Abstract
Objective
To compare rates of hospitalizations for serious infections, trends in rates from 1996 to 2011, and in-hospital mortality between patients with systemic lupus erythematosus (SLE) and those without SLE in a national sample.
Methods
We analyzed hospitalizations for pneumonia, bacteremia/sepsis, urinary tract infections, skin infections, and opportunistic infections among adults in the Nationwide Inpatient Sample. We compared rates of hospitalizations yearly among patients with SLE and the general population. We also computed odds ratios for in-hospital mortality.
Results
In 1996, the estimated number of hospitalizations for pneumonia in patients with SLE was 4382, followed by sepsis (2305), skin infections (1422), urinary tract infections (643), and opportunistic infections (370). Rates were much higher in SLE than those without SLE, with age-adjusted relative risks ranging from 5.7 (95% confidence interval (CI) 5.5, 6.0) for pneumonia to 9.8 (95% CI 9.1, 10.7) for urinary tract infection in 1996. Risks increased over time, so that by 2011, all relative risks exceeded 12.0. Overall risk of in-hospital mortality was higher in SLE only for opportunistic infections (adjusted odds ratio 1.52; 95% CI 1.12, 2.07). However, in pneumonia and sepsis, mortality risks were higher in SLE among those that required mechanical ventilation.
Conclusion
Hospitalization rates for serious infections in SLE increased substantially between 1996 and 2011, reaching over 12 times higher than in patients without SLE in 2011. Reasons for this acceleration are unclear. In-hospital mortality was higher among patients with SLE and opportunistic infections, and those with pneumonia or sepsis who required mechanical ventilation.
Serious infectious diseases are recognized as major causes of morbidity and mortality in patients with systemic lupus erythematosus (SLE), accounting for 13% to 37% of hospitalizations, 65% of avoidable hospitalizations and one-third of deaths [1–9]. Pneumonia, urinary tract, and skin infections are among the most commonly reported infections causing hospitalization in SLE, while bacteremia and sepsis, often complicated by organ failure, are leading causes of in-hospital mortality [1–5, 8–14]. The risk of serious infections in patients with SLE has been reported to be increased among those with active SLE-related inflammation and with the use of immunosuppressive medications [9–14]. Immunosuppression also increases the risk of opportunistic infections [14, 15]. These results are largely based on clinical cohorts at referral centers, and may therefore not be generalizable.
In the general population, rates of hospitalization for infections have increased markedly over the past two decades, including a 52% increase in hospitalizations for skin infections and a 190% increase for sepsis [16–18]. These increases have been attributed to the growing aged population with a higher prevalence of comorbidities, and greater use of medical interventions. Additionally, better recognition of the adverse consequences of major infections may have led to lower thresholds for hospitalization. Conversely, in-hospital mortality due to serious infections has decreased in the past decade, possibly related to the development and adoption of new guidelines for the management of serious infections [18–20]. However, it is not known if similar trends have occurred among immunosuppressed populations, including patients with SLE.
The purpose of this study was to compare relative risks and trends from 1996 to 2011 in hospitalization rates due to serious infections in hospitalizations of adults with SLE in the US with those of the general population. We also sought to determine if the risks of in-hospital mortality related to serious infections differed between patients with SLE and those without SLE. We used a national population-based sample to enhance the generalizability of the results. Further understanding of the factors contributing to infection-related mortality in SLE may lead to improvements in the clinical outcomes of these patients. We hypothesized that risks of infections and infection-related mortality would be higher in SLE than in those without SLE, but that trends in infections over time in SLE would parallel those for the general population.
METHODS
Study design and data source
We conducted a retrospective analysis using data of the Nationwide Inpatient Sample (NIS), Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality [21]. The NIS is the largest publicly available inpatient hospitalization database in the US, and includes hospital discharge abstracts from a 20% stratified probability sample of community (non-federal) hospitals. Hospitals are sampled based on geographic location, type of ownership, size, urban or rural location, and teaching status to produce a nationally-representative sample of hospitalizations. Hospitals are resampled annually.
All discharge abstracts of the selected hospitals are included, totaling approximately 8 million hospitalizations annually. Each discharge abstract contains patient demographic characteristics and disposition, type of medical insurance, hospital characteristics, and up to 25 discharge diagnoses and 15 procedures (by International Classification of Diseases-9 (ICD-9) codes). Information on medications and laboratory test results are not included. Unique patient identifiers are also not included, so the unit of analysis is therefore hospitalizations rather than patients.
We included data from 1996 to 2011. NIS data were provided through a data use agreement. This study was approved as exempted human subjects research by the NIH Office of Human Subjects Research.
Identification of study groups
We used the ICD-9 code of the primary discharge diagnosis to identify hospitalizations for which a serious infection was the primary reason for admission. We assembled five infectious hospitalization cohorts: pneumonia (ICD-9 codes 003.22, 481.0, 513.0, 480, 482, 483, 485, 486), sepsis/bacteremia (hereafter, sepsis; ICD-9 codes 038, 790.7), urinary tract infection (ICD-9 code 590), skin and soft tissue infections (ICD-9 codes 040.0, 569.61, 681, 682, 785.4, 728.86, 035), and opportunistic infections. Hospitalizations due to opportunistic infections included tuberculosis (ICD-9 codes 010–018), nontuberculous mycobacteria (031), cytomegalovirus (078.5), Epstein-Barr virus (075), herpes zoster (053), candidiasis (112.4, 112.5, 112.81, 112.83), toxoplasmosis (130), pneumocystosis (136.3), cryptococcosis (117.5), listeriosis (027.0), nocardiosis (039), aspergillosis (117.3), coccidioidomycosis (114), histoplasmosis (115), and blastomycosis (116.0). The validity of these diagnosis codes in other administrative datasets was high, with positive predictive values of 70%–100% in studies of patients with rheumatoid arthritis [22–24].
We identified hospitalizations among patients with SLE as those with any secondary diagnosis with an ICD-9 code of 710.0. All other hospitalizations were classified as occurring in patients without SLE. We limited the study to hospitalizations of those age 18 years or older, because hospitalizations for serious infections among children with SLE are very rare [25]. To assess the accuracy of secondary diagnosis codes for identifying patients with SLE in administrative data, we applied our case definition (primary diagnosis of infection and secondary diagnosis of SLE) in the California Inpatient Hospitalization data, which included unique patient identifiers from 1996 to 2000. We then examined the proportion with a subsequent acute care hospitalization with SLE as a discharge diagnosis. Among 198 patients who met the case definition in 1996, 147 were rehospitalized in 1997 – 2000. Of these, 102 (69%) had SLE as a discharge diagnosis in the subsequent hospitalization, and an additional 12 (8%) had end-stage renal disease with glomerulonephritis, both common manifestations of SLE, as discharge diagnoses.
Covariates
We abstracted data on race/ethnicity (white, black, Hispanic, other), type of medical insurance (private, public, or none), and median household income of the ZIP code of residence (provided as quartiles from high to low). We computed the Elixhauser comorbidity score from the discharge diagnoses of each hospitalization, according to published algorithms [26]. The Elixhauser score is a weighted sum of 30 comorbid conditions and has been well validated as a predictor of inhospital mortality [27]. Hospital location and teaching status were combined as urban teaching hospital, urban non-teaching hospital, and rural hospital. Hospital size was categorized by NIS as large, medium, or small, the criteria for which differed among regions and urban or rural areas, and varied slightly by year. We also used the discharge diagnosis and procedure codes to identify four patient subsets with more severe disease which may be at increased risk of mortality: nephritis (ICD-9 codes 580–584), chronic renal failure (585, 586), central nervous system disease (seizures or psychosis) (345, 780.3, 293–298), and those who had mechanical ventilation during the hospitalization (ICD-9 procedure code 967) [28].
Statistical analysis
Analysis of trends
We examined trends over the study period in two outcomes: rates of hospitalization for each type of serious infection, and in-hospital mortality proportions among those with each type of infection. For each year, we computed hospitalization rates with 95% confidence intervals (CIs) for each infection as the weighted number of hospitalizations per population. For rates among patients without SLE, we used US Census data to estimate intercensal population sizes, and age-standardized these rates across years to the 2000 Standard Population. For hospitalization rates among patients with SLE, we used the age- and sex-specific prevalence data from two recent studies to estimate the number of persons with SLE in the US, and used these as the denominators for the rates [29, 30]. The SLE hospitalization rates were also age-standardized to the 2000 Standard Population to allow comparisons with the non-SLE (general population) rates. We then computed relative risks of hospitalization as the ratio of SLE hospitalizations to non-SLE hospitalizations for each infection by year. In a sensitivity analysis, we inflated the estimated SLE population by 20% to evaluate what effect a variation of this degree would have on the relative risks.
In-hospital mortality was defined as the proportion of hospitalizations ending in death for each type of serious infection.
Analysis of risks of in-hospital mortality
We tested differences in the risk of in-hospital mortality between hospitalizations of patients with SLE and those without SLE for each of the five serious infections, using logistic regression analysis. We limited this analysis to hospitalizations of patients age 18 to 64, because in-hospital mortality in elderly patients with SLE may be determined more by comorbidities than by SLE or its treatment. We also limited this analysis to data from 2002 to 2010, to avoid pooling very early years with later years when medical care might have changed, while retaining sufficient statistical power. Based on preliminary analyses, we estimated that analysis of 6 years of data would include a sufficient number of hospitalizations with pneumonia, sepsis, and skin infections to provide adequate power (type I error = 0.05; type II error = 0.20) to detect an odds ratio of 1.50 (comparing SLE versus non-SLE groups) as statistically significant in a univariate comparison, given 1% in-hospital mortality in the non-SLE group. To increase the power to detect associations for less common infections, we increased the number of years examined to nine.
We adjusted these analyses for patient age, sex, race/ethnicity, type of medical insurance, neighborhood income, Elixhauser index, geographic region, hospital location and teaching status, hospital bed size, and year of admission. Hospitalizations with missing data for any covariate were included using an indicator variable.
For hospitalizations due to pneumonia and sepsis, we also tested interactions between SLE and nephritis, chronic renal failure, central nervous system involvement, and need for mechanical ventilation to determine if mortality risks differed by these clinical manifestations between hospitalization of those with and without SLE. We did not test interactions for other infections because their mortality risks were low. All analyses accounted for the sampling design of the NIS and were weighted to provide nationally representative estimates, using survey procedures in SAS version 9.3 (Cary, NC).
RESULTS
Hospitalization rates and trends over the study period
Pneumonia was the most common serious infection among hospitalizations of adults with SLE, with an estimated 4382 hospitalizations in 1996; this number increased to 7282 in 2011. Sepsis was the second most common serious infection, with more than a tripling of the estimated number of hospitalizations from 1996 to 2011 (from 2305 to 7633). The estimated number of hospitalizations due to skin infections also tripled (from 1422 to 4370) over this time. Hospitalizations due to urinary tract infections among patients with SLE increased from 643 to 1129, and those for opportunistic infections increased from 370 to 627. Herpes zoster was the most common opportunistic infection, accounting for 40% of these hospitalizations.
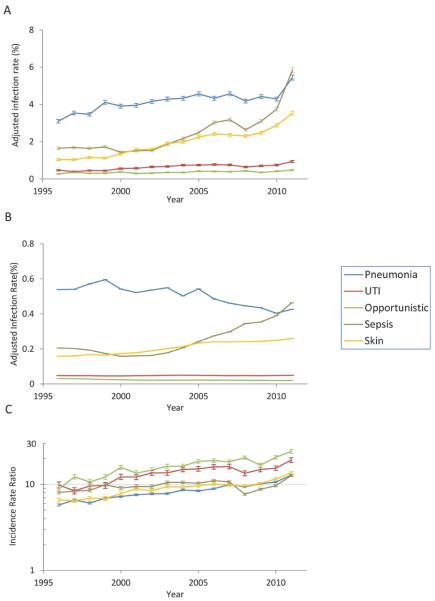
Age-adjusted rates of hospitalizations for pneumonia in SLE increased from 3.1% in 1996 to 5.4% in 2011 (Figure 1a and Supplemental table 1). The hospitalization rate for sepsis increased 3.6-fold over the study period, and exceeded that of pneumonia in 2011. The hospitalization rate for skin infection increased 3.5-fold during the study. Hospitalization rates for urinary tract and opportunistic infections were much lower than those of the other infections, and increased only slightly during the study period. Similar trends were present in patients without SLE, with the exception of a decrease in pneumonia hospitalization rates (Figure 1b and Supplemental table 1).
Figure 1.
Rates of infectious hospitalizations by calendar year among adults age 18 years or older with systemic lupus erythematosus (a) and without systemic lupus erythematosus (b). Values are percents, age-adjusted to the 2000 Standard Population. Age-adjusted relative risks of hospitalization by type of infection and calendar year in patients with systemic lupus erythematosus compared to patients without systemic lupus erythematosus (c). Error bars are 95% confidence intervals. Error bars are very close to the point estimates in (b) and are difficult to appreciate.
Relative risks of hospitalizations
In 1996, the age-adjusted risk of hospitalizations for pneumonia was 5.7 (95% confidence interval (CI) 5.5, 6.0) times higher in SLE than in the general population (Figure 1c and Supplemental table 1). The relative risk for sepsis hospitalization was 8.0 (95% CI 7.7, 8.4), while it was 6.6 (95% CI 6.2, 7.0) for skin infections, 9.8 (95% CI 9.1, 10.7) for urinary tract infection, and 8.8 (95% CI 7.9, 8.9) for opportunistic infections. In the sensitivity analysis in which we inflated the denominator of the SLE population by 20% (thereby decreasing the infection rates in SLE), the relative risks remained substantially higher in SLE (pneumonia 4.8 (95% CI 4.6, 5.0); sepsis 6.7 (95% CI 6.4, 7.0); skin infections 5.4 (95% CI 5.2, 5.8); urinary tract infections 8.2 (95% CI 7.5, 8.9); opportunistic infections 7.3 (95% CI 6.6, 8.2) in 1996).
Between 1996 to 2011, the relative risks increased for all five infections, indicating that hospitalizations for these infections increased at a faster rate in SLE than in the general population (Figure 1c). For example, the relative risk of hospitalization for opportunistic infections increased from 8.8 in 1996 to 24.1 in 2011. Over this period, the relative risk of hospitalizations for pneumonia doubled, and that for sepsis increased from 8.0 to 12.6.
In-hospital mortality
Among hospitalizations of patients with SLE, in-hospital mortality was highest among those with sepsis and opportunistic infections, and low among those hospitalized for skin and urinary tract infections (Figure 2). Estimated deaths among patients with SLE hospitalized with urinary tract infections ranged from 0 to 14 per year. In-hospital mortality generally increased during the early study years, but later decreased for all infections. Among hospitalizations for opportunistic infections, mortality was highest for those with listeriosis (27%), pneumocystosis (14%), aspergillosis (13%), nontuberculous mycobacterial infection (11%), candidiasis (11%), and cytomegalovirus (10%), and low for those with herpes zoster (0.5%).
Figure 2.

Proportion of hospitalizations among adults age 18 years or older with systemic lupus erythematosus with in-hospital mortality, by type of infection and calendar year. Results are three-year moving averages, therefore values are missing for 1996 and 2011. Error bars are 95% confidence intervals. Mortality for urinary tract infection hospitalizations are not represented because these were rare.
We compared the risk of in-hospital mortality between hospitalizations of patients with SLE and those without SLE among patients aged 18 to 64 (Supplemental table 2). The crude odds ratios for in-hospital mortality ranged from 0.95 to 1.74, but only the mortality risk of opportunistic infections was higher in SLE (Table 1). After adjustment for demographic and hospital characteristics and comorbidity, patients with SLE and opportunistic infections remained at increased risk of in-hospital mortality.
Table 1.
Risks of in-hospital mortality among hospitalizations of adults age 18 to 64 years with systemic lupus erythematosus and those without systemic lupus erythematosus in the United States from 2002 to 2010, by type of infection.
| Mortality (%) (95% CI) | ||||
|---|---|---|---|---|
| SLE | Non-SLE (reference group) | Crude Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI)* | |
| Pneumonia | 2.0 (1.7, 2.3) | 2.1 (2.0, 2.2) | 0.95 (0.82, 1.10) | 1.06 (0.92, 1.24) |
| Sepsis | 11.4 (10.6, 12.2) | 11.9 (11.7, 12.3) | 0.95 (0.87, 1.03) | 1.08 (0.99, 1.18) |
| Skin infections | 0.2 (0.1, 0.4) | 0.2 (0.1, 0.3) | 1.50 (0.91, 2.50) | 1.13 (0.67, 1.89) |
| Urinary tract infections | 0.2 (0, 0.4) | 0.1 (0, 0.2) | 1.74 (0.55, 5.50) | 1.32 (0.40, 4.30) |
| Opportunistic infections | 5.6 (4.1, 7.2) | 3.8 (3.6, 4.1) | 1.50 (1.12, 2.01) | 1.52 (1.12, 2.07) |
Adjusted for age, sex, race/ethnicity, type of medical insurance, neighborhood income, Elixhauser comorbidity score, geographic region, hospital size, hospital location and teaching status, and calendar year.
SLE= systemic lupus erythematosus
To determine if certain subgroups might be at increased risk of mortality when hospitalized with pneumonia or sepsis, we tested interactions between SLE and nephritis, chronic renal failure, central nervous system manifestations, and use of mechanical ventilation. There were no interactions with chronic renal failure or central nervous system manifestations. Use of mechanical ventilation was associated with higher risks of in-hospital mortality among patients with SLE hospitalized with either pneumonia or sepsis than among those without SLE (p < 0.0001) (Figure 3). For example, among pneumonia hospitalizations, mechanical ventilation was associated with a 21.3 fold increase in odds of mortality among hospitalizations without SLE, and a 30.3 fold increase in odds of mortality among hospitalizations with SLE. The presence of nephritis had a significant interaction with SLE status (p < 0.0001), with lower risks of mortality among hospitalizations with SLE and nephritis than among those without SLE who had nephritis (Figure 3). This may reflect differences in the severity of pneumonia and sepsis between patient groups. Among hospitalizations of patients with pneumonia and nephritis, mechanical ventilation was used in 19.5% of non-SLE cases but only 10.9% of SLE cases (age and sex-adjusted OR 2.04 (95% CI 1.75, 2.38). Similarly, among those with sepsis and nephritis, mechanical ventilation was used in 34.5% of non-SLE cases but only 24.9% of SLE cases (age and sex-adjusted OR 1.58 (95% CI 1.42, 1.75).
Figure 3.
Mortality risks in hospitalizations among adults age 18–64 years with or without systemic lupus erythematosus and nephritis hospitalized with pneumonia or sepsis (left) and subgroups with or without systemic lupus erythematosus who used mechanical ventilation among those hospitalized with pneumonia or sepsis (right). Values are odds ratios with 95% confidence intervals. The reference group in each case is that of hospitalizations among patients without systemic lupus erythematosus and without the clinical risk factor.
DISCUSSION
In this national population-based study, we found a high burden of hospitalizations for serious infections in adults with SLE. In 2011, the estimated number of hospitalizations for pneumonia and sepsis each exceeded 7000. The risk of hospitalization for these serious infections ranged from 12 to 24 times higher in patients with SLE than in the general population.
In studies of clinical cohorts of patients with SLE, the most common serious infections were pneumonia, (accounting for 25–50% of cases), followed by sepsis, skin infections, and pyleonephritis [1–5, 8–14]. Although the size of these cohorts was modest, the most prevalent types of serious infection were the same as found in our study. Because these were single-center studies, it was not clear if these results were representative of serious infections in SLE. Previous studies also did not compare rates of serious infections to those in persons without SLE. Our study confirms that pneumonia and sepsis are the most common serious infections in patients with SLE. Pneumonia, sepsis, and skin infections are also the most common serious infections requiring hospitalization in the general population [16], raising questions about how the rates of hospitalizations compare between SLE and the general population. We found strikingly high relative risks of hospitalizations for serious infections, which underscores the high burden of infections in SLE. These high risks are most likely due to more frequent use of immunosuppressive medications and reduced host resistance among patients with SLE. Renal dysfunction, hypoalbuminemia, cerebrovascular and lung disease, or diabetes mellitus and skin fragility due to corticosteroids, are among the comorbid complications of SLE that can increase infection risks.
Between 1996 and 2011, hospitalization rates increased for all five types of infections in both the SLE group and the general population, but the rates increased faster in SLE. The reasons for this are unclear. It is possible that these increases were associated with expanded use of corticosteroids and immunosuppressive medications, but there is no clear evidence that the prevalence or intensity of these treatments has changed in recent years. The acceleration in risk may also be due to changes in the features of patients with SLE. More patients with SLE are living longer [31, 32], but are also living with more comorbidities and organ damage, such as end-stage renal disease, vascular disease, chronic lung disease, or diabetes, which may increase infection risk [33–35]. More comorbidities may also increase the need for medical interventions, which in turn are associated with infection risk. Increased awareness of the potential consequences of infections in immunosuppressed patients may have also led to lower thresholds for hospitalization in recent years.
Opportunistic infections are a particular concern in patients with SLE because they are often difficult to treat and have a high mortality risk [14, 15, 36]. Opportunistic infections were uncommon in SLE, with 600 estimated hospitalizations annually. However, opportunistic infections had the highest relative risk among the five infections studied. Over the study period, this relative risk increased from 8.8 to 24.1. While this increase may be due to increased immunosuppression among patients with SLE, it may also reflect changes in the susceptibility of individuals without SLE to opportunistic infections. The latter is likely to have had some role, given the decreased prevalence of opportunistic infections among patients with human immunodeficiency virus (HIV) disease over this time. Opportunistic infections were present in 31% of HIV-related hospitalizations in 1994–1996, compared with 9.5% of HIV-related hospitalizations in 2003–2005 [37].
In-hospital mortality for all five serious infections in SLE decreased over the recent study years, which may reflect advances in diagnosis and management over time, or admission of less severely ill patients [38]. A recent meta-analysis reported the standardized mortality risk due to infections in SLE to be 5 times greater than the general population [39]. In our analysis, the risk of in-hospital mortality in SLE was generally similar to those without SLE hospitalized with the same type of infection. Therefore, the higher infection-related standardized mortality ratio in SLE is likely due to an increased frequency of infections, rather than greater severity of infections among patients with SLE. We found that hospitalizations for opportunistic infections, and those with severe pneumonia or severe sepsis as identified by the use of mechanical ventilation, were associated with higher risks of mortality among those with SLE. Patients with SLE may be less resilient in the setting of severe infection. Compared to patients with nephritis but without SLE, patients with SLE and nephritis appeared to have slightly lower risk of mortality with pneumonia or sepsis, but this may be attributable to higher proportions with less severe pneumonia or sepsis.
The strengths of our study include a large nationally representative and population-based sample, long study period that permitted examination of trends, and evaluation of multiple patient and hospital-associated covariates. However, there were several limitations. NIS provides only discharge diagnosis codes and does not include data on outpatient care or readmissions. We examined the likelihood that these hospitalizations represented unique patients, rather than readmissions, in the 2000 California Inpatient Hospital data and the 2008 New York State inpatient database. In both datasets, 93%–94% of hospitalizations of patients with SLE and a serious infection represented unique patients. Coding errors and missing data may be present, but we would not expect these to differentially affect patients with SLE and those without SLE. Up-coding of diagnoses due to higher hospital reimbursement for specific diagnoses such as sepsis may have influenced the trends in both hospitalization rates and mortality, but again would be expected to have affected the SLE group and the group without SLE similarly [40]. In addition, because NIS does not include data on microbiological tests or medications, we could not assess the potential impact of specific organisms and antibiotic choices or immunosuppressive therapy on outcomes. Lastly, given the lack of national data, we used data on SLE prevalence from two metropolitan areas to estimate the number of patients with SLE in the US.
Infections continue to be an important source of morbidity and mortality in SLE. Adoption of guidelines for the prevention and management of infections, as have been successfully used in other diseases [41, 42], are urgently needed. Vaccination programs, and changes in corticosteroid and immunosuppressive treatment strategies, may be important preventive measures [37, 43–45]. In addition, early diagnosis and treatment of serious infections, which depends on timely access to outpatient care, may help to further reduce the consequences of infections [8].
Supplementary Material
SIGNIFICANCE AND INNOVATION.
This is the first U.S. national population-based study to compare rates of serious infections in SLE to the general population.
This is the first population-based study to compare risks of infection-related mortality between patients with SLE and those without SLE.
ACKNOWLEDGEMENTS
This work was supported by the Intramural Research Program, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (ZIA AR041153). This study utilized the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health, Bethesda, MD (http://biowulf.nih.gov).
Footnotes
None of the authors has commercial or financial interests related to this work.
REFERENCES
- 1.Lee J, Dhillon N, Pope J. All-cause hospitalizations in systemic lupus erythematosus from a large Canadian referral centre. Rheumatology (Oxford) 2013;52:905–9. doi: 10.1093/rheumatology/kes391. [DOI] [PubMed] [Google Scholar]
- 2.Goldblatt F, Chambers S, Rahman A, Isenberg DA. Serious infections in British patients with systemic lupus erythematosus: hospitalisations and mortality. Lupus. 2009;18:682–9. doi: 10.1177/0961203308101019. [DOI] [PubMed] [Google Scholar]
- 3.Edwards CJ, Lian TY, Badsha H, Teh CL, Arden N, Chng HH. Hospitalization of individuals with systemic lupus erythematosus: characteristics and predictors of outcome. Lupus. 2003;12:672–6. doi: 10.1191/0961203303lu452oa. [DOI] [PubMed] [Google Scholar]
- 4.Gladman DD, Hussain F, Ibanez D, Urowitz MB. The nature and outcome of infection in systemic lupus erythematosus. Lupus. 2002;11:234–9. doi: 10.1191/0961203302lu170oa. [DOI] [PubMed] [Google Scholar]
- 5.Petri M, Genovese M. Incidence of and risk factors for hospitalizations in systemic lupus erythematosus: a prospective study of the Hopkins Lupus Cohort. J Rheumatol. 1992;19:1559–65. [PubMed] [Google Scholar]
- 6.Cervera R, Khamashta MA, Font J, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003;82:299–308. doi: 10.1097/01.md.0000091181.93122.55. [DOI] [PubMed] [Google Scholar]
- 7.Alarcón GS, McGwin G, Jr, Bastian HM, et al. Systemic lupus erythematosus in three ethnic groups. VII [correction of VIII]. Predictors of early mortality in the LUMINA cohort. LUMINA Study Group. Arthritis Rheum. 2001;45:191–202. doi: 10.1002/1529-0131(200104)45:2<191::AID-ANR173>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Ward MM. Avoidable hospitalizations in patients with systemic lupus erythematosus. Arthritis Care Res. 2008;59:162–8. doi: 10.1002/art.23346. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Irastorza G, Olivares N, Ruiz-Arruza I, Martinez-Berriotxoa A, Egurbide MV, Aguirre C. Predictors of major infections in systemic lupus erythematosus. Arthritis Res Ther. 2009;11:R109. doi: 10.1186/ar2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong SJ, Choi H, Lee HS, et al. Incidence and risk factors of infection in a single cohort of 110 adults with systemic lupus erythematosus. Scand J Infect Dis. 2009;41:268–74. doi: 10.1080/00365540902744741. [DOI] [PubMed] [Google Scholar]
- 11.Noël V, Lortholary O, Casassus P, et al. Risk factors and prognostic influence of infection in a single cohort of 87 adults with systemic lupus erythematosus. Ann Rheum Dis. 2001;60:1141–4. doi: 10.1136/ard.60.12.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosch X, Guilabert A, Pallarés L, et al. Infections in systemic lupus erythematosus: a prospective and controlled study of 110 patients. Lupus. 2006;15:584–9. doi: 10.1177/0961203306071919. [DOI] [PubMed] [Google Scholar]
- 13.Navarro-Zarza JE, Alvarez-Hernández E, Casasola-Vargas JC, Estrada-Castro E, Burgos-Vargas R. Prevalence of community-acquired and nosocomial infections in hospitalized patients with systemic lupus erythematosus. Lupus. 2010;19:43–8. doi: 10.1177/0961203309345776. [DOI] [PubMed] [Google Scholar]
- 14.Janwityanuchit S, Totemchokchyakarn K, Krachangwongchai K, Vatanasuk M. Infection in systemic lupus erythematosus. J Med Assoc Thai. 1993;76:542–8. [PubMed] [Google Scholar]
- 15.Pryor BD, Bologna SG, Kahl LE. Risk factors for serious infection during treatment with cyclophosphamide and high-dose corticosteroids for systemic lupus erythematosus. Arthritis Rheum. 1996;39:1475–82. doi: 10.1002/art.1780390906. [DOI] [PubMed] [Google Scholar]
- 16.Christensen KL, Holman RC, Steiner CA, Sejvar JJ, Stoll BJ, Schonberger LB. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025–35. doi: 10.1086/605562. [DOI] [PubMed] [Google Scholar]
- 17.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 18.Søgaard M, Nielsen RB, Schønheyder HC, Nørgaard M, Thomsen RW. Nationwide trends in pneumonia hospitalization rates and mortality, Denmark 1997–2011. Respir Med. 2014;108:1214–22. doi: 10.1016/j.rmed.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med. 2014;42:625–31. doi: 10.1097/CCM.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy MM, Dellinger RP, Townsend SR, et al. Surviving Sepsis Campaign. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38:367–74. doi: 10.1097/CCM.0b013e3181cb0cdc. [DOI] [PubMed] [Google Scholar]
- 21.HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality; Rockville, MD: 2002–2011. www.hcupus.ahrq.gov/nisoverview.jsp. [PubMed] [Google Scholar]
- 22.Schneeweiss S, Robicsek A, Scranton R, Zuckerman D, Solomon DH. Veterans' Affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol. 2007;60:397–409. doi: 10.1016/j.jclinepi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Grijalva CG, Chung CP, Stein CM, et al. Computerized definitions showed high positive predictive values for identifying hospitalizations for congestive heart failure and selected infections in Medicaid enrollees with rheumatoid arthritis. Pharmacoepidemiol Drug Safety. 2008;17:890–5. doi: 10.1002/pds.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patkar NM, Curtis JR, Teng GG, et al. Administrative codes combined with medical records based criteria accurately identified bacterial infections among rheumatoid arthritis patients. J Clin Epidemiol. 2009;62:321–7. doi: 10.1016/j.jclinepi.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knight AM, Weiss PF, Morales KH, Keren R. National trends in pediatric systemic lupus erythematosus hospitalization in the United States: 2000–2009. J Rheumatol. 2014;41:539–46. doi: 10.3899/jrheum.130592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–33. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 28.Ward MM. Development and testing of a systemic lupus-specific risk adjustment index for inhospital mortality. J Rheumatol. 2000;27:1408–13. [PubMed] [Google Scholar]
- 29.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: The Georgia Lupus Registry. Arthritis Rheumatol. 2014;66:357–68. doi: 10.1002/art.38239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somers EC, Marder W, Cagnoli P, et al. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol. 2014;66:369–78. doi: 10.1002/art.38238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urowitz MB, Gladman DD, Tom BD, Ibañez D, Farewell VT. Changing patterns in mortality and disease outcomes for patients with systemic lupus erythematosus. J Rheumatol. 2008;35:2152–8. doi: 10.3899/jrheum.080214. [DOI] [PubMed] [Google Scholar]
- 32.Bernatsky S, Boivin JF, Joseph L, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2550–7. doi: 10.1002/art.21955. [DOI] [PubMed] [Google Scholar]
- 33.Pineau CA, Bernatsky S, Abrahamowicz M, Neville C, Karp I, Clarke AE. A comparison of damage accrual across different calendar periods in systemic lupus erythematosus patients. Lupus. 2006;15:590–4. doi: 10.1177/0961203306071874. [DOI] [PubMed] [Google Scholar]
- 34.Chambers SA, Allen E, Rahman A, Isenberg D. Damage and mortality in a group of British patients with systemic lupus erythematosus followed up for over 10 years. Rheumatology (Oxford) 2009;48:673–5. doi: 10.1093/rheumatology/kep062. [DOI] [PubMed] [Google Scholar]
- 35.Mak A, Cheung MW, Chiew HJ, Liu Y, Ho RC. Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s. Semin Arthritis Rheum. 2012;41:830–9. doi: 10.1016/j.semarthrit.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Zandman-Goddard G, Shoenfeld Y. Infections and SLE. Autoimmunity. 2005;38:473–85. doi: 10.1080/08916930500285352. [DOI] [PubMed] [Google Scholar]
- 37.Buchacz K, Baker RK, Moorman AC, et al. HIV Outpatient Study (HOPS) Investigators. Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994–2005. AIDS. 2008;22:1345–54. doi: 10.1097/QAD.0b013e328304b38b. [DOI] [PubMed] [Google Scholar]
- 38.Barber C, Gold WL, Fortin PR. Infections in the lupus patient: perspectives on prevention. Curr Opin Rheumatol. 2011;23:358–65. doi: 10.1097/BOR.0b013e3283476cd8. [DOI] [PubMed] [Google Scholar]
- 39.Yurkovich M, Vostretsova K, Chen W, Aviña-Zubieta JA. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis Care Res. 2014;66:608–16. doi: 10.1002/acr.22173. [DOI] [PubMed] [Google Scholar]
- 40.Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA. 2012;307:1405–13. doi: 10.1001/jama.2012.384. [DOI] [PubMed] [Google Scholar]
- 41.Rahier JF, Magro F, Abreu C, et al. European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–68. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 42.Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated Guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58:1308–11. doi: 10.1093/cid/ciu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosca M, Tani C, Aringer M, et al. European League Against Rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies. Ann Rheum Dis. 2010;69:1269–1274. doi: 10.1136/ard.2009.117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houssiau FA, Isenberg D. Towards treating lupus nephritis without oral steroids: a dream-come-true? Ann Rheum Dis. 2013;72:1271–2. doi: 10.1136/annrheumdis-2013-203205. [DOI] [PubMed] [Google Scholar]
- 45.Kobashigawa JA. Strategies in immunosuppression after heart transplantation: is less better? Circ Heart Fail. 2011;4:111–3. doi: 10.1161/CIRCHEARTFAILURE.110.960690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.