Abstract
The association between chronic kidney disease (CKD) and cardiovascular disease (CVD) is well established, and there is mounting evidence of inter-organ crosstalk that may accelerate pathological processes and the progression of organ dysfunction in both systems. This process, termed cardiorenal syndrome (CRS) by the Acute Dialysis Quality Initiative, is considered a major health problem: patients with CKD and CVD are at much higher risk of mortality than patients with either condition alone. To date, the majority of CRS research has focused on neurohormonal mechanisms and hemodynamic alterations. However, mounting evidence suggests that abnormalities in the normal pathophysiology of the bone-mineral axis, iron, and erythropoietin play a role in accelerating CKD and CVD. The goal of this manuscript is to review the role and interrelated effects of the bone-mineral axis and anemia in the pathogenesis of chronic CRS.
Keywords: cardiorenal syndrome (CRS), bone-mineral axis, iron, erythropoietin (EPO), anemia, fibroblast growth factor 23 (FGF-23), phosphate, vitamin D, chronic kidney disease (CKD), cardiovascular disease (CVD), renal dysfunction, heart failure (HF), end-stage renal disease (ESRD)
Cardiorenal syndrome (CRS) is a group of diagnoses defined as disorders of the heart and kidney whereby dysfunction in one organ induces or worsens dysfunction in the other. The umbrella term and discrete subtypes (Box 1) were developed by the Acute Dialysis Quality Initiative (ADQI) to emphasize the bidirectional pathways and to provide context for identifying the complex pathophysiological interactions that occur in disorders that involve both the heart and kidney.1
Box 1. Discrete Subtypes of Cardiorenal Syndrome as Defined by the ADQI.
| Acute Cardiorenal Syndrome (Type 1): Acute worsening of cardiac function leading to kidney dysfunction |
| Chronic Cardiorenal Syndrome (Type 2): Chronic abnormalities in cardiac function leading to kidney dysfunction |
| Acute Renocardiac Syndrome (Type 3): Acute worsening of kidney function causing cardiac dysfunction |
| Chronic Renocardiac Syndrome (Type 4): Chronic abnormalities in kidney function leading to cardiac disease |
| Secondary Cardiorenal Syndromes (Type 5): Systemic conditions causing simultaneous dysfunction of the heart and kidney |
Abbreviation: ADQI, Acute Dialysis Quality Initiative
Despite advances in treatment of both cardiovascular and kidney disease, CRS remains a major health problem. Cardiovascular disease (CVD) is one of the most common causes of death;2 In 2011, the US Centers for Disease Control and Prevention reported that nearly 25% of all deaths in the general population were due to the disease.3 Reduced kidney function is associated with an increased risk for cardiovascular events and mortality compared with that of the general population,4 with incrementally increased risk as glomerular filtration rate declines.5,6 According to the US Renal Data System, CVD is the most common cause of death in the setting of end-stage renal disease (ESRD).7 Of note, individuals with stage 3 chronic kidney disease (CKD) are more likely to die of CVD than to progress to ESRD.8 Furthermore, patients with combined cardiovascular and kidney disease are at much higher risk of mortality than patients with either in isolation.9
Since Lindner et al10 first described the association between ESRD and CVD, a number of community-based studies, national databases, pooled analyses, and therapeutic intervention trials have sought to validate the complex relationship between CKD and atherosclerotic CVD.7,11–15 However, data specific to heart failure may be of greater value: a recent meta-analysis by Damman et al16 indicated that the prevalence of CKD in patients with heart failure exceeds 30% and is associated with an odds ratio for all-cause mortality of 2.34 (95% confidence interval [CI], 2.20–2.50).
It appears that multiple mechanisms underlie CRS, and an extensive body of literature has focused on neurohormonal mechanisms and hemodynamic alterations (Bock and Gottlieb17). Given that abnormalities in iron, the bone-mineral axis, and anemia are frequent complications of uremia (and that the management of CKD frequently involves interventions directed at these parameters), the goal of this article is to review the potential role of these factors in the pathogenesis and possible future prevention of chronic CRS (types 2 and 4).
Biochemical abnormalities in CKD
Bone-mineral axis
Deterioration in mineral homeostasis is progressive as kidney function declines, causing disruptions in serum and tissue concentrations of phosphate and calcium as well as changes in circulating levels of hormones like parathyroid hormone (PTH), vitamin D metabolites (calcidiol and calcitriol), and fibroblast growth factor 23 (FGF-23) (Figure 1). As early as CKD stage 3, the kidneys show a reduced ability to appropriately excrete phosphate, eventually resulting in hyperphosphatemia, which triggers elevations in PTH and FGF-23 concentrations. Further, impaired conversion of calcidiol to calcitriol reduces intestinal calcium absorption and increases PTH levels. As the kidney is unable to respond adequately to PTH, phosphaturia and calcium reabsorption are compromised. In addition, the kidney does not respond to FGF-23 secreted by bone, further reducing phosphate excretion and enhancing phosphate retention.18 Disruptions in the mineral and endocrine function associated with CKD are vital in controlling bone modeling and remodeling; thus, bone abnormalities are nearly ubiquitous in patients with dialysis-dependent CKD and biochemically detectable in the majority of patients with CKD stages 3 to 5.19
Figure 1. Changes in bone and mineral metabolism in chronic kidney disease.
Reduced functional kidney mass leads to phosphate retention and decreased activation of vitamin D, thus resulting in hyperphosphatemia, reduced intestinal calcium absorption, and hypocalcemia. Changes in phosphorous, calcium, and vitamin D, as well as decreased kidney responsiveness, contribute to increased parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF-23) secretion.
Iron
Because excess iron is capable of generating free radicals that are damaging to lipid membranes, proteins, and nucleic acids, circulating iron concentrations are tightly regulated. Hepcidin is a key mediator, regulating iron stores by binding and inducing internalization and degradation of ferroportin, an iron export protein. A genetic absence of hepcidin results in unchecked iron absorption via the ferroportin channels and the clinical syndrome of hemochromatosis.20 In chronic disease, hepcidin is produced at greater levels and reduces the amount of iron absorbed from the gastrointestinal tract and also traps iron in the liver and bone marrow macrophages, thereby restricting iron trafficking throughout the body, particularly in the bone marrow. Hence, both intestinal iron absorption and iron release are decreased; as such, circulating iron concentrations and iron availability to target tissues are decreased as well.21 In patients receiving dialysis, iron homeostasis is disrupted further by negative iron balance (due to blood losses from dialysis and repeated phlebotomy), depleted iron stores (resulting from increased production of red blood cells during treatment with erythropoiesis-stimulating agents), and impaired intestinal absorption of dietary iron (due to relative achlorhydria).22
Anemia
Reduced synthesis of erythropoietin (EPO; 90% of which is produced in the kidneys, 10% in liver and other organs), is considered an important cause of anemia in CKD. The main stimulus for EPO production is renal hypoxia, but this mechanism is impaired in patients with CKD.23 As kidney function declines, decreases in EPO are accompanied by a decrease in available iron (as previously described), which further hinders erythropoiesis, proliferation of erythroid precursors, and the production of EPO and EPO receptors.23 Finally, abnormalities in the availability of iron, excessive levels of inflammation, disturbance in bone and mineral homeostasis, and other factors appear to decrease bone marrow responsiveness to EPO, further exacerbating anemia as CKD progresses.24–27 Thus, the anemia of CKD, including in those with chronic kidney failure, can be characterized by elevated levels of hepcidin, impaired iron absorption and transport, reduced or inappropriately low levels of and response to EPO, and (in patients treated with hemodialysis) excess blood losses.
Relationship between the bone-mineral axis and iron
Data on possible relations between the bone-mineral axis and iron are derived from associations between hypophosphatemia and stimulated erythropoiesis in some hematopoietic disorders28,29 as well as after intravenous (IV) iron infusion.30 Iron-induced hypophosphatemia may result from increased cellular uptake of phosphate during erythropoiesis, but renal phosphate wasting appears to be an important mechanism of iron-induced hypophosphatemia.31 Clinical data have shown that administering saccharated ferric oxide32 or iron polymaltose33 results in impaired tubular phosphate handling (reabsorption).32,33 In addition to renal phosphate loss, there is also evidence for inhibition of renal 1α-hydroxylase activity and decreased 1,25-dihydroxyvitamin D concentrations. In patients receiving dialysis or with earlier stages of CKD, effects of iron administration on FGF-23 are inconsistent. In patients with non–dialysis-dependent CKD, ferric carboxymaltose induces reduction in serum phosphate concentrations that persists for 3 months together with a significant decrease in FGF-23 concentrations (though other bone metabolism parameters are unaffected).34 In patients treated with hemodialysis, IV saccharated ferric oxide increases already elevated FGF-23 concentrations without inducing hypophosphatemia and inappropriately low 1,25-dihydroxyvitamin D concentrations in the absence of functioning kidney tissue. However, these findings have not been reported consistently and could vary according to the iron preparation used.35,36 IV iron administration may also result in transient PTH suppression, most likely through its effects on FGF-23 and vitamin D by acting directly on the parathyroid.35,36
In a recent study, Bacchetta et al37 explored the possibility of a direct role for vitamin D in iron homeostasis. They found that supplementation with a single oral dose of vitamin D in healthy volunteers was associated with a 34% reduction in circulating hepcidin within 24 hours. Based on these results, Bacchetta et al suggested that vitamin D is a key regulator of the hepcidin-ferroportin axis, and highlighted vitamin D supplementation as a potential means of managing anemia in patients with low vitamin D and/or CKD.
Role of CKD-associated biochemical abnormalities in the pathogenesis of cardiorenal syndrome
Role of Phosphorus and Calcium
The altered metabolism of phosphate, vitamin D3, and iron in CKD are likely to have important contributing roles in the pathogenesis of impaired myocardial and vascular function and the development of chronic CRS. Experimental evidence indicates that high extracellular phosphate concentrations alter endothelial function38 and are toxic to vascular endothelial cells. This results in apoptosis and subsequent exposure of underlying smooth muscle cells to elevated levels of phosphate and stimulation of Pit-1 (a receptor encoded by the SLC20A1 gene).39,40 The Pit-1 receptor signals vascular smooth muscle cells to undergo transformation into osteoblastic-like cells, which secrete large quantities of calcium hydroxyapatite crystals that ultimately result in vascular calcification in the subendothelial and medial layers.38,41,42 Similarly, clinical studies have established that hyperphosphatemia is strongly associated with vascular calcification and cardiovascular mortality among individuals receiving dialysis.42 High serum phosphate levels also may inhibit calcitriol synthesis, thus stimulating the renin-angiotensin-aldosterone system (RAAS). Such stimulation induces vasoconstriction and salt/water retention, further promoting arterial stiffening.43
There is a strong association between vitamin D3 deficiency and cardiovascular complications, including high blood pressure, vascular calcification, left ventricular hypertrophy (LVH), hypertension, decreased myocardial contractility, and heart failure.43,44 In addition to their vascular effects, disturbed calcium and phosphorus may also directly induce myocardial injury and dysfunction. In experimental models of uremia, a high phosphorus diet combined with parathyroidectomy is associated with dramatic increases in LVH, myocardial fibrosis, and myocyte diameter.45,46 An important role for myocardial calcification similarly has been suggested for fetuin-A, a circulating protein synthesized in the liver that acts as a potent inhibitor of tissue mineralization. In studies of the fetuin-A knockout mouse, myocardial calcification occurs in the absence of significant vascular calcification and is associated with significant changes in cardiac output, ventricular stiffness, and myocardial collagen production and fibrosis.47
Taken together, these and other data suggest that alterations in calcium and phosphorus metabolism contribute to myocardial dysfunction and CRS in patients with reduced kidney function. Although extensive clinical data are lacking on the role of calcium and phosphorus in CRS, an elevation in the calcium-phosphorus product has been reported to be strongly associated with LVH.48 Likewise, elevations in the calcium-phosphorus product or its individual components have been described to be associated with increased risk of cardiovascular mortality in the setting of dialysis-dependent CKD.49–51 Data on the association between calcium or phosphorus concentration and heart failure outcomes are limited. However, a handful of studies are consistent with an increased risk of heart failure events among individuals with CKD and elevated calcium or phosphorus concentrations.51
Role of Vitamin D, PTH, and FGF-23
Given these associations, it seems likely that alterations in vitamin D, PTH, and FGF-23 homeostasis contribute to the development of chronic CRS. Nevertheless, the overall evidence of a direct impact of vitamin D, PTH, and FGF-23 on endothelial and vascular smooth muscle cells remains relatively sparse (although it recently was demonstrated that FGF-23 promotes cardiomyocyte hypertrophy via a Klotho-independent pathway52). In contrast, numerous experimental studies support the concept that this trio of hormones plays a role in development of myocardial hypertrophy and fibrosis in the setting of CKD. Administering vitamin D analogs, for example, markedly reduces LVH and ventricular collagen accumulation in animal models of CKD,53 while there are similar increases in fibrotic lesions and decreased expression of metalloproteinase inhibitors in vitamin D receptor–knockout mice.54,55 With regard to PTH, a convincing body of experimental evidence demonstrates that both surgical parathyroidectomy and medical parathyroidectomy (using calcimimetics or activated vitamin D) inhibit myocardial fibrosis in disease models of kidney disease.56–58 Administering FGF-23 also causes LVH, while treatment of uremic animals with an FGF-receptor blocker attenuates LVH independent of blood pressure.52 Although the mechanism underlying this finding is uncertain, FGF-23 appears to downregulate renal expression of angiotensin-converting enzyme 2, suggesting an intriguing influence on the RAAS.59
Clinical studies have confirmed that there are strong associations between this trio of hormones and myocardial pathology. For example, vitamin D deficiency has been associated with development of LVH in children with CKD.60 Similarly, high levels of PTH have been described to be independently associated with LVH and with increased concentrations of markers of cardiac damage and dysfunction (eg, troponin T and N-terminal pro–brain natriuretic peptide [NT-proBNP]).61 Abnormalities in PTH and vitamin D also have been reported to be associated with an increased risk of cardiovascular outcomes, including heart failure.62 Several studies have demonstrated an association of FGF-23 concentrations with the presence of LVH,63,64 and a graded association with the risk of hospitalization due to heart failure.65
Role of Anemia and Iron Deficiency
The prevalence of anemia may exceed 37% in patients with heart failure.66 The severity of anemia has been associated with the degree of risk of ventricular dilation, hospitalization due to heart failure, or overall mortality in patients with dialysis-dependent and earlier stages of CKD;67,68 similarly, more severe anemia has been associated with an increase in symptoms, reduced exercise capacity, and greater mortality in heart-failure populations.69 Although its role in the development of anemia may be primary, iron deficiency appears to worsen heart failure independently of anemia: a recent study showed that health-related quality of life among patients with heart failure was independently associated with iron stores but not with the presence of anemia.70
Mechanisms underlying these associations remain incompletely understood; however, the heart is rich in myoglobin, an iron-requiring protein essential in oxygen transport within the cardiomyocyte. In vitro studies suggest that iron deficiency directly impairs myocyte mechanical function71 and that iron deficiency anemia induces LVH72 characterized by myocyte hypertrophy and hypercellularity.73 Anemia also may exacerbate heart failure through a series of downstream events in which tissue hypoxia and nitric oxide release cause peripheral vasodilation and blood pressure decreases. This subsequently causes reduced kidney function, increased renal vasoconstriction, and activation of the RAAS.74,75 In turn, RAAS activation leads to fluid retention and release of NT-proBNP due to myocardial stress, ultimately amplifying progressive kidney and cardiac failure.76
Finally, iron administration may contribute to heart failure. Normally, <2% of circulating and stored iron is unbound because of its tendency to catalyze oxidative stress reactions. Lele and colleagues77 demonstrated that the heart releases catalytic iron, probably from myoglobin in the setting of acute coronary syndrome. Akrawinthawong78 has shown that free iron is released into the urine in acute kidney injury, which is followed by secretion of neutrophil gelatinase–associated lipocalin. Although transient, IV iron infusions do elevate blood levels of free iron,79 the consequences of which are unknown. In contrast, oral forms of iron are absorbed via natural transport and binding mechanisms and do not result in liberation of catalytic iron in the bloodstream.80 Thus, the absolute levels of circulating iron, its form, and route of administration may potentially play important roles in cardiovascular and renal physiology.
Role of EPO Deficiency and Resistance
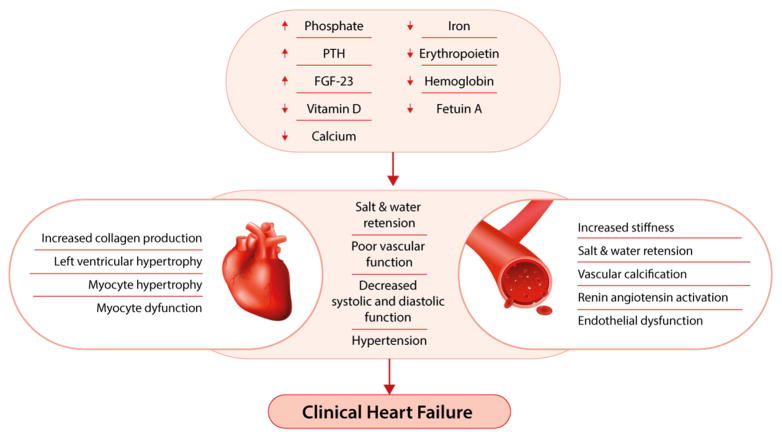
EPO, as well as its receptor (EPOR), is expressed in a number of nonerythropoietic tissues, including cardiac myocytes. There is evidence supporting a role for EPO-EPOR signaling in the modulation of physiologic responses to various types of nonerythropoietic tissue injury. For example, EPO deficiency in mice has been shown to induce cardiac hypertrophy and increase left ventricular dilatation,81 while exogenous administration of recombinant EPO confers an acute cardioprotective effect during ischemia-reperfusion injury in rats.82 In humans, several trials have demonstrated that EPO therapy of CKD-associated anemia can induce regression of LVH.83–85 However, this effect has not been universal and may be dependent on the severity of anemia.86 Moreover, multiple clinical trials have demonstrated that exogenous EPO administration is associated with increased risks for the development of heart failure, stroke, and other cardiovascular events. Such trials used high doses of EPO in the setting of EPO resistance in an attempt to achieve a higher hemoglobin target,87 and secondary analyses suggest that EPO in high doses is cardiotoxic. Thus, despite the potential benefits, restricted EPO dosing may be a prudent approach. Whether hemoglobin levels should also be limited in patients with CKD remains uncertain. The potential roles of the factors mentioned in this section are summarized in Figure 2.
Figure 2. Anemia, bone, and mineral axis factors implicated in development of heart failure.
Decreases in hemoglobin, erythropoietin, calcium, vitamin D, iron, and fetuin-A, as well as increases in serum phosphorous, parathyroid hormone (PTH), and fibroblast growth factor 23 (FGF-23), may induce myocyte hypertrophy, mechanical dysfunction, and myocardial fibrosis while simultaneously contributing to endothelial dysfunction, vascular stiffness, and vascular calcification. These changes ultimately induce systolic and diastolic function, salt and water retention, and the clinical syndrome of heart failure.
Managing cardiorenal syndrome in clinical practice
Although nephrology and cardiology societies have developed thorough and up-to-date guidelines for the management of CKD and CVD, there are no formal treatment recommendations for the management of patients with CRS due to a lack of CRS-specific trials. The overlapping interactions of the biochemical factors described in this review illustrate the bidirectional nature of chronic CRS and emphasize the need for multidisciplinary approaches in its treatment.
There is evidence that diuretics and aldosterone antagonists are effective treatments in patients with heart failure, as demonstrated by the DOSE (Diuretic Optimization Strategies Evaluation) study88,89 and RALES (Randomized Aldactone Evaluation Study).90,91 However, use of these agents in patients with CKD is limited, the result of increased risk for electrolyte disturbances such as hypokalemia or hyperkalemia.92,93
A growing body of research suggests that hyperphosphatemia is a major promoter of cardiovascular calcification in patients with CKD. Serum phosphate levels in this population can be controlled with oral phosphate binders which, in theory, could improve cardiovascular outcomes. Sevelamer, a nonabsorbable hydrogel, was the first aluminum- and calcium-free phosphate binder for hyperphosphatemia management in dialysis patients; however, evidence that sevelamer decreases calcification is limited. When compared to a calcium-based phosphate binder, sevelamer reduced the progression of coronary and aortic calcification in hemodialysis patients participating in the TTG (Treat to Goal) study.94 However, when compared to calcium acetate, sevelamer did significantly attenuate progression of these conditions in two separate studies (the CARE-2 [Calcium Acetate Renagel Evaluation 2] study and the BRiC [Phosphate Binder Impact on Bone Remodeling and Coronary Calcification] study).95,96
Despite a number of studies measuring surrogate outcomes for cardiovascular disease, to date the mortality and cardiovascular benefits of phosphate reduction remain unproven. Further, there is no solid evidence to support the superiority of a specific phosphate binder in improving cardiovascular-related outcomes. To wit, the DCOR (Dialysis Clinical Outcomes Revisited) trial, which compared the effects of calcium-based phosphate binders and sevelamer on mortality, morbidity, and hospitalizations in hemodialysis patients, did not find significant benefits to using sevelamer: over the course of 3 years, all-cause mortality (17.7 versus 17.4 deaths/100 patient years, P = 0.9) and cardiovascular mortality (9.0 vs 8.2 deaths/100 patient years, P = 0.4) were very similar for sevelamer and calcium groups.97 Comparable trends were noted for first hospitalization and cause-specific multiple hospitalizations.
In regard to vitamin D, a recent post hoc analysis of the PRIMO (Paricalcitol Capsule Benefits in Renal Failure-Induced Cardiac Morbidity) study demonstrated a lesser increase of brain natriuretic peptide and left atrial index in patients with both diabetes and CKD receiving an oral vitamin D analog (concomitantly with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers) compared to patients taking placebo.98 However, the results for the primary outcome were negative, with no impact of paricalcitol therapy on LVH.99 Furthermore, in a more recent study of individuals with CKD stages 3 to 5 and LVH at baseline, 1 μg of paricalcitol daily for 52 weeks had no effect on LV mass index or volume, ejection fraction, or diastolic function compared with placebo.100 Similarly, the EVOLVE (Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events) study of 3,883 patients receiving hemodialysis showed that cinacalcet, a calcimimetic agent, had no significant effect on the combined cardiovascular endpoint despite significant improvement in biochemical parameters of bone and mineral metabolism.101 Interestingly, however, cinacalcet use was associated with an 18% reduction in the risk of hospitalization due to heart failure (95% CI, 0.58–0.99). To our knowledge, no studies have specifically tested these therapies in individuals with CRS.
Patients with chronic heart failure often are iron deficient, even before therapy with an ESA results in gradual iron depletion. Thus, the use of IV iron before ESA therapy may help reduce the amount of ESA needed. A small number of studies have been conducted in which iron-deficient patients with chronic heart failure received IV iron. In the FAIR-HF (Ferinject Assessment in Patients with Iron Deficiency and Chronic Heart Failure) study, IV iron significantly improved symptoms of heart failure (odds ratio for improvement by one New York Heart Association functional class, 2.40; 95% CI, 1.55–3.71; P < 0.001) and exercise tolerance (6-minute walk test mean study treatment effect, 35 ± 8; P < 0.001) compared with placebo. However, there was no significant difference in secondary clinical endpoints, such as first hospitalization for cardiovascular causes or death (hazard ratio [HR], 0.61; 95% CI, 0.32–1.18; P = 0.14).102 The recent CONFIRM-HF (Ferric Carboxymaltose Evaluation on Performance in Patients With Iron Deficiency in Combination With Chronic Heart Failure) trial provides additional evidence of the potential benefits of iron therapy. Randomization to ferric carboxymaltose was associated with significant improvements in the 6-minute walk test, heart failure symptoms, and rate of hospitalization due to heart failure (HR, 0.39; 95% CI, 0.19–0.82)103.
Conversely, data from TREAT (Trial to Reduce Cardiovascular Events with Aranesp® Therapy) demonstrated that in non-dialysis patients with diabetes, CKD, and moderate anemia, darbepoetin alfa did not decrease the risk of death or cardiovascular event, congestive heart failure, nonfatal myocardial infarction, stroke, or hospitalization for myocardial ischemia compared to placebo (HR, 1.05; 95% CI, 0.94–1.17; P = 0.41).104
Available clinical evidence does not yet provide substantiated evidence for treating abnormalities associated with mineral bone disease and anemia as a means of improving outcomes of CRS. However, there are intriguing signals that merit further exploration of this area.
Conclusion
The Acute Dialysis Quality Initiative has defined CRS as disorders of the heart and kidney in which dysfunction in one organ may cause dysfunction in the other. As reviewed here, multiple lines of evidence suggest that in addition to the direct effects of uremia on cardiovascular pathophysiology, secondary changes in bone and mineral metabolism, as well as iron deficiency and anemia, have the potential to be important contributors to the pathogenesis of chronic CRS. However, this connection remains underexplored. Relatively few studies have investigated the potential for manipulating these factors to improve CRS outcomes, and results to date have been equivocal at best. Given that patients with combined CKD and CVD are at much higher risk of mortality than patients with either in isolation, CRS is a major health concern. A better understanding of the roles and interrelated effects of the biochemical factors discussed in this review, as well as more trials aimed at correcting these factors in individuals with or at risk for CRS may help inform management and treatment strategies for patients with CRS.
The authors take full responsibility for the content of and the decision to submit this manuscript, but thank Teresa A. Oblak, PhD, CMPP, of Covance Market Access Services Inc., for providing research and administrative support, copyediting, and coordination assistance (Dr. Oblak’s effort and publication-related page charges were supported by Keryx Pharmaceuticals, Inc).
Acknowledgments
Support: None.
Footnotes
Financial Disclosure: Dr Charytan has received grant/research support from, participated in speakers’ bureaus for, and/or acted as a consultant for Keryx, Tengion, Medtronic, Satellite Health Care, Dialysis Clinic, Inc, and the National Institutes of Health (grants U01DK096189, R01HL11831402, and R21DK100772). Dr Fishbane has received grant/research support from Rockwell Medical, Amgen, Keryx, and Deltanoid and acted as a consultant for Rockwell Medical, Keryx, Astra Zeneca, and Akebia. Dr Malyszko has acted as a consultant for Amgen. Dr Goldsmith has participated in speakers’ bureaus for and/or acted as a consultant for Sanofi, Keryx, Fresenius, Merck, Astellas, and Sandoz. Dr McCullough declares that he has no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.House AA, Anand I, Bellomo R, et al. Definition and classification of Cardio-Renal Syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant. 2010;25(5):1416–1420. doi: 10.1093/ndt/gfq136. [DOI] [PubMed] [Google Scholar]
- 2.Minino AM. NCHS data brief. 115. Hyattsville, MD: 2013. [Accessed November 6, 2013]. Death in the United States, 2011. Available at: http://www.cdc.gov/nchs/data/databriefs/db115.htm. [PubMed] [Google Scholar]
- 3.Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Reports. 2012;61:1–52. [PubMed] [Google Scholar]
- 4.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108(17):2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 5.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351(13):1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 6.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 7.US Renal Data System. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End Stage Renal Disease in the United States. Bethesda, MD: 2013. [Google Scholar]
- 8.Centers for Disease Control and Prevention. National Chronic Kidney Disease Fact Sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: 2010. [Accessed November 6, 2013]. Available at: http://www.cdc.gov/diabetes/pubs/pdf/kidney_factsheet.pdf. [Google Scholar]
- 9.McCullough PA, Steigerwalt S, Tolia K, et al. Cardiovascular disease in chronic kidney disease: data from the Kidney Early Evaluation Program (KEEP) Curr Diab Rep. 2011;11(1):47–55. doi: 10.1007/s11892-010-0162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindner A, Charra B, Sherrard DJ, Scribner BH. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med. 1974;290(13):697–701. doi: 10.1056/NEJM197403282901301. [DOI] [PubMed] [Google Scholar]
- 11.Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol. 2005;16(2):529–538. doi: 10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- 12.Muntner P, Hamm LL, Kusek JW, Chen J, Whelton PK, He J. The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med. 2004;140(1):9–17. doi: 10.7326/0003-4819-140-1-200401060-00006. [DOI] [PubMed] [Google Scholar]
- 13.Sim JJ, Bhandari SK, Smith N, et al. Phosphorus and risk of renal failure in subjects with normal renal function. Am J Med. 2013;126(4):311–318. doi: 10.1016/j.amjmed.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) J Am Soc Nephrol. 2003;14(12):3270–3277. doi: 10.1097/01.asn.0000100127.54107.57. [DOI] [PubMed] [Google Scholar]
- 15.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35(7):455–469. doi: 10.1093/eurheartj/eht386. [DOI] [PubMed] [Google Scholar]
- 17.Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. 2010;121(23):2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 18.Kovesdy CP, Quarles LD. The role of fibroblast growth factor-23 in cardiorenal syndrome. Nephron Clin Pract. 2013;123(3–4):194–201. doi: 10.1159/000353593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiattisunthorn K, Moe SM. Chronic kidney disease-mineral bone disorder (CKD-MBD) IBMS BoneKey. 2010;7:447–457. [Google Scholar]
- 20.Beutler E. Hemochromatosis: genetics and pathophysiology. Annu Rev Med. 2006;57:331–347. doi: 10.1146/annurev.med.57.121304.131310. [DOI] [PubMed] [Google Scholar]
- 21.Babitt JL, Lin HY. Molecular mechanisms of hepcidin regulation: implications for the anemia of CKD. Am J Kidney Dis. 2010;55(4):726–741. doi: 10.1053/j.ajkd.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malyszko J, Mysliwiec M. Hepcidin in anemia and inflammation in chronic kidney disease. Kidney Blood Press Res. 2007;30(1):15–30. doi: 10.1159/000098522. [DOI] [PubMed] [Google Scholar]
- 23.van der Putten K, Braam B, Jie KE, Gaillard CA. Mechanisms of Disease: erythropoietin resistance in patients with both heart and kidney failure. Nat Clin Pract Nephrol. 2008;4(1):47–57. doi: 10.1038/ncpneph0655. [DOI] [PubMed] [Google Scholar]
- 24.van der Putten K, Jie KE, van den Broek D, et al. Hepcidin-25 is a marker of the response rather than resistance to exogenous erythropoietin in chronic kidney disease/chronic heart failure patients. Eur J Heart Fail. 2010;12(9):943–950. doi: 10.1093/eurjhf/hfq099. [DOI] [PubMed] [Google Scholar]
- 25.Khankin EV, Mutter WP, Tamez H, Yuan HT, Karumanchi SA, Thadhani R. Soluble erythropoietin receptor contributes to erythropoietin resistance in end-stage renal disease. PLoS One. 2010;5(2):e9246. doi: 10.1371/journal.pone.0009246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiss Z, Ambrus C, Almasi C, et al. Serum 25(OH)-cholecalciferol concentration is associated with hemoglobin level and erythropoietin resistance in patients on maintenance hemodialysis. Nephron Clin Pract. 2011;117(4):c373–378. doi: 10.1159/000321521. [DOI] [PubMed] [Google Scholar]
- 27.Wagner M, Alam A, Zimmermann J, et al. Endogenous erythropoietin and the association with inflammation and mortality in diabetic chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(7):1573–1579. doi: 10.2215/CJN.00380111. [DOI] [PubMed] [Google Scholar]
- 28.Mohammed S, Knoll S, van Amburg A, 3rd, Mennes PA. Cefotetan-induced hemolytic anemia causing severe hypophosphatemia. Am J Hematol. 1994;46(4):369–370. doi: 10.1002/ajh.2830460422. [DOI] [PubMed] [Google Scholar]
- 29.Steiner M, Steiner B, Wilhelm S, Freund M, Schuff-Werner P. Severe hypophosphatemia during hematopoietic reconstitution after allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2000;25(9):1015–1016. doi: 10.1038/sj.bmt.1702407. [DOI] [PubMed] [Google Scholar]
- 30.Van Wyck DB, Mangione A, Morrison J, Hadley PE, Jehle JA, Goodnough LT. Large-dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion. 2009;49(12):2719–2728. doi: 10.1111/j.1537-2995.2009.02327.x. [DOI] [PubMed] [Google Scholar]
- 31.Sanai T, Oochi N, Okada M, Imamura K, Okuda S, Iida M. Effect of saccharated ferric oxide and iron dextran on the metabolism of phosphorus in rats. J Lab Clin Med. 2005;146(1):25–29. doi: 10.1016/j.lab.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu Y, Tada Y, Yamauchi M, et al. Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: another form of FGF23-related hypophosphatemia. Bone. 2009;45(4):814–816. doi: 10.1016/j.bone.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Schouten BJ, Hunt PJ, Livesey JH, Frampton CM, Soule SG. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab. 2009;94(7):2332–2337. doi: 10.1210/jc.2008-2396. [DOI] [PubMed] [Google Scholar]
- 34.Prats M, Font R, Garcia C, Cabre C, Jariod M, Vea AM. Effect of ferric carboxymaltose on serum phosphate and C-terminal FGF23 levels in non-dialysis chronic kidney disease patients: post-hoc analysis of a prospective study. BMC Nephrol. 2013;14(1):167. doi: 10.1186/1471-2369-14-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deger SM, Erten Y, Pasaoglu OT, et al. The effects of iron on FGF23-mediated Ca-P metabolism in CKD patients. Clin Exp Nephrol. 2013;17(3):416–423. doi: 10.1007/s10157-012-0725-0. [DOI] [PubMed] [Google Scholar]
- 36.Takeda Y, Komaba H, Goto S, et al. Effect of intravenous saccharated ferric oxide on serum FGF23 and mineral metabolism in hemodialysis patients. Am J Nephrol. 2011;33(5):421–426. doi: 10.1159/000327019. [DOI] [PubMed] [Google Scholar]
- 37.Bacchetta J, Zaritsky JJ, Sea JL, et al. Suppression of iron-regulatory hepcidin by vitamin D. J Am Soc Nephrol. 2014;25(3):564–572. doi: 10.1681/ASN.2013040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Six I, Maizel J, Barreto FC, et al. Effects of phosphate on vascular function under normal conditions and influence of the uraemic state. Cardiovasc Res. 2012;96(1):130–139. doi: 10.1093/cvr/cvs240. [DOI] [PubMed] [Google Scholar]
- 39.Di Marco GS, Konig M, Stock C, et al. High phosphate directly affects endothelial function by downregulating annexin II. Kidney Int. 2013;83(2):213–222. doi: 10.1038/ki.2012.300. [DOI] [PubMed] [Google Scholar]
- 40.Kuro OM. Klotho, phosphate and FGF-23 in ageing and disturbed mineral metabolism. Nat Rev Nephrol. 2013;9(11):650–660. doi: 10.1038/nrneph.2013.111. [DOI] [PubMed] [Google Scholar]
- 41.Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol. 2010;21(1):103–112. doi: 10.1681/ASN.2009060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanbay M, Goldsmith D, Akcay A, Covic A. Phosphate - the silent stealthy cardiorenal culprit in all stages of chronic kidney disease: a systematic review. Blood Purif. 2009;27(2):220–230. doi: 10.1159/000197562. [DOI] [PubMed] [Google Scholar]
- 43.Ronco C, Cozzolino M. Mineral metabolism abnormalities and vitamin D receptor activation in cardiorenal syndromes. Heart Fail Rev. 2012;17(2):211–220. doi: 10.1007/s10741-011-9232-8. [DOI] [PubMed] [Google Scholar]
- 44.Kienreich K, Tomaschitz A, Verheyen N, et al. Vitamin D and cardiovascular disease. Nutrients. 2013;5(8):3005–3021. doi: 10.3390/nu5083005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neves KR, Graciolli FG, dos Reis LM, Pasqualucci CA, Moyses RM, Jorgetti V. Adverse effects of hyperphosphatemia on myocardial hypertrophy, renal function, and bone in rats with renal failure. Kidney Int. 2004;66(6):2237–2244. doi: 10.1111/j.1523-1755.2004.66013.x. [DOI] [PubMed] [Google Scholar]
- 46.Custodio MR, Koike MK, Neves KR, et al. Parathyroid hormone and phosphorus overload in uremia: impact on cardiovascular system. Nephrol Dial Transplant. 2012;27(4):1437–1445. doi: 10.1093/ndt/gfr447. [DOI] [PubMed] [Google Scholar]
- 47.Merx MW, Schafer C, Westenfeld R, et al. Myocardial stiffness, cardiac remodeling, and diastolic dysfunction in calcification-prone fetuin-A-deficient mice. J Am Soc Nephrol. 2005;16(11):3357–3364. doi: 10.1681/ASN.2005040365. [DOI] [PubMed] [Google Scholar]
- 48.Regmi P, Malla B, Gyawali P, et al. Product of serum calcium and phosphorus (Ca x PO4) as predictor of cardiovascular disease risk in predialysis patients. Clin Biochem. 2014;47(1–2):77–81. doi: 10.1016/j.clinbiochem.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 50.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 51.Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant. 2009;24(5):1506–1523. doi: 10.1093/ndt/gfn613. [DOI] [PubMed] [Google Scholar]
- 52.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panizo S, Barrio-Vazquez S, Naves-Diaz M, et al. Vitamin D receptor activation, left ventricular hypertrophy and myocardial fibrosis. Nephrol Dial Transplant. 2013;28(11):2735–2744. doi: 10.1093/ndt/gft268. [DOI] [PubMed] [Google Scholar]
- 54.Rahman A, Hershey S, Ahmed S, Nibbelink K, Simpson RU. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol. 2007;103(3–5):416–419. doi: 10.1016/j.jsbmb.2006.12.081. [DOI] [PubMed] [Google Scholar]
- 55.Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol. 2007;103(3–5):521–524. doi: 10.1016/j.jsbmb.2006.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogata H, Ritz E, Odoni G, Amann K, Orth SR. Beneficial effects of calcimimetics on progression of renal failure and cardiovascular risk factors. J Am Soc Nephrol. 2003;14(4):959–967. doi: 10.1097/01.asn.0000056188.23717.e5. [DOI] [PubMed] [Google Scholar]
- 57.Amann K, Ritz E, Wiest G, Klaus G, Mall G. A role of parathyroid hormone for the activation of cardiac fibroblasts in uremia. J Am Soc Nephrol. 1994;4(10):1814–1819. doi: 10.1681/ASN.V4101814. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez-Ayala E, Avila-Diaz M, Foyo-Niembro E, Amato D, Ramirez-San-Juan E, Paniagua R. Effect of parathyroidectomy on cardiac fibrosis and apoptosis: possible role of aldosterone. Nephron Physiol. 2006;103(3):112–118. doi: 10.1159/000092244. [DOI] [PubMed] [Google Scholar]
- 59.Dai B, David V, Martin A, et al. A comparative transcriptome analysis identifying FGF23 regulated genes in the kidney of a mouse CKD model. PLoS One. 2012;7(9):e44161. doi: 10.1371/journal.pone.0044161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patange AR, Valentini RP, Gothe MP, Du W, Pettersen MD. Vitamin D deficiency is associated with increased left ventricular mass and diastolic dysfunction in children with chronic kidney disease. Pediatr Cardiol. 2013;34(3):536–542. doi: 10.1007/s00246-012-0489-z. [DOI] [PubMed] [Google Scholar]
- 61.van Ballegooijen AJ, Visser M, Kestenbaum B, et al. Relation of vitamin D and parathyroid hormone to cardiac biomarkers and to left ventricular mass (from the Cardiovascular Health Study) Am J Cardiol. 2013;111(3):418–424. doi: 10.1016/j.amjcard.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kestenbaum B, Katz R, de Boer I, et al. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol. 2011;58(14):1433–1441. doi: 10.1016/j.jacc.2011.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119(19):2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Unsal A, Kose Budak S, Koc Y, et al. Relationship of fibroblast growth factor 23 with left ventricle mass index and coronary calcificaton in chronic renal disease. Kidney Blood Press Res. 2012;36(1):55–64. doi: 10.1159/000339026. [DOI] [PubMed] [Google Scholar]
- 65.Scialla JJ, Xie H, Rahman M, et al. Fibroblast Growth Factor-23 and Cardiovascular Events in CKD. J Am Soc Nephrol. 2014;25(2):349–360. doi: 10.1681/ASN.2013050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Groenveld HF, Januzzi JL, Damman K, et al. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol. 2008;52(10):818–827. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 67.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. The impact of anemia on cardiomyopathy, morbidity, and and mortality in end-stage renal disease. Am J Kidney Dis. 1996;28(1):53–61. doi: 10.1016/s0272-6386(96)90130-4. [DOI] [PubMed] [Google Scholar]
- 68.Levin A, Thompson CR, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34(1):125–134. doi: 10.1016/s0272-6386(99)70118-6. [DOI] [PubMed] [Google Scholar]
- 69.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Borenstein J. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol. 2002;39(11):1780–1786. doi: 10.1016/s0735-1097(02)01854-5. [DOI] [PubMed] [Google Scholar]
- 70.Comin-Colet J, Enjuanes C, Gonzalez G, et al. Iron deficiency is a key determinant of health-related quality of life in patients with chronic heart failure regardless of anaemia status. Eur J Heart Fail. 2013;15(10):1164–1172. doi: 10.1093/eurjhf/hft083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldstein D, Felzen B, Youdim M, Lotan R, Binah O. Experimental iron deficiency in rats: mechanical and electrophysiological alterations in the cardiac muscle. Clin Sci (Lond) 1996;91(2):233–239. doi: 10.1042/cs0910233. [DOI] [PubMed] [Google Scholar]
- 72.Rakusan K, Cicutti N, Kolar F. Effect of anemia on cardiac function, microvascular structure, and capillary hematocrit in rat hearts. Am J Physiol Heart Circ Physiol. 2001;280(3):H1407–1414. doi: 10.1152/ajpheart.2001.280.3.H1407. [DOI] [PubMed] [Google Scholar]
- 73.Olivetti G, Quaini F, Lagrasta C, et al. Myocyte cellular hypertrophy and hyperplasia contribute to ventricular wall remodeling in anemia-induced cardiac hypertrophy in rats. Am J Pathol. 1992;141(1):227–239. [PMC free article] [PubMed] [Google Scholar]
- 74.Anand IS, Chandrashekhar Y, Wander GS, Chawla LS. Endothelium-derived relaxing factor is important in mediating the high output state in chronic severe anemia. J Am Coll Cardiol. 1995;25(6):1402–1407. doi: 10.1016/0735-1097(95)00007-Q. [DOI] [PubMed] [Google Scholar]
- 75.Anand IS, Chandrashekhar Y, Ferrari R, Poole-Wilson PA, Harris PC. Pathogenesis of oedema in chronic severe anaemia: studies of body water and sodium, renal function, haemodynamic variables, and plasma hormones. Br Heart J. 1993;70(4):357–362. doi: 10.1136/hrt.70.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Silverberg DS, Wexler D, Iaina A, Schwartz D. Correction of iron deficiency in the cardiorenal syndrome. Int J Nephrol. 2011;2011:365301. doi: 10.4061/2011/365301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lele S, Shah S, McCullough PA, Rajapurkar M. Serum catalytic iron as a novel biomarker of vascular injury in acute coronary syndromes. EuroIntervention. 2009;5(3):336–342. doi: 10.4244/v5i3a53. [DOI] [PubMed] [Google Scholar]
- 78.Akrawinthawong K, Shaw MK, Kachner J, et al. Urine catalytic iron and neutrophil gelatinase-associated lipocalin as companion early markers of acute kidney injury after cardiac surgery: a prospective pilot study. Cardiorenal Med. 2013;3(1):7–16. doi: 10.1159/000346815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kooistra MP, Kersting S, Gosriwatana I, et al. Nontransferrin-bound iron in the plasma of haemodialysis patients after intravenous iron saccharate infusion. Eur J Clin Invest. 2002;32 (Suppl 1):36–41. doi: 10.1046/j.1365-2362.2002.0320s1036.x. [DOI] [PubMed] [Google Scholar]
- 80.Kell DB. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genomics. 2009;2:2. doi: 10.1186/1755-8794-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.El Hasnaoui-Saadani R, Marchant D, Pichon A, et al. Epo deficiency alters cardiac adaptation to chronic hypoxia. Respir Physiol Neurobiol. 2013;186(2):146–154. doi: 10.1016/j.resp.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 82.Wright GL, Hanlon P, Amin K, Steenbergen C, Murphy E, Arcasoy MO. Erythropoietin receptor expression in adult rat cardiomyocytes is associated with an acute cardioprotective effect for recombinant erythropoietin during ischemia-reperfusion injury. FASEB J. 2004;18(9):1031–1033. doi: 10.1096/fj.03-1289fje. [DOI] [PubMed] [Google Scholar]
- 83.Ayus JC, Go AS, Valderrabano F, et al. Effects of erythropoietin on left ventricular hypertrophy in adults with severe chronic renal failure and hemoglobin <10 g/dL. Kidney Int. 2005;68(2):788–795. doi: 10.1111/j.1523-1755.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- 84.Silberberg J, Racine N, Barre P, Sniderman AD. Regression of left ventricular hypertrophy in dialysis patients following correction of anemia with recombinant human erythropoietin. Can J Cardiol. 1990;6(1):1–4. [PubMed] [Google Scholar]
- 85.Hayashi T, Suzuki A, Shoji T, et al. Cardiovascular effect of normalizing the hematocrit level during erythropoietin therapy in predialysis patients with chronic renal failure. Am J Kidney Dis. 2000;35(2):250–256. doi: 10.1016/s0272-6386(00)70334-9. [DOI] [PubMed] [Google Scholar]
- 86.Parfrey PS, Lauve M, Latremouille-Viau D, Lefebvre P. Erythropoietin therapy and left ventricular mass index in CKD and ESRD patients: a meta-analysis. Clin J Am Soc Nephrol. 2009;4(4):755–762. doi: 10.2215/CJN.02730608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCullough PA, Barnhart HX, Inrig JK, et al. Cardiovascular toxicity of epoetin-alfa in patients with chronic kidney disease. Am J Nephrol. 2013;37(6):549–558. doi: 10.1159/000351175. [DOI] [PubMed] [Google Scholar]
- 88.Shah RV, McNulty S, O’Connor CM, Felker GM, Braunwald E, Givertz MM. Effect of admission oral diuretic dose on response to continuous versus bolus intravenous diuretics in acute heart failure: an analysis from diuretic optimization strategies in acute heart failure. Am Heart J. 2012;164(6):862–868. doi: 10.1016/j.ahj.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kociol RD, McNulty SE, Hernandez AF, et al. Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circ Heart Fail. 2013;6(2):240–245. doi: 10.1161/CIRCHEARTFAILURE.112.969246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]) Am J Cardiol. 1996;78(8):902–907. doi: 10.1016/s0002-9149(96)00465-1. [DOI] [PubMed] [Google Scholar]
- 91.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 92.Navaneethan SD, Nigwekar SU, Sehgal AR, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2009;(3):CD007004. doi: 10.1002/14651858.CD007004.pub2. [DOI] [PubMed] [Google Scholar]
- 93.Cooper HA, Dries DL, Davis CE, Shen YL, Domanski MJ. Diuretics and risk of arrhythmic death in patients with left ventricular dysfunction. Circulation. 1999;100(12):1311–1315. doi: 10.1161/01.cir.100.12.1311. [DOI] [PubMed] [Google Scholar]
- 94.Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62(1):245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 95.Qunibi W, Moustafa M, Muenz LR, et al. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis. 2008;51(6):952–965. doi: 10.1053/j.ajkd.2008.02.298. [DOI] [PubMed] [Google Scholar]
- 96.Barreto DV, de Barreto FC, de Carvalho AB, et al. Phosphate binder impact on bone remodeling and coronary calcification--results from the BRiC study. Nephron Clin Pract. 2008;110(4):c273–283. doi: 10.1159/000170783. [DOI] [PubMed] [Google Scholar]
- 97.St Peter WL, Liu J, Weinhandl E, Fan Q. A comparison of sevelamer and calcium-based phosphate binders on mortality, hospitalization, and morbidity in hemodialysis: a secondary analysis of the Dialysis Clinical Outcomes Revisited (DCOR) randomized trial using claims data. Am J Kidney Dis. 2008;51(3):445–454. doi: 10.1053/j.ajkd.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 98.Tamez H, Zoccali C, Packham D, et al. Vitamin D reduces left atrial volume in patients with left ventricular hypertrophy and chronic kidney disease. Am Heart J. 2012;164(6):902–909. e902. doi: 10.1016/j.ahj.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 99.Thadhani R, Appelbaum E, Pritchett Y, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307(7):674–684. doi: 10.1001/jama.2012.120. [DOI] [PubMed] [Google Scholar]
- 100.Wang AY, Fang F, Chan J, et al. Effect of paricalcitol on left ventricular mass and function in CKD--the OPERA trial. J Am Soc Nephrol. 2014;25(1):175–186. doi: 10.1681/ASN.2013010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367(26):2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 102.Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361(25):2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 103.Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur Heart J. 2014 doi: 10.1093/eurheartj/ehu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361(21):2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]