Abstract
Objectives
To investigate executive function and adaptive behavior in persons with Muenke syndrome using validated instruments with a normative population and unaffected siblings as controls.
Study design
Participants in a cross sectional study included individuals with Muenke syndrome (P250R mutation in FGFR3) and their mutation negative siblings. Participants completed validated assessments of executive functioning (Behavior Rating Inventory of Executive Function; BRIEF) and adaptive behavior skills (Adaptive Behavior Assessment System; ABAS-II).
Results
Forty-four FGFR3 mutation positive individuals, median age 9 years, range 7 months to 52 years were enrolled. Additionally, 10 unaffected siblings were used as controls (5 males, 5 females, median age of 13 years, range 3 to 18 years). For the General Executive Composite scale of the BRIEF, 32.1% of the cohort had scores greater than +1.5 SD, signifying ―Potential Clinical Significance. For the General Adaptive Composite of the ABAS-II, 28.2% of affected individuals scored in the 3rd – 8th percentile of the normative population and 56.4% were below the ―Average category (less than the 25th percentile). Multiple regression analysis did not show that craniosynostosis was a predictor of BRIEF (P = 0.7) and ABAS-II scores (P = 0.7). In the sibling pair analysis, affected siblings performed significantly poorer in the BRIEF General Executive Composite and the ABAS-II General Adaptive Composite.
Conclusion
Individuals with Muenke syndrome are at an increased risk for developing adaptive and executive function behavioral changes when compared with a normative population and unaffected siblings.
Keywords: FGFR3, craniosynostosis, behavior
Craniosynostosis occurs in approximately 1 in 2,000 live births and is characterized by the premature fusion of one or more cranial sutures resulting in malformation of the skull.1 Potential consequences of abnormal skull growth include increased intracranial pressure, problems with hearing and vision, impaired blood flow in the cerebrum, and developmental delay.2,3 Muenke syndrome (OMIM 602849) constitutes the most common syndromic form of craniosynostosis, with an incidence of 1 in 30,000 births; of all patients with craniosynostosis, 8% manifest Muenke syndrome.2,4,5
Muenke syndrome is defined by the presence of a c.749 C>G FGFR3 mutation encoding a P250R substitution in the fibroblast growth factor receptor 3 protein, one of four tyrosine kinase receptors that bind fibroblast growth factors.6–7 FGFR3 is expressed during brain development, but its role in cognitive and behavioral phenotypes is still largely unknown.8–9
The classic presentation of Muenke syndrome includes uni- or bilateral coronal suture craniosynostosis, broad thumbs and toes, carpal and tarsal fusions, hearing loss, and seizures. In recent years, evidence for cognitive and behavioral differences in persons with Muenke syndrome has surfaced, yet research on this topic remains preliminary.10–15 There is also evidence that social and attention problems are more prevalent in Muenke syndrome than in the normative population or other craniosynostosis syndromes.15–16 However, studies on the cognitive, emotional, and behavioral component of the syndrome have included small numbers of patients and utilized varying tools to assess behavior and cognitive abilities. Our growing collection and experience with families known to carry the FGFR3 mutation associated with Muenke syndrome has generated increasing interest in exploring the broad spectrum of phenotypes associated with the mutation, and in particular the social and behavioral phenotypes.
This study utilized standardized tests including the Behavior Rating Inventory of Executive Function (BRIEF) and the Adaptive Behavior Assessment System® Second Edition (ABAS-II) to evaluate executive function and adaptive behaviors in individuals affected with Muenke syndrome. Executive function has been defined as “a set of interrelated functions that are responsible for purposeful, goal-directed, problem solving behavior.”17 These functions are instrumental in the process of intentionally directing or controlling one’s own behavior to achieve a certain goal or solve a problem, and include abilities such as planning and organizing a way to solve problems, initiating behavior, inhibition (controlling impulses), goal-setting, monitoring and evaluating behavior, as well as shifting from one situation or aspect of a problem to another.17 Adaptive behavior, on the other hand, entails a collection of age-appropriate skills that are needed to “adapt to” or to function independently in one’s environment. Adaptive skills are practical, everyday skills needed for “effectively and independently taking care of oneself and interacting with other people.”18
METHODS
The study was approved by the National Human Genome Research Institute (NHGRI) Institutional Review Board (05-HG-0131) at the National Institutes of Health (NIH) in Bethesda, Maryland. Participants had molecular testing and individuals carrying the FGFR3 P250R mutation were considered affected. All participants or their legal guardian provided informed consent to participate in the study. Participants completed a series of assessments and questionnaires in one of three ways: over the phone, in person at our Bethesda campus, or online via a website created for our study (http://muenkesyndrome.nhgri.nih.gov). When participants elected to complete the forms online, their responses were recorded within a secure database.
Testing
Executive function was assessed by using the Behavior Rating Inventory of Executive Function (BRIEF) with a license to use on our website purchased through Psychological Assessment Resources, Inc. (www.parinc.com). The BRIEF measures the construct of executive function in all ethnicities, 2 through 90 years of age.17 There are four versions of the BRIEF that correspond to different age groups and respondents: BRIEF, BRIEF-P (preschool version), BRIEF-SR (self-report version), and BRIEF-A (adult version). We chose to use three of the four versions: BRIEF-P for children 2–5 years, BRIEF for children 5–18 years old (parent or teacher forms); and BRIEF-A for adults 18–90 years old (self-report or informant report forms). All versions of the assessment produce clinical scales labeled Inhibit, Shift, Emotional Control, Working Memory, and Plan/Organize, as well as a Global Executive Composite (GEC), which is a summary score incorporating all clinical scales. Raw scores were converted into scaled and standard scores based on age and sex (T scores). For the normative population, the mean (T score) is 50 and the standard deviation is 10. A higher score indicates poorer executive function. BRIEF T scores are subdivided into three categories: “Average” scores less than 60 (< +1 SD), scores ranging from 60–64 (≥ +1 SD and < +1.5 SD), and scores equal to and greater than 65 (≥ +1.5 SD). According to the BRIEF manual, T scores of equal to or more than 65 are considered abnormally elevated and having potential clinical significance.19 An inconsistency scale was used to evaluate the validity of the data and indicated the extent to which the respondent answered similar BRIEF items in an inconsistent manner. If a participant’s answers were scored as “Inconsistent,” then that participant’s data were excluded from analysis.
Adaptive behavior was assessed with the Adaptive Behavior Assessment System - Second Edition (ABAS-II) with a license to use on our website purchased through Western Psychological Services (wpspublish.com). The ABAS-II is a tool designed to measure the adaptive behavior of individuals of all ethnicities between the ages of 0 and 89 years.18 There are three age groups and three response forms: a parent/primary caregiver form for young children 0–5 years old, a parent form for 5–21 years old, and an adult form for individuals 16–89 years old. The ABAS-II includes composite scores for conceptual, social, and practical domains, as well as a Global Adaptive Composite (GAC). Raw scores were converted into standardized T scores based on age. The normative population’s mean T score is 100 and the standard deviation is 15. A lower score signifies worse adaptive behavior. According to the ABAS-II manual, scores are divided into the following categories based on percentiles (%) of the normative population: very superior > 130 (≥ 98%); superior 120–129 (91–97%); above average 110–119 (75–90%); average 90–109 (25–74%); below average 80–89 (9–24%); borderline 71–79 (3–8%); extremely low 70 or less (≤2%).
In addition to collecting data on executive functioning and adaptive behavior, the study also collected data on participants’ medical, family, and school/work histories. Mutation status for all participants was determined from CLIA (Clinical Laboratory Improvement Act of 1988) approved FGFR3 mutation testing.
Statistical Analyses
For all data analysis, we used R version 3.1.2 and Microsoft Excel for Mac 2011, Version 14.4.5 with StatPlus:mac V5. Total cohort FGFR3 P250R positive participant means and standard deviation were calculated for the ABAS-II and BRIEF domains and compared with normative populations. Additionally, we compared ABAS-II and BRIEF means between probands and their age and sex matched, mutation-negative siblings; significance was performed using paired t-tests. Affected and unaffected siblings were paired to each other based on lowest difference in age. By chance, all sibling pairs except for one were the same sex. Effect size, using Cohen’s dz, was determined by comparing affected individuals (the group with unaffected siblings) with unaffected siblings.
Multiple regression analysis was performed using the BRIEF or ABAS-II score as the dependent variable and sex, age, seizure history (at least one reported seizure), craniosynostosis presence, craniosynostosis surgery history, developmental delay, and hearing loss as independent variables. Additionally, family identification as an independent variable was used to evaluate if large families affected outcomes.
RESULTS
Participants in our study (Table I) included a total of 44 affected individuals (21 males, 23 females); median age was 9 years (range of 7 months to 52 years) and 10 unaffected individuals (5 males, 5 females, median age of 13 years, range 3 to 18 years); there were a total of 10 sibling pairs. Participants self identified as 95% Caucasian, and 5% as other. The sex ratio reflects findings from the literature that males and females are equally likely to have Muenke syndrome. Of the 44 affected participants, 14 participants had a history of seizures (32.6%), 35 had craniosynostosis (83.3%), 29 participants (67.4%) had a history of at least one craniosynostosis corrective surgery, and 23 (63.9%) had a history of hearing loss (usually mild to moderate) (Table I). Of the 33 affected individuals in the cohort who were eligible for a diagnosis of attention deficit hyperactivity disorder (ADHD) (> 4 years old), 7 (21.8%) were reportedly diagnosed with ADHD (Table I), which included two affected individuals from the matched sibling groups. From the group of 10 unaffected siblings, one individual was reported as having ADHD.
Table I.
Characteristics of participants with Muenke syndrome (P250R mutation in FGFR3)
| Patient | Age at evaluation (y) |
Sex | Type of Craniosynostosis |
Seizure/s (Yes/No) |
Number of Operations |
Hearing Loss |
ADHD Diagnosis (reported) |
|---|---|---|---|---|---|---|---|
| 1 | 0.57 | M | Bicoronal | N | 0** | ? | NA |
| 2 | 0.67 | F | Bicoronal | N | 2 | 0 | NA |
| 3 | 2 | F | Bicoronal | N | 1 | 1 | NA |
| 4 | 1 | F | Unicoronal | N | 1 | 0 | NA |
| 5 | 1 | M | Unicoronal | Y | 1 | ? | NA |
| 6* | 1 | F | Unicoronal | Y | 1 | ? | NA |
| 7 | 2 | F | Bicoronal | N | 1 | 0 | NA |
| 8 | 2 | F | Unknown type | Y | 0** | 0 | NA |
| 9 | 3 | F | Bicoronal | Y | 1 | 1 | NA |
| 10 | 3 | M | Unicoronal | Y | 1 | 0 | NA |
| 11 | 4 | F | Bicoronal | N | 1 | 1 | 0 |
| 12 | 4 | M | Unknown Type | N | 0 | 1 | 0 |
| 13 | 4 | M | Bicoronal | N | 2 | 0 | 0 |
| 14 | 5 | F | Bicoronal | N | 2 | 1 | 0 |
| 15* | 6 | M | Unicoronal | N | 1 | 1 | 0 |
| 16 | 6 | M | Bicoronal | N | 1 | 1 | 1 |
| 17* | 6 | M | Bicoronal | Y | 1 | 0 | 0 |
| 18* | 7 | F | Unicoronal | Y | 1 | 1 | 0 |
| 19 | 7 | M | Bicoronal | N | 1 | 0 | 0 |
| 20* | 8 | F | Bicoronal | N | 3 | 1 | 1 |
| 21 | 8 | F | Unknown | N | Unknown | Unknown | Unknown |
| 22 | 9 | M | Bicoronal | N | 1 | Unknown | 0 |
| 23* | 9 | M | None | N | 0 | 0 | 0 |
| 24 | 9 | F | Bicoronal | N | 1 | 1 | 1 |
| 25 | 10 | F | Unicoronal | N | 1 | 1 | 0 |
| 26 | 11 | F | Unicoronal | N | 1 | 1 | 0 |
| 27 | 11 | M | Sagittal | Y | 0 | 1 | 0 |
| 28* | 11 | M | None | Y | 0 | 0 | 0 |
| 29 | 13 | M | Bicoronal | N | 1 | 1 | 1 |
| 30 | 13 | M | Unknown type | N | 1 | ? | ? |
| 31 | 14 | F | Unicoronal | N | 1 | 1 | 0 |
| 32 | 14 | F | Unicoronal | N | 3 | 0 | 0 |
| 33 | 16 | F | None | N | 0 | ? | 0 |
| 34 | 16 | M | Bicoronal | N | 1 | 1 | 1 |
| 35* | 17 | M | Unicoronal | Y | 2 | 1 | 0 |
| 36 | 17 | M | Bicoronal | N | 0 | 1 | 0 |
| 37* | 17 | M | Unknown type | Y | 2 | 1 | 1 |
| 38 | 20 | M | None | Y | 0 | 0 | 0 |
| 39* | 23 | F | None | N | 0 | 1 | 0 |
| 40 | 31 | F | None | Y | 0 | 1 | 1 |
| 41 | 37 | F | Bicoronal | N | 0 | 0 | 0 |
| 42 | 39 | M | None | N | 0 | Unknown | 0 |
| 43 | 40 | F | Bicoronal | Y | 2 | 1 | 0 |
| 44 | 52 | F | Unknown | Unknown | 0 | 1 | 0 |
Paired with a sibling without Muenke syndrome (P250R mutation in FGFR3)
Surgery planned
NA: too young for reliable ADHD diagnosis (< 4 years old).
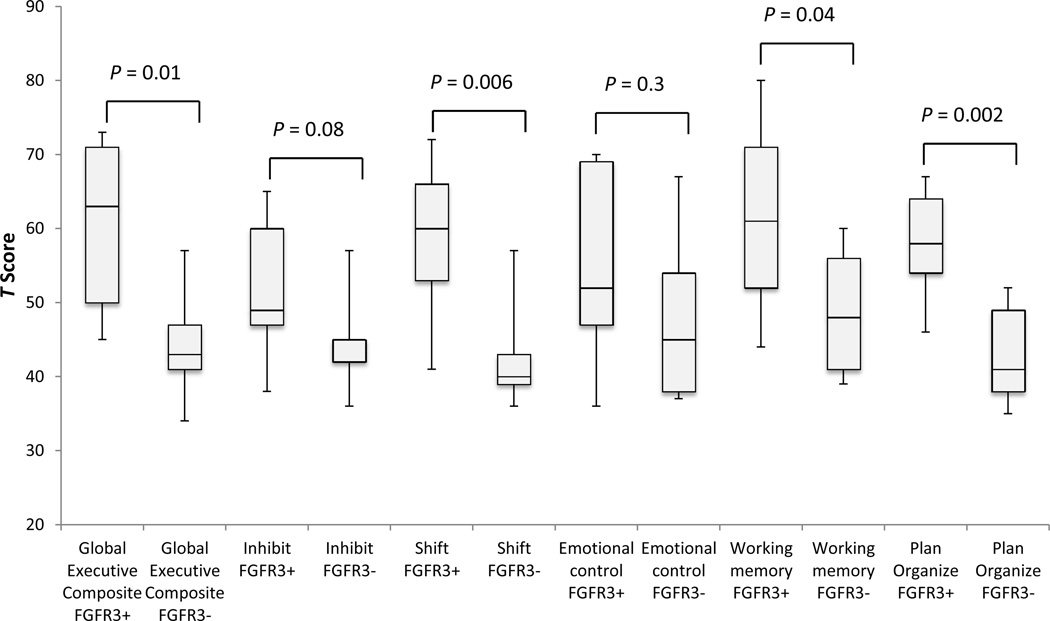
BRIEF data were available for 28 individuals: seven individuals were under two years of age and did not qualify to have a BRIEF assessment, and one participant’s BRIEF data was excluded due an “Inconsistent” score on the Inconsistency scale. In all categories, the total affected cohort had mean scores greater than the normative population. In the General Executive Composite scale (Table II), 32.1% of participants had scores greater than +1.5 SD, signifying “Potential Clinical Significance.” In the Working Memory (WM) scale, which has been associated with ADHD, the difference of means was greater than +1 SD, and 50% of participants scored +1.5 SD above the mean on the WM scale, indicating potential clinical significance. A relatively large percentage of affected participants had low levels of functioning skills in the Plan/Organize (46.5% above +1 SD), and Shift (50% above +1 SD) skill areas. For the Inhibit and Emotional Control scales, 28.6% and 25% of participants scored above +1 SD the mean, respectively. Sibling comparisons showed that there was a significant difference between affected and unaffected siblings in the Shift, Working Memory, Plan Organize, and Global Executive Composite, with effect sizes ranging from 1.5 to 1.9 (greater than 0.8 is generally considered large). Figure 1 is a box and whisker P\plot comparing affected and unaffected sibling T scores.
Table 2.
BRIEF and ABAS-II Scores for Total Cohort and Sibling Pair Comparisons. Effect sizes calculated using Cohen's dz (> 0.8 is considered large).
| Tests | Total Cohort | Sibling Pairs | |||
|---|---|---|---|---|---|
| FGFR3+ | FGFR3− |
P- value |
Cohen's dz | ||
| BRIEF | |||||
| Number of participants (n) | 28 | 9 | 9 | ||
| Global Executive Composite Mean (SD) | 58.8 (13.6) | 60.2 (10.8) | 43.8 (6.6) | 0.01 | 1.5 |
| Participants with Clinically Significant Global Executive Composite Scores* | 9 (32.1%) | ||||
| ABAS-II | |||||
| Number of participants (n) | 39 | 10 | 10 | ||
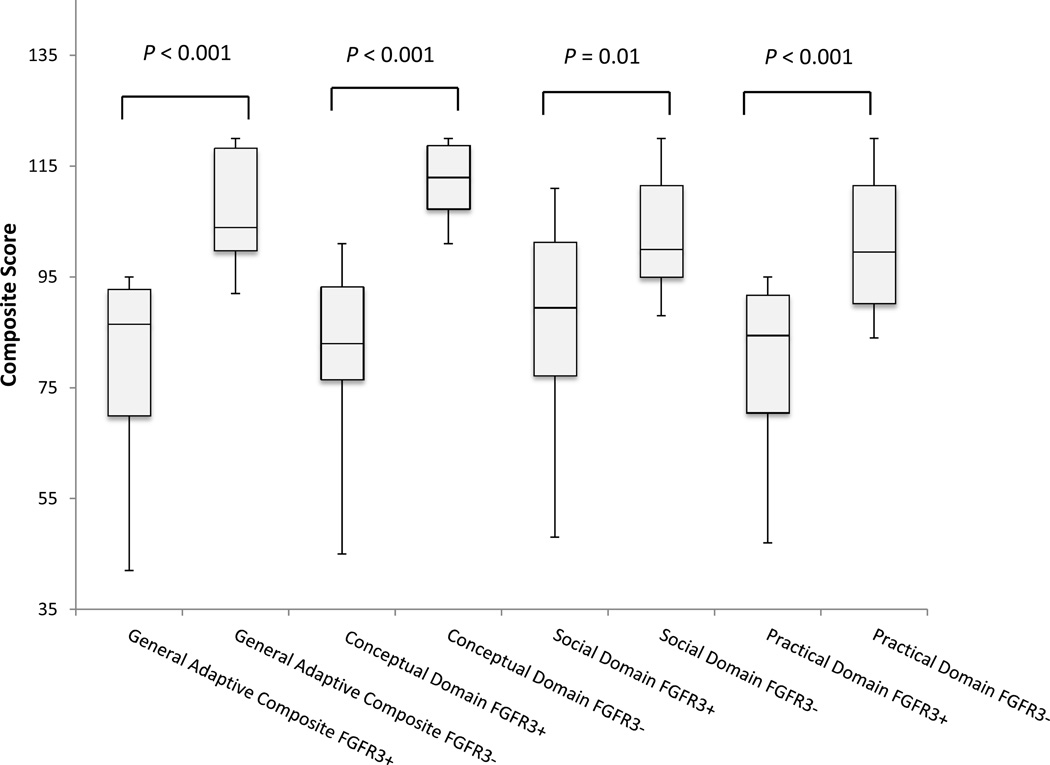
| General Adaptive Composite (SD) | 84.4 (19.3) | 79.2 (17.6) | 108.4 (10.6) | <0.001 | 2.1 |
| Participants with Below Average Scores** | 21 (53.8%) | ||||
Defined as Scores ≥ +1.5 SD
Scores < 90
Figure 1.
BRIEF T scores: Affected siblings (FGFR3+) vs. unaffected (FGFR3−) shown as Box and Whisker Plot.
Of the 44 affected individuals in the cohort, the ABAS-II was completed for 39 people. Table II summarizes the results of the ABAS-II. Means in the GAC and the other three domains of the total cohort of affected individuals were below the mean of the normative population, indicating poorer performance. Mean GAC and practical domain composite scores for affected individuals were 1 SD below the mean. Furthermore, 28.2% of affected individuals scored in the “Extremely Low” category (3rd–8th percentile of normative population) for the GAC and over 50% were below the “Average” category (less than the 25th percentile). When comparing sibling pairs, affected scores in all domains were significantly lower than unaffected (Table II; Figure 2), and effect sizes range from 1.4 to 2.7. Figure 2 is a box and whisker plot comparing affected and unaffected composite scores.
Figure 2.
ABAS-II scores: Affected siblings (FGFR3+) vs. unaffected (FGFR3−) shown as Box and Whisker Plot.
Multiple regression analysis for the BRIEF showed no significant predictive variables of the GEC when testing age, sex, seizure history, surgery history, craniosynostosis, and hearing loss (P=0.8, 0.9, 0.3, 0.8, 0.7, and 0.5, respectively). For the ABAS-II, regression analysis using the same independent variables showed that sex and age were significant predictors of the GAC (P=0.01, for both). Other P-values for seizure history, surgery history, craniosynostosis, and hearing loss were 0.7, 0.4, 0.7, and 0.1, respectively. A history of developmental delay (DD) was reported in 65% of participants with Muenke syndrome: 55% with speech/language delay, 31.6% with motor delay, and 15.8% with feeding delay. Multiple regression analysis showed, while controlling for sex, that DD history was not associated with lower performance on the BRIEF (GEC) or the ABAS-II (GAC) (P=0.05 and 0.09, respectively).
DISCUSSION
We investigated behavioral functioning in a large cohort with Muenke syndrome using standardized and validated instruments, with FGFR3 mutation negative siblings as controls, and evaluated for modifying factors such as craniosynostosis, surgery history, age, sex, and seizure history. This is the only study that we are aware that used affected FGFR3 P250R mutation positive participants who did not have craniosynostosis and sibling controls who were mutation-negative. Muenke syndrome is a molecular diagnosis based on a P250R mutation in FGFR3 and does not require craniosynostosis as a diagnostic criterion. Although previous research has examined similar constructs in craniosynostoses11,14, and case studies have collected data on behavior and development of individual patients with FGFR3-related craniosynostosis10,12–13,15–16,20, these studies have had small numbers of participants. Evaluating 13 children with Muenke syndrome, Maliepaard et al found that parents of individuals with Muenke syndrome reported higher levels of behavioral and emotional problems compared with parents of children of other craniosynostosis syndromes. Our larger study found that individuals with Muenke syndrome are at an increased risk for developing adaptive and executive function behavioral changes when compared with a normative population and unaffected siblings.
Adaptive skills as measured by the ABAS–II are practical, age-appropriate skills that are used to take independent care of oneself and interact with other people in order to function in one’s environment.18 In the ABAS-II, the General Adaptive Composite (GAC) provides the most complete measure of adaptive behavior, and is the most reliable and accurate estimate of overall adaptive functioning. Sibling pair analysis comparing 10 individuals with Muenke syndrome and their unaffected (mutation negative) siblings revealed significant differences in adaptive behavior in all four domains of the ABAS-II (Table II; Figure 2). In a separate analysis including 39 individuals with Muenke syndrome, mean adaptive functioning scores were below the normative mean and 28.2% of affected individuals were in the “Extremely Low” category, which is in the 3rd to 8th percentile of the normative population (Table II). This observation, combined with the large effect sizes from the sibling pair analysis (Table II), suggests that individuals with Muenke syndrome are at an increased risk for having deficits in adaptive functioning skills which are conceptual, social, and practical skills to function independently and meet daily environmental demands.
The BRIEF was utilized in this study to evaluate the behavioral component of executive function in which the Global Executive Composite (GEC) served as a summary score to classify levels of executive functioning. Sibling-pair analysis showed that affected siblings had significantly lower levels of executive functioning compared with their unaffected siblings (P=0.01 for GEC) (Figure 1). Analysis of the entire cohort of 28 affected participants showed that for half of the participants with Muenke syndrome, executive functioning levels (GEC) were at least one standard deviation above the normative population mean (greater scores indicating worse performance). Furthermore, 32% of the affected individuals scored greater than +1.5 SD above the normative population on in the GEC indicating potential clinical importance (Table II; Figure 1). The sibling pair analysis and the general cohort analysis both suggest that individuals with Muenke syndrome are at an increased risk of developing challenges with executive functioning compared with the general population and their siblings without Muenke syndrome. These difficulties may include trouble holding relevant information in working memory to complete a goal, directing oneself to plan and organize for future goals, and shifting one’s own attention; less prevalent problems include inhibition and directing one’s emotional responses appropriately.
The Working Memory clinical scale of the BRIEF measures a component of executive functioning, specifically, the capacity to hold information in the mind needed to complete a task such as a multistep activity or mental arithmetic.13 Parents of children with decreased scores on the Working Memory category report that their children are unable to complete tasks in the age appropriate time frame.17 Of all the affected participants in this study, half had abnormally elevated scores (> +1.5 SD) on this scale, suggesting problems with working memory that may have potential clinical importance. Sibling pair analysis also showed that affected participants performed at significantly lower levels than their unaffected siblings in Working Memory (P=0.04) (Table II; Figure 1). Children with ADHD are one group of patients with working memory deficits;21–23 the Working Memory clinical scale of the BRIEF also has predictive validity for ADHD-inattentive type.17 Although the BRIEF provides some insight into the presence of ADHD symptomology, it alone cannot be used to diagnose a patient with ADHD. We did not make a formal diagnosis of ADHD in our cohort; however, 7 individuals (21.8 %) reportedly had a history of ADHD diagnosis.
When multiple variable regression analysis was performed, age, sex, history of surgery, presence of craniosynostosis, history of seizures, and hearing loss did not affect BRIEF GEC scores, but age and sex were significantly predictive of the ABAS-II GAC scores. After adjusting for the above variables, females scored higher on the ABAS-II than males, and older individuals scored higher than younger participants on the ABAS-II. One possible explanation for this effect of age is the mode in which data were collected. Adult participants of the study completed self-report versions of the ABAS-II and the BRIEF, while parents completed the measures for the preschool and school age groups of the cohort. The two different modes of reporting may have led to a bias of older individuals being less likely to self-report problems with adaptive functioning. Additionally, adults may have acquired skills that allow them to compensate for adaptive difficulties.
Interestingly, the presence or absence of craniosynostosis did not significantly predict adaptive behavior and executive functioning., although only 9 individuals were not know to have craniosynostosis. This observation might suggest an intrinsic brain effect of the FGFR3 P250R mutation, distinct from the change in skull shape caused by craniosynostosis, consistent with FGFR3 expression pattern in the brain. FGFR3 is expressed in the developing brain and probably plays a role in cellular proliferation, differentiation, and migration8,24 Further research is needed to define the precise role of FGFR3 and the P250R mutation on brain development and behavior changes.
As a history of developmental delay was reported in 65% of participants with Muenke syndrome, we investigated the relationship between BRIEF and ABAS-II scores and developmental delay and found only a nonsignificant trend towards poorer scores in individuals with developmental delay. This study did not test for intellectual disability, which is known to affect standardized behavioral instruments. Maliepaard et al found in 13 patients with Muenke syndrome that the mean full-scale IQ (FSIQ) was in the normal range 95.2 (SD 16.4); however, 39% were found to have intellectual disability as defined as FSIQ < 85 (although, the widely accepted definition of intellectual disability is considered FSIQ <70).15 In our cohort, 4 affected participants (14.2%) were reported as having an intellectual disability.
A possible limitation of this study may be ascertainment bias. The molecular diagnosis of Muenke syndrome is often brought to the attention of clinicians when a proband with a more severe phenotype is born in a family. Therefore, there may be a proportion of undiagnosed individuals with Muenke syndrome who do not have severe clinical features and who may be not be well represented in this cohort (in which 83.3% have craniosynostosis). Another bias is a predominantly Caucasian cohort, which may reflect socioeconomic bias and access to care. Study participants were not charged for any diagnostic services provided.
Overall, the findings of this study have implications for the clinical management of children with Muenke syndrome and support the use of standardized screening tools for deficits in executive function and adaptive behavior. Although formal behavioral diagnoses were not made on participants, clinicians should be aware of the increased possibility of difficulties with executive function and behavior. Additionally, this study emphasizes the need for clinicians to extend FGFR3 testing to other family members, even in the absence of craniosynostosis, given the incomplete penetrance of Muenke syndrome; 16.7% of the study group with P250R mutations in FGFR3 did not have craniosynostosis.
Acknowledgments
Funnded by the Division of Intramural Research, National Human Genome Research Institute, National Institutes of Health, Department of Health and Human Services.
Abbreviations
- ABAS-II
Adaptive Behavior Assessment System Second Edition
- BRIEF
Behavior Rating Inventory of Executive Function
- GEC
Global Executive Composite
- GAC
Global Adaptive Composite
- ADHD
Attention deficit hyperactivity disorder
- DD
Developmental delay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Reprints: None
REFERENCES
- 1.Kimonis V, Gold JA, Hoffman TL, Panchal J, Boyadjiev SA. Genetics of craniosynostosis. Semin Pediatr Neurol. 2007 Sep;14:150–161. doi: 10.1016/j.spen.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Muenke M, Wilkie A. Craniosynostosis syndromes. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. Eighth Edition. McGraw-Hill Companies, Inc.; 2001. pp. 6117–6146. [Google Scholar]
- 3.Wilkie AO. Epidemiology and genetics of craniosynostosis. Am J Med Genet. 2000 Jan 3;90:82–84. doi: 10.1002/(sici)1096-8628(20000103)90:1<82::aid-ajmg15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Muenke M, Kress W, Collmann H, Solomon BD, editors. Monographs in Human Genetics. Volume 19: Craniosynostoses: Molecular Genetics, Principles of Diagnosis and Treatment. Vol. 19. Basel, Switzerland: Karger Publishing; 2011. [Google Scholar]
- 5.Wilkie AO, Byren JC, Hurst JA, et al. Prevalence and complications of single gene and chromosomal disorders in craniosynostosis. Pediatrics. 2010;126:e391–e400. doi: 10.1542/peds.2009-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellus GA, Gaudenz K, Zackai EH, Clarke LA, Szabo J, Francomano CA, et al. Identical mutations in three different fibroblast growth factor receptor genes in autosomal dominant craniosynostosis syndromes. Nat Genet. 1996;14:174–176. doi: 10.1038/ng1096-174. [DOI] [PubMed] [Google Scholar]
- 7.Muenke M, Gripp KW, McDonald-McGinn DM, Gaudenz K, Whitaker LA, Bartlett SP, et al. A unique point mutation in the fibroblast growth factor receptor 3 gene (FGFR3) defines a new craniosynostosis syndrome. Am J Hum Genet. 1997 Mar;60:555–564. [PMC free article] [PubMed] [Google Scholar]
- 8.Iwata T, Hevner RF. Fibroblast growth factor signaling in development of the cerebral cortex. Dev Growth Differ. 2009;51:299–323. doi: 10.1111/j.1440-169X.2009.01104.x. [DOI] [PubMed] [Google Scholar]
- 9.Robin NH. Molecular genetic advances in understanding craniosynostosis. Plast Reconstr Surg. 1999;103:1060–1070. [PubMed] [Google Scholar]
- 10.Flapper WJ, Anderson PJ, Roberts RM, David DJ. Intellectual outcomes following protocol management in Crouzon, Pfeiffer, and Muenke syndromes. J Craniofac Surg. 2009;20:1252–1255. doi: 10.1097/SCS.0b013e3181acdf9a. [DOI] [PubMed] [Google Scholar]
- 11.Bannink N, Maliepaard M, Raat H, Joosten KF, Mathijssen IM. Health-related quality of life in children and adolescents with syndromic craniosynostosis. J Plast Reconstr Aesthet Surg. 2010 Dec;63:1972–1981. doi: 10.1016/j.bjps.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 12.Bannink N, Maliepaard M, Raat H, Joosten KF, Mathijssen IM. Obstructive sleep apnea-specific quality of life and behavioral problems in children with syndromic craniosynostosis. J Dev Behav Pediatr. 2011 Apr;32:233–238. doi: 10.1097/DBP.0b013e318206d5e3. [DOI] [PubMed] [Google Scholar]
- 13.de Jong T, Maliepaard M, Bannink N, Raat H, Mathijssen IM. Health-related problems and quality of life in patients with syndromic and complex craniosynostosis. Childs Nerv Syst. 2012 Jun;28:879–882. doi: 10.1007/s00381-012-1681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapp-Simon KA, Collett BR, Barr-Schinzel MA, Cradock MM, Buono LA, Pietila KE, et al. Behavioral adjustment of toddler and preschool-aged children with single-suture craniosynostosis. Plast Reconstr Surg. 2012 Sep;130:635–647. doi: 10.1097/PRS.0b013e31825dc18b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maliepaard M, Mathijssen IM, Oosterlaan J, Okkerse JM. Intellectual, behavioral, and emotional functioning in children with syndromic craniosynostosis. Pediatrics. 2014 Jun;133:e1608–e1615. doi: 10.1542/peds.2013-3077. [DOI] [PubMed] [Google Scholar]
- 16.Escobar LF, Hiett AK, Marnocha A. Significant phenotypic variability of Muenke syndrome in identical twins. Am J Med Genet A. 2009;149A:1273–1276. doi: 10.1002/ajmg.a.32841. [DOI] [PubMed] [Google Scholar]
- 17.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Child Neuropsychol. 2000 Sep;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- 18.Harrison P, Oakland T. Adaptive Behavior Assessment System—Second edition (ABAS-II) San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 19.Gioia GA, Isquith PK, Guy SC, Kenworthy L BRIEF. Professional Manual. Lutz, FL.: PAR; 2000. Behavior Rating Inventory of Executive Function. [Google Scholar]
- 20.Doherty ES, Lacbawan F, Hadley DW, Brewer C, Zalewski C, Kim HJ, et al. Muenke syndrome (FGFR3-related craniosynostosis): expansion of the phenotype and review of the literature. Am J Med Genet A. 2007;143A:3204–3215. doi: 10.1002/ajmg.a.32078. [DOI] [PubMed] [Google Scholar]
- 21.Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. J Child Psychol Psychiatry. 1996 Jan;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- 22.Klingberg T, Forssberb H, Westerberg H. Training of working memory in children with ADHD. J Clin Exp Neuropsychol. 2002 Sep;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- 23.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005 Jun 1;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Mason I. Initiation to end point: the multiple roles of fibroblast growth factors in neural development. Nat Rev Neurosci. 2007;8:583–596. doi: 10.1038/nrn2189. [DOI] [PubMed] [Google Scholar]