Abstract
Diabetes mellitus affects the adipose tissue and mesenchymal stem cells derived from the adipose stroma and other tissues. Previous reports suggest that bone morphogenetic protein (BMP)4 is involved in diabetic complications, at the same time playing an important role in the maintenance of stem cells. In this study, we used rats transgenic for human islet amyloid polypeptide (HIP rats), a model of type 2 diabetes, to study the effect of diabetes on adipocyte-derived stem cells, referred to as dedifferentiated fat (DFAT) cells. Our results show that BMP4 expression in inguinal adipose tissue is significantly increased in HIP rats compared to controls, whereas matrix Gla protein (MGP), an inhibitor of BMP4 is decreased as determined by quantitative PCR and immunofluorescence. In addition, adipose vascularity and expression of multiple endothelial cell markers was increased in the diabetic tissue, visualized by immunofluorescence for endothelial markers. The endothelial markers co-localized with the enhanced BMP4 expression, suggesting that vascular cells play a role BMP4 induction. The DFAT cells are multipotent stem cells derived from white mature adipocytes that undergo endothelial and adipogenic differentiation. DFAT cells prepared from the inguinal adipose tissue in HIP rats exhibited enhanced proliferative capacity compared to wild type. In addition, their ability to undergo both endothelial cell and adipogenic lineage differentiation was enhanced, as well as their response to BMP4, as assessed by lineage marker expression. We conclude that the DFAT cells are affected by diabetic changes and may contribute to the adipose dysfunction in diabetes.
Keywords: Adipocytes, Dedifferentiated fat cells, Adult stem cells, Diabetes mellitus, Bone morphogenetic proteins
INTRODUCTION
The presence of diabetes mellitus is an important consideration in preparing stem cells from donors and predicting the behavior of stem cells in recipients. The reservoir of stem cells in subcutaneous adipose tissue is known to change in diabetes. Adipose-derived stem cells (ASCs), derived from the stromal vascular fraction, exhibit impaired viability and differentiation (Ferrer-Lorente et al., 2014a; Ferrer-Lorente et al., 2014b), including endothelial cell (EC) and angiogenic differentiation potential (Koci et al., 2014; Policha et al., 2014; Rennert et al., 2014). Diabetes is also known to limit the therapeutic potential of endothelial progenitor cells (EPCs) and bone marrow-derived progenitor cells (BMPCs) (Fadini et al., 2006; Li et al., 2006; Tepper et al., 2002), and has been proposed to have deleterious effects on stem cells due to oxidative stress (Saki et al., 2013).
Multiple signaling pathways, several of which are involved in stem cell maintenance, are affected by diabetes in adipose tissue, including the Notch, Wnt, fibroblast growth factors (FGF) (Ferrer-Lorente et al., 2014a; Ferrer-Lorente et al., 2014b), and the bone morphogenetic proteins (BMPs) (Dunn, 2008; Qian et al., 2013). The BMPs are important for both adipose tissue development and vascular formation (Dyer et al., 2014; Schulz and Tseng, 2009; Tang and Lane, 2012), and recent studies suggest that BMP expression is altered in response to diabetes in multiple tissues. For example, BMP2 and BMP4 are induced in diabetic aortas together with the BMP inhibitors matrix Gla protein (MGP) and Noggin (Bostrom et al., 2010; San Martin et al., 2007), BMP4 is induced in diabetic saphenous vein grafts (Hu et al., 2013), BMP4 levels are increased in the glomeruli of diabetic kidneys (Tominaga et al., 2011), and BMP2 is induced in diabetic retinopathy (Hussein et al., 2014). Conversely, a low serum level of circulating BMP7, a BMP that can be protective against vascular calcification (Li et al., 2004), is a predictor of renal progression in diabetic patients (Wong et al., 2013). In addition, BMP4-BMPR1a signaling in pancreatic beta cells is required for and augments glucose-stimulated insulin secretion (Goulley et al., 2007), and BMP4 and BMP9 affect glucose homeostasis (Chen et al., 2003; Qian et al., 2013; Zhang et al., 2010).
In this study, we used a rat model for type 2 diabetes mellitus, the most common form of diabetes, frequently associated with obesity and changes in adipose vascularity (Pasarica et al., 2009; Spencer et al., 2011). We examined the effect of progressive diabetes on adipose BMP4 expression and the characteristics of stem cells derived from white mature adipocytes, referred to as dedifferentiated fat (DFAT) cells (Jumabay et al., 2014; Matsumoto et al., 2008), focusing on cell proliferation and potential for adipogenic and endothelial cell differentiation.
METHODS
Collection of adipose tissue
The generation of rats transgenic for human islet amyloid polypeptide (HIP rats) has been previously described (Butler et al., 2004). The rats were housed individually and fed Rodent Diet 8604 (50% carbohydrate, 24% protein, and 4% fat; Harlan Teklad, Madison, WI) ad libitum. All animals were subjected to the standard 12-h light-dark cycle. The studies were reviewed by the Institutional Review Board and conducted in accordance with the animal care guidelines set by the University of California, Los Angeles. For collection of rat adipose tissue for cell isolation, HIP or wild type rats were euthanized at 2, 6, or 12 months of age by inhalation of isoflurane (5–30%) and adipose tissues were collected postmortem. The studies were reviewed and approved by the Institutional Review Board and conducted in accordance with the animal care guidelines set by the University of California, Los Angeles. The investigation conformed to the National Research Council, Guide for the Care and Use of Laboratory Animals, Eighth Edition (Washington, DC: The National Academies Press, 2011).
Isolation of adipocytes and culture of DFAT cells
Lipid-filled mature adipocytes and adipose stromal cells (ASCs) were isolated from 2 grams of rat inguinal adipose tissue as previously described (Jumabay et al., 2014). Briefly, prior to adipocyte isolation, the adipose tissue was washed repeatedly with phosphate-buffered saline (PBS) until the PBS washes were clear. After the adipocytes had been isolated, they were washed three times in culture medium (DMEM supplemented with 20% fetal bovine serum (HyClone) and 0.5% of antibiotic-antimycotic solution (Mediatech) before they were used for further analysis or culture. If the adipocytes were used for generation of DFAT cells, they were pre-incubated (floated) on top of medium in culture dishes or 50 ml plastic tubes with loosened caps for 24 hours to allow for any remaining non-adipocytes to detach and sink to the bottom. Adipocytes (30–50 μl of the top creamy layer) were then added to culture medium in 6-well plates fitted with 70 μm-filters and incubated for 5 days. DFAT cells generated from the adipocytes passed through the filters and attached to the bottom of the dishes. After 5 days, the filters with remains of the adipocytes were removed. We used a minimum of three DFAT preparations from each time point, and the cells were used between passages 0–3, mostly passage 1.
RNA analysis
Quantitative (q)PCR and RT-PCR were performed as previously described (Jumabay et al., 2012; Yao et al., 2006). The primers and probes used for qPCR for rat BMP4, rat peroxisome proliferator-activated receptor gamma (PPARgamma), rat CCAAT/enhancer-binding protein (C/EBP)alpha, rat Adiponectin, rat CD34, rat CD31, rat MGP, rat vascular endothelial growth factor (VEGF), rat VEGF receptor 2 (VEGFR2), rat VE-Cadherin, rat SRY (sex determining region Y)-box 2 (SOX2), and rat POU homeodomain protein Oct3/4 were pre-designed and obtained from Applied Biosystems (Foster City, CA) as part of Taqman® Gene Expression Assays.
Immunohistochemistry and immunocytochemistry
Immunostaining was performed as previously described in detail (Jumabay et al., 2012). Briefly, cells grown in chamber slides were fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, blocked with 10% goat serum and 1% BSA in PBS, and incubated over night at 4°C with the appropriate primary antibodies or non-specific IgG control antibodies, diluted 1:200 in 1% BSA in PBS. The next day, cells were incubated with secondary AF-488-conjugated (green fluorescence) or AF-594-conjugated (red fluorescence) goat anti-mouse or anti-rabbit secondary antibodies (Molecular Probes). The cells were washed with PBS, the nuclei stained with 4',6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich), and visualized by fluorescence microscopy. The non-specific IgG control antibodies showed no staining and are not included in the figures.
We used the following antibodies for immunostaining: hamster anti-CD31, rabbit anti-vone Willebrand Factor (vWF) (both from Dako), goat anti-BMP4, goat anti-MGP, rabbit anti-VEGF, rabbit anti-VE-Cadherin (all from Santa Cruz Biotechnology), mouse anti-Perilipin (Cell Signaling Technology).
Proliferation Assay
DFAT cells were seeded in 24-well plates at a density of 100,000 cells per well, and allowed to attach for 4–6 hours. 3H-Thymidine was added at 1 μCi/ml for 3 days, and 3H-thymidine incorporation was determined as previously described (Lee et al., 2000).
Statistical analysis
Data were analyzed for statistical significance by two-way analysis of variance with post hoc Tukey’s analysis using the GraphPad Instat® 3.0 software (GraphPad Software, San Diego, CA). P-values less than 0.05 were considered significant. All experiments were repeated a minimum of three times.
RESULTS
Diabetes mellitus enhances BMP4 expression and vascularity in adipose tissue
Our previous studies showed induction of BMP4 in diabetic aortas (Bostrom et al., 2010). Since BMP4 is essential in angiogenesis and adipogenesis (Dyer et al., 2014; Tang et al., 2004), we hypothesized that BMP4 may be induced in diabetic white adipose tissue (WAT) and contribute to changes in both tissue and adipocyte-derived stem cells.
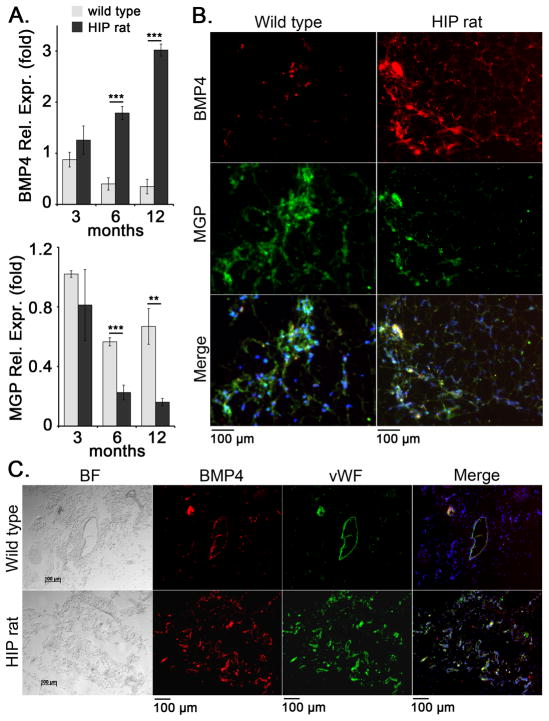
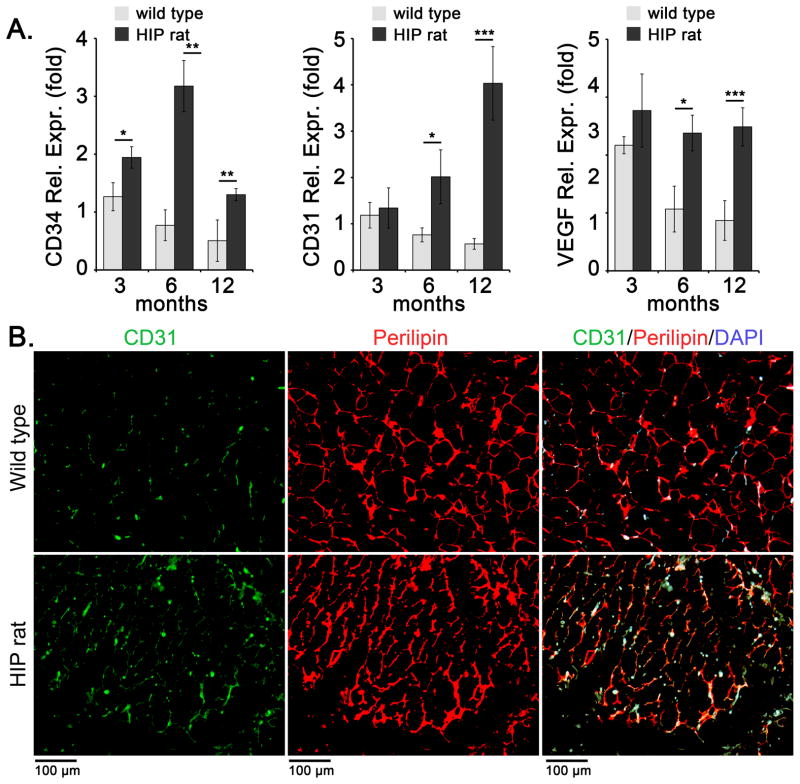
To study the effect of diabetes on expression of BMP4, MGP and endothelial lineage markers, we obtained subcutaneous inguinal WAT from HIP rats, a type 2 diabetes model (Butler et al., 2004) and wild type controls aged 3, 6, and 12 months. The glucose levels of the rats increased with age as shown in Table 1. The results showed a progressive increase in BMP4 expression by qPCR in the diabetic WAT, as compared to wild type WAT (Figure 1A). Matrix Gla protein (MGP) is a known BMP inhibitor able to counteract BMP4 in vascular endothelium (Yao et al., 2008), and its expression decreased in diabetic fat (Figure 1A). Immunostaining for BMP4 and MGP in WAT from 12 months old wild type and HIP rats confirmed the increase in BMP4 and the decrease in MGP by (Figure 1B). It also showed areas of co-localization of BMP4 and MGP (Figure 1B) supporting a close relationship between these two factors. Interestingly, the expression of Noggin, another BMP inhibitor, did not change significantly by qPCR (data not shown). DAPI was used to visualize the nuclei. We also co-stained for BMP4 and vWF (EC marker), and found that BMP4 in part co-stained with vWF, suggesting that at least some of the BMP4 expression occurs in ECs (Figure 1C). The staining suggested an overall increase in vascularity in the diabetic adipose tissue. This was further examined by qPCR and immunostaining for endothelial markers. The results showed a progressive and significant increase in CD34 (EC progenitor marker), CD31 (EC marker) and VEGF by qPCR, as compared to wild type controls (Figure 2A). The increase in CD31 was also detected by immunostaining for CD31 at 12 months of age (Figure 2B). Perilipin was used to visualize adipocytes.
Table 1.
Serum levels of glucose in wild type and HIP rats, aged 3–12 months.
| Rats | Glucose (mg/dl)3 months | Glucose (mg/dl) 6 months | Glucose (mg/dl) 12 months |
|---|---|---|---|
| Wild type | 79.0±11.5 | 99.0±7.4 | 90.3±12.8 |
| HIP | 104.7±19.9* | 139.3±38.5** | 292.1±99.8*** |
Asterisks indicate statistically significant differences between wild type and HIP rats.
<0.05,
<0.01,
<0.001, Tukey’s test (n=6 for glucose).
Figure 1. Increased expression of BMP4 and decreased expression of MGP in diabetic subcutaneous adipose tissue.
Inguinal adipose tissue was collected from wild type and HIP rats at 3, 6, and 12 months of age.
(A) Expression of BMP4 and MGP as determined by qPCR and compared to expression in wild type rat at 3 months of age.
(B, C) Expression of BMP4 and MGP (B) and BMP4 and the endothelial marker von Willebrand factor (vWF) (C) in adipose tissue as visualized by immunofluorescence. DAPI was used to visualize the nuclei.
Asterisks indicate statistically significant differences between wild type and HIP rats.
*<0.05, **<0.01, ***<0.001, Tukey’s test (n=3).
Figure 2. Enhanced expression of endothelial cell markers in diabetic subcutaneous adipose tissue.
Inguinal adipose tissue was collected from wild type and HIP rats at 3, 6, and 12 months of age.
(A) Expression of CD34, CD31, and VEGF as determined by qPCR and compared to expression in wild type rat at 3 months of age.
(B) Expression of CD31 and Perilipin in adipose tissue as visualized by immunofluorescence. DAPI was used to visualize the nuclei.
Asterisks indicate statistically significant differences between wild type and HIP rats.
*<0.05, **<0.01, ***<0.001, Tukey’s test (n=3).
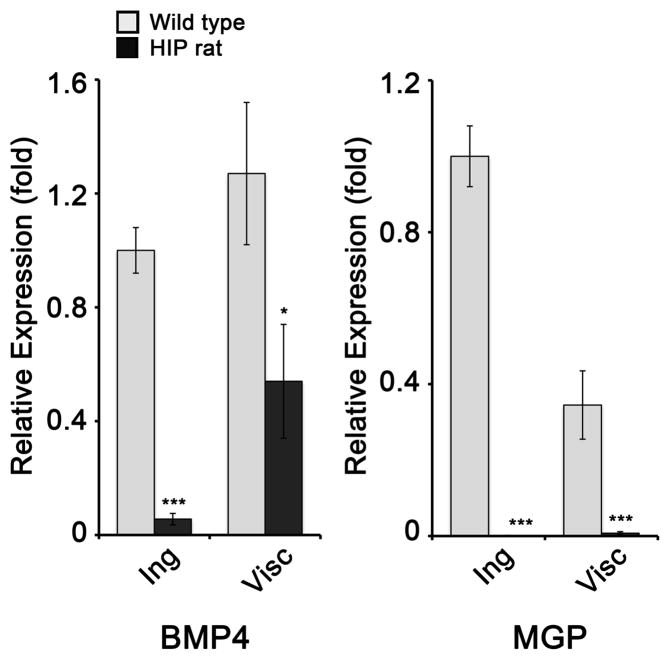
We then prepared DFAT cells from the inguinal WAT of wild type and HIP rats aged 6 months. DFAT cells are adult stem cells derived from isolated white adipocytes that spontaneously differentiate into ECs and white adipocytes (Jumabay et al., 2014; Jumabay et al., 2012; Matsumoto et al., 2008). We examined the expression of BMP4 and MGP by qPCR in DFAT cells derived from the inguinal WAT. Interestingly, BMP4 expression decreased in the HIP DFAT cells, as compared to wild type (Figure 3, left), which is opposite to the results in WAT (Figure 1A). The results for MGP, on the other hand, were similar to the findings in WAT in that MGP decreased dramatically in the HIP DFAT cells (Figure 3, right). We also examined DFAT cells derived from a different, visceral fat depot, and again found that both BMP4 and MGP expression decreased (Figure 3). Based on the co-localization of BMP4 and vWF in Figure 1C, it is possible that the BMP4 in the tissue derived from the vascular cells, which are removed prior to the preparation of DFAT cells from adipocytes.
Figure 3. Expression of BMP4 and MGP in DFAT cells.
DFAT cells were prepared from inguinal (Ing) and visceral (Visc) adipose tissue from wild type and HIP rats aged 6 months. Expression of BMP4 (left) and MGP (right) as determined by qPCR and compared to expression in wild type inguinal adipose tissue.
Asterisks indicate statistically significant differences between wild type and HIP rats.
*<0.05, ***<0.001, Tukey’s test (n=3).
Glucose and BMP4 promotes cell proliferation in DFAT cells
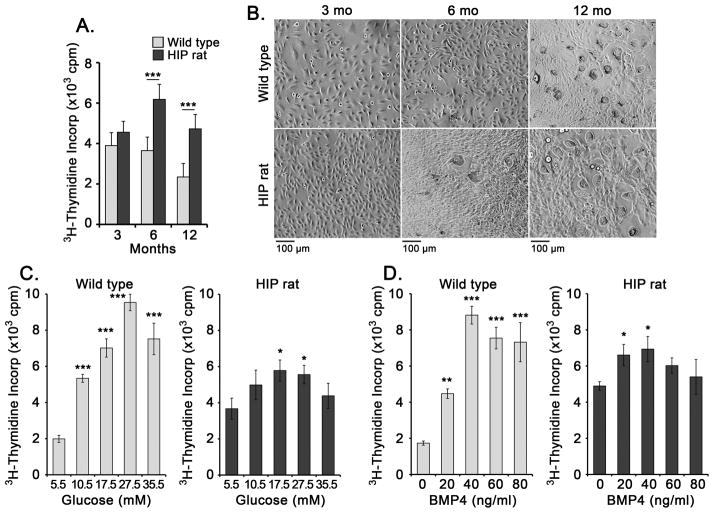
Based on the tissue results, we hypothesized that DFAT cells from diabetic rats would have an enhanced ability to proliferate and undergo endothelial and adipogenic differentiation. We prepared DFAT cells from wild type and HIP rats aged 3, 6, and 12 months and determined cell proliferation by 3H-thymidine incorporation after 3 days. The results showed significantly increased proliferation rate at 6 and 12 months of age as compared to wild type (Figure 4A,B). We then treated the DFAT cells with increasing levels of glucose (5.5–35.5 mM) or BMP4 (0–80 ng/ml) for up to 3 days. The proliferation rate of wild type DFAT cells responded significantly to both glucose and BMP4, whereas the HIP DFAT cells showed increased baseline proliferation and a diminished response to both glucose and BMP4 (Figure 4C,D).
Figure 4. Glucose and BMP4 promotes cell proliferation in DFAT cells.
DFAT cells were prepared from inguinal adipose tissue from wild type and HIP rats aged 3, 6, and 12 months.
(A) Cell proliferation as determined by 3H-thymidine incorporation for 3 days.
(B) Bright field microscopy of DFAT cells after 3 days of culture.
(C) Proliferation in response to glucose (5.5–35.5 mM) (left) or BMP4 (0–80 ng/ml) (right) as determined by 3H-thymidine incorporation.
Asterisks indicate statistically significant differences between DFAT cells from wild type and HIP rats (A), or compared to the lowest concentration of glucose or BMP4 (C).
*<0.05, **<0.01, ***<0.001, Tukey’s test (n=3).
Enhanced endothelial and adipogenic differentiation in DFAT cells from diabetic rats
We next examined spontaneous endothelial and adipogenic differentiation in the DFAT cells from wild type and HIP rats aged 6 months. The cells were allowed to differentiate in regular culture medium for up to 10 days, and expression of EC and adipogenic markers was determined. The results showed a 2–5 fold increase in the expression of the EC markers CD31, VE-cadherin and VEGF in the HIP DFAT cells by qPCR, as compared to wild type (Figure 5A). The increase was also detected by immunostaining for the respective EC markers (Figure 5B). We detected occasional cells in both wild type and HIP DFAT cells with co-staining for CD31 and perilipin (Figure 5C), supporting that the CD31-expressing cells were derived from the DFAT cells.
Figure 5. Enhanced endothelial differentiation in diabetic DFAT cells.
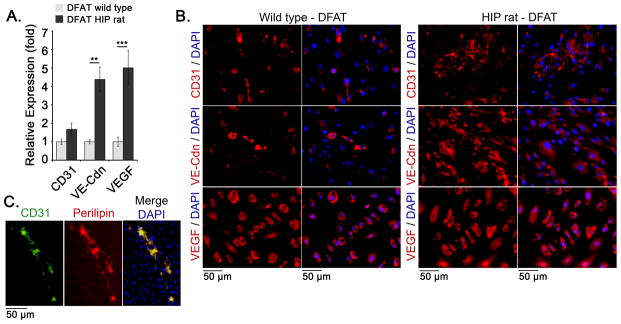
DFAT cells were prepared from inguinal adipose tissue from wild type and HIP rats aged 6 months.
(A) Expression of CD31, VE-cadherin (VE-Cdn) and VEGF, as determined by qPCR and compared to expression in wild type DFAT cells.
(B) Expression of CD31, VE-cadherin, and VEGF in DFAT cells as visualized by immunofluorescence. DAPI was used to visualize the nuclei.
(C) Co-immunofluorescence for CD31 (green) and Perilipin (red).
Asterisks indicate statistically significant differences between DFAT cells from wild type and HIP rats.
**<0.01, ***<0.001, Tukey’s test (n=3).
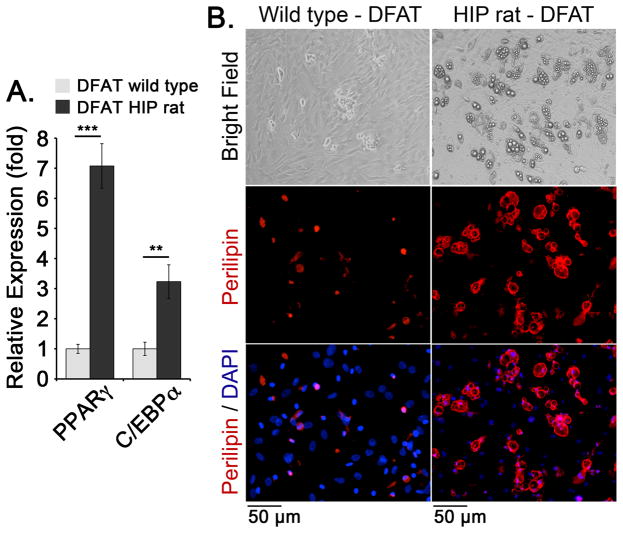
Furthermore, the results showed a 3–7 fold increase in the expression of the adipogenic markers PPARgamma and C/EBPalpha by qPCR in the diabetic DFAT cells compared to wild type DFAT cells (Figure 6A). Perilipin staining also showed an increased number of lipid-containing adipocytes in the diabetic DFAT cells (Figure 6B). Together, the results suggest that spontaneous endothelial and adipogenic differentiation is enhanced in DFAT cells derived from the diabetic animals.
Figure 6. Enhanced adipogenic differentiation in diabetic DFAT cells.
DFAT cells were prepared from inguinal adipose tissue from wild type and HIP rats aged 6 months.
(A) Expression of PPARgamma and C/EBPalpha, as determined by qPCR and compared to expression in wild type DFAT cells. DAPI was used to visualize the nuclei.
(B) Expression of Perilipin in DFAT cells as visualized by immunofluorescence. Asterisks indicate statistically significant differences between DFAT cells from wild type and HIP rats.
**<0.01, ***<0.001, Tukey’s test (n=3).
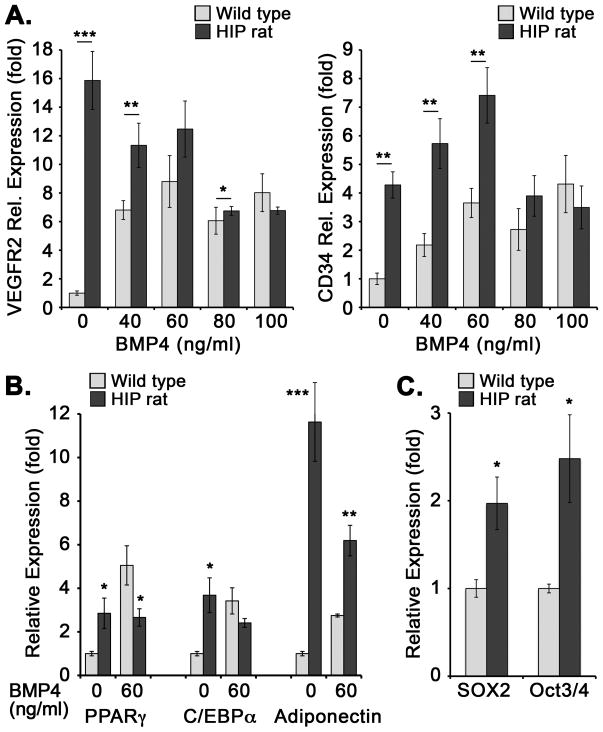
Effect of BMP4 on endothelial differentiation in DFAT cells from diabetic rats
Since BMP4 is important for endothelial differentiation (13–15), we determined the effect of BMP4 on endothelial differentiation in DFAT cells from wild type and HIP rats aged 6 months. We treated the DFAT cells with BMP4 (0–100 ng/ml) for 5 days, and determined the expression of CD34 and VEGFR2. The results showed that wild type DFAT cells increased expression of both markers in response to all concentrations of BMP4 (Figure 7A). However, the HIP DFAT cells had a higher expression of EC markers at baseline, which did increase in response to the low BMP4 concentrations (<60 ng/ml), but decreased at high BMP4 concentrations (Figure 7A). The results suggest that DFAT cells from HIP rats have a “shift” in their response to BMP4 treatment, possibly due to high BMP4 exposure in the WAT. Interestingly, the expression of adipogenic markers PPARgamma, C/EBPalpha, and Adiponectin also differed between wild type and HIP DFAT cells in response to BMP4. The HIP DFAT cells showed higher expression than wild type cells without BMP4 treatment, whereas the expression trended downwards when BMP4 (60 ng/ml) was added (Figure 7B).
Figure 7. Differentiation markers in wild type and diabetic DFAT cells in response to BMP4.
(A, B) DFAT cells were prepared from inguinal adipose tissue from wild type and HIP rats aged 6 months. The cells were treated with BMP4 (0–100 ng/ml) for 5 days, and expression was determined by qPCR and compare to wild type cells treated with no BMP4. (A) Expression of VEGFR2 (left) and CD34 (right). (B) Expression of PPARgamma, C/EBPalpha, and Adiponectin. (C) Expression of multipotent markers SOX2 and Oct3/4 in wild type and diabetic DFAT cells, passage 1, after 3 days in culture without BMP4. Asterisks indicate statistically significant differences between DFAT cells from wild type and HIP rats.
*<0.05, **<0.01, ***<0.001, Tukey’s test (n=3).
Finally, we tested the DFAT cells for expression of multipotency markers, previously detected in mouse and human DFAT cells (Jumabay et al., 2014). DFAT cells from wild type and HIP rats were tested at passage 1, which was the most common passage used in our study. The results showed increased expression of the multipotent markers SOX2 and Oct3/4 in the HIP DFAT cells compared to wild type (Figure 7C), which is consistent with the altered characteristics of the diabetic DFAT cells.
Altogether, our results show that diabetes affect BMP4 signaling in WAT and cause alterations in the characteristics of adipocyte-derived stem cells.
DISCUSSION
Our study suggests that the development of diabetes affects the adipose expression of BMP4 in rats. The BMP4 in the diabetic adipose tissue co-localized in large part with ECs, where it was associated with reduced levels of MGP. Furthermore, proliferation and ability to undergo EC and adipogenic differentiation were enhanced in DFAT cells isolated from diabetic animals.
Previous studies indicate that BMP4 stimulates both endothelial and adipogenic commitment in progenitor cells (Dyer et al., 2014; Schulz and Tseng, 2009; Tang and Lane, 2012), which in adipose tissue may give rise to both cells types (Planat-Benard et al., 2004), and could play an important role in modulating the balance between vascularity and adipogenesis. MGP is highly induced in ECs undergoing angiogenic resolution (Sharma and Albig, 2013) and preadipocytes (Mutch et al., 2009) although its role in the latter cells is unclear. We previously found that BMP4 enhanced the expression of MGP in vascular endothelium through the induction of the activin receptor-like kinase 1 (ALK1) (Yao et al., 2006), and that MGP in turn provided negative feedback regulation of BMP4. However, the MGP expression in diabetic adipose tissue responded differently in that it decreased, which might dysregulate both EC and adipogenic differentiation. The importance of adipose BMP4 is supported by other studies where BMP4 was either overexpressed or deleted from adipocytes in mice (Qian et al., 2013), which altered adipogenic differentiation in the adipose tissue. Enhanced BMP4 expression in the adipocytes caused a reduction in size and a shift towards characteristics of brown or beige adipocytes, whereas diminished BMP4 expression increased the adipocyte size (Qian et al., 2013). It is likely that diabetes affects multiple pathways that separately or together regulate the expression of BMP4 and MGP, thereby influencing adipose tissue-derived stem cells and the composition of the adipose tissue as diabetes progresses. It is also possible that BMP4 is induced in specific cell types, such as adipose ECs, which are exposed to high glucose in the blood stream, but are removed prior to DFAT cell preparation. This might explain our finding that the diabetic DFAT cells have less BMP4 expression than wild type DFAT cells. In addition, it would be consistent with our previous finding that BMP4 is induced in the aortic endothelium in diabetic animals (Bostrom et al., 2010).
Our data showed that diabetic DFAT cells had higher proliferation than control cells, but a dampened response to BMP4 and glucose, possibly due to BMP4 excess stimulation already in the tissue. On the other hand, the diabetic DFAT cells had enhanced ability to undergo endothelial and adipogenic differentiation in response to low levels of exogenous BMP4 in the culture medium, possibly due to having been primed to undergo such differentiation in vivo, prior to isolation.
The DFAT have similarities to ASCs, which are isolated from the stromal vascular fraction of adipose tissue, but constitute a more homogenous cell population than the ASCs (Jumabay et al., 2012). Indeed, our previous lineage tracing suggest that part of the ASCs may be derived from the white adipocytes (Jumabay et al., 2012). Therefore it is not surprising that the ASCs also have been shown to have altered viability and differentiation potential in diabetes (Ferrer-Lorente et al., 2014a; Ferrer-Lorente et al., 2014b), including angiogenic potential (Koci et al., 2014; Policha et al., 2014; Rennert et al., 2014). These reports together with our findings in DFAT cells have implications for pathological changes in WAT due to diabetes, and for the behavior of stem cells prepared from or delivered to diabetic patients.
Acknowledgments
Funding for this work was provided in part by NIH/NHLBI: grant number HL30568 (K.I.B), NIH/NHLBI: grant number HL81397 (K.I.B.), and NIH/NHLBI: grant number HL112839 (K.I.B.), and the American Heart Association: grant number 13SDG17190013 (M.J.).
References
- Bostrom KI, Jumabay M, Matveyenko A, Nicholas SB, Yao Y. Activation of Vascular Bone Morphogenetic Protein Signaling in Diabetes Mellitus. Circ Res. 2010;108(4):446–457. doi: 10.1161/CIRCRESAHA.110.236596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AE, Jang J, Gurlo T, Carty MD, Soeller WC, Butler PC. Diabetes due to a progressive defect in beta-cell mass in rats transgenic for human islet amyloid polypeptide (HIP Rat): a new model for type 2 diabetes. Diabetes. 2004;53(6):1509–1516. doi: 10.2337/diabetes.53.6.1509. [DOI] [PubMed] [Google Scholar]
- Chen C, Grzegorzewski KJ, Barash S, Zhao Q, Schneider H, Wang Q, Singh M, Pukac L, Bell AC, Duan R, Coleman T, Duttaroy A, Cheng S, Hirsch J, Zhang L, Lazard Y, Fischer C, Barber MC, Ma ZD, Zhang YQ, Reavey P, Zhong L, Teng B, Sanyal I, Ruben SM, Blondel O, Birse CE. An integrated functional genomics screening program reveals a role for BMP-9 in glucose homeostasis. Nat Biotechnol. 2003;21(3):294–301. doi: 10.1038/nbt795. [DOI] [PubMed] [Google Scholar]
- Dunn NR. Self-renewal made simple. Cell Stem Cell. 2008;3(1):7–8. doi: 10.1016/j.stem.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Dyer LA, Pi X, Patterson C. The role of BMPs in endothelial cell function and dysfunction. Trends in endocrinology and metabolism: TEM. 2014;25(9):472–480. doi: 10.1016/j.tem.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadini GP, Sartore S, Schiavon M, Albiero M, Baesso I, Cabrelle A, Agostini C, Avogaro A. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia. 2006;49(12):3075–3084. doi: 10.1007/s00125-006-0401-6. [DOI] [PubMed] [Google Scholar]
- Ferrer-Lorente R, Bejar MT, Badimon L. Notch Signaling Pathway Activation in Normal and Hyperglycemic Rats Differs in the Stem Cells of Visceral and Subcutaneous Adipose Tissue. Stem Cells Dev. 2014a;23(24):3034–3048. doi: 10.1089/scd.2014.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Lorente R, Bejar MT, Tous M, Vilahur G, Badimon L. Systems biology approach to identify alterations in the stem cell reservoir of subcutaneous adipose tissue in a rat model of diabetes: effects on differentiation potential and function. Diabetologia. 2014b;57(1):246–256. doi: 10.1007/s00125-013-3081-z. [DOI] [PubMed] [Google Scholar]
- Goulley J, Dahl U, Baeza N, Mishina Y, Edlund H. BMP4-BMPR1A signaling in beta cells is required for and augments glucose-stimulated insulin secretion. Cell Metab. 2007;5(3):207–219. doi: 10.1016/j.cmet.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Hu J, Liu J, Kwok MW, Wong RH, Huang Y, Wan S. Bone morphogenic protein-4 contributes to venous endothelial dysfunction in patients with diabetes undergoing coronary revascularization. Ann Thor Surg. 2013;95(4):1331–1339. doi: 10.1016/j.athoracsur.2012.12.028. [DOI] [PubMed] [Google Scholar]
- Hussein KA, Choksi K, Akeel S, Ahmad S, Megyerdi S, El-Sherbiny M, Nawaz M, Abu El-Asrar A, Al-Shabrawey M. Bone morphogenetic protein 2: a potential new player in the pathogenesis of diabetic retinopathy. Exp Eye Res. 2014;125:79–88. doi: 10.1016/j.exer.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumabay M, Abdmaulen R, Ly A, Cubberly MR, Shahmirian LJ, Heydarkhan-Hagvall S, Dumesic DA, Yao Y, Bostrom KI. Pluripotent stem cells derived from mouse and human white mature adipocytes. Stem Cells Transl Med. 2014;3(2):161–171. doi: 10.5966/sctm.2013-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumabay M, Abdmaulen R, Urs S, Heydarkhan-Hagvall S, Chazenbalk GD, Jordan MC, Roos KP, Yao Y, Bostrom KI. Endothelial differentiation in multipotent cells derived from mouse and human white mature adipocytes. J Mol Cell Cardiol. 2012;53(6):790–800. doi: 10.1016/j.yjmcc.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koci Z, Turnovcova K, Dubsky M, Baranovicova L, Holan V, Chudickova M, Sykova E, Kubinova S. Characterization of human adipose tissue-derived stromal cells isolated from diabetic patient's distal limbs with critical ischemia. Cell Biochem Funct. 2014;32(7):597–604. doi: 10.1002/cbf.3056. [DOI] [PubMed] [Google Scholar]
- Lee H, Shi W, Tontonoz P, Wang S, Subbanagounder G, Hedrick CC, Hama S, Borromeo C, Evans RM, Berliner JA, Nagy L. Role for peroxisome proliferator-activated receptor alpha in oxidized phospholipid-induced synthesis of monocyte chemotactic protein-1 and interleukin-8 by endothelial cells. Circ Res. 2000;87(6):516–521. doi: 10.1161/01.res.87.6.516. [DOI] [PubMed] [Google Scholar]
- Li T, Surendran K, Zawaideh MA, Mathew S, Hruska KA. Bone morphogenetic protein 7: a novel treatment for chronic renal and bone disease. Curr Opin Nephrol Hypertens. 2004;13(4):417–422. doi: 10.1097/01.mnh.0000133974.24935.fe. [DOI] [PubMed] [Google Scholar]
- Li TS, Furutani A, Takahashi M, Ohshima M, Qin SL, Kobayashi T, Ito H, Hamano K. Impaired potency of bone marrow mononuclear cells for inducing therapeutic angiogenesis in obese diabetic rats. Am J Physiol Heart Circ Physiol. 2006;290(4):H1362–1369. doi: 10.1152/ajpheart.00766.2005. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, Matsubara Y, Sakuma T, Satomi A, Otaki M, Ryu J, Mugishima H. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215(1):210–222. doi: 10.1002/jcp.21304. [DOI] [PubMed] [Google Scholar]
- Mutch DM, Rouault C, Keophiphath M, Lacasa D, Clement K. Using gene expression to predict the secretome of differentiating human preadipocytes. Int J Obes. 2009;33(3):354–363. doi: 10.1038/ijo.2009.3. [DOI] [PubMed] [Google Scholar]
- Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58(3):718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109(5):656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- Policha A, Zhang P, Chang L, Lamb K, Tulenko T, DiMuzio P. Endothelial differentiation of diabetic adipose-derived stem cells. J Surg Res. 2014;192(2):656–663. doi: 10.1016/j.jss.2014.06.041. [DOI] [PubMed] [Google Scholar]
- Qian SW, Tang Y, Li X, Liu Y, Zhang YY, Huang HY, Xue RD, Yu HY, Guo L, Gao HD, Sun X, Li YM, Jia WP, Tang QQ. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc Natl Acad Sci U S A. 2013;110(9):E798–807. doi: 10.1073/pnas.1215236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennert RC, Sorkin M, Januszyk M, Duscher D, Kosaraju R, Chung MT, Lennon J, Radiya-Dixit A, Raghvendra S, Maan ZN, Hu MS, Rajadas J, Rodrigues M, Gurtner GC. Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res Ther. 2014;5(3):79. doi: 10.1186/scrt468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saki N, Jalalifar MA, Soleimani M, Hajizamani S, Rahim F. Adverse effect of high glucose concentration on stem cell therapy. Int J Hematol Oncol Stem Cell Res. 2013;7(3):34–40. [PMC free article] [PubMed] [Google Scholar]
- San Martin A, Du P, Dikalova A, Lassegue B, Aleman M, Gongora MC, Brown K, Joseph G, Harrison DG, Taylor WR, Jo H, Griendling KK. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;292(5):H2073–2082. doi: 10.1152/ajpheart.00943.2006. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Tseng YH. Emerging role of bone morphogenetic proteins in adipogenesis and energy metabolism. Cytokine Growth Factor Rev. 2009;20(5–6):523–531. doi: 10.1016/j.cytogfr.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma B, Albig AR. Matrix Gla protein reinforces angiogenic resolution. Microvasc Res. 2013;85:24–33. doi: 10.1016/j.mvr.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer M, Unal R, Zhu B, Rasouli N, McGehee RE, Jr, Peterson CA, Kern PA. Adipose Tissue Extracellular Matrix and Vascular Abnormalities in Obesity and Insulin Resistance. J Clin Endocrinol Metab. 2011;96(12):E1990–E1998. doi: 10.1210/jc.2011-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. 2012;81:715–736. doi: 10.1146/annurev-biochem-052110-115718. [DOI] [PubMed] [Google Scholar]
- Tang QQ, Otto TC, Lane MD. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A. 2004;101(26):9607–9611. doi: 10.1073/pnas.0403100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106(22):2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- Tominaga T, Abe H, Ueda O, Goto C, Nakahara K, Murakami T, Matsubara T, Mima A, Nagai K, Araoka T, Kishi S, Fukushima N, Jishage K, Doi T. Activation of bone morphogenetic protein 4 signaling leads to glomerulosclerosis that mimics diabetic nephropathy. J Biol Chem. 2011;286(22):20109–20116. doi: 10.1074/jbc.M110.179382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MG, Perkovic V, Woodward M, Chalmers J, Li Q, Hillis GS, Yaghobian Azari D, Jun M, Poulter N, Hamet P, Williams B, Neal B, Mancia G, Cooper M, Pollock CA. Circulating bone morphogenetic protein-7 and transforming growth factor-beta1 are better predictors of renal end points in patients with type 2 diabetes mellitus. Kidney Int. 2013;83(2):278–284. doi: 10.1038/ki.2012.383. [DOI] [PubMed] [Google Scholar]
- Yao Y, Shahbazian A, Bostrom KI. Proline and gamma-carboxylated glutamate residues in matrix Gla protein are critical for binding of bone morphogenetic protein-4. Circ Res. 2008;102(9):1065–1074. doi: 10.1161/CIRCRESAHA.107.166124. [DOI] [PubMed] [Google Scholar]
- Yao Y, Zebboudj AF, Shao E, Perez M, Bostrom K. Regulation of bone morphogenetic protein-4 by matrix GLA protein in vascular endothelial cells involves activin-like kinase receptor 1. J Biol Chem. 2006;281(45):33921–33930. doi: 10.1074/jbc.M604239200. [DOI] [PubMed] [Google Scholar]
- Zhang H, Schulz TJ, Espinoza DO, Huang TL, Emanuelli B, Kristiansen K, Tseng YH. Cross talk between insulin and bone morphogenetic protein signaling systems in brown adipogenesis. Mol Cell Biol. 2010;30(17):4224–4233. doi: 10.1128/MCB.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]