Abstract
Purpose
Suboptimal visual acuity after endothelial keratoplasty has been attributed to increased anterior corneal high-order aberrations (HOAs). In this study, we determined anterior and posterior corneal HOAs over a range of severity of Fuchs endothelial corneal dystrophy (FECD).
Design
Cross-sectional study.
Participants
108 eyes (62 subjects) with a range of severity of FECD and 71 normal eyes (38 subjects).
Methods
All corneas were examined by using slit-lamp biomicroscopy to determine the severity of FECD versus normality. FECD corneas were categorized as mild, moderate, or advanced according to the area and confluence of guttae and the presence of clinically visible edema. Normal corneas were devoid of any guttae. Wavefront errors from the anterior and posterior corneal surfaces were derived from Scheimpflug images and expressed as Zernike polynomials through the 6th order over a 6-mm-diameter optical zone. Backscatter from the anterior 120 μm and posterior 60 μm of the cornea were also measured from Scheimpflug images and were standardized to a fixed scatter source. Variables were compared between FECD and control eyes by using generalized estimating equation models to adjust for age and correlation between fellow eyes.
Main Outcome Measures
HOAs, expressed as root-mean-square of wavefront errors, and backscatter of the anterior and posterior cornea.
Results
Total anterior corneal HOAs were increased in moderate (0.61 ± 0.27 μm, mean ± standard deviation; p =0.01) and advanced (0.66 ± 0.28 μm; p =0.01) FECD compared to controls (0.47 ± 0.16 μm). Total posterior corneal HOAs were increased in mild (0.22 ± 0.09 μm; p =0.017), moderate (0.22 ± 0.08 μm; p <0.001), and advanced (0.23 ± 0.09 μm; p <0.001) FECD compared to controls (0.16 ± 0.03 μm). Anterior and posterior corneal backscatter were higher for all severities of FECD compared to controls (p ≤0.02, anterior; p ≤0.001, posterior).
Conclusions
Anterior and posterior corneal HOAs and backscatter are higher than normal even in early stages of FECD. The early onset of HOAs in FECD might contribute to the persistence of HOAs and incomplete visual rehabilitation after endothelial keratoplasty.
Keywords: Fuchs endothelial corneal dystrophy, Scheimpflug imaging, high-order aberrations, corneal endothelium
Introduction
Fuchs endothelial corneal dystrophy (FECD) is a bilateral corneal disease characterized by focal posterior collagenous excrescences (guttae) and progressive corneal edema, resulting in reduced corneal transparency and impaired vision.1 Corneal transplantation is the only available treatment to restore corneal transparency in advanced stages of FECD. Over the last decade, endothelial keratoplasty has become the standard method of transplantation2 with good visual outcomes,3,4 but visual rehabilitation appears to be limited by persistent changes in the anterior cornea5-7 that degrade the regularity of the anterior corneal surface and increase high-order aberrations (HOA).5,8-10 Increased HOAs are associated with worse visual acuity, irrespective of the endothelial keratoplasty technique.5,7-10 However, it is unknown when in the course of FECD HOAs become abnormal. Other anterior corneal abnormalities appear early in the course of the disease, including sub-epithelial fibroblasts,11 keratocyte loss, and increased anterior corneal haze (or backscatter).12 These abnormalities persist through at least 3 years after endothelial keratoplasty.11-13 As our ability to assess and treat FECD improves with new technologies and techniques, understanding when in the course of the disease these tissues change might improve our understanding of visual limitation after endothelial keratoplasty.
In this study, we assessed changes in anterior and posterior corneal HOAs and backscatter over a range of severity of FECD and normal corneas by using Scheimpflug imaging. Non-contact Scheimpflug imaging is widely available and simple to use, and provides information about aberrations, corneal haze, and corneal thickness. We examined the relationships between HOAs, backscatter, and corneal morphologic characteristics that are typically altered in FECD, such as corneal thickness and effective endothelial cell density (ECDe).14 We hypothesized that HOAs and backscatter are increased in the anterior and posterior cornea early in FECD, and are associated with ECDe and corneal thickness.
Methods
Subjects and clinical grading
All subjects were examined by cornea specialists using slit-lamp biomicroscopy. FECD severity was graded clinically based on the area and confluence of guttae, and the presence of edema,as described previously.15,16 Corneas with 1-12 or ≥12 non-confluent central guttae (grades 1 and 2) were considered to have mild FECD; corneas with confluent guttae of 1-2-mm and 2-5-mm diameter (grades 3 and 4) were considered to have moderate FECD; and corneas with > 5-mm diameter of confluent guttae or any visible stromal or epithelial edema (grades 5 and 6) were considered to have advanced FECD. Corneas without guttae were considered normal (grade 0); control subjects were only enrolled if over age 40 years to match the age distribution of the disease group.17 Exclusion criteria for all subjects were corneal pathology (other than FECD in the FECD groups), previous ocular surgery except uncomplicated phacoemulsification with posterior chamber intraocular lens implantation, or systemic or topical medication use known to affect the cornea. The Mayo Clinic Institutional Review Board prospectively approved this study; the research followed the tenets of the Declaration of Helsinki and complied with the Health Insurance Portability and Accountability Act. Informed consent was obtained from all subjects.
Corneal aberrations
Wavefront errors over a 6-mm-diameter optical zone centered at the corneal apex were determined by using a rotating Scheimpflug camera (Pentacam HR; Oculus, Lynnwood, WA). All Scheimpflug images were checked for data acquisition errors. Wavefront errors from the anterior and posterior corneal surfaces were calculated by ray-tracing (Pentacam software version 1.20r29) and were expressed as Zernike polynomials through the 6th order. The root-mean-square (RMS) wavefront error (μm) for coma , coma-like , trefoil ( and ), , and trefoil-like aberrations were calculated from Zernike coefficients determined by the imaging software.18 Spherical aberration was expressed as . Total HOAs from 3rd to 6th order were summarized from the Zernike polynomials as . The software assumed the refractive index of the cornea was 1.3375.
Corneal backscatter
Corneal haze (backscatter) was determined from Scheimpflug image brightness. Mean backscatter of a 2-mm diameter circle centered on the apex was measured for the anterior 120 μm and posterior 60 μm of the cornea. Before each examination, the image brightness of a standardized scatter source (a custom-made titanium-embedded rigid contact lens)19 was measured to account for fluctuations in the brightness of the light source and sensitivity of the detection system over time.20 Raw corneal image brightness was adjusted according to the brightness of the reference standard. Backscatter was expressed in scatter units (SU), the concentration of a turbidity standard, AMCO Clear (AMCO Clear; GFS Chemicals, Columbus, OH), that gave the same image brightness as the corneal image.20,21
Effective endothelial cell density and corneal thickness
To account for progressive reduction of local endothelial cell density and increased area of guttae, one investigator (K.W.) determined the effective endothelial cell density (ECDe) manually.14 Central corneal endothelial images were recorded by using confocal microscopy(ConfoScan 4; Nidek Technologies, Freemont, CA) with a 20× non-contact objective as described previously.14 In brief, after several confocal scans through the endothelium, the best quality image was chosen for analysis. Local endothelial cell density was the number of contiguous cells divided by their total area and was estimated by marking one hundred adjacent cells in a circumscribed area devoid of guttae (variable frame method). The fraction of the image covered by guttae (R) in FECD subjects was estimated by using a custom image-processing program (Analyze AVW; Mayo Medical Solutions, Rochester, MN). ECDe was the product of local cell density and the fraction of image area that was devoid of guttae (1 – R). Central corneal thickness (CCT) was measured from the Scheimpflug images.
Statistical analysis
Unadjusted results were summarized as mean ± standard deviation by severity of FECD (Tables). For analytical purposes, variables were compared by using generalized estimating equation (GEE) models to account for possible correlation between fellow eyes of the same subject.22 GEE models were used to calculate population-averaged mean differences between FECD groups with respective 95% confidence intervals. Because corneal HOAs are known to change with age,23 we adjusted estimates for age as a continuous variable. We did not find that age affected central corneal backscatter, similar to a previous study,24 and thus the backscatter analysis was not adjusted for age. Relationships between variables were assessed by age-adjusted Pearson correlations with significances of correlations determined by GEE models. Contingency tables were analyzed with Fisher's exact test. Analyses were considered statistically significant if p <0.05. The minimum difference that could be detected, if indeed a difference existed, was calculated for non-significant comparisons (α =0.05, β =0.20). All analyses were performed by using Stata version 13.1 (StataCorp, College Station, TX).
Results
Subjects
One-hundred and eight corneas from 62 subjects with FECD and 71 normal corneas from 38 subjects were examined; median age was 66 years (range, 40–89 years; Table 1). Subjects with FECD were older than controls (mean difference, 7.9 years; p =0.002). Subjects with mild (p= 0.01) and moderate (p=0.03) FECD were more often pseudophakic than controls.
Table 1.
Patient characteristics
| Control | FECD | |||
|---|---|---|---|---|
| Mild | Moderate | Advanced | ||
| FECD grade | 0 | 1-2 | 3-4 | 5-6 |
| Eyes (subjects) | 71 (38) | 32 (18) | 41 (26) | 35 (25) |
| Median age (years, range) | 59 (40-80) | 74 (55-87)** | 63 (44-83) | 67 (42-89)* |
| Females (%) | 25 (66) | 13 (76) | 16 (70) | 12 (55) |
| Phakic eyes (%) | 64 (90) | 22 (69)* | 30 (73)* | 30 (86) |
| CCT, Scheimpflug [μm] | 544 ± 26 | 555 ± 29+ | 571 ± 35*** | 590 ± 43*** |
FECD = Fuchs endothelial corneal dystrophy; CCT = Central corneal thickness. Data are unadjusted mean ± standard deviation unless otherwise indicated.
p < 0.05
p < 0.01
p < 0.001 versus controls (GEE models).
Minimum detectable difference of CCT in mild FECD versus controls is 20 μm.
Corneal high-order aberrations
Data from 1 eye with FECD were excluded from analysis because of acquisition errors. Anterior corneal total HOAs were increased in moderate (p =0.01) and advanced (p =0.01) FECD compared to controls (Table 2; Figure 1). Total HOAs in mild FECD were not different compared to controls (p >0.05; minimal detectable difference, 0.15 μm). Trefoil and trefoil-like aberrations were higher in moderate FECD compared to controls (p =0.003, p =0.004, respectively; Table 2).
Table 2.
Anterior and posterior cornea high-order aberrations in Fuchs endothelial dystrophy (FECD) and control eyes
| Control | FECD | |||
|---|---|---|---|---|
| Anterior | Mild | Moderate | Advanced | |
| Total HOAs | 0.47 ± 0.16 | 0.54 ± 0.25+ | 0.61 ± 0.27* | 0.66 ± 0.28* |
| Sphere | 0.31 ± 0.11 | 0.29 ± 0.09 | 0.34 ± 0.15 | 0.37 ± 0.15 |
| Sphere-like | 0.32 ± 0.11 | 0.29 ± 0.09* | 0.34 ± 0.15 | 0.37 ± 0.15 |
| Coma | 0.22 ± 0.17 | 0.26 ± 0.22 | 0.29 ± 0.21 | 0.39 ± 0.30 |
| Coma-like | 0.23 ± 0.17 | 0.28 ± 0.21 | 0.30 ± 0.21 | 0.41 ± 0.29 |
| Trefoil | 0.10 ± 0.07 | 0.17 ± 0.22 | 0.18 ± 0.13** | 0.11 ± 0.09 |
| Trefoil-like | 0.16 ± 0.08 | 0.25 ± 0.23 | 0.29 ± 0.22** | 0.21 ± 0.13 |
| Posterior | ||||
| Total HOAs | 0.16 ± 0.03 | 0.22 ± 0.09* | 0.22 ± 0.08*** | 0.23 ± 0.09*** |
| Sphere | 0.13 ± 0.04 | 0.13 ± 0.05 | 0.13 ± 0.04 | 0.13 ± 0.04 |
| Sphere-like | 0.13 ± 0.04 | 0.13 ± 0.05 | 0.13 ± 0.04 | 0.13 ± 0.04 |
| Coma | 0.05 ± 0.03 | 0.09 ± 0.08* | 0.09 ± 0.06*** | 0.10 ± 0.07** |
| Coma-like | 0.06 ± 0.03 | 0.11 ± 0.08** | 0.10 ± 0.06*** | 0.10 ± 0.06*** |
| Trefoil | 0.03 ± 0.02 | 0.06 ± 0.05 | 0.07 ± 0.05*** | 0.09 ± 0.08*** |
| Trefoil-like | 0.06 ± 0.03 | 0.09 ± 0.05 | 0.11 ± 0.07*** | 0.12 ± 0.08*** |
High-order aberrations (HOAs) of 3rd through 6th order at 6-mm-diameter optical zone were determined by using Scheimpflug imaging (n =178). Data are unadjusted mean ± standard deviation [RMS, μm].
p < 0.05
p < 0.01
p < 0.001, versus controls (GEE models, age adjusted).
Minimum detectable difference for total anterior HOAs in mild FECD versus control is 0.15 μm (α=0.05, β=0.20).
Figure 1.
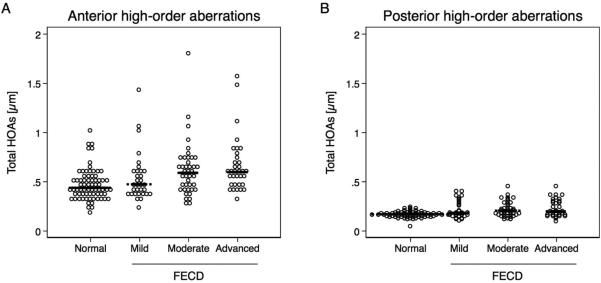
High-order aberrations (HOAs) at the anterior and posterior cornea in Fuchs endothelial corneal dystrophy (FECD, n = 107) and normal (n = 71) measured with Scheimpflug imaging. Posterior corneal HOAs were of much smaller magnitude than anterior corneal HOAs. (A) Anterior HOAs in moderate (p =0.01) and advanced (p =0.01) FECD were increased compared to controls. (B) Posterior HOAs were increased even in mild (p =0.02), moderate (p <0.001), and advanced (p <0.001) FECD compared to controls. Significance was determined with GEE models adjusted for age. Each dot represents one observation; median is indicated with black line.
Posterior corneal total HOAs were increased in mild (p =0.02), moderate (p <0.001), and advanced (p <0.001) FECD compared to controls (Table 2). Posterior coma and coma-like aberrations were higher in mild (p =0.02), moderate (p <0.001), and advanced (p =0.001) FECD compared to controls (Table 2; Figure 1). Similarly, posterior trefoil and trefoil-like aberrations were increased in moderate (p <0.001) and advanced (p <0.001) FECD compared to controls.
Lenticular status was not associated with anterior (p= 0.9) or posterior (p= 0.13) HOAs. Age was associated with anterior HOAs (p= 0.002), and thus all HOA estimates were adjusted for age.
Corneal backscatter
Anterior corneal backscatter was higher in eyes with mild FECD compared to controls (mean difference, 114 SU; 95% CI, 20–209 SU; p =0.02; Table 3, Figure 2). Anterior corneal backscatter was also increased in moderate (121 SU; 95% CI, 44–198 SU; p =0.002) and advanced (262 SU; 95%CI, 157–368 SU; p <0.001) FECD compared to controls (Table 3, Figure 2).
Table 3.
Corneal backscatter in Fuchs endothelial corneal dystrophy (FECD) and controls eyes
| Control | FECD | |||
|---|---|---|---|---|
| Mild | Moderate | Advanced | ||
| Eyes (subjects) | 21 (12) | 25 (13) | 25 (14) | 20 (13) |
| Backscatter [SU] - Anterior cornea | 1360 ± 99 | 1487 ± 165* | 1482 ± 134** | 1665 ± 250*** |
| - Posterior cornea | 670 ± 59 | 857 ± 195** | 849 ± 149*** | 1078 ± 308*** |
| ECDe [cells/mm2] | 2396 ± 298 | 1747 ± 605*** | 925 ± 575*** | 368 ± 298*** |
Backscatter was standardized to a fixed scatter source and expressed in scatter units (SU).
ECDe = Effective endothelial cell density of 100 eyes (17 control, and 31 mild, 34 moderate, and 18 advanced FECD eyes). Data are unadjusted mean ± standard deviations.
p < 0.05
p < 0.01
p < 0.001, versus controls (GEE models).
Figure 2.
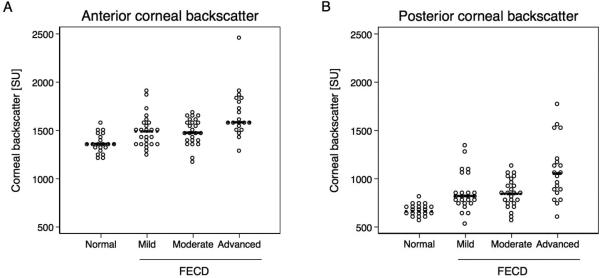
Backscatter of the anterior 120 μm and posterior 60 μm of the cornea measured in Fuchs endothelial corneal dystrophy (FECD) and normal with Scheimpflug imaging. Values are standardized to a fixed scatter source and expressed in scatter units (SU). Significance was determined with GEE models. (A) Anterior corneal backscatter was higher in eyes with mild FECD compared to controls (mean difference, 114 SU; 95% CI, 20–209 SU; p =0.02). Anterior corneal backscatter was also increased in moderate (121 SU; 95% CI, 44–198 SU; p =0.002) and advanced (262 SU; 95% CI, 157–368 SU; p <0.001) FECD compared to controls. (B) Mean posterior corneal backscatter was higher in mild (167 SU; 95% CI 68–266 SU; p =0.001), moderate (201 SU; 95% CI, 124–278 SU; p <0.001), and advanced (336 SU; 95% CI, 190–481 SU; p <0.001) FECD compared to controls. Each dot represents one observation; median is indicated with black line.
Mean posterior corneal backscatter was higher in mild (167 SU; 95% CI, 68–266 SU; p =0.001), moderate (201 SU; 95% CI, 124–278 SU; p <0.001), and advanced (336 SU; 95% CI, 190–481 SU; p <0.001) FECD compared to controls (Table 3, Figure 2).
Lenticular status was not associated with anterior (p= 1.0) or posterior (p= 1.0) backscatter. Age was not associated with anterior (p= 0.7) or posterior (p= 0.3) backscatter.
Corneal thickness
Mean central corneal thickness was 29 μm (95% CI, 15–44 μm; p <0.001) greater in moderate and 44 μm (95% CI, 28–60 μm; p <0.001) greater in advanced FECD compared to controls (Table 1). CCT in mild FECD did not differ from controls (p = 0.09; minimum detectable difference, 20 μm; Table 1).
Effective endothelial cell density
Confocal microscopy images were available for ECDe analysis in 83 eyes with FECD and 17 controls. Mean ECDe was less than ECDe in controls by 660 cells/mm2 (95% CI, 332–989 cells/mm2; p<0.001) in mild FECD, by 1449 cells/mm2 (95% CI, 1164–1733 cells/mm2; p<0.001) in moderate FECD, and by 2027 cells/mm2 (95%, CI 1772–2282 cells/mm2; p<0.001) in advanced FECD.
Relationship between metrics
Anterior HOAs were not correlated with CCT, ECDe, or corneal backscatter. Posterior HOAs were correlated with CCT (r =0.22; p =0.02), ECDe (r =–0.33; p =0.001), anterior corneal backscatter (r =0.39; p =0.001), and posterior corneal backscatter (r =0.52; p<0.001) (Table 4).
Table 4.
Relationships between high-order aberrations (HOAs) and parameters assessing Fuchs endothelial corneal dystrophy (FECD) severity
| Anterior HOAs | Posterior HOAs | Posterior backscatter | Anterior backscatter | |
|---|---|---|---|---|
| CCT | r = 0.18; p = 0.19 | r = 0.22; p = 0.02 | r = 0.44; p = 0.01 | r = 0.38; p = 0.08 |
| ECDe | r = 0.03; p = 0.68 | r = −0.33; p = 0.001 | r = −0.60; p < 0.001 | r = −0.55; p < 0.001 |
| Anterior backscatter | r = 0.01; p = 0.96 | r = 0.39; p = 0.001 | r = 0.72; p <0.001 | - |
| Posterior backscatter | r = 0.13; p = 0.20 | r = 0.52; p < 0.001 | - | - |
Pearson correlations are given for effective endothelial cell density data (ECDe, n = 100), central corneal thickness (CCT, n = 179), backscatter (n = 91), and HOAs (n = 178) with significance determined by GEE models (age-adjusted).
Discussion
Anterior corneal HOAs were increased in moderate and advanced FECD compared to controls, prior to clinically visible corneal edema, and posterior cornea HOAs and corneal backscatter were increased even in mild FECD. Increased posterior corneal HOAs and anterior and posterior corneal backscatter were associated with lower ECDe and thicker corneas in FECD. These data indicate that surface changes occur earlier than previously thought.
Increased anterior corneal HOAs in advanced FECD (with corneal edema) have been shown in previous studies.5,8-10,25,26 In fact, total anterior corneal HOAs through the 6th order in advanced FECD in this study (mean ± standard deviation, 0.66 ± 0.28 μm) were similar to those of a different group of advanced FECD eyes measured by Placido-based topography in our laboratory (0.70 ± 0.20 μm),5 indicating reproducible findings. Increased anterior corneal HOAs in FECD is not simply explained by epithelial edema, because aberrations were increased prior to the onset of clinically detectable edema, and are known to persist after endothelial keratoplasty. It is therefore likely that ultrastructural changes in the anterior cornea contribute to increased anterior aberrations. Such changes include sub-epithelial fibrosis and keratocyte depletion, 11-13,27-29 which occur in the earlier stages of the disease and might affect corneal architecture30 resulting in wavefront errors.
Increased anterior HOAs in moderate and advanced FECD were accompanied by increased HOAs at the posterior surface, including total HOAs, coma, coma-like, trefoil, and trefoil-like aberrations, in mild, moderate, and advanced FECD compared to controls. This might not be surprising in a disease in which guttae create an irregular posterior surface,1,14 but to our knowledge, these aberrations have not been previously reported in non-edematous FECD corneas. Although gross changes in posterior curvature in advanced FECD (corneas with edema), with bulging towards the anterior chamber31 that resolves with de-swelling after endothelial keratoplasty,32 are likely to contribute to posterior surface aberrations, increased posterior corneal HOAs in early stages might also be explained simply by irregularity from guttae.
We previously found that anterior corneal backscatter, measured by confocal microscopy, was increased in moderate and advanced FECD.12 In this study, we confirmed our previous findings by using Scheimpflug imaging, and also found that anterior corneal backscatter was increased in mild FECD compared to controls. We did not see this change in the earlier study, although it may have had insufficient power to detect these subtle changes because of higher variance in backscatter measured by confocal microscopy.12 Scheimpflug imaging did enable us to detect increased posterior corneal backscatter in mild, moderate, and advanced FECD compared to controls; the dominant specular reflection in confocal images limited our sensitivity to detect subtle posterior changes in the mild stages of the disease previously.12 Posterior corneal backscatter has been shown to be increased in advanced FECD when measured with a slit-lamp scatterometer.27
The associations between the optical changes (posterior HOAs and corneal backscatter) and disease severity (ECDe and CCT14,16) can be attributed to increasing area and confluence of guttae. However, we were unable to establish a similar relationship between anterior corneal HOAs and disease severity. Anterior HOAs may take longer to increase in response to a chronic state of corneal edema, or early changes may be more subtle and might not have been detected in this study.
Elevated HOAs from the anterior or poster cornea degrade the retinal image point-spread function and reduce visual acuity.26 Interestingly, the magnitude of posterior HOAs 6 months after Descemet membrane endothelial keratoplasty (0.25 ± 0.08 μm),8 was similar to that in FECD before any surgery (Table 3, Figure 1), suggesting that changes in posterior corneal architecture might persist even after removal of Descemet membrane and guttae and might contribute to incomplete visual rehabilitation. In addition, increased posterior corneal HOAs early in the course of FECD can explain decreased vision even in the absence of clinically detectable corneal edema, contrary to the traditional assumption that guttae are not visually significant.33 The magnitude of anterior corneal HOAs in FECD (Table 2) was similar to that after endothelial keratoplasty (0.65 ± 0.20 μm)5 and after non-wavefront-guided LASIK (0.65 ± 0.18 μm),5,18 indicating the potential for higher than average wavefront errors to be visually significant in FECD. Early anterior corneal abnormalities in FECD are important because they can persist after endothelial keratoplasty and contribute to poor visual outcomes. In the more extreme cases, HOAs from an irregular anterior corneal surface have required rigid contact lens correction after endothelial keratoplasty for FECD.6,7,34 Similarly, increased corneal backscatter (haze) after endothelial keratoplasty for FECD increases intraocular forward light scatter, which degrades the periphery of the point-spread function26 and manifests as disability glare and impaired contrast-sensitivity.6,12,13,27 Because many of these corneal abnormalities persist through at least 3 years after endothelial keratoplasty,5,11,13,25,27 understanding the relationship between their chronicity in FECD and post-operative recovery might offer insight into the ideal time to intervene in the disease, especially if new treatments enable earlier and safe intervention.
In this study we assumed that normal corneas and corneas with FECD had the same refractive index, and this could have affected our estimate of high-order aberrations. The effect of corneal swelling had a small effect on refractive index in animal models,35 but the effect of chronic edema in FECD is unknown.5 Another limitation of our study was that our control subjects were younger than the FECD subjects, despite attempting to recruit controls subjects of similar age. Nevertheless, we adjusted our analyses for the effect of age on HOAs; we did not detect an effect of age on corneal backscatter. The proportion of pseudophakic subjects was higher in mild and moderate FECD compared to controls, and although lenticular status did not appear to confound our results, the study was not powered to compare phakic and pseudophakic eyes. In a previous study from our laboratory, anterior HOAs did not differ in otherwise normal phakic and pseudophakic eyes,5 but it is unknown if the same would be true in eyes with FECD.
Both anterior and posterior corneal aberrations and backscatter are higher than normal in the early stages of FECD. Optical aberrations are known to degrade visual acuity, and the anatomical source of anterior aberrations, which may include subepithelial fibrosis, are likely one of the persisting and major factors for incomplete visual rehabilitation in some eyes after endothelial keratoplasty.36 The source of posterior aberrations is uncertain, and our data are not sufficient to discern whether guttae or more gross abnormalities in posterior curvature are causative. Although we have found that corneal abnormalities begin earlier in the course of FECD than when endothelial keratoplasty is typically considered,12 we cannot use these limited data to extrapolate the ideal timing of surgical intervention. Based on current knowledge of FECD and outcomes of endothelial keratoplasty, we do not advocate surgical intervention early in the course of the disease under the assumption that this will achieve the best visual outcomes. The decision for the timing of surgical intervention needs to consider the functional visual outcome as it relates to the degree of preoperative disease, tempered by data on the rate of disease progression, long-term graft survival, surgical risks and additional patient-specific factors.37,38
Précis.
Anterior corneal high-order aberrations (HOAs) increase early in the course of Fuchs endothelial corneal dystrophy. The early onset of HOAs might contribute to their persistence, and suboptimal visual acuity, after endothelial keratoplasty.
Acknowledgments
Grant information:
Supported by Research to Prevent Blindness, New York, New York (unrestricted grant to the Department of Ophthalmology and support to S.V.P. as Olga Keith Wiess Special Scholar); Dr. Werner Jackstaedt-Stiftung, Wuppertal, Germany (Research Fellowship to K.W.); Mayo Clinic Center for Translational Science Activities (grant no.: UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health, Bethesda, Maryland); and Mayo Foundation, Rochester, Minnesota. The funding organizations had no role in the design or conduct of this research.
Abbreviations and Acronyms
- FECD
Fuchs endothelial corneal dystrophy
- HOA
High-order aberration
- CCT
Central corneal thickness
- RMS
Root mean square
- SU
Scatter units
- GEE
Generalized estimating equation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation:
Presented in part at the annual meetings of the Association for Research in Vision and Ophthalmology, Orlando, Florida, May 6, 2014, and Denver, Colorado, May 3, 2015.
Conflict of Interest:
No conflicting relationship exists for any author.
References
- 1.Wilson SE, Bourne WM. Fuchs' dystrophy. Cornea. 1988;7:2–18. [PubMed] [Google Scholar]
- 2.Eye Bank Association of America 2013 Eye Banking Statistical Report. 2014 http://wwwrestoresightorg/wp-content/uploads/2014/04/2013_Statistical_Report-FINALpdf.
- 3.Li JY, Terry MA, Goshe J, et al. Three-Year Visual Acuity Outcomes after Descemet's Stripping Automated Endothelial Keratoplasty. Ophthalmology. 2012;119:1126–9. doi: 10.1016/j.ophtha.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Calvo-de-Mora M, Quilendrino R, Ham L, et al. Clinical Outcome of 500 Consecutive Cases Undergoing Descemet's Membrane Endothelial Keratoplasty. Ophthalmology. 2015;122:464–70. doi: 10.1016/j.ophtha.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Patel SV, Baratz KH, Maguire LJ, et al. Anterior corneal aberrations after Descemet stripping endothelial keratoplasty for Fuchs endothelial dystrophy. Ophthalmology. 2012;119:1522–9. doi: 10.1016/j.ophtha.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 6.Dapena I, Yeh RY, Baydoun L, et al. Potential causes of incomplete visual rehabilitation at 6 months postoperative after descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2013;156:780–8. doi: 10.1016/j.ajo.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 7.van Dijk K, Parker J, Liarakos VS, et al. Incidence of irregular astigmatism eligible for contact lens fitting after Descemet membrane endothelial keratoplasty. J Cataract Refract Surg. 2013;39:1036–46. doi: 10.1016/j.jcrs.2013.02.051. [DOI] [PubMed] [Google Scholar]
- 8.Rudolph M, Laaser K, Bachmann BO, et al. Corneal Higher-Order Aberrations after Descemet's Membrane Endothelial Keratoplasty. Ophthalmology. 2012;119:528–35. doi: 10.1016/j.ophtha.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 9.Koh S, Maeda N, Nakagawa T, et al. Characteristic Higher-Order Aberrations of the Anterior and Posterior Corneal Surfaces in 3 Corneal Transplantation Techniques. Am J Ophthalmol. 2012;153:284–90. doi: 10.1016/j.ajo.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi T, Negishi K, Yamaguchi K, et al. Effect of anterior and posterior corneal surface irregularity on vision after Descemet-stripping endothelial keratoplasty. J Cataract Refract Surg. 2009;35:688–94. doi: 10.1016/j.jcrs.2008.11.062. [DOI] [PubMed] [Google Scholar]
- 11.Patel SV, McLaren JW. In vivo confocal microscopy of Fuchs endothelial dystrophy before and after endothelial keratoplasty. JAMA Ophthalmol. 2013;131:611–8. doi: 10.1001/jamaophthalmol.2013.799. [DOI] [PubMed] [Google Scholar]
- 12.Amin SR, Baratz KH, McLaren JW, Patel SV. Corneal Abnormalities Early in the Course of Fuchs' Endothelial Dystrophy. Ophthalmology. 2014;121:2325–33. doi: 10.1016/j.ophtha.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baratz KH, McLaren JW, Maguire LJ, Patel SV. Corneal haze determined by confocal microscopy two years after Descemet stripping with endothelial keratoplasty for Fuchs corneal dystrophy. Arch Ophthalmol. 2012;130:868–74. doi: 10.1001/archophthalmol.2012.73. [DOI] [PubMed] [Google Scholar]
- 14.McLaren JW, Bachman LA, Kane KM, Patel SV. Objective assessment of the corneal endothelium in Fuchs' endothelial dystrophy. Invest Ophthalmol Vis Sci. 2014;55:1184–90. doi: 10.1167/iovs.13-13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louttit MD, Kopplin LJ, Igo RP, Jr., et al. A multicenter study to map genes for Fuchs endothelial corneal dystrophy: Baseline characteristics and heritability. Cornea. 2012;31:26–35. doi: 10.1097/ICO.0b013e31821c9b8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Repp DJ, Hodge DO, Baratz KH, et al. Fuchs' endothelial corneal dystrophy. Subjective grading versus objective grading based on the central-to-peripheral thickness ratio. Ophthalmology. 2013;120:687–94. doi: 10.1016/j.ophtha.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorenzetti DW, Uotila MH, Parikh N, Kaufman HE. Central cornea guttata. Incidence in the general population. Am J Ophthalmol. 1967;64:1155–8. [PubMed] [Google Scholar]
- 18.Calvo R, McLaren JW, Hodge DO, et al. Corneal aberrations and visual acuity after laser in situ keratomileusis: femtosecond laser versus mechanical microkeratome. Am J Ophthalmol. 2010;149:785–93. doi: 10.1016/j.ajo.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaren JW, Nau CB, Patel SV, Bourne WM. Measuring corneal thickness with the ConfoScan 4 and Z-ring adapter. Eye Contact Lens. 2007;33:185–90. doi: 10.1097/ICL.0b013e31802b3114. [DOI] [PubMed] [Google Scholar]
- 20.McLaren JW, Bourne WM, Patel SV. Standardization of corneal haze measurement in confocal microscopy. Invest Ophthalmol Vis Sci. 2010;51:5610–6. doi: 10.1167/iovs.10-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hillenaar T, Cals RHH, Eilers PHC, et al. Normative Database for Corneal Backscatter Analysis by In Vivo Confocal Microscopy. Invest Ophthalmol Vis Sci. 2011;52:7274–81. doi: 10.1167/iovs.11-7747. [DOI] [PubMed] [Google Scholar]
- 22.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 23.Guirao A, Redondo M, Artal P. Optical aberrations of the human cornea as a function of age. Journal of the Optical Society of America, A, Optics, Image Science, & Vision. 2000;17:1697–702. doi: 10.1364/josaa.17.001697. [DOI] [PubMed] [Google Scholar]
- 24.Ni Dhubhghaill S, Rozema JJ, Jongenelen S, et al. Normative values for corneal densitometry analysis by scheimpflug optical assessment. Invest Ophthalmol Vis Sci. 2014;55:162–8. doi: 10.1167/iovs.13-13236. [DOI] [PubMed] [Google Scholar]
- 25.McLaren JW, Patel SV, Bourne WM, Baratz KH. Corneal wavefront errors 24 months after deep lamellar endothelial keratoplasty and penetrating keratoplasty. Am J Ophthalmol. 2009;147:959–65. doi: 10.1016/j.ajo.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 26.Seery LS, McLaren JW, Kittleson KM, Patel SV. Retinal point-spread function after corneal transplantation for Fuchs' dystrophy. Invest Ophthalmol Vis Sci. 2011;52:1003–8. doi: 10.1167/iovs.10-5375. [DOI] [PubMed] [Google Scholar]
- 27.Patel SV, Baratz KH, Hodge DO, et al. The effect of corneal light scatter on vision after Descemet stripping with endothelial keratoplasty. Arch Ophthalmol. 2009;127:153–60. doi: 10.1001/archophthalmol.2008.581. [DOI] [PubMed] [Google Scholar]
- 28.Hecker LA, McLaren JW, Bachman LA, Patel SV. Anterior keratocyte depletion in Fuchs endothelial dystrophy. Arch Ophthalmol. 2011;129:555–61. doi: 10.1001/archophthalmol.2010.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahuja Y, Baratz KH, McLaren JW, et al. Decreased corneal sensitivity and abnormal corneal nerves in Fuchs endothelial dystrophy. Cornea. 2012;31:1257–63. doi: 10.1097/ICO.0b013e31823f7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller LJ, Pels E, Vrensen GF. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br J Ophthalmol. 2001;85:437–43. doi: 10.1136/bjo.85.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunette I, Sherknies D, Terry MA, et al. 3-D Characterization of the Corneal Shape in Fuchs Dystrophy and Pseudophakic Keratopathy. Invest Ophthalmol Vis Sci. 2011;52:206–14. doi: 10.1167/iovs.09-4101. [DOI] [PubMed] [Google Scholar]
- 32.Kwon RO, Price MO, Price FW, Jr., et al. Pentacam characterization of corneas with Fuchs dystrophy treated with Descemet membrane endothelial keratoplasty. J Refract Surg. 2010;26:972–9. doi: 10.3928/1081597X-20100212-08. [DOI] [PubMed] [Google Scholar]
- 33.Adamis AP, Filatov V, Tripathi BJ, Tripathi RC. Fuchs' endothelial dystrophy of the cornea. Surv Ophthalmol. 1993;38:149–68. doi: 10.1016/0039-6257(93)90099-s. [DOI] [PubMed] [Google Scholar]
- 34.Baydoun L, van Dijk K, Dapena I, et al. Repeat descemet membrane endothelial keratoplasty after complicated primary descemet membrane endothelial keratoplasty. Ophthalmology. 2015;122:8–16. doi: 10.1016/j.ophtha.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 35.Meek KM, Dennis S, Khan S. Changes in the refractive index of the stroma and its extrafibrillar matrix when the cornea swells. Biophys J. 2003;85:2205–12. doi: 10.1016/s0006-3495(03)74646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Dijk K, Ham L, Tse WH, et al. Near complete visual recovery and refractive stability in modern corneal transplantation: Descemet membrane endothelial keratoplasty (DMEK). Cont Lens Anterior Eye. 2013;36:13–21. doi: 10.1016/j.clae.2012.10.066. [DOI] [PubMed] [Google Scholar]
- 37.Trousdale ER, Hodge DO, Baratz KH, et al. Vision-related quality of life before and after keratoplasty for Fuchs' endothelial dystrophy. Ophthalmology. 2014;121:2147–52. doi: 10.1016/j.ophtha.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 38.Patel SV, Armitage WJ, Claesson M. Keratoplasty outcomes: Are we making advances? Ophthalmology. 2014;121:977–8. doi: 10.1016/j.ophtha.2014.01.029. [DOI] [PubMed] [Google Scholar]


