Abstract
Respiratory syncytial virus (RSV) belongs to the family Paramyxoviridae and is the single most important cause of serious lower respiratory tract infections in young children, yet no highly effective treatment or vaccine is available. To clarify the potential for an anti-G mAb, 131-2G which has both anti-viral and anti-inflammatory effects, to effectively treat RSV disease, we determined the kinetics of its effect compared to the effect of the anti-F mAb, 143-6C on disease in mice. Treatment administered three days after RSV rA2-line19F (r19F) infection showed 131-2G decreased breathing effort, pulmonary mucin levels, weight loss, and pulmonary inflammation earlier and more effectively than treatment with mAb 143-6C. Both mAbs stopped lung virus replication at day 5 post-infection. These data show that, in mice, anti-G protein mAb is superior to treating disease during RSV infection than an anti-F protein mAb similar to Palivizumab. This combination of anti-viral and anti-inflammatory activity makes 131-2G a promising candidate for treating for active human RSV infection.
Keywords: RSV, Anti-viral, RSV G protein, RSV F protein
Introduction
Respiratory syncytial virus (RSV), a pneumovirus of the family Paramyxoviridae, is a leading cause of serious lower respiratory tract disease in infants and young children worldwide (Nair et al., 2010). Unfortunately, there is still no highly effective therapeutics or licensed vaccine to prevent RSV disease. Immune prophylaxis, initially with RSV immune globulin (1998; Groothuis et al., 1993) and later with an RSV anti-F protein neutralizing monoclonal antibody (1998), was shown to be effective and is used to prevent complications of infection in high risk infants and young children (Geevarghese and Simoes, 2012). Good infection control practices can prevent the risk of nosocomial transmission in healthcare settings (Siegel et al., 2007), however effective treatment to address active infection is needed (Krilov, 2011; Ramilo et al., 2014; Tayyari and Hegele, 2012). Although ribavirin may be effective in treating RSV disease in bone marrow transplant patients (Waghmare et al., 2013), and siRNA therapy may be effective in preventing bronchiolitis obliterans in lung transplant patients (Zamora et al., 2011), effective treatment is not available for most RSV-infected patients. This lack of success in treating RSV disease may indicate insufficient anti-viral activity, or possibly the need to combine anti-viral with anti-inflammatory treatment, as suggested by a study in cotton rats (Ottolini et al., 2002).
RSV encodes eleven proteins, and among them, the F and G surface proteins induce protective immunity (Collins and Melero, 2011; Graham, 2011). The F protein induces high titers of neutralizing antibodies and better cross-protection against different RSV strains in mice than the G protein (Connors et al., 1991; Olmsted et al., 1986; Stott et al., 1987). The G protein has a substantial role in inducing and modulating the host immune response to infection (Tripp, 2004), and some of the responses appear to contribute to disease pathogenesis. The G protein has a CX3C chemokine motif in the central conserved region of the G protein that has been shown to contribute to some of these activities (Boyoglu-Barnum et al., 2013; Harcourt et al., 2006; Haynes et al., 2003; Tripp et al., 2003). The G protein CX3C motif binds to the CX3C chemokine receptor, CX3CR1, and mimics some activities of the only known CX3C chemokine, fractalkine (Tripp et al., 2001). This motif, located between aa 182–186 of the G protein, includes two of the four evolutionarily conserved cysteines at aa 173, 176, 182, and 186 which form a cysteine noose structure.
Monoclonal antibody (mAb) 131-2G (Anderson et al., 1988; Anderson et al., 1986) binds to the central conserved region of the G protein, blocks G protein binding to CX3CR1, and decreases several disease manifestations in RSV challenged mice including pulmonary inflammation and mucous production, increased airway resistance after primary infection in mice, and enhanced inflammation in RSV-challenged FI-RSV vaccinated mice (Boyoglu-Barnum et al., 2013; Haynes et al., 2009; Miao et al., 2009; Radu et al., 2010; Tripp et al., 2003). Treatment with this mAb also neutralizes virus in vivo in an Fc dependent fashion (Miao et al., 2009; Radu et al., 2010), but not in vitro (Anderson et al., 1988). Importantly, 131-2G F(ab’)2 decreases pulmonary inflammation after both primary RSV challenge or challenge in FI-RSV vaccinated mice without decreasing viral load (Miao et al., 2009; Radu et al., 2010).
We have previously shown that administration of mAb 131-2G at 3 days post infection (p.i.) neutralizes virus and decreases pulmonary inflammation by 5 days p.i. (Miao et al., 2009). The F(ab’)2 form of 131-2G similarly decreased pulmonary inflammation without effecting lung virus titers. Interestingly, 131-2G decreased pulmonary inflammation more effectively than an anti-F mAb, 143-6C, that reacts at the same antigenic site as palivizumab and like palivizumab both neutralizes RSV and inhibits RSV fusion (Anderson et al., 1988; Boyoglu-Barnum et al., 2014; DeVincenzo et al., 2014). Han et al recently reported that a humanized mAb that reacts at the same antigenic site as 131-2G also decreases airway reactivity induced by methacholine challenge and does this much more effectively than palivizumab (Han et al., 2014). These data suggest that an anti-G mAb like 131-2G might be more effective than anti-F neutralizing antibodies in treating active RSV infection. To clarify the potential for 131-2G-like antibodies to effectively treat RSV disease, we determined the kinetics of its effect compared to the effect of the anti-F mAb, 143-6C on disease in mice. Since airway disease in such an important component of human RSV disease, we studied the effect of these mAbs on virus induced airway resistance and mucus production in mice infected with RSV rA2-line19F (r19F). RSV r19F increases airway resistance and mucus productions in mice while the more commonly used RSV A2 strain does not (Boyoglu-Barnum et al., 2013; Lugo and Nahata, 1993). The results demonstrate that treatment with the anti-G protein mAb 131-2G can decrease RSV airway disease more rapidly and effectively than the anti-F protein mAb 143-6C.
MATERIALS AND METHODS
Mice
Six-to-eight weeks old, specific pathogen–free, female BALB/c mice (Charles River Laboratory, Wilmington, MA) were used in all experiments. All animal procedures were performed according to a protocol approved by Emory University (Atlanta, GA) Institutional Animal Care and Use Committee. RSV r19F was generated as described previously (Boyoglu-Barnum et al., 2013). Animal study plan was described in Figure 1.
Figure 1.
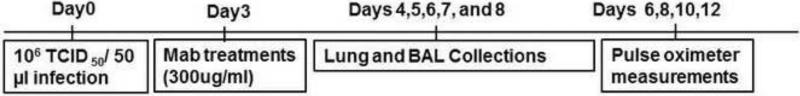
Experimental schedule for animal studies. Day indicates day relative to RSV challenge.
Quantification of lung viral load
Pulmonary viral load was assessed by measuring infectious virus in homogenized lung tissue. BeadBeater (Biospec Products, Bartlesville, OK) was used to homogenize the lungs as described (Boyoglu-Barnum et al., 2013). Virus infectivity titers were determined by a micro-infectivity assay as previously described (Anderson et al., 1985). The infectivity titer was calculated using the Reed and Muench method.
Viral RNA levels were determined by RSV real-time PCR
Total RNA was extracted from homogenized lung tissue using a Qiagen total-RNA extraction kit (Qiagen, Valencia, CA) according to the manufacturer's instructions and stored at −80°C. Quantitative real-time PCR was performed by using an AgPath-ID one-step reverse transcription (RT)-PCR kit (Applied Biosystems, Foster City, CA) and Stratagene3000 detection system (Agilent Technologies, Santa Clara, CA). Thermal cycling conditions included 10 min at 45°C, followed by 45 cycles of 15 sec at 95°C and 1 min at 55°C. The primers and probes for the RSV matrix (M) gene were (forward primer, 5’-GGC AAA TAT GGA AAC ATA GCT GAA-3’; reverse primer, 5’-TCT TTT TCT AGG ACA TTG TAY TGA ACA G-3’; probe, 5’-6-carboxyfluorescein (FAM)-TGT CCG TCT TCT ACG CCC TCG TC- black hole quencher 1 (BHQ-1)-3’). Serial dilutions of known Plaque Forming Units (PFU) of RSV RNA were used to obtain a standard curve for quantitative real-time PCR. Threshold cycles (CT) for each sample were converted to PFU equivalents/ml (PFU/ml) using the standard curve. Assays were performed for three different sets of mice.
Bronchoalveolar leuckocyte (BAL) specimens
Mice were anesthetized and euthanized by exsanguination after severing of the left axillary artery. Bronchoalveolar leuckocytes (BAL) were harvested by lavaging the lungs 3 times using 1 mL sterile 1X PBS for each wash. The BAL cells were stained for extracellular markers using a microculture staining protocol described by Tripp et al (Tripp et al., 1999). Briefly, BAL cells were blocked in Fc blocker (anti CD16/32) in 1X PBS with 1% bovine serum albumin for 15 min at 4 °C and then stained for 30 min at 4 °C in the dark with the appropriate combinations of anti-CD3 (17A2) (T cells), anti-CD4 (GK1.5)(T cells), anti-CD8 (53-6.7)(T cells), anti-CD45R/B220(RA3-6B2) (B cells), anti-CD11b (M1/70) (macrophages, dendritic cells, and monocytes), anti-mouse Ly06G/Gr-1 (RB6-8C5) (polymorphonuclear cells;PMNs), anti-mouse CD49b/Integrin alpha2 (DX5) (NK and NK T cells), and mouse isotype antibody controls (all from eBiosciences, San Diego, CA) diluted in staining buffer. The distribution and pattern of cell surface markers was determined for 30,000 lymphocyte-gated events analyzed on a BD LSRII flow cytometer (BD Biosciences, Mountain View, CA) and data analyzed using FlowJo software (TreeStar, Ashland, OR).
Pulse oximetry
Pulsus paradoxus is an abnormally large decrease in systolic blood pressure and pulse wave amplitude during inspiration and indicates increased breathing effort. The pulsus paradoxus, or breathing effort, was determined by breath-associated difference in distension of vessel walls detected by a rodent pulse oximeter (MouseOx; Starr Life Sciences Corp., Oakmont, PA) and used as an indication of airway dysfunction, as described (Boyoglu-Barnum et al., 2013). Data were analyzed using Microsoft Excel and presented in microns.
Determination of Muc5AC protein expression
Mucin-5AC protein (Muc5AC) concentration in lung tissues was assayed with an ELISA kit for mouse Mucin 5 Subtype AC (USCN Life Science Inc., Wuhan, China), according to the manufacturers protocol as previously described (Boyoglu-Barnum et al., 2013). The concentration of Muc5AC in the samples was determined by comparing the OD of the samples to the standard curve generated and expressed as fold increase in levels compared to mock-infected mice.
Histopathology
Lungs were fixed in 10% formalin for 1hr, followed by 70% ethanol and embedded in paraffin blocks. Five–micrometer sections of lung tissue were deparaffinized in xylene, rehydrated through a graded series of ethanol and stained with periodic acid-schiff (PAS) (Sigma-Aldrich, St. Louis, MO) to assess intracellular pulmonary mucin levels. PAS-stained slides were digitally scanned using a Hamamatsu Nanozoomer 2.0HT slide scanner (Meyer Instruments, Houston, TX) with a 20X objective and analyzed using ImageJ software. Fifteen to 20 fields (20X magnification) were examined per tissue section.
Multiplex cytokine analysis
Pulmonary cytokine levels were determined from the cell-free supernatant of centrifuged lung homogenates using a mouse cytokine 20-plex panel kit and the Luminex 100/200™ with xMAPTM technology (Invitrogen, Valencia, CA). The concentration of each cytokine was determined by comparison to standard curve according to the manufacturer's instructions. The threshold of detection was 1.12±0.24 pg/ml. Cytokine levels in lung homogenates were normalized to the protein (in milligrams) present in cell-free preparations of lung supernatants as measured by the BCA assay, according to the manufacturer's protocol (Thermo, Rockford, IL).
Statistical analyses
Unless otherwise indicated, groups were compared by one-way analysis of variance (ANOVA) and post-hoc Tukey's HSD test (p ≤0.05). p ≤ 0.05 was considered statistically significant. All statistical analyses were performed using the statistical package R (R Developmental Core Team 2012). Data are shown as mean ± SEM.
Results
131-2G mAb treatment reduces weight loss earlier than 143-6C
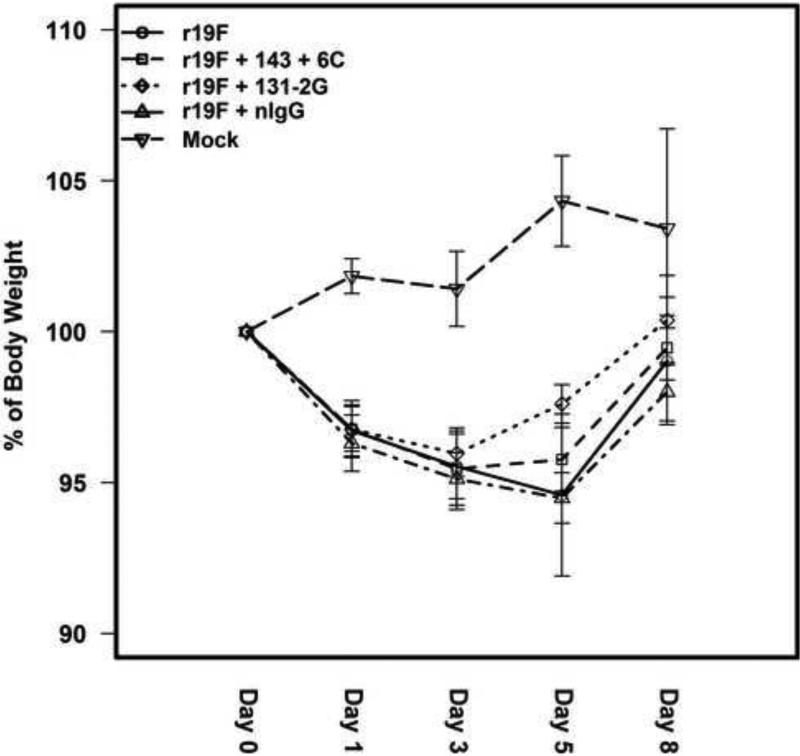
RSV infected mice treated with 131-2G mAb began to promptly reverse morbidity as determined by weight loss in infected mice (Figure 2). On day 5 pi, the weights for 131-2G treated mice were significantly (p≤0.05) higher than untreated mice (97.60±1.39% vs 94.59±5.84% of pre-infection weight, respectively). The increased weight in 131-2G treated compared to untreated, RSV infected mice persisted through day 8 pi though no longer significantly greater than for untreated mice. Mice treated with 143-6C mAb showed less reversal of weight loss with weights being greater, but not significantly (p>-0.05) greater than untreated, infected mice at day 5 pi (95.78±2.31% vs 94.59±5.84% of weight before infection, respectively) (Figure 2).
Figure 2.
The effect of treatment of RSV G protein mAb 131-2G or RSV F protein mAb 143-6C on weight change in r19F infected BALB/c mice. BALB/c mice were i.n. infected with r19F (1×106 TCID50) on day 0 and administered 300 μg of anti-G (131-2G) or anti-F (143-6C) protein mAbs on day 3 pi (n=5 mice/group). Data are means ± SEM. *, indicates significant increase on the body weight after 131-2G treatment compared to untreated, infected mice. No significant difference was detected for mAb 143-6C treated compared to untreated, infected mice. Significance (p≤0.05) was determined by one-way analysis of variance (ANOVA) and post-hoc Tukey's HSD test. Results are representative of two independent experiments.
131-2G mAb and 143-6C treatment decrease pulmonary virus titer
The effects of treatment with mAbs 131-2G and 143-6C on lung virus load were determined on days 4-8 pi. In untreated mice, virus was isolated from the lung through day 6 pi. With mAb 131-2G, virus was only isolated at 4 days pi (TCID50 log 2.69±0.09) at a titer 2.17 log-fold less than untreated mice. Live virus was not detected (TCID50 > log 2.24±1.14 is the limit of detection) at any days post treatment in mice treated with mAb 143-6C (Table 1).
Table 1.
The effect of anti-RSV G protein mAb 131-2G and anti-RSV F protein mAb 143-6C treatments on viral load of BALB/c mice. Viral titer represented as log values.
| Groups | Day4 pi | Day5 pi | Day6 pi | Day7 pi | Day8 pi |
|---|---|---|---|---|---|
| r19F | 5.84±0.78* | 5.29±0.66* | 3.14±0.82* | ND | ND |
| r19F+143-6C | ND | ND | ND | ND | ND |
| r19F+131-2G | 2.69±0.09 | ND | ND | ND | ND |
| r19F+nIgG | 5.89±0.25 | 5.31±0.129 | 3.18±0.58 | ND | ND |
| Mock | ND | ND | ND | ND | ND |
BALB/c mice were infected with r19F (1×106 TCID50) on day 0 and administered 300μg of anti-G (131-2G) or anti-F (143-6C) protein mAbs on day 3 pi (n=3 mice/group). Data are means ± SEM.
indicates significant difference in viral load for untreated, r19F challenged mice compared with mAb 131-2G and mAb 143-6C treated mice.
Significance (p<0.001) was determined by one-way analysis of variance (ANOVA) and post-hoc Tukey's HSD test. Results are representative of two independent experiments. ND indicates “Not detected”.
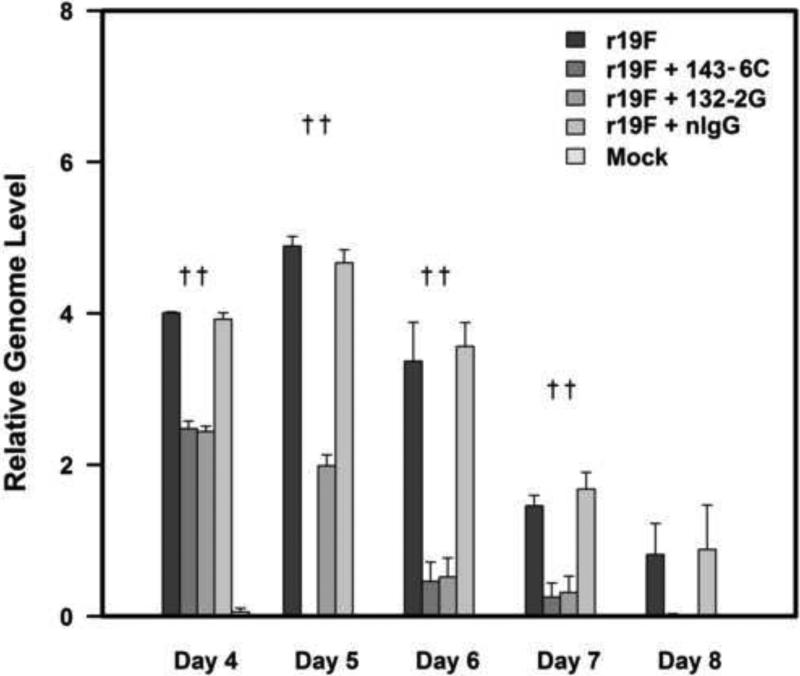
The RSV real-time PCR results for lung specimens (3 mice/group) on days 4-8 pi similarly showed that treatment with both mAbs decreased virus replication beginning immediately after treatment with 143-6C being somewhat more effective than 131-2G, i.e. no infectious virus on day 4 pi (Table 1) and lower CT values on day 5 pi (Figure 3).
Figure 3.
The level of RSV replication in lung tissue was evaluated by real-time RT-PCR (qRT-PCR) for M gene expression (relative genome level equivalent log PFU/g of lung tissue). BALB/c mice were i.n. infected with r19F (1×106 TCID50) on day 0 and administered 300 μg of anti-G (131-2G) or anti-F (143-6C) protein mAbs on day 3 pi (n=5 mice/group). Data are means ± SEM. †, indicates significant decrease for mAbs 131-2G or 143-6C treated compared to untreated, infected mice. Significance (p<0.001) was determined by one-way analysis of variance (ANOVA) and post-hoc Tukey's HSD test.
131-2G mAb treatment reduces pulmonary inflammation
The effects of treatment with mAbs 131-2G and 143-6C on pulmonary inflammation based on BAL cells on days 4,5,6,7 and 8 pi showed the peak number of cells (219×103±5.77×103) was on day 5 pi in untreated, r19F infected mice. Treatment with 131-2G showed a rapid effect of treatment with a significant (p<0.05) decrease in the total BAL cell number compared to untreated mice for all days after treatment. In contrast, treatment with 143-6C did not significantly (p≥0.05) decrease BAL cell numbers on day 4 pi, but did on the remaining days. The decrease in BAL cell numbers after 143-6C treatment was significantly (p≤0.05) less than after 131-2G treatment at all time-points (Figure 4). There also was substantial difference in the types of cells in the BAL (Table 2) between the two treatments. On day 4 pi, the greatest difference in cell types between 131-2G and 143-6C treatment was in B cells, PMNs, and NK cell numbers. By day 5 and 6 pi, the greatest difference between the two treatments was for CD3, CD4, and CD8 positive cell numbers. For example, on day 4 pi, the percent decrease compared to untreated mice for CD3− CD45R/B220+ cells (B cells) was 11.2% for 131-2G and 1.0% for 143-6C; for CD3− Ly-6G/Gr-1+ cells (polymorphonuclear cells, PMNs) 78.9% for 131-2G and 9.0% for 143-6C; and for CD49b/integrin alpha 2+ cells (NK cells) 38.8% for 131-2G and 9.3% for 143-6C for CD3. On day 6 pi, the percent decrease compared to untreated mice for CD3+ cells was 49.1% for 131-2G and 8.7% for 143-6C; for CD4+ cells 46.1% for 131-2G and 6.9% for 143-6C; and for CD8+ cells 51.3% for 131-2G and 5.39% for 143-6C.
Figure 4.
The effect of treatment of RSV G protein mAb 131-2G and RSV F protein mAb 143-6C on BAL cell number in r19F infected BALB/c mice. BALB/c mice were infected with r19F (1×106 TCID50) on day 0 and administered 300 μg of anti-G (131-2G) or anti-F (143-6C) protein mAbs on day 3 pi (n=5 mice/group). Data are means ± SEM. *, indicates significant decrease for 131-2G treated compared to untreated, infected mice: †, indicates significant decrease in cell number for mAb 143-6C treated compared to untreated, infected mice: ‡, indicates significant decrease in cell number for 131-2G treated compared to mAb 143-6C treated. Significance (p<0.05) was determined by one-way analysis of variance (ANOVA) and post-hoc Tukey's HSD test. Results are representative of two independent experiments.
Table 2.
Changes in the characteristics of the BAL cell infiltrate after treatment with the anti- G protein mAb 131-2G and the anti-F protein mAb 143-6C.
| Untreated | mAb 131-2G treated | mAb 143-6C treated | ||||
|---|---|---|---|---|---|---|
| Days(pi) | Phenotype | Mean Number Cells [103]±SE | Mean Number Cells [103]±SE | % Reduction | Mean Number Cells [103]±SE | % Reduction |
| 4 | CD3 | 42.01±0.17 | 34.56±0.53* | 17.73±0.93 | 33.86±1.20* | 19.38±3.18 |
| CD4 | 22.68±0.25 | 19.98±0.19* | 11.92±1.78 | 20.46±0.80 | 9.54±2.56 | |
| CD8 | 19.33±0.42 | 14.59±0.34* | 24.52±0.14 | 13.41±0.40** | 30.56±3.55 | |
| B cells | 8.77±0.41 | 7.79±0.05* | 11.17±0.04 | 8.68±0.08* | 1.03±0.20 | |
| Macrophage | 8.88±0.04 | 4.93±0.71* | 44.46±7.83 | 6.56±0.24** | 26.03±2.99 | |
| PMNs | 9.67±0.13 | 2.04±0.71** | 78.90±2.11 | 8.80±0.06* | 8.99±0.34 | |
| NK | 47.31±0.14 | 28.94±1.26** | 38.82±2.83 | 42.93±1.22* | 9.27±2.33 | |
| 5 | CD3 | 71.28±2.66 | 36.06±0.83* | 49.32±3.06 | 68.74±0.14 | 3.48±3.40 |
| CD4 | 31.03±0.70 | 15.79±0.13** | 49.11±0.73 | 28.66±0.26 | 7.59±2.93 | |
| CD8 | 24.88±0.05 | 9.81±0.31** | 61.49±2.41 | 24.78±0.09 | 2.82±2.64 | |
| B cells | 12.45±0.52 | 8.17±0.07* | 36.78±3.09 | 11.36±1.00 | 12.36±4.21 | |
| Macrophage | 17.43±0.31 | 6.96±0.24** | 60.03±2.09 | 14.98±0.50* | 14.00±4.40 | |
| PMNs | 6.90±0.35 | 1.97±0.09** | 67.43±4.53 | 6.11±0.58* | 19.78±3.56 | |
| NK | 71.04±3.16 | 29.31±1.43** | 58.61±3.85 | 62.99±1.56 | 11.13±6.14 | |
| 6 | CD3 | 74.83±0.79 | 38.12±0.20** | 49.05±0.27 | 68.30±2.17 | 8.74±1.93 |
| CD4 | 39.66±0.77 | 19.33±0.06** | 46.09±1.37 | 37.53±1.31 | 6.91±2.48 | |
| CD8 | 24.47±0.97 | 13.19±0.33** | 51.26±0.79 | 22.77±0.60 | 5.39±1.48 | |
| B cells | 7.36±0.09 | 4.16±0.39** | 43.36±6.11 | 6.61±0.29 | 10.19±0.77 | |
| Macrophage | 15.33±0.21 | 10.19±0.38* | 33.56±1.57 | 10.13±0.58* | 33.92±2.88 | |
| PMNs | 3.56±0.21 | 2.54±0.03* | 28.66±0.69 | 2.65±0.14* | 25.72±4.03 | |
| NK | 73.35±3.09 | 36.04±0.23** | 50.79±2.39 | 34.09±1.67** | 53.38±4.24 | |
| 7 | CD3 | 76.94±1.93 | 38.72±1.20** | 49.62±2.82 | 65.77±2.00 | 14.12±0.46 |
| CD4 | 45.70±1.41 | 23.25±0.65* | 49.06±3.00 | 40.69±1.58 | 10.99±0.71 | |
| CD8 | 25.48±4.55 | 10.69±0.64** | 49.68±3.76 | 17.13±0.33 | 19.47±0.38 | |
| B cells | 6.83±0.24 | 5.30±0.29** | 45.99±4.32 | 5.91±0.48 | 13.47±0.23 | |
| Macrophage | 15.58±0.31 | 9.44± 0.27** | 39.34±2.95 | 8.05±0.17** | 48.29±0.07 | |
| PMNs | 3.89±0.06 | 2.47±0.06** | 36.46±0.67 | 2.58±0.06** | 33.57±2.46 | |
| NK | 61.03±1.55 | 36.98±4.01** | 46.26±0.02 | 29.34±0.97** | 56.87±6.08 | |
| 8 | CD3 | 70.12±1.95 | 40.49±1.11** | 42.26±0.02 | 51.86±2.11* | 26.04±0.95 |
| CD4 | 42.17±0.97 | 24.25±0.66** | 42.50±0.25 | 30.72±1.22* | 27.17±1.21 | |
| CD8 | 17.88±0.58 | 12.08±0.21* | 32.41±0.99 | 14.99±0.61* | 16.21±0.70 | |
| B cells | 8.42±0.20 | 3.38±0.09** | 59.93±0.14 | 4.34±0.16** | 48.52±0.67 | |
| Macrophage | 11.65±0.01 | 5.78±0.22** | 50.41±1.85 | 5.38±0.17** | 53.81±1.44 | |
| PMNs | 3.52±0.10 | 1.41±0.06** | 59.94±2.92 | 1.71±0.09** | 51.41±4.17 | |
| NK | 51.95±1.12 | 19.70±0.37** | 62.08±0.10 | 23.12±1.04** | 55.51±1.05 | |
BALB/c mice were infected with r19F (1×106 TCID50) on day 0 and administered 300ug of anti-G (131-2G) or anti-F (143-6C) protein mAbs on day 3 pi (n=5 mice/group). Data are the mean total number of BAL cells by subtype at days 4-8 pi per lung: lymphocyte gate, anti-CD3+ (17A2), and anti-CD4+ (GK1.5) CD4 T cells; lymphocyte gate, CD3+ and anti-CD8+ (53-6.7) – CD8 T cells; CD3-, anti-CD45R/B220+ (RA3-6B2)- B cells; CD3-, anti-CD11b+ (M1/70)- macrophages, dendritic cells, and monocytes; CD3-, anti-mouse Ly06G/Gr-1 (RB6-8C5)- Polymorphonuclear cells (PMNs); CD3-, anti-mouse CD49b/Integrin alpha2 (DX5)- NK cell. The fold reduction is decrease in the number in treated relative to untreated mice for the cell type.
(p≤0.05, ANOVA)
(p≤0.001, ANOVA) indicates significant decrease in numbers for untreated compared to mice receiving the indicated treatments. Results are representative of two independent experiments.
131-2G mAb treatment reduces airway dysfunction earlier than 143-6C
The effects of treatment with mAbs 131-2G and 143-6C on airway dysfunction was assessed by pulsus paradoxus on days 6, 8, 10 and 12 pi. Similar to previous studies (Boyoglu-Barnum et al., 2013; Stokes et al., 2011), r19F challenged mice had significant (p≤0.05) increase in breathing effort compared to mock-infected mice on day 6 pi which increased through day 8 pi (Figure 5). Treatment with mAb 131-2G significantly (p≤0.001) decreased r19F induced breathing effort to levels similar to mock-infected mice at all time-points examined. In contrast, treatment with 143-6C did not decrease RSV r19F induced breathing effort until 8 days pi and breathing effort was greater at all times-points compared to 131-2G treated mice (Figure 5). On day 8 pi, there was a 67.21±5.84% decrease in breathing effort for 131-2G treated compared to untreated, infected mice while 143-6C treated mice had a 47.06±3.81% decrease in breathing effort (Figure 5).
Figure 5.
The effect of treatment of RSV G protein mAb 131-2G and RSV F protein mAb 143-6C on breathing effort in r19F infected BALB/c mice. BALB/c mice were infected with r19F (1×106 TCID50) on day 0 and administered 300 μg of anti-G (131-2G) or anti-F (143-6C) protein mAbs on day 3 pi (n=5 mice/group). Breath associated change in distension of peripheral arteries in microns (breathing effort) was measured at 6,8,10 and 12 days pi. Data are means ± SEM. *, indicates significant decrease (p≤0.05) for mAbs 131-2G or 143-6C treated compared to untreated, infected mice: †, indicates significant decrease (p≤0.001) for mAbs 131-2G or 143-6C treated compared to untreated, infected mice: ‡, indicates significant decrease for 131-2G treated compared to mAb 143-6C treated mice. Significance (p≤0.001) was determined by one-way analysis of variance (ANOVA) and post-hoc Tukey's HSD test. Results are representative of two independent experiments.
131-2G mAb treatment reduces pulmonary mucin production earlier than 143-6C
An increase in airway mucous is one feature of RSV disease in children (Lugo and Nahata, 1993; Quinn et al., 1985). In this study, untreated r19F challenged mice when compared to mock challenged mice showed increased mucous production on days 4-8 pi with peak increase on day 8 pi (Figure 6). There was a significant (p≤0.001) decrease in Muc5AC protein in the lungs of the mice treated with 131-2G on days 5, 6, 7 and 8 but only on days 7 and 8 for 143-6C treated mice (Figure 6).
Figure 6.
The effect of treatment of RSV G protein mAb 131-2G and RSV F protein mAb 143-6C on muc5AC protein levels in r19F virus infected BALB/c mice. BALB/c mice were infected with r19F (1×106 TCID50) on day 0 and administered 300ug of anti-G (131-2G) or anti-F (143-6C) protein mAbs on day 3 pi (n=5 mice/group). The lungs were harvested 8 days pi and lung homogenates tested for muc5AC levels. Data are means ± SEM. *, indicates significant decrease (p≤0.05) for mAbs 131-2G or 143-6C treated compared to untreated, infected mice. †, indicates significant decrease (p≤0.001) for mAbs 131-2G or 143-6C treated compared to untreated, infected mice: ‡, indicates significant decrease (p≤0.05) for 131-2G treated compared to mAb 143-6C treated. Significance was determined by one-way analysis of variance (ANOVA) and post-hoc Tukey's HSD test. Results are representative of two independent experiments.
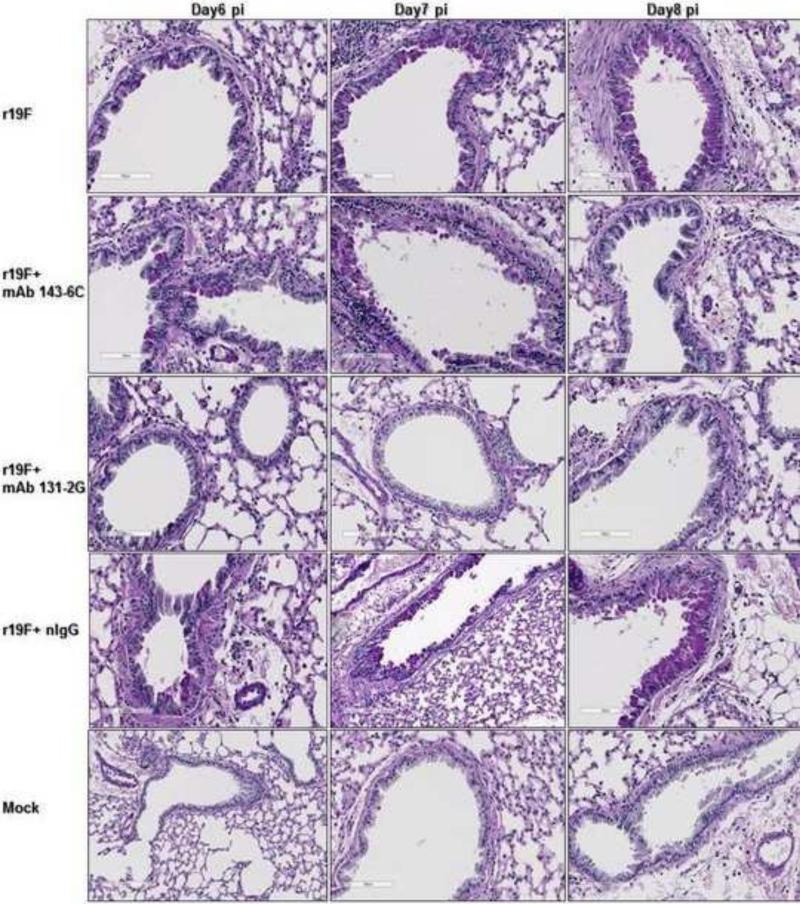
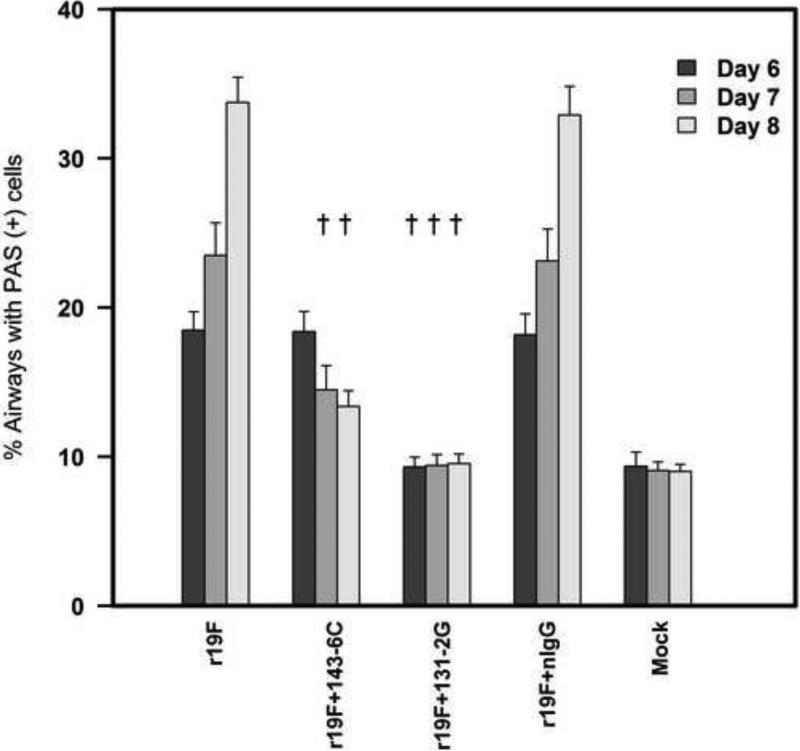
Differences in pulmonary mucous levels were also evident in lung histopathology studies by either qualitative measurements with PAS staining (Figure 7) or quantitative measurements with ImageJ analysis (Figure 8). Stained lung sections showed PAS-positive cells for untreated, or control mAb treated r19F challenged mice on days 6,7 and 8pi. Mock challenged mice and mAb 131-2G treated, r19F challenged mice did not show PAS-positive cells at any time-points while mAb 143-6C treated, r19F challenged mice showed PAS positive cells on days 6 and 7 pi (Figures 7 & 8).
Figure 7.
Mucin production associated with RSV r19F infection and RSV anti-G and F protein mAbs 131-2G and 143-6C treatment. BALB/c mice were infected with mock-infected tissue culture supernatant (mock) or 1×106 TCID50 of r19F and untreated or treated 3 days after challenge with mAbs 131-2G and 143-6C or control immune globulin (nIgG). The lungs were harvested 4-8 days pi for PAS staining. Reddish-purple color within cells lining bronchioles or alveoli indicate cells that are PAS positive and contain of mucin (n=3 mice/group). Day 6, first day with clear PAS staining, and day 8, last day studied, shown.
Figure 8.
Percentage of PAS positive cells in mouse airways. PAS-stained slides were digitally scanned using a Hamamatsu Nanozoomer 2.0HT slide scanner (Meyer Instruments, Houston, TX) with a 20X objective and analyzed using ImageJ software. Fifteen to 20 fields (20X magnification) were examined per tissue section and the percentage of PAS positive cells determined using ImageJ software. Data are means ± SEM. †, indicates significant difference (p≤0.001) for mAb 131-2G and mAb 143-6C treated mice compared untreated, r19F infected mice: ‡, indicates significant decrease (p≤0.05) for mAb 131-2G treated compared to mAb 143-6C treated, as determined by one-way analysis of variance (ANOVA) and post-hoc Tukey's HSD test compare. Results are representative of two independent experiments.
Cytokine responses
To evaluate the effect of treatment with 131-2G and 143-6C mAbs on lung inflammation, we determined cytokine and chemokine levels in lung homogenates. The most notable differences were on days 5, 6 and 7 pi when lower levels of pro-inflammatory and Th2 cytokines were detected in 131-2G treated compared to untreated r19F infected mice. For these days, mice treated with 143-6C had slightly lower levels of some pro-inflammatory and Th2 cytokines and chemokines compared to untreated mice, but the decreases were not statistically significant and less than in 131-2G treated mice (Table 3A-B). Th1-type cytokine levels were lower for 131-2G treated mice compared to untreated mice on days 4 and 5 pi and then usually higher for days 6, 7 and 8 pi. In contrast, mice treated with 143-6C tended to have a Th1-type cytokine response pattern similar to untreated mice (Table 3C). The lower level of pro-inflammatory cytokines after 131-2G treatment is consistent with fewer inflammatory cells in the lung. Since Th2 cytokines such as IL-4, IL-13, IL-5 and MCP-1 have been associated with the increased airway resistance and mucous production (Boyoglu-Barnum et al., 2013), lower levels of these cytokines in 131-2G treated mice is consistent with 131-2G treatment decreasing airway resistance and mucous production in r19F infected mice. The higher level of Th1 cytokines later during infection is consistent the shift away from a Th2 type response and toward a Th1 memory response with 131-2G treatment previously noted (Boyoglu-Barnum et al., 2014).
Table 3A.
The effect of anti-G protein mAb 131-2G and anti-F protein 143-6C treatment on Pro inflammatory cytokine/chemokine levels in r19F RSV infected mice
| Untreated | mAb 131-2G treated | mAb 143-6C treated | ||
|---|---|---|---|---|
| Days (pi) | Pro inflammatory Cytokines/chemokines | pg/gram of lung | pg/gram of lung | pg/gram of lung |
| 4 | MIP-1α | 20.45±3.17 | 14.60±3.46* | 18.33±2.18 |
| MCP-1 | 16.04±2.08 | 13.24±3.87* | 15.72±2.45 | |
| IL-1α | 42.87±5.36 | 25.96±4.43** | 27.53±3.12** | |
| IL-1β | 4.57±2.10 | 2.86±1.83 | 4.46±3.49 | |
| IL-6 | 7.52±4.99 | 5.66±4.51 | 6.72±2.64 | |
| 5 | MIP-1α | 44.01±15.07 | 19.41±15.31** | 46.00±3.88 |
| MCP-1 | 31.76±12.64 | 12.24±11.88** | 30.02±5.39 | |
| IL-1α | 109.46±41.43 | 42.75±9.84** | 95.58±31.70 | |
| IL-1β | 15.33±3.02 | 2.79±2.74** | 11.64±6.30 | |
| IL-6 | 13.60±5.99 | 6.55±1.52 | 8.50±4.83 | |
| 6 | MIP-1α | 47.41±13.03 | 24.06±5.51* | 33.77±12.12 |
| MCP-1 | 65.17±14.55 | 19.87±7.93** | 45.25±25.79 | |
| IL-1α | 96.28±26.17 | 48.34±4.21** | 56.24±11.26** | |
| IL-1β | 22.38±14.11 | 7.47±5.12** | 15.90±6.84 | |
| IL-6 | 10.56±3.51 | 6.10±0.51 | 7.61±1.02 | |
| 7 | MIP-1α | 21.98±4.73 | 25.55±8.85 | 25.96±2.24 |
| MCP-1 | 31.90±12.52 | 20.02±6.40* | 31.62±5.48 | |
| IL-1α | 42.81±7.42 | 36.05±14.80 | 50.51±9.97 | |
| IL-1β | 10.90±3.70 | 5.31±3.57 | 5.06±3.50 | |
| IL-6 | 5.63±4.47 | ND | 1.78±1.75 | |
| 8 | MIP-1α | 14.37±2.47 | 11.22±6.38 | 12.25±5.03 |
| MCP-1 | 8.07±3.78 | 6.87±3.79 | 8.94±4.56 | |
| IL-1α | 33.54±3.11 | 27.58±10.71 | 30.65±10.41 | |
| IL-1β | ND | ND | ND | |
| IL-6 | 2.75±1.97 | ND | ND | |
BALB/c mice were infected with r19F (1×106 TCID50) on day 0 and administered 300μg of anti-G (131-2G) or anti-F (143-6C) protein mAbs on day 3 pi (n=5 mice/group). The lungs were harvested on the indicated days in the Table 3A. The lung homogenates were tested by luminex multiplex assay. Data are means ± SEM.
(p≤0.05, ANOVA)
(p≤0.001, ANOVA) indicates significant changes in cytokine concentrations for untreated compared to mice receiving the indicated treatments. Results are representative of two independent experiments.
Table 3B.
The effect of anti-G protein mAb 131-2G and anti-F protein 143-6C treatment on Th2 type cytokine levels in r19F RSV infected mice.
| Untreated | mAb 131-2G treated | mAb 143-6C treated | ||
|---|---|---|---|---|
| Days (pi) | Th2 cytokines | pg/gram of lung | pg/gram of lung | pg/gram of lung |
| 4 | IL-4 | 15.21±3.19 | 14.35±2.61 | 14.00±1.77 |
| IL-5 | 10.65±0.32 | 4.67±0.77* | 9.84±0.47 | |
| IL-13 | ND | ND | ND | |
| 5 | IL-4 | 45.14±15.54 | 12.34±3.23** | 34.56±12.43 |
| IL-5 | 14.30±1.16 | 2.34±1.56** | 6.71±2.49 | |
| IL-13 | ND | ND | ND | |
| 6 | IL-4 | 30.20±5.91 | 12.11±3.83* | 24.34±9.20 |
| IL-5 | 4.55±2.38 | 1.68±0.71 | 3.01±1.45 | |
| IL-13 | 12.86±1.98 | 4.86±2.01* | 11.98±1.68 | |
| 7 | IL-4 | 19.74±3.58 | 11.33±1.60 | 11.26±4.15 |
| IL-5 | 1.82±0.62 | 0.92±0.41 | 2.00±1.16 | |
| IL-13 | 16.84±3.32 | 4.21±1.28** | 10.86±2.49 | |
| 8 | IL-4 | 4.68±2.75 | 1.25±1.09 | 2.99±2.18 |
| IL-5 | ND | ND | ND | |
| IL-13 | 28.34±5.36 | ND** | 5.68±1.42** | |
BALB/c mice were infected with r19F (1×106 TCID50) on day 0 and administered 300 μg of anti-G (131-2G) or anti-F (143-6C) protein mAbs on day 3 pi (n=5 mice/group). The lungs were harvested on the indicated days in the Table 3B. The lung homogenates were tested by luminex multiplex assay. Data are means ± SEM.
(p≤0.05, ANOVA)
(p≤0.001, ANOVA) indicates significant changes in cytokine concentrations for untreated compared to mice receiving the indicated treatments. Results are representative of two independent experiments.
Table 3C.
The effect of anti-G protein mAb 131-2G and anti-F protein 143-6C treatment on Th1 type cytokine levels in r19F RSV infected mice.
| Untreated | mAb 131-2G treated | mAb 143-6C treated | ||
|---|---|---|---|---|
| Days (p.i) | Th1 cytokines | pg/gram of lung | pg/gram of lung | pg/gram of lung |
| 4 | MIG | 132.39±62.18 | 141.26±63.57 | 122.39±69.97 |
| IFN-γ | 0.71±0.29 | 0.49±0.21 | 0.54±0.17 | |
| IP-10 | 41.75±16.07 | 10.79±2.82** | 14.28±2.43** | |
| IL-12 | 3.44±0.37 | 2.99±2.67 | 2.59±1.18 | |
| 5 | MIG | 1472.67±668.69 | 203.25±185.86** | 507.30±367.72** |
| IFN-γ | 82.04±26.35 | 11.89±5.44** | 57.82±21.07* | |
| IP-10 | 218.79±105.51 | 51.59±30.15** | 155.49±68.46 | |
| IL-12 | 3.42±1.40 | 2.54±1.76 | 2.62±0.45 | |
| 6 | MIG | 1521.28±679.05 | 2335.77±1016.70 | 697.94±388.84** |
| IFN-γ | 62.88±24.23 | 80.88±43.46 | 28.61±17.29** | |
| IP-10 | 205.40±43.72 | 232.32±77.85 | 96.85±41.80** | |
| IL-12 | 4.44±2.57 | 6.58±1.16 | 1.50±0.52 | |
| 7 | MIG | 537.76±34.49 | 1118.74±296.59** | 1069.70±496.54** |
| IFN-γ | 24.25±7.83 | 53.37±8.60* | 29.27±7.53 | |
| IP-10 | 60.77±14.30 | 141.90±36.47** | 60.77±19.81 | |
| IL-12 | 2.63±0.42 | 5.02±1.95 | 2.32±0.62 | |
| 8 | MIG | 507.39±156.93 | 408.92±230.97 | 290.84±244.74 |
| IFN-γ | 7.80±1.24 | 13.86±3.38 | 7.71±5.65 | |
| IP-10 | 29.88±5.25 | 53.74±13.96* | 36.52±20.25 | |
| IL-12 | 3.43±1.42 | 4.73±1.70 | 1.89±3.56 | |
BALB/c mice were infected with r19F (1×106 TCID50) on day 0 and administered 300 μg of anti-G (131-2G) or anti-F (143-6C) protein mAbs on day 3 pi (n=5 mice/group). The lungs were harvested on the indicated days in the Table 3B. The lung homogenates were tested by luminex multiplex assay. Data are means ± SEM.
(p≤=0.05, ANOVA)
(p≤0.001, ANOVA) indicates significant changes in cytokine concentrations for untreated compared to mice receiving the indicated treatments. Results are representative of two independent experiments.
Discussion
Respiratory syncytial virus (RSV) is a leading cause of serious lower respiratory tract disease in infants and young children worldwide, and may lead to as many as 200,000 deaths and 3-4 million hospitalizations in children <5 years of age each year (Nair et al., 2010). Though a high priority for vaccine and antiviral drug development neither a vaccine nor highly effective antiviral drug is yet available. The focus of RSV anti-viral drug development has been stopping virus replication. In a recent study of RSV challenged adults, treatment early in illness with a drug that blocks RSV fusion was highly effective in decreasing virus replication and decreasing upper respiratory tract symptoms (DeVincenzo et al., 2014). It is unknown if this, or other drugs, will also be effective in treating disease later in the course of naturally acquired infection. To have a significant effect on disease later during infection, the antiviral drug must control infection and disease quicker than the host's immune response does. Our data in mice show that the anti-F mAb 143-6C may stop virus replication quicker than the anti-G protein mAb 131-2G but 131-2G treats disease much quicker. The anti-F protein mAb 143-6C used in this study is a mouse alternative to palivizumab, i.e. it reacts at the same antigenic site and has similar activities to those of palivizumab (Anderson et al., 1988; Beeler and van Wyke Coelingh, 1989; Johnson et al., 1997). In humans palivizumab is effective for prophylaxis of RSV disease in high risk infants but neither it, nor a more potent mAb derived from palivizumab, have been effective in treating active RSV infection (Ramilo et al., 2014). Mice treated with mAb 131-2G 3 days pi with RSV r19F showed a marked decrease in all measures of disease assessed including weight loss, the number and type of pulmonary inflammatory cells, airway resistance, and mucus production several days sooner than, mice treated with 143-6C. This was evident despite 143-6C's slightly greater effect on virus replication as indicated by pulmonary virus titer and RSV RNA levels. These findings support and extend the findings described by Han et al (Han et al., 2014). In that study, RSV-infected mice treated on 2 day pi with a humanized anti–G mAb similar to 131-2G had significantly less methacholine induced airway resistance than untreated mice at days 7-10 pi. These anti-G mAb treated mice also had a lower number of BAL cell number on day 7 pi In contrast mice treated on day 2 pi with the anti-F mAb palivizumab had levels of methancholine induced airway resistance similar (days 7, 10 pi) or greater (day 14 pi) than untreated, infected mice. The anti-F mAb treated mice had similar numbers of BAL cells as untreated infected mice. We suspect that 131-2G has a more rapid effect on disease because it binds G and prevents it from inducing host responses that contribute to disease. In vivo, 131-2G has both an anti-viral effect and an anti-inflammatory effect (Miao et al., 2009; Radu et al., 2010) and it is likely that the anti-inflammatory effect explains the more rapid effect on disease. Earlier studies show that RSV G protein can enhance pulmonary inflammation and, thus, it is not surprising that binding G protein might have an anti-inflammatory effect (Andersson et al., 2000; Collarini et al., 2009; Hashimoto et al., 2004; Kauvar et al., 2010; Miao et al., 2009; Radu et al., 2010). For example, experiments in mice challenged with virus lacking G protein or administered the anti-G protein mAb before or after challenge show that presence of the G protein during infection increases weight loss, pulmonary inflammatory cells (including eosinophils in FI-RSV vaccinated mice), pulmonary mucous production, and breathing effort. This effect of G may be related to one or more previously described immune modulatory effects of G. The G protein has been associated with suppressing a number of immune responses including Toll-like receptor (TLR) 3 or 4 activity, induction of IFN-β (Shingai et al., 2008), the pro inflammatory response of lung epithelial cells (Arnold et al., 2004), lymphoproliferation (Ray et al., 2001), a number of innate responses including activation of dendritic cells (Polack et al., 2005; Ray et al., 2001), and type I IFN production by inducing suppression of cytokine signaling (SOCS) (Oshansky et al., 2009). It has also been associated with enhancing other immune responses including cytotoxic T cell responses (Bukreyev et al., 2008), pulmonary substance P levels (Tripp et al., 2000), and pulmonary Th2 cytokines.
The mechanism by which the RSV G protein modulates the host immune response and augments disease is not clear but could linked to the CX3C chemokine motif at aa 182 to 186. This motif functionally mimics some activities of the CX3C chemokine fractalkine (FKN) (Tripp et al., 2001). Some effects of the G protein have previously been linked directly to the G protein-CX3CR1 interaction including enhanced inflammation in RSV challenged FI-RSV vaccinated mice, decreased migration of CX3CR1+ T cells to the lung of infected mice, and decreased rate of breathing in mice given the G protein intravenously.
Importantly, as noted in earlier studies binding G with 131-2G F(ab’)2 has an anti-inflammatory effect without decreasing virus replication (Boyoglu-Barnum et al., 2013; Miao et al., 2009). The intact mAb used in this study however does neutralize RSV in vivo (Boyoglu-Barnum et al., 2013; Miao et al., 2009; Radu et al., 2010). Thus, in the mouse, 131-2G probably effects the course of RSV disease by both down regulating G induced inflammation and decreasing virus replication. The pulmonary cytokine and chemokine studies are consistent with and suggest some mechanisms involved in this effect of 131-2G. Treatment with 131-2G decreased the proinflammatory and Th2 cytokine/chemokine levels and increased Th1 levels significantly more effectively than mAb 143-6C. Binding G presumably deliminated its ability to increase proinflammatory and Th2 cytokines and chemokines that likely contribute to disease. Presence of the RSV G protein during infection of mice has previously been associated with increase in Th2 and proinflammatory cytokines and chemokines (Haynes et al., 2003; Maher et al., 2004; Polack et al., 2005; Tripp et al., 1999).
We hypothesize that a human mAb with activity similar to the mouse mAb 131-2G, will be effective in treating human infection. The fact that 131-2G rapdily decreases the multiple manifestations of disease in the mouse including two associated with RSV disease in humans, i.e. airway dysfucntion and increased mucous production, more rapidly than 143-6C and has both an antiviral and antiinflammatory effect, gives us hope that a human version of 131-2G maybe effective in treating human infection when other anti-RSV treatments have not. The fact that binding the G protein with antibody effectively decreases disease suggests G should also be considered as a target for anti-RSV drug development
Highlights.
Airway disease in such an important component of human RSV disease.
RSV rA2-line19F (r19F) increases airway resistance and mucus productions in mice.
The effects of mAbs 131-2G and 143-6C on airway resistance and mucus production in mice were studied.
131-2G treatment can decrease RSV airway disease more rapidly and effectively than mAb 143-6C.
Acknowledgments
Financial Support
This work was supported by NIH 1U19AI095227 grant awarded to MLM and LJA, NIH 1R01AI087798 (MLM), funding from Children's Healthcare of Atlanta, support from the Immunology Core and Flow core of Emory+Children's Pediatric Research Center, and the Emory Vaccinology Training Grant (VTP) T32 5T32AI074492-03. This study was also supported through a grant to Emory University from Trellis RSV Holdings, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflicts of interest
Larry J. Anderson has done paid consultancies on RSV vaccines for MedImmune, Inc.; Novartis Vaccines and Therapeutics; Crucell Holland B.V; and AVC, LLC. His laboratory has received funding for a study of RSV treatment in mice through a grant to Emory University from Trellis RSV Holdings, Inc. He is also co-inventor on patents held by the Centers for Disease Control and Prevention on RSV vaccines and treatment that include use of anti-G protein mAbs for treatment of RSV disease. All other authors report no potential conflicts. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- The IMpact-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- Anderson LJ, Bingham P, Hierholzer JC. Neutralization of respiratory syncytial virus by individual and mixtures of F and G protein monoclonal antibodies. Journal of virology. 1988;62:4232–4238. doi: 10.1128/jvi.62.11.4232-4238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LJ, Hierholzer JC, Bingham PG, Stone YO. Microneutralization test for respiratory syncytial virus based on an enzyme immunoassay. J Clin Microbiol. 1985;22:1050–1052. doi: 10.1128/jcm.22.6.1050-1052.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LJ, Hierholzer JC, Stone YO, Tsou C, Fernie BF. Identification of epitopes on respiratory syncytial virus proteins by competitive binding immunoassay. J Clin Microbiol. 1986;23:475–480. doi: 10.1128/jcm.23.3.475-480.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson C, Liljestrom P, Stahl S, Power UF. Protection against respiratory syncytial virus (RSV) elicited in mice by plasmid DNA immunisation encoding a secreted RSV G protein-derived antigen. FEMS Immunol Med Microbiol. 2000;29:247–253. doi: 10.1111/j.1574-695X.2000.tb01530.x. [DOI] [PubMed] [Google Scholar]
- Arnold R, Konig B, Werchau H, Konig W. Respiratory syncytial virus deficient in soluble G protein induced an increased proinflammatory response in human lung epithelial cells. Virology. 2004;330:384–397. doi: 10.1016/j.virol.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Beeler JA, van Wyke Coelingh K. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. Journal of virology. 1989;63:2941–2950. doi: 10.1128/jvi.63.7.2941-2950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyoglu-Barnum S, Chirkova T, Todd SO, Barnum TR, Gaston KA, Jorquera P, Haynes LM, Tripp RA, Moore ML, Anderson LJ. Prophylaxis with a respiratory syncytial virus (RSV) anti-G protein monoclonal antibody shifts the adaptive immune response to RSV rA2-line19F infection from Th2 to Th1 in BALB/c mice. Journal of virology. 2014;88:10569–10583. doi: 10.1128/JVI.01503-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyoglu-Barnum S, Gaston KA, Todd SO, Boyoglu C, Chirkova T, Barnum TR, Jorquera P, Haynes LM, Tripp RA, Moore ML, Anderson LJ. A respiratory syncytial virus (RSV) anti-G protein F(ab')2 monoclonal antibody suppresses mucous production and breathing effort in RSV rA2-line19F-infected BALB/c mice. Journal of virology. 2013;87:10955–10967. doi: 10.1128/JVI.01164-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukreyev A, Yang L, Fricke J, Cheng L, Ward JM, Murphy BR, Collins PL. The secreted form of respiratory syncytial virus G glycoprotein helps the virus evade antibody-mediated restriction of replication by acting as an antigen decoy and through effects on Fc receptor-bearing leukocytes. Journal of virology. 2008;82:12191–12204. doi: 10.1128/JVI.01604-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collarini EJ, Lee FE, Foord O, Park M, Sperinde G, Wu H, Harriman WD, Carroll SF, Ellsworth SL, Anderson LJ, Tripp RA, Walsh EE, Keyt BA, Kauvar LM. Potent high-affinity antibodies for treatment and prophylaxis of respiratory syncytial virus derived from B cells of infected patients. Journal of immunology (Baltimore, Md. : 1950) 2009;183:6338–6345. doi: 10.4049/jimmunol.0901373. [DOI] [PubMed] [Google Scholar]
- Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 2011;162:80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors M, Collins PL, Firestone CY, Murphy BR. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. Journal of virology. 1991;65:1634–1637. doi: 10.1128/jvi.65.3.1634-1637.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVincenzo JP, Whitley RJ, Mackman RL, Scaglioni-Weinlich C, Harrison L, Farrell E, McBride S, Lambkin-Williams R, Jordan R, Xin Y, Ramanathan S, O'Riordan T, Lewis SA, Li X, Toback SL, Lin SL, Chien JW. Oral GS-5806 activity in a respiratory syncytial virus challenge study. The New England journal of medicine. 2014;371:711–722. doi: 10.1056/NEJMoa1401184. [DOI] [PubMed] [Google Scholar]
- Geevarghese B, Simoes EA. Antibodies for prevention and treatment of respiratory syncytial virus infections in children. Antivir Ther. 2012;17:201–211. doi: 10.3851/IMP2061. [DOI] [PubMed] [Google Scholar]
- Graham BS. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol Rev. 2011;239:149–166. doi: 10.1111/j.1600-065X.2010.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis JR, Simoes EA, Levin MJ, Hall CB, Long CE, Rodriguez WJ, Arrobio J, Meissner HC, Fulton DR, Welliver RC, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. The New England journal of medicine. 1993;329:1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- Han J, Takeda K, Wang M, Zeng W, Jia Y, Shiraishi Y, Okamoto M, Dakhama A, Gelfand EW. Effects of anti-g and anti-f antibodies on airway function after respiratory syncytial virus infection. Am J Respir Cell Mol Biol. 2014;51:143–154. doi: 10.1165/rcmb.2013-0360OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt J, Alvarez R, Jones LP, Henderson C, Anderson LJ, Tripp RA. Respiratory syncytial virus G protein and G protein CX3C motif adversely affect CX3CR1+ T cell responses. Journal of immunology (Baltimore, Md. : 1950) 2006;176:1600–1608. doi: 10.4049/jimmunol.176.3.1600. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Graham BS, Ho SB, Adler KB, Collins RD, Olson SJ, Zhou W, Suzutani T, Jones PW, Goleniewska K, O'Neal JF, Peebles RS., Jr. Respiratory syncytial virus in allergic lung inflammation increases Muc5ac and gob-5. Am J Respir Crit Care Med. 2004;170:306–312. doi: 10.1164/rccm.200301-030OC. [DOI] [PubMed] [Google Scholar]
- Haynes LM, Caidi H, Radu GU, Miao C, Harcourt JL, Tripp RA, Anderson LJ. Therapeutic monoclonal antibody treatment targeting respiratory syncytial virus (RSV) G protein mediates viral clearance and reduces the pathogenesis of RSV infection in BALB/c mice. J Infect Dis. 2009;200:439–447. doi: 10.1086/600108. [DOI] [PubMed] [Google Scholar]
- Haynes LM, Jones LP, Barskey A, Anderson LJ, Tripp RA. Enhanced disease and pulmonary eosinophilia associated with formalin-inactivated respiratory syncytial virus vaccination are linked to G glycoprotein CX3C-CX3CR1 interaction and expression of substance P. Journal of virology. 2003;77:9831–9844. doi: 10.1128/JVI.77.18.9831-9844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, Dormitzer M, O'Grady J, Koenig S, Tamura JK, Woods R, Bansal G, Couchenour D, Tsao E, Hall WC, Young JF. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176:1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- Kauvar LM, Harcourt JL, Haynes LM, Tripp RA. Therapeutic targeting of respiratory syncytial virus G-protein. Immunotherapy. 2010;2:655–661. doi: 10.2217/imt.10.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krilov LR. Respiratory syncytial virus disease: update on treatment and prevention. Expert Rev Anti Infect Ther. 2011;9:27–32. doi: 10.1586/eri.10.140. [DOI] [PubMed] [Google Scholar]
- Lugo RA, Nahata MC. Pathogenesis and treatment of bronchiolitis. Clin Pharm. 1993;12:95–116. [PubMed] [Google Scholar]
- Maher CF, Hussell T, Blair E, Ring CJ, Openshaw PJ. Recombinant respiratory syncytial virus lacking secreted glycoprotein G is attenuated, non-pathogenic but induces protective immunity. Microbes Infect. 2004;6:1049–1055. doi: 10.1016/j.micinf.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Miao C, Radu GU, Caidi H, Tripp RA, Anderson LJ, Haynes LM. Treatment with respiratory syncytial virus G glycoprotein monoclonal antibody or F(ab')2 components mediates reduced pulmonary inflammation in mice. J Gen Virol. 2009;90:1119–1123. doi: 10.1099/vir.0.009308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted RA, Elango N, Prince GA, Murphy BR, Johnson PR, Moss B, Chanock RM, Collins PL. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci U S A. 1986;83:7462–7466. doi: 10.1073/pnas.83.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshansky CM, Krunkosky TM, Barber J, Jones LP, Tripp RA. Respiratory syncytial virus proteins modulate suppressors of cytokine signaling 1 and 3 and the type I interferon response to infection by a toll-like receptor pathway. Viral Immunol. 2009;22:147–161. doi: 10.1089/vim.2008.0098. [DOI] [PubMed] [Google Scholar]
- Ottolini MG, Curtis SJ, Porter DD, Mathews A, Richardson JY, Hemming VG, Prince GA. Comparison of corticosteroids for treatment of respiratory syncytial virus bronchiolitis and pneumonia in cotton rats. Antimicrob Agents Chemother. 2002;46:2299–2302. doi: 10.1128/AAC.46.7.2299-2302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack FP, Irusta PM, Hoffman SJ, Schiatti MP, Melendi GA, Delgado MF, Laham FR, Thumar B, Hendry RM, Melero JA, Karron RA, Collins PL, Kleeberger SR. The cysteine-rich region of respiratory syncytial virus attachment protein inhibits innate immunity elicited by the virus and endotoxin. Proc Natl Acad Sci U S A. 2005;102:8996–9001. doi: 10.1073/pnas.0409478102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn SF, Erickson S, Oshman D, Hayden F. Lobar collapse with respiratory syncytial virus pneumonitis. Pediatr Radiol. 1985;15:229–230. doi: 10.1007/BF02388761. [DOI] [PubMed] [Google Scholar]
- Radu GU, Caidi H, Miao C, Tripp RA, Anderson LJ, Haynes LM. Prophylactic treatment with a G glycoprotein monoclonal antibody reduces pulmonary inflammation in respiratory syncytial virus (RSV)-challenged naive and formalin-inactivated RSV-immunized BALB/c mice. Journal of virology. 2010;84:9632–9636. doi: 10.1128/JVI.00451-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramilo O, Lagos R, Saez-Llorens X, Suzich J, Wang CK, Jensen KM, Harris BS, Losonsky GA, Griffin MP. Motavizumab treatment of infants hospitalized with respiratory syncytial virus infection does not decrease viral load or severity of illness. Pediatr Infect Dis J. 2014;33:703–709. doi: 10.1097/INF.0000000000000240. [DOI] [PubMed] [Google Scholar]
- Ray R, Hoft DF, Meyer K, Brown R, Lagging LM, Belshe RB. Immunoregulatory role of secreted glycoprotein G from respiratory syncytial virus. Virus Res. 2001;75:147–154. doi: 10.1016/s0168-1702(01)00237-4. [DOI] [PubMed] [Google Scholar]
- Shingai M, Azuma M, Ebihara T, Sasai M, Funami K, Ayata M, Ogura H, Tsutsumi H, Matsumoto M, Seya T. Soluble G protein of respiratory syncytial virus inhibits Toll-like receptor 3/4-mediated IFN-beta induction. Int Immunol. 2008;20:1169–1180. doi: 10.1093/intimm/dxn074. [DOI] [PubMed] [Google Scholar]
- Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am J Infect Control. 2007;35:S65–164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes KL, Chi MH, Sakamoto K, Newcomb DC, Currier MG, Huckabee MM, Lee S, Goleniewska K, Pretto C, Williams JV, Hotard A, Sherrill TP, Peebles RS, Jr., Moore ML. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. Journal of virology. 2011;85:5782–5793. doi: 10.1128/JVI.01693-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott EJ, Taylor G, Ball LA, Anderson K, Young KK, King AM, Wertz GW. Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. Journal of virology. 1987;61:3855–3861. doi: 10.1128/jvi.61.12.3855-3861.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayyari F, Hegele RG. Identifying targets in the hunt for effective respiratory syncytial virus interventions. Expert Rev Respir Med. 2012;6:215–222. doi: 10.1586/ers.12.8. [DOI] [PubMed] [Google Scholar]
- Tripp RA. Pathogenesis of respiratory syncytial virus infection. Viral Immunol. 2004;17:165–181. doi: 10.1089/0882824041310513. [DOI] [PubMed] [Google Scholar]
- Tripp RA, Dakhama A, Jones LP, Barskey A, Gelfand EW, Anderson LJ. The G glycoprotein of respiratory syncytial virus depresses respiratory rates through the CX3C motif and substance P. Journal of virology. 2003;77:6580–6584. doi: 10.1128/JVI.77.11.6580-6584.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol. 2001;2:732–738. doi: 10.1038/90675. [DOI] [PubMed] [Google Scholar]
- Tripp RA, Moore D, Jones L, Sullender W, Winter J, Anderson LJ. Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. Journal of virology. 1999;73:7099–7107. doi: 10.1128/jvi.73.9.7099-7107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp RA, Moore D, Winter J, Anderson LJ. Respiratory syncytial virus infection and G and/or SH protein expression contribute to substance P, which mediates inflammation and enhanced pulmonary disease in BALB/c mice. Journal of virology. 2000;74:1614–1622. doi: 10.1128/jvi.74.4.1614-1622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghmare A, Campbell AP, Xie H, Seo S, Kuypers J, Leisenring W, Jerome KR, Englund JA, Boeckh M. Respiratory syncytial virus lower respiratory disease in hematopoietic cell transplant recipients: viral RNA detection in blood, antiviral treatment, and clinical outcomes. Clin Infect Dis. 2013;57:1731–1741. doi: 10.1093/cid/cit639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora MR, Budev M, Rolfe M, Gottlieb J, Humar A, Devincenzo J, Vaishnaw A, Cehelsky J, Albert G, Nochur S, Gollob JA, Glanville AR. RNA interference therapy in lung transplant patients infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2011;183:531–538. doi: 10.1164/rccm.201003-0422OC. [DOI] [PubMed] [Google Scholar]