Abstract
Background
Slow walk (gait) speed predicts functional decline, institutionalization and mortality risks in the geriatric population. A gait speed evidence base for dialysis patient outcomes is needed.
Study Design
Prospective cohort study.
Setting & Participants
752 prevalent hemodialysis (HD) patients aged 20–92 evaluated 2009–2012 in 7 Atlanta and 7 San Francisco clinics in a USRDS special study.
Predictor
Usual walk speed in meters per second, categorized as 0.6 m/s or faster (baseline n=575), <0.6 m/s (baseline n=94), and unable to perform walk test (baseline n=83).
Outcomes
Survival; hospitalization; Activities of Daily Living (ADL) difficulty; SF-36 physical function (PF).
Measurements
Cox proportional hazards models investigated gait speed and mortality over a median follow-up of 703 days. Multivariable logistic or linear regression models estimated associations of baseline gait speed with hospitalization, need for ADL assistance, and SF-36 PF score after 12-months.
Results
Participants who walked 0.6 m/s or faster had 53 (9%) deaths, those who walked <0.6 m/s had 19 (20%) deaths, and those unable to walk had 37 (44%) deaths. Adjusted mortality hazard ratios were 2.17 (95% CI, 1.19–3.98) for participants who walked <0.6 m/s and 6.93 (95% CI, 4.01–11.96) for those unable to walk, compared with participants walking 0.6 m/s or faster. After 12 months, compared with baseline walk speed 1.0 m/s or faster (n=169 participants), baseline walk speed 0.6 to <0.8 m/s (n=116) was associated with increased odds of hospitalization (OR, 2.04; 95% CI, 1.19–3.49) and ADL difficulty (OR, 3.88; 95% CI, 1.46–10.33) and with a −8.20 (95% CI, −13.57 to −2.82) estimated change in SF-36 PF score.
Limitations
Cohort not highly representative of overall US in-center HD population. Conclusions: Because walking challenges the heart, lungs, circulatory, nervous, and musculoskeletal systems, gait speed provides an informative marker of health status. The association of gait speed with HD patients’ risk for functional decline warrants continued study.
INDEX WORDS: activities of daily living (ADL) difficulty, functional status, gait speed, dismobility, physical functioning, walking ability, hemodialysis, end-stage renal disease (ESRD), hospitalization, mortality, US Renal Data System (USRDS)
Evaluation of physical functioning in the end-stage renal disease (ESRD) population is an important potential component of clinical performance measurement.1 Physical performance limitations characterize many patients with kidney disease and affect the quality of their daily lives.2,3 Moreover, recent evidence from individuals with chronic kidney disease (CKD) indicates that physical performance is associated with mortality rate.4 An evidence base for the importance, scientific acceptability, feasibility, and usability of physical performance measures in the ESRD population is critical.5
The value of measuring usual walk speed in clinical care for older persons is increasingly endorsed, and a gait speed cut point that identifies dismobility has been proposed.6 Among persons with CKD stages 2–4, Roshanravan et al. recently showed that slower gait speed predicted all-cause mortality over a median 3-year follow-up period.4 Several studies have documented that gait speed among dialysis patients is slower than would be expected based on general population values,5,7,8 but there has been no investigation of the association of gait speed with survival and other outcomes among patients undergoing dialysis. Because walking places demands on the heart and lungs, as well as the circulatory, nervous, and musculoskeletal systems, gait speed may provide a very informative marker of dialysis patients’ health. Information is needed about the predictive utility of gait speed and its potential relevance for routine clinical care.5
In this US Renal Data System (USRDS) special study, we measured baseline gait speed, ascertained survival, and assessed hospitalization, disability, and perceived physical functioning at a 12-month follow-up in a large multicenter cohort of prevalent maintenance hemodialysis (HD) patients aged 20–92 years. While acknowledging that there is no apparent threshold in graded associations between walking speed and clinical outcomes related to mobility, Cummings et al. recently defined 0.6 m/s as very slow gait speed and proposed that this cut point is a meaningful definition of dismobility. As walking speed slows below 0.6 m/s, the risk of disability and other poor health outcomes increases rapidly among older persons.6 Other working groups have proposed using cut point values of 0.8 m/s and 1.0 m/s to define slow gait speed.9,10 We hypothesized that (1) gait speed slower than 0.6 m/s would be associated with increased mortality risk among HD patients, and (2) among patients with gait speeds of 0.6 m/s or higher, slower gait speed at baseline would be associated with increased likelihood of hospitalization, need for assistance performing activities of daily living (ADLs), and lower self-reported physical functioning at a 12-month follow-up.
METHODS
Participants and Measurements
Coordinated by the USRDS, ACTIVE/ADIPOSE (A Cohort Study to Investigate the Value of Exercise in ESRD/Analyses Designed to Investigate the Paradox of Obesity and Survival in ESRD) is a multi-center study of prevalent patients receiving HD.11 Seven outpatient dialysis clinics in the Atlanta, Georgia, metropolitan area and seven outpatient dialysis clinics in the San Francisco Bay Area, California, constituted the study sites. A primary reason for exclusion of PD patients was that conducting physical performance assessments was an important component of the study, and this was more easily and economically accomplished by restricting the study participants to in-center HD patients. In addition, with a limited number of clinics as study sites, the number of PD patients potentially available for enrollment would have been small. A total of 771 prevalent HD patients were enrolled and participated in baseline assessments September 2009-September 2011. Follow-up assessments were scheduled at 12-months post-baseline. Participating clinics were affiliated with large dialysis providers, medium size providers, and academic medical centers. The median number of study participants per dialysis clinic was 50 (range, 33–99). Institutional review boards at Emory University and the University of California–San Francisco approved the study.
Eligible study participants were adults (18 years or older), English- or Spanish-speaking, treated by HD for at least 3 months, and capable of giving informed consent. Exclusion criteria were current treatment by PD or home HD; evidence of active malignancy, including brain tumor; and expected geographic relocation. Vulnerable populations (pregnant women, prisoners, persons with significant mental illness) were also excluded. Single and double amputees and patients with prior or pending transplantation were considered eligible. Among eligible patients, 85% supplied informed consent and were enrolled. Reasons most frequently given by those who declined to participate were that they were “not interested,” “too busy,” or “enrolled in another study.”
No physical performance information could be obtained at baseline for 19 of the 771 enrollees due to death, transplantation, return of kidney function, and transfer to a non-study clinic prior to their scheduled evaluation, but walking ability was ascertained at the baseline assessment for 752 study participants. In addition to 669 participants for whom walk speed was measured, we describe characteristics of the remaining 83 participants who were unable to perform the walk test; a large number of the latter participants (84%) were wheelchair dependent.
Usual walk speed of 669 patients was measured two times over a 15-feet (4.57 meters) walkway.12 Coordinators observed whether the participant used an assistive device for walking and whether an assistive device was used to perform the walk. All assessments were conducted pre-HD on the midweek treatment day.
Study coordinators also conducted a brief interview with participants and reviewed medical records. Each study site (Atlanta, San Francisco) had one primary study coordinator who conducted the majority of the assessments; the primary coordinator also trained and supervised an assistant coordinator. Consistency of measurement procedures was monitored throughout the study, using repeated demonstration/review of physical performance techniques and office quality control of recorded interview and medical record data.
During the interview, participants reported falls incurred during the past 12 months. A fall was defined as an event that resulted in a person coming to rest inadvertently on the ground, floor or other lower level.13 At each measurement time point (baseline, 12 months), ADL difficulty was assessed by participants’ report that they needed assistance or were unable to do one or more of four tasks (bathing, dressing, getting in and out of a chair, walking around home/apartment).14 Consistent with prior research, participants who needed help with (or were unable to do) any of the tasks were considered to have ADL difficulty (an indicator variable).14 Study participants also completed the 36-Item Short Form Health Survey (SF-36) Physical Functioning (PF) scale15 and the Kidney Disease Quality of Life Cognitive Function scale (KDQOL-CF);16 these measures are scored 0–100, with higher scores indicating, respectively, fewer perceived limitations in performing daily activities and better cognitive function.
Race, gender, age, and ESRD treatment initiation date were ascertained from patient report and the USRDS Medical Evidence Standard Analysis Files. Patient report was the primary source of information for race; for the small number of participants who declined to specify their race, race information was taken from the USRDS Medical Evidence file.
Comorbidities were abstracted from dialysis clinic medical records and included diabetes, chronic obstructive pulmonary disease (COPD), cancer, and cardiovascular conditions, i.e. congestive heart failure, coronary artery disease/myocardial infarction, cerebrovascular accident/transient ischemic attack, peripheral vascular disease, and other cardiac diseases (cardiac dysrhythmia, atrial fibrillation, tachycardia, pericarditis, cardiac arrest). Hemoglobin level closest to the date of the physical measurements was obtained from the dialysis clinic medical record. The three most recent systolic blood pressure readings were recorded; the average of these three values is reported. Hospitalization during the past 12 months was identified in the patient’s clinic records at baseline and again at 12 months.
Data Analysis
The average of the two trials of patients’ usual walk speed was determined. The median difference of the two walk speed values was −0.04 (interquartile range, −0.11 to 0.006) m/s. Baseline characteristics of the analytic cohort (n=752) were stratified by gait speed categories (inability to perform the walk test; <0.6, 0.6 to <0.8, 0.8 to <1.0, and ≥1.0 m/s), and characteristics of the cohort were described using mean ± standard deviation or percentage, as appropriate. The cumulative prevalence of participants with measured gait speed <0.6, <0.8, and <1.0 m/s by sex and age category was identified.
The Kaplan-Meier survival estimator was computed by baseline gait speed category. The association of baseline gait speed with mortality occurring through December 31, 2012 (most recent information available in the USRDS Standard Analysis Files), was estimated in a Cox proportional hazards model, adjusting sequentially for demographic variables (age, sex, race, education, and participant clinic) and for demographic variables plus smoking status, BMI, ESRD vintage, diabetes, COPD, cancer, cardiovascular comorbidity, hemoglobin level, cognitive function score, and history of recent falls. The interaction of age with gait speed was also tested. Only 10 participants did not have complete information for all covariates and could not be included in the fully adjusted Cox analysis. Participants began accruing risk time from the date of gait speed assessment and were censored at transplantation, change to PD, return of kidney function, withdrawal from dialysis, or the end of available follow-up time. Gait speed was evaluated in survival analyses both as a categorical and a continuous variable.
Univariate associations between baseline gait speed category (0.6 to <0.8, 0.8 to <1.0, and ≥1.0 m/s) and 12-month outcomes (new hospitalization, reported ADL difficulty, and SF-36 PF score) were first described by percentage or mean ± standard deviation. Second, the association of baseline gait speed category with hospitalization and ADL difficulty at 12 months was estimated in multivariable logistic regression models, and the association of baseline gait speed category with SF-36 PF score at 12 months was estimated in a multivariable linear regression model, adjusting for age, sex, and baseline value of the outcome measure in all analyses. Only participants with gait speeds of 0.6 m/s or faster were included in these analyses because, as noted above, it is known that even in the general population the risk of poor health outcomes and even death is substantially greater as walking speed slows below 0.6 m/s.6 Statistical analyses were conducted using SAS 9.3 (SAS Institute Inc, Cary, NC, USA).
RESULTS
As shown in Table 1, participants unable to perform the walk test had characteristics similar to those with measured gait speed <0.6 m/s, except that a higher percentage of the former were white, had peripheral vascular disease, and used an assistive walking device. Prevalence of falling in the past 12 months was 40% for participants with gait speed <0.6 m/s or who were unable to perform the walk test, while the prevalence of recent falls among participants with gait speeds 0.6 m/s and faster was 25%. The prevalence of ADL difficulty and of having been hospitalized during the past 12 months was greatest among participants with gait speed <0.6 m/s or who were unable to perform the walk test and declined as gait speed increased from 0.6 m/s to ≥1.0 m/s. Participant-reported SF-36 PF scores were lowest among participants with gait speed <0.6 m/s or who were unable to perform the walk test and became higher as gait speed increased from 0.6 m/s to ≥1.0 m/s. Participants classified in the fastest walk speed category (≥1.0 m/s) were least likely to have diabetes, COPD, or cardiovascular conditions, or to use an assistive walking device. No consistent patterns across walking ability categories were evident for educational level, smoking, ESRD vintage, cognitive function score, or predialysis systolic blood pressure, but participants unable to perform the walk test and those with gait speed <0.6 m/s had higher representation in the highest BMI category than did participants with faster walk speeds. Whether defined by <0.6, <0.8, or <1.0 m/s, the cumulative prevalence of slow gait speed among HD patients increased with age and was higher among women than among men (Table 2).
Table 1.
Characteristics of HD study participants, by measured baseline gait speed
| Unable to perform walk (n=83) |
<0.6 m/s (n=94) |
0.6–<0.8 m/s (n=139) |
0.8–<1.0 m/s (n=208) |
≥1.0 m/s (n=228) |
|
|---|---|---|---|---|---|
| Male sex | 46 | 40 | 51.8 | 63.5 | 73.7 |
| Age (y) | 64.7 (12.9) | 65.0 (12.9) | 60.1 (13.4) | 56.8 (13.5) | 50.0 (12.7) |
| Race | |||||
| White | 34 | 12 | 15.1 | 22.1 | 30.7 |
| Black | 45 | 75 | 74.8 | 64.4 | 51.3 |
| Other | 22 | 14 | 10.1 | 13.5 | 18.0 |
| At least high school | 76 | 72 | 65.9 | 82.2 | 79.0 |
| Current smoker | 9 | 14 | 25.4 | 17.8 | 18.9 |
| BMI category | |||||
| <18.5 kg/m2 | 4 | 1 | 1.4 | 1.9 | 3.1 |
| 18.5–<25 kg/m2 | 25 | 33 | 33.8 | 37.5 | 41.4 |
| 25–<30 kg/m2 | 27 | 22 | 30.2 | 32.2 | 26.4 |
| ≥30 kg/m2 | 44 | 44 | 34.5 | 28.4 | 29.1 |
| ESRD vintage (y) | 4.9 (4.3) | 4.9 (5.5) | 4.2 (4.3) | 5.8 (5.6) | 4.7 (5.0) |
| Diabetes | 77 | 65 | 56.8 | 47.6 | 36.3 |
| COPD | 13 | 12 | 12.2 | 7.7 | 2.7 |
| Cancer | 10 | 6 | 9.4 | 6.3 | 8.4 |
| CHF | 45 | 42 | 24.5 | 27.9 | 22.6 |
| CAD/MI | 45 | 44 | 25.9 | 26.4 | 18.1 |
| CVA/TIA | 20 | 15 | 10.8 | 10.1 | 5.3 |
| PVD | 37 | 8 | 10.8 | 5.3 | 4.4 |
| Other cardiac diseases | 45 | 36 | 25.2 | 26.9 | 15.5 |
| KDQoL-CF score | 86.1 (18.9) | 86.0 (16.3) | 83.0 (19.1) | 90.0 (15.2) | 90.1 (14.7) |
| Hemoglobin (g/dL) | 11.3 (1.3) | 11.5 (1.3) | 11.6 (1.3) | 11.5 (1.4) | 11.6 (1.3) |
| Predialysis SBP (mm Hg) | 145 (23) | 151 (24) | 154 (22) | 155 (25) | 150 (21) |
| Fell in past 12 mo | 39 | 40 | 33.3 | 25.0 | 19.7 |
| Hospitalized in past 12 mo | 66 | 59 | 52.5 | 44.7 | 39.0 |
| ADL difficulty reported | 73 | 22 | 16.7 | 11.1 | 4.4 |
| SF-36 PF score | 16.4 (21.5) | 32.6 (23.3) | 47.2 (27.9) | 61.6 (24.7) | 74.5 (22.4) |
| Assistive walking device | 93 | 70 | 27.5 | 8.7 | 3.5 |
| Assistive device used for walk test | NA | 51 | 10.4 | 3.9 | 0.9 |
Note: N=752. Values for categorical variables are given as percentages; values for continuous variables, as mean ± standard deviation.
Abbreviations and definitions, BMI: body mass index; CAD: coronary artery disease; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; CVA: cerebrovascular accident; ESRD: end-stage renal disease; HD: hemodialysis; KDQoL-CF: Kidney Disease Quality of Life–Cognitive Function; MI: myocardial infarction; other cardiac disease: cardiac dysrhythmia, atrial fibrillation, tachycardia, pericarditis, cardiac arrest; other race: Native American, Asian, Native Hawaiian, other Pacific Islander, other; PVD: peripheral vascular disease; SBP: systolic blood pressure; SF-36 PF: 36-Item Short-Form Health Survey Physical Function; TIA: transient ischemic attack.
Table 2.
Cumulative prevalence of slow gait speed by age and sex
| <0.6 m/s | <0.8 m/s | <1.0 m/s | |
|---|---|---|---|
| Men | |||
| age <50 y | 6% | 16.9% | 42.2% |
| age 50–64 y | 6% | 25.0% | 61.6% |
| age 65–74 y | 14% | 36.5% | 73.1% |
| age ≥75 y | 27% | 54.5% | 86.4% |
| Women | |||
| age <50 y | 5% | 23.1% | 52.3% |
| age 50–64 y | 20% | 44.7% | 80.7% |
| Age 65–74 y | 36% | 70.5% | 88.5% |
| age ≥75 y | 42% | 73.7% | 100.0% |
Note: n=669 hemodialysis patients aged 20 years or older who performed walk test.
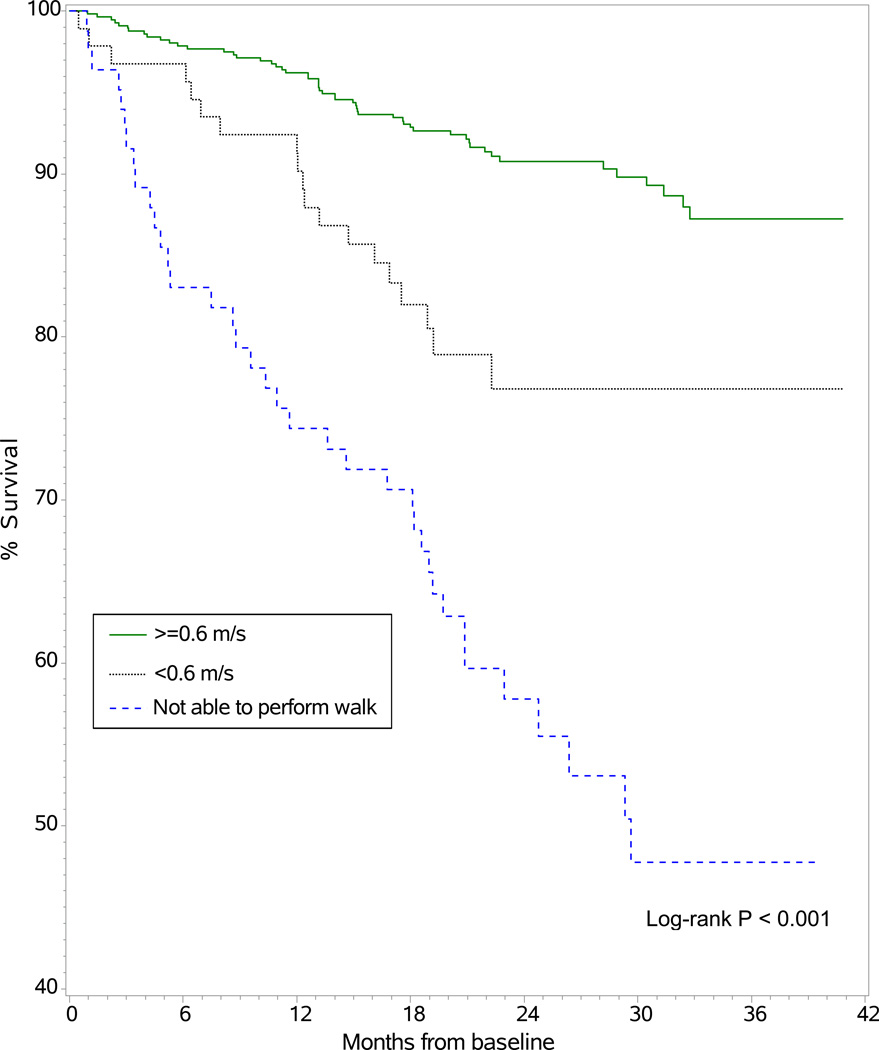
Study participants whose baseline walk speed was 0.6 m/s or faster had the best survival, followed by those whose walk speed was <0.6 m/s and then by those who were unable to perform the walk test (Figure 1). There were 53 (9%) mortality events among the 575 participants whose baseline walk speed was 0.6 m/s or faster, 19 (20%) deaths among the 94 whose baseline walk speed was <0.6 m/s and 37 (44%) deaths among the 83 who were unable to perform walk test. Compared with study participants whose walk speed was 0.6 m/s or faster, participants with walk speed <0.6 m/s were more than twice as likely to die (hazard ratio [HR], 2.46; 95% confidence interval [CI], 1.45–4.15; P < 0.001), and those who were unable to perform the walk test were more than five times more likely to die (HR, 5.84; 95% CI, 3.84–8.89; P < 0.001) [Table 3]. Results were very similar for the comparison of participants whose walk speed was <0.6 m/s with participants whose walk speed was 0.6 m/s or faster after adjusting for demographic variables, as well as in a model that adjusted for demographic variables plus ESRD vintage, comorbidities, smoking status, hemoglobin level, cognitive function score, and fall history. In the adjusted models, those unable to perform the walk test were more than six times more likely to die compared with participants whose walk speed was 0.6 m/s or faster. We tested the interaction of age and gait speed by adding the interaction term, which was not significant (P = 0.2). In addition to the results shown in Table 3, we modeled gait speed of ambulatory patients as a continuous variable; the model included gait speed and a nonambulatory/ambulatory indicator variable. Gait speed in the fully adjusted model remained associated with mortality when analyzed per incremental change in gait speed performance. Each 0.1-m/s decrement in gait speed was associated with an estimated 17% greater risk of death (HR, 1.17; 95% CI, 1.05–1.31; P = 0.004).
Figure 1.
Kaplan-Meier estimate of cumulative survival by study participants’ baseline gait speed category. For the ≥0.6 m/s, <0.6 m/s, and not able to perform walk test groups, the number of deaths/number at risk at 12 months were 21/526, 8/82, and 22/59, respectively. At 24 months, these values were 47/256, 19/33, and 34/24, respectively.
Table 3.
Cox proportional hazards models predicting association of gait speed category with all-cause mortality risk
| Gait Speed Performance | Model 1a | Model 2b | Model 3c |
|---|---|---|---|
| ≥ 0.6 m/s | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| < 0.6 m/s | 2.46 (1.45–4.15) | 2.39 (1.35–4.25) | 2.17 (1.19–3.98) |
| Unable to perform walk | 5.84 (3.84–8.89) | 6.93 (4.25–11.29) | 6.93 (4.01–11.96) |
Note: Values are given as hazard ratio (95% confidence interval). Data for prevalent hemodialysis patients through December 31, 2012.
Unadjusted for any covariates.
Included age, sex, race, education, and participant clinic (demographic variables).
Included demographic variables plus smoking status, body mass index, end-stage renal disease vintage, diabetes, chronic obstructive pulmonary disease, cancer, cardiovascular comorbidity, hemoglobin level, cognitive function score, and history of recent falls.
Outcome data at the 12-month follow-up were obtained for 466 study participants with gait speed 0.6 m/s or faster who survived and remained on in-center HD at a participating dialysis facility; their baseline characteristics were very similar to the baseline characteristics of the 575 participants with gait speed 0.6 m/s or faster shown in Table 1. In analyses adjusted for age, sex, and baseline value of the outcome, study participants with baseline gait speed 0.6 to <0.8 m/s and participants with baseline gait speed 0.8 to <1.0 m/s were twice as likely as those with baseline gait speed ≥1.0 m/s to be hospitalized during the following 12 months (ORs of 2.04 [95% CI, 1.19–3.49] and 2.05 [95% CI, 1.30–3.25], respectively). Compared with participants whose measured gait speed was ≥1.0 m/s, participants with baseline gait speed 0.6 to <0.8 m/s were almost 4 times more likely to report ADL difficulty at the 12-month follow-up (OR, 3.88; 95% CI, 1.46–10.33). On average, the PF score declined −8.20 (95% CI, −13.57 to −2.82) points from baseline to 12-months among participants with baseline gait speed 0.6 to <0.8 m/s compared with participants in the fastest gait speed category of ≥1.0 m/s (Table 4).
Table 4.
One-Year Outcomes by Baseline Gait Speed Category
| 12-mo outcome | 0.6–<0.8 m/s (n=116) |
0.8–<1.0 m/s (n=181) |
≥1.0 m/s (n=169)1 |
|
|---|---|---|---|---|
| New hospitalization | ||||
| No. (%) | 62 (53.5%) | 95 (52.5%) | 63 (37.3%) | |
| OR (95% CI)2 | 2.04 (1.19–3.49) | 2.05 (1.30–3.25) | 1.00 (reference) | |
| ADL difficulty reported | ||||
| No. (%) | 21 (18.3%) | 18 (10.1%) | 8 (4.8%) | |
| OR (95% CI)2 | 3.88 (1.46–10.33) | 2.11 (0.82–5.42) | 1.00 (reference) | |
| SF-36 PF score | ||||
| Mean ± SD | 44.8 ±29.4 | 59.7 ±26.5 | 73.3 ±24.6 | |
| Estimates (95% CI)3 | −8.20 (−13.57 to −2.82) | −4.01 (−8.45 to 0.43) | 1.00 (reference) | |
Note: n=466 participants with gait speed 0.6 m/s or faster who survived and remained on in-center HD at a participating dialysis facility at 12 months.
ADL, activities of daily living; CI, confidence interval; OR, odds ratio; l SD, standard deviation; SF-36 PF score, 36-Item Short Form Healthy Survey Physical Functioning score.
10% of patients in fastest gait speed category were not available for 12-mo follow-up due to transplantation or change to a home dialysis modality; 3.2% of patients in the two slower gait speed categories were not available for these reasons.
Estimated from logistic regression models, adjusted for age, sex and baseline value of outcome
Estimated from linear regression model, adjusted for age, sex and baseline value of outcome
DISCUSSION
Gait speed serves as a global marker of health status that can support clinical decisions “aimed at modifying…pragmatic end points.”17 However, there has been little previous study of gait speed among persons undergoing HD and of how gait speed may be associated with outcomes in this population. Participants in the ACTIVE-ADIPOSE study with gait speed <0.6 m/s and those who were unable to perform the walk test (who together were 23.5% of the study cohort) had significantly increased mortality risk compared with participants whose gait speed was 0.6 m/s or faster. In addition, HD patients with gait speeds slower than 1.0 m/s at baseline had higher odds after 12 months of having been hospitalized, of ADL difficulty, and of lower-rated PF.
Many factors may potentially contribute to gait speed decrements among HD patients and represent an important focus for further study. These factors include lower extremity pain or numbness, fractures, knee and hip replacements, and fatigue, as well as muscle atrophy. Johansen et al., who studied 38 HD patients (mean age, 55 ±15 [standard deviation] years) and 19 healthy sedentary controls (mean age, 55 ±13 years), found that HD patients walked more slowly than controls and had significant muscle atrophy. Specifically, smaller contractile cross-sectional area of the ankle dorsiflexor muscles was evident in dialysis patients, and gait speed was correlated with contractile cross-sectional area.18
Outcomes associated with gait speed have been well studied among older adults.19–23 Literature summarized by the International Task Force on Nutrition and Aging indicated that usual gait speed is predictive of older persons’ survival, disability, hospitalization or institutionalization, dementia, and falls.23 Studies of non–dialysis-dependent CKD patients as well as dialysis-dependent patients have reported increased mortality risk in individuals assessed as frail, a designation which may include slow gait speed as one defining characteristic.24–26 In addition, Roshanravan et al. recently showed that gait speed was significantly associated with all-cause mortality in 385 ambulatory persons with CKD stages 2–4 whose mean age was 61 ± 13 years.4 Each 0.1-m/s decrement in gait speed associated with a 26% higher risk for death among CKD patients over a 3-year follow-up period, in an analysis adjusted for age, sex, race, smoking, BMI, diabetes, coronary artery disease, eGFR, and study site. In a similar analysis that also included adjustment for recent falls, we found that each 0.1-m/s decrement in gait speed among maintenance HD patients who participated in the ACTIVE-ADIPOSE study associated with a 17% higher risk for death over a median follow-up of 703 days. Table 1 shows that the prevalence of recent falls ranged from 19.7% to 40.4% of patients classified by measured gait speed, and falling itself may confer mortality risk.27
Among the many strengths of the ACTIVE-ADIPOSE study is that data were from a large multi-center study cohort. Performance-based gait speed was carefully assessed, along with various patient characteristics and treatment-related factors. Measurement error in gait speed assessment appeared to be quite small. Both baseline and 12-month follow-up information was available. We focused on the association of baseline gait speed with hospitalization, ADL difficulty, and patient-reported PF after 12 months as separate outcomes, but we acknowledge that there are likely to be associations among these outcomes, in addition to their associations with gait speed. We also acknowledge that additional variables, e.g. recency of a hospitalization episode, might confound observed associations between gait speed and outcomes.
It is also important to acknowledge that recommendations for clinically relevant gait speed cut points are currently based primarily on data from white populations in the United States.6 The ACTIVE-ADIPOSE cohort is similar to the general ESRD population, but participants were limited to 7 outpatient clinics in the Atlanta area and 7 outpatient clinics in the San Francisco Bay Area, and representation of African-Americans in the cohort was greater compared with the US in-center HD population overall. Table 1 shows that the proportion of African-Americans declined with increasing gait speeds (P <0.001 based on the Cochran-Armitage Trend test). Conversely, Table 1 also shows that compared with other race groups represented in the study cohort, blacks were less likely to be unable to perform the walk test.
Maintaining walking ability is key for performing physical activity,28 an especially important goal given that walking is the most popular form of exercise in individuals with CKD.29 Achieving higher levels of ambulation, or at least slowing or preventing decline in gait speed, may enable a person to more safely negotiate household and community environments.30 The Renal Exercise Demonstration Project found that patients undergoing HD who participated in an exercise intervention had a mean change in gait speed of 0.09 ±0.15 m/s, while gait speed change in the control group who received no intervention was -0.04 ± 0.16 m/s (p =0.02),8 providing evidence that gait speed could be improved with counseling and encouragement for increased physical activity. The mean age of HD patients in that study was 56.6 ±15.6 years, and their average baseline gait speed was 0.8–0.9 m/s.
Signaling medical disturbances and risk for functional decline that may be addressed are important goals associated with gait speed assessment. Compared with patient-reported mobility difficulty, actual assessment of mobility with a gait speed test provides a quantitative marker and allows tracking of changes in mobility that could result in eventual disability. Referral to a specialist (physical therapist, clinical exercise or cardiac rehabilitation specialist) for additional evaluation and intervention might be indicated.5 The contribution of rehabilitation services to walking ability and falls among persons who require dialysis is an important area for investigation.
Gait speed is typically measured as the time it takes a person to walk at a usual pace over a measured distance (usually 4–6 m),5 while the timed up-and-go test includes rising from a chair, turning, and sitting as well as walking 3 m,20 and the Short Physical Performance Battery includes rising from a chair and three tests of static balance as well as walking 4 m.3,31 Cummings et al. note that, in a busy clinical setting, gait speed testing has the important advantages of brevity (requires approximately 2 minutes) and simplicity (no need to calculate and sum sub-section scores).6 Our study suggests that gait speed can provide an informative and potentially actionable functional status measure in the dialysis care setting, meeting the criteria of importance, scientific acceptability, feasibility, and usability. Further expansion of the evidence base will be valuable.
ACKNOWLEDGEMENTS
Support: This work was supported by National Institutes of Health contract HHSN267200715004C, ADB no. N01-DK-7-5004 (Dr Kutner). The funders of this study were consulted regarding study design and approval of the final manuscript, but were not involved in data collection or analysis. The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The results presented in this paper have not been published previously in whole or part, except in abstract format.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research area and study design: NGK, RZ, YH, PP; data acquisition: NGK, PP; data analysis/interpretation: NGK, RZ, YH, PP; statistical analysis: RZ, YH; supervision: NGK, PP. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. NGK takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
REFERENCES
- 1.National Kidney Foundation. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4, Suppl 3):S1–S154. [PubMed] [Google Scholar]
- 2.Painter P, Marcus RL. Assessing physical function and physical activity in patients with CKD. Clin J Am Soc Nephrol. 2013;8:861–872. doi: 10.2215/CJN.06590712. [DOI] [PubMed] [Google Scholar]
- 3.Reese PP, Cappola AR, Shults J, Townsend RR, Gadegbeku CA, Anderson C, et al. Physical performance and frailty in chronic kidney disease. Am J Nephrol. 2013;38(4):307–315. doi: 10.1159/000355568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roshanravan B, Robinson-Cohen C, Patel KV, Ayers E, Littman AJ, de Boer IH, et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol. 2013;24:822–830. doi: 10.1681/ASN.2012070702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Painter P, Marcus R. Physical function and gait speed in patients with chronic kidney disease. Nephrol Nurs J. 2013;40(6):529–538. [PubMed] [Google Scholar]
- 6.Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility—giving mobility clinical visibility. JAMA. 2014;311(20):2061–2062. doi: 10.1001/jama.2014.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansen KL, Chertow GM, Silva M, Carey S, Painter P. Determinants of physical performance in ambulatory patients on hemodialysis. Kidney Int. 2001;60:1586–1591. doi: 10.1046/j.1523-1755.2001.00972.x. [DOI] [PubMed] [Google Scholar]
- 8.Painter PL, Carlson L, Carey S, Paul SM, Myll J. Physical functioning and health-related quality-of life-changes with exercise training in hemodialysis patients. Am J Kidney Dis. 2000;35(3):482–492. doi: 10.1016/s0272-6386(00)70202-2. [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US. Renal Data System. USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the US. Chapter 9 Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [Google Scholar]
- 12.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. for the Cardiovascular Health Study Research Group. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 13.Buchner DM, Hornbrook MC, Kutner NG, Tinetti ME, Ory MG, Mulrow CD, et al. the FICSIT Group. Development of the common data base for the FICSIT trials. J Am Geriatr Soc. 1993;41:297–308. doi: 10.1111/j.1532-5415.1993.tb06708.x. [DOI] [PubMed] [Google Scholar]
- 14.Gill TM, Gahbauer EA. Evaluating disability over discrete periods of time. J Gerontol A Biol Sci Med Sci. 2008;63A:588–594. doi: 10.1093/gerona/63.6.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36) Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 16.Kurella M, Luan J, Yaffe K, Chertow GM. Validation of the Kidney Disease Quality of Life (KDQOL) cognitive function subscale. Kidney Int. 2004;66:2361–2367. doi: 10.1111/j.1523-1755.2004.66024.x. [DOI] [PubMed] [Google Scholar]
- 17.Cesari M. Role of gait speed in the assessment of older patients. JAMA. 2011;305(1):93–94. doi: 10.1001/jama.2010.1970. [DOI] [PubMed] [Google Scholar]
- 18.Johansen KL, Shubert T, Doyle J, Soher B, Sakkas GK, Kent-Braun JA. Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int. 2003;63(1):291–297. doi: 10.1046/j.1523-1755.2003.00704.x. [DOI] [PubMed] [Google Scholar]
- 19.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viccaro LJ, Perera S, Studenski SA. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J Am Geriatr Soc. 2011;59:887–892. doi: 10.1111/j.1532-5415.2011.03336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montero-Odasso M, Shapira M, Soriano ER, Varela M, Kaplan R, Camera LA, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60A(10):1304–1309. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- 22.Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. 2007;55(11):1727–1734. doi: 10.1111/j.1532-5415.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 23.Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people: an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelm-Leen ER, Hall YN, Tamura MK, Chertow GM. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122:664–671. doi: 10.1016/j.amjmed.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roshanravan B, Khatri M, Robinson-Cohen C, Levin G, Patel KV, deBoer IH, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60(6):912–921. doi: 10.1053/j.ajkd.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAdams-DeMarco MA, Law A, Salter ML, Boyarsky B, Gimenez L, Jaar BG, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61:896–901. doi: 10.1111/jgs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdel-Rahman EM, Turgut F, Turkmen K, Balogun RA. Review: Falls in elderly hemodialysis patients. Q J Med. 2011;104:829–838. doi: 10.1093/qjmed/hcr108. [DOI] [PubMed] [Google Scholar]
- 28.Hadley EC. Testing interventions to preserve walking ability: Progress against disability, one step at a time. J Gerontol A Biol Sci Med Sci. 2007;62A(8):834–836. doi: 10.1093/gerona/62.8.834. [DOI] [PubMed] [Google Scholar]
- 29.Chen I-R, Wang S-M, Liang C-C, Kuo H-L, Chang C-T, Liu J-H, et al. Association of walking with survival and RRT among patients with CKD stages 3–5. Clin J Am Soc Nephrol. 2014;9(7):1183–1189. doi: 10.2215/CJN.09810913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braden H. Self-selected gait speed: A critical clinical outcome. [Accessed June 11, 2014];Lower Extremity Review. 2012 Nov; http://lermagazine.com/article/self-selected-gait-speed-a-critical-clinical-outcome. [Google Scholar]
- 31.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance Battery. J Gerontol A Biol Sci Med Sci. 2000;55A(4):M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]