Summary
Aside from its role in cell membrane integrity, cholesterol is a key component in steroid hormone production. The vital functions of steroid hormones such as estrogen, testosterone, glucocorticoids (Gcrts) and mineralocorticoids (Mnrts) in perinatal and adult life are well understood; however, their role during early embryonic development remains largely unexplored. Here we show that siRNA-mediated perturbation of steroid hormone production during mesoderm formation has important consequences on cardiac differentiation in mouse embryonic stem cells (mESC). Both Gcrts and Mnrts are capable of driving cardiac differentiation in mESC. Interestingly, the Gcrt receptor is widely expressed during gastrulation in the mouse, and is exclusively localized in the nuclei - and thus active - in visceral endoderm cells, suggesting that it functions much earlier than previously anticipated. We therefore studied Gcrt signaling in mESC as a model of the gastrulating embryo, and found that Gcrt signaling regulates expression of the transcription factor Hnf4a and the secreted Nodal and BMP inhibitor Cer1 in the early visceral endoderm. RNAi-mediated knockdown of Gcrt function blocked cardiomyocyte differentiation, with limited effects on other cardiovascular cell types including vascular endothelial cells and smooth muscle. Furthermore, the cardiogenic effect of Gcrts required Hnf4a and paracrine Cer1. These results establish a novel function for cholesterol-derived steroid hormones and identify Gcrt signaling in visceral endoderm cells as a regulator of Cer1 and cardiac fate.
Keywords: embryonic stem cells, embryo, cholesterol, glucocorticoid signaling, visceral endoderm, mouse, cardiogenesis
Introduction
Cholesterol is an essential structural component of the cell membrane and is needed for cell shape and motility. Cholesterol is also an important source for the production of steroid hormones such as the glucocorticoids (Gcrt), mineralocorticoids (Mnrt), estrogen and testosterone, which are produced by a series of cytochrome class enzymes (Han et al., 2014). Interestingly, very little is known about the function of cholesterol derivatives during early mammalian development, despite the fact that the two cholesterol modifying enzymes Cyp11a1 and Cyp17a1 are prominently expressed and active in the visceral endoderm of gastrulating mouse embryos (Bair and Mellon, 2004; Korgun et al., 2003). Moreover, Cyp17a1 is essential for early development since deletion of the Cyp17a1 gene in the mouse results in death by embryonic day 7 (Bair and Mellon, 2004). In addition, mRNA for the receptor Nr3c1 of the cholesterol-derived Gcrt is enriched in the endoderm of the rat embryo (Korgun et al., 2003). Gcrts comprise a class of steroid hormones that are important for adult life (Kadmiel and Cidlowski, 2013; Newton, 2000). Synthetized in the adrenal cortex of adults, Gcrts reach most organs through the bloodstream. Once within cells, Gcrts bind to the nuclear receptor Nr3c1, causing receptor dimerization and translocation to the nucleus, where the ligand-receptor complex interacts with Gcrt response elements in the promoters of Gcrt target genes (Kadmiel and Cidlowski, 2013; Newton, 2000). Functionally, Gcrts are best known for maintaining glucose levels in adults, thereby providing a steady supply of ATP and pyruvate, both essential energy components for the cell. Gcrts are also important repressors of inflammation through a negative feedback loop of the immune system (Kadmiel and Cidlowski, 2013; Newton, 2000). Very little is known about Gcrt function during early mammalian embryonic development, and to our knowledge, a role in cell fate specification is unexplored.
Here, we show that cholesterol metabolites play an important role in cardiac differentiation in mouse embryonic stem cells (mESC). Gcrt receptor expression in the early mouse embryo and its exclusive localization to cell nuclei within the visceral endoderm then prompted us to further investigate a possible role for this class of cholesterol-derived steroid hormones. As the visceral endoderm is an important signaling center for the formation of anterior structures including head and heart (Arai et al., 1997; Perea-Gomez et al., 2002), we studied Gcrt function in differentiating mESC as an in vitro model of the developing mouse embryo. We found that Gcrt controls expression of Cerberus-1 (Cer1), a secreted inhibitor of Nodal and BMP signaling, with an essential role in cardiac differentiation in amphibians and ESC (Cai et al., 2013; Foley et al., 2007). These studies suggest that cholesterol metabolites such as the Gcrts may function much earlier in embryonic development than previously anticipated and furthermore indicate that Gcrts regulate the heart-inducing properties of the anterior visceral endoderm.
Results and Discussion
Cholesterol-derived metabolites regulate cardiac differentiation
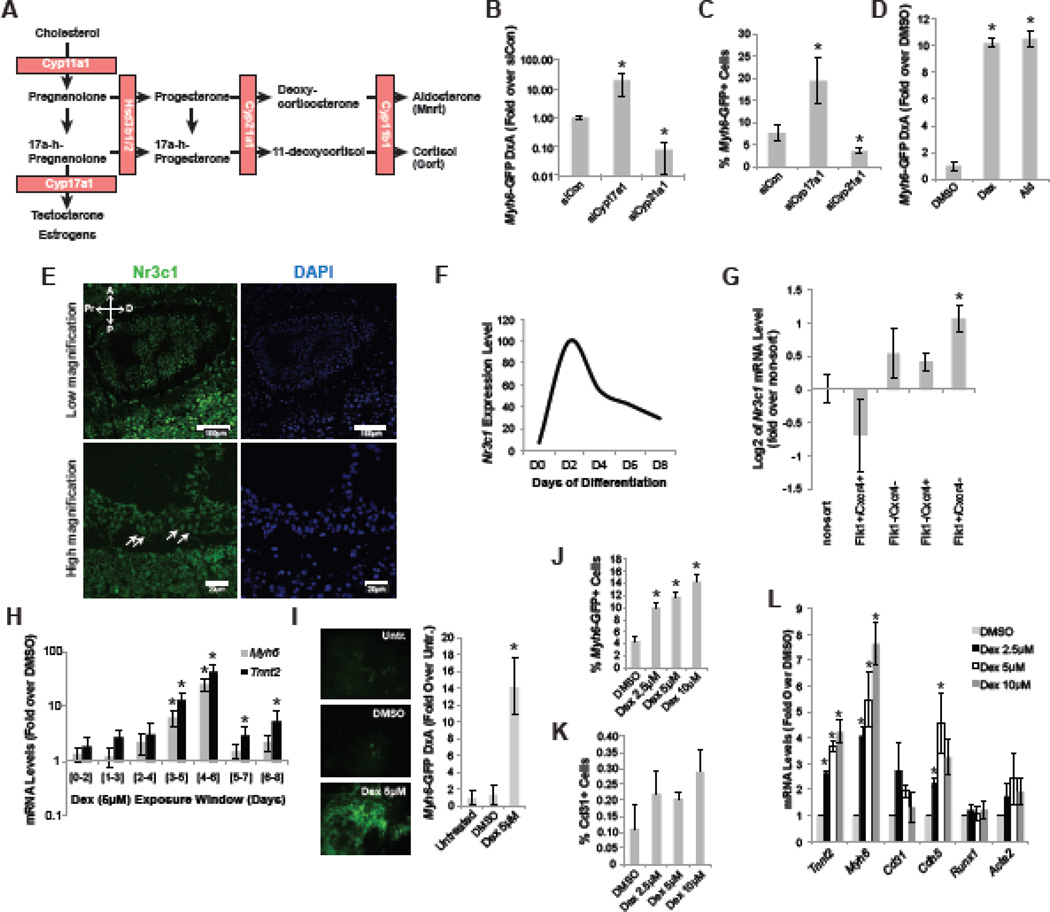
Cholesterol is metabolized into a number of steroid hormone classes, including testosterone, estrogen, mineralocortocoids (Mnrt) and glucocorticoids (Gcrt) through a series of catalytic steps, which are each regulated by a selective enzyme (Figure 1A)(Han et al., 2014). Cyp17a1 is responsible for the generation of testosterone and estrogen, while Cyp21a1 and Cyp11b1 are essential for Mnrt and Gcrt production (Han et al., 2014). Given their early expression in the visceral endoderm (VE)( Bair and Mellon, 2004), we hypothesized that cholesterol metabolites may have a role in early germ layer specification. Since the VE is a crucial player in cardiac development, we tested the contribution of the various metabolites during cardiac differentiation in mESC. Through siRNA-mediated knockdown of Cyp17a1, the production of testosterone and estrogen would be blocked and through knockdown of Cyp21a1 Gcrt and Mnrt would no longer be synthetized. We therefore studied functional alteration of these two genes during mesoderm formation and patterning in mESC, a stage equivalent to the expression of Cyp17a1 and Cyp21a1 in the embryo. When introduced in mESC at day 3 of spontaneous ndifferentiation in serum (an assay with minimal cardiac differentiation to allow a larger dynamic range), the siRNA to Cyp17a1 increased cardiac differentiation as observed with the cardiac specific Myh6-GFP mESC reporter cell line, suggesting that increased Mnrt and Gcrt production promotes cardiac differentiation (Figure 1B,C). Indeed, knockdown of Cyp21a1, the enzyme responsible for Mnrt and Gcrt production, reduced cardiac differentiation significantly (Figure 1B,C). We then treated serum-differentiated mESC with Aldosterone (Ald, a Mnrt) and Dexamethasone (Dex, a synthetic Gcrt) to confirm the contribution of both steroid classes to cardiac differentiation, and as predicted by the siRNA data, both Ald and Dex promoted cardiac differentiation (Figure 1D) when added at day 3 of differentiation.
Figure 1. Cholesterol derivatives control cardiac differentiation in mESC.
(A) Schematic representation of the key steps of cholesterol metabolism that result in the production of testosterone, estrogens, mineralocorticoids (Mnrt) and glucocorticoids (Gcrt). Enzymes required for the sub-steps are indicated in orange boxes. (B–C) Myh6-GFP expression level (GFP density multiplied by area, DxA) and Myh6-GFP flow cytometry analysis, respectively, at day 15 of differentiation after introduction of the indicated siRNAs at day 3 of differentiation. (D) Myh6-GFP expression level (GFP density multiplied by area, DxA) at day 15 of differentiation after treatment with DMSO, Dexamethasone (Dex) or Aldosterone (Ald) at day 3 of differentiation. (E) Nr3c1 is expressed ubiquitously in E6.5-E7 mouse embryos. White arrows indicate nuclear expression of Nr3c1 in the outer visceral endoderm layer. (F) Nr3c1 gene expression time course in mESC spontaneously differentiated in serum-containing medium, quantified by RT-qPCR. (G) RT-qPCR analysis for Nr3c1 expression in sorted mESC-derived day 5 populations including mesoderm (Flk1+/Cxcr4−), endoderm (Flk1−/Cxcr4+), ectoderm (Flk1−/Cxcr4−) and cardiogenic mesoderm (Flk1+/Cxcr4+)(Cai et al., 2013; Morrison et al., 2008; Nelson et al., 2008). (H) RT-qPCR analysis for cardiac genes Myh6 and Tnnt2 of day 12 ESC cultures differentiated in serum. Dexamethasone (Dex) treatment was given at the indicated time windows. (I) Representative images of Myh6-GFP reporter activity on day 15 of differentiation after treatment with indicated molecules at day 3 and image-based quantification of Myh6-GFP total area (A) and intensity (D). (J–K) Myh6-GFP and Cd31 flow cytometry analysis of day 15 cultures treated with Dex at day 3 of differentiation, respectively. (L) RT-qPCR analysis of cardiovascular markers on day 15 samples after treatment with different doses of Dex. Asterisk indicates p<0.05 relative to controls.
As previous studies had shown expression of the Gcrt receptor Nr3c1 in the early embryo (Korgun et al., 2003), we confirmed that Nr3c1 is indeed ubiquitously expressed in the gastrulating mouse embryo (Figure 1E), indicating an important role for Gcrt at this time. Intriguingly, Nr3c1 is exclusively localized in the nuclei of cells in the outer most cell layer of the embryo, which corresponds to the visceral endoderm (Figure 1E). Nuclear receptors such as Nr3c1 translocate to the nucleus when bound by their ligand (Kadmiel and Cidlowski, 2013; Newton, 2000), and, therefore, nuclear localization suggested that Nr3c1 was exclusively active in the VE during gastrulation. We thus returned to mESCs as a simplified model of the developing embryo to further study the role of Gcrt signaling during gastrulation stages.
In mESC cultures, Nr3c1 mRNA is most abundantly expressed between days 2 and 4 of differentiation, which corresponds to Nr3c1 expression in E6.5-7.5 stages of the embryo when germ layers segregate and early cell fate is specified (Figure 1F) (Murry and Keller, 2008). Also equivalent to the embryo, Nr3c1 mRNA expression was broadly detectable in mesoderm, endoderm and ectoderm populations, which were FACS sorted from day 5 differentiating mESC cultures (Figure 1G, Supp Fig 1A)(Cai et al., 2013; Morrison et al., 2008; Nelson et al., 2008). Given the expression pattern of Nr3c1 in differentiating mESC, we then performed a wider Dex exposure series over time to delineate the precise time window for cardiac induction. RT-qPCR analysis for the cardiac markers Myh6 and Tnnt2 clearly demonstrate that Dex is most active between days 3 and 6 of differentiation (Figure 1H). Dex drives an increase in cardiomyocyte purity but does not affect the relative percentage of endothelial cells, suggesting that its effect is cardiac specific (Figure I–K, Supp Fig 2A). RT-qPCR analysis confirmed these findings, demonstrating that cardiac fate was clearly induced in a concentration dependent manner (Tnnt2 and Myh6) (Figure 1L). Endothelial marker (Cd31 and Cdh5) expression decreased slightly, but is most likely of limited significance as the abundance of endothelial cells in the cultures was low (<0.3%) (Figure 1K). Furthermore, Dex did not affect gene expression of hematopoietic (Runx1) or smooth muscle lineage markers (Acta2) (Figure 1L). Together, these data indicate that the cholesterol-derived Gcrts are able to specifically control cardiac differentiation from mouse ESC.
Glucocorticoids drive Cer1 expression in visceral endoderm cells
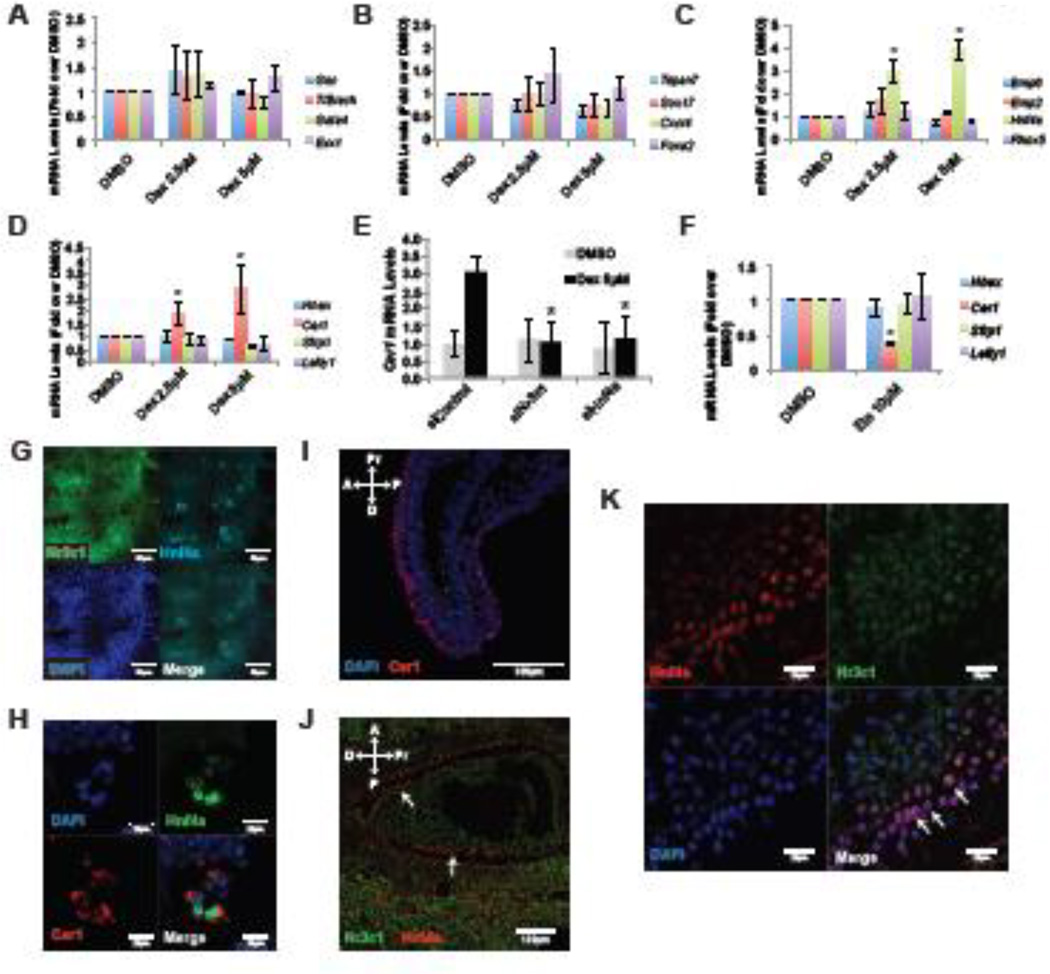
To probe the underlying mechanism of cardiac differentiation by Gcrts, we stimulated Gcrt signaling with Dex at day 3 of differentiation, when Nr3c1 mRNA levels were high. After 48 hours of treatment, RT-qPCR analysis of fate specific markers revealed no effect on mesoderm (Gsc, T/Brach, Gata4 or Evx1) or definitive endoderm (Tspan7, Sox17, Cxcr4 or Foxa2), suggesting that Gcrt signaling is not involved in early germ layer specification (Figure 2A,B). Since nuclear expression of Nr3c1 in the VE of the mouse embryo indicated local Gcrt activity, we profiled genes known to be expressed in the VE. Dex upregulated Cer1, encoding a dual Nodal/BMP antagonist (Belo et al., 2000), and Hnf4a, which is directly regulated by Gcrt through response elements in its promoter (Bailly et al., 2001)(Figure 2C,D). In contrast, general VE markers (Bmp6, Emp2, Rhox5, Hhex, Lefty1 and Sfrp1) were unaffected, suggesting that Gcrts do not regulate VE fate per se, but play a selective role within the VE (Figure 2C,D). Indeed, siRNAs against Nr3c1 revealed that it is required for Dex to induce Cer1 (Figure 2E). Furthermore, siRNA knockdown of Hnf4a demonstrated that it is needed for Dex to induce Cer1 (Figure 2E), exposing a cascade from Dex to Cer1, involving Nr3c1 and Hnf4a.
Figure 2. Gcrt signaling is active in the visceral endoderm.
(A–D) RT-qPCR analysis of day 5 serum-containing differentiation cultures after treatment with indicated concentration of Dexamethasone (Dex) at day 3 of differentiation. (E) Cer1 expression analysis by RT-qPCR of day 5 cultures after transfection with indicated siRNAs and treatment with indicated molecules at day 3. (F) RT-qPCR analysis for indicated genes of day 5 serum-differentiated ESC cultures that were treated with Etomidate (Eto) at day 3 of differentiation. (G–H) Immunostaining of day 5 serum-differentiated ESC cultures in the presence of Dex for indicated proteins and nuclear DAPI staining. (I–K) Immunostaining of E6.5-E7 mouse embryos for indicated proteins and nuclear DAPI staining. Panel N is a magnified view from embryos similar to panel M. Arrows indicate nuclear Nr3c1 and Hnf4a overlap in the visceral endoderm layer.
Scale bars represent indicated size. Abbreviations: A, anterior; D, distal; P, posterior; Pr, proximal. Asterisk indicates p<0.05 relative to controls.
We then asked if endogenous Gcrt signaling was also involved in the induction of Cer1 expression by treating differentiating mESC cultures with Etomidate (Eto), a Gcrt production inhibitor that prevents activity of Cyp11b1, a key enzyme in Gcrt production (Wagner et al., 1984; Zolle et al., 2008). Eto (10µM) given at day 3 exclusively blocked Cer1 expression, but did not affect other VE markers (Figure 2F). Moreover, siRNA knockdown of Hnf4a or Nr3c1 decreased Cer1 mRNA in absence of Dex (Supp Fig 1B). Together, these findings implicate Gcrt signaling in the developmental regulation of Cer1.
Gcrt or Dex might induce Cer1 via Hnf4a either within the same cell or cell-non-autonomously through paracrine interactions. To resolve these possibilities, we examined co-expression of Nr3c1, Hnf4a and Cer1 in differentiating mESC cultures. As in embryos, most cells of differentiation day 5 mESC cultures express Nr3c1, and Hnf4a-positive cell clusters are apparent in a subset of Nr3c1 expressing cells (Figure 2G). At higher magnification, Cer1 is observed only in Hnf4a-positive cells (Figure 2H), suggesting Cer1 is only expressed in VE cells marked by Hnf4a. Indeed, if a non-VE specific mechanism were to exist, every cell expressing Nr3c1 would upregulate Cer1 under Dex-treated conditions, which is not the case (Figure 2G,H). In addition, sorting of endoderm cells from mESC cultures further illustrates that Hnf4a and Cer1 are co-expressed in the same cell population (Supp Fig 1A,C). Importantly, in E6.5-E7.5 embryos, Cer1 and Hnf4a are co-expressed in the VE cells that have Nr3c1 localized to the nucleus (Figure 2I–K). Therefore, we conclude that Gcrt signaling may act cell-autonomously within the VE to regulate the production of the secreted antagonist Cer1.
Glucocorticoid signaling controls cell fate specification through Hnf4a and Cer1
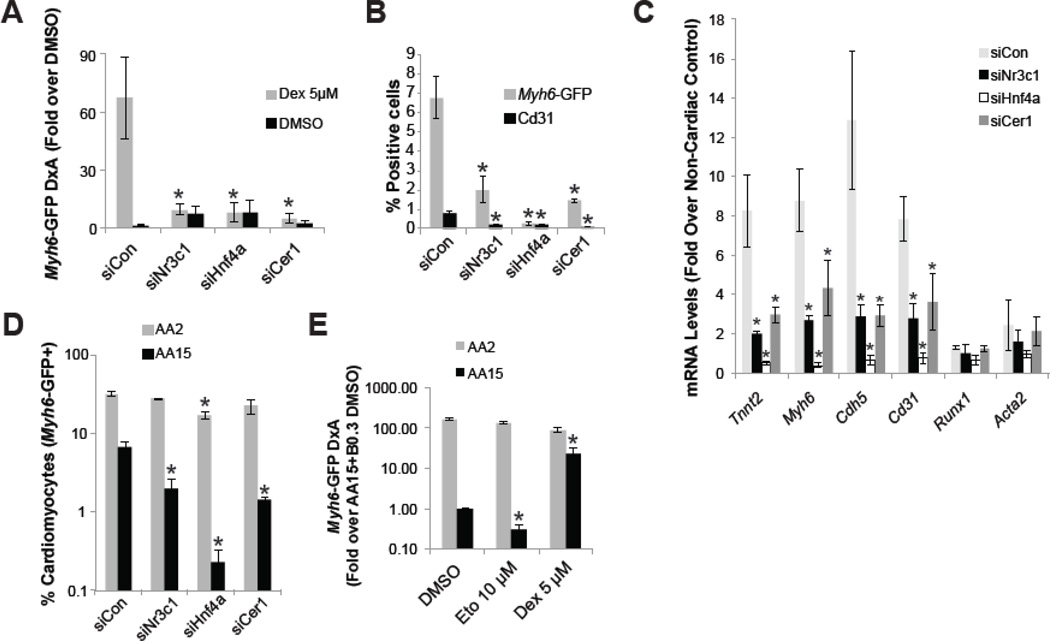
The VE, and more specifically the anterior portion of the VE (aVE), is an important organizing center during early embryogenesis in mammals that patterns anterior structures (Arai et al., 1997; Kimura et al., 2000; Perea-Gomez et al., 2002; Perea-Gomez et al., 2001). Meticulous removal of the aVE in the mouse or its equivalent in chick (Hensen’s node) or amphibians (Organizer) prevents heart induction, illustrating the importance for the aVE in cell fate specification (Arai et al., 1997; Nascone and Mercola, 1995; Schultheiss et al., 1995). Since Gcrt appears to be active in the VE, we tested involvement of Hnf4a and Cer1 in cardiac differentiation downstream of Gcrt. We transfected siRNAs or infected shRNAs for each component, establishing that a Nr3c1-Hnf4a-Cer1 cascade mediates the pro-cardiac effect of Dex (Figure 3A; Supp Fig 3A–C). We then asked if the Nr3c1-Hnf4a-Cer1 cascade was required for cardiac differentiation in serum-free embryoid body-based mESC cultures in the absence of Dex. siRNA-mediated knockdown of Nr3c1, Hnf4a or Cer1 effectively blocked cardiac differentiation in this setting, suggesting that endogenous Gcrt signaling is involved in specifying cardiac fate (Figure 3B–C, Supp Fig 2B).
Figure 3. Gcrt signaling activity in the visceral endoderm promotes cardiac fate.
(A) Day 12 image-based analysis of the Myh6-GFP reporter in mESC differentiation in serum in the presence of Dex and/or siRNA from day 3 of differentiation. (B–C) Flow cytometry analysis of Myh6-GFP and Cd31 and RT-qPCR analysis, respectively, in serum-free differentiation day 15 cultures exposed to 15ng/ml Activin A (AA) at day 2 and siRNA at day 3. (D) Flow cytometry analysis of Myh6-GFP in serum-free differentiation day 15 cultures, with Activin A (AA) titration at day 2 as indicated and siRNA introduced at day 3. (E) Image-based analysis of Myh6-GFP in serum-free differentiation day 15 cultures, with Activin A titration at day 2 as indicated and small molecules introduced at day 3. Asterisk indicates p<0.05 relative to controls.
We thus far established that cardiac differentiation induced by Dex is controlled by visceral endoderm factors, which suggests that Dex is driving a cell non-autonomous effect on cardiac differentiation through the endoderm. As discussed above (See Figure 2), our results indeed suggest that Dex is only acting on a small number of endoderm cells. To confirm that paracrine signaling from the endoderm may be responsible for the cardiac effect of Gcrt signaling, we studied the effects of modulation of Gcrt signaling in cultures that had limited endoderm formation in comparison with endoderm-containing cultures. Through titration of Activin A during day 2 to day 4 of differentiation, cultures can be enriched for endoderm or mesoderm, using high and low Activin A concentrations respectively (D'amour et al., 2005; Gadue et al., 2006). Indeed, cultures exposed to 15ng/ml Activin A, and thus endoderm enriched (Supp Fig 3D), yield less mesoderm derivatives than cultures exposed to 2ng/ml Activin A (siCon histogram bars, Figure 3D), as indicated by the 5-fold difference in cardiomyocyte abundance. Under endoderm-enriched conditions, individual components of the Gcrt-Nr3c1-Hnf4a-Cer1 cascade are each required for cardiomyocyte differentiation (AA15, Figure 3D). In contrast, cardiomyocyte differentiation is significantly less sensitive to siRNA-mediated knockdown of Nr3c1, Hnf4a and Cer1 when mES cells are differentiated towards mesoderm at the expense of endoderm (AA2, Figure 3D). Furthermore, Dex and Eto respectively increased and suppressed cardiomyocyte induction only under endoderm-enriched conditions (Figure 3E). These findings demonstrate that the need of Gcrt signaling for cardiac fate is lost when endoderm is replaced by mesoderm; thus implying that Gcrts are driving cardiac fate through a paracrine mechanism in the endoderm.
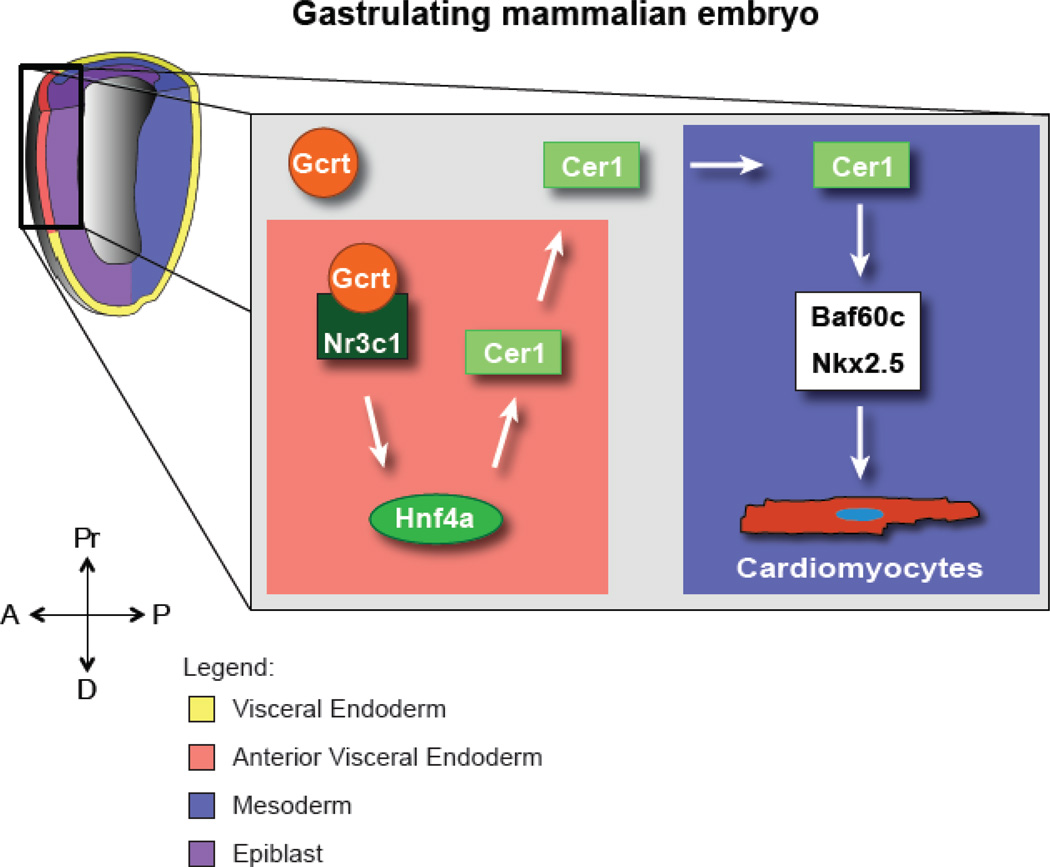
These studies are the first demonstration that cholesterol derivatives such as glucocorticoids regulate cell fate during very early development. Our data support the model that Gcrt signaling occurs in the VE of E6.5-E7 embryos, inducing Hnf4a, which then drives Cer1 expression. Cer1 then controls cardiac fate through the chromatin remodeling protein Baf60c by titrating Nodal signaling (Figure 4)(Cai et al., 2013). Although neither the Nr3c1 nor Cer1 homozygous null transgenic mice have a clear cardiac phenotype in vivo (Belo et al., 2000; Cole et al., 1995), our findings suggest that Gcrt signaling in the aVE might nonetheless play an important role in development. It is well established that the aVE is essential to induce the neural plate (Perea-Gomez et al., 2002) and heart (Arai et al., 1997; Nascone and Mercola, 1995; Schultheiss et al., 1995), and the absence of a pronounced effect in the null mice may be explained by the following two reasons. First, the promiscuity between Gcrt and Mnrt and their receptors should be considered when assessing the importance of Nr3c1 in vivo function during early development (Hellal-Levy et al., 1999; Rogerson et al., 1999; Yoshikawa et al., 2002), and may thus explain why Nr3c1−/− mutants are viable with no evident cardiac defect. Indeed, we did observe that Mnrt signaling also promotes cardiac differentiation. Further investigation of the role of Mnrt signaling is however needed to understand how Gcrt and Mnrt may interact during cardiac differentiation in ESC. Second, compensation or redundancy with Lefty1 might rescue the Cer1 null embryos. Lefty1 is under-expressed during mesoderm formation in mESCs as compared to embryos (Cai et al., 2013), suggesting that Lefty1 might compensate for the loss of Cer1 in the in vivo situation. Interestingly, double homozygous Lefty1/Cer1 null mice have defective neural plate and heart development (Perea-Gomez et al., 2002), suggestive of mutual compensation possibly driven by feedback from high Nodal/Activin signaling on Cer1 (Supp Fig 3D). Gcrts do not induce Lefty1; thus, the Gcrt- Nr3c1-Hnf4a-Cer1 and Nodal/Lefty1 cascades may exist as mutually reinforcing mechanisms to fine-tune Nodal signaling to correctly specify anterior cell fates including the heart. An implication of our study is that altered Gcrt and possibly Mnrt signaling could affect heart formation during embryonic development in utero.
Figure 4. Gcrt regulates Cer1 in the visceral endoderm to control cardiac fate in the underlying mesoderm.
Proposed model of Gcrt activity in cell fate specification during early embryogenesis based on mESC findings. A, anterior; D, distal; P, posterior; Pr, proximal.
Materials and Methods
More detailed materials and methods are made available as supplementary information.
Mouse ESC culture and differentiation
CGR8 mouse ESC were maintained on gelatin-coated dishes with Leukemia inhibitory factor (LIF, Millipore). In presence of serum, cells were differentiated as described previously (Willems et al., 2012). Dexamethasone (Sigma), Aldosterone (Sigma) or Etomidate (Sigma) was added at day 3 or as indicated. siRNAs (Ambion) were transfected at day 3 of differentiation using Lipofectamine RNAiMax (Life Technologies)(Supp Fig 4).
Serum-free differentiation was performed through an embryoid body (EB) step. At day 2 EB were dissociated and reaggregated in the presence of growth factors to specify particular lineages. Activin A (R&D Systems) and BMP4 (R&D Systems) were titrated depending on the fate choice (see supplementary information). At day 3 EB were dissociated again, and plated in the presence of siRNA and Lipofectamine RNAiMax or small molecules. Assays were then continued with Activin A and BMP4. At day 5 media was replaced with media containing IWR1 analogs (Lanier et al., 2012; Willems et al., 2011).
Samples were processed as indicated for RT-qPCR, flow cytometry or high content imaging. For high content imaging, plates were loaded onto an automated microscope (Incell 1000, GE Healthcare) and images were processed using Cyteseer (Vala Sciences Inc.). GFP reads are represented as the GFP area (A) multiplied by the GFP intensity (D).
Immunostaining on embryos and cells
Decidua containing embryos at day E6.5 through E7.5 were dissected and flash frozen prior to cryosectioning. Slides were stained as described in supplement. Images were acquired in a LSM510 Zeiss confocal microscope. Cells from differentiation cultures were processed similarly after fixation in paraformaldehyde.
Flow cytometry and cell sorting
Cells were dissociated to single cell suspensions and analyzed on a FACSCanto or LSRFortessa (BD Biosciences) for eGFP under control of the Myh6 promoter. For extracellular staining, cells were incubated for 30 minutes with antibodies as listed in the supplement. Prior to analysis, cells were incubated with propidium iodide (PI) to label non-viable cells. Data analysis was performed using FlowJo (Treestar). Measured events were gated for PI negative populations (exclusion of dead cells) and/or forward/side scatter (exclusion of debris and aggregates) before producing histograms or dot plots. Samples for sorting where processed similarly and were run on a FACS Vantage-Diva sorter (BD Biosciences).
RNA extraction and RT-qPCR
RT-qPCR was performed following MIQE guidelines as described in supplement (Bustin et al., 2009). Briefly, RNA was extracted with Trizol and reverse transcribed with the Quantitect RT kit (Qiagen) according to the included instructions. qPCR reactions were run on an ABI 7900 (Life Technologies) using iTaq Universal Sybr Green Master mix (Bio-Rad). Primer sequences are made available in the RTPrimerDB (http://www.rtprimerdb.org/)(Pattyn et al., 2003) and primer ID numbers are listed in Supplementary Table 1. RT-qPCR data was standardized and analyzed before statistical analysis as described previously (Willems et al., 2008).
Statistical analysis
All experiments were analyzed in at least three biological replicates and were tested for significance by a Student’s t-test. Data in the figure is represented as mean with error bars indicating SEM, with asterisks indicating a p-value < 0.05.
Supplementary Material
Highlights.
Cholesterol metabolites such as glucocorticoids control cardiac differentiation in mouse embryonic stem cells
Glucocorticoids control cardiac specification through Hnf4a dependent Cer1 production
These results may have important implications for elevated or reduced glucocorticoid levels during the development of the heart during gestation in utero
Acknowledgements
The authors would like to thank Drs. Fred Levine and Marcia Dawson for helpful discussions, Yoav Altman for FACS and Caroline Kemp for assistance in graphic design. This research was supported by grants from the NIH (R37HL059502, R01HL108176 to MM; and P30 CA030199 and P30 AR061303 to SBMRI Functional Genomics and Cytometry); the California Institute for Regenerative Medicine (CIRM) (RC1-000132 to MM); the Mayo Clinic Center for Regenerative Medicine (to AMF and AT) and the Fondation Leducq (to AT and MM). In addition, we are grateful for a Doctoral Fellowship from the Science and Technology Foundation of the Portuguese Ministry of Science Technology and Higher Education (to JCT) and a Howard Hughes Med-into-Grad Program fellowship (to JCT), a CIRM (TG2-01162) postdoctoral fellowship (to EW) and American Heart Association Postdoctoral fellowships (to EW and AMF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
Experiments were performed and analyzed by JCT, AMF, WC and EW after an initial concept of JCT, MM and EW. The manuscript was written and edited by JCT, AMF, WC, AT, MM and EW. Major funding was obtained by AT and MM.
References
- Arai A, Yamamoto K, Toyama J. Murine cardiac progenitor cells require visceral embryonic endoderm and primitive streak for terminal differentiation. Dev Dyn. 1997;210:344–353. doi: 10.1002/(SICI)1097-0177(199711)210:3<344::AID-AJA13>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Bailly A, Torres-Padilla ME, Tinel AP, Weiss MC. An enhancer element 6 kb upstream of the mouse HNF4alpha1 promoter is activated by glucocorticoids and liver-enriched transcription factors. Nucleic Acids Research. 2001;29:3495–3505. doi: 10.1093/nar/29.17.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair SR, Mellon SH. Deletion of the Mouse P450c17 Gene Causes Early Embryonic Lethality. Molecular and Cellular Biology. 2004;24:5383–5390. doi: 10.1128/MCB.24.12.5383-5390.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belo JA, Bachiller D, Agius E, Kemp C, Borges AC, Marques S, Piccolo S, De Robertis EM. Cerberus-like is a secreted BMP and nodal antagonist not essential for mouse development. genesis. 2000;26:265–270. [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real- Time PCR Experiments. Clinical Chemistry. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Cai W, Albini S, Wei K, Willems E, Guzzo RM, Tsuda M, Giordani L, Spiering S, Kurian L, Yeo GW, et al. Coordinate Nodal and BMP inhibition directs Baf60c-dependent cardiomyocyte commitment. Genes & Development. 2013;27:2332–2344. doi: 10.1101/gad.225144.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schütz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes & Development. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- D'amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nature Biotechnology. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- Foley A, Korol O, Timmer A, Mercola M. Multiple functions of Cerberus cooperate to induce heart downstream of Nodal. Developmental Biology. 2007;303:57–65. doi: 10.1016/j.ydbio.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han TS, Walker BR, Arlt W, Ross RJ. Treatment and health outcomes in adults with congenital adrenal hyperplasia. Nat Rev Endocrinol. 2014;10:115–124. doi: 10.1038/nrendo.2013.239. [DOI] [PubMed] [Google Scholar]
- Hellal-Levy C, Couette B, Fagart J, Souque A, Gomez-Sanchez C, Rafestin-Oblin M. Specific hydroxylations determine selective corticosteroid recognition by human glucocorticoid and mineralocorticoid receptors. FEBS Letters. 1999;464:9–13. doi: 10.1016/s0014-5793(99)01667-1. [DOI] [PubMed] [Google Scholar]
- Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in health and disease. Trends in Pharmacological Sciences. 2013;34:518–530. doi: 10.1016/j.tips.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura C, Yoshinaga K, Tian E, Suzuki M, Aizawa S, Matsuo I. Visceral endoderm mediates forebrain development by suppressing posteriorizing signals. Developmental Biology. 2000;225:304–321. doi: 10.1006/dbio.2000.9835. [DOI] [PubMed] [Google Scholar]
- Korgun ET, Dohr G, Desoye G, Demir R, Kayisli UA, Hahn T. Expression of insulin, insulin-like growth factor I and glucocorticoid receptor in rat uterus and embryo during decidualization, implantation and organogenesis. Reproduction. 2003;125:75–84. doi: 10.1530/rep.0.1250075. [DOI] [PubMed] [Google Scholar]
- Lanier M, Schade D, Willems E, Tsuda M, Spiering S, Kalisiak J, Mercola M, Cashman JR. Wnt inhibition correlates with human embryonic stem cell cardiomyogenesis: a structure-activity relationship study based on inhibitors for the Wnt response. J Med Chem. 2012;55:697–708. doi: 10.1021/jm2010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison G, Oikonomopoulou I, Migueles R, Soneji S, Livigni A, Enver T, Brickman J. Anterior Definitive Endoderm from ESCs Reveals a Role for FGF Signaling. Cell Stem Cell. 2008;3:402–415. doi: 10.1016/j.stem.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Murry CE, Keller G. Differentiation of Embryonic Stem Cells to Clinically Relevant Populations: Lessons from Embryonic Development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Nascone N, Mercola M. An inductive role for the endoderm in Xenopus cardiogenesis. Development. 1995;121:515–523. doi: 10.1242/dev.121.2.515. [DOI] [PubMed] [Google Scholar]
- Nelson TJ, Faustino RS, Chiriac A, Crespo-Diaz R, Behfar A, Terzic A. CXCR4 +/FLK-1 +Biomarkers Select a Cardiopoietic Lineage from Embryonic Stem Cells. Stem Cells. 2008;26:1464–1473. doi: 10.1634/stemcells.2007-0808. [DOI] [PubMed] [Google Scholar]
- Newton R. Molecular mechanisms of glucocorticoid action: what is important? Thorax. 2000;55:603–613. doi: 10.1136/thorax.55.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn F, Speleman F, De Paepe A, Vandesompele J. RTPrimerDB: the real-time PCR primer and probe database. Nucleic Acids Research. 2003;31:122–123. doi: 10.1093/nar/gkg011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea-Gomez A, Rhinn M, Ang SL. Role of the anterior visceral endoderm in restricting posterior signals in the mouse embryo. Int. J. Dev. Biol. 2001;45:311–320. [PubMed] [Google Scholar]
- Perea-Gomez A, Vella FDJ, Shawlot W, Oulad-Abdelghani M, Chazaud C, Meno C, Pfister V, Chen L, Robertson E, Hamada H, et al. Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Developmental Cell. 2002;3:745–756. doi: 10.1016/s1534-5807(02)00321-0. [DOI] [PubMed] [Google Scholar]
- Rogerson FM, Dimopoulos N, Sluka P, Chu S, Curtis AJ, Fuller PJ. Structural Determinants of Aldosterone Binding Selectivity in the Mineralocorticoid Receptor. Journal of Biological Chemistry. 1999;274:36305–36311. doi: 10.1074/jbc.274.51.36305. [DOI] [PubMed] [Google Scholar]
- Schultheiss TM, Xydas S, Lassar AB. Induction of avian cardiac myogenesis by anterior endoderm. Development. 1995;121:4203–4214. doi: 10.1242/dev.121.12.4203. [DOI] [PubMed] [Google Scholar]
- Wagner RL, White PF, Kan PB, Rosenthal MH, Feldman D. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N. Engl. J. Med. 1984;310:1415–1421. doi: 10.1056/NEJM198405313102202. [DOI] [PubMed] [Google Scholar]
- Willems E, Cabral-Teixeira J, Schade D, Cai W, Reeves P, Bushway PJ, Lanier M, Walsh C, Kirchhausen T, Izpisua Belmonte JC, et al. Small Molecule-Mediated TGF-β Type II Receptor Degradation Promotes Cardiomyogenesis in Embryonic Stem Cells. Cell Stem Cell. 2012;11:242–252. doi: 10.1016/j.stem.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems E, Leyns L, Vandesompele J. Standardization of real-time PCR gene expression data from independent biological replicates. Analytical Biochemistry. 2008;379:127–129. doi: 10.1016/j.ab.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Willems E, Spiering S, Davidovics H, Lanier M, Xia Z, Dawson M, Cashman J, Mercola M. Small-Molecule Inhibitors of the Wnt Pathway Potently Promote Cardiomyocytes From Human Embryonic Stem Cell-Derived Mesoderm. Circulation Research. 2011;109:360–364. doi: 10.1161/CIRCRESAHA.111.249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa N, Makino Y, Okamoto K, Morimoto C, Makino I, Tanaka H. Distinct Interaction of Cortivazol with the Ligand Binding Domain Confers Glucocorticoid Receptor Specificity: CORTIVAZOL IS A SPECIFIC LIGAND FOR THE GLUCOCORTICOID RECEPTOR. Journal of Biological Chemistry. 2002;277:5529–5540. doi: 10.1074/jbc.M107946200. [DOI] [PubMed] [Google Scholar]
- Zolle IM, Berger ML, Hammerschmidt F, Hahner S, Schirbel A, Peric-Simov B. New Selective Inhibitors of Steroid 11β- Hydroxylation in the Adrenal Cortex. Synthesis and Structure–Activity Relationship of Potent Etomidate Analogues. J Med Chem. 2008;51:2244–2253. doi: 10.1021/jm800012w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.