Abstract
In mammalian physiology, lactation follows pregnancy. Disruption of this physiology is associated with long-term adverse maternal health outcomes, including higher risks of later life obesity, type 2 diabetes, metabolic syndrome, hypertension, and cardiovascular disease. Multiple mechanisms likely contribute to these associations, including the metabolic demands breastfeeding, modulation of stress reactivity, and confounding by other health behaviors. At the same time, evidence suggests that maternal metabolic health entering pregnancy affects lactation performance. In this paradigm, adverse lactation outcomes may be a marker for underlying maternal disease risk. Understanding these relationships has important clinical and policy implications for women's health.
Introduction
In mammalian physiology, lactation follows pregnancy. Breastfeeding provides the infant with specific and innate immune factors, as well as macro- and micronutrients, to support growth and development. To meet these nutritional needs, mothers expend approximately 500 calories per day,1 deriving energy from their fat stores accumulated during pregnancy.
Disruption of this physiology is associated with adverse maternal health outcomes. In observational studies, lack of breastfeeding is associated with greater postpartum weight retention,2 and increased rates of later-life obesity,3 diabetes,4,5,6,7 hypertension,8,9 metabolic syndrome,10,11 and cardiovascular disease.12,13. However, women with pregravid obesity are less likely to initiate and sustain breastfeeding,14,15 and recent work implicates insulin resistance in the pathogenesis of low milk supply.16,17,18 Thus, while breastfeeding may prevent development of the metabolic syndrome, preexisting metabolic dysregulation may also prevent breastfeeding.
Disentangling cause and effect has important implications for public health. To the extent that breastfeeding is a modifiable risk factor metabolic disease, strategies to enable women to breastfeed may improve women's health. However, if metabolic disease is a risk factor for breastfeeding difficulties, women at metabolic risk may require additional support to enable them to meet their infant's nutritional needs.
This paper will review both human and animal studies linking lactation with maternal metabolic health, explore potential mechanisms, and discuss implications for clinical care and future research.
Lactation and maternal metabolic health
In observational studies, lactation is associated with differences in maternal metabolic health. Several authors have quantified this relationship among women with gestational diabetes, who are at increased risk of long-term metabolic disease. Kjos et al.19 compared postpartum glucose and lipid values among 809 Latina women with gestational diabetes. In models adjusted for age, body mass index, and insulin use during pregnancy, those who were breastfeeding at the time of their oral glucose tolerance test had lower fasting (93 ± 13 vs. 98 ± 17 mg/dL, p <.01) and post-load (124 ± 41 vs. 134 ± 49 mg/dL, p<.01) glucose values at 4 to 12 weeks postpartum. Lactating women were less likely to have diabetes mellitus (4.2% vs. 9.4%, p=.01) and had higher HDL cholesterol, (48 ± 11 vs. 44 ± 10, p<.01) compared with non-lactating women. Gunderson et al20 measured postpartum glucose tolerance among 522 women with gestational diabetes at 6–9 weeks postpartum. They found that women who were exclusively or mostly breastfeeding had lower fasting glucose levels than exclusively or mostly formula-feeding women, with a mean difference of about 5 mg/dL. Two-hour post-load values were similar in breastfeeding and formula feeding mothers, but breastfeeding mothers had greater insulin sensitivity. In a secondary analysis among women who were lactating,21 the authors compared women who breastfed their infants during the 2-hour oral glucose tolerance test (OGTT) with those who did not. Fasting values were similar in the two groups, but women who breastfed during the OGTT had lower post-load glucose levels (mean difference −6.2, 95% CI −11.5, −1.0) as well as lower post-load insulin levels than those who did not. These results provide evidence that lactogenesis is associated with mobilization of glucose through non-insulindependent mechanisms.
The protective association between breastfeeding and metabolic disease appears to persist after weaning. Gunderson et al. measured the association between lifetime breastfeeding and development of metabolic syndrome among women in the CARDIA cohort study,10. Among women with a history of gestational diabetes, breastfeeding for >9 months was associated with a markedly lower risk of metabolic syndrome than breastfeeding 0–1 months. (MV-Adj OR 0.14, 95% CI 0.04–0.55), in models adjusting for baseline demographics, body mass index, metabolic syndrome components and physical activity. Ziegler at al22 measured progression to Type 2 Diabetes in a prospective cohort study of women with gestational diabetes in Germany (N=304). Among women who were islet-antibody negative (N=272), breastfeeding for at least 3 months was associated with a lower rate of progression to type 2 diabetes (MV-Adj HR 0.54, 95% CI 0.34–0.85), compared with breastfeeding for ≤ 3months.
Lactation is similarly associated with reduced metabolic disease risk in the general population. Breastfeeding mobilizes approximately 500 kcal per day,1 and greater duration and intensity of breastfeeding is associated with reduced postpartum weight retention.2 In a randomized controlled trial of introduction of supplementary foods at 4 months vs. continued exclusive breastfeeding, women allocated to exclusive breastfeeding lost more weight than women allocated to supplementation, supporting a causal association between breastfeeding intensity and maternal weight loss.23
These differences in weight loss appear to persist after weaning. In the UK Millions Women Study, women who breastfed ≥ 6 months per birth had a lower BMI after menopause than women who breastfed < 6 months per birth, stratified by parity.3 Longer duration of breastfeeding is also associated with reduced central adiposity. In a secondary analysis of the Study of Women's Health Across the Nation (SWAN) cohort, Ram et al. found that longer lifetime breastfeeding duration was associated with smaller waist circumference, as well as lower prevalence of metabolic syndrome.11 McClure et al. analyzed a subset of the SWAN cohort that underwent CT-scans to evaluate subcutaneous and visceral adiposity.24 They found that women who never breastfed or did not breastfeed each child at least 3 months had greater visceral adiposity than women who consistently breastfed for > 3 months after each birth.
Longer lifetime lactation is also associated with lower incidence of type 2 diabetes. In the Nurses' Health Study cohorts, lifetime duration of lactation was associated with reduced likelihood of incident type 2 diabetes in the 15 years since a woman's last birth (HR per year of lifetime lactation, 0.85, 95% CI 0.73–0.99 in NHS and 0.86, 0.79–0.93 in NHS II).6 Longer lactation has similarly been associated with reduced diabetes risk in the Women's Health Initiative,12 the Reproductive Risk factors for Incontinence Study at Kaiser,5 the Shanghai Women's Health Study,7 and the EPICPotsdam Study.4
Never or curtailed breastfeeding is also associated with an increased risk of incident hypertension. In the Nurses' Health Study II,9 breastfeeding the first child < 3 months was associated with a increased risk of incident hypertension, compared with ≥ 12 months of breastfeeding. Similar results have been reported among premenopausal women in Korea8 and in the Women's Health Initiative.12 Lactation history is also associated with cardiovascular disease risk, with lower incidence of myocardial infarction in the Nurses' Health Study13 and reduced prevalent cardiovascular disease in the Women's Health Initiative.12
If these associations are causal, current suboptimal breastfeeding rates contribute to substantial morbidity for women's health. In a Monte Carlo Simulation Model, Bartick et al25 modeled burden of diabetes, hypertension and myocardial infarction for a hypothetical population of women born in a single year. Disease burden, premature death, and costs were modeled under optimal breastfeeding conditions, assuming that 90% of women breastfed for 12 months after each birth, and under current conditions for the US population in 2008. In these models, suboptimal breastfeeding was associated with 53,847 excess cases of hypertension (95% CI 43,836 to 64,596), 4,482 excess cases of diabetes (95% CI −791 to 8022) and 13,946 excess cases of myocardial infarction (95% CI 6318–21,090).
Potential mechanisms
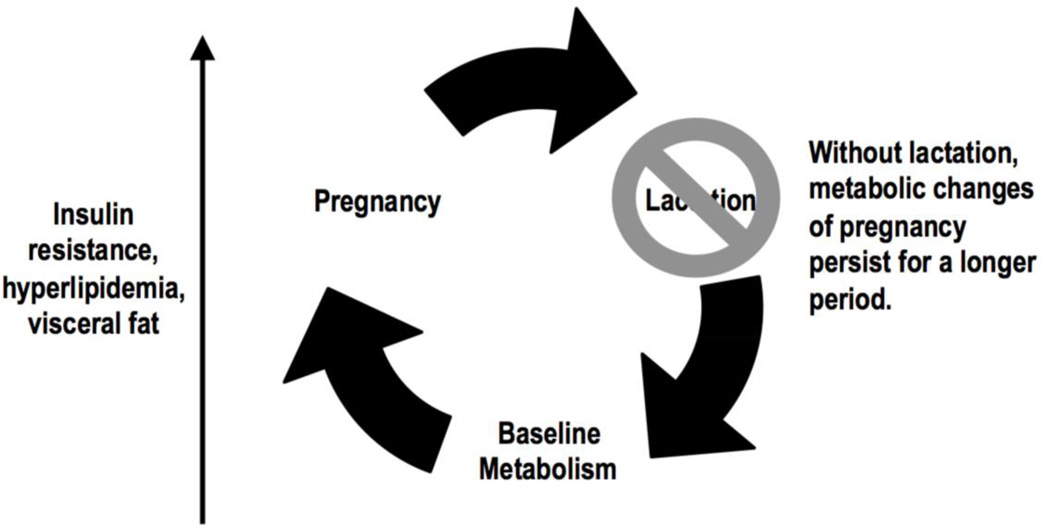
Several mechanisms may explain the association between lactation duration and maternal health. The Reset Hypothesis26 (Figure 1) proposes that fat stores accumulate during pregnancy in anticipation of the metabolic needs of the lactating mother. When lactation does not occur, these metabolic changes persist, with adverse consequences for maternal health. This concept is consistent with evolutionary models suggesting that lactation evolved to allow mammals to nourish their young in the setting of unreliable food supplies.27 Animal models support a causal association between lactation and retained fat stores: in a murine model, dams who did not lactate had greater visceral, but not subcutaneous fat stores at 2 months postpartum than dams who lactated.28
Figure 1.
The 'Reset' Hypothesis
Lactation may also affect maternal health through effects on stress reactivity. Several studies have found that lactating women have reduced HPA-axis activation29 and greater vagal tone,29 compared with non-lactating women. These effects appear to vary with time since last feeding. In a study of lactating women, those who breastfed 30 minutes before a standardized social stressor had diminished HPA response, compared with women who breastfed 100 minutes before.30 Among mothers engaged in an aggression challenge task,31 those who were breastfeeding had higher aggression levels, but lower systolic and diastolic blood pressure, than mothers who were formula feeding, suggesting that breastfeeding may be associated with reduced cardiovascular stress in the face of threat. To the extent that these differences persist after weaning, lactation may improve maternal health through moderation of stress reactivity.
Caveats
However, studies linking lactation with improved maternal health are confounded by other health factors. Mothers who breastfeed differ from mothers who formula feed; they are wealthier, better educated, less likely to smoke, and more likely to engage in other beneficial health behaviors.32,43,34 Thus, residual or unmeasured confounding, rather than breastfeeding itself, may explain observed associations between breastfeeding and health outcomes.
In addition, pre-pregnancy obesity is associated with reduced breastfeeding initiation and duration.14 In the Danish National Birth Cohort, Baker et al. found that overweight and obese women were more likely to discontinue full and any breastfeeding, adjusting for maternal age, parity, education, mode of delivery, and smoking during the postpartum period.15 Maternal obesity is also associated with delayed onset of lactogenesis following delivery,35 and with increased risk of disrupted lactation, defined as early, undesired weaning attributed to lactation dysfunction.36 Among normal BMI women, 9.0% experienced disrupted lactation, compared with 13.0% of overweight and 14.4 percent of obese women (MV-adjusted OR 1.52, 95% CI 1.06–2.17 for overweight; 1.73, 95% CI 1.16–2.60 for obese women, compared with normal weight women).
Several mechanisms may play a role in associations between obesity and early cessation of breastfeeding. Maternal metabolic status during pregnancy may affect breast development. In a sample of women with polycystic ovary syndrome, Vanky et al37 found that women with no breast growth in pregnancy, indexed by no change in bra size, had higher first trimester body mass index, systolic blood pressure, and fasting insulin, and they gained less weight during pregnancy. Women with no breast growth reported shorter duration of exclusive and any breastfeeding. Glucose homeostasis in the third trimester has also been correlated with feeding outcomes. In a small sample (N=16), insulin:glucose ratio and adiponectin measured at the time of the 28-week glucose loading test was associated with onset of milk production: women with a less favorable metabolic profile during pregnancy had later onset of lactogenesis,18 which is a risk factor for breastfeeding cessation.38 Differences in insulin resistance may act at the level of the lactocyte. Lemay et al16 compared gene expression in the milk fat globule transcriptome of women with and without low milk supply, and found differences in expression of PTPRF, which blocks the action of insulin to stimulate milk production. This action suggests a mechanism through which insulin resistance may contribute to reduced milk synthesis. Pituitary hormones may also play a role: Rasmussen et al39 found that pregravid overweight was associated with reduced prolactin response in the early postpartum period. Taken together, these data suggest that premature cessation of breastfeeding may be a marker for underlying metabolic dysregulation.
Perinatal depression and anxiety disorders may also mediate associations between lactation and maternal metabolic disease. Several authors have reported associations between perinatal depression and metabolic complications of pregnancy.40,41,42,43 Depression and anxiety are also associated with premature cessation of lactation.36,44 An underlying psychobiological vulnerability may, therefore, predispose women to both metabolic complications of pregnancy and early weaning.45
Among women with gestational diabetes, greater maternal glucose intolerance is associated with both infant and maternal breastfeeding difficulties. Bromicker et al. measured neonatal sucking patterns on day 3 of life among infants born to women with insulin-controlled GDM, diet-controlled GDM, and normal controls, and they found that exposure to insulin-controlled GDM was associated with 5.2 fewer bursts and 42 fewer sucks during the 5-minute observation period, compared with normal controls. In the Study of Women, Infant Feeding and Type 2 Diabetes after GDM pregnancy (SWIFT) cohort, Matias et al46 found that pregravid BMI > 30 kg/m2 and treatment of GDM with insulin were independently associated with delayed onset of lactogenesis. Interestingly, delayed lactogenesis was also associated with poor infant latch, suggesting that the differences in oromotor function may play a role in delayed onset of lactogenesis.
Implications for Clinical Care
In observational studies, longer duration of breastfeeding is associated with reduced risk of metabolic disease. Lactation mobilizes energy stores for the infant, and evidence from animal studies supports a causal effect of lactation on maternal metabolism. These data suggest that enabling women to breastfeed may facilitate weight loss and reduce metabolic disease risk.
Several interventions have been tested to increase breastfeeding among high-BMI women. Chapman et al. randomized women with BMI ≥ 27 to a peer counselor intervention47 and found that women allocated to the intervention when compared to controls were more likely to be breastfeeding at 2 weeks, and their infants were less likely to be hospitalized in the first 6 months of life. There were no other differences in breastfeeding outcomes. Of note, control mothers could elect to enroll in the prenatal clinic's free lactation peer-support program. Carlsen et al. tested an International Board-Certified Lactation Consultants (IBCLC) phone support intervention for obese women in Denmark,48 and found that the intervention significantly increased duration of exclusive breastfeeding (median 120 days vs. 41 days). Bonuck et al. tested an integrated pre- and post-natal IBCLC intervention among 990 women, two-thirds of whom were overweight or obese.49 Lactation consultants met with mothers during 2 routine prenatal visits as well as during their maternity stay, and they contacted women by phone and provided home visits as needed after birth. The intervention increased rates of high-intensity breastfeeding at three months postpartum from 11 to 21%. These data suggest that proactive support of overweight and obese women may improve breastfeeding outcomes. Elements of the Bonuck breastfeeding intervention have been incorporated into an ongoing trial of a pre- and post-natal intervention to optimize outcomes among women with gestational diabetes.50
To enable women to breastfeed, maternity care providers should discuss a family's feeding plans early in prenatal care and provide practical anticipatory guidance and clinical care for breastfeeding complications, as needed.51 Providers should also facilitate maternity care practices that enable women to achieve their infant feeding goals, such as initiating breastfeeding within an hour of birth, giving only breast milk, rooming in, breastfeeding on demand, avoiding pacifiers, and fostering breastfeeding support groups after discharge.52
Such support is particularly important for women at increased risk of metabolic disease, given the association between obesity and breastfeeding difficulties. It may be helpful to emphasize the value of a nurturing relationship at the breast, in the event that exclusive breastfeeding is not possible. Maternal and pediatric care providers should coordinate care for these dyads with qualified lactation professionals, such as International Board-Certified Lactation Consultants. Embedding lactation consultants within obstetric and pediatric care may be sustainable now that the Affordable Care Act requires coverage of lactation support services.53
Implications for Future
Research Observational data demonstrate that pregravid metabolic risk factors, including insulin resistance and elevated body mass index, are associated with lower breastfeeding rates. Moreover, associations between early weaning and metabolic disease suggest that Adverse Lactation Outcomes (ALO), like Adverse Pregnancy Outcomes (APO), may be risk factors for future maternal metabolic disease. Research is needed to determine the mechanisms underlying these associations and to design and implement interventions that will enable more women to meet their infant feeding goals. Obstetric studies should routinely collect data on breastfeeding outcomes in order to advance this work. Such data should include standardized measures of breastfeeding intention,54 onset of lactogenesis,55 breastfeeding intensity,56 and reasons for weaning57 (Appendix). Incorporating assessment of lactation outcomes into epidemiologic studies is a prerequisite for determining the extent to which Adverse Lactation Outcomes predict long-term metabolic disease.
Translational research is also needed to define the physiologic mechanisms underlying lack of breast growth during pregnancy, delayed onset of lactogenesis and low milk production, which may mediate associations between maternal metabolic disease and early cessation of breastfeeding. The majority of research on mammary gland function has been performed in animals, and the extent to which these models apply to women is unclear, as detailed in a recent conference.17 Neville et al. note that research is imperative to "understand the mechanism by which obesity affects mammary gland development, milk secretion, and milk composition."
Conclusion
Both epidemiologic studies and animal models suggest that lactation may be a modifiable risk factor for maternal metabolic disease in later life. If this association is causal, investment in programs and policies that enable women to breastfeed may significantly improve women's health. Research is needed to elucidate the mechanisms linking premature weaning with maternal metabolic dysfunction, as well as to identify and implement strategies that enable women at metabolic risk to achieve their breastfeeding goals, thereby improving health across two generations.
Supplementary Material
Acknowledgments
Supported in part by R01HL109216-01A1, R01 HD073220-01, and R21DK092750-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Butte NF, Wong WW, Hopkinson JM. Energy Requirements of Lactating Women Derived from Doubly Labeled Water and Milk Energy Output. J. Nutr. 2001;131(1):53–58. doi: 10.1093/jn/131.1.53. [DOI] [PubMed] [Google Scholar]
- 2.Baker JL, Gamborg M, Heitmann BL, Lissner L, Sorensen TI, Rasmussen KM. Breastfeeding reduces postpartum weight retention. Am J Clin Nutr. 2008;88(6):1543–1551. doi: 10.3945/ajcn.2008.26379. [DOI] [PubMed] [Google Scholar]
- 3.Bobrow KL, Quigley MA, Green J, Reeves GK, Beral V. Persistent effects of women's parity and breastfeeding patterns on their body mass index: results from the Million Women Study. International journal of obesity. 2012 doi: 10.1038/ijo.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jager S, Jacobs S, Kroger J, Fritsche A, Schienkiewitz A, Rubin D, Boeing H, Schulze MB. Breast-feeding and maternal risk of type 2 diabetes: a prospective study and meta-analysis. Diabetologia. 2014;57(7):1355–1365. doi: 10.1007/s00125-014-3247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwarz EB, Brown JS, Creasman JM, Stuebe A, McClure CK, Van Den Eeden SK, Thom D. Lactation and maternal risk of type 2 diabetes: a population-based study. Am J Med. 2010;123(9):863 e1–863 e6. doi: 10.1016/j.amjmed.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of Type 2 Diabetes. JAMA. 2005;294(20):2601–2610. doi: 10.1001/jama.294.20.2601. [DOI] [PubMed] [Google Scholar]
- 7.Villegas R, Gao YT, Yang G, Li HL, Elasy T, Zheng W, Shu XO. Duration of breast-feeding and the incidence of type 2 diabetes mellitus in the Shanghai Women's Health Study. Diabetologia. 2008;51(2):258–266. doi: 10.1007/s00125-007-0885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SY, Kim MT, Jee SH, Yang HP. Does long-term lactation protect premenopausal women against hypertension risk? A Korean women's cohort study. Prev Med. 2005;41(2):433–438. doi: 10.1016/j.ypmed.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 9.Stuebe AM, Schwarz EB, Grewen K, Rich-Edwards JW, Michels KB, Foster EM, Curhan G, Forman J. Duration of lactation and incidence of maternal hypertension: a longitudinal cohort study. Am J Epidemiol. 2011;174(10):1147–1158. doi: 10.1093/aje/kwr227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunderson EP, Jacobs DR, Jr, Chiang V, Lewis CE, Feng J, Quesenberry CP, Jr, Sidney S. Duration of Lactation and Incidence of the Metabolic Syndrome in Women of Reproductive Age According to Gestational Diabetes Mellitus Status: A 20-Year Prospective Study in CARDIA--The Coronary Artery Risk Development in Young Adults Study. Diabetes. 2010;59(2):495–504. doi: 10.2337/db09-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ram KT, Bobby P, Hailpern SM, Lo JC, Schocken M, Skurnick J, Santoro N. Duration of lactation is associated with lower prevalence of the metabolic syndrome in midlife-SWAN, the study of women's health across the nation. Am J Obstet Gynecol. 2008;198(3):268.e1–268.e6. doi: 10.1016/j.ajog.2007.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarz EB, Ray RM, Stuebe AM, Allison MA, Ness RB, Freiberg MS, Cauley JA. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113(5) doi: 10.1097/01.AOG.0000346884.67796.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stuebe AM, Michels KB, Willett WC, Manson JE, Rexrode K, Rich-Edwards JW. Duration of lactation and incidence of myocardial infarction in middle to late adulthood. Am J Obstet Gynecol. 2009a;200(2):138 e1–138 e8. doi: 10.1016/j.ajog.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amir L, Donath S. A systematic review of maternal obesity and breastfeeding intention, initiation and duration. BMC Pregnancy and Childbirth. 2007;7(1):9. doi: 10.1186/1471-2393-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker JL, Michaelsen KF, Sorensen TI, Rasmussen KM. High prepregnant body mass index is associated with early termination of full and any breastfeeding in Danish women. Am J Clin Nutr. 2007;86(2):404–411. doi: 10.1093/ajcn/86.2.404. [DOI] [PubMed] [Google Scholar]
- 16.Lemay DG, Ballard OA, Hughes MA, Morrow AL, Horseman ND, Nommsen-Rivers LA. RNA sequencing of the human milk fat layer transcriptome reveals distinct gene expression profiles at three stages of lactation. PLoS ONE. 2013;8(7):e67531. doi: 10.1371/journal.pone.0067531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neville MC, Anderson SM, McManaman JL, Badger TM, Bunik M, Contractor N, Crume T, Dabelea D, Donovan SM, Forman N, Frank DN, Friedman JE, German JB, Goldman A, Hadsell D, Hambidge M, Hinde K, Horseman ND, Hovey RC, Janoff E, Krebs NF, Lebrilla CB, Lemay DG, Maclean PS, Meier P, Morrow AL, Neu J, Nommsen-Rivers LA, Raiten DJ, Rijnkels M, Seewaldt V, Shur BD, Vanhouten J, Williamson P. Lactation and Neonatal Nutrition: Defining and Refining the Critical Questions. Journal of Mammary Gland Biology and Neoplasia. 2012 doi: 10.1007/s10911-012-9261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nommsen-Rivers LA, Dolan LM, Huang B. Timing of stage II lactogenesis is predicted by antenatal metabolic health in a cohort of primiparas. Breastfeeding medicine : the official journal of the Academy of Breastfeeding Medicine. 2012;7(1):43–49. doi: 10.1089/bfm.2011.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjos SL, Henry O, Lee RM, Buchanan TA, Mishell DR., Jr The effect of lactation on glucose and lipid metabolism in women with recent gestational diabetes. Obstet Gynecol. 1993;82(3):451–455. [PubMed] [Google Scholar]
- 20.Gunderson EP, Hedderson MM, Chiang V, Crites Y, Walton D, Azevedo RA, Fox G, Elmasian C, Young S, Salvador N, Lum M, Quesenberry CP, Lo JC, Sternfeld B, Ferrara A, Selby JV. Lactation intensity and postpartum maternal glucose tolerance and insulin resistance in women with recent GDM: the SWIFT cohort. Diabetes Care. 2012b;35(1):50–56. doi: 10.2337/dc11-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunderson EP, Crites Y, Chiang V, Walton D, Azevedo RA, Fox G, Elmasian C, Young S, Salvador N, Lum M, Hedderson MM, Quesenberry CP, Lo JC, Ferrara A, Sternfeld B. Influence of breastfeeding during the postpartum oral glucose tolerance test on plasma glucose and insulin. Obstet Gynecol. 2012a;120(1):136–143. doi: 10.1097/AOG.0b013e31825b993d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziegler AG, Wallner M, Kaiser I, Rossbauer M, Harsunen MH, Lachmann L, Maier J, Winkler C, Hummel S. Long-term protective effect of lactation on the development of type 2 diabetes in women with recent gestational diabetes mellitus. Diabetes. 2012;61(12):3167–3171. doi: 10.2337/db12-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewey KG, Cohen RJ, Brown KH, Rivera LL. Effects of Exclusive Breastfeeding for Four versus Six Months on Maternal Nutritional Status and Infant Motor Development: Results of Two Randomized Trials in Honduras. J Nutr. 2001;131(2):262–267. doi: 10.1093/jn/131.2.262. [DOI] [PubMed] [Google Scholar]
- 24.McClure CK, Catov J, Ness R, Schwarz EB. Maternal visceral adiposity by consistency of lactation. Matern Child Health J. 2012;16(2):316–321. doi: 10.1007/s10995-011-0758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartick MC, Stuebe AM, Schwarz EB, Luongo C, Reinhold AG, Foster EM. Cost Analysis of Maternal Disease Associated With Suboptimal Breastfeeding. Obstet Gynecol. 2013;122(1):111–119. doi: 10.1097/AOG.0b013e318297a047. [DOI] [PubMed] [Google Scholar]
- 26.Stuebe AM, Rich-Edwards JW. The reset hypothesis: lactation and maternal metabolism. Am J Perinatol. 2009b;26(1):81–88. doi: 10.1055/s-0028-1103034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dall SR, Boyd IL. Evolution of mammals: lactation helps mothers to cope with unreliable food supplies. Proc Biol Sci. 2004;271(1552):2049–2057. doi: 10.1098/rspb.2004.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poole AT, Vincent KL, Olson GL, Patrikeev I, Saade GR, Stuebe A, Bytautiene E. Effect of lactation on maternal postpartum cardiac function and adiposity: a murine model. Am J Obstet Gynecol. 2014 doi: 10.1016/j.ajog.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altemus M, Deuster PA, Galliven E, Carter CS, Gold PW. Suppression of hypothalmic-pituitary-adrenal axis responses to stress in lactating women. J Clin Endocrinol Metab. 1995;80(10):2954–2959. doi: 10.1210/jcem.80.10.7559880. [DOI] [PubMed] [Google Scholar]
- 30.Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, Hellhammer DH. Effects of Suckling on Hypothalamic-Pituitary-Adrenal Axis Responses to Psychosocial Stress in Postpartum Lactating Women. J Clin Endocrinol Metab. 2001;86(10):4798–4804. doi: 10.1210/jcem.86.10.7919. [DOI] [PubMed] [Google Scholar]
- 31.Hahn-Holbrook J, Holt-Lunstad J, Holbrook C, Coyne SM, Lawson ET. Maternal Defense: Breast Feeding Increases Aggression by Reducing Stress. Psychological Science. 2011;22(10):1288–1295. doi: 10.1177/0956797611420729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck LF, Morrow B, Lipscomb LE, Johnson CH, Gaffield ME, Rogers M, Gilbert BC. Prevalence of selected maternal behaviors and experiences, Pregnancy Risk Assessment Monitoring System (PRAMS), 1999. MMWR Surveill Summ. 2002;51(2):1–27. [PubMed] [Google Scholar]
- 33.Pesa JA, Shelton MM. Health-enhancing behaviors correlated with breastfeeding among a national sample of mothers. Public Health Nurs. 1999;16(2):120–124. doi: 10.1046/j.1525-1446.1999.00120.x. [DOI] [PubMed] [Google Scholar]
- 34.Weiser TM, Lin M, Garikapaty V, Feyerharm RW, Bensyl DM, Zhu BP. Association of maternal smoking status with breastfeeding practices: Missouri, 2005. Pediatrics. 2009;124(6):1603–1610. doi: 10.1542/peds.2008-2711. [DOI] [PubMed] [Google Scholar]
- 35.Nommsen-Rivers LA, Chantry CJ, Peerson JM, Cohen RJ, Dewey KG. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am J Clin Nutr. 2010;92(3):574–584. doi: 10.3945/ajcn.2010.29192. [DOI] [PubMed] [Google Scholar]
- 36.Stuebe AM, Horton BJ, Chetwynd E, Watkins S, Grewen K, Meltzer-Brody S. Prevalence and Risk Factors for Early, Undesired Weaning Attributed to Lactation Dysfunction. J Womens Health (Larchmt) 2014b;23(5):404–412. doi: 10.1089/jwh.2013.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanky E, Nordskar JJ, Leithe H, Hjorth-Hansen AK, Martinussen M, Carlsen SM. Breast size increment during pregnancy and breastfeeding in mothers with polycystic ovary syndrome: a follow-up study of a randomised controlled trial on metformin versus placebo. BJOG : an international journal of obstetrics and gynaecology. 2012;119(11):1403–1409. doi: 10.1111/j.1471-0528.2012.03449.x. [DOI] [PubMed] [Google Scholar]
- 38.Brownell E, Howard CR, Lawrence RA, Dozier AM. Delayed onset lactogenesis II predicts the cessation of any or exclusive breastfeeding. The Journal of Pediatrics. 2012;161(4):608–614. doi: 10.1016/j.jpeds.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rasmussen KM, Kjolhede CL. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics. 2004;113(5):e465–e471. doi: 10.1542/peds.113.5.e465. [DOI] [PubMed] [Google Scholar]
- 40.Bogaerts AF, Van den Bergh BR, Witters I, Devlieger R. Anxiety during early pregnancy predicts postpartum weight retention in obese mothers. Obesity (Silver Spring) 2013 doi: 10.1002/oby.20352. [DOI] [PubMed] [Google Scholar]
- 41.Herring SJ, Rich-Edwards JW, Oken E, Rifas-Shiman SL, Kleinman KP, Gillman MW. Association of postpartum depression with weight retention 1 year after childbirth. Obesity. 2008;16(6):1296–1301. doi: 10.1038/oby.2008.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kozhimannil KB, Pereira MA, Harlow BL. Association between diabetes and perinatal depression among low-income mothers. JAMA : the journal of the American Medical Association. 2009;301(8):842–847. doi: 10.1001/jama.2009.201. [DOI] [PubMed] [Google Scholar]
- 43.Pedersen P, Baker JL, Henriksen TB, Lissner L, Heitmann BL, Sorensen TI, Nohr EA. Influence of psychosocial factors on postpartum weight retention. Obesity (Silver Spring) 2011;19(3):639–646. doi: 10.1038/oby.2010.175. [DOI] [PubMed] [Google Scholar]
- 44.Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Paediatr. 2007;96(4):590–594. doi: 10.1111/j.1651-2227.2007.00184.x. [DOI] [PubMed] [Google Scholar]
- 45.Meltzer-Brody S, Stuebe A. The long-term psychiatric and medical prognosis of perinatal mental illness. Best practice & research. Clinical obstetrics & gynaecology. 2014;28(1):49–60. doi: 10.1016/j.bpobgyn.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matias SL, Dewey KG, Quesenberry CP, Jr, Gunderson EP. Maternal prepregnancy obesity and insulin treatment during pregnancy are independently associated with delayed lactogenesis in women with recent gestational diabetes mellitus. Am J Clin Nutr. 2014;99(1):115–121. doi: 10.3945/ajcn.113.073049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chapman DJ, Morel K, Bermudez-Millan A, Young S, Damio G, Perez-Escamilla R. Breastfeeding education and support trial for overweight and obese women: a randomized trial. Pediatrics. 2013;131(1):e162–e170. doi: 10.1542/peds.2012-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlsen EM, Kyhnaeb A, Renault KM, Cortes D, Michaelsen KF, Pryds O. Telephone-based support prolongs breastfeeding duration in obese women: a randomized trial. Am J Clin Nutr. 2013;98(5):1226–1232. doi: 10.3945/ajcn.113.059600. [DOI] [PubMed] [Google Scholar]
- 49.Bonuck K, Stuebe A, Barnett J, Labbok MH, Fletcher J, Bernstein PS. Effect of primary care intervention on breastfeeding duration and intensity. Am J Public Health. 2014;104(Suppl 1):S119–S127. doi: 10.2105/AJPH.2013.301360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berry DC, Neal M, Hall EG, Schwartz TA, Verbiest S, Bonuck K, Goodnight W, Brody S, Dorman KF, Menard MK, Stuebe AM. Rationale, design, and methodology for the optimizing outcomes in women with gestational diabetes mellitus and their infants study. BMC Pregnancy and Childbirth. 2013;13(1):184. doi: 10.1186/1471-2393-13-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stuebe AM. Enabling Women to Achieve Their Breastfeeding Goals. Obstetrics & Gynecology. 2014a;123(3):643–652. doi: 10.1097/AOG.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 52.DiGirolamo AM, Grummer-Strawn LM, Fein SB. Effect of Maternity-Care Practices on Breastfeeding. Pediatrics. 2008;122(Supplement_2):S43–S49. doi: 10.1542/peds.2008-1315e. [DOI] [PubMed] [Google Scholar]
- 53.United States Breastfeeding Committee and National Breastfeeding Center. Model Policy: Payer Coverage of Breastfeeding Support and Counseling Services, Pumps and Supplies. 2014 from http://www.usbreastfeeding.org/Portals/0/Publications/Model-Policy-Payer-Coverage-Breastfeeding-Support.pdf. [Google Scholar]
- 54.Nommsen-Rivers LA, Dewey KG. Development and validation of the infant feeding intentions scale. Matern Child Health J. 2009;13(3):334–342. doi: 10.1007/s10995-008-0356-y. [DOI] [PubMed] [Google Scholar]
- 55.Chapman DJ, Perez-Escamilla R. Maternal Perception of the Onset of Lactation Is a Valid, Public Health Indicator of Lactogenesis Stage II. J. Nutr. 2000;130(12):2972–2980. doi: 10.1093/jn/130.12.2972. [DOI] [PubMed] [Google Scholar]
- 56.Li R, Fein SB, Grummer-Strawn LM. Association of Breastfeeding Intensity and Bottle-Emptying Behaviors at Early Infancy With Infants' Risk for Excess Weight at Late Infancy. Pediatrics. 2008;122(Supplement_2):S77–S84. doi: 10.1542/peds.2008-1315j. [DOI] [PubMed] [Google Scholar]
- 57.Odom EC, Li R, Scanlon KS, Perrine CG, Grummer-Strawn L. Reasons for earlier than desired cessation of breastfeeding. Pediatrics. 2013;131(3):e726–e732. doi: 10.1542/peds.2012-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.