Abstract
Objectives
To determine whether antiretroviral (ARV) medications can be detected in meconium from 2nd or 3rd trimester, labor and delivery (L&D), or postnatal exposures.
Study design
Twenty ARV medications were quantified by LC-MS/MS in 598 meconium samples from uninfected infants born to pregnant women with HIV enrolled in the Pediatric HIV/AIDS Cohort Study.
Results
ARV detection in meconium following 3rd trimester exposure was 85.7–94.4% for all ARVs except stavudine (0%, n=2), likely due to low doses and a high limit for quantification. Of 107 samples with some 2nd trimester only ARV exposures, meconium was positive for only lopinavir, tenofovir, or efavirenz in 11.8–14.3% of exposed neonates; administration of these ARVs occurred between gestational weeks 25–28 in the positive samples. Days without lopinavir or tenofovir before delivery significantly correlated with decreasing concentrations of tenofovir and lamivudine in meconium. Concentrations significantly correlated with increasing gestational age among infants with continuous 2nd and 3rd trimester exposure. Zidovudine given during L&D or for neonatal prophylaxis was detected in 95.1% and 94.6% of meconium samples, respectively.
Conclusions
Changes in ARV treatments during pregnancy offered a unique opportunity to investigate ARV detection in meconium. ARVs in meconium primarily reflect 3rd trimester ARV exposures, although 6 of 107 2nd trimester only exposures were detected. Zidovudine administration during L&D was detected in meconium indicating potential urine contamination or rapid incorporation into meconium. These data will improve interpretation of meconium drug test results.
Keywords: pregnancy, labor and delivery, HIV, zidovudine, PHACS
Meconium begins to form in utero during the 12th gestational week and accumulates thereafter.1, 2 It is the specimen of choice for assessing fetal drug exposure,3–11 offering advantages over neonatal urine with easier collection from diapers and a longer window for detection, primarily reflecting 3rd trimester fetal drug exposures.7, 11
Drug disposition in meconium is poorly understood and determining the time frame during gestation when drug exposure can be detected in meconium (the window of drug detection), is difficult. Most meconium forms in the final weeks before delivery,12 when fetal growth, and fetal/placental blood and nutrient transport increase exponentially.13, 14 There is minimal information about meconium detection of 2nd trimester fetal drug exposure. Our group previously evaluated opiate and cocaine meconium detection windows with 3 times-weekly urine collections to assess drug exposure timing.7, 11 We identified when drug relapse occurred, and concluded meconium reliably detected only 3rd trimester drug use.7, 11
Interpretation of drug concentrations in meconium also may be complicated drug administration during labor and delivery (L&D). In 10 women who received 50–200 mg meperidine during labor, meconium was positive for meperidine in all infants, and 3 infants also were positive for normeperidine.15 These results may be explained by contamination of meconium with neonatal urine, rapid drug incorporation into meconium close to birth, or decreased P-glycoprotein expression late in pregnancy.16 Zidovudine (AZT) is often administered during L&D of mothers with HIV and to infants exposed to HIV postnatally.17 Utilizing maternal and neonatal AZT medication chart information provided a unique opportunity to investigate drug incorporation into meconium during L&D.
We evaluated windows of drug detection in meconium and determined whether meconium could detect antiretrovirals (ARVs) that werestopped or started during the 2nd or 3rd trimester. We also evaluated detection and quantification of AZT in meconium following maternal administration during L&D and/or infant postnatal administration.
Methods
The Surveillance Monitoring for ARV Toxicities (SMARTT) study of the Pediatric HIV/AIDS Cohort Study (PHACS) enrolled children exposed to HIV but not infected and their mothers infected with HIV who were prescribed ARVs during pregnancy to investigate long-term prenatal exposure effects of ARV.18 Mothers and infants enrolled in SMARTT’s Dynamic cohort between 22 weeks gestation and 1 week after birth. Institutional Review Boards at each site and the Harvard T.H. Chan School of Public Health approved the protocol, with maternal written informed consent.
Meconium was collected at one or more time points within 72 h of birth; multiple diaper collections were combined until transitional stool. Prior to 2011, meconium was refrigerated at study sites, and all specimens were frozen (≤−20°C) until analysis (0–6 years). Beginning in 2011, meconium was frozen immediately after collection. Our novel liquid chromatography tandem mass spectrometry method quantified 20 ARV markers in 0.25 g meconium with limits of quantification (LOQ) from 10–500 ng/g.19 Sixteen parent ARVs (abacavir, ABC; amprenavir; atazanavir, ATV; darunavir, DRV; efavirenz, EFV; emtricitabine, FTC; lamivudine, 3TC; lopinavir, LPV; nelfinavir, NFV; nevirapine, NVP; raltegravir, RAL; ritonavir, RTV; saquinavir, SQV; stavudine, d4T; tenofovir, TFV; AZT) and four metabolites (ABC-carboxylate; ABC-glucuronide; NFV hydroxy-tert-butylamide, M8; AZT-glucuronide) were measured, representing >99% of SMARTT ARV exposures.20
During validation, stability of ARV in meconium was investigated to determine if initial storage temperatures adversely affected quantification of ARV in meconium; all quantitative analytes were >82% stable under refrigerated (72 h 4°C) and frozen (triplicate −20°C freeze/thaw cycles) conditions.19 Meconium concentrations in ARV in samples collected before 2011 (n=240) were compared with those collected in 2011 or later (n=358) by a Mann-Whitney test to further evaluate stability.
Statistical Analyses
ARV prescription between 15–28 gestational weeks defined 2nd trimester exposure and >28 weeks through delivery defined 3rd trimester exposure. Among infants whose mothers were prescribed 3rd trimester ARVs, group differences between samples with and without missed 3rd trimester detection in meconium were evaluated with Mann Whitney tests.
For ARVs with multiple 2nd trimester-only detections, the association between days off the ARV pre-delivery and meconium concentration was investigated. Analysis included women with only 2nd trimester use and those with any 3rd trimester use. First, square-root, natural log, and log10 transformations were evaluated to normalize meconium ARV concentrations. A linear regression model was built for normalized meconium concentrations and maternal days off ARV. Exposure duration (days) was added a priori. Potential confounders (maternal tobacco, alcohol, or illicit drug use during pregnancy) were added to the model individually and retained when the effect estimate for the association between days off ARV and meconium concentration changed ≥15%.
Associations between gestational age and ARV concentrations in meconium were investigated using linear regression for infants whose mothers were maintained continuously on the same ARV (entire 2nd and 3rd trimesters, with ≤3 days off drug). Sufficient data (n=21–213) were available for 6 ARVs: TFV, FTC, 3TC, LPV, RAL, and RTV. For meconium TFV, maternal tenofovir disoproxil fumarate (TDF), a widely used TFV prodrug, was considered. RTV is commonly prescribed with other protease inhibitors (PIs) as a pharmacokinetic boosting agent; when RTV was the sole PI in a mother’s regimen, samples were excluded from this analysis (n=4). Potential confounders (maternal HIV RNA copies/mL before L&D, and maternal tobacco, alcohol or illicit drug use during pregnancy) were evaluated separately and retained in the adjusted model when the effect estimate for the association between gestational age and meconium concentration changed ≥15%.
To investigate meconium detection of AZT with L&D and infant prophylaxis, 7 exposure categories were considered: (1) maternal 3rd trimester, L&D, and neonatal prophylaxis; (2) only 3rd trimester and L&D; (3) only L&D and neonatal prophylaxis; (4) only maternal 3rd trimester and neonatal prophylaxis; (5) only L&D; (6) only neonatal prophylaxis; and (7) only 3rd trimester. Meconium AZT detection prevalence and concentrations are reported and Kruskal-Wallis chi-square tests assessed median concentration differences of AZT and AZT-glucuronide between groups. Significant associations were described by P<0.05.
Results
Meconium ARV Detection in Third Trimester
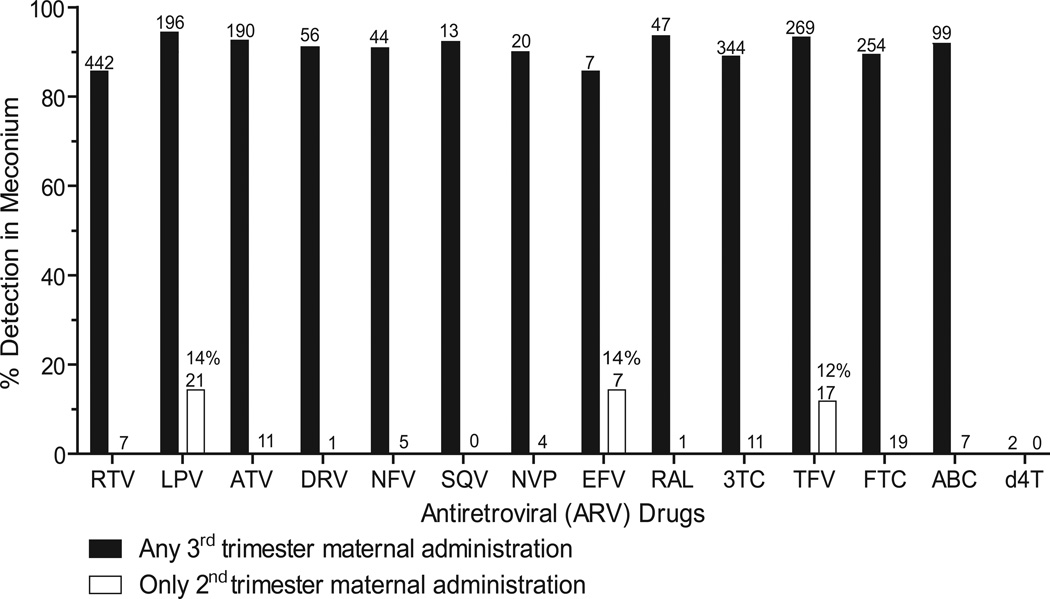
Of 1750 SMARTT Dynamic cohort infants enrolled through October 1, 2013, ARVs were quantified in 598 meconium samples. Maternal medication histories indicated 3rd trimester exposure to ARVs in 587 meconium samples (11 participants had no 3rd trimester ARV medication data available). ARV drug detection in third trimester meconium was 85.7–94.4% for ABC, ATV, DRV, EFV, FTC, 3TC, LPV, NFV, NVP, RAL, RTV, SQV, and TFV (Figure and Table I; Table I available at www.jpeds.com). There was no difference in percent drug detection between 2nd and 3rd trimester exposure and 3rd trimester exposure alone. AZT was considered separately due to other common exposure routes including maternal L&D administration and neonatal prophylaxis. No amprenavir exposure occurred in our population.
Figure.
Antiretroviral (ARV) drug detection in meconium following any 3rd trimester or only 2nd trimester maternal ARV prescription. Numbers above bars indicate total meconium specimens with 3rd or 2nd trimester exposure. 2nd trimester drug detection only occurred in some samples with LPV, EFV, and TFV exposure. Zidovudine (AZT) meconium drug detection data not shown (see text). No amprenavir exposure cases were included in this population. RTV, ritonavir; LPV, lopinavir, ATV, atazanavir; DRV, darunavir; NFV, nelfinavir; SQV, saquinavir; NVP, nevirapine; EFV, efavirenz; RAL, raltegravir; 3TC, lamivudine; TFV, tenofovir; FTC, emtricitabine; ABC, abacavir; d4T, stavudine.
Table 1.
Drug detection rates for ARVs in meconium in 587 3rd trimester and 107 only 2nd trimester ARV exposed samples
| ARVa | Infants with any 3rd trimester exposurebc |
3rd Trimester meconium ARV drug detection (%) |
Infants with only 3rd trimester exposurebc |
Proportion with meconium ARV drug detection (%) |
Median (range) positive meconium concentration from infants with only 3rd trimester exposure, ng/g |
Infants with some 2nd and 3rd trimester exposurebc |
Proportion with meconium ARV drug detection (%) |
Median (range) positive meconium concentration from infants with 2nd and 3rd trimester exposure, ng/g |
Infants with only 2nd trimester exposurebc |
Proportion with meconium ARV drug detection (%) |
Median (range) positive meconium concentration from infants with 2nd trimester exposure, ng/g |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease inhibitors (PIs) | |||||||||||
| RTV | 442 | 85.7 | 31 | 27 (87.1) | 201 (13 – 4302) | 411 | 352 (85.6) | 368 (11 – 30349) | 7 | 0 | |
| LPV | 196 | 94.4 | 25 | 23 (92.0) | 24800 (46 – 149602) | 171 | 162 (94.7) | 38553 (10 – 546720) | 21 | 3 (14.3) | 177 (29 – 2401) |
| ATV | 190 | 92.6 | 14 | 14 (100) | 9026 (139 – 143018) | 176 | 162 (92.1) | 16929 (29 – 125352) | 11 | 0 | |
| DRV | 56 | 91.1 | 3 | 2 (66.7) | 578 (156 – 1000) | 53 | 49 (92.5) | 17900 (222 – 240517) | 1 | 0 | |
| NFV | 44 | 90.9 | 2 | 2 (100) | 1822 (484 – 3160) | 42 | 38 (90.5) | 10292 (11 – 314133) | 5 | 0 | |
| SQV | 13 | 92.3 | 1 | 1 (100) | 85432 | 12 | 11 (91.7) | 16620 (114 – 36200) | 0 | ||
| Non-nucleoside analog reverse transcriptase inhibitors (NNRTIs) | |||||||||||
| NVP | 20 | 90.0 | 1 | 1 (100) | 1451 | 19 | 17 (89.5) | 3734 (77 – 17465) | 0 | ||
| EFV | 7 | 85.7 | 5 | 4 (80.0) | 2555 (1776 – 40853) | 2 | 2 (100) | 5056 (2096 – 8016) | 7 | 1 (14.3) | 356 |
| Integrase inhibitors | |||||||||||
| RAL | 47 | 93.6 | 17 | 16 (94.1) | 2545 (67 – 16178) | 30 | 28 (93.3) | 1912 (38 – 21280) | 1 | 0 | |
| Nucleoside/Nucleotide analog reverse transcriptase inhibitors (NRTIs) | |||||||||||
| 3TC | 344 | 89.0 | 21 | 19 (90.5) | 1993 (80 – 11745) | 323 | 287 (88.9) | 2101 (55 – 40345) | 11 | 0 | |
| TFV | 269 | 93.3 | 25 | 21 (84.0) | 2160 (37 – 37162) | 244 | 230 (94.3) | 7800 (11 – 89655) | 17 | 2 (11.8) | 79 (22 – 135) |
| FTC | 254 | 89.4 | 22 | 18 (81.8) | 700 (35 – 4268) | 232 | 209 (90.1) | 991 (15 – 57126) | 19 | 0 | |
| ABC | 99 | 91.9 | 7 | 5 (71.4) | 417 (234 – 3748) | 92 | 86 (93.5) | 882 (10 – 12873) | 7 | 0 | |
| d4T | 2 | 0 | 0 | 2 | 0 (0) | 0 | |||||
RTV, ritonavir; LPV, lopinavir, ATV, atazanavir; DRV, darunavir; NFV, nelfinavir; SQV, saquinavir; NVP, nevirapine; EFV, efavirenz; RAL, raltegravir; 3TC, lamivudine; TFV, tenofovir; FTC, emtricitabine; ABC, abacavir; d4T, stavudine; Data for zidovudine (AZT) meconium drug detection shown separately due to other common exposure routes, including labor and delivery (L&D) and neonatal administration. No amprenavir exposure examples examined in this sample population.
Complete exposure duration for the entire listed trimester was not required for group inclusion here.
Column sets are not exclusive. Infants in each column set may overlap.
There was no detection of prescribed 3rd trimester ARVs in 107 meconium samples (Table II). When an ARV exposure was not detected, other ARVs were detected in 28.4–75% of samples (Table 2 II). Only two specimens with 3rd trimester d4T exposure were negative, although all other 3rd trimester ARVs were detected in these samples. We investigated whether these missed detections in meconium may have resulted from poor maternal medication adherence, suggested by plasma HIV RNA >400 copies/mL at L&D, or elevated LOQs when <0.25 g meconium was available. In our 107 samples with some missed 3rd trimester ARV exposures, plasma HIV RNA at L&D was >400 copies/mL in 47 (43.9%) samples, and limited available meconium for testing occurred in 13 (12.1%). Specimens positive for the ARVs that were missed in other samples generally had low concentrations near our LOQs (Table I). In 90 of these 107 samples (including 12 of 13 (92.3%) low volume specimens and 37 of 47 (78.7%) high viral load samples), other ARVs in the sample were correctly identified. Median (range) infant birth weight and gestational age for these 107 infants were 2890 g (1705–4530) and 38.0 weeks (31.9–41.0), respectively; these were significantly lower than medians observed among infants with 3rd trimester detection in meconium (3023 g [1370–5195], 38.4 weeks [29.0–42.1]; P<0.01, both comparisons). Median RTV, 3TC, and TFV exposure duration among samples with missed 3rd trimester exposure detection in meconium were significantly lower than median exposure durations among samples with successful 3rd trimester detection (P≤0.01, all 3 comparisons).
Table 2.
Meconium antiretroviral (ARV) detection frequency among 107 infants and proportion of missed detection of 3rd trimester prescribed ARVs
| Meconium ARVa |
Number of infants with 3rd trimester exposure |
Missed ARV meconium detection (n, %) |
|---|---|---|
| SQV | 4 | 1 (25.0) |
| TFV | 67 | 18 (26.9) |
| LPV | 37 | 11 (29.7) |
| ATV | 47 | 14 (29.8) |
| EFV | 3 | 1 (33.3) |
| RAL | 9 | 3 (33.3) |
| DRV | 14 | 5 (35.7) |
| NFV | 10 | 4 (40.0) |
| ABC | 18 | 8 (44.4) |
| FTC | 60 | 27 (45.0) |
| 3TC | 63 | 38 (60.3) |
| NVP | 3 | 2 (66.7) |
| RTV | 88 | 63 (71.6) |
| d4T | 2 | 0 (100) |
SQV, saquinavir; TFV, tenofovir; LPV, lopinavir; ATV, atazanavir; EFV, efavirenz; RAL, raltegravir; DRV, darunavir; NFV, nelfinavir; ABC, abacavir; FTC, emtricitabine; 3TC, lamivudine; NVP, nevirapine; RTV, ritonavir; d4T, stavudine. Zidovudine (AZT) meconium drug detection shown separately due to other common exposure routes, including labor and delivery (L&D) and neonatal administration. No amprenavir exposure examples examined in this sample population.
The 4 ARV metabolites were always detected in meconium with parent ARVs. In the 94 ABC-positive samples, 82 were ABC-carboxylate-positive and 79 ABC-glucuronide-positive, with median (range) metabolite/parent concentration ratios of 4.2 (0.09–39.7) and 0.12 (0.02–3.3), respectively. NFV’s hydroxyl metabolite M8 was detected in all 42 NFV-positive meconium samples, with a median M8/NFV ratio of 4.3 (0.07–74.3).
With the 2011 procedural change to immediately freeze meconium after collection, we compared missed drug detection in samples collected before and after 2011. ARV meconium concentrations were not significantly different between samples collected before 2011 and those collected after 2011 for all analytes, except AZT (P=0.011). This suggests the 2011 procedural change from initial meconium refrigeration to rapid meconium freezing did not impact meconium ARV concentrations, except AZT. Additionally, missed meconium detection of 3rd trimester ARV exposure did not occur more often in specimens collected before 2011 (P=0.51).
Second Trimester Meconium ARV Detection
Separate from the 107 samples with missed detection in meconium of 3rd trimester that were prescribed ARVs, there were another 107 samples with exposure to ARVs only during the 2nd trimester, including 21 LPV, 19 FTC, 17 TDF, 11 ATV, 11 3TC, 7 ABC, 7 EFV, 7 RTV, 5 NFV, 1 DRV, and 1 RAL exposures. Only 3 LPV, 2 TDF, and 1 EFV exposures were detected (Table III). In these 6 samples, maternal days off the ARV before delivery were 57–92 days, documenting meconium ARV detection of exposures between gestational weeks 25–28 (Table III).
Table 3.
Second trimestera antiretroviral (ARV) exposure detected in meconium from 6 infants
| Infant | Maternal Lopinavir (LPV) prescription start |
Maternal LPV prescription end |
Infant gestational age (GA) |
Meconium LPV, ng/g |
|---|---|---|---|---|
| 1 | 147 days before delivery (GA:15 weeks) | 57 days before delivery (GA: 27.9 weeks) | 252 days (36.0 weeks) | 2401 |
| 2 | 142 days before delivery (GA: 18.29 weeks) | 91 days before delivery (GA: 25.6 weeks) | 270 days (38.6 weeks) | 177 |
| 3 | 138 days before delivery (GA: 19.29 weeks) | 92 days before delivery (GA: 25.9 weeks) | 273 days (39.0 weeks) | 29 |
| Maternal TDFb prescription start | Maternal TDF prescription end | Infant GA | Meconium Tenofovir (TFV), ng/g | |
| 4 | 253 days before delivery (GA: 0 weeks) | 65 days before delivery (GA: 26.9 weeks) | 253 days (36.1 weeks) | 135 |
| 5 | 159 days before delivery (GA: 15.29 weeks) | 75 days before delivery (GA: 27.3 weeks) | 266 days (38.0 weeks) | 22 |
| Maternal Efavirenz (EFV) prescription start | Maternal EFV prescription end | Infant GA | Meconium EFV, ng/g | |
| 6 | 90 days before delivery (GA: 25.71 weeks)c | 84 days before delivery (GA: 26.6 weeks) | 270 days (38.6 weeks) | 356 |
2nd trimester defined as the gestational period of >15 and ≤28 weeks.
TDF: tenofovir disproxil fumarate, a bioavailable prodrug of tenofovir (TFV)
Infant 6’s mother was prescribed EFV for 6 days at the end of the 2nd trimester.
A linear relationship between maternal days off LPV and TDF before delivery and meconium concentrations was observed. The estimated square-root LPV concentration change with each additional day off LPV before delivery was −1.23 (95% confidence interval (CI): −1.9, −0.49; P<0.01). Estimated square-root TFV concentration change in meconium with each additional maternal day off TDF before delivery was −0.36 (95% CI: −0.59, −0.14; P<0.01). Cigarette, alcohol, or illicit drug use during pregnancy did not significantly impact these associations.
Meconium Concentrations Increase with Gestational Age
Significant associations between gestational age and ARV concentrations in meconium were documented for 3TC and TFV and remained significant after adjusting for maternal HIV RNA at L&D >1000 copies/mL (Table IV; available at www.jpeds.com). For each increased gestational age day, the estimated square-root meconium 3TC and TFV concentration change was 1.24 and 1.04, respectively. For meconium FTC, LPV, RAL, and RTV, positive but non-significant associations were generally observed with gestational age (Table IV). Meconium concentrations were normalized with square-root (TFV, 3TC, LPV, RAL) or log10 transformations (FTC, RTV). Cigarette, alcohol, or illicit drug use during pregnancy did not significantly impact these associations.
Table 4.
Effect estimates for gestational age and meconium antiretroviral (ARV) concentrations in infants who were exposed continuously during the 2nd and 3rd trimesters
| Meconium ARVa |
N | Proportion with meconium ARV detection |
Gestational Age Median (IQR), days, weeks |
Unadjusted Model Estimateb (95% CI) |
P- value |
Adjusted Model Estimatebc (95% CI) |
P- value |
|---|---|---|---|---|---|---|---|
| 3TC | 128 | 93.8 % | 267 (263.5 – 276) 38.1 (37.6 – 39.4) |
1.27 (0.75, 1.80) | <0.01 | 1.24 (0.71, 1.77) | <0.01 |
| FTC | 145 | 93.8 % | 269 (265 – 276) 38.4 (37.9 – 39.4) |
0.011 (−0.002, 0.024) | 0.10 | 0.007 (−0.005, 0.018) | 0.26 |
| LPV | 67 | 97.0 % | 269 (263 – 274) 38.4 (37.6 – 39.1) |
3.24 (−0.68, 7.16) | 0.10 | 2.55 (−1.28, 6.38) | 0.19 |
| RAL | 21 | 90.5 % | 271 (266 – 276) 38.7 (38.0 – 39.4) |
0.67 (−1.32, 2.65) | 0.49 | 0.50 (−1.43, 2.42) | 0.60 |
| RTVd | 213 | 89.2 % | 269 (264 – 275) 38.4 (37.7 – 39.3) |
0.006 (−0.007, 0.018) | 0.39 | 0.001 (−0.011, 0.013) | 0.83 |
| TFV | 153 | 96.1 % | 269 (264 – 276) 38.4 (37.7 – 39.4) |
1.22 (0.40, 2.05) | <0.01 | 1.04 (0.25, 1.83) | 0.01 |
3TC, lamivudine; FTC, emtricitabine; LPV, lopinavir; RAL, raltegravir; RTV, ritonavir; TFV, tenofovir
Estimates represent changes in square-root (TFV, 3TC, LPV, RAL) or log10 transformed (FTC, RTV) meconium concentrations for each increase in gestational age day.
All models adjusted for maternal HIV RNA at labor and delivery (L&D) >1000 copies/mL.
Only boosted RTV considered; 4 unboosted RTV prescription cases excluded.
CI indicates confidence interval
Neonatal and L&D AZT Meconium Detection
Table V describes AZT and AZT-glucuronide meconium detection and concentrations by AZT exposure category in 596 infants. Overall, when maternal 3rd trimester AZT prescription occurred, it also was administered during L&D and/or orally to the infant after birth; this exposure yielded a 96.3% detection rate in meconium (Table V). L&D AZT administration occurred in 94.8% of tested infants. Neonatal AZT prophylaxis was more common, occurring in 98.7% of infants. All except 33 (5.5%) meconium samples from infants exposed to AZT by any route were AZT-positive. Median AZT meconium concentrations were significantly different between exposure groups (P=0.004), although median AZT-glucuronide concentrations were not (P=0.46). Median AZT-glucuronide/AZT ratios were commonly >1 (Table V). No information was available on AZT L&D administration timing relative to delivery, or L&D and neonatal AZT dose.
Table 5.
Zidovudine (AZT) and AZT-glucuronide meconium detection and concentrations by type of AZT exposure in 596 samples
| 7 AZT exposure categories |
N (% of total 596) |
AZT positive meconium n (%) |
Median (range) positiveb meconium AZT concentrations, ng/g |
Median (range, n) positivec meconium AZT-glucuronide concentrations, ng/g |
Median (range) positive meconium AZT-glucuronide/AZT ratio |
|---|---|---|---|---|---|
| Maternal 3rd trimester, L&Da, and neonatal prophylaxis | 323 (54.2%) | 312 (96.6%) | 3292 (102 – 81468) | 7337 (501 – 783582, n=235) | 1.9 (0.06 – 29.3) |
| Maternal 3rd trimester and L&D (no neonatal prophylaxis) | 3 (0.5%) | 2 (66.7%) | 1327 (996 – 1657) | 1575 (1248 – 1903, n=2) | 1.3 (0.8 – 1.9) |
| L&D and neonatal prophylaxis (no 3rd trimester AZT) | 238 (39.8%) | 222 (93.3%) | 1464 (103 – 136595) | 6225 (531 – 105280, n=127) | 1.6 (0.2 – 41.4) |
| Maternal 3rd trimester and neonatal prophylaxis (no L&D) | 2 (0.3%) | 2 (100%) | 1755 (385 – 3125) | 1548 (n=1) | 0.5 |
| L&D only | 3 (0.5%) | 3 (100%) | 2888 (563 – 20165) | 18005 (2263 – 33746, n=2) | 1.2 (0.8 – 1.7) |
| Neonatal prophylaxis only | 27 (4.5%) | 22 (81.5%) | 1413 (189 – 22620) | 4338 (500 – 57823, n=14) | 1.1 (0.4 – 5.3) |
| Maternal 3rd trimester only | 0 |
Labor and delivery (L&D) administration
AZT LOQ 100 ng/g; median AZT meconium concentrations were significantly different between exposure groups (Kruskal-Wallis chi-square statistic 18.9, P=0.004).
AZT-glucuronide LOQ 500 ng/g; median AZT-glucuronide meconium concentrations were not significantly different between exposure groups (Kruskal-Wallis chi-square 5.7, P=0.46).
Discussion
We describe drug detection windows with meconium, offering an improved study design compared with previous detection window research in meconium. Previously, these detection windows were studied by collecting other biological samples to assess drug exposure and timing of relapses.7, 11, 16–18 Our novel analytical method allowed meconium quantification of ARVs from 4 diverse drug classes in a single sample, offering substantial improvements in assessing drug detection windows compared with previous studies and providing additional clinical relevance. ARV regimen changes during pregnancy offered a unique opportunity to investigate disposition of ARVs in meconium.
Drug deposition in meconium occurs from drug passing through the fetal gastrointestinal tract, bile secretions, or swallowed amniotic fluid containing fetal urine. Drug concentrations may relate to amount and timing of maternal drug consumption and the time interval between last drug use and birth, due to non-linear meconium accumulation late in gestation.
Our undetected 3rd trimester drug exposures may be explained by poor medication compliance,21 low meconium volume (higher LOQs), shorter exposure durations, earlier delivery and lower gestational age, and/or individual differences in placental transfer, maternal/fetal metabolism, and infant meconium accumulation late in gestation (as large amounts of drug-negative meconium accumulate close to birth4, 22). Although our study was unique with detailed medication prescription records, we cannot state that mothers were compliant in taking medications. In our samples with some missed ARV detection, other 3rd trimester prescribed ARVs were correctly identified, suggesting longer ARV exposures, higher ARV doses, greater placental transfer, and/or maternal/fetal genetic metabolism variability may have contributed to differential detection. Our novel analytical method quantified ARVs in meconium; however, our d4T LOQ may be too high for accurate d4T detection in meconium.
Meconium analysis detected 2nd trimester only ARV exposure, but in only 6 of 107 samples. Although the detected drugs, LPV, TFV, and EFV, are from different ARV drug classes, they had the highest median concentrations in meconium observed within their class (Table I). Our findings confirm previous preclinical2, 23, 24 and clinical4, 7, 22 research that meconium primarily reflects 3rd trimester fetal drug exposure, although in a few circumstances 2nd trimester drug detection is feasible. Previous investigations of 2nd trimester drug detection in meconium relied on early-gestation postmortem meconium collection2 and animal models.23 Our study is a clinical study based on medication prescription to show 2nd trimester drug exposures can be detected in meconium collected from neonates after birth.
LPV meconium concentrations were the highest ARV concentrations observed (Table I), and LPV was the most commonly detected 2nd trimester ARV exposure. LPV is highly protein bound and demonstrates low (0.2–3.3%) placental transfer;25 however, as P-glycoprotein expression and plasma protein binding decrease during pregnancy, increased drug transfer may occur.16, 26 In addition, national guidelines recommend 2nd and 3rd trimester LPV doses should increase 1.5-fold.17 All LPV exposures in our study resulted from co-formulated LPV/RTV administration. RTV inhibits LPV metabolism via CYP 3A4. Even with low placental transfer, boosting LPV resulted in high fetal exposure, as demonstrated by high meconium concentrations.
Greater gestational ages correlated to higher drug concentrations in meconium. Square-root meconium 3TC and TFV concentrations significantly increased 1.24- and 1.04-fold, respectively, for every increase in gestational age days, indicating meconium 3TC and TFV may accumulate quadratically with gestational age. Our missed detection of 3rd trimester exposure in samples with significantly earlier gestational ages and lower birth weight supports these findings.
This research validated disposition of ARV treatments during L&D into meconium. Clinically, these data suggest cautious interpretation of drugs found in meconium when commonly given during L&D. In all 3 administrations of AZT during L&D AZT only samples, meconium AZT was detected, although the concentration range was large. These results verify 1994 findings with meperidine detection in meconium with administration during L&D.15 Detection of L&D administered drugs may be explained by rapid meconium drug incorporation immediately prior to birth,12 decreased placental drug efflux late in gestation,16 or neonatal urine contamination in diapers. AZT-glucuronide was detected in 2 of these 3 samples; metabolites may have resulted from maternal or neonatal metabolism.
Thirty-three (5.5%) meconium specimens from AZT-exposed infants were negative for AZT (Table V). Most occurred in infants with no 3rd trimester exposure. The large concentration ranges (with some concentrations near LOQs) for AZT and AZT-glucuronide in meconium from infants with 3rd trimester, L&D, and neonatal AZT administration, could explain the negative results found with less exposure. These AZT meconium results indicate possible rapid meconium incorporation when the fetus/infant experienced only L&D or neonatal administration; this rapid incorporation may be determined by additional maternal/neonatal factors, such as genetic polymorphisms, and dose timing and duration.
AZT-glucuronide concentrations were investigated as a possible means to increase detection of AZT; however, our results indicate AZT-glucuronide was always detected with AZT. AZT-glucuronide/AZT in meconium were commonly >1, indicating possible fetal AZT-glucuronide formation or placental transfer of maternal AZT-glucuronide and neonatal uptake of the phase 2 metabolite. Placental transfer of AZT was previously shown to be high17 and AZT pharmacokinetic studies in pregnant baboons and monkeys indicated similar maternal and fetal AZT-glucuronide/AZT ratios and AZT-glucuronide plasma concentrations following maternal steady-state AZT infusion.27, 28 These data suggest maternal AZT-glucuronide is effectively transferred across the placenta as previously reported for other glucuronides.29, 30 Fetal glucuronidation capacity is generally limited, but increases during a neonate’s first 3 months are log-linear until adult levels are achieved.31 Small amounts of fetal metabolism may have contributed to the substantial fetal AZT-glucuronide concentrations, as observed in fetal baboon AZT administration studies.27, 29 Contrasting poor fetal glucuronidation ability, intestinal deglucuronidation with beta-glucuronidase is higher during fetal and neonatal periods compared with early childhood.32 Thus, AZT-glucuronide/AZT concentration in meconium may be the result of placental transfer, fetal AZT-glucuronide formation, and intestinal cleavage of maternal AZT-glucuronide. No metabolites of ARV were detected without the parent compounds, suggesting parent drug quantification may be sufficient to detect these intrauterine ARV exposures.
Acknowledgments
We thank the children and families for their participation in PHACS.
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism (through cooperative agreements with the Harvard T.H. Chan University School of Public Health [HD052102] and Tulane University School of Medicine [HD052104]. Data management services were provided by Frontier Science and Technology Research Foundation, Boston, MA (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc, Rockville, MD (PI: Julie Davidson). The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or US Department of Health and Human Services.
List of abbreviations in order cited
- L&D
labor and delivery
- AZT
zidovudine
- ARV
antiretroviral
- SMARTT
Surveillance Monitoring for ARV Toxicities Study
- PHACS
Pediatric HIV/AIDS Cohort Study
- LOQ
limit of quantification
- ABC
abacavir
- ATV
atazanavir
- DRV
darunavir
- EFV
efavirenz
- FTC
emtricitabine
- 3TC
lamivudine
- LPV
lopinavir
- NFV
nelfinavir
- NVP
nevirapine
- RAL
raltegravir
- RTV
ritonavir
- SQV
saquinavir
- d4T
stavudine
- TFV
tenofovir
- M8
NFV hydroxy-tert-butylamide
- TDF
TFV disoproxil fumarate
- CI
confidence interval
Appendix
The following individuals are members of PHACS:
Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL: Ram Yogev, MD, Margaret Ann Sanders, MPH, Kathleen Malee, PhD, Scott Hunter, PhD; Baylor College of Medicine, Houston, TX: William Shearer, MD, PhD, Mary Paul, MD, Norma Cooper, MA, Lynnette Harris, PhD; Bronx Lebanon Hospital Center, Bronx, NY: Murli Purswani, MBChB, Emma Stuard, MD, Anna Cintron, PsyD; Children's Diagnostic & Treatment Center, Fort Lauderdale, FL: Ana Puga, MD, Dia Cooley, MEd, Patricia Garvie, PhD, James Blood, MSW; New York University School of Medicine, New York, NY: William Borkowsky, MD, Sandra Deygoo, MS CCRD, Helen Rozelman, PhD; Rutgers-New Jersey Medical School, New Brunswick, NJ: Arry Dieudonne, MD, Linda Bettica, BSN, Susan Adubato, PhD; St. Jude Children's Research Hospital, Arlington, VA: Katherine Knapp, MD, Kim Allison, RN, Megan Wilkins, PhD; San Juan Hospital/Department of Pediatrics, San Juan, PR: Midnela Acevedo-Flores, MD, Lourdes Angeli-Nieves, MD, Vivian Olivera, PhD; SUNY Downstate Medical Center, Brooklyn, NY: Hermann Mendez, MD, Ava Dennie, R-PAC, Susan Bewley, PhD; Tulane University Health Sciences Center, New Orleans, LA: Chi Dola, MD, Robert Maupin, MD, Karen Craig, RN, MSN, CCRC, Patricia Sirois, PhD; University of Alabama, Birmingham, AL: Marilyn Crain, MD, Newana Beatty, BA, Dan Marullo, PhD; University of California, San Diego, CA: Stephen Spector, MD, Kim Norris, RN, BSN, Sharon Nichols, PhD; University of Colorado Denver Health Sciences Center, Denver, CO: Elizabeth McFarland, MD, Jenna Wallace, MSW, Carrie Chambers, BSN; University of Florida, Jacksonville, FL: Mobeen Rathore MBBS (MD), Kristi Stowers, BSH, Ann Usitalo, PhD; University of Illinois, Chicago, IL: Kenneth Rich, MD, Lourdes Richardson, MSN, Renee Smith, PhD; University of Miami, Miami, FL: Gwendolyn Scott, MD, Claudia Florez, MD, Elizabeth Willen, PhD; University of Southern California, Los Angeles, CA: Toni Frederick, PhD, MSPH, Mariam Davtyan, MPH, Susan Kerenyi, PsyD; University of Puerto Rico Medical Center, San Juan, PR: Zoe Rodriguez, MD, Ibet Heyer, RN BSN, Nydia Scalley Trifilio, MS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Gray T, Huestis M. Bioanalytical procedures for monitoring in utero drug exposure. Anal Bioanal Chem. 2007;388:1455–1465. doi: 10.1007/s00216-007-1228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrea EM, Jr, Romero A, Knapp DK, Ostrea AR, Lucena JE, Utarnachitt RB. Postmortem drug analysis of meconium in early-gestation human fetuses exposed to cocaine: clinical implications. J Pediatr. 1994;124:477–479. doi: 10.1016/s0022-3476(94)70379-5. [DOI] [PubMed] [Google Scholar]
- 3.Himes SK, Concheiro M, Scheidweiler KB, Huestis MA. Validation of a novel method to identify in utero ethanol exposure: simultaneous meconium extraction of fatty acid ethyl esters, ethyl glucuronide, and ethyl sulfate followed by LC-MS/MS quantification. Anal Bioanal Chem. 2014;406:1945–1955. doi: 10.1007/s00216-013-7600-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Himes SK, Stroud LR, Scheidweiler KB, Niaura RS, Huestis MA. Prenatal tobacco exposure, biomarkers for tobacco in meconium, and neonatal growth outcomes. J Pediatr. 2013;162:970–975. doi: 10.1016/j.jpeds.2012.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson J, Kirchner HL, Xue W, Minnes S, Singer LT, Bearer CF. Fatty acid ethyl esters in meconium are associated with poorer neurodevelopmental outcomes to two years of age. J Pediatr. 2008;152:788–792. doi: 10.1016/j.jpeds.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray TR, Barnes AJ, Huestis MA. Effect of hydrolysis on identifying prenatal cannabis exposure. Anal Bioanal Chem. 2010;397:2335–2347. doi: 10.1007/s00216-010-3772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray TR, Choo RE, Concheiro M, Williams E, Elko A, Jansson LM, et al. Prenatal methadone exposure, meconium biomarker concentrations and neonatal abstinence syndrome. Addiction. 2010;105:2151–2159. doi: 10.1111/j.1360-0443.2010.03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray TR, Eiden RD, Leonard KE, Connors GJ, Shisler S, Huestis MA. Identifying prenatal cannabis exposure and effects of concurrent tobacco exposure on neonatal growth. Clin Chem. 2010;56:1442–1450. doi: 10.1373/clinchem.2010.147876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray TR, Kelly T, LaGasse LL, Smith LM, Derauf C, Grant P, et al. New meconium biomarkers of prenatal methamphetamine exposure increase identification of affected neonates. Clin Chem. 2010;56:856–860. doi: 10.1373/clinchem.2009.139055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray TR, Shakleya DM, Huestis MA. A liquid chromatography tandem mass spectrometry method for the simultaneous quantification of 20 drugs of abuse and metabolites in human meconium. Anal Bioanal Chem. 2009;393:1977–1990. doi: 10.1007/s00216-009-2680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kacinko SL, Jones HE, Johnson RE, Choo RE, Huestis MA. Correlations of maternal buprenorphine dose, buprenorphine, and metabolite concentrations in meconium with neonatal outcomes. Clin Pharmacol Ther. 2008;84:604–612. doi: 10.1038/clpt.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burd L, Hofer R. Biomarkers for detection of prenatal alcohol exposure: a critical review of fatty acid ethyl esters in meconium. Birth Defects Res A Clin Mol Teratol. 2008;82:487–493. doi: 10.1002/bdra.20464. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. J Anim Sci. 1995;73:1839–1851. doi: 10.2527/1995.7361839x. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds LP, Caton JS, Redmer DA, Grazul-Bilska AT, Vonnahme KA, Borowicz PP, et al. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J Physiol. 2006;572:51–58. doi: 10.1113/jphysiol.2005.104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morales V, Knapp DK, Utarnachitt R, Utarnachitt R, Ostrea EM. Analysis of meconium will detect intrapartum drug use: clinical implications. Pediatr Res. 1994;35:87A. [Google Scholar]
- 16.Nanovskaya TN, Nekhayeva IA, Hankins GD, Ahmed MS. Transfer of methadone across the dually perfused preterm human placental lobule. Am J Obstet Gynecol. 2008;198:126, e1–e4. doi: 10.1016/j.ajog.2007.06.073. [DOI] [PubMed] [Google Scholar]
- 17.Department of Health and Human Services; [Accessed March 28, 2014]. Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States (Updated March 2014) Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/perinatalgl.pdf. [Google Scholar]
- 18.Williams PL, Seage GR, 3rd, Van Dyke RB, Siberry GK, Griner R, Tassiopoulos K, et al. A trigger-based design for evaluating the safety of in utero antiretroviral exposure in uninfected children of human immunodeficiency virus-infected mothers. Am J Epidemiol. 2012;175:950–961. doi: 10.1093/aje/kwr401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Himes SK, Scheidweiler KB, Tassiopoulos K, Kacanek D, Hazra R, Rich K, et al. Development and validation of the first liquid chromatography-tandem mass spectrometry assay for simultaneous quantification of multiple antiretrovirals in meconium. Anal Chem. 2013;85:1896–1904. doi: 10.1021/ac303188j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griner R, Williams PL, Read JS, Seage GR, 3rd, Crain M, Yogev R, et al. In utero and postnatal exposure to antiretrovirals among HIV-exposed but uninfected children in the United States. AIDS Patient Care STDS. 2011;25:385–394. doi: 10.1089/apc.2011.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsui D. Adherence with drug therapy in pregnancy. Obstet Gynecol Int. 2012;2012:796590. doi: 10.1155/2012/796590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray TR, Eiden RD, Leonard KE, Connors G, Shisler S, Huestis MA. Nicotine and metabolites in meconium as evidence of maternal cigarette smoking during pregnancy and predictors of neonatal growth deficits. Nicotine Tob Res. 2010;12:658–664. doi: 10.1093/ntr/ntq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silvestre MA, Lucena JE, Roxas R, Jr, Evangelista ES, Ostrea EM., Jr Effects of timing, dosage, and duration of morphine intake during pregnancy on the amount of morphine in meconium in a rat model. Biol Neonate. 1997;72:112–117. doi: 10.1159/000244473. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura KT, Ayau EL, Uyehara CF, Eisenhauer CL, Iwamoto LM, Lewis DE. Methamphetamine detection from meconium and amniotic fluid in guinea pigs depends on gestational age and metabolism. Dev Pharmacol Ther. 1992;19:183–190. doi: 10.1159/000457483. [DOI] [PubMed] [Google Scholar]
- 25.Ceccaldi PF, Gavard L, Mandelbrot L, Rey E, Farinotti R, Treluyer JM, et al. Functional role of p-glycoprotein and binding protein effect on the placental transfer of lopinavir/ritonavir in the ex vivo human perfusion model. Obstet Gynecol Int. 2009;2009:726593. doi: 10.1155/2009/726593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet. 2004;43:1071–1087. doi: 10.2165/00003088-200443150-00002. [DOI] [PubMed] [Google Scholar]
- 27.Garland M, Szeto HH, Daniel SS, Tropper PJ, Myers MM, Stark RI. Placental transfer and fetal metabolism of zidovudine in the baboon. Pediatr Res. 1998;44:47–53. doi: 10.1203/00006450-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Patterson TA, Binienda ZK, Lipe GW, Gillam MP, Slikker W, Jr, Sandberg JA. Transplacental pharmacokinetics and fetal distribution of azidothymidine, its glucuronide, and phosphorylated metabolites in late-term rhesus macaques after maternal infusion. Drug Metab Dispos. 1997;25:453–459. [PubMed] [Google Scholar]
- 29.Garland M, Abildskov KM, Kiu TW, Daniel SS, Weldy P, Stark RI. Placental transfer and fetal elimination of morphine-3-beta-glucuronide in the pregnant baboon. Drug Metab Dispos. 2008;36:1859–1868. doi: 10.1124/dmd.108.021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matlow JN, Lubetsky A, Aleksa K, Berger H, Koren G. The transfer of ethyl glucuronide across the dually perfused human placenta. Placenta. 2013;34:369–373. doi: 10.1016/j.placenta.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Onishi S, Kawade N, Itoh S, Isobe K, Sugiyama S. Postnatal development of uridine diphosphate glucuronyltransferase activity towards bilirubin and 2-aminophenol in human liver. Biochem J. 1979;184:705–707. doi: 10.1042/bj1840705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antonowicz I, Shwachman H, Sotoo I. Beta-galactosidase and beta-glucuronidase activities in intestinal mucosa of infants and children. Pediatrics. 1971;47:737–744. [PubMed] [Google Scholar]