Abstract
Objective
To describe the administration of sedatives and analgesics at the end of life in a large cohort of infants in North American neonatal intensive care units (NICUs).
Study design
Data on mortality and sedative and analgesic administration were obtained from infants who died from 1997–2012 in 348 NICUs managed by the Pediatrix Medical Group. Sedatives and analgesics of interest included opioids (fentanyl, methadone, morphine), benzodiazepines (clonazepam, diazepam, lorazepam, midazolam), central alpha-2 agonists (clonidine, dexmedetomidine), ketamine, and pentobarbital. We used multivariable logistic regression to evaluate the association between administration of these drugs on the day of death and infant demographics and illness severity.
Results
We identified 19,726 infants who died. Of these, 6188 (31%) received a sedative or analgesic on the day of death; opioids were most frequently administered, 5366/19,726 (27%). Administration of opioids and benzodiazepines increased during the study period, from 16/283 (6%) for both in 1997 to 523/1465 (36%) and 295/1465 (20%) in 2012, respectively. Increasing gestational age, increasing postnatal age, invasive procedure within 2 days of death, more recent year of death, mechanical ventilation, inotropic support, and antibiotics on the day of death were associated with exposure to sedatives or analgesics.
Conclusions
Administration of sedatives and analgesics increased over time. Infants of older gestational age and those more critically ill were more likely to receive these drugs on the day of death. These findings suggest that drug administration may be driven by severity of illness.
Keywords: infant deaths, palliative care, comfort drugs
End-of-life care for dying patients is central to the provision of quality health care.1 Research efforts have sought to understand current management and identify best practices of end-of-life care.1 Although most of these efforts focus on critically ill adults, systematic data are urgently needed to guide end-of-life care for children and their families.1-4 Existing data suggest that high-quality end-of-life care for children includes interventions for relief of pain and other symptoms, most often by pharmacotherapy.2,3,5,6 Increasing recognition of pain in the neonatal period, historical evidence of limited administration of analgesia to infants undergoing painful procedures, and the high incidence of infant deaths compared to pediatric deaths outside this period offer unique opportunities to evaluate drug administration to infants at the end of life.7-9
Data on drug administration at the end of life in North American neonatal intensive care units (NICUs) are few.9-14 In a 4-center study (2 in the United States and 1 each in Canada and the Netherlands), providers most frequently administered opioids, 116/151 (77%), and benzodiazepines, 61/151 (41%), to dying infants.11 At each center, providers increased doses of opioids or benzodiazepines as the time of death approached; however, there was great variability among the centers in the doses given to achieve infant comfort.11 In 4 U.S. studies, providers also variably administered opioids or benzodiazepines during the process of ventilator withdrawal or withholding in infants.9,12-14 Variable drug administration may be due to patient- or provider-level characteristics.15-20 Here, we describe the administration of sedatives and analgesics at the end of life to a large cohort of infants in NICUs in North America and seek to determine the patient characteristics that influence this drug administration.
METHODS
Study Cohort
We identified all infants who died from 1997 to 2012 in 348 NICUs managed by the Pediatrix Medical Group. Data were obtained from the Pediatrix Medical Group Clinical Data Warehouse. The Pediatrix Clinical Data Warehouse prospectively captures data entered from history and physicals, daily notes, and discharge notes. These data include maternal history and demographics, administered drugs, laboratory results, culture results, and diagnoses. Drug dosing, intervals, and indications were not recorded.
Definitions
Sedatives and analgesics of interest included opioids (fentanyl, methadone, morphine), benzodiazepines (clonazepam, diazepam, lorazepam, midazolam), central alpha-2 agonists (clonidine, dexmedetomidine), ketamine, and pentobarbital. Infants were classified as exposed to a drug of interest if there was documentation of drug administration on the day of death or on either of the last 2 days of life. Infants who had a documented drug start date within 7 days of death but no documented end date were also presumed to be exposed to drugs of interest on the day of death. We evaluated infant severity of illness by exposure to antibiotics, inotropes, and mechanical ventilation on the day of death, and exposure to an invasive procedure within 2 days of death. We categorized NICUs based on average annual discharges (low volume, <300 infants; medium volume, 301–600 infants; large volume, >600 infants).
Statistical Methods
We used standard summary statistics including counts, percentages, and medians with interquartile ranges to describe the study variables. We determined the number of infants exposed to sedatives and analgesics on the day of death, on either of the last 2 days of life, and at any time during their hospitalization. We compared the proportion of infants exposed to sedatives and analgesics on the day of death, on either of the last 2 days of life, and at any time during their hospitalization by gestational age, NICU volume, and year of death using chi-square tests of association. We performed univariable logistic regressions to evaluate the association between (1) sedative and analgesic exposure on the day of death, on either of the last 2 days of life, and at any time during their hospitalization, and (2) the following variables: gestational age, postnatal age, race/ethnicity, gender, invasive procedure within 2 days of death, and inotrope, antibiotic, and ventilator exposure on the day of death. For multivariable modeling, we included all covariates that may be clinically associated with sedative and analgesic exposure on the day of death. The final model included random effects for NICU site and the following covariates: race/ethnicity, gestational age, postnatal age, invasive procedure within 2 days of death, year of death, and inotropic support, antibiotic exposure, and ventilator status on the day of death.
We performed a sensitivity analysis, limiting our cohort to infants admitted to the NICU for at least 2 days. STATA 12 (College Station, TX) was used to perform the statistical analysis. A P < .05 was considered statistically significant for all tests. The study was approved by the Duke University Institutional Review Board without the need for written informed consent as the data were collected without identifiers.
RESULTS
Demographics
We identified 19,726 infants who died during their NICU admission from 1997 to 2012. The median gestational age at birth, birth weight, and postnatal age on the day of death were 26 weeks (interquartile range 24, 32), 820 g (615, 1641), and 8 days (2, 21), respectively. Of the 19,726 infants, 6188 (31%) received a sedative or analgesic on the day of death, 6601 infants (33%) received a sedative or analgesic within the last 2 days of life, and 9538 (48%) received a sedative or analgesic at any point during their hospitalization. The median gestational age and birth weight were higher in infants who received sedatives and analgesics on the day of death compared to those who did not, 27 weeks (24, 33) vs. 26 weeks (24, 32) (P < 0.001), and 861 g (633, 1790) vs. 800 g (605, 1570) (P < 0.001), respectively (Table I). Of the 19,726 infants, 5366 (27%) received an opioid on the day of death, and 3142 (16%) received a benzodiazepine on the day of death (Table II; online).
Table I.
Demographics
| No sedative or analgesic exposure on day of death (N = 13,538) |
Sedative or analgesic exposure on day of death (N = 6188) |
Sedative or analgesic exposure during hospitalization (N = 9538) |
|
|---|---|---|---|
| Gestational age | |||
| <28 | 7868 (58) | 3391 (55) | 5488 (58) |
| 28-33 | 2661 (20) | 1319 (21) | 2044 (21) |
| >33 | 2988 (22) | 1469 (24) | 2354 (25) |
| Male | 7562 (56) | 3558 (57) | 5443 (57) |
| Birth weight (g) | |||
| <750 | 5863 (43) | 2402 (39) | 3965 (42) |
| 750-999 | 2140 (16) | 1014 (17) | 1608 (17) |
| 1000-1499 | 1655 (12) | 812 (14) | 1180 (12) |
| ≥1500 | 3786 (28) | 1947 (31) | 2768 (29) |
| Postnatal age (days) | |||
| 1 | 2551 (19) | 565 (9) | 1558 (16) |
| 2-7 | 5051 (37) | 2719 (44) | 2410 (25) |
| 8-28 | 3740 (28) | 1877 (30) | 3181 (33) |
| >28 | 2092 (15) | 1027 (17) | 2365 25) |
| Postmenstrual age | |||
| <28 | 5960 (44) | 2476 (38) | 3530 (37) |
| 28-33 | 3507 (26) | 1748 (29) | 2752 (29) |
| ≥33 | 3946 (29) | 1955 (33) | 3221 (34) |
| Race/ethnicity | |||
| White | 5753 (42) | 2684 (40) | 4116 (43) |
| Black | 3237 (24) | 1391 (22) | 2239 (23) |
| Hispanic | 3225 (24) | 1588 (26) | 2349 (25) |
| Other | 674 (5) | 317 (5) | 486 (5) |
| Antibiotic supporta | 4612 (34) | 2881 (47) | |
| Inotropic supporta | 3703 (27) | 2605 (42) | |
| Procedureb | 266 (2) | 320 (5) | |
| Ventilation supporta | 11,492 (85) | 5800 (94) |
Values are expressed as n (%).
On the day of death.
Within 2 days of death.
Table II.
Type of sedative and analgesic exposure
| Day of death (N = 6188) |
|
|---|---|
| Opioids | 5366 (87) |
| Fentanyl | 3509 (57) |
| Morphine | 2103 (34) |
| Methadone | 55 (<1) |
| Benzodiazepines | 3142 (51) |
| Midazolam | 2258 (36) |
| Lorazepam | 943 (15) |
| Diazepam | 75 (1) |
| Clonazepam | 5 (<1) |
| Other | |
| Clonidine | 0 |
| Dexmedetomidine | 4 (<1) |
| Ketamine | 4 (<1) |
| Pentobarbital | 58 (<1) |
Values are expressed as n (%).
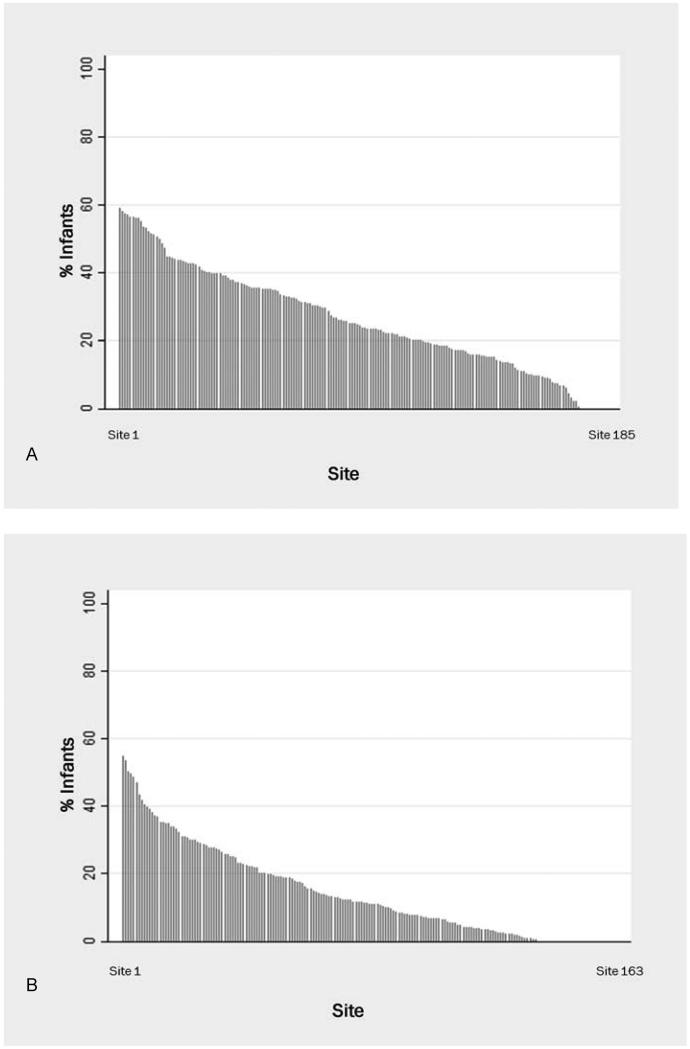
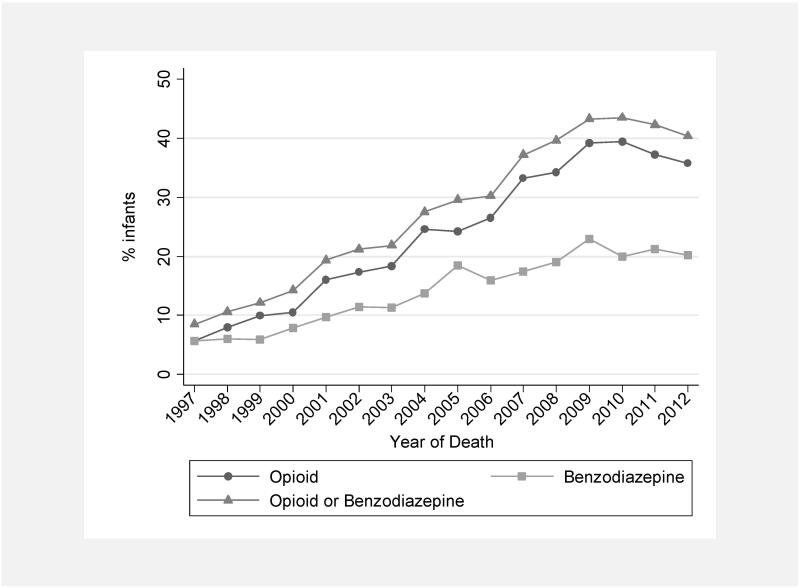
The use of sedatives or analgesics varied widely across centers and over time. The proportion of infants exposed to a sedative or analgesic on the day of death varied across NICUs (median 18%, interquartile range 0, 34) (Figure 1). Centers with the highest use administered sedatives and analgesics to approximately 60% of dying infants. The overall use of opioids on the day of death increased during the study period, from 16/283 infants (6%) in 1997 to 523/1465 infants (36%) in 2012 (P < 0.001) (Figure 2). The overall use of benzodiazepines on the day of death also increased from 16/283 (6%) in 1997 to 295/1465 (20%) in 2012 (P < .001).
Figure 1.
Use of opioids (A) and benzodiazepines (B) on day of death among all sites with >10 deaths.
Figure 2.
Exposure to opioids and benzodiazepines by year of death (exposure on the day of death).
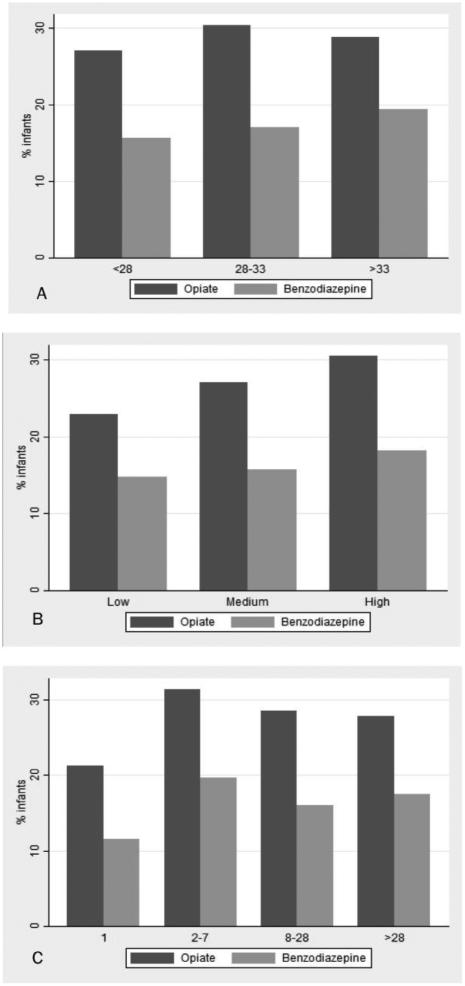
On multivariable analysis, exposure to sedatives or analgesics on the day of death was associated with increasing gestational age, increasing postnatal age, invasive procedure within 2 days of death, more recent year of death, mechanical ventilation, inotropic support, and antibiotics on the day of death (Table III, Figure 3; online).
Table III.
Predictors of sedative and analgesic exposure on the day of death
| OR (95% CI) | ||
|---|---|---|
| Antibiotics on the day of death | 1.75 (1.63, 1.89) | |
| Gestational age (weeks) | ||
| <28 | Reference | |
| 28-33 | 1.32 (1.21, 1.44) | |
| >33 | 1.51 (1.38, 1.68) | |
| Year of death | 1.15 (1.13, 1.16) | |
| Inotropic support | 1.65 (1.53, 1.77) | |
| Invasive procedure within 2 days of death | 1.98 (1.63, 2.39) | |
| Mechanical ventilation | 2.06 (1.82, 2.34) | |
| Postnatal age (days) | ||
| <3 | Reference | |
| ≥3 | 1.64 (1.53, 1.77) | |
Figure 3.
Exposure to opioids and benzodiazepines on the day of death by (A) gestational age (weeks) at birth, (B) average annual center volume, and (C) postnatal age (days) at time of death.
Sensitivity Analysis
We repeated our analysis limiting our cohort to infants admitted for at least 2 days. This included 16,197 (82%) infants from the original cohort of 19,726. Of the 16,197 infants, 5504 (34%) received a sedative or analgesic on the day of death, 5917 (37%) received a sedative or analgesic within the last 2 days of life, and 8830 (55%) received a sedative or analgesic at any point during their hospitalization. Of the 1172 infants who died in 2012, 527 (45%) received a sedative or analgesic on the day of death, 550 (47%) received a sedative or analgesic within the last 2 days of life, and 770 (66%) received a sedative or analgesic at any point during their hospitalization. The demographics of these infants were similar to the original cohort. Infants who received a sedative or analgesic were more likely to have died more recently, be of older gestational age, be on inotropic support, be receiving antibiotics, and to have undergone an invasive procedure in the 2 days prior to death.
DISCUSSION
We found that sedative and analgesic administration to dying infants is limited but increased over time. Limited administration of these drugs has also been shown in the setting of painful procedures, with only one-fifth of painful procedures in 430 infants performed with initiation of analgesic therapy.7 Limited sedative and analgesic use both prior to death and during painful procedures may be explained by numerous factors, including perception of physiology not conducive to experiencing pain (i.e., severe brain injury or comatose),15,21 sudden and unexpected infant death or death in code situations, limited data regarding pharmacokinetics and safety of sedatives and analgesics in infant populations,22 few validated tools to assess pain and no tools to assess suffering in dying infants,23 health care team characteristics and interactions,24 and fear of litigation and hastening of death.11 In addition, data from 2 large randomized controlled trials suggested little improvement in analgesia and no change in the risk of poor neurologic outcome with continuous infusion of opioids during mechanical ventilation.25-27 These findings may have also contributed to the perception that infants should not be exposed to opioids, even in the setting of death.
Our results also demonstrate that, over the last 15 years, the proportion of infants who died and were exposed to sedatives or analgesics on the day of death increased over time. The increasing use over time may reflect increased public interest and knowledge about palliative care and potential deficiencies that exist;1,28 dissemination of evidence that premature infants have the anatomic, neurophysiological, and hormonal components necessary to experience pain and that the experience of pain in the newborn period may be associated with significant short-term morbidity and poor long-term neurodevelopmental outcomes;29-35 and an increase over time in postnatal age at death.36 Similar to the association between older postnatal age and receipt of sedatives and analgesics, we also found that infants of older gestational age were more likely to be exposed to these drugs on the day of death. This finding is consistent with previous evidence that infants of lower birth weight (<800 g) were less likely to receive drugs for comfort at the end of life.13 Limited drug administration for younger and smaller infants may reflect larger societal values of infant personhood and be a consequence of limited methods for detecting discomfort and suffering in the most premature infants.37
In contrast to our results, a recent study of end-of-life care of 151 infants in 4 tertiary NICUs found that the majority (79–97%) of infants received drugs for comfort in the 48 hours before their deaths.11 Similarly, other studies of small cohorts of infants in North American and European NICUs have reported frequent administration of sedatives and analgesics during withdrawal of life support.9,16,17,38 Evidence also suggests that providers frequently administer sedatives and analgesics to dying children.10 In a study of 53 patients at 3 teaching hospitals, 87% received sedatives and analgesics prior to death.21 Similarly, anticipation and treatment of symptoms during withdrawal of life support in adult patients is a near universally accepted phenomenon, with most controversy regarding what doses of sedatives and analgesics are medically, culturally, and legally acceptable in this setting.2,5,39 In a study of 60 adult patients in a Dutch intensive care unit, 80% received opioids and 67% received sedatives during withdrawal of life support.40 Those who did not receive drugs were more likely to have acutely devastating neurologic disease.40
The differences between the results of available studies and our findings may be multifactorial. Previous work has suggested significant variation among neonatologists in end-of-life administration of analgesics and sedatives, and these variations differ by country or region, often resulting from the influence of culture and local law.11,19,41-45 Similarly, our results demonstrate significant variation in end-of-life use of sedatives and analgesics by site. Unlike our study, other studies on neonatal end-of-life sedative and analgesic administration involve ≤4 centers. The results of these smaller studies may represent the practice of individual providers.
Our study is limited by collection of medications entered into the daily progress note on the day of death. We acknowledge that we may miss medications administered at the bedside but not recorded in the medical record during the last stages of death or withdrawal of support. This limitation likely leads to underreporting of drug administration. However, in our study, drug administration was approximately 60% in some centers, which is similar to findings in smaller studies.36
Also among our limitations, the database used for this study also lacks dosing information that could reveal escalation of drug dosing in an attempt to provide comfort, does not provide method of drug administration (e.g., continuous infusion or intermittent bolus doses), and cannot characterize intent of the provider in drug administration. We were also not able to differentiate infants who died because of withdrawal or withholding of life-sustaining therapies from those who died suddenly or without a specific plan for the end of life.18 The distinction between death by decision and unexpected death would certainly influence outcomes and promote improved comparability between our study and others.18 However, we assume that the distribution of mode of death in our cohort is similar to that in other studies. In prior studies, withdrawal or withholding of life support accounts for deaths in up to 73% of infants in tertiary NICUs, and death due to physiologic instability or requiring cardiopulmonary resuscitation outside of the delivery room occurs in less than 10% of deaths in NICUs.14,18,46
Strengths of our study include the characterization of sedative and analgesic use at the end of life in the largest cohort of infants to date, characterization of sedative and analgesic administration in the general care of dying patients and not solely in the setting of withdrawal of life support, and determination of differences in center practice regarding sedative and analgesic management.
Opportunities exist to optimize end-of-life care for infants. Optimized care may be accomplished through the development of standardized protocols or formalized palliative care teams specific to the end-of-life needs in infants. However, more information is needed to identify the optimal components of such protocols and teams, and to develop a standard of care for infants at the end of life. Pertinent information may include the following: (1) reasons for limited sedative and analgesic administration to our most vulnerable pediatric patients; (2) methods to determine suffering in all infants, including at the end of life, and in extremely premature infants, those with hypoxic encephalopathy, and those with congenital anomalies; (3) reasons for variation in end-of-life sedative and analgesic administration among NICUs, including region of the United States, composition of medical providers, and state and local laws; (4) changes in drug dosing surrounding death; and (5) physician intent in drug administration at the end of life.
Acknowledgments
Funding Source: Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number UL1TR001117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, which had no role in study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the manuscript for publication. The first author wrote the first draft of the manuscript (no honorarium/grant/specific payment received).
Dr Zimmerman receives research support from the National Institute of General Medical Sciences (5T32GM086330-03 [PIs: Brouwer, Benjamin, Watkins]). Dr Hornik receives salary support for research from the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR001117). Dr. Ku receives research support from the National Institute of Child Health and Human Development (5T32GM086330-03 [PIs: Brouwer, Benjamin, Watkins]). Dr Laughon receives support from the U.S. government for his work in pediatric and neonatal clinical pharmacology (Government Contract HHSN267200700051C, PI: Benjamin under the Best Pharmaceuticals for Children Act) and from the National Institute of Child Health and Human Development (K23HD068497). Dr Smith receives salary support for research from the NIH and the National Center for Advancing Translational Sciences of the NIH (HHSN267200700051C, HHSN275201000003I, and UL1TR001117); he also receives research support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp).
Abbreviation
- NICU
Neonatal intensive care unit
Footnotes
Conflicts of Interest: The other authors have no financial disclosures relevant to this article.
REFERENCES
- 1.Rubenfeld GD, Curtis JR. End-of-Life Care in the ICU Working Group. End-of-life care in the intensive care unit: a research agenda. Crit Care Med. 2001;29:2001–6. doi: 10.1097/00003246-200110000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Truog RD, Campbell ML, Curtis JR, Haas CE, Luce JM, Rubenfeld GD, et al. Recommendations for end-of-life care in the intensive care unit: a consensus statement by the American College [corrected] of Critical Care Medicine. Crit Care Med. 2008;36:953–63. doi: 10.1097/CCM.0B013E3181659096. [DOI] [PubMed] [Google Scholar]
- 3.Truog RD, Meyer EC, Burns JP. Toward interventions to improve end-of-life care in the pediatric intensive care unit. Crit Care Med. 2006;34(suppl 11):S373–9. doi: 10.1097/01.CCM.0000237043.70264.87. [DOI] [PubMed] [Google Scholar]
- 4.Institute of Medicine . When children die: improving palliative and end-of-life care for children. The National Academies Press; Washington, DC: 2003. [Google Scholar]
- 5.Truog RD, Brock DW, White DB. Should patients receive general anesthesia prior to extubation at the end of life? Crit Care Med. 2012;40:631–3. doi: 10.1097/CCM.0b013e3182413b8a. [DOI] [PubMed] [Google Scholar]
- 6.Billings JA. Humane terminal extubation reconsidered: the role for preemptive analgesia and sedation. Crit Care Med. 2012;40:625–30. doi: 10.1097/CCM.0b013e318228235d. [DOI] [PubMed] [Google Scholar]
- 7.Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008;300:60–70. doi: 10.1001/jama.300.1.60. [DOI] [PubMed] [Google Scholar]
- 8.Walther FJ. Withholding treatment, withdrawing treatment, and palliative care in the neonatal intensive care unit. Early Hum Dev. 2005;81:965–72. doi: 10.1016/j.earlhumdev.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Partridge JC, Wall SN. Analgesia for dying infants whose life support is withdrawn or withheld. Pediatrics. 1997;99:76–9. doi: 10.1542/peds.99.1.76. [DOI] [PubMed] [Google Scholar]
- 10.Carter BS, Howenstein M, Gilmer MJ, Throop P, France D, Whitlock JA. Circumstances surrounding the deaths of hospitalized children: opportunities for pediatric palliative care. Pediatrics. 2004;114:e361–6. doi: 10.1542/peds.2003-0654-F. [DOI] [PubMed] [Google Scholar]
- 11.Janvier A, Meadow W, Leuthner SR, Andrews B, Lagatta J, Bos A, et al. Whom are we comforting? An analysis of comfort medications delivered to dying neonates. J Pediatr. 2011;159:206–10. doi: 10.1016/j.jpeds.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Abe N, Catlin A, Mihara D. End of life in the NICU. A study of ventilator withdrawal. MCN Am J Matern Child Nurs. 2001;26:141–6. doi: 10.1097/00005721-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Matthews AL, O’Conner-Von S. Administration of comfort medication at end of life in neonates: effects of weight. Neonatal Netw. 2008;27:223–7. doi: 10.1891/0730-0832.27.4.223. [DOI] [PubMed] [Google Scholar]
- 14.Wall SN, Partridge JC. Death in the intensive care nursery: physician practice of withdrawing and withholding life support. Pediatrics. 1997;99:64–70. doi: 10.1542/peds.99.1.64. [DOI] [PubMed] [Google Scholar]
- 15.Schultz M, Loughran-Fowlds A, Spence K. Neonatal pain: a comparison of the beliefs and practices of junior doctors and current best evidence. J Paediatr Child Health. 2010;46:23–8. doi: 10.1111/j.1440-1754.2009.01612.x. [DOI] [PubMed] [Google Scholar]
- 16.van der Heide A, van der Maas PJ, van der Wal G, Kollée LA, de Leeuw R. Using potentially life-shortening drugs in neonates and infants. Crit Care Med. 2000;28:2595–9. doi: 10.1097/00003246-200007000-00069. [DOI] [PubMed] [Google Scholar]
- 17.Verhagen AA, Dorscheidt JH, Engels B, Hubben JH, Sauer PJ. Analgesics, sedatives and neuromuscular blockers as part of end-of-life decisions in Dutch NICUs. Arch Dis Child Fetal Neonatal Ed. 2009;94:F434–8. doi: 10.1136/adc.2008.149260. [DOI] [PubMed] [Google Scholar]
- 18.Verhagen AA, Janvier A. The continuing importance of how neonates die. JAMA Pediatr. 2013;167:987–8. doi: 10.1001/jamapediatrics.2013.3065. [DOI] [PubMed] [Google Scholar]
- 19.Lago PM, Piva J, Garcia PC, Troster E, Bousso A, Sarno MO, et al. End-of-life practices in seven Brazilian pediatric intensive care units. Pediatr Crit Care Med. 2008;9:26–31. doi: 10.1097/01.PCC.0000298654.92048.BD. [DOI] [PubMed] [Google Scholar]
- 20.Piva J, Lago P, Othero J, Garcia PC, Fiori R, Fiori H, et al. Evaluating end of life practices in ten Brazilian paediatric and adult intensive care units. J Med Ethics. 2010;36:344–8. doi: 10.1136/jme.2009.035113. [DOI] [PubMed] [Google Scholar]
- 21.Burns JP, Mitchell C, Outwater KM, Geller M, Griffith JL, Todres ID, et al. End-of-life care in the pediatric intensive care unit after the forgoing of life-sustaining treatment. Crit Care Med. 2000;28:3060–6. doi: 10.1097/00003246-200008000-00064. [DOI] [PubMed] [Google Scholar]
- 22.Ward RM, Benitz WE, Benjamin DK, Jr., Blackmon L, Giacoia GP, Hudak M, et al. Criteria supporting the study of drugs in the newborn. Clin Ther. 2006;28:1385–98. doi: 10.1016/j.clinthera.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Gibbins S, Stevens BJ, Yamada J, Dionne K, Campbell-Yeo M, Lee G, et al. Validation of the Premature Infant Pain Profile-Revised (PIPP-R) Early Hum Dev. 2014;90:189–93. doi: 10.1016/j.earlhumdev.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Latimer MA, Johnston CC, Ritchie JA, Clarke SP, Gilin D. Factors affecting delivery of evidence-based procedural pain care in hospitalized neonates. J Obstet Gynecol Neonatal Nurs. 2009;38:182–94. doi: 10.1111/j.1552-6909.2009.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simons SH, van Dijk M, van Lingen RA, Roofthooft D, Duivenvoorden HJ, Jongeneel N, et al. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA. 2003;290:2419–27. doi: 10.1001/jama.290.18.2419. [DOI] [PubMed] [Google Scholar]
- 26.Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, et al. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363:1673–82. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- 27.Aranda JV, Carlo W, Hummel P, Thomas R, Lehr VT, Anand KJ. Analgesia and sedation during mechanical ventilation in neonates. Clin Ther. 2005;27:877–99. doi: 10.1016/j.clinthera.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 28.A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT) JAMA. 1995;274:1591–8. The SUPPORT Principal Investigators. [PubMed] [Google Scholar]
- 29.Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav. 1999;66:627–37. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anand KJS, Sippell WG, Aynsleygreen A. Randomized trial of fentanyl anesthesia in preterm babies undergoing surgery—effects on the stress response. Lancet. 1987;1:243–8. doi: 10.1016/s0140-6736(87)90065-1. [DOI] [PubMed] [Google Scholar]
- 31.Anand KJ, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonate. 2000;77:69–82. doi: 10.1159/000014197. [DOI] [PubMed] [Google Scholar]
- 32.Giannakoulopoulos X, Sepulveda W, Kourtis P, Glover V, Fisk NM. Fetal plasma cortisol and beta-endorphin response to intrauterine needling. Lancet. 1994;344:77–81. doi: 10.1016/s0140-6736(94)91279-3. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald M, Millard C, McIntosh N. Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anaesthesia. Pain. 1989;39:31–6. doi: 10.1016/0304-3959(89)90172-3. [DOI] [PubMed] [Google Scholar]
- 34.Anand KJ, Brown MJ, Causon RC, Christofides ND, Bloom SR, Aynsley-Green A. Can the human neonate mount an endocrine and metabolic response to surgery? J Pediatr Surg. 1985;20:41–8. doi: 10.1016/s0022-3468(85)80390-0. [DOI] [PubMed] [Google Scholar]
- 35.Anand KJ, Carr DB. The neuroanatomy, neurophysiology, and neurochemistry of pain, stress, and analgesia in newborns and children. Pediatr Clin North Am. 1989;36:795–822. doi: 10.1016/s0031-3955(16)36722-0. [DOI] [PubMed] [Google Scholar]
- 36.Dupont-Thibodeau A, Langevin R, Janvier A. Later rather than sooner: the impact of clinical management on timing and modes of death in the last decade. Acta Paediatr. 2014 doi: 10.1111/apa.12747. doi:10.1111/apa.12747. [DOI] [PubMed] [Google Scholar]
- 37.Christoffersen-Deb A. Viability: a cultural calculus of personhood at the beginnings of life. Med Anthropol Q. 2012;26:575–94. doi: 10.1111/maq.12008. [DOI] [PubMed] [Google Scholar]
- 38.Provoost V, Cools F, Bilsen J, Ramet J, Deconinck P, Vander Stichele R, et al. The use of drugs with a life-shortening effect in end-of-life care in neonates and infants. Intensive Care Med. 2006;32:133–9. doi: 10.1007/s00134-005-2863-2. [DOI] [PubMed] [Google Scholar]
- 39.Rocker GM, Heyland DK, Cook DJ, Dodek PM, Kutsogiannis DJ, O'Callaghan CJ. Most critically ill patients are perceived to die in comfort during withdrawal of life support: a Canadian multicentre study. Can J Anaesth. 2004;51:623–30. doi: 10.1007/BF03018407. [DOI] [PubMed] [Google Scholar]
- 40.Epker JL, Bakker J, Kompanje EJO. The use of opioids and sedatives and time until death after withdrawing mechanical ventilation and vasoactive drugs in a Dutch intensive care unit. Anesth Analg. 2011;112:628–34. doi: 10.1213/ANE.0b013e31820ad4d9. [DOI] [PubMed] [Google Scholar]
- 41.Moro T, Kavanaugh K, Okuno-Jones S, Vankleef JA. Neonatal end-of-life care: a review of the research literature. J Perinat Neonatal Nurs. 2006;20:262–73. doi: 10.1097/00005237-200607000-00015. [DOI] [PubMed] [Google Scholar]
- 42.Lago PM, Piva J, Kipper D, Garcia PC, Pretto C, Giongo M, et al. [Life support limitation at three pediatric intensive care units in southern Brazil]. Limitacao de suporte de vida em tres unidades de terapia intensiva pediatrica do sul do Brasil. J Pediatr (Rio J) 2005;81:111–7. [PubMed] [Google Scholar]
- 43.Garros D, Rosychuk RJ, Cox PN. Circumstances surrounding end of life in a pediatric intensive care unit. Pediatrics. 2003;112:e371. doi: 10.1542/peds.112.5.e371. [DOI] [PubMed] [Google Scholar]
- 44.Moore P, Kerridge I, Gillis J, Jacobe S, Isaacs D. Withdrawal and limitation of life-sustaining treatments in a paediatric intensive care unit and review of the literature. J Paediatr Child Health. 2008;44:404–8. doi: 10.1111/j.1440-1754.2008.01353.x. [DOI] [PubMed] [Google Scholar]
- 45.Drake R, Frost J, Collins JJ. The symptoms of dying children. J PainSymptom Manage. 2003;26:594–603. doi: 10.1016/s0885-3924(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 46.Fontana MS, Farrell C, Gauvin F, Lacroix J, Janvier A. Modes of death in pediatrics: differences in the ethical approach in neonatal and pediatric patients. J Pediatr. 2013;162:1107–11. doi: 10.1016/j.jpeds.2012.12.008. [DOI] [PubMed] [Google Scholar]