Abstract
Fanconi anemia is a genetic bone marrow failure syndrome. Current treatment options are suboptimal and do not prevent the eventual onset of aplastic anemia requiring bone marrow transplantation. We previously showed that resveratrol, an antioxidant and an activator of the protein deacetylase Sirt1, enhanced hematopoiesis in Fancd2 mutant mice and improved the impaired stem cell quiescence observed in this disease. Given that Sirt1 is important for the function of hematopoietic stem cells, we hypothesized that Sirt1 activation may improve hematopoiesis. Indeed, Fancd2−/− mice and wild-type mice treated with the selective Sirt1 activator SRT3025 had increased numbers of hematopoietic stem and progenitor cells, platelets and white blood cells. SRT3025 was also protective against acetaldehyde-induced hematopoietic damage. Unlike resveratrol, however, SRT3025 did not affect stem cell quiescence, suggesting distinct mechanisms of action. Conditional deletion of Sirt1 in hematopoietic cells did not abrogate the beneficial effects of SRT3025, indicating that the drug did not act by directly stimulating Sirt1 in stem cells, but must be acting indirectly via extra-hematopoietic effects. RNASeq transcriptome analysis revealed the down-regulation of Egr1-p21 expression, providing a potential mechanism for improved hematopoiesis. Overall, our data indicate that SRT3025 or related compounds may be beneficial in Fanconi anemia and other bone marrow failure syndromes.
Keywords: Bone Marrow Failure, Fanconi Anemia, Hematopoietic Stem Cell, Sirt1, Hematopoiesis, Egr1, p21
1. Introduction
Fanconi anemia (FA) is a genetic disorder characterized by bone marrow failure, myelodysplastic syndrome, and acute myeloid leukemia. The primary cause of mortality and morbidity is aplastic anemia (Kutler et al., 2003). Treatment with anabolic androgens such as oxymetholone is currently the only small molecule therapy with known benefit, but does not completely correct the disease (Shimamura and Alter, 2010). Hematopoietic stem cell (HSC) transplantation is the only curative therapy available to FA patients with marrow aplasia (Shimamura and Alter, 2010).
FA patients have fewer CD34+ cells in their bone marrow and suffer from additional progressive loss of CD34+ cells as they get older (Ceccaldi et al., 2011; Kelly et al., 2007). We and others have found that Fancd2−/− mice recapitulate important hematopoietic defects characteristic of FA, including fewer hematopoietic stem and progenitor cells (HSPC) in the bone marrow, reduced long-term HSC repopulating capacity, and low platelet counts in peripheral blood (Parmar et al., 2010; Zhang et al., 2010). We therefore have used this FA murine model to test candidate compounds for therapeutic efficacy on hematopoiesis and cancer prevention (Zhang et al., 2008, 2010, 2014). We found that the red wine ingredient resveratrol helped to maintain Fancd2 mutant c-Kit+Sca-1+Lin− (KSL) cells in quiescence (Zhang et al., 2010).
Although resveratrol was initially thought to act primarily by activating the protein deacetylase Sirt1 (Lagouge et al., 2006), additional modes of action have been described more recently (Pangeni et al., 2014; Park et al., 2012). Sirt1 has been shown to be important in the function of hematopoietic stem cells by several groups (Rimmele et al., 2014; Singh et al., 2013). Sirt1 deletion in the blood lineages causes increased DNA damage and accelerated aging of stem cells. We therefore reasoned that pharmacological activation of Sirt1 beyond ground state levels might be beneficial in FA. Small molecules capable of stimulating the enzymatic activities of Sirt1 and other sirtuins have been developed and show beneficial effects in multiple animal models (Sinclair and Guarente, 2014). In particular, they show promise in tumor prevention and in the treatment of metabolic syndrome (Hubbard and Sinclair, 2014; Kabra et al., 2009; Minor et al., 2011; Miranda et al., 2014).
In the current study, we tested whether the potent Sirt1 activator SRT3025 (Miranda et al., 2014), a molecule that is structurally unrelated to resveratrol, could enhance hematopoiesis in Fancd2−/− mice.
2. Materials and Methods
2.1 Mice
Fancd2 or Fancc mutant mice and ROSA26 transgenic mice were maintained on the 129S4 background (Friedrich and Soriano, 1991; Houghtaling et al., 2003; Whitney et al., 1996). Sirt1 transgenic mice with a floxed STOP cassette were crossed with CMV-Cre mice (Jackson Labs, stock #006054) to induce the removal of the STOP cassette (Price et al., 2012). The resulting mouse strain (Sirt1 OE) over-expresses Sirt1 in all tissues. Sirt1 exon 4-floxed mice and Vav1-Cre mice were purchased from Jackson Labs (stocks #008041 and #008610) and crossed to generate blood-specific Sirt1 knockout mice. Both Sirt1 over-expressing mice and blood-specific knockout mice were maintained on a pure C57BL/6J background. The SRT3025 diet was made by mixing SRT3025 (provided by Sirtris, a GSK company, Cambridge, MA, USA) with common rodent diet at 3.18g/kg diet (Research Diets Inc., New Brunswick, NJ, USA). Oxymetholone was purchased from Sigma (Saint Louis, MO, USA) and mixed with standard rodent chow at 300 mg/kg diet (Bio-Serv, NJ, USA). Each diet was administered upon weaning (3 - 4 weeks of age) and the treatment continued for 6 months unless specified otherwise. All animals were treated in accordance with the guidelines of the Institutional Animal Care and Use Committee.
2.2 Flow cytometry
Bone marrow cells were isolated from the femora and tibiae of the mice and stained as described previously (Zhang et al., 2010). The KSL antibody cocktail contains anti-mouse c-Kit, Sca-1, and lineage markers (CD3e, CD4, CD5, CD8a, B220, Ter119, NK1.1, Mac1, Gr1).
For analysis of CD34−KSL cells, cells were stained with FITC- conjugated anti-mouse CD34 along with the KSL antibody cocktail. For the analysis of CD48−CD150+CD135−CD34−KSL cells, PE-conjugated anti-mouse CD135 and CD34 antibodies were added to the KSL antibody mixture, along with FITC-conjugated anti-mouse CD48 and Brilliant Violet 421-conjugated anti-mouse CD150.
2.3 Serial bone marrow transplantation
Bone marrow cells were isolated from femora and tibiae of the donor mice and mixed with competing ROSA26 bone marrow cells for transplantation. Fancd2−/− cells were mixed with ROSA26 cells at an 8:1 ratio, whereas Fancd2+/+ cells were mixed with ROSA26 at a 1:1 ratio. Four million mixed cells were used for retro-orbital injection of recipient Fancc−/− mice lethally irradiated with 1200 rad of irradiation (delivered as two split doses 4 hours apart). Six months post-transplantation, peripheral blood was collected from each recipient to determine repopulation efficiency. Also, bone marrow cells were isolated from three recipients per group and transplanted into lethally irradiated Fancc−/− mice (4 million cells per secondary recipient). Peripheral blood was collected from each secondary recipient twenty weeks after transplantation to determine the secondary repopulation potential.
DNA was purified from the recipients’ peripheral blood and used for quantitative real-time PCR analysis on a LightCycler from Roche. Each original donor's contribution to peripheral blood was calculated. PCR primers are listed in supplemental Table 1.
2.4 RNA-Seq
RNA was isolated from double sorted KSL cells using Trizol reagent (Invitrogen), followed by RNAeasy Mini Kit (Qiagen). Each sample represented total mRNA isolated from pooled KSL cells of 4 individual mice. Three samples per condition were processed for library construction using TruSeq RNA Sample Preparation Kit (Illumina).
Libraries were sequenced as 51-base-length reads on an Illumina HiSeq 2000 genome analyzer. Bowtie short read aligner software was used to map all reads to the mouse reference genome (version mm9) (Langmead et al., 2009). Data was analyzed using PoissonSeq analysis package (Li et al., 2012).
The RNA-Seq raw data have been deposited into GEO gene expression database at NCBI. The accession number is GSE60941.
2.5 Complete blood count
Blood samples were collected in EDTA-coated capillary tubes and used for complete blood count assay on a Hemavet 950FS Multi-species Hematology System (Drew Scientific Inc., Dallas, TX, USA).
2.6 Colony-forming unit-spleen assay
Recipient (8 - 12 weeks old wild-type) mice were irradiated with a split dose of 1100 rad one day before transplantation. Bone marrow cells from each donor mouse (40 thousand cells for Fancd2−/− donor or 20 thousand for other donor) were injected into each recipient mouse. 12 days after transplantation, spleens were taken and fixed with Bouin's fixative solution.
2.7 Statistical analysis
The two-tailed, unpaired student's t-test was used for statistical analysis. A P value less than 0.05 was considered significant.
3. Results
3.1 Dietary SRT3025 administration enhances hematopoiesis
To determine whether SRT3025 can influence hematopoiesis, cohorts of Fancd2−/− and wild-type mice were given either SRT3025-supplemented rodent chow or placebo for 6 months starting at 1 month of age. The dose of SRT3025 was the same as a previous rodent study reporting beneficial effects on a metabolic syndrome from SRT3025 administration (Miranda et al., 2014). At harvest, we collected blood samples and determined that SRT3025 concentrations in the plasma of the treated animals ranged between 1.5 and 3.6 μM, whereas no SRT3025 was detected in the placebo controls. Fancd2−/− mice at this age have relatively normal hematological parameters of peripheral blood except for lower platelet counts and higher mean corpuscular volume (MCV) (Zhang et al., 2015), the same two phenotypes that are common in FA patients and usually precede the onset of cytopenia in other lineages (Shimamura and Alter, 2010). A complete blood count (CBC) revealed that chronic SRT3025 treatment significantly increased platelet counts and white blood cell counts (P<0.05 in all cases, Figure 1A) and reduced MCV levels (P≤0.01 for both genotypes, Table 1) in both Fancd2−/− and wild-type mice. All other hematologic parameters remained unchanged after SRT3025 treatment for both genotypes (Table 1). To compare the efficacy of SRT3025 with the widely used drug oxymetholone, we then performed a 6-month administration of oxymetholone on a cohort of Fancd2−/− and wild-type mice. The dose of oxymetholone was chosen to be equivalent to 80% of the maximum dose for human FA patients (Shimamura and Alter, 2010). We recently discovered that, after a chronic administration of Oxymetholone at this dose for 17 months, both Fancd2−/− and wild-type mice exhibited clearly improved hematologic parameters, including platelet counts, red blood cell counts, hematocrit, and hemoglobin levels, compared with the placebo-treated controls (Zhang et al., 2015). However, the shorter 6 month treatment was insufficient to improve these hematologic parameters (Supplemental Table 2). These results suggest that SRT3025 acted faster than oxymetholone to improve hematopoiesis.
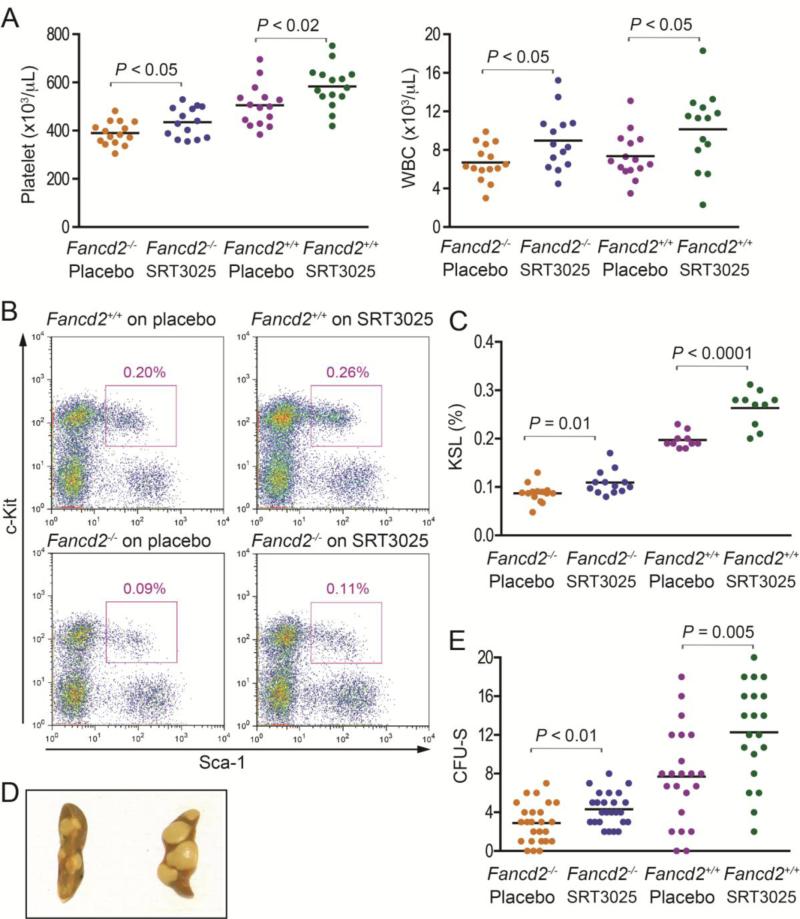
Figure 1. Effects of SRT3025 on hematopoiesis.
A). Peripheral blood counts in SRT3025-treated Fancd2−/− and wild-type mice and their placebo-treated controls. n=14-17 for each group. B). Representative flow cytometric profiles of KSL cell frequencies in bone marrow after 6-months of SRT3025 administration. The cells were gated on PI−Lin− first. The purple box indicates KSL cell subset. The percentages on the profiles were the average KSL cell frequencies of pooled multiple mice for each group. C). KSL cell frequencies in the bone marrow. The KSL percentage refers to the proportion of KSL cells among all nucleated bone marrow cells, n=14 for either SRT3025 or placebo group of Fancd2−/− mice, and n=10 for either SRT3025 or placebo group of Fancd2+/+ mice. D). Representative pictures of the CFU-S assay; E). Quantification of the CFU-S results. Data represent 8 donors for each group with 2 – 4 recipients for each donor. For comparison purposes, all the data were normalized to 40, 000 bone marrow cell input and shown on the figure.
Table 1.
Complete blood counts in SRT3025-treated Fancd2–/– and Fancd2+/+ mice
| Fancd2–/– Placebo | Fancd2–/– SRT3025 | P | Fancd2+/+ Placebo | Fancd2+/+ SRT3025 | P | |
|---|---|---|---|---|---|---|
| WBCs, ×103/μL | 6.7 ± 0.5 | 9.0 ± 0.8 | 0.02 | 7.4 ± 0.6 | 10.1 ± 1.0 | 0.03 |
| RBCs, ×106/μL | 9.9 ± 0.1 | 10.1 ± 0.1 | 0.24 | 10.0 ± 0.1 | 10.1 ± 0.1 | 0.45 |
| Hemoglobin, g/dL | 15.1 ± 0.1 | 15.0 ± 0.1 | 0.52 | 15.0 ± 0.2 | 14.8 ± 0.1 | 0.28 |
| Hematocrit, % | 53.6 ± 0.6 | 54.2 ± 0.7 | 0.58 | 54.0 ± 0.7 | 53.6 ± 0.8 | 0.71 |
| MCV, fL | 54.7 ± 0.4 | 52.7± 0.5 | <0.01 | 54.0 ± 0.3 | 52.8 ± 0.3 | 0.01 |
| Platelets, ×103/μL | 390 ± 12 | 435 ± 17 | 0.04 | 505 ± 22 | 584 ± 23 | 0.02 |
Data were pooled results from multiple mice (14 – 15 mice each group) and presented as mean value ± SEM. WBCs denotes white blood cells; RBCs, red blood cells; MCV, mean corpuscular volume.
Next, we examined the bone marrow of the mice treated with SRT3025. The bone marrow cellularity in either Fancd2−/− or wild-type mice showed no difference between SRT3025-treated animals and placebo-treated controls (data not shown). However, flow cytometry analysis demonstrated that the fractions of KSL cells, an immunophenotypically defined HSPC population, in SRT3025-treated Fancd2−/− mice and wild-type mice were 22.2% (P=0.01) and 30.0% (P<0.0001) larger than those in their placebo-treated controls, respectively (Figure 1B & 1C).
Consistent with the increased frequencies of HSPCs in SRT3025-treated bone marrow, the colony-forming unit-spleen (CFU-S) assay also confirmed that SRT3025-treated Fancd2−/− and wild-type bone marrow cells formed 48.3% (P<0.01) and 59.7% (P=0.005) more macroscopic splenic colonies than placebo-treated Fancd2−/− and wild-type controls, respectively (Figure 1D & 1E), suggesting an improvement in the number and function of progenitors in SRT3025-treated bone marrow.
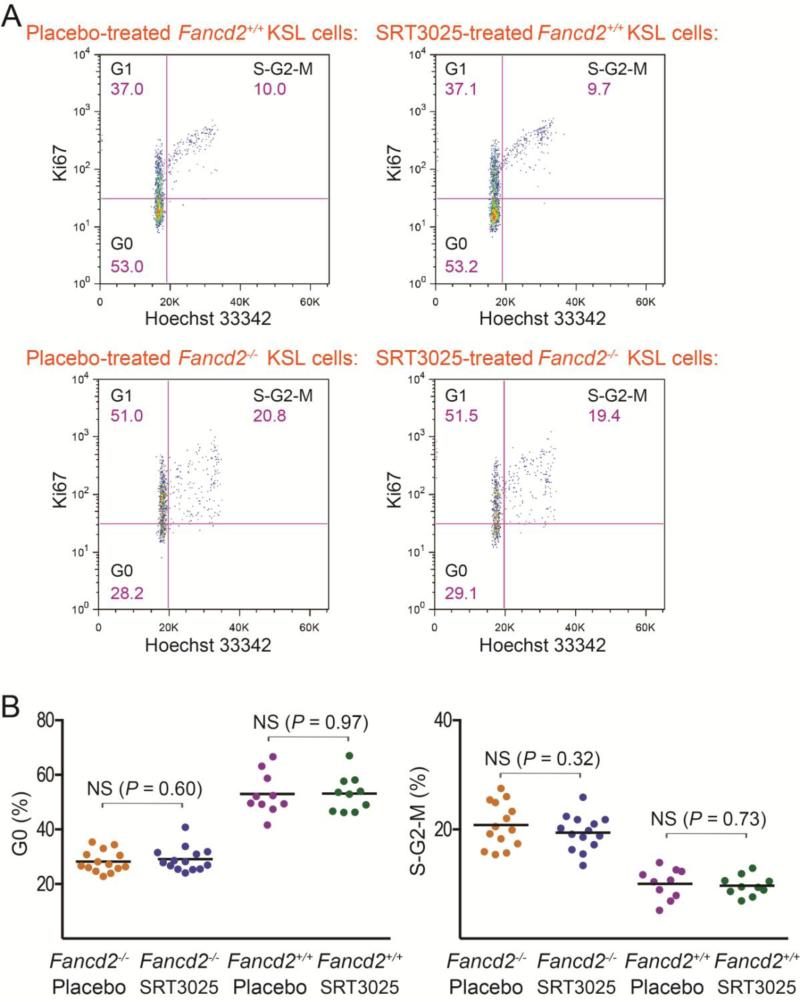
One possible mechanism for HSPC expansion is an elevated level of proliferation. To determine the impact of SRT3025 on the rate of HSPC proliferation, we examined the cell cycle profiles of KSL cells from SRT3025-treated mice. Hoechst 33342 staining (for DNA content) and intracellular Ki67 staining (for cycling cells, not for quiescent G0 cells) were used in combination to measure cell cycle phases (Wilson et al., 2008). As shown in Figure 2A & 2B, the average percentages of quiescent G0 KSL cells in SRT3025-treated Fancd2−/− and Fancd2+/+ mice were 29.1% and 53.2%, respectively, similar to the average G0 percentages of 28.3% and 53.0% observed in placebo-treated Fancd2−/− and Fancd2+/+ mice, respectively. Likewise, the average S-G2-M proportions of KSL cells showed no significant differences between SRT3025-treated mice and their placebo-treated controls either. These results indicate that SRT3025 treatment did not change the cell cycle status of HSPCs. It is, therefore, unlikely that the increase of HSPCs after SRT3025 administration was caused by an elevated rate of proliferation.
Figure 2. Effects of SRT3025 on HSPC proliferation.
A). Representative cell cycle profiles of KSL cells from SRT3025-treated mice and their gender-matched placebo-treated littermate controls. Hoechst 33342 (for DNA content) and anti-mouse Ki67 (for G0/G1 discrimination) were used together to distinguish cells in the G0, G1, and S-G2-M phases of the cell cycle. The shown percentage for each gate was the average percentage for each group of mice. B). Statistical quantification of cell cycle results for the bone marrow KSL cells. n=14 for either SRT3025 or placebo group of Fancd2−/− mice, and n=10 for either SRT3025 or placebo group of Fancd2+/+ mice. NS denotes not significant.
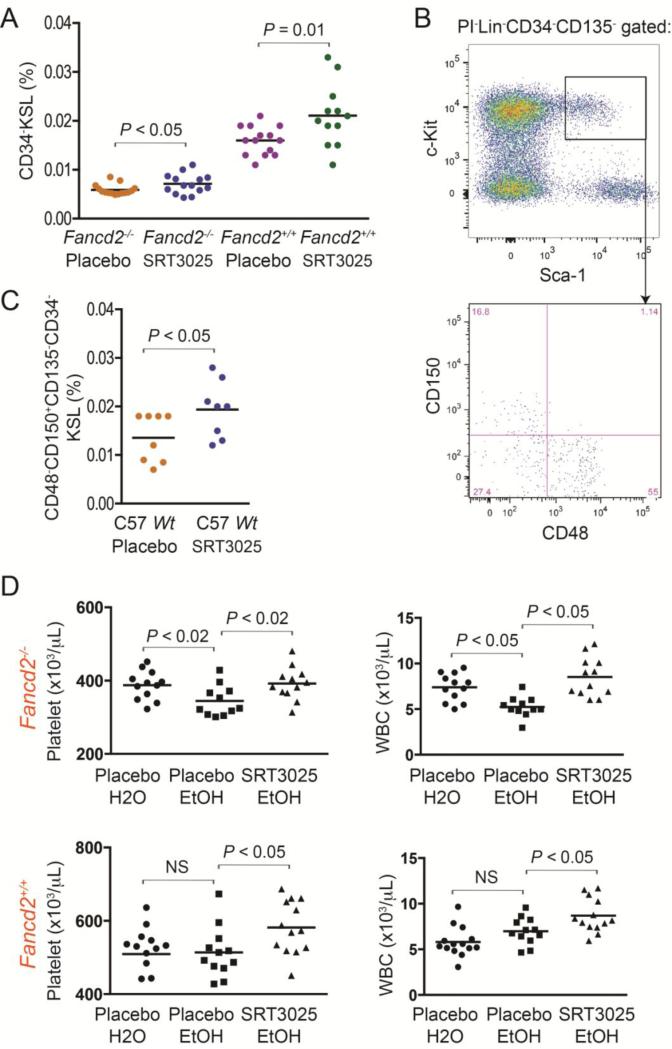
We then measured the number of the more primitive CD34−KSL cells by flow cytometry and noticed that the bone marrow cells in SRT3025-treated Fancd2−/− and wild-type mice had 0.0071% and 0.021% CD34−KSL cells, respectively, significantly more than the 0.0059% and 0.016% CD34−KSL cells observed in their placebo-treated controls (Figure 3A).
Figure 3. Frequency and repopulating capacity of long-term HSCs after SRT3025 administration.
A). CD34−KSL cell frequencies in the bone marrow. Data were pooled results from 12 – 15 mice for each group. B). Representative flow cytometric profiles of CD48−CD150+CD135−CD34−KSL cells. C). CD48−CD150+CD135−CD34−KSL cell frequencies in whole bone marrow from SRT3025- or placebo-treated wild-type C57BL/6J mice (n=8 each). Wt denotes wild-type. D). Hematopoietic effects of SRT3025 on Fancd2−/− and wild-type mice under chronic ethanol treatment. CBC data collected after 3 months of 5% ethanol treatment. Data are pooled results from 12 to 14 mice in each group. NS denotes not significant.
To further assess SRT3025's effect on the size of the long-term HSC pool, we also treated a cohort of 1-month old wild-type C57BL/6J mice for 6 months with SRT3025. We took advantage of Slam and other markers, which are well characterized in C57BL/6J strain background, and examined the most primitive long-term HSC population, defined phenotypically as CD48−CD150+CD135−CD34−KSL cells (Figure 3B) (Wilson et al., 2008). We found that, after 6 months’ SRT3025 administration, the frequencies of CD48−CD150+CD135−CD34−KSL cells in SRT3025-treated C57BL/6J wild-type mice were also significantly higher than those in placebo-treated wild-type controls (P < 0.05, Figure 3C). Collectively, these results demonstrate that chronic SRT3025 administration expands the HSC and progenitor pool and improves hematological parameters in peripheral blood.
3.2 SRT3025 protects Fancd2−/− mice on chronic ethanol treatment from acetaldehyde-induced damage to the hematopoietic system
It has recently been shown that aldehyde toxicity in hematopoietic stem cells contributes to stem cell depletion in FA patients and mouse models (Garaycoechea et al., 2012; Langevin et al., 2011). The ability of SRT3025 to expand the HSPC pool prompted us to test whether SRT3025 administration could help to handle aldehyde toxicity. Since metabolism of alcohol converts ethanol to acetaldehyde as an intermediate metabolite, we chose to use ethanol to generate acetaldehyde in vivo. A cohort of 6-month old Fancd2−/− and wild-type mice were given regular water or 5% ethanol for 3 months. The mice were also simultaneously treated with either SRT3025-supplemented or placebo diet. As shown in Figure 3D, compared with the control mice under regular water treatment, three months of ethanol treatment caused a significant decrease in both platelet and white blood cell counts in Fancd2−/− mice fed with placebo diet, while other hematologic parameters were not significantly affected. More importantly, the Fancd2−/− mice being given ethanol and SRT3025 concurrently were able to maintain normal hematologic parameters (Figure 3D). These results suggest that SRT3025 administration may offset acetaldehyde-induced hematologic damage in Fancd2−/− mice. In contrast to Fancd2−/− mice, wild-type mice were not sensitive to ethanol treatment, which is generally in line with previous reports (Langevin et al., 2011). Also, in the absence of ethanol-induced damage, SRT3025 still boosted both platelet and white blood cell counts in wild-type mice, suggesting that the protective effect of SRT3025 on the hematopoietic system was not specific to aldehyde detoxification, probably due to the expansion of the HSPC pool.
3.3 SRT3025 administration does not increase or impair HSC repopulating potential
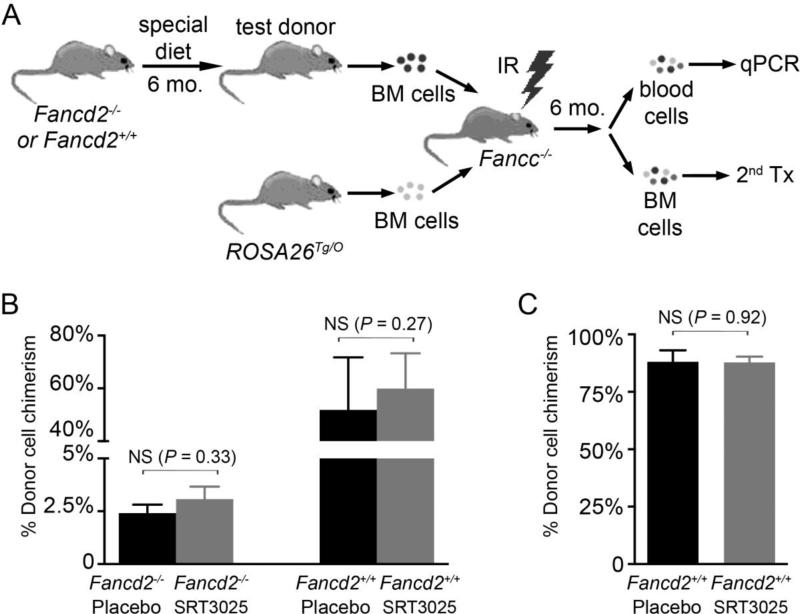
The functional properties of long-term HSCs from SRT3025-treated mice were assessed next using an in vivo competitive repopulation assay. As depicted in Figure 4A, bone marrow cells from a test donor were mixed with ROSA26Tg/O genetically marked bone marrow (as competitors) and then transplanted into a lethally irradiated Fancc−/− mouse. Quantitative real-time PCR was performed 6 months post-transplantation to evaluate each donor's contribution to the hematopoietic repopulation in the primary recipients. After 6 month's SRT3025 treatment, HSCs from SRT3025-treated mice exhibited hematopoietic reconstitution capacities equivalent to those from placebo-treated controls (Figure 4B). We then preformed serial transplantation assays, comparing the bone marrow cells harvested from these primary recipients. Twenty weeks after secondary transplantation, similar secondary hematopoietic reconstitution potential was observed for bone marrow cells originally derived from both SRT3025-treated and placebo-treated Fancd2+/+ mice (Figure 4C). Consistent with the severely impaired HSC function in Fancd2−/− mice, no stable chimerism (0.1% or more) was detected in secondary recipients that received bone marrow cells originally derived from either SRT3025-treated or placebo-treated Fancd2−/− primary donors (date not shown). These results indicate that long term SRT3025 administration did not permanently increase or decrease HSC repopulating potential.
Figure 4. Serial transplantation experiment.
A). Strategy used in the in vivo competitive repopulation experiment. BM, IR, Tx, and mo denote bone marrow, irradiation, transplantation, and month, respectively. B). Repopulating potential of bone marrow cells from SRT3025- and placebo-treated mice. % chimerism refers to the percentage of test donor-derived cells in all donor-derived cells. For Fancd2−/− donors, the data represent 6 – 7 donors for either SRT3025 or placebo group with 3 recipients from each donor; for Fancd2+/+ donors, the data represent 5 donors each group with 2 – 3 recipients from each donor. NS denotes not significant. Data are presented as mean ± SEM. C). Secondary repopulating potential of bone marrow cells from SRT3025- and placebo-treated mice. Results from multiple recipients (n=5 for original placebo-treated Fancd2+/+ donors and n=9 for original SRT3025-treated Fancd2+/+ donors) were pooled together for each experimental group.
3.4 SRT3025 does not work by direct activation of Sirt1 in hematopoietic cells
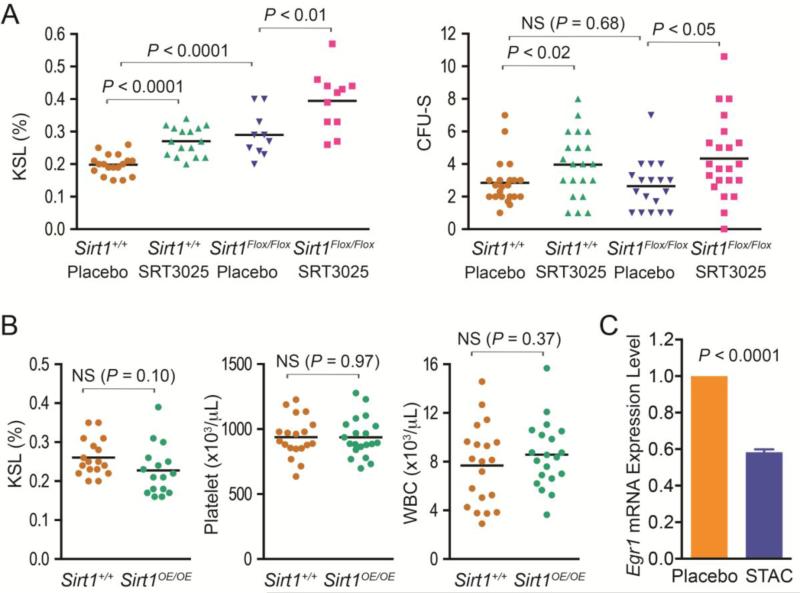
The HSPC expansion effect of SRT3025 differed from our previous observation that Sirt1 activator resveratrol did not change the size of the HSPC pool but helped to maintain Fancd2 mutant KSL cells in quiescence. These distinct effects of resveratrol and SRT3025 prompted us to question whether SRT3025 influenced hematopoiesis through the mediation of Sirt1 activation. To answer this question, we utilized a Sirt1 exon 4-floxed mouse strain and Vav1-Cre transgenic mice to generate blood tissue-specific Sirt1 conditional knockout mice (Sirt1Flox/Flox Vav1-CreTg/O), in which the deletion of Sirt1 exon 4 is dependent on the blood cell-specific expression of Cre recombinase driven by Vav1 promoter (Ogilvy et al., 1998). Deletion of Sirt1 Exon 4 disrupts Sirt1 catalytic activities and mimics Sirt1-null phenotypes (Cheng et al., 2003). qPCR with exon 4-specific primers confirmed that Sirt1 expression in bone marrow cells from the conditional knockout mice was undetectable. We then gave cohorts of one-month old Sirt1Flox/Flox Vav1-CreTg/O and Sirt1+/+ Vav1-CreTg/O mice either SRT3025-supplemented rodent chow or placebo for 6 months. At harvest, we observed that, as compared with placebo-treated wild-type mice, placebo-treated Sirt1Flox/Flox Vav1-CreTg/O mice had higher KSL frequencies but similar CFU-S forming capacities in their bone marrow cells (Figure 5A), indicating that the absence of Sirt1 caused an expansion of HSPC compartment but provided no functional improvement to these cells. These results were in agreement with the recent observation that Sirt1 ablation promotes aberrant HSPC expansion and loss of HSC (Rimmele et al., 2014; Singh et al., 2013). Surprisingly, flow cytometry analysis revealed that SRT3025 administration significantly expanded KSL compartment in both Sirt1 conditional knockout mice (by 36.2%, P<0.01) and wild-type controls (by 37.0%, P<0.0001) (Figure 5A). We also noticed an increase of CFU-S forming ability in SRT3025-treated Sirt1 conditional knockout mice (by 64.4%, P<0.05) and wild-type controls (by 39.8%, P<0.02) (Figure 5A). Collectively, these results indicate that SRT3025's beneficial effects are not dependent on the presence of Sirt1 in blood cells themselves.
Figure 5. Effects of SRT3025 on hematopoiesis after conditional deletion of Sirt1 in blood cells or transgenic overexpression.
A). Characterization of hematopoiesis in blood-specific Sirt1 knockout mice (Sirt1Flox/Flox) vs. unrecombined controls (Sirt1+/+). All mice carried the Vav1-CreTg/O driver allele. For KSL frequencies, data represent 10 – 18 mice for each group. CFU-S results represent 6 donors for each group with 2 to 4 recipients for each donor. NS denotes not significant. B). Characterization of hematopoiesis in Sirt1-overexpressing transgenic mice. n=16-17 each for KSL frequencies,; n =20 each for compete blood counts. C). SRT3025 suppressed Egr1 gene expression in wild-type KSL cells. RNA input was normalized based on glyceraldehyde 3-phosphate dehydrogenase mRNA expression. The Egr1 expression level in the KSL cells of placebo-treated mice was set at 1 as a reference. Data represents the average (± SEM) of 10 placebo-treated or 12 SRT3025-treated mice.
3.5 Transgenic overexpression of Sirt1 does not phenocopy the effects of SRT3025
It is possible that SRT3025 indirectly influenced hematopoiesis by the activation of Sirt1 in tissues other than blood. To further address this issue, we also utilized a Sirt1 overexpressing transgenic (Sirt1 OE) mouse model to investigate the effect of global Sirt1 overexpression on hematopoiesis. In Sirt1 OE mice, the Sirt1 gene is constitutively overexpressed in all the tissues, i.e. 3 to 7-fold in bone marrow and 13 to 15-fold in liver (data not shown). Interestingly, we found that Sirt1 OE mice did not show a larger KSL population and displayed similar levels of platelet and white blood cell counts as controls (Figure 5B).
3.6 RNA-Seq transcriprome analysis reveals a possible mechanism for SRT3025's hematopoiesis-enhancing effect
To further investigate potential molecular mechanisms underlying the HSPC expansion effect of SRT3025, we used transcriptome analysis to understand the gene expression changes in HSPCs after SRT3025 administration. KSL cells were sorted from bone marrow cells via flow cytometry after 3 months of SRT3025 administration on wild-type mice and used for RNA-Seq gene expression analysis (Wang et al., 2009). Three individual samples (each being a pooled sample from 4 mice) were processed for each experimental condition, with combined reads (aligned to unique RefSeq genes) per condition in the range of 80 million. As shown in Table 2, six genes were significantly down-regulated by more than 1.5 fold in SRT3025-treated wild-type KSL cells, whereas no genes were significantly up-regulated. Most interestingly, the cell cycle regulator p21 was down-regulated by 2.1 fold after SRT3025 administration. Moreover, the transcription factor early growth response-1 (Egr1) known to control p21 transcription (Choi et al., 2008; Min et al., 2008) was also down-regulated (Supplemental Table 3). Egr1 is enriched in HSPCs as compared with whole bone marrow cells in our previous RNA-Seq transcriptome analysis of KSL cells (Zhang et al., 2015) and another recent study (Min et al., 2008). To test whether the transcriptional down-regulation of Egr1 indeed occurs after SRT3025 administration, we performed qRT-PCR on RNA samples isolated from KSL cells. As shown in Figure 5C, the Egr1 mRNA levels in the KSL cells of SRT3025-treated wild-type mice were significantly lower than those in placebo-treated controls. These results suggest that Egr1 suppression might be the upstream event that leads to the transcriptional down-regulation of p21 in response to SRT3025 down-regulation.
Table 2.
Genes differentially changed in wild-type HSPCs in response to SRT3025 administration
| Gene | Symbol | Fold change | P value | aq value |
|---|---|---|---|---|
| p21 | Cdkn1a | -2.1 | <0.001 | <0.001 |
| thrombospondin 1 | Thbs1 | -3.0 | <0.001 | <0.001 |
| myosin light chain 2 precursor, variant 2 | Myl10 | -1.8 | <0.001 | <0.001 |
| fos-like antigen 2 | Fosl2 | -1.8 | <0.001 | 0.044 |
| immunoglobulin J chain | Igj | -2.3 | <0.001 | 0.044 |
| tight junction protein Magil | Magi1 | -3.0 | <0.001 | 0.044 |
Note:
q value represents false discovery rate. The q value cut-off was set at 0.05. Genes are sorted according to the q values.
4. Discussion
FA patients have fewer HSPCs and suffer from progressive HSPC loss. Fancd2−/− mice faithfully recapitulate this phenotype and are born with a smaller HSPC pool. In this study, we found that oral administration of the Sirt1 activator SRT3025 partially corrected this hematopoietic defect by expanding the HSPC compartment. This modest expansion was shown not only by quantitation of immunophenotypically defined stem cells, but also functionally in the CFU-S assay. A larger stem cell and progenitor pool could potentially lead to an increase in peripheral blood counts of multiple lineages. Indeed, we observed a significant increase in platelet and white blood cell counts after only 6 months of chronic SRT3025 administration in both Fancd2−/− and wild type mice. From a therapeutic standpoint, the improvement of peripheral blood counts at this early time point is rather promising. In a similarly designed study with the same strain of FA mice, it took 17 months of oxymetholone therapy before peripheral blood counts improved (Zhang et al., 2015).
The benefits of SRT3025 on hematopoiesis were not specific to FA. As a matter of fact, the compound had a larger effect on both the expansion of HSPC pool and the increase of platelet counts in wild-type mice than in Fancd2−/− mice. This is probably due to the severe pre-existing functional impairment of Fancd2−/− HSCs demonstrated by multiple investigators (Garaycoechea et al., 2012; Parmar et al., 2010; Zhang et al., 2010). Since its efficacy is genotype independent, SRT3025 might be useful in the treatment of bone marrow failure diseases other than FA. For example, mutations in the checkpoint regulator Atr cause a phenotype similar to FA with HSPC loss in mice and humans (Seckel syndrome) (Ruzankina et al., 2007). Similarly, Atm−/− mice also fail to maintain a normal stem cell capacity (Takubo et al., 2008).
Our studies shed light on the possible mechanism(s) behind SRT3025's actions. First, SRT3025 did not act through the direct activation of Sirt1 in hematopoietic cells. Conditional ablation of the Sirt1 gene in all hematopoietic cells did not prevent the expansion of HSPC by SRT3025. Second, constitutive and systemic transgenic overexpression of Sirt1 did not cause increases in HSPC number. Consistently, the known Sirt1 activator resveratrol did not change the size of the HSPC pool and had a rather distinct effect on hematopoiesis in the same Fancd2−/− mouse model (Zhang et al., 2010). These findings could indicate that SRT3025 increases the stem cell pool by an off-target effect completely unrelated to its potent Sirt1 activation in vitro. No such off-target effects of this drug are known. Third, the beneficial effects on hematopoiesis from SRT3025 were correlated with the expansion of HSPCs. The size of HSPC population is maintained by a balance of survival, proliferation, and self-renewal (Alenzi et al., 2009). However, the proliferation rate and repopulating potential of HSPCs were unchanged after SRT3025 administration. In light of the recent finding that an exacerbated p53/p21 DNA damage response limits HSPC survival to maintain genome integrity and suppress tumors (Ceccaldi et al., 2012), it is possible that SRT3025 enhanced the survival of HSPCs. Finally, the transcriptome analysis of HSPCs in SRT3025-treated mice supported this hypothesis. SRT3025 suppressed the transcription of p21, one of the critical p53-target genes. We showed previously that siRNA knock-down of p21 mimicked p53 suppression and rescued the functional defects of FA patients’ CD34+ cells, suggesting p21 as one mediator of p53-driven HSC elimination (Ceccaldi et al., 2012). However, complete genetic ablation of p21 in Fancd2−/− mice failed to rescue the hematopoietic defects of Fancd2−/− mice and pointed out an indispensable role of p21 in maintaining the normal size of the HSPC pool (Zhang et al., 2013). While these findings appear contradictory at first glance, the apparently opposite outcomes might be explained by the difference between partial down-regulation and complete ablation of p21 activity. Therefore, it is possible that the transcriptional suppression of p21 by SRT3025 contributes to the compound's beneficial effects on hematopoiesis.
More interestingly, our data suggest that SRT3025 might suppress p21 transcription through the down-regulation of Egr1. Egr1 modulates p21 gene expression and also plays important functions in HSPC development and proliferation (Krishnaraju et al., 2001; Min et al., 2008; Ragione et al., 2003). Within the bone marrow, Egr1 expression is highly enriched in hematopoietic stem and progenitor cells (Min et al., 2008; Zhang et al., 2015). Systemic deletion of Egr1 gene in mice alters the expression of multiple genes including Bmi-1, Cdk4, and p21, and causes enhanced HSC proliferation and premature HSC exhaustion in serial transplantation (Min et al., 2008). Under our experimental conditions, SRT3025-induced Egr1 down-regulation only affected p21 gene expression and did not impair HSC secondary hematopoietic reconstitution potential in serial transplantation. It has been proved that lower levels of p53/p21 expression in a subset of FA patients lead to milder bone marrow deficiency (Ceccaldi et al., 2011). More importantly, recent work showed that the transcriptional activation of p21 by Egr1 is independent of p53 (Choi et al., 2008). Given the detrimental tumorigenic effects of p53 loss, SRT3025-mediated Egr1-p21 down-regulation might be able to at least prevent the progressive HSPC elimination in FA patients without compromising p53 activity that is key to genome integrity. It is temping to propose that targeting Egr1-p21 signaling might represent a new venue in the future design of therapeutic regimens for FA patients.
Supplementary Material
Highlights.
SRT3025 administration expands HSPCs and boosts blood counts;
SRT3025 works in both wild-type and Fanconi anemia mice;
SRT3025 administration down-regulates Egr1-p21 expression in HSPCs.
Acknowledgments
We appreciate the support from Sirtris and the helpful advice from Dr. James Ellis. We thank Ngoc Pham for her help with flow cytometry, Dr. Rafael de Cabo for his help with SirT1 OE mice, and Kevin Watanabe-Smith and Eric Benedetti for animal care. This work was supported by NIH grant RO1AG028731 and The Glenn Medical Foundation (to DAS) and NIH grant P01 HL048546 (to MG).
Abbreviations
- FA
Fanconi anemia
- HSPC
hematopoietic stem and progenitor cell
- HSC
hematopoietic stem cell
- KSL
c-Kit+Sca-1+Lin−
- CBC
complete blood count
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship
Contributions: Q.S.Z. designed the study, performed research, analyzed and interpreted data, and wrote the manuscript; M.D., K.S., and L.M. performed research; C.P. helped with the bioinformatics analysis of RNA-Seq data; D.A.S. provided Sirt1 transgenic mice; and M.G. designed the study, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure:
D.A.S. is a consultant to GlaxoSmithKline. All other authors declare they have no financial interest related to this work.
References
- Alenzi FQ, Alenazi BQ, Ahmad SY, Salem ML, Al-Jabri AA, Wyse RK. The haemopoietic stem cell: between apoptosis and self renewal. The Yale journal of biology and medicine. 2009;82:7–18. [PMC free article] [PubMed] [Google Scholar]
- Ceccaldi R, Briot D, Larghero J, Vasquez N, Dubois d'Enghien C, Chamousset D, Noguera ME, Waisfisz Q, Hermine O, Pondarre C, et al. Spontaneous abrogation of the G(2)DNA damage checkpoint has clinical benefits but promotes leukemogenesis in Fanconi anemia patients. J Clin Invest. 2011;121:184–194. doi: 10.1172/JCI43836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccaldi R, Parmar K, Mouly E, Delord M, Kim JM, Regairaz M, Pla M, Vasquez N, Zhang QS, Pondarre C, et al. Bone Marrow Failure in Fanconi Anemia Is Triggered by an Exacerbated p53/p21 DNA Damage Response that Impairs Hematopoietic Stem and Progenitor Cells. Cell Stem Cell. 2012;11:36–49. doi: 10.1016/j.stem.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BH, Kim CG, Bae YS, Lim Y, Lee YH, Shin SY. p21 Waf1/Cip1 expression by curcumin in U-87MG human glioma cells: role of early growth response-1 expression. Cancer Res. 2008;68:1369–1377. doi: 10.1158/0008-5472.CAN-07-5222. [DOI] [PubMed] [Google Scholar]
- Friedrich G, Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- Garaycoechea JI, Crossan GP, Langevin F, Daly M, Arends MJ, Patel KJ. Genotoxic consequences of endogenous aldehydes on mouse haematopoietic stem cell function. Nature. 2012;489:571–575. doi: 10.1038/nature11368. [DOI] [PubMed] [Google Scholar]
- Houghtaling S, Timmers C, Noll M, Finegold MJ, Jones SN, Meyn MS, Grompe M. Epithelial cancer in Fanconi anemia complementation group D2 (Fancd2) knockout mice. Genes Dev. 2003;17:2021–2035. doi: 10.1101/gad.1103403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard BP, Sinclair DA. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends in pharmacological sciences. 2014;35:146–154. doi: 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabra N, Li Z, Chen L, Li B, Zhang X, Wang C, Yeatman T, Coppola D, Chen J. SirT1 is an inhibitor of proliferation and tumor formation in colon cancer. J Biol Chem. 2009;284:18210–18217. doi: 10.1074/jbc.M109.000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PF, Radtke S, von Kalle C, Balcik B, Bohn K, Mueller R, Schuesler T, Haren M, Reeves L, Cancelas JA, et al. Stem cell collection and gene transfer in Fanconi anemia. Mol Ther. 2007;15:211–219. doi: 10.1038/sj.mt.6300033. [DOI] [PubMed] [Google Scholar]
- Krishnaraju K, Hoffman B, Liebermann DA. Early growth response gene 1 stimulates development of hematopoietic progenitor cells along the macrophage lineage at the expense of the granulocyte and erythroid lineages. Blood. 2001;97:1298–1305. doi: 10.1182/blood.v97.5.1298. [DOI] [PubMed] [Google Scholar]
- Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, Hanenberg H, Auerbach AD. A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood. 2003;101:1249–1256. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Langevin F, Crossan GP, Rosado IV, Arends MJ, Patel KJ. Fancd2 counteracts the toxic effects of naturally produced aldehydes in mice. Nature. 2011;475:53–58. doi: 10.1038/nature10192. [DOI] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome biology. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Witten DM, Johnstone IM, Tibshirani R. Normalization, testing, and false discovery rate estimation for RNA-sequencing data. Biostatistics (Oxford, England) 2012;13:523–538. doi: 10.1093/biostatistics/kxr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min IM, Pietramaggiori G, Kim FS, Passegue E, Stevenson KE, Wagers AJ. The transcription factor EGR1 controls both the proliferation and localization of hematopoietic stem cells. Cell Stem Cell. 2008;2:380–391. doi: 10.1016/j.stem.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin YK, Canto C, Scheibye-Knudsen M, et al. SRT1720 improves survival and healthspan of obese mice. Scientific reports. 2011;1:70. doi: 10.1038/srep00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda MX, van Tits LJ, Lohmann C, Arsiwala T, Winnik S, Tailleux A, Stein S, Gomes AP, Suri V, Ellis JL, et al. The Sirt1 activator SRT3025 provides atheroprotection in Apoe−/− mice by reducing hepatic Pcsk9 secretion and enhancing Ldlr expression. European heart journal. 2014 doi: 10.1093/eurheartj/ehu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvy S, Elefanty AG, Visvader J, Bath ML, Harris AW, Adams JM. Transcriptional regulation of vav, a gene expressed throughout the hematopoietic compartment. Blood. 1998;91:419–430. [PubMed] [Google Scholar]
- Pangeni R, Sahni JK, Ali J, Sharma S, Baboota S. Resveratrol: review on therapeutic potential and recent advances in drug delivery. Expert opinion on drug delivery. 2014;11:1285–1298. doi: 10.1517/17425247.2014.919253. [DOI] [PubMed] [Google Scholar]
- Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar K, Kim J, Sykes SM, Shimamura A, Stuckert P, Zhu K, Hamilton A, Deloach MK, Kutok JL, Akashi K, et al. Hematopoietic stem cell defects in mice with deficiency of Fancd2 or Usp1. Stem Cells. 2010;28:1186–1195. doi: 10.1002/stem.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragione FD, Cucciolla V, Criniti V, Indaco S, Borriello A, Zappia V. p21Cip1 gene expression is modulated by Egr1: a novel regulatory mechanism involved in the resveratrol antiproliferative effect. J Biol Chem. 2003;278:23360–23368. doi: 10.1074/jbc.M300771200. [DOI] [PubMed] [Google Scholar]
- Rimmele P, Bigarella CL, Liang R, Izac B, Dieguez-Gonzalez R, Barbet G, Donovan M, Brugnara C, Blander JM, Sinclair DA, et al. Aging-like Phenotype and Defective Lineage Specification in SIRT1-Deleted Hematopoietic Stem and Progenitor Cells. Stem cell reports. 2014;3:44–59. doi: 10.1016/j.stemcr.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood reviews. 2010;24:101–122. doi: 10.1016/j.blre.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair DA, Guarente L. Small-molecule allosteric activators of sirtuins. Annual review of pharmacology and toxicology. 2014;54:363–380. doi: 10.1146/annurev-pharmtox-010611-134657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Williams CA, Klarmann K, Burkett SS, Keller JR, Oberdoerffer P. Sirt1 ablation promotes stress-induced loss of epigenetic and genomic hematopoietic stem and progenitor cell maintenance. The Journal of experimental medicine. 2013;210:987–1001. doi: 10.1084/jem.20121608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takubo K, Ohmura M, Azuma M, Nagamatsu G, Yamada W, Arai F, Hirao A, Suda T. Stem cell defects in ATM-deficient undifferentiated spermatogonia through DNA damage-induced cell-cycle arrest. Cell Stem Cell. 2008;2:170–182. doi: 10.1016/j.stem.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney MA, Royle G, Low MJ, Kelly MA, Axthelm MK, Reifsteck C, Olson S, Braun RE, Heinrich MC, Rathbun RK, et al. Germ cell defects and hematopoietic hypersensitivity to gamma-interferon in mice with a targeted disruption of the Fanconi anemia C gene. Blood. 1996;88:49–58. [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Zhang QS, Benedetti E, Deater M, Schubert K, Major A, Pelz C, Impey S, Marquez-Loza L, Rathbun RK, Kato S, et al. Oxymetholone Therapy of Fanconi Anemia Suppresses Osteopontin Transcription and Induces Hematopoietic Stem Cell Cycling. Stem Cell Reports. 2015;4:90–102. doi: 10.1016/j.stemcr.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QS, Eaton L, Snyder ER, Houghtaling S, Mitchell JB, Finegold M, Van Waes C, Grompe M. Tempol protects against oxidative damage and delays epithelial tumor onset in Fanconi anemia mice. Cancer Res. 2008;68:1601–1608. doi: 10.1158/0008-5472.CAN-07-5186. [DOI] [PubMed] [Google Scholar]
- Zhang QS, Marquez-Loza L, Eaton L, Duncan AW, Goldman DC, Anur P, Watanabe-Smith K, Rathbun RK, Fleming WH, Bagby GC, et al. Fancd2−/− mice have hematopoietic defects that can be partially corrected by resveratrol. Blood. 2010;116:5140–5148. doi: 10.1182/blood-2010-04-278226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QS, Marquez-Loza L, Sheehan AM, Watanabe-Smith K, Eaton L, Benedetti E, Major A, Schubert K, Deater M, Joseph E, et al. Evaluation of resveratrol and N-acetylcysteine for cancer chemoprevention in a Fanconi anemia murine model. Pediatric blood & cancer. 2014;61:740–742. doi: 10.1002/pbc.24780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QS, Watanabe-Smith K, Schubert K, Major A, Sheehan AM, Marquez-Loza L, Newell AE, Benedetti E, Joseph E, Olson S, et al. Fancd2 and p21 function independently in maintaining the size of hematopoietic stem and progenitor cell pool in mice. Stem cell research. 2013;11:687–692. doi: 10.1016/j.scr.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.