Abstract
Objective
To assess the vision-related quality of life in a cohort of patients with ocular graft-versus-host disease (GVHD).
Design
Prospective study.
Participants
Eighty-four patients diagnosed with chronic ocular GVHD
Methods
We assessed the vision-related quality of life with the 25-item National Eye Institute Visual Function Questionnaire (NEI-VFQ-25). The symptoms of ocular GVHD were assessed using the Ocular Surface Disease Index (OSDI) and Symptom Assessment in Dry Eye (SANDE) questionnaires.
Main outcome measures
We assessed vision-related quality of life with NEI-VFQ-25 and compared the scores obtained from patients with ocular GVHD to those from a healthy population. In the ocular GVHD population, we also evaluated the associations between the NEI-VFQ-25 and dry eye symptoms measured by OSDI and SANDE questionnaires, age, duration of disease, best-corrected visual acuity, corneal fluorescein staining, tear break-up time, and Schirmer test.
Results
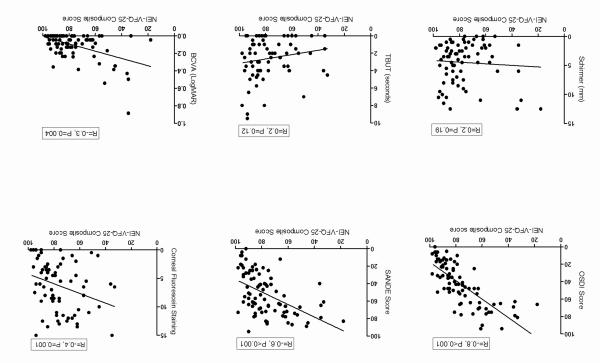
The mean composite NEI-VFQ-25 score in patients with ocular GVHD was 76.5 ± 17. Compared to healthy subjects, ocular GVHD patients reported reduced scores on all NEI-VFQ-25 subscales (each P < 0.001) with exception of color vision (P = 0.11). The NEI-VFQ-25 composite scores significantly correlated with OSDI (R = −0.81, P < 0.001), SANDE (R = −0.56, P < 0.001), corneal fluorescein staining (R = −0.36, P = 0.001) and best-corrected visual acuity (R = −0.30, P = 0.004).
Conclusion
Patients with ocular GVHD experience measurable impairment of vision-related quality of life. This study highlights the impact of ocular GVHD on the vision-related quality of life, and hence the importance of comprehensive diagnosis and treatment of this condition.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative treatment for various malignant and non-malignant hematological disorders. Medical advances have increased the frequency of transplants and survival rates,1,2 and long-term complications after HSCT have consequently become key determinants of the overall quality of life in these patients. Graft-versus-host disease (GVHD), a condition occurring after allogeneic HSCT when donor-derived immune cells recognize and attack the recipient tissues,3 is a major cause of morbidity that compromises patients’ quality of life (QOL).4 Manifestations of graft-versus-host disease can be seen in various organs including skin, gastrointestinal tract, liver, lungs, oral mucosa, or eyes.5
Ocular involvement presents in 40-60% of patients undergoing allogeneic HSCT.6 Ocular GVHD generally manifests as dry eye disease with symptoms of ocular discomfort, pain, redness, grittiness, and blurred vision. The clinical signs include conjunctival hyperemia, corneal epitheliopathy, meibomian gland dysfunction, conjunctival and corneal scarring, stromal ulceration, and symblepheron.7,8 Dry eye disease has been shown to affect quality of life in other settings, but there is limited information regarding the impact of manifestations of ocular GVHD on vision-related quality of life.9-12
The 25 item National Eye Institute Visual Function Questionnaire (NEI-VFQ-25) is used to assess patients’ perceptions of their visual function and the impact of an eye disease on their quality of life.13 The NEI VFQ-25 has been used to assess vision-related quality of life in various eye diseases such as cataract, macular degeneration, glaucoma, ocular chemical burns, diabetic retinopathy, uveitis, dry eye disease, and low vision.11,14-18 The purpose of this study was to evaluate vision-related quality of life in a large cohort of patients with a diagnosis of ocular GVHD using the NEI-VFQ-25. Additionally, we evaluated the association between the measured quality of life and the signs and symptoms of ocular GVHD.
Methods
One hundred consecutive patients with ocular GVHD examined at the Cornea Service, Massachusetts Eye and Ear Infirmary, Boston MA, were included in this prospective study. The study was approved by the Institutional Review Board and followed the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients. The inclusion criteria required patients to have a diagnosis of chronic ocular GVHD confirmed by an ophthalmologist, be older than 18 years, and comprehend the English language. All the patients satisfied the National Institutes of Health (NIH) criteria for diagnosis of ocular GVHD, which require a distinctive affectation of chronic GVHD in an organ different than the eye accompanied by one of the following ocular manifestations: 1) new ocular sicca documented with a bilateral Schirmer test averaging ≤ 5 mm, or 2) a new onset of ocular sicca by slit-lamp examination with a bilateral Schirmer test averaging 6-10 mm.5
All the participants self-responded the NEI-VFQ-25 questionnaire once the basic instructions were provided by the research staff.13 This questionnaire consists of 25 vision-targeted questions representing 11 subscales that include: general vision, difficulty with near vision activities, difficulty with distance vision activities, ocular pain, limitations in social functioning due to vision, role limitations due to vision, dependency on others due to vision, mental health symptoms due to vision, driving difficulties, limitations with peripheral vision, and color vision. Additionally the NEI-VFQ-25 includes one question that assesses the patient’s general health. The overall composite score for the NEI-VFQ-25 is calculated by averaging the scores of all the subscales with the exception of the general health question. The score of each subscale is represented by the average of the responses to questions answered in each subscale section. Both the composite and subscale scores range from 0 to 100, where higher scores indicate better quality of life.
Symptoms of ocular surface disease were assessed with the following two questionnaires: Ocular Surface Disease Index (OSDI) and Symptom Assessment in Dry Eye (SANDE).19,20 The OSDI questionnaire consists of 12 questions measuring the frequency of dry eye symptoms, visual impact of dry eye, and triggers; each question was graded on a scale from 0 (“none of the time”) to 4 (“all of the time”). The total OSDI score was calculated according to the questionnaire’s algorithm with a total score ranging from 0 to 100 where higher scores indicate greater disability.20 The SANDE questionnaire comprises two questions measuring the frequency and severity of dry eye symptoms; each of these two items were assessed on a 100 mm visual analog scale and scored from 0 to 100. The total SANDE score was calculated as the square-root of the product of the two item scores, and ranges from 0 to 100 with higher scores indicating greater disability.19 Additionally, we recorded the time of hematopoietic stem cell transplantation, duration of ocular GVHD, and assessed the following clinical parameters at the same visit when the questionnaires were administered: Snellen best-corrected visual acuity (BCVA), Schirmer I test with anesthesia, tear break-up time (TBUT), and corneal fluorescein staining (CFS; National Eye Institute grading system).21 We excluded 16 patients who had other ocular comorbidities unrelated to ocular GVHD that could potentially affect visual function, such as cataract with BCVA of 20/30 or worse (8 patients), glaucoma (1), macular or retinal disorders (4), amblyopia (1), hemianopia (1), and intraocular surgery within one month (1). There were no patients with any history of refractive surgery.
Data are presented as the mean ± standard deviation (SD) and range for continuous variables, and percentages for categorical variables. The NEI-VFQ-25 composite and subscale scores were computed according to the published algorithms.22 The NEI-VFQ-25 subscale mean scores from patients with ocular GVHD were compared to the mean scores of 122 healthy subjects from the original NEI-VFQ-25 developmental work using the Welch’s unpaired t-test.13 The healthy control population included 75 women (62%) and 47 men (38%) older than 21 years (mean 59±14), with no evidence of underlying eye disease other than refractive errors correctable to ≥20/25 in the worse eye. The median visual acuity in the better eye was 20/20 (range 20/13-20/100).13,16 We evaluated the association of the QOL scores with patients’ age, dry eye symptoms, duration of ocular GVHD; and with the average of both eyes for best-corrected visual acuity, corneal fluorescein staining, tear break-up time, and Schirmer test using the Spearman’s coefficient of correlation. Visual acuities were measured with a standardized Snellen chart and converted to LogMAR values at the time of the analysis. A two-sided P value of < 0.05 was considered statistically significant.
Results
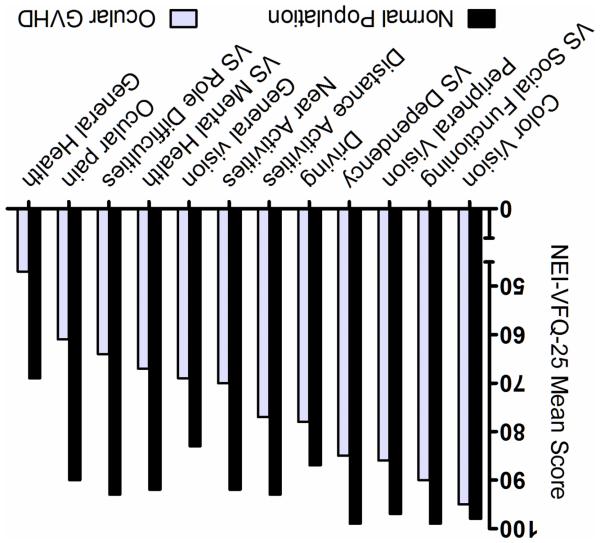
The final analysis included 84 patients (56 men and 28 women) with a mean age of 56 ± 12 years (range 22 to 74). The patients’ transplant characteristics are shown in Table 1. The mean BCVA, average of both eyes, was 0.08 ± 0.13 LogMAR (Snellen equivalent 20/24, median 20/22, range 20/20-20/150) and the mean BCVA in the better eye was 0.04 ± 0.09 LogMAR (Snellen 20/22, median 20/20, range 20/20-20/60). The mean dry eye clinical signs were: Schirmer test 4.5 ± 3.6 mm (range 0-12.5), TBUT 2.6 ± 2.3 seconds (range 0-9.5), and CFS 6.3 ± 4.4 (range 0-15). The mean OSDI score was 42.5 ± 24.1 (range 0-94) and the mean SANDE score was 52.2 ± 24.2 (range 0-95). The mean NEI-VFQ-25 composite and subscale scores are shown in Table 2. The mean composite score was 76.5 ± 17 (18-98). The comparison between the mean subscales’ scores in patients with ocular GVHD and the healthy population of reference is shown in Figure 1. The mean age between the studied GVHD population (56 ± 12) and the reference population (59 ± 14) was comparable (P = 0.11). With the exception of color vision, the patients with ocular GVHD presented with significantly lower scores on all NEI-VFQ-25 subscales when compared to the reference population (P < 0.001). The subscales that scored particularly low (less quality of life) with a difference of more than 10 points as compared to the healthy subjects were: ocular pain, vision specific role limitations, vision specific mental health symptoms, difficulty with near vision, distance vision, general vision activities, vision specific dependency, peripheral vision, and general health.
Table 1.
Characteristics of the study population
| Characteristic | |
|---|---|
| Age mean ± SD years | 56 ± 12 |
| Gender (%) | |
| Male | 56 (67) |
| Female | 28 (33) |
| Primary Disorder (%) | |
| Acute myeloid leukemia | 33 (39) |
| Acute lymphoid leukemia | 6 (7) |
| Chronic myeloid leukemia | 5 (6) |
| Chronic lymphoid leukemia | 10 (12) |
| Myelodysplastic syndrome | 11 (13) |
| Non-Hodgkin Lymphoma | 13 (16) |
| Hodgkin Lymphoma | 1 (1) |
| Multiple myeloma | 3 (4) |
| Others | 2 (2) |
| Duration since HSCT in days mean ± SD | 1348 ± 1002 |
| Duration since diagnosis of ocular GVHD in days mean ± SD | 873 ± 955 |
SD: standard deviation; HSCT: hematopoietic stem cell transplantation; GVHD: graft-versus-host disease.
Table 2.
25-item National Eye Institute Visual Function Questionnaire Scores in Patients with Ocular Graft-versus-host Disease
| Subscale (no. of questions in each subscale) |
Mean ± SD | Range |
|---|---|---|
| Composite Score | 76.5 ± 17 | 18-98 |
| Ocular pain (2) | 60.8 ± 24 | 0-100 |
| VS Role difficulties (2) | 64.5 ± 31 | 0-100 |
| VS Mental health (4) | 67.2 ± 26 | 6-100 |
| General vision (1) | 69.1 ± 16 | 20-100 |
| Near activities (3) | 69.6 ± 21 | 17-100 |
| Distance activities (3) | 77.1 ± 21 | 8-100 |
| Driving* (3) | 76.9 ± 21 | 0-100 |
| VS Dependency (3) | 85.3 ± 24 | 8-100 |
| Peripheral vision (1) | 85.4 ± 23 | 0-100 |
| VS Social functioning (2) | 90.1 ± 18 | 13-100 |
| Color vision (1) | 94.9 ± 16 | 0-100 |
| General Health (1) | 46.7 ± 22 | 0-100 |
Score range from 0 to 100 (where 100 represent the best quality of life). VS: vision specific.
Six participants did not drive for reasons other than vision difficulties.
Figure 1.
Comparison of the mean National Eye Institute Visual Function Questionnaire-25 scores between ocular graft-versus-host disease patients (n = 84) and a healthy reference population from the article by Mangione et al.13 (n = 122). VS: vision-specific. P value was < 0.001 in all the subscales except for the color vision subscale (P = 0.11).
OSDI and SANDE questionnaires showed a statistically significant correlation with the NEI-VFQ-25 composite score (Figure 2; OSDI: R = −0.81, P < 0.001, SANDE: R= −0.56, P < 0.001); and with the majority of the QOL subscales’ scores (Table 3), showing a significant association between increased dry eye symptoms and decreased quality of life scores. Among the dry eye clinical parameters, there was a modest but statistically significant correlation between the NEI-VFQ-25 composite score and corneal fluorescein staining (Figure 2; R = −0.36, P = 0.001). CFS also correlated significantly with most subscales of the NEI-VFQ-25 (Table 3), showing that increased corneal fluorescein staining is associated with reduced quality of life scores. The best-corrected visual acuity was also statistically significantly correlated with the quality of life composite score (Figure 2; R = −0.30, P = 0.004) and with the majority of the NEI-VFQ-25 subscales (Table 3). Schirmer test and TBUT were not correlated with the quality of life scores (Figure 2, Table 3). There was no correlation between the composite quality of life score and patients’ age (R = 0.15, P = 0.15) or duration of ocular GVHD (R = 0.01, P = 0.85). Correlations adjusted for age and sex are presented as supplementary data in table 4 (available at http://www.aaojournal.org).
Figure 2.
Correlations of the National Eye Institute Visual Function Questionnaire-25 (NEI-VFQ-25) score with the clinical symptoms and signs. OSDI: Ocular Surface Disease Index; SANDE: Symptoms Assessment in Dry Eye; TBUT: tear break-up time; BCVA: best corrected visual acuity; LogMAR: logarithm of minimum angle of resolution; R: Spearman’s correlation coefficient. Higher scores in the NEI-VFQ-25 indicate better quality of life (note the negative correlations with OSDI, SANDE, corneal fluorescein staining, and BCVA).
Table 3.
Correlations of NEI-VFQ-25 Scores with Symptoms and Clinical Parameters in patients with Ocular Graft-versus-host Disease
| NEI-VFQ-25 Scales | OSDI | SANDE | CFS | TBUT | Schirmer | BCVA |
|---|---|---|---|---|---|---|
| Composite score | −0.8* | −0.6* | −0.4* | 0.2 | 0.2 | −0.3* |
| Ocular pain | −0.7* | −0.6* | −0.3* | 0.2 | 0.1 | −0.2 |
| VS Role difficulties | −0.6* | −0.5* | −0.2 | 0.2 | 0.1 | −0.2* |
| VS Mental function | −0.8* | −0.5* | −0.4* | 0.2 | 0.2 | −0.3* |
| General vision | −0.5* | −0.2* | −0.4* | 0.1 | 0.1 | −0.3* |
| Near activities | −0.7* | −0.5* | −0.4* | 0.1 | 0.2 | −0.2* |
| Distance activities | −0.6* | −0.6* | −0.3* | 0.1 | 0.1 | −0.4* |
| Driving | −0.4* | −0.2 | −0.2 | 0.1 | 0.2 | −0.3* |
| VS Dependency | −0.6* | −0.4* | −0.3* | 0.1 | 0.0 | −0.2 |
| Peripheral vision | −0.4* | −0.2* | −0.1 | 0.1 | −0.1 | −0.2 |
| VS Social function | −0.5* | −0.5* | −0.1 | 0.1 | 0.0 | −0.3* |
| Color vision | −0.3* | −0.2* | −0.0 | −0.1 | −0.1 | −0.1 |
| General Health | −0.2 | −0.2 | −0.0 | 0.1 | −0.1 | −0.1 |
NEI-VQ-25: 25-item National Eye Institute Visual Function Questionnaire; SANDE: Symptoms Assessment in Dry Eye; OSDI: Ocular Surface Disease Index; CFS: corneal fluorescein staining; TBUT: tear break-up time; BCVA: best-corrected visual acuity; VS: vision specific. Higher scores in the NEI-VFQ-25 indicate better quality of life (note the negative correlations with OSDI, SANDE, CFS, and BCVA).
P<0.05
Discussion
The present study demonstrates that patients with ocular GVHD experience significant impairment in vision-related quality of life when compared to the healthy population. We found that patients’ vision-related quality of life is strongly associated with symptoms of ocular dryness, and modestly associated with the degree of corneal epitheliopathy.
Ocular GVHD presents mainly with dry eyes and ocular surface inflammation and, unsurprisingly, ocular pain was the most affected variable among the eleven subscales of the NEI-VFQ-25 questionnaire. This observation is in accordance with previous reports on QOL in dry eye disease.11,23,24 The mean scores in the subscales of role difficulties, near vision activities, distance vision activities, general vision, dependency, vision specific mental health, and peripheral vision, were particularly low with a difference of more than 10 points when compared to the mean scores in the healthy reference population.13 Additionally, a study from 2012, reported a mean composite QOL score of 94 in a cohort of 71 healthy controls.11 A difference of 5 to 10 points in the NEI-VFQ-25 has been reported to be clinically significant in previous studies.25,26 The low scores on the different subscales suggest that, beyond the ocular discomfort from the ocular surface disease, patients with ocular GVHD experience significant problems with various activities of the daily living. Riemans et al. showed significantly decreased scores in ocular pain, role difficulties, general vision, and social functioning in 14 patients with ocular GVHD compared to post-HSCT patients without ocular GVHD.12 Our observations in a larger cohort of 84 patients confirm these observations and additionally show a severe effect on the vision-related mental health subscale, with a difference of 25 points compared to healthy subjects. Vision-related mental health is assessed by questions regarding worry and frustration because of the patient’s eye problems. Our finding is in accord with previous reports of impaired mental health, anxiety and depression scores in patients with dry eye disease,11,23 and perhaps suggests the potential benefit of patient counseling as part of a comprehensive management of ocular GVHD.
Our results demonstrate that the composite quality of life score in patients with ocular GVHD (score 76.5) is impaired to a similar degree as has been observed among patients with Sjogren’s syndrome related dry eye (73).27 The ocular pain observed in this study (61) was comparable to pain scores reported in patients with ocular chemical burns (58) evidencing the severity of the ocular discomfort experienced by these patients.15 Other ocular conditions in which the NEI-VFQ-25 QOL composite score has been used to assess vision-related quality of life are: posterior subcapsular cataract (score 81),28 open angle glaucoma (84),28 dry eye disease (88, 82, 85),24,29,30 age-related macular degeneration (73),31 macular telangiectasia (77),32 proliferative diabetic retinopathy (83),33 diabetic macular edema (83),33 anterior herpetic uveitis (88),34 ocular chemical burns (40),15 and Steven Johnson syndrome (49).35 The subscales related to role difficulties, mental health and dependency in patients with ocular GVHD show either comparable or more severely affected scores than other incapacitating eye diseases such as glaucoma, cataract, anterior herpetic uveitis, and branch retinal vein occlusion.13,34,36 This observation highlights the extent of the limitations experienced by patients with ocular GVHD performing vision-demanding activities.
We noted a strong association between the NEI-VFQ-25 and the OSDI scores (R = −0.81) and a moderate association between the NEI-VFQ-25 and the SANDE scores (R = −0.56). The OSDI is perhaps the most commonly used dry eye questionnaire to measure symptoms of ocular surface disease. Although the OSDI is considered as an ocular surface disease-specific symptom questionnaire, it gathers additional information regarding limitations in performing common vision-related activities. Some of the OSDI questions address topics related to those in the NEI-VFQ-25, and this may explain the strong correlation between the two scales. Vitale et al. reported a moderate correlation between the OSDI and NEI-VFQ-25 (R= −0.61) in patients with Sjogren’s syndrome.37 The present findings confirm that the OSDI not only provides dry eye symptom information, but may also provide valuable information to judge the overall vision-related QOL in patients with ocular GVHD. This is particularly important since quality of life is not commonly evaluated and thus clinicians could utilize the OSDI to indirectly infer their patients’ quality of life. The SANDE questionnaire is a short and sensitive tool to assess dry eye symptoms,38 and the lower degree of correlation between the SANDE and NEI-VFQ composite score (R = −0.56), as compared to OSDI, is likely because the SANDE scale is designed to measure ocular surface discomfort symptoms and not other activities of daily living.
Corneal epitheliopathy, measured by corneal fluorescein staining, is the only dry eye clinical parameter that significantly correlates with the QOL composite score and the majority of its subscales. Since a smooth corneal surface and a stable tear film are critical factors in forming clear images,39 the epithelial damage caused by ocular surface disease leading to corneal surface irregularities may cause optical disturbances that impair visual function.40 A modest level of association between CFS and OSDI scores was previously reported by our group,41 and by Le et al.,23 who also noted a significant association between CFS and the composite NEI-VFQ-25 QOL score. These findings would suggest that therapeutic strategies that effectively target corneal fluorescein staining could lead to an improvement of vision-related quality of life in these patients. The pathophysiology of ocular GVHD involves an immune reaction of the transplanted cells against the host tissue, which leads to inflammation in the conjunctiva and lacrimal and meibomian glands, therefore manifesting with dry eye syndrome features.7 Hence, our data linking CFS to reduced vision-related quality of life in patients with ocular GVHD may be applicable to other types of dry eye.
A limitation of this study entails, arguably, the patients’ incapacity to fully discriminate between their perception of disability from multiple systemic affectations and the ocular disease. Although the NEI-VFQ-25 is designed to evaluate the vision-related quality of life, it relies on patients’ perception of disability and patients undergoing bone marrow transplantation have several systemic health conditions that may also influence their responses. In this study, we lacked a control group and relied on the data from the NEI-VFQ-25 developmental study control group, as other investigators have done previously.14,17,18,32,42 The main objective of this manuscript is to describe the vision-related quality of life in patients with ocular GVHD, thus comparing their QOL scores with healthy subjects from a historical healthy control group was intended only to provide context for interpretation of the observed QOL scores in patients with ocular GVHD. Due to the nature of such comparisons (using summary rather than individual data), some possible differences between these populations could not be directly accounted for, and hence results from this comparison should be interpreted with caution. It would also be of interest to compare QOL indicators among groups of GVHD patients with different morbidities. A limitation of some questionnaires that rely on patients’ perception of symptoms, such as the NEI-VFQ-25, is their incapacity to assess further improvement in patients who reach near the maximum score despite considerable disease. Therefore, after clinical recovery (e.g., after treatment) the room for further score improvement is limited, this ceiling effect was present in some of the patients evaluated in this study.
In summary, this study shows that patients with chronic ocular GVHD experience significant impairment in multiple domains of vision-related quality of life as compared to the healthy population of reference. Strong associations are noted between symptoms of ocular surface disease and vision-related quality of life, while corneal fluorescein staining is modestly associated with quality of life scores. The findings from this study reveal the great impact of ocular GVHD on the vision-related quality of life and highlight the multiple facets of dysfunction faced by survivors after bone marrow transplantation.
Supplementary Material
Précis.
Patients with ocular graft-versus-host disease experience significant impairment of vision-related quality of life compared with healthy controls. Additionally, symptoms of dry eye and corneal epitheliopathy show significant correlation with the quality of life measurements.
Acknowledgments
The authors thank Dr. Susanne Eiglmeier for her assistance in the preparation of this manuscript.
Financial Support: This work was supported by the National Eye Institute, National Institutes of Health (Grant no: K24-EY019098) and Research to Prevent Blindness. The funding organizations had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting presentation: This study was partly presented at the annual meeting of the Association for Research in Vision and Ophthalmology, May 2014, Orlando, Florida.
Conflict of Interest: The authors have no proprietary or commercial interest in any materials discussed in this article.
References
- 1.Appelbaum FR. Hematopoietic-cell transplantation at 50. N Engl J Med. 2007;357:1472–5. doi: 10.1056/NEJMp078166. [DOI] [PubMed] [Google Scholar]
- 2.Gratwohl A, Baldomero H, Aljurf M, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303:1617–24. doi: 10.1001/jama.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kansu E. The pathophysiology of chronic graft-versus-host disease. Int J Hematol. 2004;79:209–15. doi: 10.1532/ijh97.04015. [DOI] [PubMed] [Google Scholar]
- 4.Pallua S, Giesinger J, Oberguggenberger A, et al. Impact of GvHD on quality of life in long-term survivors of haematopoietic transplantation. Bone marrow transplantation. 2010;45:1534–9. doi: 10.1038/bmt.2010.5. [DOI] [PubMed] [Google Scholar]
- 5.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of blood and marrow transplantation. 2005;11:945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Shikari H, Antin JH, Dana R. Ocular Graft-versus-Host Disease: A Review. Surv Ophthalmol. 2013;58:233–51. doi: 10.1016/j.survophthal.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Hessen M, Akpek EK. Ocular graft-versus-host disease. Curr Opin Allergy Clin Immunol. 2012;12:540–7. doi: 10.1097/ACI.0b013e328357b4b9. [DOI] [PubMed] [Google Scholar]
- 8.Kim SK. Update on ocular graft versus host disease. Curr Opin Ophthalmol. 2006;17:344–8. doi: 10.1097/01.icu.0000233952.09595.d8. [DOI] [PubMed] [Google Scholar]
- 9.Miljanovic B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143:409–15. doi: 10.1016/j.ajo.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchino M, Schaumberg DA. Dry Eye Disease: Impact on Quality of Life and Vision. Current ophthalmology reports. 2013;1:51–7. doi: 10.1007/s40135-013-0009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Gong L, Chapin WJ, Zhu M. Assessment of vision-related quality of life in dry eye patients. Invest Ophthalmol Vis Sci. 2012;53:5722–7. doi: 10.1167/iovs.11-9094. [DOI] [PubMed] [Google Scholar]
- 12.Riemens A, Te Boome LC, Kalinina Ayuso V, et al. Impact of ocular graft-versus-host disease on visual quality of life in patients after allogeneic stem cell transplantation: questionnaire study. Acta ophthalmologica. 2014;92:82–7. doi: 10.1111/aos.12047. [DOI] [PubMed] [Google Scholar]
- 13.Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–8. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 14.Hariprasad SM, Mieler WF, Grassi M, et al. Vision-related quality of life in patients with diabetic macular oedema. Br J Ophthalmol. 2008;92:89–92. doi: 10.1136/bjo.2007.122416. [DOI] [PubMed] [Google Scholar]
- 15.Le Q, Chen Y, Wang X, Li Y, Hong J, Xu J. Vision-related quality of life in patients with ocular chemical burns. Invest Ophthalmol Vis Sci. 2011;52:8951–6. doi: 10.1167/iovs.11-8355. [DOI] [PubMed] [Google Scholar]
- 16.Mangione CM, Lee PP, Pitts J, et al. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ). NEI-VFQ Field Test Investigators. Arch Ophthalmol. 1998;116:1496–1504. doi: 10.1001/archopht.116.11.1496. [DOI] [PubMed] [Google Scholar]
- 17.Naik RK, Rentz AM, Foster CS, et al. Normative comparison of patient-reported outcomes in patients with noninfectious uveitis. JAMA ophthalmology. 2013;131:219–25. doi: 10.1001/2013.jamaophthalmol.102. [DOI] [PubMed] [Google Scholar]
- 18.Cahill MT, Banks AD, Stinnett SS, Toth CA. Vision-related quality of life in patients with bilateral severe age-related macular degeneration. Ophthalmology. 2005;112:152–8. doi: 10.1016/j.ophtha.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 19.Schaumberg DA, Gulati A, Mathers WD, et al. Development and validation of a short global dry eye symptom index. Ocular surface. 2007;5:50–7. doi: 10.1016/s1542-0124(12)70053-8. [DOI] [PubMed] [Google Scholar]
- 20.Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–21. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 21.Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995;21:221–32. [PubMed] [Google Scholar]
- 22.Mangione CM. NEI VFQ-25 Scoring Algorithm. National Eye Institute; Rockville, MD: 2000. Available at: ( http://www.rand.org/health/surveys_tools/vfq.html.) [Google Scholar]
- 23.Le Q, Ge L, Li M, et al. Comparison on the vision-related quality of life between outpatients and general population with dry eye syndrome. Acta ophthalmologica. 2014;92:e124–132. doi: 10.1111/aos.12204. [DOI] [PubMed] [Google Scholar]
- 24.Nichols KK, Mitchell GL, Zadnik K. Performance and repeatability of the NEI-VFQ-25 in patients with dry eye. Cornea. 2002;21:578–83. doi: 10.1097/00003226-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Globe DR, Wu J, Azen SP, Varma R. The impact of visual impairment on self-reported visual functioning in Latinos: The Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1141–9. doi: 10.1016/j.ophtha.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Miskala PH, Hawkins BS, Mangione CM, et al. Responsiveness of the National Eye Institute Visual Function Questionnaire to changes in visual acuity: findings in patients with subfoveal choroidal neovascularization--SST Report No. 1. Arch Ophthalmol. 2003;121:531–9. doi: 10.1001/archopht.121.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizuno Y, Yamada M, Miyake Y. Association between clinical diagnostic tests and health-related quality of life surveys in patients with dry eye syndrome. Jpn J Ophthalmol. 2010;54:259–65. doi: 10.1007/s10384-010-0812-2. [DOI] [PubMed] [Google Scholar]
- 28.Wu SY, Hennis A, Nemesure B, Leske MC, Barbados Eye Studies Group Impact of glaucoma, lens opacities, and cataract surgery on visual functioning and related quality of life: the Barbados Eye Studies. Invest Ophthalmol Vis Sci. 2008;49:1333–8. doi: 10.1167/iovs.07-1252. [DOI] [PubMed] [Google Scholar]
- 29.Labiris G, Katsanos A, Fanariotis M, et al. Psychometric properties of the Greek version of the NEI-VFQ 25. BMC Ophthalmol. 2008;8:4. doi: 10.1186/1471-2415-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Q, Zhou X, Ge L, Wu L, Hong J, Xu J. Impact of dry eye syndrome on vision-related quality of life in a non-clinic-based general population. BMC Ophthalmol. 2012;16:12–22. doi: 10.1186/1471-2415-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orr P, Rentz AM, Margolis MK, et al. Validation of the National Eye Institute Visual Function Questionnaire-25 (NEI VFQ-25) in age-related macular degeneration. Invest Ophthalmol Vis Sci. 52:3354–9. doi: 10.1167/iovs.10-5645. 201;18. [DOI] [PubMed] [Google Scholar]
- 32.Clemons TE, Gillies MC, Chew EY, et al. The National Eye Institute Visual Function Questionnaire in the Macular Telangiectasia (MacTel) Project. Invest Ophthalmol Vis Sci. 2008;49:4340–6. doi: 10.1167/iovs.08-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein R, Moss SE, Klein BE, Gutierrez P, Mangione CM. The NEI-VFQ-25 in people with long-term type 1 diabetes mellitus: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 2001;119:733–40. doi: 10.1001/archopht.119.5.733. [DOI] [PubMed] [Google Scholar]
- 34.Hoeksema L, Los LI. Vision-related quality of life in herpetic anterior uveitis patients. PloS one. 2014;9:e85224. doi: 10.1371/journal.pone.0085224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaido M, Yamada M, Sotozono C, et al. The relation between visual performance and clinical ocular manifestations in Stevens-Johnson syndrome. Am J Ophthalmol. 2012;154:499–511. doi: 10.1016/j.ajo.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 36.Awdeh RM, Elsing SH, Deramo VA, et al. Vision-related quality of life in persons with unilateral branch retinal vein occlusion using the 25-item National Eye Institute Visual Function Questionnaire. Br J Ophthalmol. 2010;94:319–23. doi: 10.1136/bjo.2007.135913. [DOI] [PubMed] [Google Scholar]
- 37.Vitale S, Goodman LA, Reed GF, Smith JA. Comparison of the NEI-VFQ and OSDI questionnaires in patients with Sjogren's syndrome-related dry eye. Health Qual Life Outcomes. 2004;2:44. doi: 10.1186/1477-7525-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gulati A, Sullivan R, Buring JE, et al. Validation and repeatability of a short questionnaire for dry eye syndrome. Am J Ophthalmol. 2006;142:125–31. doi: 10.1016/j.ajo.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 39.Ridder WH, 3rd, Tomlinson A, Huang JF, Li J. Impaired visual performance in patients with dry eye. Ocul Surf. 2011;9:42–55. doi: 10.1016/s1542-0124(11)70009-x. [DOI] [PubMed] [Google Scholar]
- 40.Kaido M, Matsumoto Y, Shigeno Y, et al. Corneal fluorescein staining correlates with visual function in dry eye patients. Invest Ophthalmol Vis Sci. 2011;52:9516–22. doi: 10.1167/iovs.11-8412. [DOI] [PubMed] [Google Scholar]
- 41.Amparo F, Jin Y, Hamrah P, et al. What is the value of incorporating tear osmolarity measurement in assessing patient response to therapy in dry eye disease? Am J Ophthalmol. 2014;157:69–77. doi: 10.1016/j.ajo.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balcer LJ, Baier ML, Kunkle AM, et al. Self-reported visual dysfunction in multiple sclerosis: results from the 25-Item National Eye Institute Visual Function Questionnaire (VFQ-25) Mult Scler. 2000;6:382–5. doi: 10.1177/135245850000600604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.