Abstract
Background & Aim
Histologic analysis of liver biopsies allows for grading and staging of nonalcoholic fatty liver disease (NAFLD). We performed a longitudinal study to investigate the long-term prognostic relevance of histologic features for patients with NAFLD.
Methods
We performed a retrospective analysis of 619 patients diagnosed with NAFLD from 1975 through 2005 at medical centers in the United States, Europe, and Thailand. Patients underwent laboratory and biopsy analyses, and were examined every 3–12 months after their diagnosis. Outcomes analyzed were overall mortality, liver transplantation, and liver-related events. Cumulative outcomes were compared by log-rank analysis. Cox proportional-hazards regression was used to estimate adjusted hazard ratios (HR). Time at risk was determined from the date of liver biopsy to the date of outcome or last follow-up examination.
Results
Over a median follow-up of 12.6 years (range 0.3–35.1), 193 of the patients (33.2%) died or underwent liver transplantation. Features of liver biopsies significantly associated with death or liver transplantation included fibrosis stage 1 (HR, 1.88; 95% CI, 1.28, 2.77), stage 2 (HR, 2.89; 95% CI, 1.93, 4.33), stage 3 (HR, 3.76; 95% CI, 2.40, 5.89), and stage 4 (HR, 10.9; 95% CI, 6.06, 19.62) compared with stage 0, as well as age (HR, 1.07; 95% CI, 1.05, 1.08), diabetes (HR, 1.61; 95% CI, 1.13, 2.30), current smoking (HR, 2.62; 95% CI, 1.67, 4.10) and statin use (HR, 0.32; 95% CI, 0.14, 0.70). Twenty-six patients (4.2%) developed liver-related events; fibrosis stage 3 (HR 14.2 [95% CI 3.38, 59.68]) and stage 4 (HR 51.5 [95% CI 9.87, 269.2]) compared to stage 0, were significantly associated with the events. Patients with fibrosis, regardless of steatohepatitis or NAFLD activity score, had shorter survival times than patients without fibrosis.
Conclusions
In a longitudinal study of patients with NAFLD, fibrosis stage, but no other histologic features of steatohepatitis, were independently associated with long-term overall mortality, liver transplantation, and liver-related events.
Keywords: NASH, prognosis, the PRELHIN study, prediction
Nonalcoholic fatty liver disease (NAFLD) represents the most common chronic liver condition around the world. The disease encompasses a wide range of liver pathology with some patients presenting with steatosis and no additional features of liver injury whereas others present with nonalcoholic steatohepatitis (NASH) with or without fibrosis or cirrhosis [1]. This range of liver pathology does not necessarily imply that individuals with steatosis are at risk for NASH or advanced fibrosis or that those with NASH will inevitably progress to cirrhosis. Nevertheless, some patients with NAFLD may develop cirrhosis and die from complications of portal hypertension, liver failure and hepatocellular cancer (HCC) [2–5] if liver transplantation is not performed. Currently, NAFLD represents a common cause of liver transplantation, and even the second most common cause of liver transplantation in large medical centers [6]. Despite this clinical reality, the long-term prognosis of patients with NAFLD remains incompletely elucidated. When compared to the general population of same age and sex, patients with NAFLD have a significantly higher mortality [7]. However, the long-term prognosis is not the same across the spectrum of the disease. Patients with steatosis and minimal or no additional features of liver injury may follow a relatively benign clinical course with overall mortality similar to the general population of similar age and sex [8] whereas, in patients with NASH the long-term mortality seems greater than in the general population of same age and sex [9]. Unfortunately, studies on NASH confirmed by liver biopsy reported to date [9,10] included small number of patients, and used a definition of NASH that is different from currently accepted criteria [11,12] precluding meaningful conclusions [13,14].
Liver biopsy in patients with suspected NAFLD allows confirming the diagnosis, and more importantly grading and staging the disease. The NAFLD activity score (NAS) proposed by the NASH Clinical Research Network (CRN) is an accepted scoring system to grade three histological features namely steatosis, hepatocellular ballooning, and lobular inflammation [11]. The NAS is composed of features of active injury that are potentially reversible in the short term, and thus recommended for use in clinical trials. Fibrosis is not included as a component of the NAS. Although the NAS correlates with the presence or absence of NASH, the diagnosis of NASH is based, not on the raw NAS but on the interpretation by the histopathologist of the presence and pattern of specific histological abnormalities on liver biopsy [12]. Although fibrosis may be present in the absence of NASH, it is a worrisome feature on liver biopsy in NAFLD as indicates a more advanced liver disease. In addition, portal inflammation which is not a component of the NAS, has been associated with more severe histological injury and fibrosis in cross-sectional studies suggesting portal inflammation may be indicative of a worse prognosis [15,16]. However, it remains uncertain what long-term prognostic information can be obtained from grading the different histological features and from staging the disease in an individual patient with NAFLD. Thus, the aim of this study was to determine the long-term prognostic relevance of liver histology in NAFLD (the PRELHIN study), namely steatosis, lobular inflammation, portal inflammation, hepatocyte ballooning, NAS, NASH, and fibrosis stage in a large number of patients with NAFLD.
PATIENTS AND METHODS
This is a longitudinal, international, multicenter cohort study. Patients were retrospectively identified by reviewing the pathology database at each participating center of subjects with the pathology diagnosis of steatosis, steatohepatitis, or fatty liver. After an extensive review of the patients′ medical records including all notes from clinic visits, laboratory and imaging data and liver biopsy reports, only those with the diagnosis of NAFLD were included in the analysis as described below. They were untreated, consecutively biopsied patients that met the eligibility criteria, and were recruited from 1975 to 2005 from medical centers located in 6 different countries: United States, Denmark, Australia, Iceland, Thailand, and Scotland. The time period 1975 to 2005 was chosen to have a 30-year ascertainment period, and appropriate duration of follow-up for the last patient recruited. Follow-up was extended up to the end of 2012. In more than 90% of cases included the liver biopsy was performed due to persistent elevation of liver enzymes in patients with confirmed fatty infiltration of the liver detected on imaging studies. The liver biopsy was performed to confirm the diagnosis of NAFLD after appropriate exclusion of liver disease of other etiology, such as alcohol-induced or drug-induced liver disease, autoimmune or viral hepatitis, and cholestatic or metabolic/genetic liver disease. These other liver diseases were excluded using specific clinical, laboratory, radiographic, and/or histological criteria. All patients had a negative history of alcohol abuse as indicated by a weekly ethanol consumption of <140 g in women, and <210 g in men. History of alcohol consumption was specifically investigated by interviewing the patients and in many cases also by interviewing close relatives during both the first and follow-up visits. Subjects in whom an alcohol history was not provided were excluded. Serology for viral hepatitis B and C was performed prior to liver biopsy or during follow-up visits in all patients and all tested negative. The study was approved by appropriate regulatory bodies at each participating center and all patients had given consent for participation in medical research.
A total of 859 patients were initially identified (Mayo Clinic n=385, Denmark n=169; Australia n=119; Iceland n=107; Thailand n=46; and Scotland n=33). Extensive clinical and laboratory data were collected at the time the liver biopsy was performed. A complete medical history and physical examination was undertaken in all patients. The ethnicity (Hispanic or Latino, and not Hispanic or Latino) and race (white, Asian, Black or African American, American Indian/Alaska native, Native Hawaiian or other Pacific Islander) of the patients were determined based on the categories proposed by the United States Department of Health and Human Services Public Health Service [17]. Body mass index (BMI) was calculated using the formula: weight (in kilograms)/height (in meters2). Laboratory evaluation included routine liver biochemistry (alanine aminotransferase [ALT] and aspartate aminotransferase [AST] levels, total bilirubin, albumin, alkaline phosphatase, and gamma glutamyl transpeptidase); complete blood count; fasting lipids; fasting glucose; serum ferritin; transferrin saturation; viral serology for hepatitis B and C infection; autoantibodies; alpha 1 antitrypsin levels and phenotype; and ceruloplasmin levels. Data on medications use were collected from review of medical records.
Liver Histology
The liver biopsy slides of these 859 patients were shipped for pathology centrally reading and scoring by Dr. David Kleiner at the National Cancer Institute who was unaware of the patients' clinical and laboratory data. Particular attention was placed to the quality of the liver tissue and stainsing. In cases of slides with faded staining, new cuts of stored paraffin-embedded liver tissue and stains were obtained; when no stored tissue was available, the coverslip of the original slide was removed and the tissue re-stained. Thus, all liver biopsy slides were of appropriate quality for confident grading and staging of the liver biopsy features. The grade and stage of the several histological features was based on the scoring system proposed by the NASH CRN as described in supplemental Table 1 [11]. A threshold of 5% of hepatocytes showing steatosis was required for the diagnosis of NAFLD [11,12]. To control for biopsy size, the length of the biopsy was measured with a hand ruler, and the number of portal areas on one cross-section was counted. Only those liver biopsies that the histopathologist deemed of appropriate size and had sufficient number of portal tracts allowing confident grading and staging were included. Biopsies showing evidence of a second histological process were also excluded. The grade of the several histological features was scored on H&E stained tissue. The stage of fibrosis was scored on Masson's Trichrome stained tissue. The NAS was recorded as the unweighted sum of the scores for steatosis (0–3), lobular inflammation (0–3), and ballooning (0–2), thus ranging from 0 to 8. Based on the NAS, three NAS categories were created: NAS ≤ 2, NAS 3–4, and NAS ≥ 5 [11]. Portal inflammation was graded on a scale 0–2 [15]. The diagnosis of NASH as determined by the reporting histopathologist was recorded and categorized as non-NASH, borderline/suspicious NASH, or definitive NASH as proposed [12]. Fibrosis was staged on a 0–4 scale [11].
Follow-up
Patients were followed-up at 3–12 month intervals after the diagnosis of NAFLD. At each visit, a complete medical history and physical examination was performed along with routine laboratory work-up to follow their liver disease. Patients with advanced fibrosis or cirrhosis underwent endoscopy screening for gastroesophageal varices and screening for HCC at regular intervals following standard of care recommendations or guidelines in place at specific times as proposed by liver societies [18,19]. The decision to perform upper GI endoscopy and liver imaging during the follow up of patients with no or earlier (stage 1–2) fibrosis was left up to the treating physician in each medical center. Giving the lack of approved treatment for NAFLD, treatment recommendations specifically for NAFLD during the study period were similar in all centers and consisted of the standard recommendations to achieve and maintain appropriate body weight with increased physical activity and dietary changes. No specific dietary intervention or specific type of physical activity were used. Individuals who did not reach an outcome and whose health status was unknown for more than 12 months of reviewing their medical records were considered lost to follow-up. No patients received treatment with pioglitazone, vitamin E, or pentoxifylline at any point during the study duration, and no patients underwent bariatric surgery.
Statistical analysis
Data are presented as median (interquartile range [IQ]) or otherwise specified, and number (percentage) of patients with a condition. The outcomes analyzed were 1) overall mortality (death from any cause) or liver transplantation (whichever occurred first); and 2) liver-related events (ascites [detected clinically and/or on imaging], gastroesophageal varices/bleeding [confirmed by endoscopy], portosystemic encephalopathy [diagnosed clinically], and HCC [detected on imaging and/or biopsy]).
Cumulative overall mortality/liver transplantation and liver-related events during follow-up were calculated using Kaplan–Meier analysis and compared by log-rank testing. The liver biopsy features that were analyzed by Kaplan-Meier (unadjusted) analysis were steatosis grade, lobular inflammation grade, portal inflammation grade, ballooning grade, NAS category (≤ 2, 3–4, and ≥ 5), NASH category (non-NASH, borderline/suspicious NASH, and definitive NASH), and fibrosis stage (stage 0, 1, 2, 3, 4). Fibrosis stage 1A, 1B and 1C [11] were combined as stage 1 as there was no a significant difference in mortality rate among the tree groups (log-rank test, p = 0.5). Borderline zone 3 and zone 1 patterns [11] were combined as borderline NASH as there was no a significant difference in mortality rate between the two groups (log-rank test, p = 0.1).
Univariate (unadjusted), and multivariate (adjusted) hazard rate ratio (HR) estimates (relative risk) for outcomes were calculated by Cox proportional hazard regression analysis to control for the effect of potential risk factors (confounders), while taking into consideration varying lengths of follow-up. When possible, two models were created for each outcome analyzed; model 1 included the liver biopsy features that were statistically significant (p<0.05) by univariate analysis; and model 2 included variables from model 1 plus variables that may potentially affect the outcomes such as age, gender, race, BMI, diabetes, hypertension, use of statins for at least 6 months, site, and smoking. Since it is reasonable to assume that the relationship between risk factors and outcomes may not remain stable over a long recruitment period, calendar year of liver biopsy was considered as a variable in the Cox regression models as was the liver biopsy size to control for the potential effect of biopsy size on the outcomes. Variables independently associated with the outcomes analyzed were identified by stepwise forward selection procedure using a threshold of p < 0.1 for variable selection. Time at risk (T0) was from the date of liver biopsy to the date of outcome or last follow-up. Patients known to have, or who developed, any of the outcomes within 3 months of the liver biopsy procedure were eliminated a priori from the analysis. Analyses were performed using IBM SPSS Statistics version 21.0 (IBM Corporation, Armonk, NY) software.
RESULTS
Baseline characteristics
Of the 859 patients, 240 were excluded (supplemental Figure 1) leaving 619 patients for analysis. Supplemental Figure 2 illustrates the number of liver biopsies performed by calendar year period. Table 1 describes the baseline clinical and laboratory characteristics of the 619 patients. The median age was 49 years (IQ range 38–60), and the median BMI was 30.7 kg/m2 (IQ range 26.4–36.5). There was a predominance of white race, about two thirds were women, and about a third of patients suffered from diabetes or hypertension. Table 2 describes the liver biopsy features of the patient population. The median NAS was 3.0 (IQ range 2–5) with a mean (±SD) of 3.6 (1.7) (range 1–8). There was a similar proportion of patients within the three NAS categories; over a half had non-NASH, and almost a third had definitive NASH. About a half did not have fibrosis on liver biopsy, a third had fibrosis stage 1–2, and 11.5% had advanced (stage 3–4) fibrosis.
Table 1.
Clinical, demographic and laboratory characteristics of the patient population
| Variable | Total (n = 619) |
|---|---|
| Age (years) | 49 (38,60) |
| Gender | |
| Female | 387 (62.5) |
| Male | 232 (37.5) |
| Ethnic group | |
| Hispanic | 1 (0.2) |
| Non-Hispanic | 618 (99.8) |
| Race group | |
| White | 545 (88) |
| Asian | 45 (7.3) |
| Black, or African American | 1 (0.2) |
| More than one race | 28 (4.5) |
| Body mass index (kg/m2) | 30.7 (26.4,36.5) |
| Diabetes (yes) | 232 (37.5) |
| Hypertension (yes) | 190 (30.7) |
| Statins use (yes) | 63 (10.2) |
| Smoking | |
| Former | 72 (11.6) |
| Current | 54 (8.7) |
| ALT (IU/L) | 74 (45, 121) |
| AST (IU/L) | 38 (27,65) |
| AST/ALT ratio | 0.7 (0.5, 0.9) |
| Total bilirubin (mg/dL) | 0.6 (0.4,0.8) |
| Albumin (g/dL) | 4.2 (3.8,4.5) |
| Alkaline phosphatase (IU/L) | 198 (146,288) |
| Platelet (x109) | 251 (196,308) |
| Glucose (mg/dL) | 101 (91, 124) |
| Triglycerides (mg/dL) | 173 (121, 259) |
| Total Cholesterol (mg/dL) | 209 (174,239) |
| HDL-cholesterol (mg/dL) | 43 (36,51) |
FOOTNOTE Data are presented as median (interquartile range) and number (percentage) of patients with a condition.
Table 2.
Liver biopsy characteristics of the patient population
| Variable | Total (n = 619) |
|---|---|
| Steatosis | |
| 0 | 0 |
| 1 | 269 (43.5) |
| 2 | 204 (33) |
| 3 | 146 (23.6) |
|
| |
| Lobular inflammation | |
| 0 | 31 (5) |
| 1 | 423 (68.3) |
| 2 | 110 (17.8) |
| 3 | 55 (8.9) |
|
| |
| Portal inflammation | |
| 0 | 157 (25.4) |
| 1 | 376 (60.7) |
| 2 | 86 (13.9) |
|
| |
| Ballooning | |
| 0 | 389 (62.8) |
| 1 | 150 (24.2) |
| 2 | 80 (12.9) |
|
| |
| NAFLD activity score, category | |
| 1–2 | 205 (33.1) |
| 3–4 | 247 ( 39.9) |
| 5–8 | 167 (27) |
|
| |
| NASH category | |
| Non-NASH | 335 (54.1) |
| Borderline/suspicious zone 3 pattern | 94 (15.2) |
| Borderline/suspicious zone 1 pattern | 11 (1.8) |
| Definitive NASH | 179 (28.9) |
|
| |
| Fibrosis stage | |
| 0 | 322 (52) |
| 1A | 55 (8.9) |
| 1B | 37 (6) |
| 1C | 49 (7.9) |
| 2 | 85 (13.7) |
| 3 | 53 (8.6) |
| 4 | 18 (2.9) |
FOOTNOTE Data are presented as number (percentage) of patients with a condition.
Long-term follow-up
The median duration of follow-up of the 619 patients was 12.6 years (range 0.3 to 35.1 years) for a total of 7,799 person-years of follow-up. A total of 97 (15.7%) patients were lost to follow up and they had a mean (±SD) follow-up of 6.0 (5.5) years. Type and number of outcomes in the total cohort are described in Table 3. A total of 193 (33.2%) patients reached the outcome of mortality/liver transplantation. Cardiovascular events followed by non-liver malignancy, and complications of cirrhosis (along with HCC and liver transplantation) were the three most common causes of death. Twenty-six (4.2%) patients developed a total of 39 liver-related events. Development of gastroesophageal varices with or without bleeding, ascites and portosystemic encephalopathy were the predominant liver-related events.
Table 3.
Liver events, and causes of death
| Outcome | Number |
|---|---|
| Death or OLT | (n = 193) |
| Cardiovascular disease | 74 (38.3%) |
| Non-liver cancer | 36 (18.7%) |
| Cirrhosis complications | 15 (7.8%) |
| HCC | 2 (1%) |
| Liver transplantation | 1 (0.5%) |
| Infections | 15 (7.8) |
| Other | 35 (18.1%) |
| Pulmonary | 5 |
| Autoimmune disease | 4 |
| Renal failure | 4 |
| Accidents/trauma | 10 |
| Pancreatitis | 2 |
| Non-variceal Gl bleeding | 4 |
| Surgery complications | 2 |
| Others | 4 |
| Unknown | 15 (7.8) |
|
| |
| Liver events | (n = 26) a |
| Gastroesophageal varices/bleeding | 12 (46%) |
| Ascites | 9 (34.6%) |
| Portosystemic encephalopathy | 6 (23.1%) |
| Spontaneous bacterial peritonitis | 3 (11.5%) |
| Hepatocellular cancer | 3 (11.5%) |
| Hepatopulmonary syndrome | 2 (7.7%) |
| Hepatorenal syndrome | 4 (15.4%) |
26 patients developed a total of 39 liver-related events.
Long-term outcomes based on liver histology
The cumulative probability (unadjusted) obtained by Kaplan-Meier analysis of mortality/liver transplantation and liver-related events through the different liver biopsy features is described in Table 4, and illustrated in supplemental Figures 3A to 3G, and 4A to 4G. There was a significantly different survival free of liver transplantation and survival free of liver-related events according to ballooning grade, portal inflammation grade, the NASH categories and fibrosis stage, but not among steatosis grade, lobular inflammation grade, or NAS categories.
Table 4.
Cumulative probability (unadjusted) of outcomes by liver biopsy category
| Liver Biopsy Features | Mortality/liver transplantation (n = 619) |
Liver-related events (n = 615) |
||||
|---|---|---|---|---|---|---|
| Events (n) | Unadjusted cumulative (%)a | P value | Events (n) | Unadjusted cumulative(%)a | P value | |
| Steatosis, grade | 0.607 | 0.509 | ||||
| 1 | 91/269 | 33.8 | 12/269 | 4.5 | ||
| 2 | 56/204 | 27.4 | 6/202 | 3.0 | ||
| 3 | 46/146 | 31.5 | 8/144 | 5.6 | ||
|
| ||||||
| Lobular inflammation, grade | 0.399 | 0.526 | ||||
| 0 | 11/31 | 35.5 | 0/30 | 0 | ||
| 1 | 127/423 | 30.0 | 17/421 | 4.0 | ||
| 2 | 38/110 | 34.5 | 6/109 | 5.5 | ||
| 3 | 17/55 | 30.9 | 3/55 | 5.4 | ||
|
| ||||||
| Portal inflammation, grade | <0.001 | <0.001 | ||||
| 0 | 40/157 | 25.5 | 3/156 | 1.9 | ||
| 1 | 110/376 | 29.3 | 13/374 | 3.5 | ||
| 2 | 43/86 | 50.0 | 10/85 | 11.8 | ||
|
| ||||||
| Ballooning, grade | <0.001 | <0.001 | ||||
| 0 | 110/389 | 28.3 | 10/387 | 2.6 | ||
| 1 | 49/150 | 32.7 | 8/150 | 5.3 | ||
| 2 | 34/80 | 42.5 | 8/78 | 10.3 | ||
|
| ||||||
| NAS category | 0.186 | 0.106 | ||||
| 1–2 | 61/205 | 29.8 | 6/205 | 2.9 | ||
| 3–4 | 75/247 | 30.4 | 9/245 | 3.7 | ||
| 5–8 | 57/167 | 34.1 | 11/165 | 6.7 | ||
|
| ||||||
| NASH category | <0.001 | <0.001 | ||||
| Non-NASH | 85/335 | 25.4 | 4/334 | 1.2 | ||
| Borderline/suspicious | 42/105 | 40 | 7/104 | 6.7 | ||
| Definitive NASH | 66/179 | 36.9 | 15/177 | 8.5 | ||
|
| ||||||
| Fibrosis stage | <0.001 | <0.001 | ||||
| 0 | 74/322 | 23.0 | 5/321 | 1.6 | ||
| 1 | 42/141 | 29.8 | 4/141 | 2.8 | ||
| 2 | 36/85 | 42.3 | 6/85 | 7.1 | ||
| 3 | 27/53 | 50.9 | 7/51 | 13.7 | ||
| 4 | 14/18 | 77.8 | 4/17 | 23.5 | ||
Estimated using Kaplan-Meier analysis
Fibrosis stage correlated significantly with portal inflammation grade (r = 0.62; p < 0.001), ballooning grade (r = 0.60; p < 0.001) and NASH category (r = 0.70; p < 0.001). Among the 462 patients with portal inflammation grade 1 or 2, 204 (44.2%) did not have fibrosis (stage 0) on liver biopsy whereas the remaining 258 (55.8%) had increased (stage 1-4) fibrosis (Chi-squared 53.6; p < 0.001). Among the 230 patients with ballooning grade 1 or 2, only 45 (19.6%) did not have fibrosis (stage 0) on liver biopsy, whereas the remaining 185 (80.4%) had increased (stage 1-4) fibrosis (Chi-squared 154.4; p < 0.001). Among the 284 patients with borderline or definitive NASH, only 43 (15.1%) did not have fibrosis (stage 0) on liver biopsy whereas the reaming 241 (84.9%) had increased (stage 1-4) fibrosis (Chi-squared 285.9; p < 0.001).
The univariate-unadjusted hazard ratios for each histological feature for the outcome of mortality/liver transplantation are described in supplemental Table 2 and the multivariate-adjusted hazard ratios are described in Table 5. Fibrosis stage was the only histological feature independently associated with this outcome along with age, presence of diabetes, current smoking, and use of statins. The univariate-unadjusted hazard ratios for each histological feature for the outcome of liver related events are described in supplemental Table 3 and the multivariate-adjusted hazard ratios are described in Table 6. Fibrosis stage was the only variable independently associated with this outcome.
Table 5.
Multivariate-adjusted hazard ratios and 95% CI′s of outcome mortality/liver transplantation
| Hazard Ratio | 95% CI of HR | P value | |
|---|---|---|---|
|
| |||
| Model 1 | |||
|
| |||
| Fibrosis, stage 0 | 1 (reference) | ||
| Fibrosis, stage 1 | 2.07 | 1.40, 3.08 | <0.001 |
| Fibrosis, stage 2 | 3.02 | 2.0, 4.56 | <0.001 |
| Fibrosis, stage 3 | 3.97 | 2.50, 6.30 | <0.001 |
| Fibrosis, stage 4 | 11.97 | 6.47, 22.12 | <0.001 |
|
| |||
| Model 2 | |||
|
| |||
| Fibrosis, stage 0 | 1 (reference) | ||
| Fibrosis, stage 1 | 1.82 | 1.18, 2.81 | 0.007 |
| Fibrosis, stage 2 | 1.91 | 1.20, 3.03 | 0.007 |
| Fibrosis, stage 3 | 1.90 | 1.16, 3.12 | 0.01 |
| Fibrosis, stage 4 | 6.35 | 3.35, 12.04 | <0.001 |
|
| |||
| Age (years) | 1.07 | 1.05, 1.08 | <0.001 |
|
| |||
| Diabetes (yes) | 1.60 | 1.11, 2.30 | 0.01 |
|
| |||
| Smoking, Never | 1 (reference) | ||
| Former | 1.11 | 0.71, 1.73 | 0.640 |
| Current | 2.62 | 1.67, 4.10 | <0.001 |
|
| |||
| Statins use (yes) | 0.32 | 0.15, 0.71 | 0.005 |
Model 1 includes the liver biopsy features that were significant by univariate analysis (supplemental Table 2) plus calendar year of liver biopsy and biopsy size.
Model 2 includes variables from model 1 plus age, gender, race, BMI, diabetes, hypertension, statins use, site, smoking.
Table 6.
Multivariate-adjusted hazard ratios and 95% CI's of outcome liver-related events
| Hazard Ratio | 95% Cl of HR | P value | |
|---|---|---|---|
| Model 1 | |||
|
| |||
| Fibrosis, stage 0 | 1 (reference) | ||
| Fibrosis, stage 1 | 2.31 | 0.62, 8.66 | 0.213 |
| Fibrosis, stage 2 | 6.68 | 2.02, 22.06 | 0.002 |
| Fibrosis, stage 3 | 13.42 | 4.24, 42.55 | <0.001 |
| Fibrosis, stage 4 | 52.89 | 13.31, 210.15 | <0.001 |
|
| |||
| Model 2 | |||
|
| |||
| Fibrosis, stage 0 | 1 (reference) | ||
| Fibrosis, stage 1 | 2.38 | 0.63, 8.91 | 0.198 |
| Fibrosis, stage 2 | 7.51 | 2.26, 24.94 | 0.001 |
| Fibrosis, stage 3 | 13.78 | 4.35, 43.65 | <0.001 |
| Fibrosis, stage 4 | 47.46 | 11.94, 188.61 | <0.001 |
Model 1 includes the liver biopsy features that were significant by univariate analysis (supplemental Table 3) plus calendar year of liver biopsy and biopsy size.
Model 2 includes variables from model 1 plus age, gender, race, BMI, diabetes, hypertension, statins use, site, smoking.
Liver-related mortality/liver transplantation
Deaths due to complications of cirrhosis, HCC or liver transplantation occurred in 18/193 (9.3%) patients (Table 2). The cumulative probability (unadjusted) obtained by Kaplan-Meier analysis showed a significantly different survival free of liver transplantation according to ballooning grade (log-rank test = 19.3; p < 0.001), portal inflammation grade (log-rank test = 28.8; p < 0.001), NASH categories (log-rank test = 26.2; p < 0.001), and fibrosis stage (log-rank test = 71; p < 0.001), but not among steatosis grade, lobular inflammation grade, or NAS categories. By multivariate-adjusted Cox regression, fibrosis stage 1-2 (HR, 11.2 [95% CI, 1.33, 93.47]; p < 0.03), and fibrosis stage 3-4 (HR, 85.79 [95% CI, 10.93, 673.30]; p < 0.001) as compared to fibrosis stage 0 were the only prognostic factors associated with liver-related mortality/liver transplantation.
Long-term outcomes of individuals without advanced fibrosis
Since patients with stage 3 and 4 fibrosis are expected to have a higher mortality or need liver transplantation, and expected to develop more liver-related events we did an additional analysis of the long-term outcomes of individuals with earlier disease. For this, the 71 patients with advanced fibrosis (stage 3 or 4) were eliminated leaving a total of 548 patients without (stage 0) or mild (stage 1-2) fibrosis. Among them, 152 (27.7%) reached the outcome of mortality/liver transplantation. The cumulative probability (unadjusted) obtained by Kaplan-Meier analysis of mortality/liver transplantation on this cohort is described in supplemental Table 4. There was a significant difference among ballooning grade, NASH categories and fibrosis stage. By multivariate Cox regression analysis, again fibrosis stage was the only histological variable independently associated with the outcome of mortality/liver transplantation along with age, diabetes, current smoking, and statin use (supplemental Table 5).
Fifteen (2.7%) patients had a liver-related event on follow-up. The cumulative (unadjusted) probability obtained by Kaplan-Meier analysis of liver-related events (supplemental Table 4) showed a significant difference among NASH categories and fibrosis stage. When both variables competed in a multivariate Cox regression model, fibrosis stage but not NASH category was independently associated with the outcome of liver-related events. The HR for fibrosis stage 2 as compared to fibrosis stage 0 was 7.47 (95% CI, 2.25, 24.74; p=0.001) whereas fibrosis stage 1 was not significantly different from fibrosis stage 0 (HR, 2.50 [95% CI, 0.66, 9.39]; p=0.2).
Long-term outcomes of individuals with NASH vs. non-NASH
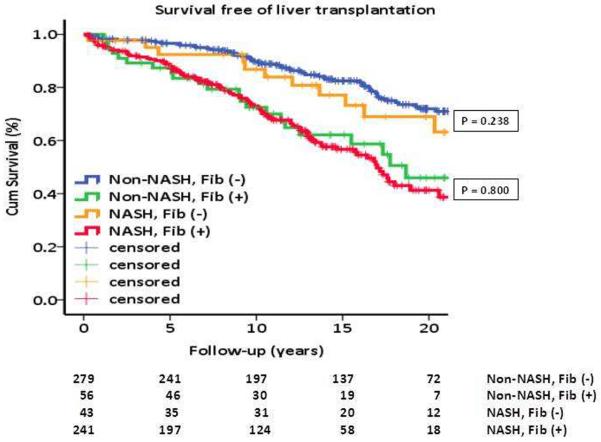
Since NASH as compared to non-NASH is perceived as being associated with a worse prognosis, additional analyzes were done separately in non-NASH and NASH (borderline plus definitive) while considering the presence or absence of fibrosis. Survival free of liver transplantation in patients with non-NASH was significantly lower in those with fibrosis as compared to those without fibrosis (p < 0.001); in the NASH group a similar significantly lower survival free of liver transplantation was found among those with fibrosis as compared to those with without fibrosis (p = 0.018) (see supplemental Table 6). This lower survival free of liver transplantation in patients with increased fibrosis regardless of having NASH or non-NASH is illustrated in Figure 1. Regarding the outcome of liver-related events, no significant difference was found although there was a trend (p = 0.07) for lower survival free of liver-related events among those with NASH and fibrosis as compared to those with NASH without fibrosis (supplemental Table 6).
Figure 1.
Survival free of liver transplantation grouped by NASH and non-NASH and separated by presence or absence of fibrosis. Survival free of liver transplantation was similar in those without fibrosis and similar among those with fibrosis regardless of having or not the diagnosis of NASH (see also supplemental table 6).
DISCUSSION
Our study demonstrates that: 1) fibrosis stage independently, and regardless of presence or severity of other histological features is the most relevant liver biopsy feature associated with overall- and liver-related mortality/liver transplantation or liver-related events. This effect was seen even with the earliest stages of fibrosis detectable by microscopic examination, and even when individuals with advanced (fibrosis stage 3-4) disease were excluded. 2) Presence of fibrosis rather than the histological diagnosis of NASH or non-NASH is associated with a higher mortality and higher rate of liver-related events; and 3) the NAS does not on its own provide any long-term prognostic information. In addition, our results validate the pathologists' practice of staging fibrosis on biopsy into multiple categories and provide prognostic meaning for early stage disease –something that has not been demonstrated in other chronic liver diseases.
The results of our study are directly relevant for patient management, counseling and monitoring. The absence of liver fibrosis on liver biopsy indicates a lower likelihood of dying or developing liver-related complications within one or two decades. On the contrary, presence of fibrosis identifies the subgroup of individuals with NAFLD that need to make a concerted effort to achieve and maintain appropriate body weight, optimize metabolic control and be offered enrolment in clinical trials aimed at preventing fibrosis development or reducing fibrosis progression. In our patient population, 38% were diabetic, 31% had hypertension, 20% were current or former smokers, and the most common cause of death was cardiovascular (38%). Although these associations with NAFLD and NASH are not novel (6), we were able to demonstrate that diabetes and smoking, along with fibrosis, age and absence of statin use contribute to mortality and underscore the need for a comprehensive approach to patient management.
The results of this study are also relevant for the design and interpretation of clinical trials. Improvement in the NAS is often recommended and used as the primary end-point to determine treatment efficacy in treatment trials [20]. In our study, the NAS on its own did not provide any long-term prognostic information, although some preliminary data suggest an association of NAS improvement with fibrosis regression in the short (two years) term [21]. Thus, more data are needed to determine whether improvement in the NAS improves or not the long-term prognosis in NAFLD when NAS improvement is not associated with improvement or resolution of fibrosis or at least delay in fibrosis progression [22].
It is frequently claimed that NASH but not simple steatosis is associated with a worse long-term prognosis. The results of our study would suggest that this dictum is not true although the fact that only 43 patients diagnosed with definite or borderline NASH had fibrosis stage 0 limits the strength of this observation. Long-term prognosis in our cohort depended less upon a diagnosis of NASH or non-NASH than on whether or not the liver biopsy shows fibrosis (Figure 1). Thus, for the purpose of assessing the long-term prognosis, the focus should be on fibrosis stage while other histological findings may have importance for understanding disease pathophysiology and short-term disease progression/regression as noted above. Most patients with non-NASH have steatosis with little or no inflammation; of the 335 patients with non-NASH in our study, 248 (74%) had fibrosis stage 0, ballooning grade 0, and grade 0 or 1 for portal or lobular inflammation. The etiology of fibrosis in patients with non-NASH is not entirely clear, although we would hypothesize that these cases represent a form of NASH in remission. Placebo-treated groups in clinical trials of NASH have demonstrated that a significant minority of patients may lose the diagnostic changes of NASH (23).
Our study reproduced observations from cross-sectional studies that portal inflammation correlates significantly with advanced liver fibrosis [15,16]. Our study extends these observations to demonstrate that while portal inflammation is not of significant long-term prognostic relevance when adjusted for the severity of liver fibrosis, it did show significance in univariate analysis. Ballooning injury showed a similar pattern with univariate significance for the outcomes analyzed that was also lost when adjusted by liver fibrosis. While the long-term prognostic significance of portal inflammation, ballooning and NASH category was lost when fibrosis stage was considered, our univariate analysis demonstrated an association between these features and long-term outcomes suggesting that such features may play a larger role in short-term disease progression, an idea supported by recent data from the NASH CRN [24,25].
The main strengths of our study are the large number of patients included; the long-term follow up averaging more than 12 years per patient and up to 35 years in some cases; complete follow-up in the vast majority of patients (84.3%); having a liver biopsy confirming the diagnosis of NAFLD in every case; having a single experienced liver pathologist grading and staging the liver biopsy features exclusively for the purpose of this study; and the use of a scoring system for grading and staging the biopsy features that is currently widely accepted in NAFLD. A new histological scoring system in NAFLD has recently been proposed [26], but studies are needed to determine their long-term prognostic relevance.
Our study has some limitations most of which are inherent to retrospective studies including the lack of a specific treatment protocol with diet and exercise similar in all centers, and the lack of a specific protocol for patient follow-up with regards to endoscopy and imaging procedures in non-cirrhotic patients, and thus it is possible that the number of liver-related events in our study was underestimated. Also, a 30-year ascertainment period witnessed many changes in the management of the three most common causes of death in our cohort, and although all multivariate analyses were adjusted by calendar year of liver biopsy, we were not able to adjust for specific treatment modalities other than statin use. Of note, our cohort included only one patient who underwent liver transplantation, although 17 individuals died of liver disease, suggesting that co-morbid conditions may have precluded transplantation. Cirrhosis due to NASH is becoming a more common indication for liver transplantation and it is possible that the use of liver transplantation may be higher in a modern prospectively followed cohort. Our study is also limited by the overrepresentation of white race and underrepresentation of some ethnic/racial groups including Hispanics and Blacks/African Americans. Finally, while having an adequate liver biopsy available for review is one of the strengths of our study, it creates a potential accrual bias that is difficult to control for, as it was a key to our study design.
In conclusion, liver fibrosis is the most important liver biopsy feature associated with increased overall- and liver-related mortality and increased likelihood of developing liver-related complications. This association of liver fibrosis with long-term outcomes is independent of presence and severity of other histological features and the NAFLD activity score. Presence and severity of fibrosis regardless of the diagnosis of NASH dictates the long-term prognosis in patients with NAFLD. These results are relevant for patient counseling and monitoring, and for the interpretation and design of clinical trials.
Supplementary Material
ACKNOWLEDGMENT
We thank Dr. Oscar Arauz, School of Medicine and Pharmacology, University of Western Australia, Perth, Western Australia, Australia; and Dr. Ananya Pongpaibul, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand for their invaluable help in collecting the data for this study. Drs. Arauz and Pongpaibul did not receive compensation in association with his contribution to this article.
Funding source: This study was supported by a National Institute of Health R01 DK82426 grant. This study was supported in part by the Intramural Research Program of the NIH, National Cancer Institute. The sponsor played no role in the study design or the collection, analysis, and interpretation of data.
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NAS
NAFLD activity score
- ALT
alanine aminotransferases
- AST
aspartate aminotransferases
- BMI
body mass index
- HCC
hepatocellular cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None to declare
REFERENCES
- 1.Bellentani S, Marino M. Epidemiology and natural history of nonalcoholic fatty liver disease (NAFLD) Ann Hepatol. 2009;8(Suppl 1):S4–S8. [PubMed] [Google Scholar]
- 2.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–666. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 3.Mendes FD, Suzuki A, Sanderson SO, Lindor KD, Angulo P. Prevalence and indicators of portal hypertension in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10:1028–1033. doi: 10.1016/j.cgh.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, Adams LA, Charatcharoenwitthaya P, Topping JH, Bugianesi E, Day CP, George J. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208–16. doi: 10.1002/hep.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384–1391. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 6.Agopian VG, Kaldas FM, Hong JC, Whittaker M, Holt C, Rana A, Zarrinpar A, Petrowsky H, Farmer D, Yersiz H, Xia V, Hiatt JR, Busuttil RW. Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg. 2012;256:624–633. doi: 10.1097/SLA.0b013e31826b4b7e. [DOI] [PubMed] [Google Scholar]
- 7.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A. Angulo P The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Dam-Larsen S, Becker U, Franzmann MB, Larsen K, Christoffersen P, Bendtsen F. Final results of a long-term, clinical follow-up in fatty liver patients. Scand J Gastroenterol. 2009;44:1236–1243. doi: 10.1080/00365520903171284. [DOI] [PubMed] [Google Scholar]
- 9.Ekstedt M, Franzen LE, Mathiensen UI, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 10.Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, Goodman Z. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53:1874–1882. doi: 10.1002/hep.24268. [DOI] [PubMed] [Google Scholar]
- 11.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ, Nonalcoholic Steatohepatitis Clinical Research Network Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 12.Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA, NASH Clinical Research Network (CRN) Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunt EM, Kleiner DE, Behling C, Contos MJ, Cummings OW, Ferrell LD, Torbenson MS, Yeh M. Misuse of scoring systems. Hepatology. 2011;54:369–370. doi: 10.1002/hep.24347. [DOI] [PubMed] [Google Scholar]
- 14.Angulo P. Diagnosing steatohepatitis and predicting liver-related mortality in patients with NAFLD: two distinct concepts. Hepatology. 2011;53:1792–1794. doi: 10.1002/hep.24403. [DOI] [PubMed] [Google Scholar]
- 15.Brunt EM, Kleiner DE, Wilson LA, Unalp A, Behling CE, Lavine JE, Neuschwander-Tetri BA, NASH Clinical Research Network Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49:809–820. doi: 10.1002/hep.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rakha EA, Adamson L, Bell E, Neal K, Ryder SD, Kaye PV, Aithal GP. Portal inflammation is associated with advanced histological changes in alcoholic and non-alcoholic fatty liver disease. J Clin Pathol. 2010;63:790–795. doi: 10.1136/jcp.2010.079145. [DOI] [PubMed] [Google Scholar]
- 17. [Accessed 3/6/2015];Non-competing continuation progress report (PHS 2590): US. Department of Health and Human Services Public Health Service. 2014 http://grants.nih.gov/grants/funding/2590/phs2590.pdf.
- 18.de Franchis R. Revising consensus in portal hypertension: Report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762–768. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Bruix J, Sherman M. Management of Hepatocellular Carcinoma: An Update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–353. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunt EM, Kleiner DE, Wilson L, Unalp A, Tonascia J, Neuschwander-Tetri B. Histologic predictors of improvement in fibrosis in NASH: results from the Clinical Research Network PIVENS Trial. Hepatology. 2012;56(4 Suppl):887A. [Google Scholar]
- 22.Angulo P. Clinical trials: Trial design in NASH—realities and challenges. Nat Rev Gastroenterol Hepatol. 2011;8:424–425. doi: 10.1038/nrgastro.2011.125. [DOI] [PubMed] [Google Scholar]
- 23.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunt EM, Belt PH, Wilson L, Guy CD, Yeh MM, Kowdley KV, Sanyal AJ, Neuschwander-Tetri BA, Kleiner DE. Progression to bridging fibrosis in non-alcoholic fatty liver disease over 4 years in the NASH CRN. Hepatology. 2013;58(4 Suppl):495A. [Google Scholar]
- 25.Kleiner DE, Brunt EM, Wilson L, Belt PH, Guy CD, Yeh MM, Kowdley KV, Sanyal AJ. Neuschwander-Tetri BA Fibrosis Progression in Adult Non-Alcoholic Fatty Liver Disease: Association with Severity of Histological Features. Mod Pathol. 2014;27(S2):423A. [Google Scholar]
- 26.Bedossa P, FLIP pathology consortium Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60:565–575. doi: 10.1002/hep.27173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.