Abstract
Our recent results demonstrated that bile acids facilitate virus escape from the endosomes into the cytoplasm for successful replication of porcine enteric calicivirus (PEC). We report a novel finding that bile acids can be substituted by cold treatment for endosomal escape and virus replication. This endosomal escape by cold treatment or bile acids is associated with ceramide formation by acid sphingomyelinase (ASM). ASM catalyzes hydrolysis of sphingomyelin into ceramide, which is known to destabilize lipid bilayer. Treatment of LLC-PK cells with bile acids or cold led to ceramide formation, and small molecule antagonists or siRNA of ASM blocked ceramide formation in the endosomes and significantly reduced PEC replication. Inhibition of ASM resulted in the retention of PEC, feline calicivirus or murine norovirus in the endosomes in correlation with reduced viral replication. These results suggest the importance of viral escape from the endosomes for the replication of various caliciviruses.
Introduction
Viruses in the Caliciviridae family are small non-enveloped viruses of 27–35 nm diameters with a single-stranded, positive-sense RNA genome of 7–8 kb. There are at least five genera in the Caliciviridae family: norovirus and sapovirus cause enteric infections in humans and animals, whereas lagovirus, vesivirus and nebovirus cause a range of diverse diseases mainly in animals (Green, 2007). Human norovirus in the Norovirus genus accounts for about 60% of gastroenteritis cases and cause 21 million cases of gastroenteritis and 800 deaths annually in the United States alone (Hall et al., 2012; Scallan et al., 2011). However, research on human norovirus has been hampered by the inability of the virus to grow in cell culture (Duizer et al., 2004; Herbst-Kralovetz et al., 2013). The difficulty in culturing human norovirus is thought to be related to the early stage of viral replication including virus uptake and/or uncoating process, because transfection of norovirus RNA into cultured cells was shown to lead to virus replication and release of viral particles into the medium (Guix et al., 2007). While it is reported that many caliciviruses utilize host endosomal trafficking system for entry into host cells (Gerondopoulos et al., 2010; Perry and Wobus, 2010; Stuart and Brown, 2006), little is known about virus entry pathway of human norovirus.
After viruses are internalized into cells via the endocytic pathways, they must escape from the endosomal compartments to the cytoplasm to initiate replication (Hogle, 2002; Kielian and Rey, 2006; Moyer and Nemerow, 2011). Enveloped viruses utilize fusion machinery in their envelop protein which fuses with the cellular membrane to release the viral genome into cytosol of host cells (Kielian and Rey, 2006). The activation of fusion protein in the endosomes is mediated by environmental factors including low pH, interaction with receptor (and co-receptor), endosomal proteolysis, or combination of any these factors (Chandran et al., 2005; Earp et al., 2005; Eckert and Kim, 2001; Feng et al., 1996; Heinz and Allison, 2000; Matsuyama et al., 2004; Mothes et al., 2000; Skehel et al., 1982). Non-enveloped viruses lack the fusion protein, but some viruses are known to utilize lytic factors for membrane disruption and penetration into cell cytoplasm (Moyer and Nemerow, 2011).
Acid sphingomyelinase (ASM) catalyzes hydrolysis of sphingomyelin to ceramide. Ceramide has emerged as an important mediator of diverse cellular effects from various stress stimuli including bacterial or viral infection, as well as ionizing radiation, UV light and heat (Gulbins and Kolesnick, 2002, 2003; He et al., 2003; Montes et al., 2008; Stancevic and Kolesnick, 2010). Modulation of the biophysical properties of membranes by ceramide has been reported to be associated with formation of small rafts that fuse together to form large ceramide-enriched membrane platforms, changes in membrane fluidity and permeability, facilitation of membrane fusion, or promotion of macropinocytosis (Basáñez et al., 1997; Gulbins et al., 2004; Gulbins and Kolesnick, 2002; Montes et al., 2002; Siskind and Colombini, 2000), formation of channels large enough for proteins to cross membranes or cause lipid flip-flop (Contreras et al., 2009; Samanta et al., 2011). Ceramide or ASM has also been shown to be required for entry of measles virus, rhinovirus, Japanese encephalitis virus and Ebolavirus (Avota et al., 2011; Grassmé et al., 2005; Miller et al., 2012; Tani et al., 2010). Our previous reports have shown that bile acids facilitate the endosomal escape of porcine enteric calicivirus (PEC, a sapovirus) (Shivanna et al., 2014a), but the exact mechanism involved is not yet well understood. In this study, we demonstrated that cold treatment (4°C for 1 h) during PEC entry into host cells resulted in ceramide formation in the endosomes and viral endosomal escape and replication in the absence of bile acid. Furthermore, PEC alone did not lead to ceramide formation in the endosomes while bile acid- or cold-shock treatment resulted in ASM-mediated ceramide formation in LLC-PK cells. Interestingly, feline calicivirus (FCV, a vesivirus) or murine norovirus-1 (MNV-1, a norovirus), that grows in the absence of bile acids in cell culture, induced ceramide formation in the target cells. Blocking of ceramide formation by inhibiting ASM activity significantly reduced the replication of PEC, FCV or MNV-1 in the cells. We further showed that inhibition of ASM activity lead to retention of PEC, FCV and MNV-1 in the cells by confocal microscopy, which suggests the crucial role of ceramide formation by ASM in viral endosomal escape during the early stage of calicivirus replication.
Materials and methods
Cells and viruses
PEC Cowden strain was propagated in LLC-PK cells in the presence of glycochenodeoxycholic acid (GCDCA, 100 μM) (Chang et al., 2004) in Eagle’s Minimal Essential Medium (MEM) supplemented with 5% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. FCV Urbana strain was propagated in CRFK cells in MEM containing 5% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. MNV-1 was propagated in RAW264.7 cells in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. The cultured viruses were concentrated by centrifugation at 27,000 rpm through a 40% w/v sucrose cushion at 4°C for 2 h in a SW27 rotor. The obtained pellet was resuspended in serum free MEM and stored in −80°C.
Reagents and antibodies
GCDCA and bromoethylamine hydrobromide was purchased from Sigma-Aldrich (St Louis, MO). AY9944, an ASM inhibitor, was purchased from Santa Cruz Biotech (Santa Crus, CA) Other ASM inhibitors including fluoxetine, desipramine, chlorpromazine were purchased from Sigma-Aldrich. For confocal microscopy, anti-PEC/cowden antibodies raised in swine (Chang et al., 2005), anti-FCV antibodies raised in guinea pig (Sosnovtsev and Green, 1995), anti-MNV-1 antibodies raised in guinea pig (Wobus et al., 2004), mouse IgM anti-ceramide antibody from Enzo Life Sciences, Inc (Farmingdale, NY) and rabbit polyclonal IgG anti-Rab7 antibodies purchased from Santa Cruz Biotech were used as the primary antibodies. The secondary antibodies used include PerCP-Cy5.5 anti-rabbit from Santa Cruz Biotech, FITC anti-swine IgG and FITC anti-mouse purchased from Kirkegaard and Perry Lab (Gaithersburg, MD) and FITC-anti-guinea pig IgG from Sigma-Aldrich. Additional PEC antibodies including anti-2AB, RdRp or VPg were described previously (Chang et al., 2005).
Infection and replication of PEC
Confluent LLC-PK cells were inoculated with PEC at an MOI of 50 in the absence or presence of GCDCA (100 μM) and incubated at 4°C or 37°C for 1 h. Following 1 h incubation, the plates were immediately transferred to 37°C, and incubated for additional 1 h. Then, virus infected cells were thoroughly washed twice with PBS, replenished with MEM containing 5 % FBS, and further incubated at 37°C for 12 h or other indicated time. Viral replication was quantified by real-time qRT-PCR (Shivanna et al., 2014a) or by the TCID50 assay (Reed and Muench, 1938). Total RNA was extracted from the cells (before extensive cell lysis) for real time qRT-PCR or the cells were disrupted with 3 cycles of freezing/thawing, and supernatant was used for virus titration by the TCID50 assay. Viral replication by cold treatment was also evaluated with immunofluorescence assay (IFA). Cells were inculated with PEC at an MOI of 50, incubated at 4°C or 37°C for 1 h, and then incubated at 37°C for 12 h. The infected cells were fixed with chilled methanol, and stained with PEC antibody (anti-2AB, RdRp or VPg) and FITC-conjugated rabbit anti-guinea pig IgG antibody.
Inhibition of ASM by siRNA or small molecule inhibitors
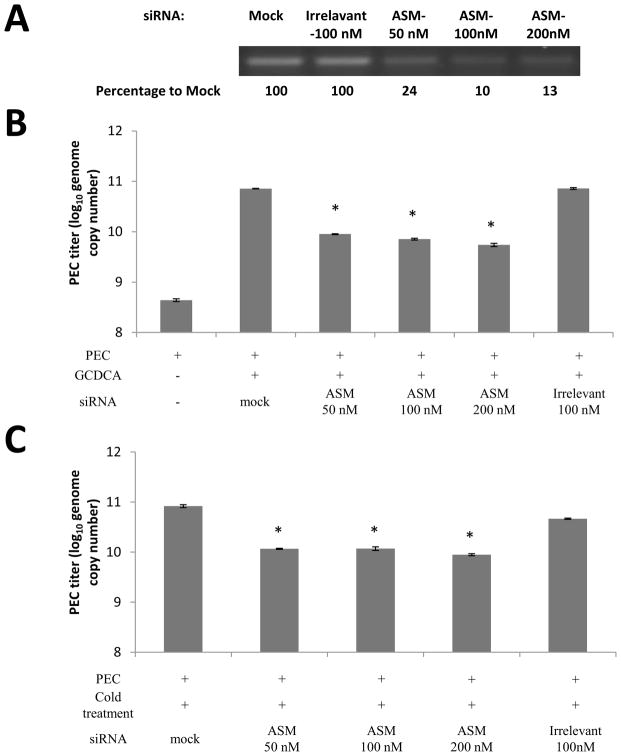
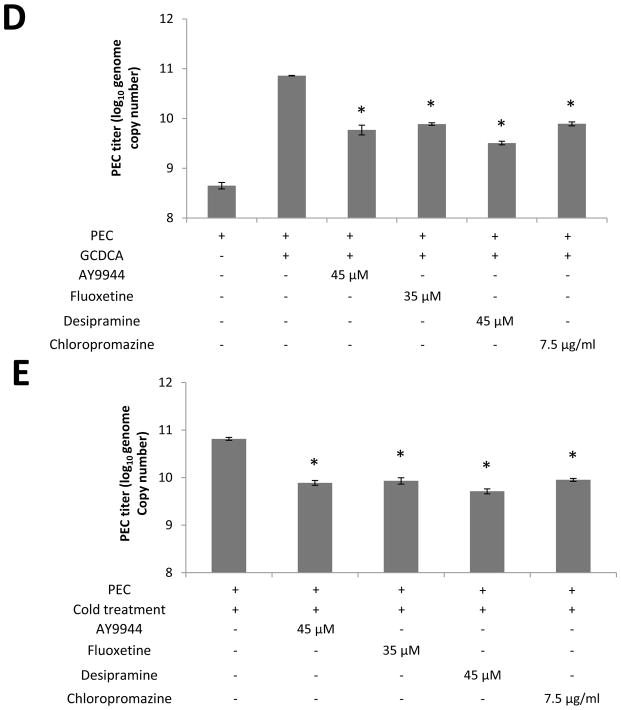
ASM siRNA and primers/probes for qRT-PCR for ASM were designed based on the porcine ASM gene (GenBank accession No. XM_005656607) and synthesized by Integrated DNA technology (Coralville, IA). The porcine ASM siRNAs were 5′-rArUrUrCrGrGrUrArArUrArArUrUrCrCrArG and 5′-rGrCrUrGrGrArGrCrUrGrGrArUrUrArU. For siRNA studies, one-day old LLC-PK cells were transfected with mock (transfection agent), irrelevant siRNA (negative control from Qiagen) or ASM siRNA, followed by incubation at 37°C for 48 h. Following transfection and incubation, ASM specific mRNA levels were quantified by real-time qRT-PCR using primers: F-5′-CCTTCGCACCCTCAGAATC, R- 5′-CAGAAGTTCTCACGGGAACAA, and Probe: 5′-56-FAM/ATTGAGAGA/ZEN/GATGAGGCGGAGGC/3IABkFQ/-3′. The RT-PCR products were also run on agarose gels to measure ASM mRNA levels. At 48 h post transfection, cells were inoculated with PEC at an MOI of 50 in the presence of GCDCA (100 μM, 37 °C) or treated with cold (4°C) for 1 h. Cells were then further incubated at 37 °C for 1 h, and washed with PBS. After washing, fresh media was added to cells and virus infected cells were further incubated for 1 h (for confocal study) or 12 h (for viral replication study). Inhibitors of ASM including AY9944, fluoxetine, desipramine and chlorpromazine were also used to examine their effects on ceramide formation and viral replication. For this inhibition study, optimal concentration of each ASM inhibitor was determined by examining the cytotoxic effects and the effects on ceramide formation and virus replication: 45 μM for AY9944, 35 μM for fluoxetine, 45 μM for desipramine, and 7.5 μg/ml for chlorpromazine. LLC-PK cells were treated with each compound at 37°C for 1 h. Cells were then inoculated with PEC at an MOI of 50 and further incubated at 37°C in the presence of GCDCA (100 μM) for 1 h or subject to cold treatment at 4°C for 1 h. Following 1 h incubation, cells were washed with PBS and fresh media were added to cells with or without an inhibitor. Cells were then further incubated at 37°C for 1 h (for confocal study) or 12 h (viral replication study).
Inactivation of FCV and MNV-1
The harvested virus suspension was centrifuged at 3000 rpm for 15 min to remove cell debris. The resulting supernatant virus suspension was mixed with fresh binary ethylenimine (BEI) at the ratio of 9:1 (0.1 M solution of bromoethylamine hydrobromide in 0.2N NaOH cyclized to BEI by incubating at 37°C for 1h in a water bath). The treated virus suspension was stirred for 24 h at 37°C. BEI is then inactivated by adding fresh filter sterilized sodium thiosulfate (1.0 M solution) at a rate of 30 ml per liter to make 0.03 M concentration. The mixture was mixed for 2–4 h at 37°C and the pH was adjusted to 6.6–7.2 with 4 N HCl. The inactivated virus suspension is then concentrated by spinning at 27,000 rpm through a 40% sucrose cushion at 4°C for 2 h in a SW27 rotor. The resulting pellet was resuspended in MEM and stored at 4°C. Inactivation of FCV or MNV-1 was confirmed by inoculating CRFK or RAW264.7 cells with the inactivated viruses.
FCV or MNV-1 infection
To study ceramide formation by FCV or MNV-1 infections, confluent CRFK or RAW267.4 cells were inoculated with mock-medium (Mock) or high MOI of FCV (50 MOI) or MNV-1 (30 MOI), respectively, and incubated at 37°C for 1 h. Inactivated FCV or MNV-1 was inoculated to CRFK and RAW267.4 (both target and non-target cells) at the same MOI used with live viruses. To test ASM activity on ceramide formation, inhibitors of ASM including AY9944, fluoxetine, desipramine and chlorpromazine were used for FCV or MNV-1 infection studies. Briefly, confluent CRFK and RAW267.4 cells were pretreated with each ASM inhibitor (AY9944 45 μM, fluoxetine 35 μM, desipramine 45 μM, chlorpromazine 7.5 μg/ml) at 37°C for 1 h. At this concentration, no cytotoxicity was observed. Cells were then inoculated with FCV (MOI 50) or MNV-1 (MOI 30) in presence of the inhibitor at 37°C for 1h. Following 1 h incubation, virus-infected cells were washed twice with PBS and replenished with fresh MEM containing the same concentration of ASM inhibitor. Cells were further incubated at 37°C for 12 h and the replication of FCV or MNV-1 was determined by the TCID50 assay.
Confocal microscopy
For confocal microscopy, cells were fixed in 4% paraformaldehyde (Sigma-Aldrich) in PBS (pH 7.4) at room temperature (RT) for 15 min, permeabilized with 0.1% Triton ×100 in PBS for 10 min at RT, washed three times with PBS, and incubated in blocking buffer (PBS containing 0.5% bovine serum albumin) for 15 min. Also fixed cells without permeabilization were prepared as a control. The cells were then incubated with primary antibodies specific to the capsid protein of PEC (1:200), MNV-1 (1:200) or FCV (1:200), or Rab7 (1:200) at 37°C to probe PEC, MNV-1, FCV or endosomes, respectively. After 2 h incubation at 37°C, cells were washed three times with PBS and further incubated at 37°C for 2 h with appropriate secondary antibodies. Cellular DNA was stained with sytox orange (0.5 μM in 0.9% NaCl). Coverslips were mounted in Prolong Gold antifade reagent (Molecular Probes), and the cells were scanned with a confocal microscope LSM 510 (Zeiss, Oberkochen, Germany) using a 100× oil-immersion objective. The images were analyzed by ImageJ software 1.47 (http://imagej.nih.gov/ij/), and merged images were prepared. For the colocalization of ceramide and Rab7, colocalization analysis was performed using JACoP and colocalization-MBF plugins for ImageJ software. Single channel images were thresholded by Costes’ auto threshold method and the Manders split correlation coefficient for colocalization was then determined for each image.
Statistical analysis
All the results shown are the means and the standard errors of the means from at least three independent experiments. The effects of ASM siRNA and ASM inhibitors were analyzed by two-tailed Student’s t-test. P value <0.05 was considered statistically significant. Confocal images shown are representative of at least three independent experiments.
Results
Cold treatment induces PEC replication without bile acids
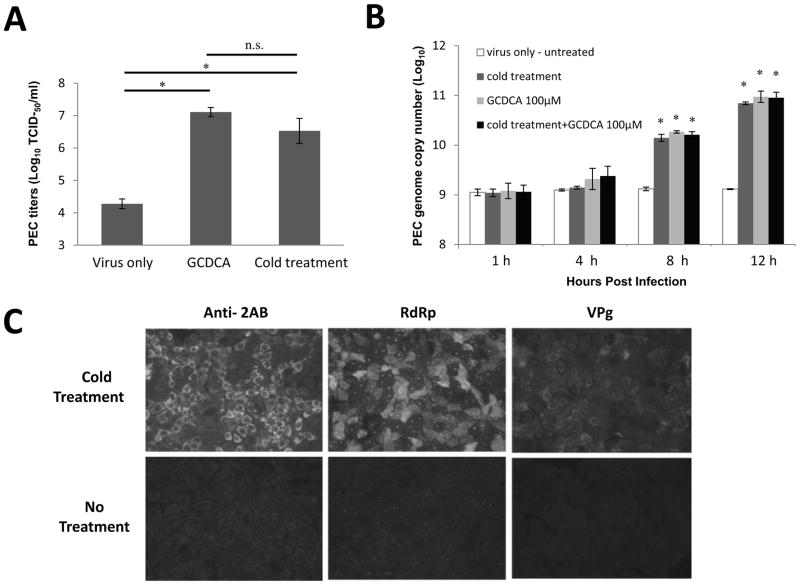
When cells were infected with PEC at a high MOI (50) and incubated at 4°C for 1 h during virus entry, replication of PEC was evident by virus titration and IFA assay without bile acids (Figure 1A–C). Virus titration by real time qRT-PCR or the TCID50 method demonstrated efficient PEC replication by cold treatment that is comparable to bile acid-mediated replication (Figure 1A and B). Virus replication kinetics measured by real time qRT-PCR at 1, 4, 8, and 12 h post infection (pi) were not different between cold treatment, bile acid treatment or combination of two treatments (Figure 1B). While PEC RNA levels remain stagnant without any treatment, treatment of cells with cold or bile acids led to an approximately 10-fold increase of virus copy number at 8 h and up to a 100-fold increase at 12 h compared to the untreated control (Figure 1B). Similar PEC replication kinetics was observed with PEC at a lower MOI of 5. However, detection of viral replication was not consistent probably due to the small number of viral progeny since only single cycle virus replication was allowed by one-time cold treatment in our studies (data not shown). The IFA assay also showed PEC replication induced by cold treatment (Figure 1C). At 12 h pi, the expression of viral proteins, 2AB, polymerase or VPg, was evident in the virus-infected cells treated with cold or bile acids. However, without cold or bile acid treatment, PEC infection even at 50 MOI did not yield any evidence for expression of the viral proteins (Figure 1C).
Figure 1. Effects of cold treatment in PEC replication and viral escape from the endosomes.
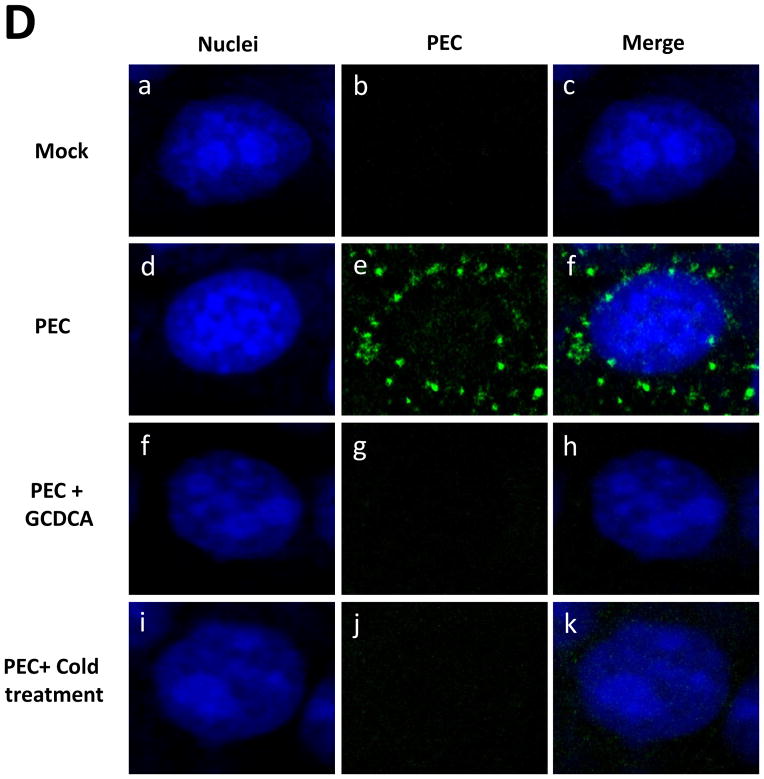
A) Confluent LLC-PK cells were infected with PEC (MOI 50) with no treatment (virus only), GCDCA (100 μM) or cold treatment (4°C, 1 h) and virus replication was determined by TCID50 method after 16 h. An asterisk indicates a significant difference (p < 0.05) between the groups, and n.s. no significance difference. B) LLC-PK cells were inoculated with PEC at an MOI of 50 with GCDCA (100 μM) or cold treatment (4°C, 1 h). PEC RNA was quantified by qRT-PCR at 1, 4, 8 and 12 h post virus infection. An asterisk indicates a significant difference (p < 0.05) compared to virus only (untreated) group. C) LLC-PK cells were inoculated with PEC (MOI 50) and incubated at 4°C (cold treatment) or 37°C (control) for 1 h. Cells were fixed at 12 h and expression of 2AB, polymerase (RdRp) or VPg was determined. D) Confocal laser scanning microscopic examination on PEC entry by various treatments. LLC-PK cells grown on Lab-Tek II CC2 chamber slides were infected either with mock (medium) or PEC (MOI 50) with no treatment (PEC), GCDCA (100 μM) or cold treatment (4°C, 1 h). Following 1 h incubation, cells were incubated for additional 1 h at 37°C. Cells were then fixed for confocal microscopy. Nuclei were stained with sytox orange (5μM) (pseudo colored blue), and PEC was probed with swine polyclonal anti-PEC primary antibodies and detected by FITC labelled goat-anti-swine antibody (green). Merged images for nuclei and PEC were prepared.
We have previously reported that bile acids facilitate viral escape from the endosomes for replication of PEC (Shivanna et al., 2014a). Thus we examined if cold treatment is also involved in viral endosomal escape using the confocal microscopy. In our previous report (Shivanna et al., 2014a), PEC particles disappeared from the endosomes at 1 h pi in the presence of bile acids leading to virus replication. However, PEC particles remained in the endosomes without bile acids and failed to escape from the endosomes for virus replication. In this study, we observed the same phenomenon of disappearance of PEC particles at 1 h with cold or bile acid treatment, which is in contrast to the detection of PEC particles at 1 h pi without any treatment (Figure 1D).
Bile acids or cold treatment induce ceramide formation through activation of ASM
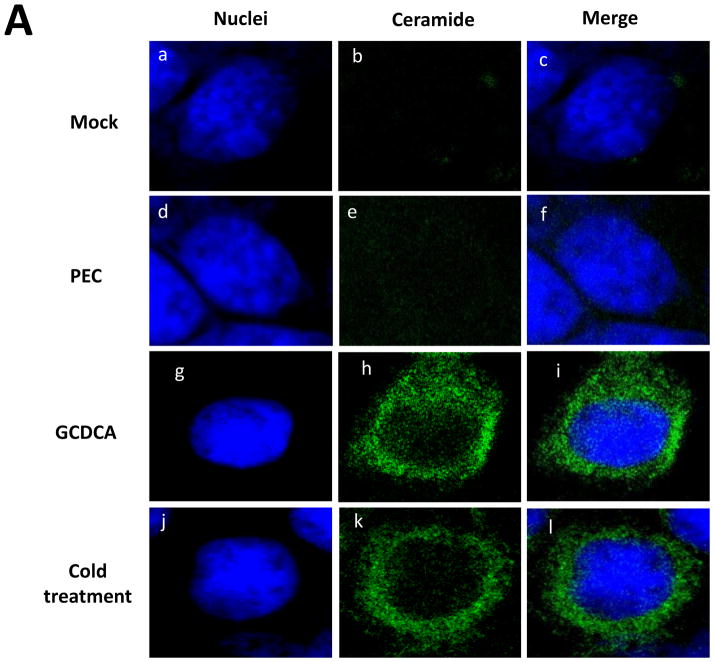
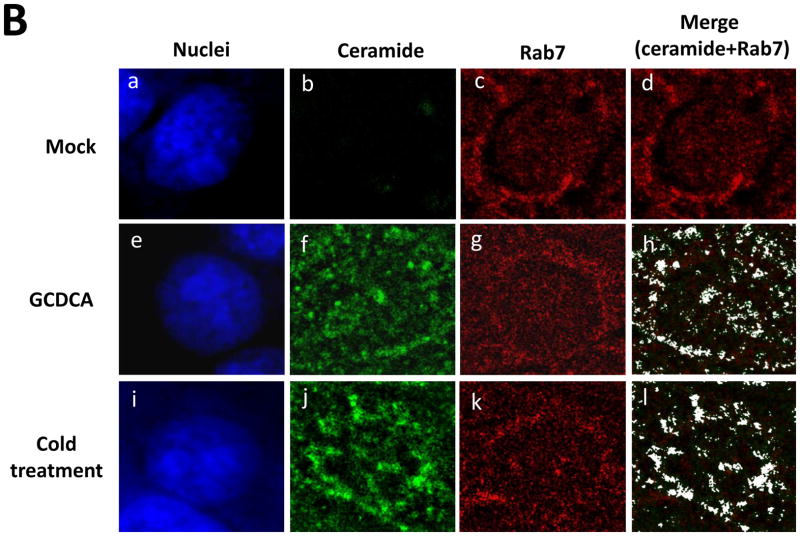
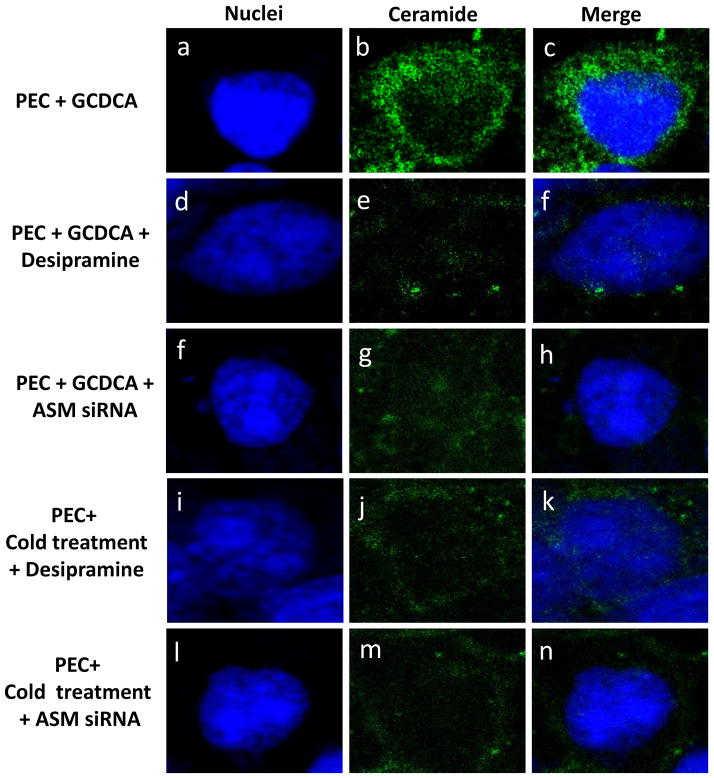
Using confocal microscopy, we first examined if GCDCA or cold treatment induce ceramide formation in the cells. Untreated, un-infected LLC-PK cells had little ceramide (Figure 2A, a–c). Likewise, ceramide was not detected in PEC-infected (50 MOI) or untreated cells (Figure 2A, d–f). However, GCDCA or cold treatment induced remarked ceramide formation in the cells (Figure 2A, g–l). Ceramide induced by GCDCA or cold treatment co-localized with the endosomal marker Rab7 (red) (Manders split colocalization coefficient of > 0.90) (Figure 2B, h and l). Treatment of non-permeabilized cells with GCDCA or cold temperature showed little fluorescence signals for ceramide, indicating that ceramide formation may not occur on the cytoplasmic membranes. Inhibition of ASM activity by desipramine (Kornhuber et al., 2010) or ASM siRNA drastically reduced ceramide formation by GCDCA or cold treatment, indicating that ceramide formation induced by bile acids or cold treatment is mediated by ASM activity (Figure 3). Reduction in ASM mRNA levels in cells transfected with various concentrations of ASM siRNA was confirmed with real time qRT-PCR (> 90 % with 100 or 200 nM of ASM siRNA) or regular RT-PCR (up to 87 % as shown in Figure 4A).
Figure 2. Ceramide formation by bile acids or cold treatment in LLC-PK cells.
Ceramide formation by bile acids or cold treatment in LLC-PK cells. A) LLC-PK cells were incubated with medium (Mock) (a–c), PEC (MOI 50) (d–f), GCDCA (100 μM, at 37 °C) (g–i) or cold treatment (4°C for 1 h) (j–l). Cells were transferred to 37°C, and incubated for 1 h. Then, cells were fixed and probed with mouse IgM anti-ceramide antibody and goat-anti-rabbit antibody (green) for ceramide or stained with sytox orange (5 μM, pseudo colored blue) for nuclei, and merged images were prepared. B) LLC-PK cells were treated with Mock, GCDCA or cold and fixed as in Figure 2A. Rab7 was probed with rabbit polyclonal anti-Rab7 with PerCP-Cy5.5 labelled goat-anti-rabbit antibody. Colocalization images of ceramide (green) with Rab7 (red) were prepared from cross-sections above nuclei for better resolution and analyzed using ImageJ. Colocalization of ceramide with Rab7 appears in white in the merged images.
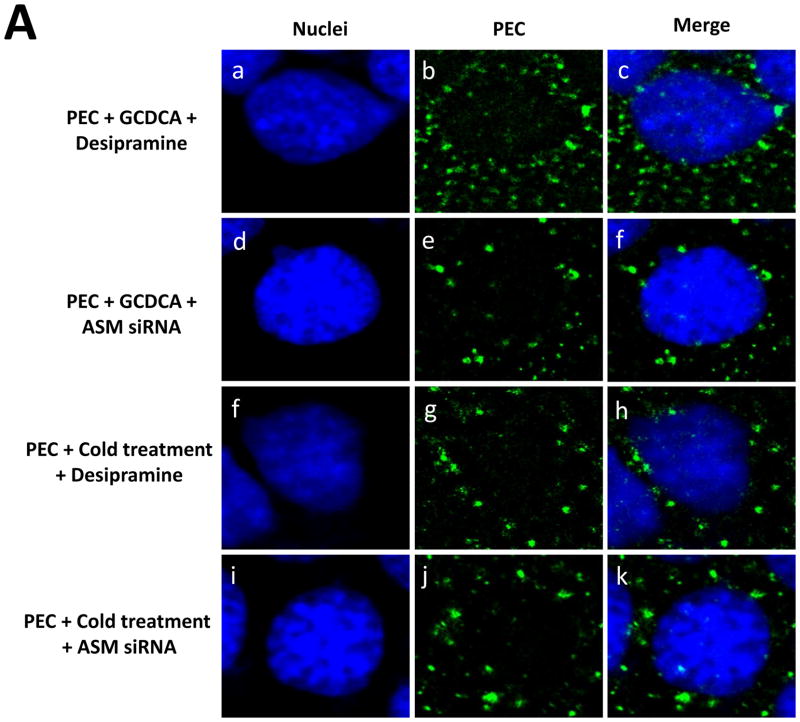
Figure 3. Effects of ASM on ceramide formation in LLC-PK cells by GCDCA or cold treatment.
Confluent cells were inoculated with PEC (50 MOI) and subject to GCDCA (100 μM, 37 °C for 1 h, a–h) or cold treatment (4 °C for 1 h, i–n) in the presence or absence of an ASM inhibitor (desipramine, 40 μM) or ASM siRNA (200 nM, transfected at 48 h prior virus infection) for 1 h. Cells were then further incubated at 37 °C for 1 h and were fixed for confocal microscopy. Individual or merged images for nuclei (blue) and ceramide (green) were prepared.
Figure 4. Effects of siRNA or inhibitors of ASM on PEC replication.
A) After the transfection of ASM-specific (at various concentrations) or irrelevant siRNA, ASM mRNA was amplified by RT-PCR for 25 cycles, and the RT-PCR products on agarose gel were quantified by densitometric analysis using imageJ software. B–C) LLC-PK cells were transfected with mock, irrelevant siRNA or ASM siRNA and incubated for 48 h. Then cells were inoculated with PEC at an MOI of 50 and treated with GCDCA (100 μM, 37°C for 1 h) or cold (4°C for 1h). After further incubated at 37°C for 1h, cells were washed with PBS, and incubated at 37°C for 12 h. Viral replication was determined by real time qRT-PCR. D–E). Confluent LLC-PK cells were pretreated with various ASM inhibitors at different concentrations at 37°C for 1 h, followed by inoculation with PEC (MOI 50) with GCDCA (100 μM, 37°C for 1 h, D) or cold (4°C for 1 h, E). Following 1 h incubation, cells were further incubated at 37°C for 1 h and washed with PBS. Cells were then added with fresh media containing inhibitors and further incubated for 12 h, at which time, viral replication was quantified by real time qRT-PCR. An asterisk indicates a significant difference between the groups (p < 0.05).
ASM activity is required for productive calicivirus replication
ASM inhibitors or ASM siRNA were used to demonstrate the requirement of ASM activity in PEC replication. Transfection of ASM siRNA in cells treated with GCDCA or cold led to significant reduction in PEC genome by 0.9~1.1 or 0.85~0.97-fold (log10) compared to un-transfected cells treated with GCDCA or cold, respectively (Figure 4B and C). However, transfection with irrelevant siRNA did not have any effect on PEC replication. Various ASM inhibitors, AY9944, fluoxetine, desipramine and chlorpromazine, showed similar inhibitory effects on virus replication (Figure 4D and E): each ASM inhibitor reduced PEC replication in cells treated with GCDCA or cold with a reduction in PEC genome by 0.96~1.35 or 0.86~1.10-fold (log10) compared to the cells treated with GCDCA or cold without an inhibitor, respectively (Figure 4D and E).
FCV or MNV-1 infections induce ceramide formation in the target cells and inhibition of ASM significantly reduces their replication
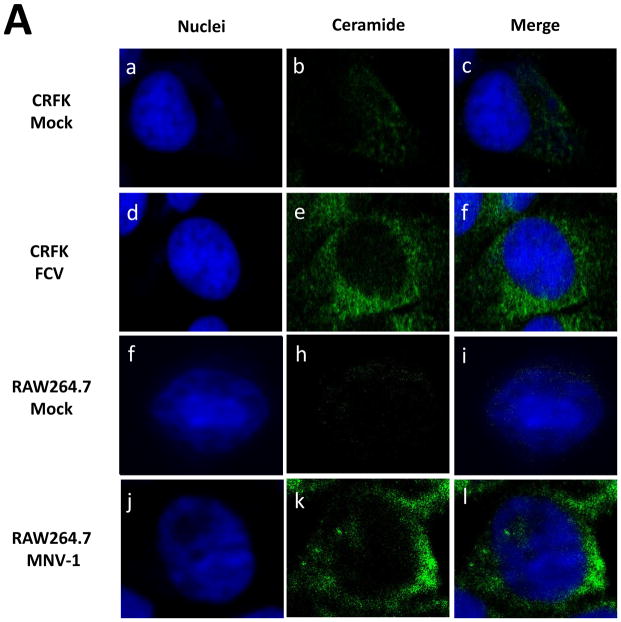
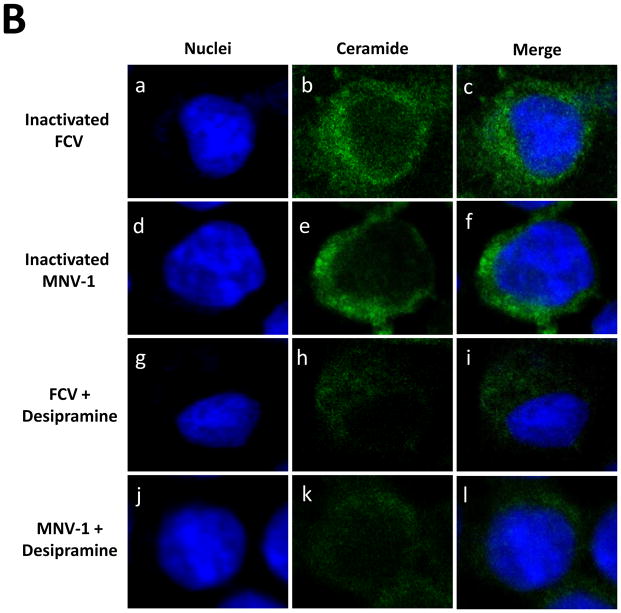
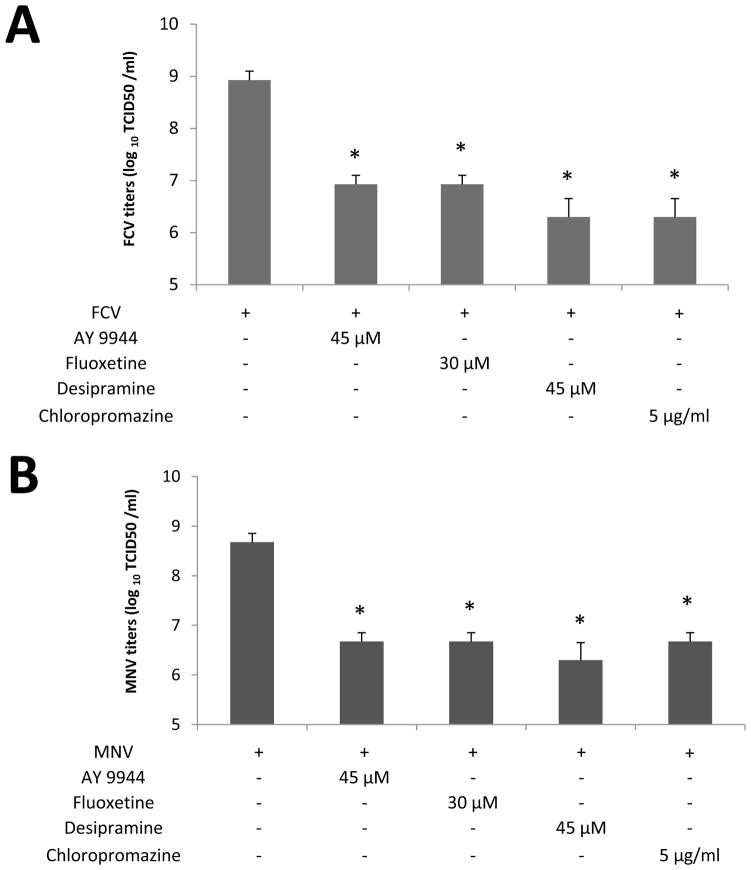
Since FCV or MNV-1 does not require bile acids for replication, we hypothesized that FCV or MNV-1 infection itself induces ceramide formation during virus entry process. Incubation of CRFK or RAW267.4 cells with FCV (MOI 50) or MNV-1 (MOI 30) at 37°C for 1 h, respectively, led to prominent ceramide formation in the permeabilized cells (Figure 5A, d–f and i–k). However, little ceramide was detected in uninfected cells (Figure 5A, b and h), when viruses were inoculated to non-permissive cells (e.g. FCV in RAW264.7 cells) or in non-permeabilized cells. Interestingly, BEI-inactivated FCV or MNV-1 also induced ceramide formation in their respective permissive cells (Figure 5B, b, e). As shown for live viruses, desipramine also blocked ceramide formation by inactivated FCV or MNV-1 in CRFK or RAW264.7 cells, respectively (Figure 5B, h and k). Inhibition of ceramide formation by ASM inhibitors also reduced FCV or MNV-1 replication: ASM inhibitors decreased FCV or MNV-1 replication by 100~375.1- or 100~211.3-fold in TCID50 titers, respectively (Figure 6A and B).
Figure 5. Ceramide formation by incubation of cells with live or inactivated FCV or MNV-1.
A) CRFK or RAW264.7 cells were inoculated with medium (Mock, a–c or f–h), FCV (50 MOI, d–f) or MNV-1 (30 MOI, i–k) and incubated at 37°C for 1 h. Cells were then fixed for confocal microscopy. B) CRFK or RAW264.7 cells were incubated with inactivated FCV (50 MOI equivalent, a–c) or inactivated MNV-1 (30 MOI equivalent, d–f) or cells were infected with FCV (50 MOI) or MNV-1 (30 MOI) with or without desipramine (45 μM) at 37 °C for 1 h. Cells were then fixed and stained with sytox orange (5 μM, pseudo colored blue) for nuclei or probed with mouse IgM anti-ceramide antibody and goat-anti-rabbit antibody (green) for ceramide. Merged images of ceramide (green) with nuclei (blue) were prepared.
Figure 6. The effects of ASM inhibitors in the replication of FCV or MNV-1.
Confluent CRFK or RAW264.7 cells were pretreated with various ASM inhibitors at different concentrations at 37°C for 1 h, followed by inoculation with FCV (MOI 50) or MNV-1 (30 MOI), respectively. Cells were then incubated further at. 37°C for 1h, washed with PBS. After washing, fresh media containing the same inhibitors were added to the cells. Cells were then further incubated at 37°C for 12 h. Viral replication was assessed with titration with TCID50 method. An asterisk indicates a significant difference between the groups (p < 0.05)
Ceramide formation is required for entry of calicivirus into host cells
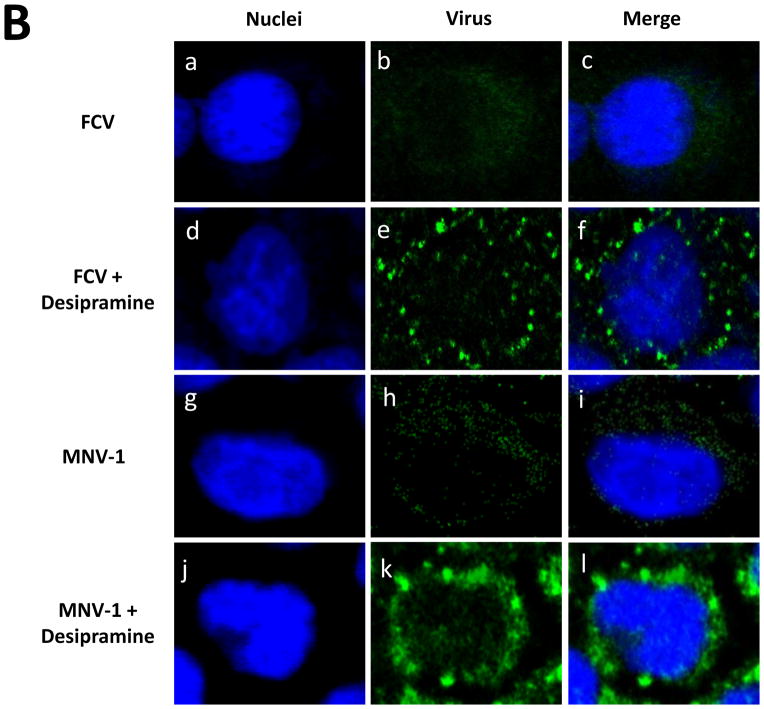
We have previously reported that endosomal uptake and subsequent endosomal escape of PEC and MNV-1 is critical for virus replication by showing that PEC and MNV-1 particles colocalized with the endosomal marker Rab7 disappears at 1 h pi, leading to virus replication, while PEC or MNV-1 remaining in the endosomes failed to lead to virus replication (Shivanna et al., 2014b). In this study, we tested the hypothesis that ceramide formation, which is known to trigger membrane destabilization, may facilitate escape of calicivirus from the endosomes during virus entry. Inhibition of ASM by an inhibitor or siRNA led to retention of fluorescence signals for PEC even in the presence of GCDCA or cold treatment at 1 h pi (Figure 7A), which correlated with reduced PEC replication (Figure 4B–E). Without ASM inhibition, fluorescence signals for PEC capsid protein disappeared in the cells treated with GCDCA or cold (Figure 1D, f–k), and the location of the PEC particles was reported to be in the endosomes in our previous report (Shivanna et al., 2014a, b). Similar phenomenon was observed with confocal studies using FCV or MNV-1: the fluorescence signals for their capsid proteins disappeared in the cells at 1 h pi (Figure 7B, a–c for FCV and g–i for MNV-1) when ASM activity was intact. However, marked accumulation of FCV or MNV-1 capsid proteins were observed in CRFK or RAW267.4 cells, respectively, in the presence of an ASM inhibitor (Figure 7Bd–f or j–l). This observation also correlates well with the reduction in FCV or MNV-1 replication by ASM inhibitors (Figure 6). These results suggest the requirement of ceramide formation by ASM for successful viral escape from the endosomes during the entry of these caliciviruses.
Figure 7. Effects of ASM inhibition on the detection of PEC, FCV or MNV-1 during virus entry by confocal microscopy.
A). LLC-PK cells were inoculated with PEC (50 MOI) and treated with GCDCA (100 μM, 37 °C for 1 h) or cold (4 °C for 1 h) with desipramine (45 μM) or ASM siRNA (200 nM) as described in the text. After additional incubation at 37 °C for 1 h, cells were fixed for confocal microscopy. B). CRFK or RAW264.7 cells were inoculated with either FCV or MNV-1, respectively with or without desipramine (45 μM). Fixed cells were probed with PEC, FCV or MNV-1 antibody with the secondary antibody (green) for virus as described above or in text. Merged images of virus (green) with nuclei (blue) were prepared.
Discussion
While a recent report suggested that human noroviruses may infect B cells (Jones et al., 2014), there is no practical cell culture system for the important enteric pathogens. Virus entry step is implicated in the inability of human norovirus to propagate in cell culture since transfection of human norovirus RNA into cultured cells produces infectious virus progeny (Guix et al., 2007). PEC RNA, once delivered into the cells by transfection, is also capable of producing infectious viruses, indicating that virus entry is the limiting step in PEC replication (Shivanna et al., 2014a). However, PEC is able to propagate in the cells in the presence of bile acids, which suggest that bile acids are involved in PEC entry. We have recently demonstrated that bile acids facilitate viral endosomal escape from the endosomes and PEC particles are trapped in the endosomes without bile acids (Shivanna et al., 2014a). The requirement of bile acids is unique for PEC replication and other cultivable caliciviruses, FCV and MNV-1, does not require any supplement in cell culture for virus propagation (Kreutz and Seal, 1995; Perry and Wobus, 2010; Stuart and Brown, 2006). These findings illustrate that understanding the molecular basis of viral entry process may lead to the development of a better cell culture model for human noroviruses and other uncultivable enteric viruses.
Our initial attempts to synchronize PEC entry using high MOI (> 10) into LLC-PK cells by incubating the cells inoculated with viruses at 4°C and then at 37°C resulted in unexpected PEC replication in the absence of bile acids (Figure 1). Since single cold treatment was applied to PEC culture, high MOI infections made it easy to detect the expression of PEC non-structural proteins (Figure 1C), however, PEC replication can be initiated with cold treatment at various MOI. As it was observed with bile acids that facilitated the endocytic escape of PEC in cells (Shivanna et al., 2014a), cold treatment of cells incubated with PEC allowed disintegration of PEC particles and subsequent virus replication. These findings suggest that bile acids and cold treatment may trigger the same endocytic escape mechanism in LLC-PK cells.
ASM activation is induced in response to various cellular stress stimuli such as cold temperature, irradiation and infection to produce ceramide which is involved in various signaling pathways (Montes et al., 2008; Zeidan and Hannun, 2010; Zhang et al., 2001). ASM-mediated ceramide formation has also been reported to play an important role in the replication of some viruses (Avota et al., 2011; Grassmé et al., 2005; Miller et al., 2012; Tani et al., 2010). Since bile acids and cold treatment are able to induce ASM activation (Gupta et al., 2004; Montes et al., 2008) and ceramide formation is known to be important in the replication of multiple viruses, we hypothesized that ASM activation and ceramide production is involved in cold- or bile acids-mediated PEC entry and replication. Using the confocal microscopy, we found that cold treatment or bile acids induced ceramide formation in the endosomal-like compartments, whereas PEC alone had little effect (Figure 2A and B). This ceramide formation by bile acids or cold temperature and PEC replication required intact ASM activity, which was demonstrated by the inhibition studies using ASM inhibitors and siRNA in LLC-PK cells (Figures 3 and 4).
Since ASM-mediated ceramide formation is found to be important in endocytic escape and replication of PEC, we extended our studies to other caliciviruses, FCV and MNV-1. FCV and MNV-1 utilize endocytic pathway for entry and readily propagate in cell culture without any supplement (Gerondopoulos et al., 2010; Perry and Wobus, 2010; Stuart and Brown, 2006). However, it is not yet known what factors are involved during endosomal escape of these caliciviruses. In this study, incubation of live or inactivated FCV or MNV-1 with CRFK or RAW267.4 cells, respectively, at 37°C for 1 h led to ceramide formation (Figure 5A and B). However, there was no evidence of ceramide formation when viruses were incubated with the cells that do not allow virus replication (non-permissive cells). These results suggest that ceramide formation occurs prior to or in the absence of active virus replication in permissive cells (Figure 5B). As was the case with PEC, ceramide formation and virus replication was mediated by ASM activation as demonstrated by ASM inhibitor studies (Figures 5B and 6). These results on PEC, FCV and MNV-1 indicate that ASM-mediated ceramide formation by virus infection itself or other extraneous factors is critical for successful virus replication for these caliciviruses.
Next, the role of ASM-mediated ceramide formation in virus entry was studied. On confocal microscopy, virus particles disappeared in the cytoplasm at 1 h pi and virus replication successfully ensued when ASM activity was not inhibited. However, PEC, FCV or MNV-1 particles are still observed at 1 h pi when ASM activity was blocked by an inhibitor or siRNA (Figure 7). Previously, we have reported that absence of bile acids in the culture media leads to PEC retention in the endosomes and failed virus replication in LLC-PK cells, which indicate that bile acids are required for endocytic escape of PEC into cytoplasm (Shivanna et al., 2014a). Findings from the previous and current studies indicate that calicivirus entry requires activation of ASM which induces ceramide formation in the endosomes and ceramide formation is involved in viral endosomal escape.
The importance of ASM and ceramide in virus replication has been previously reported (Grassmé et al., 2005; Jan et al., 2000; Miller et al., 2012; Tani et al., 2010). Semliki Forest virus requires the presence of ceramide in the cell membrane for virus-cell fusion (Nieva et al., 1994). ASM function is required for enhancement of viral uptake of Ebolavirus, Measles virus and rhinoviruses, as well as for other virus-induced cellular effects (Avota et al., 2011; Grassmé et al., 2005; Miller et al., 2012). Since formation of ceramide platforms was shown to alter membrane fluidity and permeability to form large channels or cause lipid flip-flop along with its hydrophobic protein interaction site (Contreras et al., 2009; Krönke, 1999; Montes et al., 2002; Samanta et al., 2011), it is speculated that modification of lipid organization in the cellular membranes may contribute to viral translocation into the cytoplasm. In this study, we made a novel finding that caliciviruses, PEC, MNV-1 and FCV, require ASM-mediated ceramide formation for productive virus replication in cell culture system. While we found that bile acids or cold treatment triggers ceramide formation in PEC-infected cells, it is not yet known how the interaction between MNV-1 or FCV and host cell leads to ASM activation. The cellular receptor(s) for PEC or MNV-1 are not well known to date but the receptor for FCV was identified as feline junctional adhesion molecule (fJAM-A) (Ossiboff and Parker, 2007). JAM-A is a multifunctional cell surface protein and JAM-A of murine or human origin forms a complex with phosphokinase C (PKC) (Iden et al., 2012). Since some PKC isoforms are capable of activating ASM, it can be speculated that interaction of FCV and fJAM-A may lead to activation of ASM through a PKC isoform. However, the mechanisms of ASM activation by virus-cell interaction remain to be determined.
In summary, we demonstrated that activation of ASM by bile acids (PEC) or virus (FCV or MNV-1) led to ceramide formation on the endosomal membranes, facilitating viral endosomal escape, and inhibition of this event significantly reduced viral replication. Virus entry for caliciviruses is a complex process involving uncoating of virus capsid proteins and translocation of genetic material across the endosomal membrane into cytoplasm for initiation of virus replication. We have recently reported that caliciviruses such as PEC, FCV and MNV-1 require cathepsin L activity and adequate endosomal environment (low pH) for endocytic viral escape and successful virus replication (Shivanna et al., 2014b). Our previous findings and the results from this study indicate that calicivirus entry into host cells is multi-step processes involving cleavage of capsid proteins by cellular protease and ceramide formation, which eventually leads to release of viral genome into cytoplasm. How these events are coordinated during calicivirus entry process warrants further studies. Nevertheless, these results suggest that a common mechanism is utilized by caliciviruses during virus entry into host cells and may contribute to our understanding of calicivirus entry process.
Highlights.
Bile acids or cold treatment facilitate the replication of porcine enteric calicvirus by triggering endosomal escape.
Ceramide formation by acid sphingomyelinase is important in the replication of caliciviruses
Inhibitors of acid sphingomyelinase result in the calicivirus retention in endosomes during entry.
Acknowledgments
We would like to thank David George and Dr. Daniel L. Boyle for technical assistance. This work was supported by NIH grant, R01AI109039.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avota E, Gulbins E, Schneider-Schaulies S. DC-SIGN Mediated Sphingomyelinase-Activation and Ceramide Generation Is Essential for Enhancement of Viral Uptake in Dendritic Cells. PLoS Pathog. 2011;7:e1001290. doi: 10.1371/journal.ppat.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basáñez G, Ruiz-Argüello MB, Alonso A, Goñi FM, Karlsson G, Edwards K. Morphological changes induced by phospholipase C and by sphingomyelinase on large unilamellar vesicles: a cryo-transmission electron microscopy study of liposome fusion. Biophys J. 1997;72:2630–2637. doi: 10.1016/S0006-3495(97)78906-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. Endosomal Proteolysis of the Ebola Virus Glycoprotein Is Necessary for Infection. Science. 2005;308:1643–1645. doi: 10.1126/science.1110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KO, Sosnovtsev SS, Belliot G, Wang Q, Saif LJ, Green KY. Reverse genetics system for porcine enteric calicivirus, a prototype sapovirus in the Caliciviridae. Journal of virology. 2005;79:1409–1416. doi: 10.1128/JVI.79.3.1409-1416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KO, Sosnovtsev SV, Belliot G, Kim Y, Saif LJ, Green KY. Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1. Proc Natl Acad Sci U S A. 2004;101:8733–8738. doi: 10.1073/pnas.0401126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras FX, Sánchez-Magraner L, Alonso A, Goñi FM. Transbilayer (flip-flop) lipid motion and lipid scrambling in membranes. FEBS Letters. 2009;584:1779–1786. doi: 10.1016/j.febslet.2009.12.049. [DOI] [PubMed] [Google Scholar]
- Duizer E, Schwab KJ, Neill FH, Atmar RL, Koopmans MPG, Estes MK. Laboratory efforts to cultivate noroviruses. Journal of General Virology. 2004;85:79–87. doi: 10.1099/vir.0.19478-0. [DOI] [PubMed] [Google Scholar]
- Earp LJ, Delos SE, Park HE, White JM. The many mechanisms of viral membrane fusion proteins. Curr Top Microbiol Immunol. 2005;285:25–66. doi: 10.1007/3-540-26764-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert DM, Kim PS. MECHANISMS OF VIRAL MEMBRANE FUSION AND ITS INHIBITION. Annual Review of Biochemistry. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Gerondopoulos A, Jackson T, Monaghan P, Doyle N, Roberts LO. Murine norovirus-1 cell entry is mediated through a non-clathrin-, non-caveolae-, dynamin- and cholesterol-dependent pathway. J Gen Virol. 2010;91:1428–1438. doi: 10.1099/vir.0.016717-0. [DOI] [PubMed] [Google Scholar]
- Grassmé H, Riehle A, Wilker B, Gulbins E. Rhinoviruses infect human epithelial cells via ceramide-enriched membrane platforms. J Biol Chem. 2005;280:26256–26262. doi: 10.1074/jbc.M500835200. [DOI] [PubMed] [Google Scholar]
- Green KY. Caliciviruses: The Noroviruses. 5. Lippincott Williams & Wilkins; Philadelphia: 2007. [Google Scholar]
- Guix S, Asanaka M, Katayama K, Crawford SE, Neill FH, Atmar RL, Estes MK. Norwalk Virus RNA Is Infectious in Mammalian Cells. Journal of virology. 2007;81:12238–12248. doi: 10.1128/JVI.01489-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbins E, Dreschers S, Wilker B, Grassmé H. Ceramide, membrane rafts and infections. J Mol Med (Berl) 2004;82:357–363. doi: 10.1007/s00109-004-0539-y. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Kolesnick R. Acid Sphingomyelinase-derived Ceramide Signaling in Apoptosis. In: Quinn P, Kagan V, editors. Phospholipid Metabolism in Apoptosis. Springer; US: 2002. pp. 229–244. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- Gupta S, Natarajan R, Payne SG, Studer EJ, Spiegel S, Dent P, Hylemon PB. Deoxycholic Acid Activates the c-Jun N-terminal Kinase Pathway via FAS Receptor Activation in Primary Hepatocytes: ROLE OF ACIDIC SPHINGOMYELINASE-MEDIATED CERAMIDE GENERATION IN FAS RECEPTOR ACTIVATION. Journal of Biological Chemistry. 2004;279:5821–5828. doi: 10.1074/jbc.M310979200. [DOI] [PubMed] [Google Scholar]
- Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. The Roles of Clostridium difficile and Norovirus Among Gastroenteritis-Associated Deaths in the United States, 1999–2007. Clinical Infectious Diseases. 2012;55:216–223. doi: 10.1093/cid/cis386. [DOI] [PubMed] [Google Scholar]
- He X, Okino N, Dhami R, Dagan A, Gatt S, Schulze H, Sandhoff K, Schuchman EH. Purification and characterization of recombinant, human acid ceramidase. Catalytic reactions and interactions with acid sphingomyelinase. J Biol Chem. 2003;278:32978–32986. doi: 10.1074/jbc.M301936200. [DOI] [PubMed] [Google Scholar]
- Heinz FX, Allison SL. Structures and mechanisms in flavivirus fusion. In: Maramorosch K, Murphy FA, Shatkin AJ, editors. Advances in Virus Research. Vol. 55. 2000. pp. 231–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst-Kralovetz MM, Radtke AL, Lay MK, Hjelm BE, Bolick AN, Sarker SS, Atmar RL, Kingsley DH, Arntzen CJ, Estes MK, Nickerson CA. Lack of norovirus replication and histo-blood group antigen expression in 3-dimensional intestinal epithelial cells. Emerg Infect Dis. 2013;19:431–438. doi: 10.3201/eid1903.121029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogle JM. Poliovirus cell entry: common structural themes in viral cell entry pathways. Annu Rev Microbiol. 2002;56:677–702. doi: 10.1146/annurev.micro.56.012302.160757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iden S, Misselwitz S, Peddibhotla SSD, Tuncay H, Rehder D, Gerke V, Robenek H, Suzuki A, Ebnet K. aPKC phosphorylates JAM-A at Ser285 to promote cell contact maturation and tight junction formation. J Cell Biol. 2012;196:623–639. doi: 10.1083/jcb.201104143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan JT, Chatterjee S, Griffin DE. Sindbis Virus Entry into Cells Triggers Apoptosis by Activating Sphingomyelinase, Leading to the Release of Ceramide. Journal of virology. 2000;74:6425–6432. doi: 10.1128/jvi.74.14.6425-6432.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, Gonzalez-Hernandez MB, Iovine NM, Wobus CE, Vinje J, Tibbetts SA, Wallet SM, Karst SM. Enteric bacteria promote human and mouse norovirus infection of B cells. Science. 2014;346:755–759. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M, Rey FA. Virus membrane-fusion proteins: more than one way to make a hairpin. Nat Rev Micro. 2006;4:67–76. doi: 10.1038/nrmicro1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber J, Tripal P, Reichel M, Mühle C, Rhein C, Muehlbacher M, Groemer TW, Gulbins E. Functional Inhibitors of Acid Sphingomyelinase (FIASMAs): a novel pharmacological group of drugs with broad clinical applications. Cell Physiol Biochem. 2010;26:9–20. doi: 10.1159/000315101. [DOI] [PubMed] [Google Scholar]
- Kreutz LC, Seal BS. The pathway of feline calicivirus entry. Virus Research. 1995;35:63–70. doi: 10.1016/0168-1702(94)00077-p. [DOI] [PubMed] [Google Scholar]
- Krönke M. Biophysics of ceramide signaling: interaction with proteins and phase transition of membranes. Chem Phys Lipids. 1999;101:109–121. doi: 10.1016/s0009-3084(99)00059-6. [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Delos SE, White JM. Sequential Roles of Receptor Binding and Low pH in Forming Prehairpin and Hairpin Conformations of a Retroviral Envelope Glycoprotein. Journal of virology. 2004;78:8201–8209. doi: 10.1128/JVI.78.15.8201-8209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ME, Adhikary S, Kolokoltsov AA, Davey RA. Ebolavirus Requires Acid Sphingomyelinase Activity and Plasma Membrane Sphingomyelin for Infection. Journal of virology. 2012;86:7473–7483. doi: 10.1128/JVI.00136-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes LR, Lopez DJ, Sot J, Bagatolli LA, Stonehouse MJ, Vasil ML, Wu BX, Hannun YA, Goni FM, Alonso A. Ceramide-enriched membrane domains in red blood cells and the mechanism of sphingomyelinase-induced hot-cold hemolysis. Biochemistry. 2008;47:11222–11230. doi: 10.1021/bi801139z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes LR, Ruiz-Argüello MB, Goñi FM, Alonso A. Membrane restructuring via ceramide results in enhanced solute efflux. J Biol Chem. 2002;277:11788–11794. doi: 10.1074/jbc.M111568200. [DOI] [PubMed] [Google Scholar]
- Mothes W, Boerger AL, Narayan S, Cunningham JM, Young JA. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell. 2000;103:679–689. doi: 10.1016/s0092-8674(00)00170-7. [DOI] [PubMed] [Google Scholar]
- Moyer CL, Nemerow GR. Viral weapons of membrane destruction: variable modes of membrane penetration by non-enveloped viruses. Current Opinion in Virology. 2011;1:44–49. doi: 10.1016/j.coviro.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieva JL, Bron R, Corver J, Wilschut J. Membrane fusion of Semliki Forest virus requires sphingolipids in the target membrane. EMBO J. 1994;13:2797–2804. doi: 10.1002/j.1460-2075.1994.tb06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossiboff RJ, Parker JS. Identification of regions and residues in feline junctional adhesion molecule required for feline calicivirus binding and infection. Journal of virology. 2007;81:13608–13621. doi: 10.1128/JVI.01509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JW, Wobus CE. Endocytosis of murine norovirus 1 into murine macrophages is dependent on dynamin II and cholesterol. Journal of virology. 2010;84:6163–6176. doi: 10.1128/JVI.00331-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. The American Journal of Hygiene. 1938;27:493–497. [Google Scholar]
- Samanta S, Stiban J, Maugel TK, Colombini M. Visualization of ceramide channels by transmission electron microscopy. Biochim Biophys Acta. 2011;1808:1196–1201. doi: 10.1016/j.bbamem.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States--major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivanna V, Kim Y, Chang K-O. The crucial role of bile acids in the entry of porcine enteric calicivirus. Virology. 2014a;456–457:268–278. doi: 10.1016/j.virol.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivanna V, Kim Y, Chang K-O. Endosomal acidification and cathepsin L activity is required for calicivirus replication. Virology. 2014b;464–465:287–295. doi: 10.1016/j.virol.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind LJ, Colombini M. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J Biol Chem. 2000;275:38640–38644. doi: 10.1074/jbc.C000587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel JJ, Bayley PM, Brown EB, Martin SR, Waterfield MD, White JM, Wilson IA, Wiley DC. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc Natl Acad Sci U S A. 1982;79:968–972. doi: 10.1073/pnas.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnovtsev S, Green KY. RNA Transcripts Derived from a Cloned Full-Length Copy of the Feline Calicivirus Genome Do Not Require VpG for Infectivity. Virology. 1995;210:383–390. doi: 10.1006/viro.1995.1354. [DOI] [PubMed] [Google Scholar]
- Stancevic B, Kolesnick R. Ceramide-rich platforms in transmembrane signaling. FEBS Lett. 2010;584:1728–1740. doi: 10.1016/j.febslet.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart AD, Brown TDK. Entry of Feline Calicivirus Is Dependent on Clathrin-Mediated Endocytosis and Acidification in Endosomes. Journal of virology. 2006;80:7500–7509. doi: 10.1128/JVI.02452-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H, Shiokawa M, Kaname Y, Kambara H, Mori Y, Abe T, Moriishi K, Matsuura Y. Involvement of ceramide in the propagation of Japanese encephalitis virus. Journal of virology. 2010;84:2798–2807. doi: 10.1128/JVI.02499-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2004;2:e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan YH, Hannun YA. The acid sphingomyelinase/ceramide pathway: biomedical significance and mechanisms of regulation. Curr Mol Med. 2010;10:454–466. doi: 10.2174/156652410791608225. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mattjus P, Schmid PC, Dong Z, Zhong S, Ma WY, Brown RE, Bode AM, Schmid HH, Dong Z. Involvement of the acid sphingomyelinase pathway in uva-induced apoptosis. J Biol Chem. 2001;276:11775–11782. doi: 10.1074/jbc.M006000200. [DOI] [PMC free article] [PubMed] [Google Scholar]