Abstract
Normal pregnancy requires increased uterine endothelial cell driven vasodilation that is related to increases in sustained Ca2+ signaling via increased connexin 43 (Cx43) gap junction function. Preeclampsia, a hypertensive disorder of pregnancy associated with endothelial dysfunction, is also linked with down regulation of Ca2+ driven vasodilator production and increased levels of vascular endothelial growth factor (VEGF). Cx43 function can be acutely down-regulated by phosphorylation of multiple inhibitory residues and VEGF is known to promote phosphorylation of Cx43. Herein, we show that VEGF-165 promotes Cx43 phosphorylation at Ser-279/282 and Tyr-265 residues and blocks pregnancy-adapted Ca2+ signaling in ovine uterine artery endothelial cells (UAEC). Pharmacologic Src and ERK kinase pathway inhibitors (PP2 and U0126) reverse these phosphorylations and rescue Ca2+ signaling. We also report a nutraceutical Src inhibitor, t10,c12 conjugated linoleic acid (10,12 CLA) rescues Ca2+ signaling in UAEC and therefore may have therapeutic potential for preeclampsia.
Keywords: VEGF, pregnancy, gap junction, CLA, Src, Ca2+
1. INTRODUCTION
Adaptation of the uterine vasculature during pregnancy is mediated in part by increased angiogenesis and increased vasodilation, and is critical to lowering vascular resistance and increasing blood flow through the uteroplacental unit (Bird, et al. 2003; Sladek, et al. 1997). This increased flow serves to deliver nutrients and gasses to the growing fetus (Sladek et al. 1997). Our laboratory has extensively studied the mechanisms by which pregnancy enhances endothelial vasodilation, and particularly pregnancy-dependent changes in the signaling pathways that drive NO (nitric oxide) production. Early in defining pregnancy adaptation of endothelial vasodilator production, Bird et al (Bird, et al. 2000) observed that uterine artery endothelial cells from pregnant ewes (P-UAEC) had an enhanced ability to activate eNOS (endothelial nitric oxide synthase) over those from nonpregnant ewes (NP-UAEC) in response to multiple agonists, even after being cultured to passage 4 (roughly 14 days outside of the pregnant environment). By passage 4, eNOS protein expression (which is elevated in fresh uterine endothelium from pregnant ewes when compared to nonpregnant uterine arteries) had become nearly indistinguishable between P- and NP-UAEC. Additional studies on ATP-stimulated Ca2+ signaling determined that the increased NO output was due to an increased capacity of P-UAEC to sustain elevated [Ca2+]i (intracellular free Ca2+ concentration) in the form of periodic, transient, and synchronized Ca2+ bursts and that this was also observed in more of the cells. These bursts are a product of CCE (capacitative Ca2+ entry), utilizing TRPC3 (transient receptor potential channel 3) interaction with IP3R2 (inositol 1,4,5-trisphosphate receptor 2) (Gifford, et al. 2006), and are permitted only when UAEC are able to communicate with each other through Cx43 (connexin 43) gap junctions. This is illustrated by the loss of ATP-stimulated Ca2+ bursts after treatment with the Cx43-competitive peptide, GAP27 (Morschauser, et al. 2014; Yi, et al. 2010b). Thus, we termed pregnancy-adaptive programming to describe the programmed Ca2+ signaling adaptations in the form of sustained Ca2+ bursts that stimulate increased eNOS activity in P-UAEC over that of NP-UAEC.
An insufficient drop in uterine vascular resistance is observed with the onset of preeclampsia, a condition primarily characterized by maternal hypertension that presents significant risk to mother and child. A failure to demonstrate a pregnancy-enhanced ability to produce vasodilators is a hallmark of the preeclamptic condition (Bird et al. 2003). Recently, we (Bird, et al. 2013) put forth a model of both pregnancy adaptation and disease-related failure whereby control of Cx43 function or lack thereof can be explained by the hormonal milieu and the associated endothelial cell signaling of healthy or diseased pregnancies. The model proposes that in normal pregnancy, factors known to circulate in abundance in pregnancy such as cAMP, cGMP, and estrogen, as well as mechanical signals such as shear stress, may be able to signal to the endothelium to upregulate Cx43 gap junction function. As a consequence, there is an increase in the capacity of the endothelial tissue to produce an enhanced Ca2+ response to vasodilatory agonists, which then allows enhanced vasodilator production. Conversely, the hormonal environment of preeclampsia shares similarities with that of a wound site including abnormally high levels of growth factors and cytokines (reviewed in (Bird et al. 2013)) known to signal through kinases such as PKC (protein kinase C), Src, and ERK (extracellular-signal-regulated kinase) to phosphorylate Cx43. Phosphorylation of Cx43 at multiple c-terminal amino acid residues such as Ser-279/282, Tyr-265, Ser-368, and Ser-262 are targeted by the aforementioned signaling pathways, and have been described as inhibitory phosphorylations (Lampe and Lau 2000). The phorbol ester, PMA (phorbol myristic acid), is commonly used as a receptor-independent inhibitor of gap junction function (Lampe 1994; Sirnes, et al. 2008; van der Zandt, et al. 1990) and signals through PKC, Src, and ERK in UAEC (Bird et al. 2013). Indeed, when P-UAEC are exposed to PMA, sustained phase Ca2+ burst responses to ATP are dramatically reduced (Bird et al. 2013; Cale and Bird 2006), and this is associated with the phosphorylation of Cx43 at both positions Ser-279/282 via the ERK-1/2 pathway and Tyr-265 via the Src pathway. Furthermore, a sub-maximal dose of PMA, which gives rise to a physiologic level of inhibition of sustained ATP Ca2+ response (defined as a level of inhibition to approach the lesser function of NP-UAEC), can be completely reversed by the Src family kinase inhibitor PP2 [amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo (3,4d) pyrimidine]. This underscores both the susceptibility of Cx43 to endocrine inhibition in P-UAEC and the reversibility of the observed loss of Ca2+ burst function (Bird et al. 2013).
Such studies provide proof of principle for the model, but the question now is if the endocrine factors elevated above normal in preeclamptic subjects have a similar ability to promote the observed endothelial dysfunction sufficient to reverse pregnancy adaptation, and to do so by phosphorylation and closure of Cx43. Some evidence in ovine endothelium that one particular hormone of wounding, vascular endothelial growth factor (VEGF), can achieve acute inhibition of Ca2+ bursting and so NO output in intact pregnant (P) uterine artery endothelium (UA Endo) down to the level of that in nonpregnant (NP)-UA Endo has been reported by Yi et al (Yi, et al. 2011). In that study, a 30-minute pretreatment with 10 ng/ml VEGF-165 reversed the pregnancy-adapted Ca2+ and NO responses to ATP in UA Endo; however, no examination of corresponding Cx43 phosphorylation or of the signaling pathways that mediate the response to VEGF were undertaken. In this study we now seek to examine the molecular basis behind VEGF-165 inhibition of ATP-stimulated Ca2+ bursting responses in ovine endothelium and clearly establish whether phosphorylation of Cx43 in response to VEGF can indeed overwhelm the otherwise pregnancy-enhanced function in P-UAEC. Furthermore, this study specifically aims to address three hypotheses: 1) VEGF-165 pretreatment inhibits subsequent ATP-stimulated Ca2+ bursts in P-UAEC down to levels approaching that of NP-UAEC; 2) inhibitory phosphorylations of Cx43 due to activation of ERK and Src pathways by VEGF-165 correlate closely with the observed inhibition of subsequent ATP-stimulated Ca2+ bursts; and 3) agents which prevent such inhibitory phosphorylations through pretreatment of P-UAEC with MEK/ERK or Src inhibitors also achieve ‘rescue’ of normal ATP-stimulated Ca2+ bursts in VEGF-165-pretreated P-UAEC. We then conclude this study by testing a potential therapeutic agent, t10,c12 conjugated linoleic acid (10,12 CLA), which has been recently described as a c-Src inhibitor (Shahzad 2013). As natural substance found in meat and dairy products from grass-fed ruminants (Dhiman, et al. 1999) it may be a candidate for rescue of Cx43 function via Src inhibition, and therefore rescue sustained Ca2+ signaling in UAEC. Though more commonly studied for its anti-tumor properties and effects on lipid metabolism, we ask herein whether 10,12 CLA may provide a nutraceutical alternative to pharmacologic approaches to endothelial directed therapy for hypertensive disorders in pregnancy, such as preeclampsia.
2. MATERIALS AND METHODS
2.1 Materials
Unless noted otherwise, MEM and all other cell culture reagents were purchased from Life Technologies. For [Ca2+]i imaging studies, 35-mm dishes with glass coverslip windows were purchased from MatTek Corp. Fura-2 AM and Pluronic F127 were obtained from Life Technologies. CaCl2 was from EMD Milllipore. ATP (disodium salt), PMA and all other chemicals, unless noted otherwise, were from Sigma. VEGF-165 and PlGF (placental growth factor) were from R & D Systems, Inc. VEGF-E, a product of recombinant orf virus, was from Angio-Proteiomie. VEGFRi; (VEGF receptor tyrosine kinase inhibitor; 4-[(4’-chloro-2’-fluoro)phenylamino]-6,7-dimethoxyquinazoline, a reasonably selective inhibitor of VEGFR-2 over VEGFR-1; IC50 = 100 nM and 2 uM, respectively) was purchased from EMD Millipore. PP2 (a selective inhibitor of Src family kinases) was from Enzo Life Sciences. U0126 (a selective inhibitor of MEK, a kinase known to directly phosphorylate ERK1 and 2) was from Promega Corp. CLA isomers were initially provided by Dr. Mark Cook and Dr. Manish Patankar. Subsequent isomers were purchased from Matreya LLC, with no difference in data between the two sources.
2.2 Isolation of uterine artery endothelial cells
Procedures for animal handling and protocols for experimental procedures were approved by the University of Wisconsin-Madison Research Animal Care Committees of both the School of Medicine and Public Health and the College of Agriculture and Life Sciences and followed the recommended American Veterinary Medicine Association guidelines for humane treatment and euthanasia of laboratory farm animals. Uterine arteries were obtained from mixed Western breed non-pregnant sheep and pregnant ewes at 120–130 days of gestation during non-survival surgery. UAEC were prepared as previously described (Bird et al. 2000; Grummer, et al. 2009) and stored in liquid nitrogen. For all subsequent experiments, cells were grown in MEM containing 20% FBS, 1% penicillin-streptomycin, and 4 ug/ml gentamycin.
2.3 Fura-2 [Ca2+]i imaging studies
Pooled passage 4 cells were grown in 35-mm glass-bottom microwell dishes until they reached 100% confluence. Cells were then loaded with Fura-2 by adding 10 µM Fura-2AM in the presence of 0.05% Pluronic F127 in 1 ml Krebs buffer (25 mM HEPES, pH 7.4, 125 mM NaCl, 5 mM KCl, 1 mM MgSO4, 1 mM KH2PO4, 6 mM glucose, 2 mM CaCl2) for 60 min at 37° C. Cells were washed with Krebs buffer and incubated as in (Yi et al. 2010b). Fura-2 loading was verified by viewing at 380 nm UV excitation on a Nikon inverted microscope (InCyt Im2, Intracellular Imaging, Inc.) with PixelFly camera (Cooke). The cells were incubated with ATP and the data was recorded for many cells simultaneously as in (Yi et al. 2010b). After the initial ATP treatment, the dish was washed while maintaining the focus on each individual cell. Where noted, an antagonist pretreatment was made to the experimental buffer after the wash step. Then, after a 5-min baseline, VEGF or vehicle was added and recorded for 30 min before a subsequent treatment of 100 uM ATP was added while recording continued. The second ATP treatment was allowed to proceed for 30 min. Ca2+ bursts were counted for the “before” and “after” ATP treatments. Those cells which produced 3 or more Ca2+ bursts during the initial ATP treatment were included in the data set because initial and second bursts are no different between P- and NP-derived cells. Before and after comparisons data for each cell burst numbers were compared in this data set.
2.4 Western blot analyses
UAEC were passaged to 60-mm dishes and maintained for 24–48 hours until approximately 80% confluent. Antagonists were added 20 min prior to 30-min agonist treatment. At the end of stimulation media was quickly aspirated and cells snap frozen in liquid N2. Cells were solubilized in lysis buffer [50 mM HEPES (pH 7.5), 4 mM Na4P2O7-10 H2O, 100 mM NaCl, 10 mM EDTA, 10 mM NaF, 2 mM Na3(VO4)2, 1 mM phenylmethylsulfonylfluoride, 1% Triton X-100, 5 ug/ml leupeptin, and 5 ug/ml aprotinin], briefly sonicated, and centrifuged at 12,000 × g for 5 min. Subsequent western blotting of extracted proteins and detection of phosphorylated proteins using phosphorylation-state specific antibodies were achieved using ECL detection exactly as described previously (Grummer et al. 2009; Sullivan, et al. 2006). For this study, phosphorylation of Cx43 was detected on 4 separate membranes using the following antibodies: Ser-279/282 Cx43 (#sc-12900-R; Santa Cruz Biotechnology, Inc.) at 1:1300; Tyr-265 Cx43 (#sc-17220-R; Santa Cruz Biotechnology) at 1:750; Ser-368 Cx43 (#3841, Millipore Corp.) at 1:1000; Ser-262 Cx43 (#sc-17218-R, Santa Cruz Biotechnology) at 1:1300. Total Cx43 (#C6214; Sigma) was detected using antibody at 1:5000. For all rabbit primary antibodies, goat anti-rabbit HRP-conjugated secondary antibody (#7074, Cell Signaling Technology) was used at 1:3000. The CT1 antibody (used at 1:2500; see supplementary data) was obtained from the Fred Hutchinson Cancer Research Center (Seattle, WA); the secondary antibody (used at 1:3000) was rabbit anti-mouse IgG HRP-conjugated F(ab’)2 (#AQ160P, Millipore Corp.). Phosphorylation of ERK 1/2 was measured using ERK 1/2 Thr-202/Tyr-204 (#4377, Cell Signaling Technology) at 1:2500. To ensure consistent protein loading, antibodies were normalized to levels of Hsp90 (#4874, Cell Signaling Technology) at 1:2500.
2.5 Statistical analysis
Data are presented as means ± S.E.M. and were analyzed by Student's t-test or analysis of variance, as appropriate (Figures 1, 3, 4, 5, 6). For Figures 2 and 7, paired t-test was used for each cell against itself within a single treatment group, and a Mann-Whitney Rank-Sum test was used against vehicle control. A value of P<0.05 was considered statistically significant.
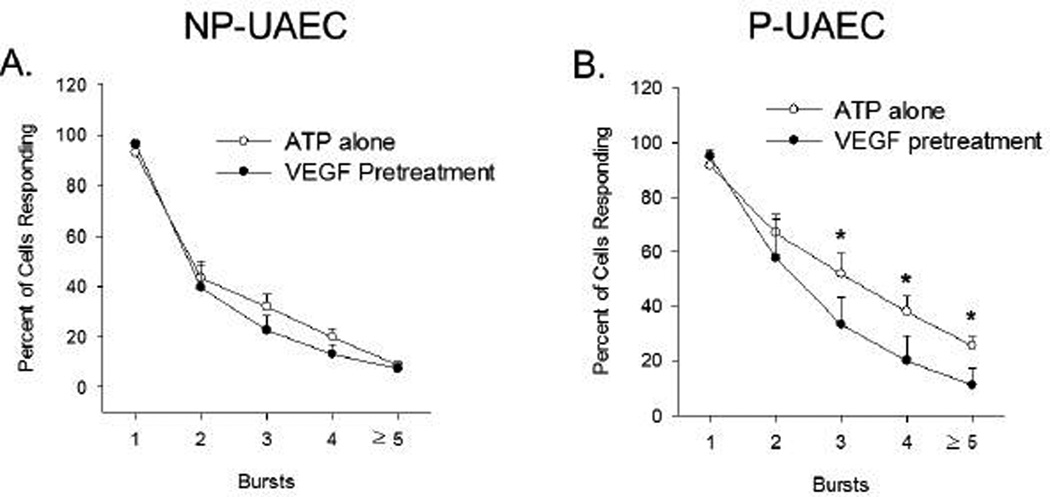
Figure 1. Percent of UAEC showing each successive burst in response to ATP with or without VEGF-165 pretreatment.
Stimulation of Ca2+ bursts by 100 uM ATP for 30 min is not changed by 30 min pretreatment with 10 ng/ml VEGF-165 in NP-UAEC (Panel A, n=5–8 dishes). However, stimulation of Ca2+ bursts by ATP is significantly reduced for bursts 3 or more by 30-min pretreatment with 10 ng/ml VEGF-165 in P-UAEC (Panel B, n=5–8 dishes). Results are shown as Means ± S.E.M., * P<0.05 by Student’s t-test.
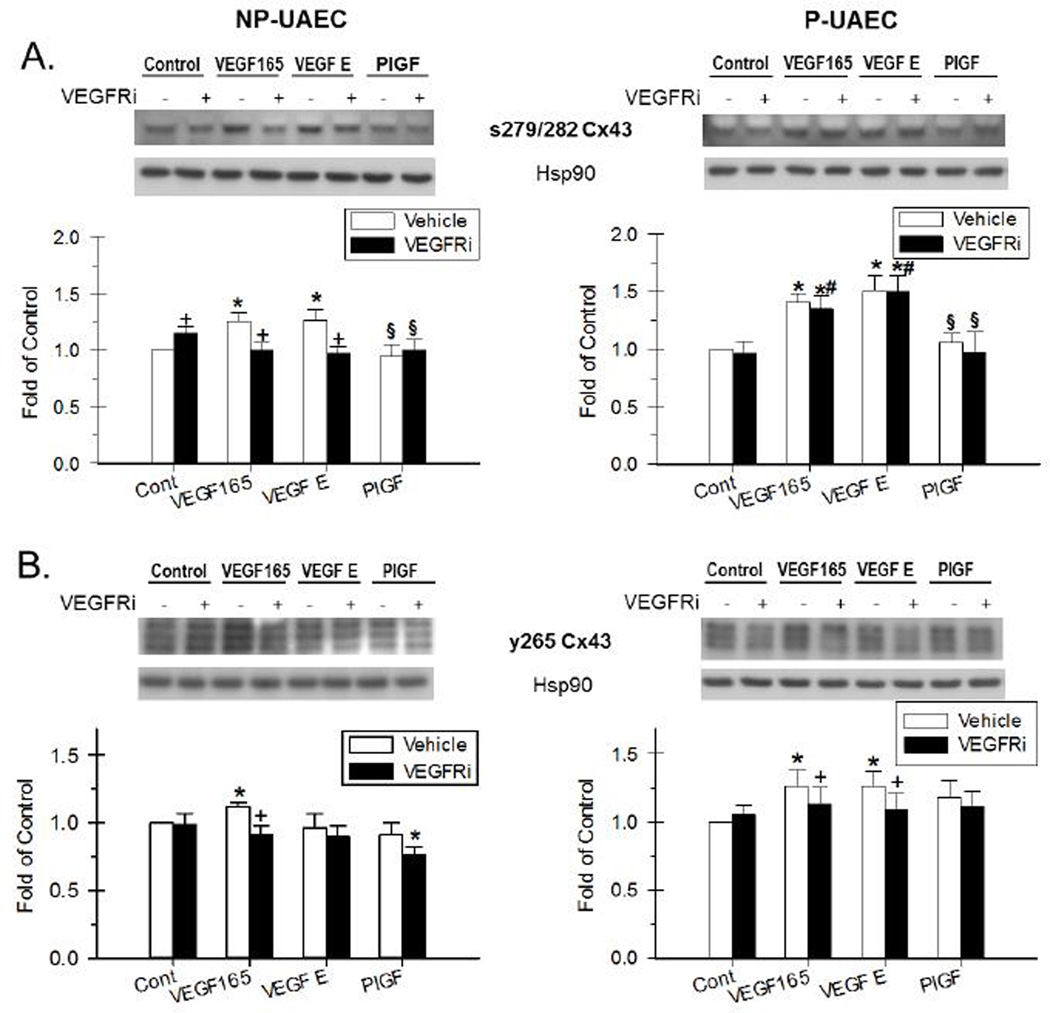
Figure 3. Effect of VEGF-165, VEGF-E and PlGF and the inhibition by VEGFR inhibitor on Cx43 phosphorylation.
NP-UAEC and P-UAEC (left and right graphs, respectively) were pretreated for 20 min with 1 uM VEGFR inhibitor, followed by a 30-min treatment with 10 ng/ml VEGF-165, 100 ng/ml VEGF-E or 100 ng/ml PlGF. Results were normalized to Hsp90 protein and expressed as fold change over unstimulated control. Phospho-specific western blot analyses were performed, and representative blots are shown. Values shown are Means ± S.E.M. of 6 independent experiments (* P<0.05 compared with control; + P<0.05 compared with same agonist treatment; # P<0.05 compared with NP-UAEC; § P<0.05 compared with VEGF-165 treatment). A. Response of Ser-279/282 Cx43. B. Response of Tyr-265 Cx43.
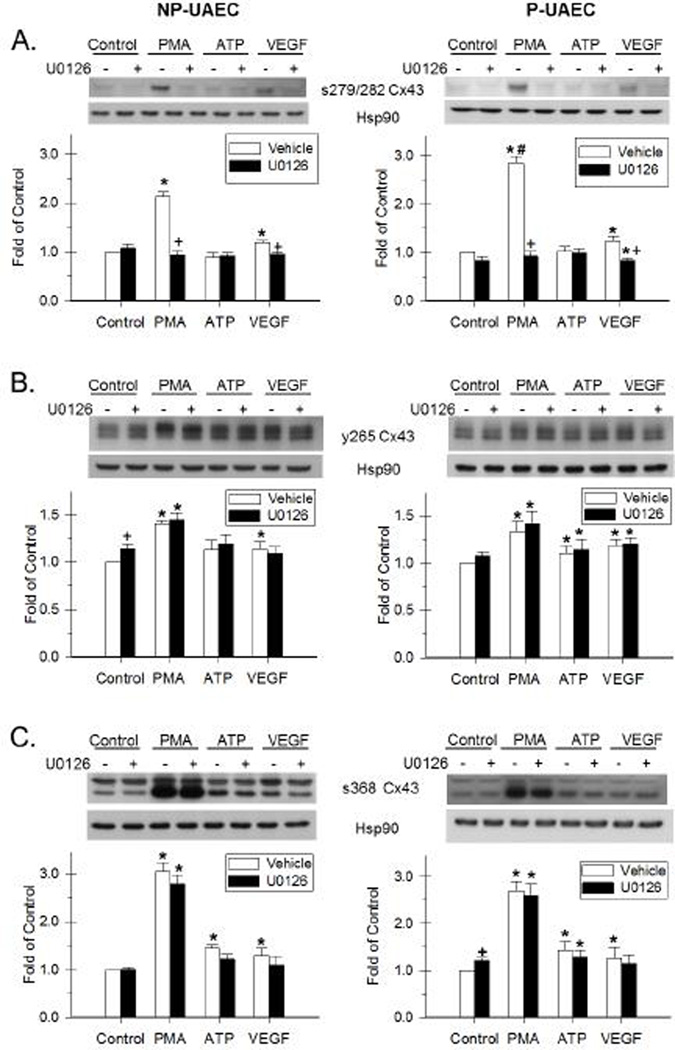
Figure 4. Effect of PMA, ATP and VEGF-165 and the inhibition by U0126 on Cx43 phosphorylation.
NP-UAEC and P-UAEC (left and right graphs, respectively) were pretreated for 20 min with 10 uM U0126, followed by a 30-min treatment with 10 nM PMA, 100 uM ATP or 10 ng/ml VEGF. Results were normalized to Hsp90 protein and expressed as fold change over unstimulated control. Phospho-specific western blot analyses were performed, and representative blots are shown. Values shown are Means ± S.E.M. of 5–6 independent experiments (* P<0.05 compared with control; + P<0.05 compared with same agonist treatment; # P<0.05 compared with NP-UAEC). A. Response of Ser-279/282 Cx43. B. Response of Tyr-265 Cx43. C. Response of the 42 kDa band of s368 Cx43.
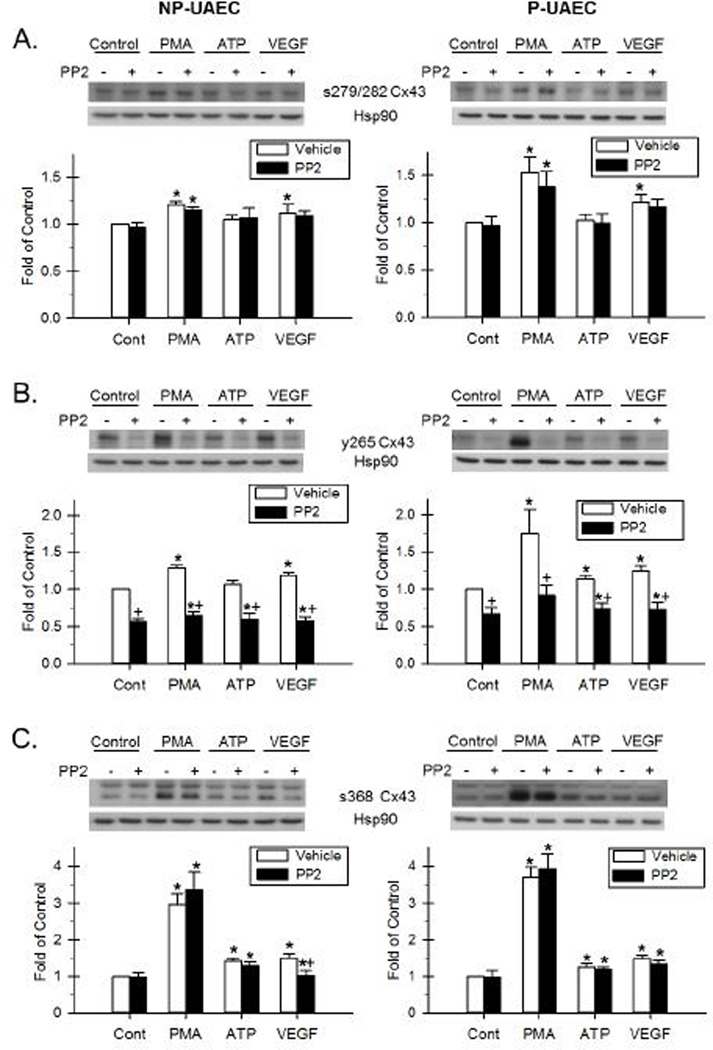
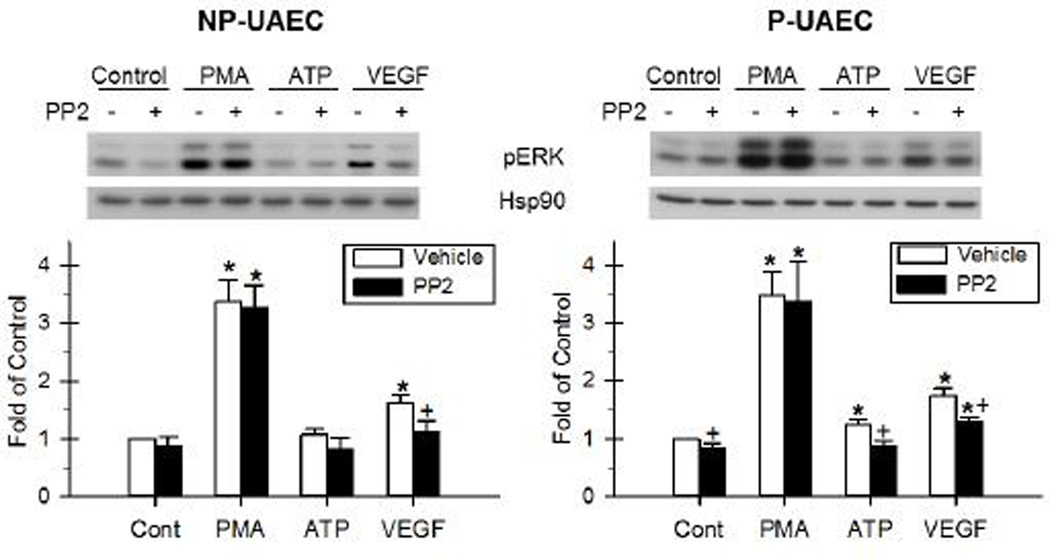
Figure 5. Effect of PMA, ATP and VEGF-165 and the inhibition by PP2 on Cx43 phosphorylation.
NP-UAEC and P-UAEC (left and right graphs, respectively) were pretreated for 20 min with 10 uM PP2, followed by a 30-min treatment with 10 nM PMA, 100 uM ATP or 10 ng/ml VEGF. Results were normalized to Hsp90 protein and expressed as fold change over unstimulated control. Phospho-specific western blot analyses were performed, and representative blots are shown. Values shown are Means ± S.E.M of 6–7 independent experiments (* P<0.05 compared with control; + P<0.05 compared with same agonist treatment). A. Response of Ser-279/282 Cx43. B. Response of Tyr-265 Cx43. C. Response of the 42 kDa band of s368 Cx43.
Figure 6. Effect of PMA, ATP and VEGF-165 and the inhibition by PP2 on ERK phosphorylation.
NP-UAEC and P-UAEC (left and right graphs, respectively) were pretreated for 20 min with 10 uM PP2, followed by a 30-min treatment with 10 nM PMA, 100 uM ATP or 10 ng/ml VEGF. Results were normalized to Hsp90 protein and expressed as fold change over unstimulated control. Graph illustrates p44-ERK. Phospho-specific western blot analysis was performed, and representative blots are shown. Values shown are Means ± S.E.M. of 6–7 independent experiments (* P<0.05 compared with control; + P<0.05 compared with same agonist treatment).
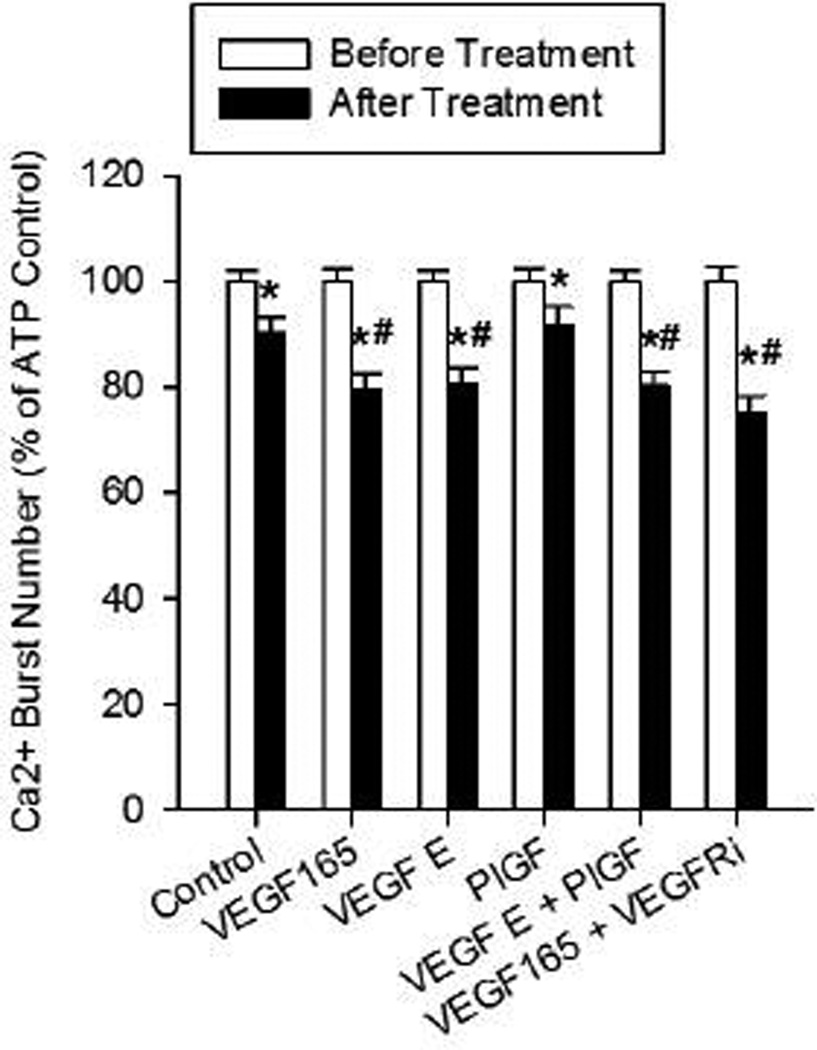
Figure 2. VEGF-165 inhibits ATP-stimulated Ca2+ bursts through VEGFR-2 regions outside of the endogenous kinase domain.
Experiments were performed by stimulating and recording a group of P-UAEC with ATP (100 uM) for 30 min, washing and re-stimulating the same cells with ATP again after a 30-min pre-incubation with the following VEGFR binding agonists: VEGF-165 (10 ng/ml), VEGF-E(100 ng/ml), PlGF (100 ng/ml), VEGF-E+PlGF combined, or VEGF-165+VEGFR-2 kinase inhibitor (VEGFRi, 1 uM) combined pretreatment. Data are mean burst numbers ± S.E.M., and restricted to cells showing 3 or more bursts upon initial ATP stimulation. Statistics were performed on raw data. For control, n=263; for VEGF-165, n=261; for VEGF-E, n=191; for PlGF, n=228; for PlGF+VEGF-E, n=238; for VEGF-165+VEGFRi, n=202 (individual cells gathered from 5–8 dishes). Significant differences from first ATP treatment (before) are shown by * P<0.0001, second ATP control (after) are shown by # P<0.005. Comparisons versus internal control are by paired t-test comparisons between “after” groups from different treatments are by rank sum test. Dashed line indicates the equivalent level of Ca2+ bursts in NP-UAEC upon ATP stimulation for reference.
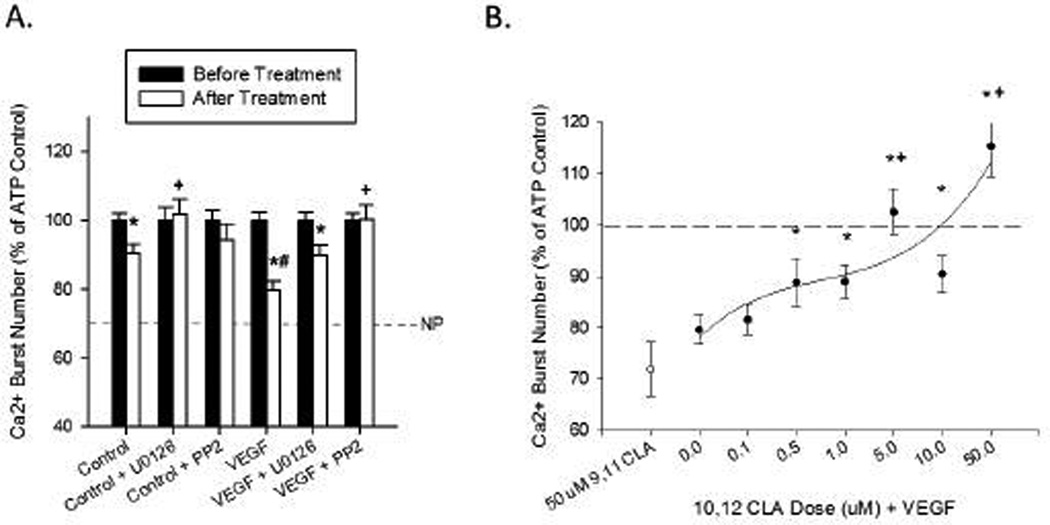
Figure 7. Inhibition of ERK and Src signaling pathways ‘rescues’ inhibition of ATP-stimulated Ca2+ bursts by VEGF-165.
For panel A, these data are presented in the same manner as in Figure 2 and the control and VEGF-165 bars are re-plotted for comparison. Kinase inhibitors (U0126: 10 uM, 20 min; PP2: 10 uM, 20 min) were added after the wash step and remained in the dish during VEGF-165 (10 ng/ml, 30 min) and subsequent ATP (100 uM, 30 min) treatment. Significant differences from first ATP treatment (before) are shown by * P<0.001, second ATP control (after) are shown by # P<0.05 for significant decrease, + P<0.05 for significant increase. Dashed line indicates the equivalent level of Ca2+ bursts in NP-UAEC upon ATP stimulation for reference. In panel B, a dose response for 10,12 CLA were used as Src inhibiting Ca2+ burst rescue agents in the same manner as U0126 and PP2 in panel A, with 9,11 CLA used as a control (10,12 CLA: 0.1 uM – 50 uM); 9,11 CLA: 50 uM, 30 min). Significant improvement over VEGF-165 pretreated cells (0.0 uM 10,12 CLA) is shown by * P<0.05, significant improvement from ATP control is shown by + P<0.01. Dashed line indicates full Ca2+ burst recovery. For both panels, data are mean ± S.E.M, and restricted to cells showing 3 or more bursts initially. Statistics were performed on raw data. For control, n=263; for control+U0126, n=120; for control+PP2, n=122; for VEGF-165, n=261; for VEGF-165+U0126, n=253; for VEGF-165+PP2, n=183; for VEGF-165+9,11 CLA, n=91; for VEGF-165 + 0.1 uM 10,12 CLA, n=152; for VEGF-165 + 0.5uM 10,12 CLA, n=125; for VEGF-165 + 1uM 10,12 CLA, n=165; for VEGF-165 + 5uM 10,12 CLA, n=147;for VEGF-165 + 10uM 10,12 CLA, n=145; for VEGF-165+ 50uM 10,12 CLA, n=124 (individual cells from 3–8 dishes). Comparisons versus internal control are by paired t-test; comparisons between “after” groups from different treatments are by rank sum test.
3. RESULTS
3.1 VEGF-165 inhibits pregnancy-adapted programming in P-UAEC to a level indistinguishable from NP-UAEC via VEGFR-2
Figure 1 shows the frequency of ATP-stimulated Ca2+ bursts in NP-vs P-UAEC with or without VEGF pretreatment. Pretreating P-UAEC with 10 ng/ml VEGF-165 for 30 min reduced sustained burst responses to a level equivalent to that of non-pretreated NP-UAEC. Pretreatment of NP-UAEC with VEGF had no further inhibitory effect on burst numbers.
Because VEGF-165 does not further inhibit ATP-stimulated Ca2+ bursts in NP-UAEC, more detailed examination of agonist and antagonist effects on Ca2+ bursts was only performed on P-UAEC. VEGF-165 binds both VEGFR-1 and VEGFR-2, so to assess which receptor(s) are responsible for inhibiting the ATP-stimulated Ca2+ burst response the VEGFR-1 specific agonist PlGF and the VEGFR-2 specific agonist VEGF-E were used as previously (Grummer et al. 2009). This approach revealed (Figure 2) that VEGFR-2 is responsible for inhibiting the ATP-stimulated Ca2+ burst response, as pretreatment with VEGF-E (19.3% inhibition) reproduced the effectiveness of VEGF-165 (20.4%), while PlGF (8.3%) had no further effect beyond the modest inhibition of ATP on its own response (9.7%). Further, VEGF-E and PlGF together (19.7%) did not synergize to further inhibit ATP to a greater extent than VEGF-165 or VEGF-E alone. Of note, when the VEGF-165 was used in the presence of the VEGFR-2 kinase inhibitor, there was no reversal of the inhibition of ATP-stimulated Ca2+ bursts in P-UAEC, suggesting residual signaling from VEGFR-2 exists which is not blocked by VEGFR-2 kinase inhibitor. (Figure 2).
3.2 Phosphorylation of Cx43
In order to assess the possibility that VEGF inhibition of ATP-stimulated Ca2+ bursts may be mediated by inhibitory phosphorylations on the c-terminal tail of Cx43, we ran western blot analyses for site-specific phosphorylations known to be inhibitory to gap junction function. The sites most widely accepted to be inhibitory to Cx43 function, as well as the kinase most likely to be responsible for each particular phosphorylation site, are as follows: Ser-279/282 (ERK1/2), Tyr-265 (Src), Ser-368 (PKC) and Ser-262 (PKC) (Lampe and Lau 2000). We began by analyzing the ability of VEGF family peptides to phosphorylate each key residue and whether the VEGFR-2 kinase inhibitor blocked each phosphorylation event. We then moved on to examine in more detail ERK and Src pathway specificity (see discussion for more on PKC) to PMA, VEGF-165 and ATP (control) for each phosphorylation site in turn.
3.2.1 Role of VEGFR-1 vs VEGFR-2 in phosphorylation of Cx43 at inhibitory residues
Western blot analyses corresponding to total Cx43, Ser-279/282, Tyr-265, Ser-368, and Ser-262 phospho-Cx43 have been previously reported in UAEC (Bird et al. 2013). The agonist effects herein on each of the multiple bands for the various phospho-Cx43 sites are tabulated (Supplement Tables S1 and S2); the phosphorylation of the Ser-279/282 and Tyr-265 sites most relevant to this discussion are shown in Figure 3.
Phosphorylation of Ser-279/282 was significantly stimulated at 30 min by VEGF-165 and VEGF-E, but not PlGF pretreatment in both P-UAEC and NP-UAEC (Figure 3A). Of note, pretreatment with VEGFR-2 kinase inhibitor also completely inhibited Ser-279/282 phosphorylation by both VEGF-165 and VEGF-E in NP-UAEC, but had no effect on Ser-279/282 phosphorylation in response to either agonist in P-UAEC.
Phosphorylation of Tyr-265 by VEGF family peptides is shown in Figure 3B. Consistent with our previous studies using PMA, (Bird et al. 2013), the bands illustrated at approximately 80 kDa, are similar to those demonstrated in other studies (Yao, et al. 2000), and are much more prominent than those at 42–46 kDa. We have also previously shown the 80 kDa bands are eliminated with alkaline phosphatase treatment (Bird et al. 2013). In P-UAEC, a significant Tyr-265 phosphorylation was detected after stimulation with VEGF-165 and VEGF-E. Of note, only VEGF-165 stimulated phosphorylation of Tyr-265 in NP-UAEC, while PlGF had no effect in either cell type. For VEGF-165 and VEGF-E in P-UAEC, Tyr-265 phosphorylation was significantly reduced by pretreatment with VEGFR-2 kinase inhibitor. In NP-UAEC, VEGF-165 pretreatment in the presence of VEGFR-2 kinase inhibitor reduced Tyr-265 phosphorylation to basal levels.
3.3 Comparative effects of PMA, VEGF and ATP on phosphorylation of Cx43 at inhibitory residues
3.3.1 Phosphorylation of Cx43 at Ser-279/282
After a 30-min PMA treatment, there was an increase in Ser-279/282 phosphorylation in NP-UAEC, and a greater increase observed in P-UAEC (Figure 4A and 5A, Tables S3 and S4). VEGF-165 also stimulated a significant increase in Ser-279/282 phosphorylation in both P-UAEC and NP-UAEC, although to a lower level than that stimulated by PMA. This contrasts the effect of similar treatment with ATP, which resulted in no detectable Ser-279/282 phosphorylation in either cell type at 30 min.
Because phosphorylation of Cx43 at positions Ser-279/282 is widely believed to be mediated by the ERK-1/2 pathway (Warn-Cramer, et al. 1996), we then used inhibitors of two signaling pathways commonly thought to mediate inhibitory Cx43 phosphorylations, U0126 (MEK/ERK) and, as a control, PP2 (Src family kinase). U0126 abolished the PMA- and VEGF-165-stimulated phosphorylation of Ser-279/282 in both P-UAEC and NP-UAEC (Figure 4A, Tables S3 and S4). In contrast, PP2 had no significant effect on either PMA- or VEGF-165-stimulated Ser-279/282 phosphorylation in either P-UAEC or NP-UAEC (Figure 5A).
3.3.2 Phosphorylation of Cx43 at Tyr-265
In P-UAEC, a significant Tyr-265 phosphorylation was detected after stimulation with PMA, VEGF-165, and ATP (Figure 4B and 5B, Tables S3 and S4). Only PMA and VEGF-165 stimulated significant changes in phosphorylation of Tyr-265 in NP-UAEC. Like the Ser-279/282 site above, PMA was the strongest stimulant of Tyr-265 phosphorylation in both P-UAEC and NP-UAEC. VEGF-165 and ATP were weaker stimulants of Tyr-265 phosphorylation than PMA in P-UAEC.
The Tyr-265 residue of Cx43 is considered a Src site (Kanemitsu, et al. 1997; Lin, et al. 2001), and as such we again used the signaling pathway inhibitors above. This time we expected the Src family kinase inhibitor, PP2, to be effective in reversing agonist-stimulated Tyr-265 phosphorylation while U0126 would serve as the negative control. Indeed, PP2 was effective in inhibiting PMA-, VEGF-165-, and ATP-stimulated Tyr-265 phosphorylation in P-UAEC as well as the PMA- and VEGF-165-stimulated Tyr-265 phosphorylation in NP-UAEC (Figure 5B). In addition, PP2 blocked basal Tyr-265 phosphorylation in both P-UAEC and NP-UAEC. The MEK/ERK inhibitor, U0126, had no effect on PMA-, VEGF-165-, or ATP-stimulated Tyr-265 phosphorylation in either P-UAEC or NP-UAEC (Figure 4B, Tables S3 and S4).
3.3.3 Phosphorylation of Cx43 at Ser-368
The phosphorylation of Ser-368 in UAEC in response to PMA, VEGF-165, and ATP is shown in Figures 4C and 5C (details in Tables S3 and S4). Two bands, at approximately 42 and 46 kDa, are found with this antibody. The lower band responds robustly to PMA treatment in comparison to ATP and VEGF, but this band is not eliminated with alkaline phosphatase treatment (Bird et al. 2013). In both NP- and P-UAEC, phosphorylation of the 46-kDa band was stimulated by PMA. U0126 significantly reversed PMA stimulation of the 46-kDa band only in P-UAEC. Phosphorylation of the 42-kDa band was also increased in P-UAEC and NP-UAEC when treated with PMA. ATP and VEGF also stimulated phosphorylation of this 42-kDa band in NP- and P-UAEC. Pretreatment with U0126 had no significant effect on the phosphorylation of the 42-kDa band of Ser-368 by any agonist in either cell type. Pretreatment with PP2 alone was successful in reversing the VEGF-165 stimulation of the 42-kDa band in NP-UAEC.
3.3.4 Phosphorylation of Cx43 at Ser-262
As seen in Tables S3 and S4, the phosphorylation of Ser-262 (32 kDa band, which is eliminated with alkaline phosphatase treatment), was stimulated by PMA in NP-UAEC and by PMA and VEGF-165 in P-UAEC, while pretreatment with ATP had no effect on Ser-262 phosphorylation in either cell type. The MEK/ERK inhibitor U0126 reversed Ser-262 phosphorylation by both PMA and VEGF-165. U0126 alone mildly inhibited Ser-262 phosphorylation in the NP-UAEC control.
3.4 Evidence for Crosstalk between Src and E RK signaling pathways
Previous unpublished studies from our laboratory on UAEC have suggested that a component of ERK signaling could be downstream of Src activation. We therefore ran western analysis for ERK phosphorylation after treatment with PMA, VEGF-165, and ATP for 30 min in the presence or absence of the Src family kinase inhibitor PP2. In P-UAEC, PMA, VEGF-165, and ATP all stimulated significant levels of ERK phosphorylation, with PMA being a strong stimulant and VEGF-165 and ATP being modest in comparison (Figure 6). However, when PP2 was applied as a pretreatment, PMA stimulation of ERK phosphorylation was unaffected while VEGF-165 and ATP stimulation of ERK phosphorylation was significantly reduced. Of note, VEGF stimulation of ERK phosphorylation was only partially reduced by PP2, while ATP stimulation was fully reversed to the level of PP2 alone. In NP-UAEC, only PMA and VEGF-165 stimulated significant ERK phosphorylation at 30 min. As in P-UAEC, the PMA stimulation of ERK phosphorylation in NP-UAEC was unaffected by PP2 pretreatment. VEGF-165 stimulation of ERK phosphorylation in NP-UAEC was significantly reversed by PP2 pretreatment, and although the mean response did not return fully to baseline, it was not significantly different from control.
3.5 VEGF-165 inhibition of ATP-stimulated Ca2+ bursts is ‘rescued’ by blocking MEK/ERK and Src signaling pathways
In order to confirm cause and effect between Cx43 phosphorylation and inhibition of Ca2+ bursting in P-UAEC, we attempted to ‘rescue’ pregnancy-adapted function in P-UAEC from inhibition by VEGF pretreatment. Given the ability of U0126 (10 uM) and PP2 (10 uM) to block Cx43 phosphorylation at Ser-279/282 and Tyr-265, we applied these doses of each inhibitor to Fura-2 loaded cells that were treated with an initial 100uM ATP for 30 min, then subsequently treated with 10 ng/ml VEGF for 30 min followed by a second 100 uM ATP for an additional 30 min. In each case, pregnancy-adapted ATP-stimulated Ca2+ burst responses were restored (Figure 7A). The MEK/ERK inhibitor, U0126 rescued ATP-stimulated Ca2+ bursts back to a level consistent with a repeat stimulation with ATP alone (10.2%), while the Src family kinase inhibitor, PP2, was even more efficient at rescuing ATP-stimulated Ca2+ bursts, returning mean burst numbers back to initial ATP pre-stimulation levels (+0.03%). Additionally, U0126 alone significantly improved ATP-stimulated Ca2+ burst responses on repeat stimulation.
In order to test the potential viability of Src inhibition as a therapeutic strategy for restoring sustained Ca2+ signaling in endothelial cells in hypertensive pregnancy, we then applied a 10,12 CLA dose response as a pretreatment in an attempt to rescue Ca2+ bursts from VEGF-165 mediated inhibition of ATP-stimulated Ca2+ responses (Figure 7B). In P-UAEC, 10,12 CLA pretreatment doses as low as 0.5 uM restored Ca2+ burst responses to ATP in the presence of VEGF-165 to ATP control levels (~90%). Doses of 5uM and 50 uM 10,12 CLA result in significant improvement over ATP control, and the regression fit suggests anything over 5 uM 10,12 CLA will fully rescue VEGF-165-inhibited ATP-stimulated Ca2+ bursts. The c9,t11 isomer of CLA did not inhibit Src (Shahzad 2013) and likewise had no ability to restore Ca2+ bursting in response to ATP in the presence of VEGF-165, even at 50 uM.
4. DISCUSSION
ATP is one of many GPCR-linked agonists that control endothelial vasodilation, but there are also other classes of agonists that impact on endothelial cells, including most notably the growth factor VEGF. VEGF is capable of activating PLC-gamma in UAEC and so when UA Endo are treated with VEGF, it is no surprise a detectable Ca2+ and NO response are both observed (Boeldt, et al. 2014; Yi et al. 2011). Nonetheless, when UA Endo are treated with VEGF in this way, the subsequent ATP-stimulated Ca2+ and NO response is reduced to a level approaching that in NP-UAEC, which is far below that made up for by the modest VEGF-stimulated Ca2+ and NO response (Yi et al. 2011). Thus, VEGF appears to have a paradoxical effect on uterine artery endothelium, as it both immediately promotes vasodilation in its own right but subsequently inhibits far more the production of vasodilators in response to GPCR such as P2Y2 coupled to PLC-beta.
We have recently reported (Boeldt et al. 2014) that the signaling mechanisms regulating the promotion of VEGF-stimulated Ca2+ responses and vasodilator production act through VEGFR-2 kinase-mediated activation of PLC-gamma in a manner involving Src, but also reported in Grummer et al (Grummer et al. 2009) that VEGFR-2 can apparently activate ERK-1/2 independently of VEGFR-2 kinase domain or activation of eNOS. Given the ability of VEGF-165 to inhibit subsequent ATP-stimulated Ca2+ responses and NO output (Yi et al. 2010b; Yi et al. 2011), it may be there are further divergent signaling pathways responsible for these negative effects of VEGF on GPCR-stimulated vasodilator production. Certainly these inhibitory effects may be relevant given VEGF-165 is a physiologic agonist elevated in pregnancy and even more so in preeclamptic pregnancies (Chung, et al. 2004; Sitras, et al. 2009). In this study we follow up on the UA Endo studies of Yi et al (Yi et al. 2011) that show VEGF pretreatment inhibits subsequent ATP Ca2+ responses by extending them to UAEC. Using primary UAEC in culture also allows us to use pharmacological tools to go beyond the simple question of whether pregnancy-enhanced Ca2+ burst responses to ATP is inhibited by VEGF pretreatment, and to now ask how this occurs. Figure 8 summarizes our proposed mechanism for the effects of VEGF on Cx43 inhibitory phosphorylations which mediate inhibition of subsequent ATP-stimulated Ca2+ bursts. We also show the likely actions of potential therapeutic targets that rescue P-UAEC from the adverse VEGF effects. The following paragraphs further detail the evidence with which the proposed model was constructed.
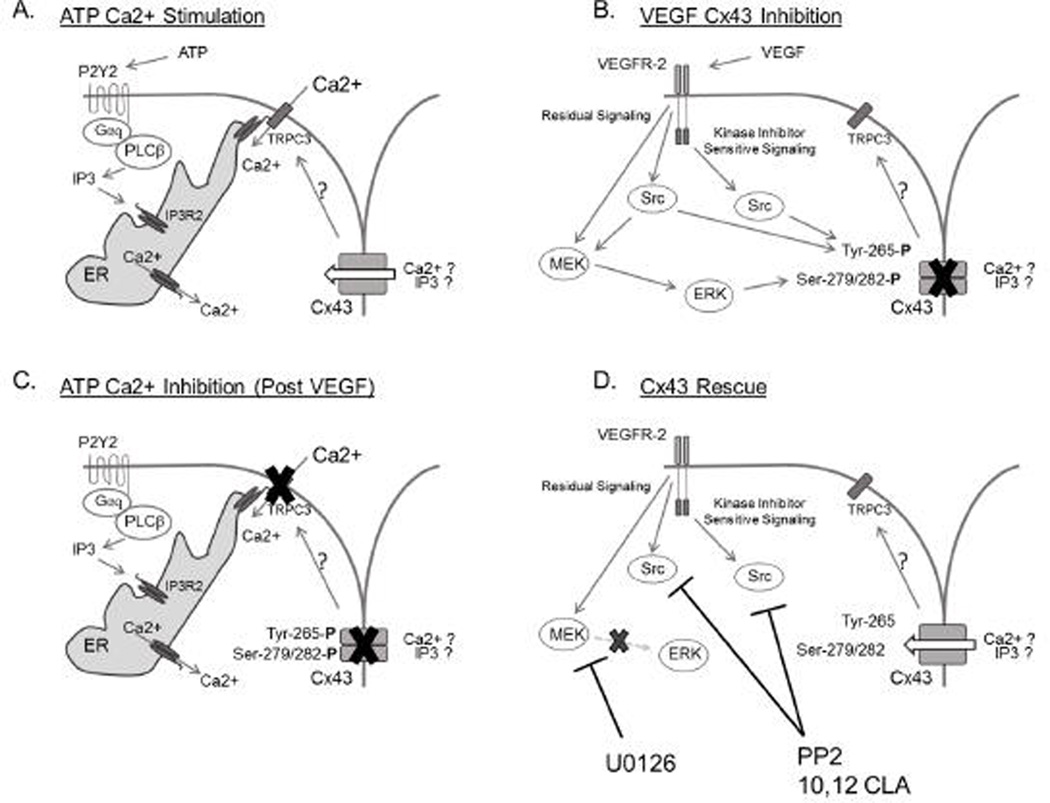
Figure 8. Proposed mechanism for ATP stimulation, VEGF inhibition, and subsequent recovery of sustained Ca2+ responses in P-UAEC.
Panel A depicts a typical ATP-stimulated Ca2+ response, as described previously (Boeldt, et al. 2011; Yi, et al. 2010a). Most notably, the initial Ca2+ response consists of IP3-regulated endoplasmic reticulum Ca2+ release into the cytosol. When the endoplasmic reticulum Ca2+ levels are sufficiently low, IP3R2 interacts with TRPC3 at the cell surface, opening it and allowing extracellular Ca2+ influx. This process is potentiated by Cx43 function through an as yet unknown mechanism, though likely candidates include IP3 or Ca3+ gating in close proximity to TRPC3. Panel B is now derived from previous studies (Grummer et al. 2009) and those described here concerning VEGF-stimulated signaling responsible for acute inhibitory phosphorylation of Cx43 at Ser-279/282 and Tyr-265. Of note, Src family kinase signaling originates from both classic kinase domain signaling and ‘residual’ VEGFR-2 signaling. Our data in particular suggests MEK/ERK signaling is derived exclusively from ‘residual’ VEGFR-2 signaling, through direct signaling or through secondary events downstream of a Src kinase. Panel C shows the consequence of VEGF-stimulated Cx43 phosphorylation on subsequent ATP-stimulated Ca2+ responses. The phosphorylation of Cx43 at Ser-279/282 and Tyr-265 render Cx43 non-functional and thus the transmission of signals necessary for sustained phase Ca2+ responses are lost. The result is a normal initial Ca2+ response to ATP stimulation through release from the ER, but a significant reduction in the sustained-phase Ca2+ bursts that follow. In panel D, mechanisms of action for pharmacological inhibitors of MEK/ERK and Src family kinases are shown. Blockage of MEK/ERK and Src kinases prevents inhibitory phosphorylation of Cx43 at Ser-279/282 and Tyr-265 and so prevents the inhibitory activity of VEGF pretreatment and restores sustained-phase Ca2+ bursts in response to ATP.
Specific to our first stated hypothesis that VEGF-165 pretreatment inhibits subsequent ATP-stimulated Ca2+ bursts in P-UAEC, when P-UAEC were pretreated with VEGF-165, there was also a decrease in frequency of Ca2+ bursts upon subsequent ATP treatment. In NP-UAEC, there was no further significant decrease in ATP-stimulated burst numbers after VEGF-165 pretreatment. The net result was that after a simple 30-min pre-exposure of P-UAEC to VEGF-165, there was a complete loss of the pregnancy-adapted Ca2+ burst response to ATP stimulation, just as in UA Endo (Yi et al. 2011). When Yi et al (Yi et al. 2011) pretreated intact UA Endo with VEGF-165, they reported subsequent inhibition of ATP-stimulated Ca2+ bursts in vessels derived from pregnant sheep, but this was not so apparent in vessels from luteal nonpregnant sheep that already showed lower levels of function. Thus, the results of this study in primary cell culture are in agreement with equivalent work on intact vessels.
We also have identified which VEGF receptor subtype mediates inhibition of the ATP-stimulated Ca2+ response. Our findings that i) VEGFR-1 and VEGFR-2 agonist VEGF-165 inhibited ATP-stimulated Ca2+ bursts by ~20%, and this was reproduced with the VEGFR-2-specific agonist VEGF-E; ii) the VEGFR-1-specific agonist PlGF had no effect; and iii) the VEGF-E and PlGF combination had no further effect over that of VEGF-E alone all suggest the VEGF-165 inhibition of ATP-stimulated Ca2+ bursts must be mediated through VEGFR-2. From a therapeutic standpoint this is unfortunate, as the stimulatory activation of PLC-gamma by VEGF and the activation of eNOS are both through the same VEGFR2 (Boeldt et al. 2014). However, in contrast to acute Ca2+ signaling and eNOS activation by VEGF (Boeldt et al. 2014), this VEGF inhibition of subsequent ATP-stimulated Ca2+ bursts was not sensitive to the VEGFR-2 kinase inhibitor, raising the possibility that the signal may propagate from a region of the receptor that is not the endogenous kinase domain, and thus may still be a unique target for therapy. Alternatively, the VEGFR-2 kinase inhibitor is not fully effective at blocking all signal emanating from the endogenous kinase domain. The VEGFR-2 kinase inhibitor insensitive activity will therefore be referred to as ‘residual’ VEGFR-2 signaling below. The former suggestion is possible in P-UAEC, given Grummer et al (Grummer et al. 2009) have previously reported that ERK phosphorylation is also insensitive to the VEGFR-2 kinase inhibitor. It is also possible ERK signaling may be a critical mediator of VEGF-165 inhibition of ATP-stimulated Ca2+ bursts, since ATP has previously been shown to be a strong stimulant of ERK phosphorylation (Bird et al. 2000; Di, et al. 2001), and ATP pretreatment alone also slightly inhibited its own subsequent response by ~10%. Further examination of the relationship between Cx43 phosphorylation and inhibition, and the failure of the MEK inhibitor to be as effective a rescue agent as PP2 suggest this is not the major or at least the sole inhibitory mechanism (see below) and will require further study.
Because both PMA and VEGF-165 stimulate various kinase signaling pathways that can result in different degrees of inhibitory phosphorylations on Cx43 (ERK (at Ser-279/282), Src (at Tyr-265), and PKC (at Ser-368 and Ser-262)), a comparison of the effects of PMA with VEGF and the less inhibitory pretreatment with ATP allowed us to confirm our second stated hypothesis. We now show that VEGF-165-stimulated phosphorylation of Cx43 at key residues via activation of MEK/ERK and Src signals does indeed correlate most closely with inhibition of the subsequent ATP-stimulated Ca2+ bursts in P-UAEC. Not only did we test this by observing phosphorylation events, but also confirmed cause-and-effect using kinase-targeted inhibitors as rescue agents for subsequent Ca2+ burst responses to ATP. These data are in agreement with studies in both P-UAEC and other cells (Lampe and Lau 2000) which suggest multiple kinases target Cx43 amino acid residues, as well as with our previous studies which showed inhibition of pregnancy-adapted ATP-stimulated Ca2+ bursts in P-UAEC occur with GAP27 and PMA treatment (Bird et al. 2013; Morschauser et al. 2014; Yi et al. 2010b).
Although the PKC sites were thoroughly investigated using phospho-specific antibodies, we did not pursue selective PKC inhibition to reverse these phosphorylations or to rescue subsequent ATP-stimulated Ca2+ bursts (more on Ca2+ burst rescue below). This is because UAEC have been shown to express many PKC isoforms, and isoform-specific inhibitors have proven unreliable in this particular cell type. In addition, it is unlikely the PKC-targeted phosphorylation sites were strongly phosphorylated by the classical PKCalpha pathway because VEGF-165 acting through PLC-gamma is not a particularly strong stimulant of IP3-dependent Ca2+ responses compared to ATP; and yet ATP pretreatment is not as strong an inhibitor as VEGF-165 on any subsequent ATP response. We therefore restricted our pharmacologic inhibitor list to U0126 (MEK/ERK inhibitor) and PP2 (Src family kinase inhibitor) for this particular study, and both inhibitors appear pathway-specific in our hands. We took PKC involvement to be critical only if we observed the positive phosphorylation of Ser-262 or Ser-368 of Cx43 and also a failure to rescue subsequent bursting in response to ATP by U0126 or PP2. This condition of failure to rescue was never achieved using VEGF-165 as a pretreatment, indirectly suggesting PKC is not a major mediator of physiologic agonist-dependent Cx43 inhibition. In contrast to the physiologic agonists, pharmacologic inhibition with 10 nM PMA (a known PKC activator) did indeed result in phosphorylations at these two sites and also failed to show complete rescue in response to U0126 or PP2. Thus in UAEC the standard PKC stimulant PMA clearly mediates, at least in part, inhibition of Cx43 via PKC, exactly as we expected, but physiologic inhibition by VEGF is not predominantly dependent on this pathway.
After a 30-min treatment with VEGF-165, we detected significant phosphorylation of four Cx43 phosphorylation sites. Phosphorylation of Ser-279/282 proved to be a ‘residual’ VEGFR-2 signaling target mediated by ERK, as confirmed by its sensitivity to U0126 but not PP2. Phosphorylation of Tyr-265 was at least partially dependent on classic VEGFR-2 kinase inhibitor sensitive signaling and likely mediated via Src, as confirmed by its sensitivity to PP2 but not U0126. Phosphorylation of Ser-386 was classically VEGR-2 kinase inhibitor sensitive and not mediated via ERK or Src signaling, as indicated by resistance to inhibition by U0126 or PP2. Phosphorylation of Ser-262 resulted from ‘residual’ VEGFR-2 signaling and was ERK mediated, as indicated by some effect of U0126 but not PP2. Nonetheless, while these data suggests Cx43 phosphorylation is indeed observed in a manner consistent with the combined actions of ERK, Src and PKC, the additional finding that U0126 and PP2 are also highly effective at achieving such substantial rescue of Ca2+ bursting function from VEGF pretreatment suggests the ERK and Src pathways to be more physiologically relevant in the VEGF inhibition of Cx43 function than PKC.
The Cx43 phosphorylation data set presented is consistent with our stated third hypothesis that a causal relationship between these pathways results in a loss of burst function, as is the additional observation that pretreatment with MEK/ERK or Src inhibitors successfully ‘rescues’ normal levels of ATP-stimulated Ca2+ bursts in VEGF-165 pretreated P-UAEC. Given this is the case, then it may be that targeting these Cx43 inhibitory kinases could provide a basis for therapy of diseases such as preeclampsia, which are characterized by reduced endothelial NO output and are associated with increased circulating VEGF and other agonists known to stimulate the ERK and Src pathways (Bird et al. 2013). Given that treatment of P-UAEC with the MEK/ERK inhibitor U0126 before addition of the VEGF-165 pretreatment and subsequent ATP treatment restored ATP-stimulated Ca2+ bursts back to ATP control levels (~90% of initial ATP levels), and that treatment of P-UAEC with the Src family kinase inhibitor PP2 before addition of the VEGF-165 pretreatment and subsequent ATP treatment restored ATP-stimulated Ca2+ burst levels beyond that of ATP control levels to initial ATP pretreatment levels (100%), then both kinase inhibitors are viable candidates for fully reversing the VEGF-165 inhibition of subsequent ATP-stimulated Ca2+ responses. That either U0126 or PP2 are successful further suggests (i) both Ser-279/282 and Tyr-265 phosphorylation events may need to occur and phosphorylation of a single residue is not sufficient alone to fully explain the observed inhibition of bursting via VEGFR-2; or (ii) that both MEK/ERK-1/2 and Src are in a linear pathway and inhibitory ‘residual’ VEGFR-2 signaling via ERK-1/2-mediated Ser-279/282 phosphorylation is important and may have an impact on targeting of future therapeutic compounds to restore pregnancy-adapted function in subjects with preeclampsia. Although the partial inhibition of VEGF-stimulated ERK-1/2 activation by PP2 in P-UAEC suggests a Src family kinase member in part mediates VEGF activation of the MEK/ERK-1/2 pathway, further consideration of the greater function of P-UAEC after PP2 treatment suggests the involvement of both Ser-279/282 and the Tyr-265 sites in contributing to functional inhibition of Cx43 by VEGFR-2. PP2 is also more efficient at restoring ATP-stimulated Ca2+ bursts than U0126 after ATP pretreatment, which in turn suggests ATP could also be achieving modest inhibition of its own Ca2+ burst response through inhibitory Src family kinase-mediated phosphorylation of Tyr-265; and such a response to ATP pretreatment is observed to a limited extent. Consistent with this, Tyr-265 phosphorylation was only reversed by PP2 and there was no ATP-stimulated phosphorylation of the ERK mediated sites, Ser-279/282 or s262. We conclude then that PP2 is most likely rescuing Ca2+ bursting otherwise inhibited by VEGF-165 or indeed ATP pretreatment by reversing the corresponding Tyr-265 phosphorylation in each case. The fact ATP- or VEGF-stimulated phosphorylation of Tyr-265 still occurred in the presence of U0126 treatment explains why rescue by U0126 alone is less effective, and thus resulted in a slight residual inhibition of subsequent ATP Ca2+ burst responses.
Finally, the 10,12 isomer of CLA is a natural byproduct of fermentation of grass in the ruminant gut and so is often found in pasture-fed ‘organic’ dairy products (Dhiman et al. 1999). It has also been shown to be a c-Src inhibitor in a cancer cell line (Shahzad 2013). In this cancer cell line, full inhibition of c-Src is achieved at 7 uM 10,12 CLA (Shahzad 2013). According to one study, background levels of circulating total CLA in the plasma are around 5.5 uM (Sato, et al. 2011) but 10,12 CLA is undetectable. After three weeks of being fed a diet rich in 10,12 CLA, plasma concentrations of total CLA reaching 55uM and 10,12 CLA levels reaching 20uM are achievable (Sato et al. 2011). We therefore used 10, 12 CLA as a potential Ca2+ burst rescue agent in VEGF-165 pretreated P-UAEC, acting as a nutraceutical alternative to PP2. The complete rescue, ranging from return to ATP control (at 0.5 uM) to significant improvement over control (5uM and above), suggests 10, 12 CLA may be a viable therapeutic for preeclampsia or possibly for other pregnancy-related hypertensive disorders of endothelial origin. An alternate CLA isomer (c9,t11) was used as a isomeric control, as it has no described Src inhibiting properties (Shahzad 2013), and the lack of response confirms the isomer specificity of 10, 12 CLA as a rescue agent. This is a novel observation as CLA preparations are widely used and at least at limited dose are generally regarded as safe for use in humans by the United States Food and Drug Administration (2007). Furthermore, a therapeutic dose of 10,12 CLA is easily achievable by changes in diet alone make this a practical therapeutic strategy. Clearly further studies will be needed to establish safety in human pregnancy, but one study has already shown promising results using CLA in conjunction with Ca2+ supplementation to reduce pregnancy induced hypertension (Herrera, et al. 2005). Nonetheless this study establishes an important ‘proof of principle’ that 10,12 CLA can rescue endothelial cell function from growth factor inhibition, and when combined with the demonstration of a method of quantitative screening of potential therapeutics (rescue effects on sustained Ca2+ bursts), we feel our collective findings represent a highly significant first step to future therapy.
Supplementary Material
Highlights.
VEGF-165 promotes inhibitory phosphorylations at s279/282 and y265 on Cx43 in UAEC
VEGF-165 pretreatment blocks pregnancy-enhanced Ca2+ burst responses in UAEC
Src and ERK inhibitors reverses Cx43 phosphorylations and restores Ca2+ bursts
The nutriceutical Src inhibitor, 10,12 CLA rescues Ca2+ bursts in UAEC
10,12 CLA may have therapeutic potential for preeclampsia
ACKNOWLEDGEMENTS
We would like to thank Terrance Phernetton for assistance in animal preparation. We would also like to thank Dr. Cook and Dr. Patankar for additional discussion on the use of CLA.
FUNDING
This work was funded by the National Institutes of Health [grant awards HL079020, HD38843 and HD069181]. This paper reports partial fulfillment of the requirements for DSB towards a Ph.D. in the University of Wisconsin Endocrinology and Reproductive Physiology Training Program. DSB was also supported by NIH T32 predoctoral training award [T32HD41921] and the University of Wisconsin School of Medicine and Public Health Shapiro Award.
Abbreviations used
- P
pregnant
- NP
non-pregnant
- UAEC
uterine artery endothelial cells
- UA-Endo
uterine artery endothelium on intact vessel segment
- Cx43
connexin 43
- NO
nitric oxide
- eNOS
endothelial nitric oxide synthase
- [Ca2+]i
intracellular free Ca2+ concentration
- TRPC3
transient receptor potential channel 3
- IP3R2
inositol 1,4,5-trisphosphate receptor 2
- CCE
capacitative Ca2+ entry
- PKC
protein kinase C
- ERK
extracellular-signal-regulated kinase
- VEGF
vascular endothelial growth factor
- CLA
conjugated linoleic acid
- PlGF
placental growth factor
- VEGFRi
VEGF receptor 2 kinase inhibitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- GRAS Notification for Conjugated Linoleic Acid (CLA)-Rich Oil for Use in Certain Foods. 2007 [Google Scholar]
- Bird IM, Boeldt DS, Krupp J, Grummer MA, Yi FX, Magness RR. Pregnancy, programming and preeclampsia: gap junctions at the nexus of pregnancy-induced adaptation of endothelial function and endothelial adaptive failure in PE. Curr Vasc Pharmacol. 2013;11:712–729. doi: 10.2174/1570161111311050009. [DOI] [PubMed] [Google Scholar]
- Bird IM, Sullivan JA, Di T, Cale JM, Zhang L, Zheng J, Magness RR. Pregnancy-dependent changes in cell signaling underlie changes in differential control of vasodilator production in uterine artery endothelial cells. Endocrinology. 2000;141:1107. doi: 10.1210/endo.141.3.7367. [DOI] [PubMed] [Google Scholar]
- Bird IM, Zhang L, Magness RR. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. American journal of physiology. Regulatory, integrative and comparative physiology. 2003;284:R245. doi: 10.1152/ajpregu.00108.2002. [DOI] [PubMed] [Google Scholar]
- Boeldt DS, Grummer MA, Magness RR, Bird IM. Altered VEGF-stimulated Ca2+ signaling in part underlies pregnancy-adapted eNOS activity in UAEC. J Endocrinol. 2014;223:1–11. doi: 10.1530/JOE-14-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeldt DS, Yi FX, Bird IM. eNOS activation and NO function: pregnancy adaptive programming of capacitative entry responses alters nitric oxide (NO) output in vascular endothelium--new insights into eNOS regulation through adaptive cell signaling. The Journal of endocrinology. 2011;210:243. doi: 10.1530/JOE-11-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cale JM, Bird IM. Dissociation of endothelial nitric oxide synthase pho sphorylation and activity in uterine artery endothelial cells. 2006;290:H1433. doi: 10.1152/ajpheart.00942.2005. [DOI] [PubMed] [Google Scholar]
- Chung JY, Song Y, Wang Y, Magness RR, Zheng J. Differential expression of vascular endothelial growth factor (VEGF), endocrine gland derived-VEGF, and VEGF receptors in human placentas from normal and preeclamptic pregnancies. The Journal of clinical endocrinology and metabolism. 2004;89:2484. doi: 10.1210/jc.2003-031580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman TR, Anand GR, Satter LD, Pariza MW. Conjugated linoleic acid content of milk from cows fed different diets. J Dairy Sci. 1999;82:2146–2156. doi: 10.3168/jds.S0022-0302(99)75458-5. [DOI] [PubMed] [Google Scholar]
- Di T, Sullivan JA, Magness RR, Zhang L, Bird IM. Pregnancy-specific enhancement of agonist-stimulated ERK-1/2 signaling in uterine artery endothelial cells increases Ca(2+) sensitivity of endothelial nitric oxide synthase as well as cytosolic phospholipase A(2) Endocrinology. 2001;142:3014. doi: 10.1210/endo.142.7.8278. [DOI] [PubMed] [Google Scholar]
- Gifford SM, Yi FX, Bird IM. Pregnancy-enhanced store-operated Ca2+ channel function in uterine artery endothelial cells is associated with enhanced agonist-specific transient receptor potential channel 3-inositol 1,4,5-trisphosphate receptor 2 interaction. The Journal of endocrinology. 2006;190:385. doi: 10.1677/joe.1.06773. [DOI] [PubMed] [Google Scholar]
- Grummer MA, Sullivan JA, Magness RR, Bird IM. Vascular endothelial growth factor acts through novel, pregnancy-enhanced receptor signalling pathways to stimulate endothelial nitric oxide synthase activity in uterine artery endothelial cells. The Biochemical journal. 2009;417:501. doi: 10.1042/BJ20081013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera JA, Shahabuddin AK, Ersheng G, Wei Y, Garcia RG, Lopez-Jaramillo P. Calcium plus linoleic acid therapy for pregnancy-induced hypertension. Int J Gynaecol Obstet. 2005;91:221–227. doi: 10.1016/j.ijgo.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Kanemitsu MY, Loo LW, Simon S, Lau AF, Eckhart W. Tyrosine phosphorylation of connexin 43 by v-Src is mediated by SH2 and SH3 domain interactions. The Journal of biological chemistry. 1997;272:22824. doi: 10.1074/jbc.272.36.22824. [DOI] [PubMed] [Google Scholar]
- Lampe PD. Analyzing phorbol ester effects on gap junctional communication: a dramatic inhibition of assembly. The Journal of cell biology. 1994;127:1895. doi: 10.1083/jcb.127.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Archives of Biochemistry and Biophysics. 2000;384:205. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- Lin R, Warn-Cramer BJ, Kurata WE, Lau AF. v-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. The Journal of cell biology. 2001;154:815. doi: 10.1083/jcb.200102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morschauser TJ, Ramadoss J, Koch JM, Yi FX, Lopez GE, Bird IM, Magness RR. Local effects of pregnancy on connexin proteins that mediate Ca2+-associated uterine endothelial NO synthesis. Hypertension. 2014;63:589–594. doi: 10.1161/HYPERTENSIONAHA.113.01171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Shinohara N, Honma T, Ito J, Arai T, Nosaka N, Aoyama T, Tsuduki T, Ikeda I. The change in conjugated linoleic acid concentration in blood of Japanese fed a conjugated linoleic acid diet. J Nutr Sci Vitaminol (Tokyo) 2011;57:364–371. doi: 10.3177/jnsv.57.364. [DOI] [PubMed] [Google Scholar]
- Shahzad MMK. Chronic Neurobehavioral Stress Promotes Resistance to Chemotherapy in Epithelial Ovarian Carcinoma. 2013 [Google Scholar]
- Sirnes S, Leithe E, Rivedal E. The detergent resistance of Connexin43 is lost upon TPA or EGF treatment and is an early step in gap junction endocytosis. Biochemical and biophysical research communications. 2008;373:597. doi: 10.1016/j.bbrc.2008.06.095. [DOI] [PubMed] [Google Scholar]
- Sitras V, Paulssen RH, Gronaas H, Leirvik J, Hanssen TA, Vartun A, Acharya G. Differential placental gene expression in severe preeclampsia. Placenta. 2009;30:424. doi: 10.1016/j.placenta.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. The American Journal of Physiology. 1997;272:R441. doi: 10.1152/ajpregu.1997.272.2.R441. [DOI] [PubMed] [Google Scholar]
- Sullivan JA, Grummer MA, Yi FX, Bird IM. Pregnancy-enhanced endothelial nitric oxide synthase (eNOS) activation in uterine artery endothelial cells shows altered sensitivity to Ca2+, U0126, and wortmannin but not LY294002--evidence that pregnancy adaptation of eNOS activation occurs at multiple levels of cell signaling. Endocrinology. 2006;147:2442. doi: 10.1210/en.2005-0399. [DOI] [PubMed] [Google Scholar]
- van der Zandt PT, de Feijter AW, Homan EC, Spaaij C, de Haan LH, van Aelst AC, Jongen WM. Effects of cigarette smoke condensate and 12-O-tetradecanoylphorbol-13-acetate on gap junction structure and function in cultured cells. Carcinogenesis. 1990;11:883. doi: 10.1093/carcin/11.6.883. [DOI] [PubMed] [Google Scholar]
- Warn-Cramer BJ, Lampe PD, Kurata WE, Kanemitsu MY, Loo LW, Eckhart W, Lau AF. Characterization of the mitogen-activated protein kinase phosphorylation sites on the connexin-43 gap junction protein. The Journal of biological chemistry. 1996;271:3779. doi: 10.1074/jbc.271.7.3779. [DOI] [PubMed] [Google Scholar]
- Yao J, Morioka T, Oite T. PDGF regulates gap junction communication and connexin43 phosphorylation by PI 3-kinase in mesangial cells. Kidney international. 2000;57:1915. doi: 10.1046/j.1523-1755.2000.00041.x. [DOI] [PubMed] [Google Scholar]
- Yi FX, Boeldt DS, Bird IM. Pregnancy Induced Reprogramming of Endothelial Function in Response to ATP: Evidence for Post Receptor Ca2+ Signaling Plasticity. In: Gerasimovskaya E, Kaczmarek E, editors. Extracellular ATP and Adensosine as the Regulators of Endothelial Cell Function. Springer Publications; 2010a. p. 197. [Google Scholar]
- Yi FX, Boeldt DS, Gifford SM, Sullivan JA, Grummer MA, Magness RR, Bird IM. Pregnancy enhances sustained Ca2+ bursts and endothelial nitric oxide synthase activation in ovine uterine artery endothelial cells through increased connexin 43 function. Biology of reproduction. 2010b;82:66. doi: 10.1095/biolreprod.109.078253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi FX, Boeldt DS, Magness RR, Bird IM. Ca2+ i signaling vs. eNOS expression as determinants of NO output in uterine artery endothelium: relative roles in pregnancy adaptation and reversal by VEGF165. American journal of physiology. Heart and circulatory physiology. 2011;300:H1182. doi: 10.1152/ajpheart.01108.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.