted.
The current project analyzed data from a clinical trial in which adult, daily smokers were randomized to one of five pharmacotherapy regimens or placebo (Piper et al., 2009), plus brief individual smoking cessation counseling. This efficacy trial evaluated the following smoking cessation treatments: nicotine patch, nicotine lozenge, bupropion, the combination of nicotine patch and lozenge, and the combination of bupropion and nicotine lozenge, relative to one another and to placebo (Piper et al., 2009). Based on this and other recent trials, it appears as though combination nicotine replacement therapy yields the highest short- and long-term (6-month) abstinence rates and is superior to monotherapies and placebo (Cahill, Stevens; Perera, & Lancaster, 2013; Piper et al., 2009; Smith et al., 2009). The original efficacy trial (Piper et al., 2009) focused on binary abstinence outcomes at various time points (i.e., cessation in the first week of the quit attempt, 1 week, 8 weeks, or 6-months post-quit). A subsequent analysis focused on progression through cessation milestones (Shiffman et al., 2006) and found treatment effects on initial abstinence, lapse occurrence and latency, and relapse occurrence and latency (Japuntich, Piper, Leventhal, Bolt, & Baker, 2011). The current study extends these analyses by examining treatment effects on complex patterns of smoking during the first month of a quit attempt, when most lapses and relapses have occurred and treatments have their largest effects (McCarthy et al., 2008a; Piasecki et al., 2002; Piper et al., 2009). We anticipated that we would find a class of smokers who never quit (i.e., whose probabilities of abstinence remained near 0% across the first month of the quit attempt), a class of smokers who quit immediately and stayed quit (i.e., whose probabilities of abstinence remained near 100%), and some intermediate groups of smokers of particular interest who were engaged in efforts to change their smoking status, but struggling to do so. We also examined the extent to which membership in latent classes based on first-month smoking was predictive of later (six-month) biochemically verified abstinence. We expected that groups who struggled to achieve or maintain abstinence in the first month would be less likely to be abstinent five months later.
The project also examined how treatment and theoretically- and clinically-relevant individual differences were related to the smoking pattern classes identified by RMLCA. In terms of covariates, we focused on variables identified in previous research as risk or protective factors for relapse (e.g., nicotine dependence, quitting self-efficacy). This analysis served simultaneously to validate the class solution, by demonstrating associations between class membership and known relapse risk factors, and to determine the extent to which these variables might be useful in identifying smokers at risk for smoking in the first weeks of a cessation attempt. Thus, the study aimed to identify distinct patterns of smoking early in a quit attempt, and to identify treatment effects on and individual differences associated with those patterns.
Method
Data for the current study were drawn from the Piper et al. (2009) double-blind randomized clinical trial of smoking cessation pharmacotherapy regimens. A total of 1,504 adult, daily smokers were randomized to one of the following treatment conditions: placebo (n=189), patch (n=262), lozenge (n=260), bupropion SR (n=264), patch and lozenge combination (n=267), bupropion SR and lozenge combination (n=262). The placebo condition was smaller by design based on a priori power analyses. All participants were offered six sessions of brief (10–20 minute) smoking cessation counseling designed to provide support, psychoeducation, and problem solving assistance.
Participants
Participants were recruited between 2005 and 2007 via mass media advertisements seeking adult daily smokers for a smoking cessation treatment and health outcomes study in Madison and Milwaukee, WI. To be eligible for the study, participants had to be at least 18 years of age, smoke at least 10 cigarettes per day for at least six months, have an expired carbon monoxide (CO) level of at least 10 parts per million (ppm), and be motivated to quit smoking. Smokers were excluded for use of other tobacco, current use of bupropion, current psychotic disorder, or any contraindication to any one of the study medications, pregnancy or breast feeding, or living with someone in the study.
Procedures
Study procedures (approved by the Institutional Review Boards at the University of Wisconsin and Aurora Health Care) are described in detail elsewhere (Piper et al., 2009, 2010). Briefly, randomization to medication condition occurred after all screening and baseline assessment. Randomization was double blinded and blocked on gender and self-identified race (White or other-than-White). All medications were administered in accordance with package inserts and recommended dosage schedules. For this reason, treatment duration was 8 weeks post-quit, except for nicotine lozenge (alone or in combination therapies), which extended for 12 weeks post-quit. All nicotine replacement treatments began on a target quit day; bupropion treatment began 1 week pre-quit. Medication adherence was very good, with participants taking more than 70% of study medication (as assessed by medication counts at visits) for all medications except the lozenge (67% of doses taken, Piper et al., 2009). Counseling sessions were offered 3 weeks pre-quit, 1 week pre-quit, on the target quit day set by investigators, and 1, 2, and 4 weeks thereafter. Retention in treatment and across follow-ups was excellent (92% of participants were retained through the end of treatment visits and 90% completed the 6-month follow-up visit).
Measures
Cigarette dependence
Several pen-and-paper scales and items assessing cigarette dependence were administered in the initial screening sessions. These include items assessing smoking heaviness (cigarettes smoked per day, on average) and years smoked. Cigarette dependence scales administered included the Fagerström Test of Nicotine Dependence, now called the Fagerström Test of Cigarette Dependence (FTCD; Fagerström, 2012; Heatherton et al., 1991; scores range from 0 to 10 with scores above 5 indicating moderate dependence), the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68; Piper et al., 2004; rated on 7-point scales), and the Nicotine Dependence Syndrome Scale (NDSS; Shiffman, Waters, & Hickcox, 2004; total score on this scale is a factor score based on raw scores weighted by factor loadings from the development sample for the measure). For the WISDM-68, we computed a summary score for primary dependence motives (by taking the mean of scores on 4 of the 13 subscales of the WISDM-68: tolerance, craving, automaticity, and loss control) and a separate summary score for secondary dependence motives by taking the mean of the remaining 9 subscales (Piasecki, Piper, & Baker, 2010). Both primary and secondary WISDM-68 scores range from 1 to 7. On these scales, higher scores indicate greater dependence. These measures of dependence have been shown to be predictive of relapse (Piper et al., 2006; 2008; Shiffman et al., 2004). All three measures were included because they tap different aspects of dependence and all have evidence of validity, without clear evidence of superiority of one measure.
Quitting History
Cessation history, including number of quit attempts and longest period of abstinence, have been shown to predict cessation outcomes (Borland et al., 2010; Gilpin, Pierce, & Falkin, 1997; Hyland et al., 2006; Hymowitz et al., 1997; Partos, Borland, Yong, Hyland, & Cummings, 2013). Participants reported the number of past quit attempts and the longest duration of past abstinence. There were 38 extreme outliers reporting 20 to 99 serious past quit attempts. These extreme values were truncated at 20. After truncation, the data were still significantly positively skewed, so all values were increased by 1 (so the minimum value was 1) and log-transformed.
The longest duration of past abstinence was assessed as a categorical variable with seven levels ranging from less than a day to more than a year. To preserve the richness of this ordinal data (vs. turning this into a binary variable) without adding excessive complexity to our models (with several contrast-coded variables), this categorical variable was collapsed into a single ordinal variable. For each level of the categorical variable, the new ordinal variable took the value corresponding to the mid-point of its interval, in days (e.g., 1 to 3 months was set to 60 days; see Weisberg, 1991).
Self-efficacy
Quitting self-efficacy is a validated predictor of short- and long-term abstinence outcomes (e.g., Gwaltney, Metrik, Kahler, & Shiffman, 2009). Subjects were asked at baseline “If you try to quit smoking within the next 30 days, how likely is it that you will be successful?” and responded on a 7-point scale ranging from “not at all” to “extremely” likely. Single self-efficacy items predict abstinence and mediate treatment effects (Gwaltney et al., 2009; McCarthy et al., 2008b).
Baseline craving
Cigarette craving has been shown to predict initial cessation (e.g., Bolt, Piper, Theobald, & Baker, 2012) and distal outcomes (McCarthy, Piasecki, Fiore, & Baker, 2006). Craving was measured using the Wisconsin Smoking Withdrawal Scale (WSWS) which contains a reliable, four-item subscale assessing craving (Cronbach’s alpha 0.88 to 0.90; Welsch et al., 1999). Items were rated on a five-point scale ranging from 0, strongly disagree, to 4, strongly agree.
Baseline sleep disturbance
Research has linked sleep problems to increased smoking (Hamidovic & de Wit, 2009). Baseline sleep problems were assessed using a five-item subscale from the WSWS (Cronbach’s alpha 0.90 to 0.93; Welsch et al., 1999). The subscale contains items such as “My sleep has been troubled” and “I awaken from sleep frequently during the night” and is rated on the same 0 to 4 scale as the craving subscale.
Lifetime history of mood disorders
Modules assessing mood, anxiety, substance abuse, eating, and attention deficit disorders from The Composite International Diagnostic Interview (CIDI; Kessler & Ustün, 2004; World Health Organization, 1990) were administered at baseline. Both lifetime and past-year (but not necessarily current) major depression or dysthymia diagnoses were available. We focused on lifetime history of depressive or dysthymic disorder to see if we would replicate previous findings that lifetime diagnostic status predicts abstinence rates (Glassman et al., 1988) using our RMLCA approach.
Demographics
Participants reported their age, gender, self-identified race, level of education, annual household income, marital status, and employment status at baseline. Education and income were both assessed using categorical options. Subjects endorsed one of six levels of education ranging from kindergarten or less to 4 years of college or more. For annual household income, subjects selected one of seven categories ranging from less than $10,000 per year to $75,000 or more per year. As with the longest past quit attempt, these variables were recoded into ordinal scales by setting the value to the midpoint of the range chosen.
Smoking Status
At each visit, participants completed time-line follow-back assessments of the amount smoked each day since the last visit (Brown et al., 1998). Daily smoking status (any smoking vs. none) for each of the first 27 days of the quit attempt was used as the repeated indicator of latent class in the RMLCA. The data were largely complete, with 96.1% of the sample retained for the current analyses having complete data and fewer than 3% missing data on any given day. A binary indicator was used in favor of a smoking heaviness indicator because total abstinence was common in the sample as a whole (with a mean probability of total abstinence roughly 70% per day and 73.6% of participants abstinent on more than half of the first 27 days). Also, models with more complex multinomial indicators could not be estimated for all 27 days of interest. Six-month smoking status was intent-to-treat (10% missing) seven-day point-prevalence abstinence based on self-reported total abstinence from tobacco in the past 7 days confirmed with a CO level below 10 ppm (Benowitz et al., 2002).
Data Analysis Plan
All models were estimated using SAS version 9.3 (SAS Institute Inc., Cary, NC) via Proc LCA and in Mplus version 7.0 (Muthén & Muthén, 1998–2012) using maximum likelihood estimation with 200 random starts. The results from the two programs were convergent. The SAS results are presented below, except where noted. The RMLCA is essentially a latent variable measurement model in which categorical latent class is defined by an individual’s response to each item (e.g., smoking status each day) across time. Individuals with similar patterns of responding are expected to be members of the same latent class. The parameters of the latent class model help to define the latent classes: (1) latent class proportions indicate how many people are expected to be in each class; and (2) the probabilities of responding to an item, given latent class (probabilities closer to 1.0 indicate a strong correspondence between latent class membership and endorsement of the item). It is assumed that the latent class variable explains all of the variation between variables within each class (i.e., the variables are uncorrelated within each class), an assumption called conditional independence. The current study uses a repeated measures extension of the basic latent class model described by Lanza and Collins (2006).
In the current study the item indicators were daily smoking status (0 = no smoking, 1 = smoking) across the first 27 days of treatment. A 27-day-period was selected because this was when we anticipated the greatest instability in daily smoking status and going beyond this number of repeated measures caused estimation problems. The goal of the RMLCA was to determine the number of patterns (i.e., classes) that would be necessary to explain the variation in smoking status over time. We estimated 1 to 7 classes, and the optimal number of classes was determined by multiple indices of model fit and classification precision: Bayesian Information Criteria (BIC; Nylund, Asparouhov, & Muthen, 2006; Schwarz, 1978), the sample size adjusted BIC (aBIC, Sclove, 1987), bootstrapped likelihood ratio test (BLRT; McLachlan & Peel, 2000; Nylund et al., 2006), classification precision (defined by entropy, a summary measure of the estimated posterior class probabilities), and interpretability of latent classes. The BLRT, which tests the improvement in fit for each additional estimated class, was estimated in Mplus. We began model testing using unconditional models (without covariates) and then added treatment contrasts and covariates as predictors of latent class membership. All continuous covariates were z-score standardized (mean = 0, standard deviation = 1). Class prevalence and item probabilities were compared across all models to assess stability of the class estimates with covariates included. Missing data in daily smoking status was accommodated using maximum likelihood estimation, which allows for all available data to be used in estimating the variance-covariance matrix. Individuals with missing data on any of the covariates (n = 17; 1.2% of the total sample) were excluded from covariate analyses.
After identifying the number of latent classes, conditional RMLCA models including all treatment and baseline covariates were fit. A set of 5 conceptually-based orthogonal contrasts was used to assess for treatment effects on latent class membership. First, all active treatments were compared against placebo medication. Next, the patch and lozenge combination, which produced higher 6-month abstinence rates than each of the monotherapies and longer survival to lapse or relapse than other treatments (Piper et al., 2009), was compared against all other active treatments (patch alone, lozenge alone, bupropion alone, and the combination of bupropion and lozenge). Third, a composite of patch only and the combination of bupropion and lozenge treatment was compared to a composite of lozenge only and bupropion only conditions. This contrast was chosen because the early abstinence rates (through the end of treatment) between the pairs of conditions to be combined in each composite were similar to one another and the survival curves prior to smoking 3 days in a row were very similar within these pairs of conditions (Piper et al., 2009). The fourth contrast compared patch only to the bupropion and lozenge combination to assess the extent to which these 2 conditions, which had similar early abstinence rates and relapse survival curves, differed from one another. The fifth contrast compared bupropion monotherapy with lozenge monotherapy to see the extent to which these 2 monotherapies with similar outcomes and survival differed from one another.
Conditional models also included baseline covariates such as nicotine dependence. In light of the collinearity of the multiple nicotine dependence measures, and in an effort to protect family-wise error, we computed factor scores on a single dependence factor defined by total FTCD and NDSS total scores, and WISDM-68 primary and secondary scale total scores. In initial conditional models, this composite factor score was screened as a covariate. Because the composite was significantly related to class membership, the individual scales were then included in the conditional RMLCA models in an effort to identify which scales drove the composite effect.
In the distal outcome analyses, rates of 7-day point prevalence abstinence confirmed by an expired CO level below 10 ppm at the 6-month post-quit follow-up were examined in the latent classes using marginal means in models containing all treatment contrasts and significant covariates. In RMLCA, individuals cannot be classified definitively into one and only one class; each case was assigned posterior probabilities of membership in each of the latent classes and these probabilities were used to inform class membership for the distal outcome analysis.
Results
In the current study, 1433 of the 1504 (95.3%) who provided at least 1 day of smoking data after a target quit day were included in analyses. The remaining 71 (4.7%) enrollees did not provide even 1 day of post-quit smoking status data, which eliminated them from our models. There were more women (n=876, 58.2 %) than men (n=628, 41.8%) in the sample and the majority of participants self-identified as White (n=1258, 83.6%) with a smaller sample of African-American individuals (n=204, 13.6%). As reported in Piper et al., 2010, a total of 1,080 (73.5%) subjects in the full sample (1,470 of whom completed the Composite International Diagnostic Interview (CIDI); Kessler & Ustün, 2004; World Health Organization, 1990) had a CIDI-identified lifetime diagnosis of any mood, anxiety, or substance use disorder (in addition to nicotine dependence), 263 (17.9%) had a lifetime diagnosis of a mood disorder, 579 (39.4%) had a lifetime diagnosis of an anxiety disorder, and 817 (55.6%) had a lifetime substance use disorder. Other sample characteristics are summarized in Table 1.
Table 1.
Summary of covariate values.
| Covariate | Mean | SD |
|---|---|---|
| Age in years | 44.80 | 11.06 |
| Annual household income in $1,000s | 47.47 | 24.81 |
| Years of education | 13.71 | 1.77 |
| Fagerström Test of Cigarette Dependence (FTCD) | 5.39 | 2.13 |
| Nicotine Dependence Syndrome Scale | −0.23 | 0.91 |
| Wisconsin Inventory of Smoking Dependence Motives-68: Primary scales | 4.92 | 1.18 |
| Wisconsin Inventory of Smoking Dependence Motives-68: Secondary scales | 3.79 | 1.08 |
| Cigarettes per day | 21.41 | 8.95 |
| Years Smoked | 26.41 | 11.24 |
| Number of past quit attempts | 4.92 | 4.41 |
| Longest period abstinent in days | 80.84 | 99.36 |
| Past mood disorder (1=yes, 0=no lifetime mood disorder) | 0.18 | 0.38 |
| Baseline self-efficacy | 5.60 | 1.38 |
| Baseline Wisconsin Smoking Withdrawal Scale: Craving subscale | 2.45 | 0.81 |
| Baseline Wisconsin Smoking Withdrawal Scale: Sleep subscale | 1.87 | 1.00 |
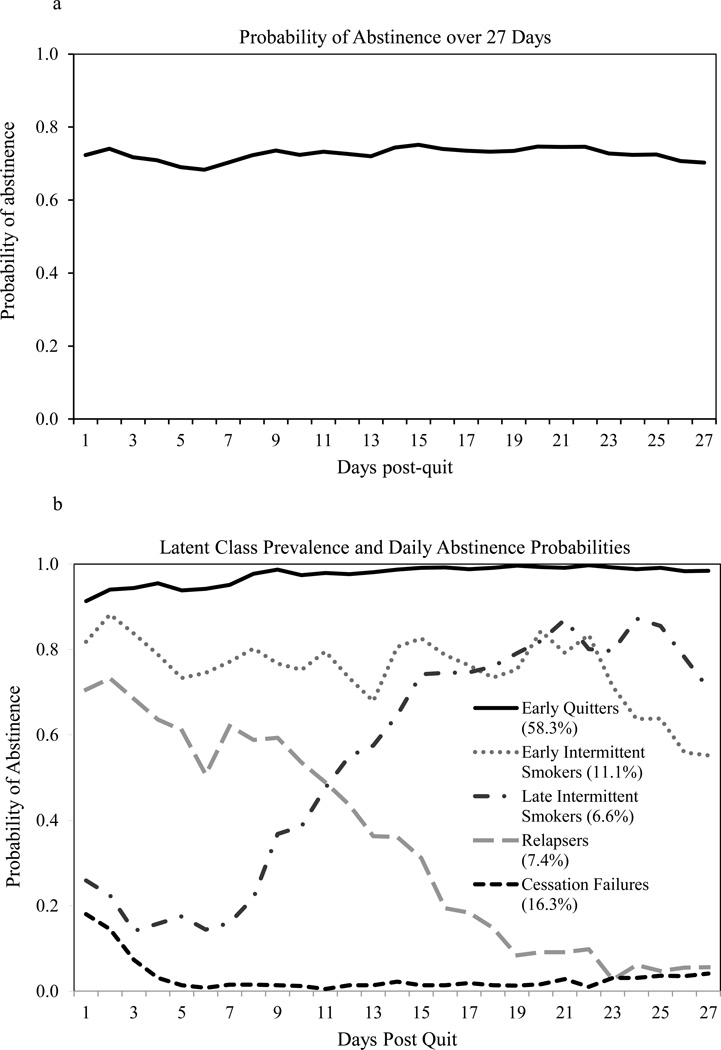
The probability of abstinence was roughly 70% on each of the 27 days (See Figure 1a). Only 35.5% of individuals were continuously abstinent across 27 days, however. Another 9.6% smoked all 27 days. Thus, more than half of individuals varied in smoking status across time.
Figure 1.
a. Estimated probability of abstinence (from 0, smoking, to 1, abstinent) from cigarettes by day for the first 27 days of a quit attempt assuming all smokers belong to a single latent class. The full sample (N=1433) with any post-quit smoking data was included in this analysis.
b. Estimated probability of abstinence (rho) from cigarettes by day for the first 27 days of a quit attempt, given latent class membership, in the unconditional 5-class model. Class prevalences are noted in parantheses below the class labels in the figure legend. The full sample (N=1433) with any post-quit smoking data was included in this analysis. Note that the Intermittent smoker classes are so named because daily abstinence probabilities in these classes are intermediate between abstinence and smoking, suggesting smoking on some but not all days. The Early Intermittent class is so named because smoking probabilities in this class suggest a reduction of smoking days beginning on the target quit day. The Late Intermittent class is so named because abstinence probabilities do not increase markedly in this class until the second week of the quit attempt.
Unconditional RMLCA
The 27 days of smoking status were used to examine RMLCA models with 1–7 classes. The difference in BIC/aBIC model fit and the BLRT between models indicated that the improvement in model fit (as reflected by a drop in BIC/aBIC) with each additional class began to slow at the 5-class model (see Table 2). Because the model fit indices improved with 6 and 7 classes, we examined these solutions closely. We found that the additional 1–2 classes in these models were small (with prevalences at or below 5%), had patterns of daily smoking status highly similar to those in the 5-class solution, and were highly similar to the classes in the 5-class solution in terms of covariate profiles and 6-month abstinence rates. Based on reduction in BIC/aBIC, interpretability, and clinical utility we selected the 5-class model as the final model.
Table 2.
Model fit indices for unconditional models with 1–7 classes (N = 1433).
| Latent Classes |
Log- likelihood |
Parameters | BIC | Difference in BIC |
aBIC | Difference in aBIC |
Entropy | BLRT |
|---|---|---|---|---|---|---|---|---|
| 1 | −22293 | 27 | 44783 | -- | 44697 | -- | 1.00 | -- |
| 2 | −12177 | 55 | 24753 | 20029 | 24579 | 20118 | 0.99 | p<.001 |
| 3 | −10670 | 83 | 21943 | 2810 | 21679 | 2900 | 0.97 | p<.001 |
| 4 | −10112 | 111 | 21029 | 914 | 20677 | 1002 | 0.96 | p<.001 |
| 5 | −9839 | 139 | 20688 | 341 | 20247 | 430 | 0.95 | p<.001 |
| 6 | −9615 | 167 | 20443 | 245 | 19912 | 335 | 0.95 | p<.001 |
| 7 | −9469 | 195 | 20353 | 90 | 19733 | 179 | 0.93 | p<.001 |
Notes:
BIC is the Bayesian information criterion, a measure of model fit; smaller values indicated better fit.
aBIC is the BIC adjusted for sample size; smaller values again indicate better fit.
Entropy is a measure of the accuracy of classification of participants in latent classes and of class differentiation; higher values indicate better classification.
BLRT is the Bootstrap likelihood ratio test, a test of the significance of differences in model fit with the addition of one more latent class; p<.05 indicates a significant change in model fit with a change in the number of latent classes.
The 5-class model (Figure 1b) included a class of individuals with high abstinence probabilities through 27 days (which we have named Early Quitters, 58.3% estimated prevalence), a class who likely never quit (Cessation Failures, 16.5%), a class who achieved and then maintained a heightened but intermediate probability of abstinence (ranging from 0.55 to 0.88 across days) suggestive of smoking on some days (Early Intermittent Smokers, 11.1%), and two classes that evinced diverging patterns of smoking over time, Relapsers (7.4%) and Late Intermittent Smokers (6.6%), crossing in opposite directions around day 11. Both the Early and Late Intermittent Smokers appeared to smoke intermittently (i.e., they reduced the number of days smoked). Early Intermittent smokers limited smoking to rare days immediately after the target quit day whereas Late Intermittent smokers had only rare instances of abstinence until the second week of the quit attempt, when abstinence probabilities climbed and began to exceed 0.5.
Conditional RMLCA – Treatment Effects
Treatment effects were evaluated in models containing all covariates shown in Table 3 and described below. The latent class structure and prevalences were stable, with only modest changes in these estimates, in the conditional (Table 3) and unconditional (Figure 1b) models. To identify treatment effects on class membership, we first compared each other class against the Early Quitters to see how membership in the less successful classes compared with this reference class in terms of the planned treatment contrasts and covariates (Table 3, Panel A). Then, a further set of contrasts was conducted with Cessation Failures as the reference group to see how the intermediate classes differed from these least successful quitters (Table 3, Panel B). A final set of contrasts was run with Relapsers as the reference group to examine differences among the intermediate classes (Table 3, Panel C).
Table 3.
Odds ratios with 95% Confidence Interval [CI] of latent class membership predictors and χ2 tests of differences in 6-month abstinence rates among latent classes.a
| Covariates |
Early Intermittent Smoker (11.5%) |
Late Intermittent Smoker (6.5%) |
Relapser (7.3%) |
Cessation Failure (16.4%) |
|---|---|---|---|---|
| Panel A: Reference Group: Early Quitter (58.2%) | ||||
| All Active Tx vs. Placebo | 0.66 [0.48, 0.90]* | 0.64 [0.43, 0.96]* | 0.61 [0.42, 0.89]* | 0.37 [0.29, 0.47]* |
| Patch+Loz vs. Other Active Tx | 0.80 [0.61, 1.05] | 0.78 [0.55, 1.10] | 0.96 [0.71, 1.30] | 0.52 [0.39, 0.68]* |
| Patch or Bup+Loz vs. Bup or loz | 0.69 [0.51, 0.94]* | 0.72 [0.49, 1.05] | 0.70 [0.49, 1.02] | 0.59 [0.45, 0.77]* |
| Patch vs. Bup+Loz | 1.18 [0.95, 1.47] | 0.82 [0.62, 1.09] | 1.31 [1.00, 1.71] | 0.93 [0.76, 1.13] |
| Bup vs. Loz | 0.91 [0.74, 1.12] | 0.77 [0.59, 1.00] | 0.87 [0.67, 1.12] | 0.97 [0.82, 1.16] |
| FTCD | 1.10 [0.97, 1.25] | 1.10 [0.94, 1.29] | 1.42 [1.21, 1.66]* | 1.32 [1.18, 1.48]* |
| Longest period abstinent in days | 0.80 [0.70, 0.92]* | 0.74 [0.61, 0.89]* | 0.74 [0.63, 0.88]* | 0.78 [0.69, 0.88]* |
| Number of past quit attempts | 0.95 [0.83, 1.08] | 1.08 [0.91, 1.27] | 0.89 [0.77, 1.04] | 0.81 [0.73, 0.91]* |
| Baseline Self-efficacy | 1.03 [0.90, 1.18] | 0.89 [0.76, 1.04] | 1.10 [0.94, 1.30] | 0.81 [0.73, 0.90]* |
| Baseline WSWS Sleep scale | 1.17 [1.03, 1.32]* | 1.08 [0.92, 1.26] | 1.28 [1.10, 1.49] | 1.12 [1.01, 1.26]* |
| Minority (1=Minority, 0=White) | 1.29 [0.90, 1.84] | 1.74 [1.17, 2.60]* | 1.62 [1.11, 2.37]* | 2.04 [1.55, 2.67]* |
| Intercept | 0.20 [0.17, 0.23]* | 0.10 [0.09, 0.12]* | 0.11 [0.09, 0.13]* | 0.23 [0.21, 0.27]* |
| Panel B: Reference Group: Cessation Failure (16.4%) | ||||
| All Active Tx vs. Placebo | 1.78 [1.28, 2.49]* | 1.74 [1.15, 2.65]* | 1.66 [1.12, 2.45]* | |
| Patch+Loz vs. Other Active Tx | 1.55 [1.09, 2.21]* | 1.51 [0.99, 2.28] | 1.85 [1.26, 2.71]* | |
| Patch or Bup+Loz vs. Bup or loz | 1.18 [0.82, 1.68] | 1.21 [0.79, 1.87] | 1.19 [0.79, 1.81] | |
| Patch vs. Bup+Loz | 1.27 [0.98, 1.66] | 0.88 [0.64, 1.22] | 1.41 [1.04, 1.92]* | |
| Bup vs. Loz | 0.93 [0.73, 1.18] | 0.79 [0.59, 1.05] | 0.89 [0.67, 1.18] | |
| FTCD | 0.83 [0.72, 0.97]* | 0.83 [0.69, 1.00] | 1.07 [0.90, 1.28] | |
| Longest period abstinent in days | 1.03 [0.87, 1.22] | 0.95 [0.77, 1.17] | 0.95 [0.78, 1.16] | |
| Number of past quit attempts | 1.16 [1.00, 1.35] | 1.32 [1.10, 1.59]* | 1.10 [0.93, 1.30] | |
| Baseline Self-efficacy | 1.27 [1.10, 1.48]* | 1.09 [0.93, 1.29] | 1.36 [1.14, 1.62]* | |
| Baseline WSWS Sleep scale | 1.04 [0.89, 1.20] | 0.96 [0.80, 1.14] | 1.14 [0.96, 1.35] | |
| Minority (1=Minority, 0=White) | 0.63 [0.43, 0.93]* | 0.86 [0.56, 1.32] | 0.80 [0.53, 1.20] | |
| Intercept | 0.84 [0.71, 1] | 0.44 [0.36, 0.54]* | 0.47 [0.39, 0.58]* | |
| Panel C: Reference Group: Relapser (7.3%) | ||||
| All Active Tx vs. Placebo | 1.07 [0.69, 1.67] | 1.05 [0.63, 1.76] | ||
| Patch+Loz vs. Other Active Tx | 0.84 [0.57, 1.23] | 0.81 [0.53, 1.26] | ||
| Patch or Bup+Loz vs. Bup or loz | 0.99 [0.63, 1.54] | 1.02 [0.61, 1.69] | ||
| Patch vs. Bup+Loz | 0.90 [0.65, 1.25] | 0.62 [0.43, 0.90]* | ||
| Bup vs. Loz | 1.05 [0.77, 1.42] | 0.89 [0.63, 1.25] | ||
| FTCD | 0.78 [0.64, 0.94]* | 0.78 [0.63, 0.96]* | ||
| Longest period abstinent in days | 1.08 [0.88, 1.33] | 0.99 [0.78, 1.26] | ||
| Number of past quit attempts | 1.06 [0.88, 1.27] | 1.21 [0.98, 1.49] | ||
| Baseline Self-efficacy | 0.94 [0.77, 1.14] | 0.81 [0.65, 0.99]* | ||
| Baseline WSWS Sleep scale | 0.91 [0.76, 1.09] | 0.84 [0.68, 1.03] | ||
| Minority (1=Minority, 0=White) | 0.79 [0.50, 1.27] | 1.07 [0.64, 1.78] | ||
| Intercept | 1.77 [1.43, 2.19]* | 0.93 [0.72, 1.19] | ||
Class prevalances are slightly different in the conditional versus unconditional (Figure 1b) models;
p < 0.05;
Tx=Treatment; Patch=Nicotine patch; Loz=Nicotine lozenge; Bup=Bupropion SR; Patch+Loz=Combination nicotine patch and lozenge; Bup+Loz=Combination Bupropion SR and nicotine lozenge
Treatment predicted class membership. As shown in Table 3A, those receiving any active medication were less likely than those receiving placebo to be in a smoking class (i.e., actively treated participants were significantly more likely to be Early Quitters than each of the other classes). Actively treated participants had 63% lower odds (OR=.37) of being a Cessation Failure rather than an Early Quitter than did placebo-treated participants, for example. Receiving combination patch and lozenge treatment roughly halved odds of being a Cessation Failure rather than Early Quitter, relative to the other active treatment conditions (bupropion+lozenge, bupropion, lozenge, or patch). In addition, Patch monotherapy and the bupropion and lozenge combination were superior to bupropion or lozenge monotherapy in promoting Early Quitting over Cessation Failure or Early Intermittent Smoking (Table 3A).
Participants receiving active medication were more than 1.6 times more likely than placebo-treated participants to be in the Early or Late Intermittent Smoking or Relapse classes rather than Cessation Failures (Table 3B). Combination patch and lozenge treatment promoted early change in smoking (Early Intermittent Smoking or Relapse) rather than Cessation Failure better than did other active treatments, but did not significantly differentiate Cessation Failures and Late Intermittent Smokers (Table 3B). Patch monotherapy increased the odds of Relapse rather than Cessation Failure better than did bupropion+lozenge (Table 3B). Patch monotherapy (vs. bupropion+lozenge) was also associated with decreased odds of Late Intermittent Smoking versus Relapse (Table 3C). No other significant treatment effects were detected.
Conditional RMLCA – Covariate Effects
First, a model with all candidate covariates and the treatment contrasts was tested. Covariates were then trimmed from the model one-by-one using log-likelihood difference testing, with each covariate evaluated for the degree of decrement in model fit. The final, best-fitting model retained treatment variables and the following covariates: longest duration of past abstinence (past abstinence), self-efficacy, FTCD, number of past quit attempts (truncated and log-transformed), baseline sleep disturbance (sleep), and minority status (minority).
As shown in Table 3A, Early Quitters differed from at least one of the other classes on every covariate and they differed significantly from Cessation Failures on every covariate. For example, the likelihood of being classified as a Cessation Failure, rather than an Early Quitter, was doubled for subjects who identified as non-White, compared to those who identified as White (Table 3A: OR = 2.04). Minority status was also associated with greater risk of Relapse and Late Intermittent Smoking rather than Early Quitting. Longer past abstinence, more past quit attempts, and higher self-efficacy had protective effects, as they were associated with roughly 20% lower odds of being Cessation Failures rather than Early Quitters. Longer past abstinence also significantly reduced the odds of being in all other smoking classes rather than an Early Quitter. In contrast, higher FTCD scores increased the odds of being classified as a Cessation Failure or Relapser rather than Early Quitter. Baseline sleep disturbance also increased risk of being a Cessation Failure or an Early Intermittent Smoker, rather than an Early Quitter.
Table 3B shows the differences between all intermediate classes and Cessation Failures. FTCD scores and minority status differentiated Early Intermittent Smokers and Cessation Failures, with higher FTCD scores and minority status associated with lower odds of reducing smoking. More past quit attempts increased the odds of being a Late Intermittent Smoker rather than a Cessation Failure. Higher self-efficacy increased the odds of Relapse or Early Intermittent Smoking rather than Cessation Failure.
Table 3C shows the differences between Relapsers and the two other intermediate classes. Early Intermittent Smokers and Late Intermittent Smokers differed from Relapsers in terms of FTCD. Higher FTCD scores were associated with 22% lower odds of being in an Intermittent smoking class rather than Relapse. Higher self-efficacy was related to reduced odds of classification as a Late Intermittent Smoker rather than a Relapser (i.e., Relapsers had higher baseline self-efficacy than Late Intermittment Smokers, Table 3B, who were intermediate in self-efficacy between Cessation Failures, Table 3B, and Relapsers, Table 3C).
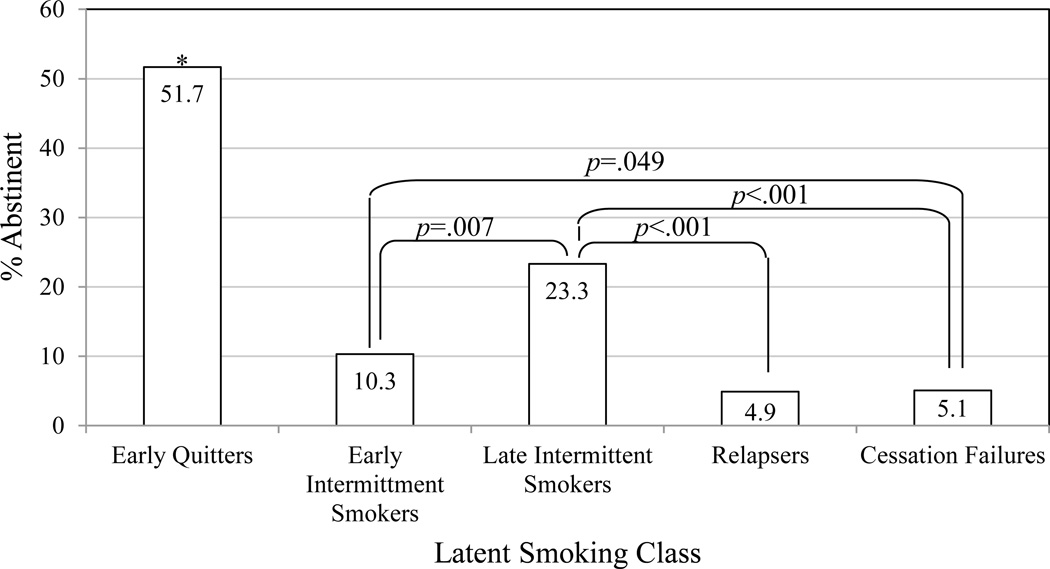
Distal Outcomes
The overall CO-confirmed 7-day point prevalence abstinence rate 6-months post-quit was 34.0% (481 of 1416 cases with complete data on covariates). Individuals were assigned to latent classes based on posterior probabilities of membership in each class. Model entropy was high (entropy = 0.95), indicating that classification was adequately precise and individuals, on average, tended to have a high probability of likely class membership in only one class. An examination of marginal means in abstinence across the latent classes (Figure 2) revealed that abstinence rates were highest among the Early Quitters, followed by Late Intermittent Smokers, Early Intermittent Smokers, and then Cessation Failures and Relapsers. Chi-squared tests of these abstinence rates showed that Early Quitters had significantly higher rates than did all others (all ps<0.0001). Cessation Failures had lower abstinence rates than either Late (p<0.001) or Early Intermittent Smokers (p=.049), but not Relapsers (p=.929). Relapsers had significantly lower abstinence rates than did Late (p<.001), but not Early Intermittent Smokers (p=.116). In addition, Late Intermittent Smokers had significantly higher abstinence rates than did Early Intermittent Smokers (p=0.007).1
Figure 2.
Estimates of CO-confirmed, seven-day, point-prevalence abstinence from smoking six months following a target quit day. These are estimated marginal means of abstinence among latent classes in a model containing covariates.
* Early Quitter abstinence rates differ significantly from each of the other latent classes at p<.0001
Discussion
The latent classes identified in this analysis suggest that there are multiple trajectories of abstinence (or smoking) during the first weeks of a quit attempt. These classes of trajectories appear to be valid and meaningful, as they are significantly associated with treatment, covariates, and 6-month abstinence in anticipated ways. All pharmacotherapies improved the odds of membership in an early quitting class, rather than a smoking class, better than did placebo, and the patch and lozenge combination was particularly helpful in reducing cessation failure. Results also confirmed that nicotine dependence, quitting history, and self-efficacy are important to success in quitting, and suggest that pre-quit sleep problems may be another important risk factor for difficulty quitting, along with minority status.
A substantial proportion of smokers exhibited unstable abstinence probabilities across the first weeks of quitting. Three quarters of the subjects fell into two extreme groups, Early Quitters and Cessation Failures, who exhibited fairly stable (after the first week) and extreme daily abstinence probabilities, and also differed in distal abstinence status. Roughly one-quarter of quitters, in contrast, showed substantial shifts in abstinence probabilities over the succeeding weeks. Relapsers were likely to be confident and abstinent initially, but then became more likely to smoke, ending up with the same 5% six-month abstinence rate as Cessation Failures who never quit. Late Intermittent Smokers, conversely, show the potential for recovery from a poor early course that resembled that of Cessation Failures. Despite low initial abstinence and low confidence relative to those who quit early (Early Quitters and Relapsers), Late Intermittent Smokers went on to achieve an impressive 23% six-month abstinence rate, significantly higher than in other classes with immediate change in smoking and similar overall abstinence probabilities collapsed over days (Early Intermittent Smokers and Relapsers). The ability to recover from initial difficulty may be a marker for later success. It may reflect persistence, motivation, mobilization of support, or other mechanisms that could be examined in the future.
The two groups most extreme in terms of abstinence probabilities, Early Quitters and Cessation Failures, differed significantly by treatment and in baseline characteristics. Active treatment, particularly nicotine patch and combination treatments, decreased the risk of Cessation Failure relative to Early Quitting. Cessation Failures were more nicotine dependent, had lower self-efficacy, and reported worse baseline sleep than did Early Quitters. Cessation Failures also had fewer lifetime quit attempts and shorter past abstinence than did Early Quitters. This stands in contrast to previous research showing that more past-year quit attempts predict lower odds of successful quitting (Partos et al., 2013). Recent quit attempts may indicate risk for current failure in a way that the more distal attempts assessed in this study did not. Minority individuals were twice as likely to be Cessation Failures versus Early Quitters, and this did not appear to be accounted for by income or education, as the minority status covariate was significant in initial models containing these non-significant variables. These findings support the validity of the class solution and extend our knowledge, both in linking baseline sleep disturbances to difficulty quitting completely, and in demonstrating that the risk factors act on the early trajectory of abstinence during a quit attempt.
Other notable findings include examples of daily abstinence probability divergence and convergence among groups. Some groups started on similar paths but then diverged. Cessation Failures and Late Intermittent Smokers had similar, low abstinence probabilities in week one, but diverged thereafter as the Late Intermittent Smokers increased abstinent days while Cessation Failures approached zero abstinence probability. Active treatment may have facilitated this divergence, as those on active medication (vs. placebo) were more likely to recover from initial failure in the intermittent class rather than remain smoking daily. Greater success in past quit attempts also predicted late intermittent smoking rather than Cessation Failure.
In addition, Quitters, Early Intermittent Smokers, and Relapsers all achieved abstinence probabilities above 0.7 in the first days of the quit attempt, but then diverged, such that Early Quitters maintained abstinence, Relapsers showed a precipitous fall in abstinence, and Early Intermitted Smokers showed a slow decline in volatile daily abstinence probabilities. Treatment seemed to affect this divergence, as well. Active treatment reduced the risk of relapse or inconsistency in abstinence (relative to placebo), rather than consistent abstinence. Receiving the patch or the combination of bupropion and lozenge also protected against Early Intermittent Smoking (rather than consistent abstinence) better than did bupropion or lozenge monotherapies. In addition, nicotine dependence was positively associated with risk of Relapse, relative to both consistent and inconsistent abstinence (i.e., Early Quitting and Early Intermittent Smoking). Greater past abstinence duration was also linked to reduced risk of Early Intermittent Smoking rather than consistent abstinence (Early Quitting). Interestingly, baseline sleep disturbance was associated with greater risk of inconsistency in abstinence, as this increased the odds of being an Early Intermittent Smoker rather than an Early Quitter.
Other groups started with different behavior, but then converged on the same endpoints (at 27 days and 6-months) by different paths. Most notably, Relapsers and Cessation Failures who, combined, comprise nearly one-quarter of the sample, had very low abstinence probabilities at 27 days and 6-months post-quit, although Relapsers started the quit attempt with high abstinence probabilities. Treatment appears to have contributed to the differences in initial cessation in these two classes, as any treatment (vs. placebo), combination patch and lozenge treatment (vs. other active treatment), and patch treatment (vs. bupropion+lozenge), all increased the odds of Relapse (marked by early abstinence) relative to Cessation Failure (marked by smoking from the outset). Greater baseline self-efficacy was also associated with Relapse versus Cessation Failure, which suggests that confidence may foster initial success in quitting. Such confidence may be a double-edged sword, as confidence was also greater among Relapsers than among Late Intermittent Smokers. Clinically, it may be important to boost self-efficacy to reduce cessation failure, but also to prevent overconfidence that may increase risk of later relapse.
The results also add to our understanding of the comparative efficacy of the five pharmacotherapies tested. Conventional analyses have already demonstrated the benefits of active (vs. placebo) pharmacotherapy (Japuntich et al., 2011; Piper et al., 2009), and the superiority of combination therapy (Smith et al., 2009), particularly the patch and lozenge combination (Piper et al., 2009) in promoting long-term abstinence. Patch monotherapy and bupropion and lozenge combination therapy were also known to slow progression to relapse following an initial lapse (Japuntich et al., 2011). The current results suggest that all active treatments, and particularly patch and combination treatments, differentiate the least and most optimal patterns of abstinence (Cessation Failure and Early Quitting) and also promote complete rather than intermittent initial abstinence. Interestingly, nicotine patch therapy seems to be better at promoting initial abstinence (Relapse vs. Cessation Failure) than bupropion and lozenge combination treatment, but does not seem to foster recovery (Late Intermittent Smoking vs. Relapse) as well as bupropion and lozenge. This suggests that adaptive treatments (e.g., Rose & Behm, 2014) may be indicated, such that those who relapse on the patch may benefit from a switch to a new treatment to support recovery from relapse.
Taken together, these findings suggest that the latent classes are meaningful. The significant associations observed with treatment and known risk and protective factors also mitigate concern that the latent classes of smoking trajectories obtained are an artifact of the approach used. Latent class approaches have previously been criticized for consistently yielding 4-class models of alcohol use trajectories that resemble a "cat's cradle," in which one class consistently uses heavily, one class consistently abstains, one starts low and increases use, and one starts with heavy use and then decreases use (Sher, Jackson, & Steinley, 2011). Although we observed a pattern similar, if slightly more complex than this, in the current study, we also found considerable evidence to support the validity of the solution. The distal outcome analysis, in particular, suggests that the trajectory in daily abstinence probabilities in the first weeks of a quit attempt conveys important information about long-term abstinence probabilities. Even when mean abstinence probabilities are similar, as in Relapsers and Late Intermittent Smokers, or when day-27 abstinence probabilities are similar, as for Early and Late Intermittent Smokers, the pattern of change over the first month seems to convey important information about later smoking status. We also used a non-linear approach that could capture arbitrarily complex patterns of daily smoking and still found that adding additional classes told us little above and beyond the five class solution adopted. The cat’s cradle may be ubiquitous because it captures real processes of change, and the pattern of change observed early in a quit attempt may predict lasting change in ways that can inform adaptive treatments in the future.
The current results suggest that adaptive treatment strategies for smoking cessation based on early smoking patterns may need to allow enough time for patterns to reach stable states. In this sample, 14% of smokers achieved fairly stable smoking probabilities only after two to three weeks of quitting efforts. A grace period of two to three weeks may help detect ability to maintain abstinence (to differentiate Early Quitters from Relapsers) and ability to recover from initial smoking (to differentiate Cessation Failures from Late Intermittent Smokers) that may influence distal outcomes. Notably, the standard that the Food and Drug Administration uses to assess smoking cessation outcome in drug studies typically allows a grace period of two weeks, but then requires continuous abstinence thereafter. Our findings suggest that the initial grace period is needed to allow many smokers to achieve a stable state of abstinence.
Limitations
We used retrospective, self-reported, binary daily smoking status (rather than smoking heaviness) as the repeated indicator of latent status in this analysis. Such data are subject to deliberate deception and recall bias. There is little reason to expect that these sources of error would affect the patterns seen over time, however. Treating smoking as binary in the analysis may also mask important patterns in smoking heaviness. It is possible to create latent classes based on smoking heaviness, but not for 27 days in RMCLA due to convergence problems. Identifying latent classes based on smoking heaviness patterns over shorter periods of time (e.g., 7 days) would be valuable complements to the current approach. In addition, the current data came from a treatment-seeking, heavy-smoking sample, with all receiving counseling and most receiving active pharmacotherapy. It may be that results would not generalize to self-quitters or a sample differing in nicotine dependence levels. It is likely that class prevalence would change in lower-dependence and less motivated samples, and different classes might appear. Results may also fail to generalize to a more diverse population. Although the current sample was fairly balanced in terms of gender and diverse in terms of education and income, only 2% of the sample self-identified as neither White nor African American. Latent class mixture models can be influenced by sample-specific patterns of responding and replicating the findings in an independent sample is important for increasing confidence in the results. We also have not explored the full range of covariates that may be associated with daily smoking status latent class. Psychopathology other than mood disorders and social or contextual variables may also predict latent class. Examining additional covariates, and time-varying covariates, in particular, could yield useful insights into the process of cessation.
Conclusions
Results from this RMLCA suggest that distinct and valid classes can be identified from binary smoking data in the first few weeks of a quit attempt and used to test treatment effects in novel ways. Daily smoking latent class membership was related to known relapse risk factors and treatment in ways that support the validity of the latent class structure. The differences that emerged among the intermediate classes of smokers in terms of abstinence probability trajectories over time, covariate relations, and distal outcomes suggest that there are meaningful differences among groups of smokers who look similar in point-prevalence snapshots of abstinence. Such differences can only be examined, understood, and targeted in treatment if smoking outcomes are assessed in ways that better match the diversity and complexity of cessation responses observed in this study. Similar RMLCA approaches may be of use in the study of change processes in other areas (e.g., other behaviors targeted for change in treatment).
PUBLIC HEALTH SIGNIFICANCE.
This study showed that tracking daily smoking early in an attempt to quit smoking can help identify those at risk for long-term smoking and can test ways treatments influence early smoking patterns.
Acknowledgments
The data for this study were collected as part of a previously reported randomized clinical trial (Piper et al., 2009). This work was supported by National Institute on Drug Abuse Grant R01DA033303 awarded to Drs. McCarthy and Shiffman. The data were collected in a randomized clinical trial (Clinical Trial Registry # NCT00332644) funded by National Institute on Drug Abuse Grant 5P50DA019706.
GlaxoSmithKline donated active and placebo medication for the randomized clinical trial of five smoking cessation pharmacotherapies that generated the data analyzed in the present study. Dr. McCarthy has also received discounts on nicotine lozenge purchases from GlaxoSmithKline in the past. Dr. Shiffman has consulted to GlaxoSmithKline on smoking cessation products. GlaxoSmithKline played no role in the design, implementation, analysis, or reporting of the parent trial or the current study.
Footnotes
To confirm these results, we used a newly developed inclusive approach to distal outcome analysis that corrects for underestimation of relations between latent classes and distal outcomes by estimating the latent class solution with the distal outcome (six-month abstinence) included as the sole covariate using Proc LCA and the distal outcomes analysis macro in SAS (Bray, Lanza, & Tan, in press). Results (N=1433) yielded similar estimates of abstinence rates in each class, despite a small difference in sample size (N difference=17) due to the inclusion of only distal outcome as a covariate in the analysis. The overall abstinence rate was 34.3% and abstinence rates for latent classes were estimated at: 59.0% for Early Quitters, 21.4% for Late Intermittent Smokers, 11.7% for Early Intermittent Smokers, 4.9% for Relapsers, and 5.0% for Cessation Failures.
Contributor Information
Danielle E. McCarthy, Rutgers, the State University of New Jersey, Department of Psychology and Institute for Health, Health Care Policy and Aging Research, 112 Paterson St., New Brunswick, NJ 08901, demccart@rci.rutgers.edu
Lemma Ebssa, Rutgers, the State University of New Jersey, Institute for Health, Health Care Policy and Aging Research, 112 Paterson St., New Brunswick, NJ 08901, lemma@rci.rutgers.edu.
Katie Witkiewitz, University of New Mexico, 2650 Yale Blvd SE, MSC 11-6280, Albuquerque, NM 87106, katiew@unm.edu.
Saul Shiffman, University of Pittsburgh, Department of Psychology, Bellefield Professional Building, 130 N. Bellefield Ave., Pittsburgh, PA 15260-2695, shiffman@pinneyassociates.com.
References
- Amato L, Minozzi S, Davoli M, Vecchi S. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database of Systematic Reviews. 2011:10. doi: 10.1002/14651858.CD004147.pub4. Art. No. CD004147. [DOI] [PubMed] [Google Scholar]
- Benowitz N, Jacob P, Ahijevych K, Jarvis MJ, Hall S, LeHouzec J, Hansson A, et al. Biochemical verification of tobacco use and cessation: SRNT subcomittee on biochemical verification. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Bolt DM, Piper ME, Theobald WE, Baker TB. Why two smoking cessation agents work better than one: The role of craving suppression. Journal of Consulting and Clinical Psychology. 2012;80:54–65. doi: 10.1037/a0026366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller MO, Moos RH. Marijuana discontinuation, anxiety symptoms, and relapse to marijuana. Addictive Behaviors. 2009;34:782–785. doi: 10.1016/j.addbeh.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Borland R, Yong HH, Balmford J, Cooper J, Cummings KM, O’Connor RJ, Fong GT. Motivational factors predict quit attempts but not maintenance of smoking cessation: Findings from the International Tobacco Control Four country project. Nicotine & Tobacco Research. 2010;12:s4–s11. doi: 10.1093/ntr/ntq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annual Review of Clinical Psychology. 2007;3:257–284. doi: 10.1146/annurev.clinpsy.3.022806.091455. [DOI] [PubMed] [Google Scholar]
- Bray BC, Lanza ST, Tan X. Eliminating bias in classify-analyze approaches for latent class analysis. Structural Equation Modeling: A Multidisciplinary Journal. doi: 10.1080/10705511.2014.935265. (in press). Retrieved from https://methodology.psu.edu/media/techreports/12-118.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors. 1998;12:101–112. [Google Scholar]
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database of Systematic Reviews. 2013;5 doi: 10.1002/14651858.CD009329.pub2. Art. No.: CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Quitting smoking among adults-United States, 2001–2010. MMWR Morbidity & Mortality Weekly Report. 2011;60:1513–1519. Retrieved from http://www.cdc.gov/ [PubMed] [Google Scholar]
- Chen X, Li X, Stanton B, Moa R, Sun Z, Zhang H, Qu M, Thomas R. Patterns of cigarette smoking among students from 19 colleges and universities in Jiangsu Province, China: a latent class analysis. Drug and Alcohol Dependence. 2004;76:153–163. doi: 10.1016/j.drugalcdep.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Ciraulo DA, Dong Q, Silverman BL, Gastfriend DR, Pettinati HM. Early treatment response in alcohol dependence with extended-release naltrexone. Journal of Clinical Psychiatry. 2008;69:190–195. doi: 10.4088/jcp.v69n0204. [DOI] [PubMed] [Google Scholar]
- Collins LM, Lanza ST. Latent Class and Latent Transition Analysis: With Applications in the Social, Behavioral, and Health Sciences. Hoboken, NJ: Wiley; 2010. [Google Scholar]
- Deiches JF, Baker TB, Lanza S, Piper ME. Early lapses in a cessation attempt: Lapse contexts, cessation success, and predictors of early lapse. Nicotine & Tobacco Research. 2013;15:1883–1891. doi: 10.1093/ntr/ntt074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerström Test for Cigarette Dependence. Nicotine & Tobacco Research. 2012;14:75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Wewers ME. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- Furberg H, Sullivan PF, Maes H, Prescott CA, Lerman C, Bulik C, Kendler KS. The types of regular cigarette smokers: A latent class analysis. Nicotine & Tobacco Research. 2005;7:351–360. doi: 10.1080/14622200500124917. [DOI] [PubMed] [Google Scholar]
- Gilpin EA, Pierce JP, Farkas AJ. Duration of smoking abstinence and success in quitting. Journal of the National Cancer Institute. 1997;89:572–576. doi: 10.1093/jnci/89.8.572. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Stetner F, Walsh T, Raizman PS, Fleiss JL, Cooper MA, Covey LS. Heavy smokers, smoking cessation, and clonidine: Results of a double-blind, randomized trial. JAMA. 1988;259:2863–2866. [PubMed] [Google Scholar]
- Guo B, Aveyard P, Fielding A, Sutton S. Using latent class and latent transition analysis to examine the transtheoretical model staging algorithm and sequential stage transition in adolescent smoking. Substance Use & Misuse. 2009;44:2028–2042. doi: 10.3109/10826080902848665. [DOI] [PubMed] [Google Scholar]
- Gwaltney CJ, Metrik J, Kahler CW, Shiffman S. Self-efficacy and smoking cessation: A meta-analysis. Psychology of Addictive Behaviors. 2009;23:56–66. doi: 10.1037/a0013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidovic A, de Wit H. Sleep deprivation increases cigarette smoking. Pharmacology Biochemistry and Behavior. 2009;93:263–269. doi: 10.1016/j.pbb.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell PT, Trenz RC, Scherer M, Martins SS, Latimer WW. A latent class approach to treatment readiness corresponds to a transtheoretical ("Stages of Change") model. Journal of Substance Abuse Treatment. 2013;45:249–256. doi: 10.1016/j.jsat.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Herd N, Borland R. The natural history of quitting smoking: findings from the International Tobacco Control (ITC) Four Country Survey. Addiction. 2009;104:2075–2087. doi: 10.1111/j.1360-0443.2009.02731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Solomon LJ, Fingar JR, Naud S, Helzer JE, Callas PW. The natural history of efforts to stop smoking: A prospective cohort study. Drug and Alcohol Dependence. 2012;128:171–174. doi: 10.1016/j.drugalcdep.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Solomon LJ, Naud S, Fingar JR, Helzer JE, Callas PW. Natural history of attempts to stop smoking. Nicotine & Tobacco Research. 2014 doi: 10.1093/ntr/ntu052. article published online April 9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland A, Borland R, Li Q, Yong H-H, McNeill A, Fong GT, Cummings KM. Individual-level predictors of cessation behaviours among participants in the International Tobacco Control (ITC) Four Country Survey. Tobacco Control. 2006;15:iii83–iii94. doi: 10.1136/tc.2005.013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymowitz N, Cummings KM, Hyland A, Lynn WR, Pechacek TF, Hartwell TD. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tobacco Control. 1997;6:S57–S62. doi: 10.1136/tc.6.suppl_2.s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japuntich SJ, Piper ME, Leventhal AM, Bolt DM, Baker TB. The effect of five smoking cessation pharmacotherapies on Smoking Cessation Milestones. Journal of Consulting and Clinical Psychology. 2011;79:34–42. doi: 10.1037/a0022154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery RW, Epstein LH, Wilson GT, Drewnowski A, Stunkard AJ, Wing RR. Long-term maintenance of weight loss: Current status. Health Psychology. 2000;19:5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation: who will quit with and without the nicotine patch. Journal of the American Medical Association. 1994;271:589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Ustün TB. The World Mental Health (WMH) Survey Initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) International Journal of Methods in Psychiatric Research. 2004;13:93–121. doi: 10.1002/mpr.168. Retrieved from http://www.hcp.med.harvard.edu/ncs/ftpdir/Kessler%20Ustun%20WMHCIDI.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza ST, Collins LM. A mixture model of discontinuous development in heavy drinking from ages 18 to 30: The role of college enrollment. Journal of Studies on Alcohol. 2006;67:552–561. doi: 10.15288/jsa.2006.67.552. [DOI] [PubMed] [Google Scholar]
- Li Y, Wileyto EP, Heitjan DF. Statistical analysis of daily smoking status in smoking cessation clinical trials. Addiction. 2011;106:2039–2046. doi: 10.1111/j.1360-0443.2011.03519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke SE, Gall LC, Norman GJ. Attrition and adherence rates of sustained vs. intermittent exercise interventions. Annals of Behavioral Medicine. 2011;42:197–209. doi: 10.1007/s12160-011-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: An electronic diary study. Journal of Abnormal Psychology. 2006;115:454–466. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Fiore MC, Baker TB. A randomized, controlled clinical trial of bupropion SR and individual smoking cessation counseling. Nicotine & Tobacco Research. 2008a;10:717–729. doi: 10.1080/14622200801968343. [DOI] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Baker TB. Psychological mediators of bupropion SR treatment for smoking cessation. Addiction. 2008b;103:1521–1533. doi: 10.1111/j.1360-0443.2008.02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan GJ, Peel D. Finite Mixture Models. New York: John Wiley; 2000. [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic mental illness: Implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction. 2006;101:212–222. doi: 10.1111/j.1360-0443.2006.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Seventh Edition. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. 2006 Retrieved from http://www.statmodel.com/recpapers.shtml. [Google Scholar]
- Ossip-Klein DJ, Bigelow G, Parker SR, Curry S, Hall S, Kirkland S. Task Force 1: Classification and assessment of smoking behavior. Health Psychology. 1986;5:s3–s11. [PubMed] [Google Scholar]
- Partos TR, Borland R, Yong H-H, Hylund A, Cummings KM. The quitting rollercoaster: How recent quitting history affects cessation outcomes (Data from the International Tobacco Control 4-Country Cohort Study) Nicotine & Tobacco Research. 2013;15:1578–1587. doi: 10.1093/ntr/ntt025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EN, Hughes JR. The day-to-day process of stopping or reducing smoking: A prospective study of self-changers. Nicotine & Tobacco Research. 2009;11:1083–1092. doi: 10.1093/ntr/ntp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Fiore MC, McCarthy DE, Baker TB. Have we lost our way? The need for dynamic formulations of smoking relapse proneness. Addiction. 2002;97:1093–1108. doi: 10.1046/j.1360-0443.2002.00216.x. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Piper ME, Baker TB. Tobacco dependence: insights from investigations of self-reported motives. Current Directions in Psychological Science. 2010;19:395–401. doi: 10.1177/0963721410389460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, McCarthy DE, Baker TB. Assessing tobacco dependence: A guide to measure evaluation and selection. Nicotine & Tobacco Research. 2006;8:339–351. doi: 10.1080/14622200600672765. [DOI] [PubMed] [Google Scholar]
- Piper ME, McCarthy DE, Bolt DM, Smith SS, Lerman C, Fiore MC, Baker TB. Assessing dimensions of the nicotine dependence phenotype: An evaluation of the Nicotine Dependence Syndrome Scale (NDSS) and the Wisconsin Inventory of Smoking Dependence Motives (WISDM) Nicotine & Tobacco Research. 2008;10:1009–1020. doi: 10.1080/14622200802097563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: The Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) Journal of Consulting and Clinical Psychology. 2004;72:139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, Baker TB. A randomized placebo-controlled clinical trial of five smoking cessation pharmacotherapies. Archived of General Psychiatry. 2009;66:1253–1262. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fleming MF, Bittrich AA, Brown JL, Baker TB. Psychiatric disorders in smokers seeking treatment for tobacco dependence: Relations with tobacco dependence and cessation. Journal of Consulting and Clinical Psychology. 2010;78:13–23. doi: 10.1037/a0018065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm. American Journal of Psychiatry. 2014;11:1199–1205. doi: 10.1176/appi.ajp.2014.13050595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JS, Chassin L, Presson C, Sherman SJ, Stein MD, Col N. A latent class typology of young women smokers. Addiction. 2007;102:1310–1319. doi: 10.1111/j.1360-0443.2007.01889.x. [DOI] [PubMed] [Google Scholar]
- Schwarz Gideon E. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- Sclove LS. Application of model-selection criteria to some problems in multivariate analysis. Psychometrika. 1987;52:333–343. [Google Scholar]
- Sher KJ, Jackson KM, Steinley D. Alcohol use trajectories and the ubiquitous cat’s cradle: Cause for concern? Journal of Abnormal Psychology. 2011;120:322–335. doi: 10.1037/a0021813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, Clark DB. Analyzing milestones in smoking cessation: illustration in a nicotine patch trial in adult smokers. Journal of Consulting and Clinical Psychology. 2006;74:276–285. doi: 10.1037/0022-006X.74.2.276. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ, Hickcox M. The Nicotine Dependence Syndrome Scale: a multidimensional measure of nicotine dependence. Nicotine & Tobacco Research. 2004;6:327–348. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Smith SS, McCarthy DE, Japuntich S, Christiansen B, Piper ME, Jorenby DE, Jackson TC. Randomized effectiveness trial of five smoking cessation pharmacotherapies combined with quit line counseling referral in primary care clinics. Archives of Internal Medicine. 2009;169:2148–2155. doi: 10.1001/archinternmed.2009.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead LF, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2012;10 doi: 10.1002/14651858.CD008286.pub2. Art. No.: CD008286. [DOI] [PubMed] [Google Scholar]
- Steidmann D, Manber R, Blasey C, Markowitz JC, Klein DN, Rothbaum BO, Thase, Arnow BA. Directing critical decision points in psychotherapy and psychotherapy + medication for chronic depression. Journal of Consulting and Clinical Psychology. 2013;81:783–792. doi: 10.1037/a0033250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Annals of Behavioral Medicine. 1994;16:199, 202. [Google Scholar]
- Storr CL, Zhou H, Liang K-Y, Anthony JC. Empirically derived latent classes of tobacco dependence syndromes observed in recent-onset tobacco smokers: Epidemiological evidence from a national probability sample survey. Nicotine & Tobacco Research. 2004;6:533–545. doi: 10.1080/14622200410001696493. [DOI] [PubMed] [Google Scholar]
- Sutfin EL, Reboussin BA, McCoy TP, Wolfson M. Are college student smokers really a homogeneous group? A latent class analysis of college student smokers. Nicotine & Tobacco Research. 2009;11:444–454. doi: 10.1093/ntr/ntp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake DS. A latent class analysis of nicotine-dependence criteria and use of alternate tobacco. Journal of Studies on Alcohol and Drugs. 2008;69:709–717. doi: 10.15288/jsad.2008.69.709. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Health Benefits of Smoking Cessation. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1990. DHHS Publication No. (CDC) 90-8416. Retrieved from http://profiles.nlm.nih.gov/NN/B/B/C/T/ [Google Scholar]
- Weisberg H. Central tendency and variability. In: Lewis-Beck MS, editor. Quantitative Applications in the Social Sciences: Vol. 83. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology. 1999;7:354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Wileyto EP, Patterson F, Niaura R, Epstein LH, Brown RA, Audrain-McGovern J, Lerman C. Recurrent event analysis of lapse and recover in a smoking cessation clinical trial using bupropion. Nicotine & Tobacco Research. 2005;7:257–268. doi: 10.1080/14622200500055673. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Composite International Diagnostic Interview. Geneva, Switzerland: World Health Organization; 1990. [Google Scholar]
- Xian H, Scherrer JF, Madden PA, Lyons MJ, Tsuang M, True WR, Eisen SA. Latent class typology of nicotine withdrawal: genetic contributions and association with failed smoking cessation and psychiatric disorders. Psychological Medicine. 2005;35:409–419. doi: 10.1017/s0033291704003289. [DOI] [PubMed] [Google Scholar]