Abstract
Objective
Management of meals and mealtime behavior is often challenging for parents of young children with type 1 diabetes. Parent functioning related to diabetes care may directly affect mealtime behaviors and glycemic control. This study evaluated associations among diabetes-specific parent functioning, parent and child mealtime behaviors, and glycemic control.
Methods
Parents of young children with type 1 diabetes (n=134) completed self-report measures assessing diabetes-specific functioning (hypoglycemia fear, diabetes self-efficacy, diabetes-related quality of life) and child and parent mealtime behaviors. Hemoglobin A1c and percentage of blood glucose values out of range (<70 mg/dL or >200 mg/dL) over a 30 day period were abstracted from medical charts as indicators of glycemic control. Structural equation modeling was utilized to evaluate predictors and related outcomes of child and parent mealtime behavior.
Results
The proposed model fit the data very well. More frequent problematic child mealtime behaviors were associated with poorer glycemic control; however, more frequent problematic parent mealtime behaviors were marginally associated with better glycemic control. Poorer diabetes-specific parent functioning was associated with more frequent problematic child and parent mealtime behaviors.
Conclusions
Problematic child mealtime behaviors, such as disruptive behavior, present a significant risk for poorer glycemic control. Parents may engage in ineffective mealtime management strategies in an effort to meet glycemic recommendations and avoid hyperglycemia and hypoglycemia. Future research will help to determine if parents may benefit from specific, developmentally-appropriate behavioral strategies to manage meals and snacks and promote optimal diabetes management.
Keywords: type 1 diabetes, young children, mealtime behavior, glycemic control
Introduction
The age of onset of type 1 diabetes (T1D) is shifting to younger ages (Harjutsalo, Sjoberg, & Tuomilehto, 2008), leading to a rapid increase in the prevalence of T1D in young children (Vehik et al., 2007). When a young child is diagnosed with T1D, parents must assume primary responsibility for diabetes management, including frequent blood glucose (BG) level monitoring, insulin administration, diet and physical activity supervision, and hypoglycemia and hyperglycemia identification and treatment (Smaldone & Ritholz, 2011; Sullivan-Bolyai, Knafl, Deatrick, & Grey, 2003). Maintenance of near-normal glycemic levels in young children is critical for short-term and long-term health outcomes, as this developmental period is characterized by rapid physical, neurological, and cognitive growth (Desrocher & Rovet, 2004).
Negative parent perceptions about their abilities to manage T1D add to the burden of responsibility for parents of young children and can affect daily diabetes care (S. Sullivan-Bolyai, Deatrick, Gruppuso, Tamborlane, & Grey, 2003). Constructs contributing to diabetes-specific parent functioning include worry about hypoglycemia, parental confidence in their ability to manage T1D, and satisfaction with T1D care. Diabetes-specific parent functioning and perceived burden may also be influenced by demographic and medical characteristics, such as child age or insulin regimen. For example, parents of younger children and parents of young children on intensive insulin regimens report greater diabetes-related stress than parents of older children and those on conventional insulin regimens (Herbert et al., in press; Stallwood, 2005; Streisand, Swift, Wickmark, Chen, & Holmes, 2005).
Research has not consistently found links between diabetes-specific parent functioning and health outcomes (Jaser, Whittemore, Ambrosino, Lindemann, & Grey, 2009; Mitchell et al., 2009). Patton and colleagues found that higher parent-reported hypoglycemia worry was associated with higher mean daily BG levels but not A1c (Patton, Dolan, Henry, & Powers, 2007). Hilliard and colleagues found no significant associations between parent disease-specific stress and glycemic control (Hilliard, Monaghan, Cogen, & Streisand, 2011). In comparison, Stallwood found that higher parent-reported diabetes-specific stress was associated with better glycemic control (Stallwood, 2005). It is possible that diabetes-specific parent functioning may exert greater influence on daily behaviors enacted to manage diabetes, which in turn impact glycemic control. One possible candidate is the management of meals and mealtime behavior, which is likely influenced by parent functioning and, more importantly, has significant implications for glycemic control. Relations among diabetes-specific parent functioning, mealtime behaviors and glycemic control have not been examined to date.
Mealtime problems, such as maladaptive or disruptive behaviors and unpleasant mealtime interactions, are common in young children (Berlin, Davies, Lobato, & Silverman, 2009; Patton, Dolan, Chen, & Powers, 2013; Powers et al., 2002). Frequently endorsed mealtime problems by parents of young children include getting up from the table during meals, prolonged meal duration, food refusal, tantrums, and food selectivity (Crist & Napier-Phillips, 2001). Mealtime problems affect up to 45% of typically-developing children (Berlin et al., 2009; Manikam & Perman, 2000), and may be as or more prevalent among young children with T1D. Parents of young children with T1D endorse significant stress related to mealtime management and, as a result, may engage in problematic or ineffective mealtime management strategies, including issuing direct and indirect commands, engaging in coercive behavior, and physically prompting feeding (Patton, Dolan, & Powers, 2008; Powers et al., 2002). An observational study found that problematic parent and child mealtime behaviors occurred during nearly half of an observed meal's duration in young children with T1D (Patton, Dolan, & Powers, 2008).
Difficulties with mealtime management may directly impact glycemic control. For example, eating less than expected can lead to severe hypoglycemia, particularly if insulin is administered prior to meals as recommended, whereas eating more than expected may require extra insulin or additional monitoring to avoid hyperglycemia (American Diabetes Association, 2013). Frequent problematic child and parent mealtime behaviors can interfere with food intake and have been associated with poorer glycemic control (Patton, Williams, Dolan, Chen, & Powers, 2009; Patton, Dolan, & Powers, 2006). More frequent mealtime behavior problems have also been associated with worse adherence to healthy T1D dietary practices, yet, in one study, more frequent parent mealtime behavior problems were also associated with better T1D dietary adherence. Parent mealtime functioning was not directly related to glycemic control (Patton et al., 2013). A pilot intervention targeting nutrition and parent behavior resulted in improved mealtime behavior and lower mean BG levels, even though dietary content was unchanged (Patton, Odar, Midyett, & Clements, 2014). Many of these studies were preliminary and had relatively small, homogenous samples, ranging from 8 to 59 young children with T1D. Additional research is needed to clarify these relations and understand the specific factors that may influence mealtime behaviors and practices among parents and young children with T1D, particularly in samples large enough for statistical modeling.
This cross-sectional study sought to characterize diabetes-specific parent functioning, including parental hypoglycemia fear, self-efficacy for diabetes management, and quality of life, in relation to parent and child mealtime behavior and glycemic control in young children with T1D. Most studies have attempted to explore these complex relations in isolation with small or mixed samples that include both young and school-age children (Jaser et al., 2009; Powers et al., 2002); thus, this study further aimed to model interrelated factors associated with glycemic control with structural equation modeling in a relatively large sample of young children with T1D. We predicted that poorer diabetes-specific parent functioning (i.e., more hypoglycemic fear, lower perceived efficacy for diabetes management, and poorer diabetes quality of life) would be associated with more frequent parent and child mealtime behavior problems, which would in turn be associated with poorer glycemic control (i.e. greater percentage of BG levels out of range, higher A1c).
Methods
Participants
Primary caregivers of young children with T1D were recruited from three tertiary diabetes care sites and enrolled in a randomized controlled trial (RCT) of a behavioral intervention designed to promote parental adjustment and management of T1D. Eligibility criteria for the RCT included: primary caregiver (hereafter referred to as “parent”) of a child age 1-6 diagnosed with T1D for at least 6 months, English fluency, and absence of child or parent major chronic illness or developmental disability. Following baseline data collection, RCT participants completed an in-person orientation session, five telephone-based intervention or education sessions, and follow-up questionnaires at three time points (1-month, 6-months, and 12-months post completion of phone sessions). Only baseline data were used for this study.
Procedure
Institutional review board approval was obtained from each of the three recruitment sites. Eligible participants were identified through clinic list review and were mailed an informational letter detailing study procedures. Participants who did not opt-out of further contact were contacted by phone by a trained research assistant to assess eligibility for and interest in study participation. Interested participants provided verbal consent, completed baseline assessment measures by telephone, met with a trained research assistant to complete written informed consent and an in-person orientation session, and were randomized to the intervention group or education-only control group. Hemoglobin A1c values and the most recent 30 days of BG values were taken from medical chart reviews and clinic downloads of glucometers.
Two-hundred eighty-five parents were initially mailed letters explaining the larger intervention study procedures. Of the 285 parents, 66 (23%) were unable to be contacted, 16 (6%) were ineligible, and 36 (13%) declined to participate, resulting in 167 parents (58%) who initially agreed to participate. Of the 167 parents who initially agreed, 134 parents (80%) completed baseline questionnaires and consent procedures. All participants received a modest gift card or personal check for completion of baseline assessment measures ($50).
Study Measures
Demographic and medical questionnaire
Parents completed a study-specific General and Medical Information Questionnaire to report demographic and medical information, including parent and child age, gender, race, marital status, child diabetes regimen, illness duration, and history of meeting with a dietitian.
Glycemic control
Hemoglobin A1c is the most widely accepted measure of glycemic control and provides an average glucose level from the past 2-3 months (American Diabetes Association, 2013). A1c values were collected as part of routine clinical care and were abstracted from the medical chart for the clinic visit closest to the date of baseline questionnaire completion. All assays were conducted with the DCA 2000 Analyzer and used high performance liquid chromatography to assure comparability among subjects (Tamborlane et al., 2005).
Glycemic control was also assessed by evaluation of the most recent 30 days of BG data. Glucometers were downloaded as a part of routine clinical care at each recruitment site. If a glucometer was not downloadable, 30 days of printed participant-downloaded data or handwritten data (by study research assistant) were obtained at the clinic visit closest to baseline questionnaire completion. For the purposes of this study, the percentage of BG values less than 70 mg/dL and greater than 200 mg/dL (the recognized range for hypoglycemia and hyperglycemia) in a 30 day period was calculated for each participant.
Mealtime Behaviors
The Behavioral Pediatrics Feeding Assessment Scale (BPFAS) is a well-validated parent-report questionnaire that assesses a range of behaviors associated with feeding and mealtime in young children (Crist & Napier-Phillips, 2001). Two subscales assess: 1) child behaviors associated with meals (25 items), such as disruptive behaviors, 2) parent behaviors/practices and emotions associated with meals (10 items), such as using threats to encourage eating or making an alternate food if the first food is refused. Parents rate the frequency of the target behavior on a 5 point Likert scale from 1 = “never” to 5 = “always;” higher scores indicate more frequent mealtime difficulties. The BPFAS has been widely used with general samples of young children (Crist & Napier-Phillips, 2001) and young children with chronic illness (Mitchell, Powers, Byars, Dickstein, & Stark, 2004; Patton et al., 2009). Internal consistency estimates among this sample were good (Child Behavior Frequency α = .83; Parent Behavior Frequency α = .71).
Hypoglycemia fear
The Hypoglycemia Fear Survey - Parents of Young Children (HFS-PYC) (Cox, Irvine, Gonder-Frederick, Nowacek, & Butterfield, 1987; Patton et al., 2007) is a 27-item measure that assesses parents’ fear about their young child experiencing a low BG level. The HFS-PYC Worry subscale consists of 16 items rated on a 5-point Likert scale from1 = “never” to 5 = “always;” higher scores indicate greater worries related to hypoglycemia fear. In a sample of young children, Patton et al. (2007) reported acceptable internal consistency scores for the Worry subscale (worry α = .89). Internal consistency estimates among this sample were good (HFS-Worry α = .92).
Self-efficacy for diabetes care
The Self-Efficacy for Diabetes Scale for Parents (SED-P) is a 22-item measure of parents’ confidence in their ability to perform daily tasks related to diabetes management (Streisand et al., 2005), adapted from the original Self-Efficacy for Diabetes Scale (Grossman, Brink, & Hauser, 1987). Parents rate their confidence in performing specific T1D-related tasks on a 5-point Likert scale ranging from 1 = “very sure I can’t” to 5 = “very sure I can”. Higher scores indicate greater parent perceived self-efficacy in caring for their child's T1D. Previous studies have found good internal consistency (α = .90), reliability, and construct validity estimates. Internal consistency for this sample was acceptable (α = .78). To maintain consistency with the scoring of other measures of diabetes-specific parent functioning, this scale was reverse scored so that higher SED-P scores indicated lower perceived self-efficacy for T1D management.
Quality of life
The Parents Diabetes Quality of Life Questionnaire (PDQOL) (Vandagriff, Marrero, Ingersoll, & Fineberg, 1992) is a self-report questionnaire designed to assess parents’ diabetes-specific quality of life. Three subscales assess: 1) parent satisfaction with T1D management and care; 2) direct impact of T1D on parental lifestyle, and 3) worries related to T1D. Each subscale is scored on a 5-point Likert scale from 1 = “very satisfied” to 5 = “very dissatisfied;” 1 = “never” to 5 = “all the time;” and 0 = “does not apply” to 4 = “all the time,” respectively. Subscale scores are summed to create a total QOL score; higher scores indicate poorer QOL. The measure was reported by Vandagriff et al. (1992) to be both reliable and valid (α = .64-.89). Internal consistency estimates for the total score in the current sample were good (α = .89).
Data Analytic Plan
Descriptive and correlational analyses were conducted using SPSS version 21. Model estimation was conducted using Mplus 7.1 (Muthen & Muthen, 1998-2012). The conceptual model proposed for evaluation in this study is depicted in Figure 1. A1c is the primary outcome of interest. Parent-report on measures of diabetes-specific functioning, including hypoglycemia fear – worry subscale, self-efficacy for diabetes, and diabetes-related quality of life, were used as indicators of the latent variable of diabetes-specific parent functioning. Parent reports on the two frequency subscales of the Behavioral Pediatrics Feeding Assessment Scale - Child Behavior Frequency and Parent Behavior Frequency - were included as well. It is hypothesized that child and parent mealtime behaviors indirectly influence A1c through daily glycemic control, as indicated by percent of daily BG levels out of range (<70 and >200 mg/dL). In addition to a direct effect on A1c, diabetes-specific parent functioning is hypothesized to have three specific indirect effects on A1c through: 1) frequency of parent problematic mealtime behaviors and percent of daily BG levels out of range; 2) frequency of child problematic mealtime behaviors and percent of daily BG levels out of range; and 3) percent of daily BG levels out of range. Demographic and medical variables that were significantly correlated with variables of interest (child age, child race, parent marital status, and child medical regimen) were included in the model as covariates.
Figure 1. Structural Equation Model.
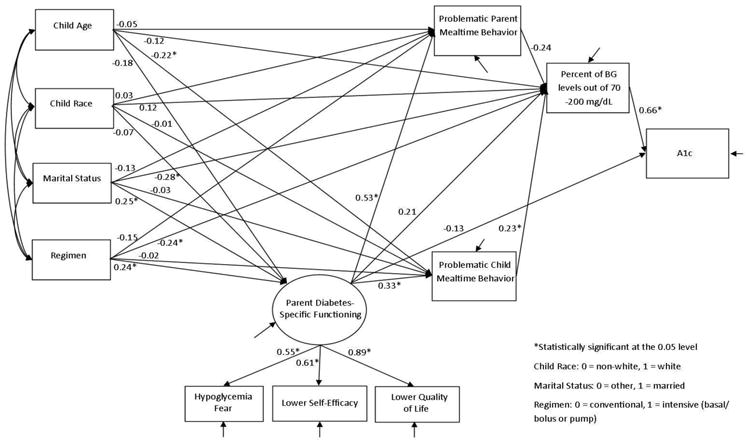
Before statistical modeling, Mardia multivariate skewness and kurtosis tests (Mardia, 1974; Mardia, Kent, & Bibby, 1979) were conducted to test the assumption of multivariate normality in the data. The results show that the Mardia Kurtosis statistic was not significant (p = 0.3178), but the Mardia Skewness statistic was significant (p = 0.0106), indicating violation of multivariate normality assumption. As such, a robust maximum likelihood estimator (MLR) was applied for model estimation (Muthen, 1998-2004). MLR is a sandwich estimator providing robust standard errors and model χ2 statistic when data do not have normal distributions. In model estimation, the full information maximum likelihood (FIML) approach uses every piece of information in the observed data for analysis with the much less restrictive assumption of missing at random (MAR) that allows missingness to be related to both observed outcomes and covariates (Finkbeiner, 1979). Model fit was evaluated using model Chi-square statistic and model fit indices, including root mean square error of approximation (RMSEA), 90% confidence interval of RMSEA, close-fit test P-value, comparative fit index (CFI), Tucker-Lewis index (TLI), and standardized root mean square residual (SRMR).
Results
Participants
One hundred and thirty four parents (90% female) completed baseline data collection. Children were between ages 1-6 (M age = 5.32 ± 1.34 years) and had been diagnosed with T1D for an average of 1.99 years (± 1.24 years). Mean glycemic control was 8.13% (± 0.88), falling above the current American Diabetes Association recommended A1c level ≤ 7.5% for youth (Chiang, Kirkman, Laffel, & Peters, 2014). Forty-three percent of participants reported meeting with a dietitian within the past year; 36% reported meeting with a dietitian at diagnosis only. See Table 1 for demographic details.
Table 1. Participant Characteristics (n = 134).
| % | Mean | SD | Range (possible range) |
|
|---|---|---|---|---|
| Parent age (years) | 36.80 | 5.93 | 22.21-60.05 | |
| Parent gender (% female) | 90% | |||
| Child age (years) | 5.32 | 1.34 | 2.01-6.98 | |
| Child gender (% female) | 49% | |||
| Child race (% Caucasian) | 78% | |||
| Parent Marital Status (% married) | 84% | |||
| Household income (%≥$50,000) | 76% | |||
| Disease Duration (years) | 1.99 | 1.24 | 0.54-5.95 | |
| Regimen (% basal-bolus or pump) | 72% | |||
| Hypoglycemia Fear – Worry Subscale | 39.86 | 11.64 | 16-72 (16-80) | |
| Self-Efficacy for Diabetes – Parent (R)* | 37.51 | 7.09 | 22-56 (22-110) | |
| Parent Diabetes Quality of Life | 80.58 | 17.03 | 36-130 (28-196) | |
| Child Mealtime Problems – Frequency | 46.84 | 9.80 | 29-76 (25-135) | |
| Parent Mealtime Feelings/Strategies - Frequency | 18.78 | 4.92 | 10-31 (10-50) | |
| Hemoglobin A1c | 8.13% | 0.88 | 6.40–11.00% | |
| Percent of BG values <70 and >200 mg/dL | 51.91% | 13.01 | 9.10-76.00% |
The SED-P was reverse scored
Descriptive Statistics and Correlations
Mean scores were calculated for all study variables (see Table 1). Report of frequency of child (M = 46.84) and parent (M = 18.78) mealtime concerns using the BPFAS were comparable to the mean child (range = 44.9 - 50.0) and parent (range = 16.0-20.6) mealtime concerns reported by other samples of young children with T1D (Patton et al., 2009; Powers et al., 2002). Similarly, mean hypoglycemia worry (M = 39.86) scores were comparable to hypoglycemia worry scores (range = 38.0 - 44.3) in similar samples (Patton et al., 2007; Patton, Dolan, Henry, & Powers, 2008). Self-efficacy for diabetes care (M (prior to reverse scoring) = 94.49) is comparable to a sample of fathers of young children with T1D (M = 92.0) and higher than reports from primarily mothers of older children with T1D (M = 78.86) (Mitchell et al., 2009; Streisand et al., 2005). Parent diabetes-related quality of life scores (M = 80.58) were comparable to parents of school-age children (M = 86.54) (Vandagriff et al., 1992).
Bivariate correlations or point-biserial correlations (when one variable was categorical) were conducted to evaluate relations among demographic characteristics, parent-report measures, and glycemic control indicators. Demographic characteristics that were significantly related to predictors and outcomes in the proposed model (child age, child race, parent marital status, and insulin regimen) were included in the model. Younger child age was associated with decreased diabetes self-efficacy (r = -.20) and greater frequency of child mealtime problems (r = -.28); ps<.05. Caucasian race was associated with lower percentage of BG levels out of range (r = -.26) and lower A1c (r = -.24); ps<.01. Additionally, marital status (married) was associated with lower percentage of BG levels out of range (r = -.28) and lower A1c (r = -.34); ps<.01. Use of an intensive insulin regimen was significantly associated with poorer diabetes-related quality of life (r = .25) but a lower percentage of daily BG levels out of range (r = -.22); ps<.05. Other demographic characteristics were not included in the model because they were either not significantly related to any outcomes of interest (e.g., child gender) or their pattern of significance with the outcomes of interest was inconsistent (e.g., disease duration was only associated with percentage of BG levels out of range).
Structural Equation Modeling
The proposed model fit the data well. The CFI and TLI were greater than 0.90, and SRMR was less than 0.08, all of which indicate a good model fit. The model Chi-square was not statistically significant (p = 0.06), indicating that the proposed model could not be rejected. The overall model fit index, as indicated by RMSEA, was 0.06, which is well below the cutoff point for model fit (0.08). Although the upper bound (0.10) of the RMSEA 90% confidence interval was slightly larger than 0.08, the Close-Fit Test (p = 0.31) could not reject the hypothesis of RMSEA ≤ 0.05.
The factor loadings of the three diabetes-specific parent functioning indicators ranged from 0.55 to 0.89, all greater than the conventional cutoff point of 0.30 and supporting the creation of a latent variable. The estimated path coefficients are reported in Figure 1. As expected, the percentage of BG values outside the target range of 70 – 200 mg/dL positively related to A1c (0.66, p < 0.001). The frequency of problematic child mealtime behaviors also positively related to the percentage of BG values out of range (0.23, p = 0.04); that is, a greater frequency of child mealtime problems was associated with a greater percentage of BG levels out of range. The model supported the hypothesized indirect effect of more frequent problematic child mealtime behaviors relating to a greater percentage of BG values out of range and, in turn, poorer A1c (p = 0.049). In comparison, the frequency of problematic parent mealtime behaviors demonstrated a trending association with the percentage of BG values out of range (-0.24, p = 0.05); more frequent problematic parent practices during meals were marginally associated with a lower percentage of BG levels out of range. The model supported the indirect effect of more frequent problematic parent mealtime behaviors marginally relating to a lower percentage of BG values out of range and, in turn, better A1c values (p = 0.06). Diabetes-specific parent functioning positively related to both problematic child (0.33, p < 0.001) and parent (0.53, p < 0.001) mealtime behaviors but had no significant direct effect on glycemic control indices. Poorer diabetes-specific parent functioning related to more frequent problematic child and parent mealtime behaviors.
Demographic factors were also evaluated in the model. Child age had a negative effect on the frequency of problematic child mealtime behaviors (-0.22, p = 0.003); younger child age was associated with more frequent child mealtime problems. Parent marital status had a positive effect on diabetes-specific parent functioning (0.25, p = 0.010) and a negative effect on the percentage of BG values out of range (-0.28, p = 0.001); married parents reported poorer diabetes-specific functioning but a lower percentage of BG values out of range. Child medical regimen also had a positive effect on diabetes-specific parent functioning (0.24, p = 0.020) and a negative effect on the percentage of BG values out of range (-0.28, p = 0.003); intensive regimens were associated with poorer diabetes-specific parent functioning but a lower percentage of BG values out of range.
Discussion
This study is one of the first to evaluate interrelated factors associated with glycemic control via structural equation modeling in a relatively large sample of parents of young children with T1D. Results extend the current literature by linking mealtime behaviors with indicators of both parent emotional functioning and glycemic control. More frequent problematic child mealtime behaviors were associated with poorer glycemic control, which suggests that addressing disruptive child mealtime behaviors and other mealtime concerns is of critical importance in efforts to improve glycemic control in young children. Worse diabetes-specific parent functioning, including more hypoglycemia worry, lower self-efficacy for diabetes-related tasks, and poorer diabetes-related quality of life, was significantly associated with more frequent problematic child and parent mealtime problems but not directly associated with A1c, highlighting the complex relations between parent functioning and glycemic control in young children with T1D.
Current findings support the hypothesis that mealtime behavior is associated with both parent functioning and glycemic control in young children with T1D. As suggested by smaller samples using observational and parent-reported data (Patton et al., 2009; Powers et al., 2002), more frequent problematic child mealtime behaviors were associated with greater percentage of BG values out of range and, in turn, poorer A1c. The current results extend these findings by adding parent indicators of diabetes-specific functioning and mealtime behavior. More frequent problematic parent mealtime behaviors were marginally associated with a lower percentage of BG values out of range and, in turn, showed a trend toward better A1c values. Thus, greater frequency of problematic child behaviors was detrimental, but the relation between greater frequency of problematic parent behaviors and glycemic control was less clear. It is possible that this relation is driven by other factors in the model, as parent mealtime behaviors were not correlated with BG values out of range or A1c in preliminary analyses. However, research has also demonstrated that parents of young children with T1D work harder to manage meals as compared to parents of healthy children and it is possible that this effort is reflected in both more frequent positive and negative mealtime behaviors (Patton, Dolan, & Powers, 2008). Additional longitudinal studies that further explore this trend are warranted.
Greater parent stress and effort during meals has consequences. Parents with poorer diabetes-specific functioning report more frequent parent and child mealtime problems. Although poorer diabetes-specific functioning was not associated directly with glycemic control, its effect on glycemic control was indirect and better represented by the more proximal indicator of mealtime problems. Comprehensive biopsychosocial models of meals in young children without T1D recognize the dynamic dyadic interactions inherent in mealtime management and the importance of assessing caregiver variables, including stress and efficacy, in the mealtime process (Berlin et al., 2009; Davies, Satter, Berlin, Sato, & Silverman, 2006). Evaluation of mealtime behavior in young children with T1D is a core component of diabetes education for this age group and should also include an assessment of diabetes-specific parent functioning as well as parent mealtime practices. Since these data are cross-sectional, the direction of this association is unknown. Reducing hypoglycemia worry and increasing parental efficacy related to diabetes care may contribute to improved parent and child mealtime behaviors. Parents may require additional skills or support to implement behavioral strategies associated with the promotion of healthy eating and management of mealtime behavior in young children, particularly in light of disruptive child mealtime behaviors.
Key demographic characteristics were associated with adverse health indicators in this sample. Younger age was associated with report of more frequent child mealtime behavior problems. Mealtime behavior problems likely vary, and may change, with age, especially as children develop self-regulation and communication skills and assume more responsibility for self-feeding. Younger children are more likely to engage in crying, tantrums, and spitting out food during meals, whereas older children are more likely to leave the table or delay eating during meals (Crist & Napier-Phillips, 2001). Parents of toddlers may require specialized instruction to manage nutritional needs related to T1D and to manage food refusal and other behavior problems. Parents who were married or who had children on intensive insulin regimens also reported worse diabetes-specific functioning; however, their children were also less likely to have out-of-range BG levels or elevated A1c values. Other studies have also found a relation between more intensive, demanding insulin regimens (such as insulin pumps) and diabetes-specific burden (Monaghan, Herbert, Cogen, & Streisand, 2012) but also greater satisfaction with diabetes care (Churchill, Ruppe, & Smaldone, 2009). Thus, the increased parent demands of an intensive regimen may be a worthwhile trade for some parents seeking tighter glycemic control. It is unclear why married parents reported worse diabetes-specific functioning. It is possible that married parents perceive a greater impact on family activities or engage in more frequent disagreements with a spouse about diabetes care (Sullivan-Bolyai, Rosenberg, & Bayard, 2006). This should be further evaluated in future research.
Increased parent stress has been associated with better diabetes outcomes in older children with T1D (Helgeson, Becker, Escobar, & Siminerio, 2012; Stallwood, 2005), suggesting that parents may sacrifice their own quality of life in an effort to manage their child's daily diabetes care. It may be possible to decrease parent stress while also maintaining appropriate involvement in a daily T1D care regimen for young children. Pilot data for behavioral interventions with this population suggest that providing parents with skills and support related to problem solving, accessing social support, and managing nutrition and mealtime misbehavior in young children with T1D may decrease parent stress and child problematic behaviors (Monaghan, Hilliard, Cogen, & Streisand, 2011; Patton et al., 2014). However, the impact of such interventions on glycemic control in this unique population has been variable. It will be important to examine the associations among mealtime behavior and glycemic control over time in ongoing RCTs.
Interpretation of the results of this study is limited by a cross-sectional design. All data used in the current analyses were drawn from baseline data; therefore, no conclusions related to the direction of these relations can be made. The study's sample size is a significant strength that allows for statistical modeling to evaluate interrelations among the variables of interest. However, the model was developed based, in part, on theory and prior empirical research, and the variables selected for inclusion in the study do not represent all aspects of parent and child functioning. Additionally, alternate models were not evaluated. It is possible that parent and child mealtime behaviors influence both diabetes-specific parent functioning and glycemic control, rather than mealtime behavior problems serving as a more proximal manifestation of diabetes-specific parent functioning. Examination of changes in diabetes-specific parent functioning, mealtime problems, and glycemic control over time, starting closer to T1D diagnosis, will provide greater insight into potential intervention targets. Parent report on the BPFAS was similar to that of other samples of young children with T1D but not significantly worse than a normative sample of healthy controls (Crist & Napier-Phillips, 2001; Patton et al., 2009). However, although mealtime problems may not be more prevalent, research suggests that they are more problematic, as parents of young children with T1D have unique challenges associated with scheduling and selecting meals to best match prescribed T1D regimens (Patton, Dolan, & Powers, 2006). Further, children is this sample displayed a high degree of glycemic variability, and over 50% of BG values in a 30 day period fell outside of the recommended range for young children and only 24% of participants met current glycemic targets of an A1c level ≤7.5% (Chiang et al., 2014). The study sample may limit generalizability of the study findings, as data are drawn from parent-report from one caregiver and parents enrolled in the trial are primarily middle-to-upper class, married mothers. The study sample also may have been restricted by the study design, as participants were enrolled in a longitudinal intervention study. Use of direct observation of mealtime behaviors in young children with T1D may provide a more nuanced understanding of how specific mealtime problems manifest in young children with T1D and their parents and the immediate impact of mealtime problems on glucose levels.
Increasing parental support and instruction related to management of mealtime behaviors is a potentially modifiable factor that may improve glycemic control. The American Diabetes Association (2013) recommends that all children with T1D participate in an annual nutritional consultation; however, this recommendation is often not met. In our own sample, less than half of participants reported meeting with a dietitian within the past year. More surprisingly, a third reported only meeting with a dietitian at diagnosis, a time when T1D eating habits are not yet well-established and new routines have not yet been created. Annual or even more frequent dietitian appointments may be beneficial. In addition to nutritional content of meals, parents may benefit from direct, tailored behavioral support related to managing child behavior and related stress during meals, such as how to address picky eating or food refusal. Parental concerns and stressors are likely to change over time as children age, and different strategies may be more or less effective at each developmental stage. Integrated multidisciplinary clinics that offer patients and families an opportunity to meet with a dietitian and behavioral health specialist in addition to the physician can provide targeted support and may represent best practices in diabetes care.
Parents of young children are vulnerable to diabetes-specific distress that may impact daily T1D management behaviors, including mealtime behaviors, and health outcomes (Jaser et al., 2009; Stallwood, 2005). Further evaluation of parental needs and stressors associated with daily meals and snacks can determine the most effective strategies for promotion of healthy mealtime behaviors in this population, and parents of young children with T1D may benefit from supportive interventions addressing mealtime concerns. Enhancing effective parent and child behaviors associated with meals and mealtime management from an early age may contribute to positive, long-term nutrition and health outcomes in young children with T1D.
Acknowledgments
This work was support by The National Institute of Diabetes and Digestive and Kidney Diseases, Grant Number R01DK080102, awarded to Randi Streisand, PhD.
Maureen Monaghan, PhD, is supported by the National Institute of Diabetes and Digestive and Kidney Diseases, Grant Number K23DK099250.
References
- American Diabetes Association. Standards of medical care in diabetes- 2013. Diabetes Care. 2013;36(1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin KS, Davies WH, Lobato DJ, Silverman AH. A biopsychosocial model of normative and problematic pediatric feeding. Children's Health Care. 2009;38:263–282. doi: 10.1080/02739610903235984. [DOI] [Google Scholar]
- Chiang JL, Kirkman MS, Laffel LMB, Peters AL. Type 1 Diabetes Through the Life Span: A Position Statement of the American Diabetes Association. Diabetes Care. 2014;37(7):2034–2054. doi: 10.2337/dc14-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill J, Ruppe R, Smaldone A. Use of continuous insulin infusin pumps in young children with type 1 diabetes: A systematic review. Journal of Pediatric Health Care. 2009;23(3):173–179. doi: 10.1016/j.pedhc.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Cox DM, Irvine A, Gonder-Frederick LA, Nowacek G, Butterfield J. Fear of hypoglycemia: Quantification, validation and utilization. Diabetes Care. 1987;10(5):617–621. doi: 10.2337/diacare.10.5.617. [DOI] [PubMed] [Google Scholar]
- Crist WP, Napier-Phillips AB. Mealtime behaviors of young children: A comparison of normative and clinical data. Journal of Developmental & Behavioral Pediatrics. 2001;22(5):279–286. doi: 10.1097/00004703-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Davies WH, Satter E, Berlin KS, Sato AF, Silverman AH. Reconceptualizing feeding and feeding disorders in interpersonal context: The case for a relational disorder. Journal of Family Pychology. 2006;20(3):409–417. doi: 10.1037/0893-3200.20.3.409. [DOI] [PubMed] [Google Scholar]
- Desrocher M, Rovet J. Neurocognitive correlates of type 1 diabetes mellitus in childhood. Child Neuropsychology. 2004;10(1):36–52. doi: 10.1076/chin.10.1.36.26241. [DOI] [PubMed] [Google Scholar]
- Finkbeiner C. Estimation for the multiple factor model when data are missing. Psychometrika. 1979;44:409–420. doi: 10.1007/bf02296204. [DOI] [Google Scholar]
- Grossman H, Brink S, Hauser S. Self-efficacy in adolescent girls and boys with insulin-dependent diabetes mellitus. Diabetes Care. 1987;10(3):324–329. doi: 10.2337/diacare.10.3.324. [DOI] [PubMed] [Google Scholar]
- Harjutsalo V, Sjoberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: A cohort study. The Lancet. 2008;371(9626):1777–1782. doi: 10.1016/S0140-6736(08)60765-5. [DOI] [PubMed] [Google Scholar]
- Helgeson VS, Becker D, Escobar O, Siminerio L. Families with children with diabetes: Implications of parent stress for parent and child health. Journal of Pediatric Psychology. 2012;37(4):467–478. doi: 10.1093/jpepsy/jsr110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert L, Clary L, Owen V, Monaghan M, Alvarez V, Streisand R. The impact of school/daycare functioning and fear of hypoglycemica on quality of life in caregivers of young children with type 1 diabetes. Journal of Clinical Nursing. 2014 doi: 10.1111/jocn.12658. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Hilliard ME, Monaghan M, Cogen FR, Streisand R. Parent stress and child behaviour among young children with type 1 diabetes. Child Care Health Dev. 2011;37(2):224–232. doi: 10.1111/j.1365-2214.2010.01162.x. [DOI] [PubMed] [Google Scholar]
- Jaser S, Whittemore R, Ambrosino JM, Lindemann E, Grey M. Coping and psychological adjustment in mothers of young children with type 1 diabetes. Children's Health Care. 2009;38(2):91–106. doi: 10.1080/02739610902813229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikam R, Perman JA. Pediatric feeding disorders. Journal of Clinical Gastroenterology. 2000;30:34–46. doi: 10.1097/00004836-200001000-00007. [DOI] [PubMed] [Google Scholar]
- Mardia KV. Applications of some measures of multivariate skewness and kurtosis in testing normality and robustness studies. Sankhya: The Indian Journal of Statistics. 1974;36:115–128. Series B, Pt 2. [Google Scholar]
- Mardia KV, Kent JT, Bibby JM. Multivariate Analysis. London: Academic Press; 1979. [Google Scholar]
- Mitchell MJ, Powers SW, Byars KC, Dickstein S, Stark LJ. Family functioning in young children with cystic fibrosis: Observations of interactions at mealtime. Journal of Developmental & Behavioral Pediatrics. 2004;25(5):335–346. doi: 10.1097/00004703-200410000-00005. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Hilliard ME, Mednick L, Henderson C, Cogen FR, Streisand R. Stress among fathers of young children with type 1 diabetes. Families, Systems, & Health. 2009;27(4):314–324. doi: 10.1037/a0018191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan M, Herbert LJ, Cogen FR, Streisand R. Sleep behaviors and parent functioning in young children with type 1 diabetes. Children's Health Care. 2012;41(3):246–259. doi: 10.1080/02739615.2012.685385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan M, Hilliard M, Cogen F, Streisand R. Supporting parents of very young children with type 1 diabetes: results from a pilot study. Patient Education and Counseling. 2011;82(2):271–274. doi: 10.1016/j.pec.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen B. Mplus Technical Appendices. Los Angeles, CA: Muthen & Muthen; 1998-2004. [Google Scholar]
- Muthen L, Muthen B. Mplus User's Guide. Los Angeles: Muthen & Muthen; 1998-2012. [Google Scholar]
- Patton S, Dolan L, Powers S. Differences in family mealtime interactions between young children with type 1 diabetes and controls: Implications for behavioral intervention. Journal of Pediatric Psychology. 2008;33(8):885–893. doi: 10.1093/jpepsy/jsn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton S, Odar C, Midyett L, Clements M. Pilot study results for a novel behavior plus nutrition intervention for caregivers of young children with type 1 diabetes. Journal of Nutrition Education and Behavior. 2014;46(5):429–433. doi: 10.1016/j.jneb.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton S, Williams L, Dolan L, Chen M, Powers S. Feeding problems reported by parents of young children with type 1 diabetes on insulin pump therapy and their associations with children's glycemic control. Pediatric Diabetes. 2009;10(7):455–460. doi: 10.1111/j.1399-5448.2009.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton SR, Dolan LM, Chen M, Powers SW. Dietary adherence and mealtime behaviors in young children with type 1 diabetes on intensive insulin therapy. Journal of the Academy of Nutrition and Dietetics. 2013;113(2):258–262. doi: 10.1016/j.jand.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton SR, Dolan LM, Henry R, Powers SW. Parental fear of hypoglycemia: young children treated with continuous subcutaneous insulin infusion. Pediatric Diabetes. 2007;8(6):362–368. doi: 10.1111/j.1399-5448.2007.00242.x. [DOI] [PubMed] [Google Scholar]
- Patton SR, Dolan LM, Henry R, Powers SW. Fear of hypoglycemia in parents of young children with type 1 diabetes mellitus. Journal of Clinical Psychology in Medical Settings. 2008;15(3):252–259. doi: 10.1007/s10880-008-9123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton SR, Dolan LM, Powers SW. Mealtime interactions relate to dietary adherence and glycemic control in young children with type 1 diabetes. Diabetes Care. 2006;29(5):1002–1006. doi: 10.2337/diacare.2951002. [DOI] [PubMed] [Google Scholar]
- Powers SW, Byars KC, Mitchell MJ, Patton SR, Standiford DA, Dolan LM. Parent report of mealtime behavior and parenting stress in young children with type 1 diabetes and in healthy control subjects. Diabetes Care. 2002;25(2):313–318. doi: 10.2337/diacare.25.2.313. [DOI] [PubMed] [Google Scholar]
- Smaldone A, Ritholz MD. Perceptions of parenting children with type 1 diabetes diagnosed in early childhood. Journal of Pediatric Health Care. 2011;25(2):87–95. doi: 10.1016/j.pedhc.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallwood L. Influence of caregiver stress and coping on glycemic control of young children with diabetes. Journal of Pediatric Health Care. 2005;19(5):293–300. doi: 10.1016/j.pedhc.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Streisand R, Swift E, Wickmark T, Chen R, Holmes C. Pediatric parenting stress among parents of children with type 1 diabetes: the role of self-efficacy, responsibility, and fear. Journal of Pediatric Psychology. 2005;30(6):513–521. doi: 10.1093/jpepsy/jsi076. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Deatrick J, Gruppuso P, Tamborlane W, Grey M. Constant vigilance: Mothers' work parenting young children with type 1 diabetes. Journal of Pediatric Nursing. 2003;18(1):21–29. doi: 10.1053/jpdn.2003.4. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Knafl K, Deatrick J, Grey M. Maternal management behaviors for young children with Type 1 diabetes. MCN: The American Journal of Maternal/Child Nursing. 2003;28(3):160–166. doi: 10.1097/00005721-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Rosenberg R, Bayard M. Fathers' reflections on parenting young children with type 1 diabetes. MCN: American Journal of Maternal Child Nursing. 2006;31(1):24–31. doi: 10.1097/00005721-200601000-00007. [DOI] [PubMed] [Google Scholar]
- Tamborlane W, Kollman C, Steffes M, Ruedy K, Dongyuan X, Beck R, DirecNet Study Group Comparison of fingerstick hemoglobin A1c levels assayed by DCA 2000 with the DCCT/EDIC central laboratory assay: Results of a Diabetes Reseach in Children Network (DirecNet) study. Pediatric Diabetes. 2005;6(1):13–16. doi: 10.1111/j.1399-543X.2005.00088.x. [DOI] [PubMed] [Google Scholar]
- Vandagriff JL, Marrero DG, Ingersoll GM, Fineberg NS. Parents of children with diabetes: What are they worried about? Diabetes Educator. 1992;18(4):299–302. doi: 10.1177/014572179201800407. [DOI] [PubMed] [Google Scholar]
- Vehik K, Hamman R, Lezotte D, Norris J, Klingensmith G, Bloch C, Dabelea D. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care. 2007;30(3):503–509. doi: 10.2337/dc06-1837. [DOI] [PubMed] [Google Scholar]


