Abstract
Objective
Despite the potentially life-saving effects of stem cell transplant (SCT), many transplant patients experience traumatic stress reactions due to mortality threat, interpersonal isolation, financial and occupational loss, and invasive medical procedures. Emerging evidence suggests that traumarelated stress symptoms (TSS) predict significant health complications following SCT. The aim of the current prospective study was to examine TSS in the acute aftermath of SCT as a predictor of neutrophil recovery following SCT, a crucial component of immune defense against infection.
Methods
Fifty-one autologous SCT recipients were assessed for TSS 7 days after SCT. Patients’ absolute neutrophil counts were collected from medical charts for the first 30 days following SCT. Hierarchical linear growth modeling was used to test the hypothesis that TSS at day 7 would be associated with delayed recovery of neutrophil counts from days 9 to 30 post SCT, that is, when neutrophil counts began to recover.
Results
As hypothesized, TSS measured 7 days after SCT was significantly associated with slower neutrophil recovery even after pre-existing TSS, depression, distress related to physical symptoms, and potential medical confounds were statistically controlled. Exploratory analyses showed that of the TSS symptom clusters, re-experiencing symptoms and hyperarousal symptoms predicted neutrophil recovery, whereas avoidance symptoms did not.
Conclusion
Though traumatic stress symptoms may be a normative response to SCT, our findings suggest that TSS following SCT may interfere with neutrophil recovery and overall health. These results provide further insight as to potential mechanisms by which traumatic stress translates to poor medical outcomes for SCT patients.
Background
Cancer diagnosis and treatment is often highly stressful [1] and traumatic [2]; this may be particularly true for the treatment of cancers that require stem cell transplants (SCTs). It is estimated that trauma-related stress symptoms (TSS) may affect upwards of 40% of SCT survivors [3]. SCT utilizes chemotherapy and/or radiation therapy to destroy cancerous cells as well as native bone marrow that are then replaced with hematopoietic stem cells from the patient in an autologous transplant [4]. Along with the potential for extending life, the procedure entails significant discomfort and uncertainty. In addition, psychosocial functioning is frequently compromised by extended periods of isolation, occupational and family role changes, and financial strain [5]. Although psychological distress has been shown to predict shorter survival in the years following SCT [6–8], the precise mechanisms by which psychological distress relates to mortality is largely unknown [9]. Given the high rates of TSS in SCT survivors, we sought to explore the impact of TSS on immune reconstitution among SCT patients during the first month of treatment when risk for medical trauma is increased and access to normal coping resources is limited.
Several aspects of SCT and the acute recovery period may increase or exacerbate TSS. Complications ranging from nausea and fatigue to life-threatening infection and organ failure are risks during SCT [8,10]. Patients may also experience mucositis (i.e., severe mouth sores, oral pain), decreased appetite, diarrhea, anemia, and bleeding. Physical symptoms are often most severe at the ‘nadir’ or when blood counts are at their lowest, usually occurring in the first weeks of treatment [11]. This culmination of fear of mortality, uncertainty, and severe physical distress can evoke significant TSS for many patients, which is characterized by re-experiencing of traumatic memories, avoidance, and hyperarousal. Moreover, hospitalization is required for 2–3 weeks following the SCT while blood counts recover and discharge is often dependent on blood count recovery. The fact that hospitalization is both lengthy and occurs for an uncertain period can interfere with patients’ common coping resources and processes, including social support and normal routines that could otherwise be beneficial for regulating distress [5]. During this time, when patients’ coping resources are acutely constrained, they are repeatedly exposed to stressful and aversive medical procedures and related stimuli that may elicit TSS [3,12]. Thus, TSS in the period following SCT may be an important period of vulnerability.
Preliminary evidence suggests that TSS may be a critical predictor of immune recovery in SCT patients. In the early weeks and months following SCT, hematopoietic progenitor cells replace diseased tissues and circulating blood cells. During this critical window, the patient’s immune system has been severely impaired secondary to high-dose chemotherapy; thus, the natural defense against infection is absent or weak. A critical goal for the transplant is the differentiation of hematopoietic progenitor cells to neutrophil cells that form the foundation of innate immunity against infection. The bone marrow is particularly sensitive to activation of the sympathetic nervous system (SNS) [13], and new evidence from animal models indicates that the SNS and catecholamines play critical roles in the regulation of hematopoietic progenitor cells [14,15]. The potential risk to immune system reconstitution may be especially high for SCT recipients experiencing TSS because TSS is associated with stress-related activation of the hypothalamic–pituitary–adrenal axis and SNS, which in turn has been associated with immune dysregulation [16–18] including alterations in cellular immunity [19]. In particular, re-experiencing and hyperarousal are associated with elevated SNS activity and catecholamine secretion [20,21] that could potentially interfere with normal egress of hematopoietic progenitor cells [8,20,21].
Although TSS and depression are both associated with traumatic health circumstances, altered immune function [22–24], and poorer SCT outcomes, they may have independent impacts on immune recovery at the level of stress reactivity and immunological mechanisms. In the context of anxiety disorders, comorbid depressive symptoms may have a dampening effect on emotional and physiological reactivity [25,26]. Moreover, multivariate research has indicated that PTSD symptoms were positively associated with the inflammatory biomarker C-reactive protein, whereas depression was negatively associated with C-reactive protein in the multivariate model [22]. Although TSS as measured by the Impact of Events Scale has been linked to cellular immune function in the context of mass trauma [23], less is known how these processes might occur during SCT when the immune system is recovering. As such, there is a need to analyze the independent associations of TSS with immune recovery during SCT while also accounting for depressive symptoms, but no studies to date have investigated the contribution of TSS independent of depression symptoms to neutrophil engraftment.
The data reported below are part of a longitudinal design examining the impact of stress and coping on immune functioning and health status of individuals undergoing autologous SCT to treat malignancy. It was hypothesized that higher levels of TSS during the acutely stressful and physically demanding phase of the transplant process would be associated with a decelerated rate of neutrophil recovery in the following weeks of treatment. Neutrophil counts are the focus in determining adequate engraftment following bone marrow transplant; this is an internationally recognized benchmark utilized by bone marrow transplant (BMT) centers all over the world, and this data is collected as a basic requirement of reporting outcomes for all BMT patients under the Center for International Blood and Marrow Transplant Research (CIBMTR) and National Marrow Donor Program (NMDP). Specifically, it was expected that TSS measured proximally to the nadir, 7 days after SCT, would be significantly associated with a decelerated rate of neutrophil recovery from days 9 to 30 after SCT. We further hypothesized that TSS near the nadir would be significantly associated with neutrophil recovery over and above pre-existing TSS prior to transplant, depression symptoms during transplant, distress related to acute physical symptoms, and potential medical confounds such as risk status and cancer diagnosis. Finally, we conducted exploratory analyses to examine whether specific TSS symptom clusters would have a stronger association with rates of neutrophil recovery.
Methods
Participants and procedure
The institutional review board of Rush University Medical Center approved this study. Participants for the current study were adults (18 years of age and older) with a cancer diagnosis who were medically evaluated and cleared for SCT. Potential participants were approached upon inpatient admission to the SCT unit where informed consent was obtained. Participants were excluded from the study if they had already undergone a previous SCT, were receiving an additional or tandem transplant, were receiving a donor leukocyte infusion, were not proficient in reading or writing in English, had a pre-existing HIV diagnosis, or were currently taking medication for an active comorbid autoimmune disorder. Eligible participants were given a packet of self-report paper and pencil measures upon admission to the hospital prior to SCT (baseline) and day 7 after SCT. Neutrophil counts and medical variables were collected from participants’ medical charts.
A total of 68 participants who underwent autologous SCT were enrolled in the study. Of these 68 participants, 17 were removed from analyses because of missing data on primary predictors (n =10), withdrawal (n = 2) failure to follow study protocols (n = 2), changes and impairment in mental status (n = 2), and complications from arthritis (n = 1). The retained sample of 51 participants was 54.9% male with a mean age of 55.10 years (standard deviation (SD) = 12.04, range = 26–74) at the time of transplant. Of the participants, 63% identified as Caucasian, 21.6% identified as African American or black, 11.8% identified as Hispanic, 2.0% identified as Native American, and 2.0% identified as other ethnicity. At the time of baseline measurement, 64.7% of participants identified themselves as being married or otherwise partnered, 15.7% identified as being single, 17.6% identified as being divorced, and 2.0% identified as being widowed. Overall, the sample was well educated, with 98.0% of participants completing a high school education and 60.8% of participants having gone on to earn an advanced collegiate or technical degree. Participants of this study were being treated for non-Hodgkin’s lymphoma (n = 22, 43.1%), multiple myeloma (n = 23, 45.1%), and Hodgkin’s lymphoma (n =6, 11.8%).
Measures
Demographics and health and behavior questionnaire
A survey administered prior to transplant collected demographic information including gender; marital status; number of children under age 18 years; employment status; education; ethnic/racial identification; household income; current medications for pain, sleep, depression, or anxiety; and current cigarette/nicotine, alcohol, recreational drug, and caffeine use.
Medical covariates
Aspects of the medical status known to affect neutrophil recovery were collected from the participants’ medical records. These variables were treated as covariates and included cancer diagnosis, the liquid volume of the SCT dose, cytomegalovirus (CMV) status prior to transplant, CMV status following transplant, and physician-rated level of risk coded as low, medium, or high.
The Impact of Events Scale—Revised
The Impact of Events Scale—Revised (IES-R) is a 22-item measure of psychological distress in response to traumatic stressors [27]. This scale is designed to capture DSM-IV PTSD symptoms and can be broken into three symptom clusters: re-experiencing, hyperarousal, and avoidance. Participants were asked to indicate the degree to which they experienced symptoms in response to their illness and SCT on a five-point Likert scale (0 = not at all; 4 = extremely). Sample items include the following: ‘Pictures about it popped into my mind’, ‘I had dreams about it’, and ‘Any reminder brought back feelings about it’. The measure has been shown to demonstrate concurrent and discriminate validity [28]. In the current study, the measure demonstrated excellent internal consistency for the overall scale at baseline (α = 0.95) and day 7 after SCT (α = 0.95). Subscales were internally consistent at baseline and day 7 after SCT for re-experiencing (α = 0.91, 0.91), avoidance, (α = 0.85, 0.87), and hyperarousal scales (α = 0.87, 0.90).
The Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression Scale (HADS) is a 14-item measure of the presence and severity of common symptoms of anxiety and depression [29]. This measure is frequently used in medical populations, as it avoids asking about somatic symptoms of anxiety and depression that may be directly associated with illness or treatment. Validation studies support the internal consistency, factor structure, and validity of the measure [30]. The seven-item depression scale was used as a predictor in study analyses. The depression scale was internally consistent in the current sample at baseline (α = 0.68) and day 7 after SCT (α = 0.85).
Cancer Treatment and Distress Scale
The Cancer Treatment and Distress Scale (CTXD) is 28-item measure of cancer-related distress [31]. Patients rate the extent they experienced distress using a four-point Likert scale (0 = none to 3 = severe). Items included the following: ‘Getting through chemotherapy, radiation therapy or other treatments’, ‘Difficulty eating or tasting food’, ‘Mouth or throat sores’, ‘Procedures I have to go through’, and ‘Nausea and vomiting’. For the current analysis, the acute symptoms subscale was used to adjust models for distress related to acute cancer symptoms that may be confounded with TSS. The scale was internally consistent at day 7 after SCT (α =0.88).
Neutrophil count
Patients’ absolute neutrophil counts were collected from patients’ medical charts for the first 30 days following SCT. Normal neutrophil counts range from 1500 to 8000 cells/μL [32].
Analyses
All analyses were conducted in SPSS version 20 [33]. Descriptive statistics were computed to characterize the sample and variables of interest. Given the number of individuals excluded because of missing data, we calculated Little’s MCAR [34] to establish the extent to which data were missing completely at random. This analysis was non-significant χ2(3,63)=1.91, p > 0.05, suggesting that the data met criteria for missing completely at random and that missingness would not bias the estimates of study analyses. Following the example of McGregor and colleagues [17], mixed modeling with fixed and random effects for intercept and slope was used to model growth trajectories of patients’ absolute neutrophil counts between days 9 and 30. This window was selected because day 9 represented the start of neutophil recovery after transplant for this study population. In total, 492 neutrophil counts were obtained from the 51 identified participants. Missing data were estimated with maximum likelihood estimation procedures. As with McGregor and colleagues [17], we rejected the use of an unstructured covariance matrix as this reduces statistical power. Because neutrophil levels tended to rise and then stabilize following a hyperbolic function, a log transformation was applied to the time variable to more accurately model the nonlinear shape of neutrophil recovery [35]. When plotted in mathematical space, the log transformation has the function of modeling the nonlinear neutrophil recovery over time. In this nonlinear function, the rate of neutrophil recovery decelerates over time. Refer to figure 1 for an illustration of the predicted growth curve models for one SD above and below the mean. TSS scores were centered at the grand mean and entered as a predictor of initial neutrophil count and slope, as was depression. Covariates included gender, filgrastim treatment, stem cell dose, physician rated risk, and CMV status prior to and following transplant.
Figure 1.
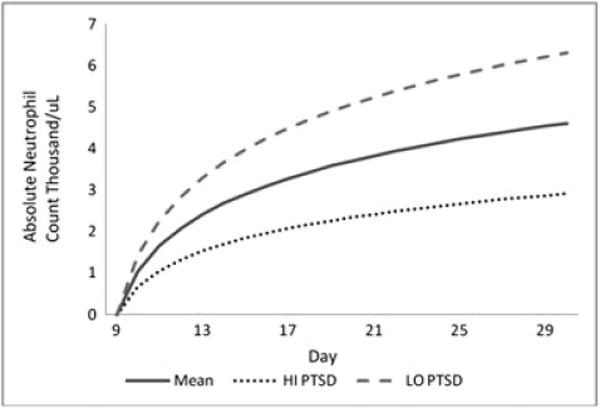
Neutrophil recovery days 9 to 30 post transplant. Mean–mean day 7 IES-R score; HI PTSD–one SD above mean day 7 IES-R score; LO PTSD–one SD below mean day 7 IES-R score
Results
Overall, participants reported significant distress before and after SCT. The mean score on the IES-R scale at baseline was 16.74 (SD = 15.18) and 20.53 (SD = 18.22) at day 7 after SCT. On the basis of a suggested clinical cutoff of 33, approximately 13% of the sample screened positive for significant TSS at baseline, and this frequency increased to 17% by day 7 after SCT [36]. The mean score on the HADS depression scale was 3.42 (SD = 2.53) at baseline and was 7.43 (SD = 4.73) at day 7 after SCT. On the basis of a suggested clinical cutoff of 8 for clinical depression [37], 4% screened positive for significant depressive symptoms at baseline with approximately 42% reporting significant depressive symptoms at day 7 after SCT.
Unconditional growth model
Two unconditional models were used to evaluate the overall pattern of neutrophil reconstitution. We began with a linear model, but visual inspection of the data suggested a hyperbolic smoothing of neutrophil recovery and a nonlinear model with the time variable log transformed. The log transformed model provided better fit to the data (linear model 1 AIC = 2891.767 versus nonlinear model 2 AIC = 2836.510) and was retained for conditional analyses.
Conditional growth models
Conditional models were used to evaluate the relationship between TSS scores and the rate of neutrophil recovery. We investigated the associations between TSS at day 7 after SCT and neutrophil recovery. In the first conditional model, TSS scores assessed at day 7 after SCT were unrelated to initial neutrophil status (B = 0.01 SE = 0.02, p = 0.79) and tended to be associated with a shallower slope of neutrophil recovery (B = −0.02 SE = 0.01, p = 0.05). The second conditional model included the medical covariates described earlier, distress related to acute symptoms, depression symptoms, and TSS prior to SCT (Table 1). In this fully adjusted model, TSS at day 7 was not significantly related to initial neutrophil status (B = 0.02 SE = 0.03, p = 0.48) but was significantly associated with a shallower slope of neutrophil recovery (B = −0.03 SE = 0.01, p = 0.03). Finally, this model was recomputed with non-significant covariates removed to conserve power; TSS at day 7 remained a significant predictor of neutrophil recovery with a similar sized effect. Figure 1 depicts the neutrophil status at the mean TSS score at day 7 after SCT, and one SD above and below the mean. Simple slope analyses indicated that the slope of neutrophil recovery was approximately twice the rate among individuals with PTSD symptoms one SD below the mean (B = 1.94 S = 0.28, p < 0.001), compared with individuals with PTSD symptoms one SD below the mean (B = 1.02 SE = 0.28, p < 0.001). TSS were negatively associated with estimated neutrophil counts at day +30 (B = −0.09 SE = 0.03, p < 0.01).
Table 1.
Conditional growth curve model with TSS predictor and covariates
| Parameter | Estimate | SE | T | Wald Z | Significance | 95% confidence interval
|
|
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| Intercept | 2.43 | 1.22 | 1.99 | 0.06 | −0.06 | 4.92 | |
| IES-R +7 | 0.02 | 0.03 | 0.71 | 0.48 | −0.03 | 0.07 | |
| HADS +7 | −0.17 | 0.10 | −1.77 | 0.08 | −0.36 | 0.02 | |
| Slope | 1.48 | 0.18 | 8.08 | <0.01 | 1.12 | 1.85 | |
| IES-R +7 slope | −0.03 | 0.01 | −2.19 | 0.03 | −0.05 | −0.00 | |
| HADS +7 slope | 0.06 | 0.05 | 1.33 | 0.19 | −0.03 | 0.16 | |
| CTXD +7 | 0.08 | 0.07 | 1.04 | 0.30 | −0.07 | 0.22 | |
| IES-R baseline | 0.01 | 0.02 | 0.45 | 0.67 | −0.04 | 0.06 | |
| Graft volume | −0.00 | 0.00 | −0.15 | 0.88 | −0.00 | 0.00 | |
| Filgrastim | −0.97 | 1.63 | −0.60 | 0.55 | −4.31 | 2.36 | |
| MM | −1.91 | 0.98 | −1.96 | 0.06 | −3.89 | 0.07 | |
| NHL | −0.60 | 0.85 | −0.70 | 0.49 | −2.33 | 1.13 | |
| Low risk | −0.47 | 0.58 | −0.80 | 0.43 | −1.65 | 0.72 | |
| Med risk | −0.41 | 0.58 | −0.71 | 0.25 | −1.58 | 0.76 | |
| CMV pre | −1.18 | 1.02 | −1.16 | 0.25 | −3.23 | 0.87 | |
| CMV post | −0.47 | 0.59 | −0.80 | 0.43 | −1.66 | 0.73 | |
| Gender | 0.40 | 0.46 | 0.86 | 0.40 | −0.54 | 1.33 | |
| Variances | |||||||
| Residual | 6.57 | 0.46 | 14.41 | 0.00 | 5.74 | 7.53 | |
| Intercept | 0.19 | 0.67 | 0.28 | 0.78 | 0.00 | 191.60 | |
| Time | 0.60 | 0.26 | 2.29 | 0.02 | 0.26 | 1.42 | |
IES-R +7, day 7 Impact of Events Scale—Revised; HADS +7, day 7 Hospital Anxiety and Depression Scale; IES-R +7 slope, deviation from overall slope for each unit increase in day 7 IES-R; HADS +7 Slope, deviation from overall slope for each unit increase in day 7 HADS; CTXD +7, day 7 Cancer Treatment and Distress Scale; IES-R Baseline, day of admission Impact of Events Scale—Revised; MM, multiple myeloma; NHL, non-Hodgkins lymphoma; Low risk, physician-rated level of risk coded as low; Med risk, physician-rated level of risk coded as medium; CMV pre, cytomegalovirus positive prior to transplant; CMV post, cytomegalovirus positive following transplant.
We then repeated the conditional growth models using the IES-R subscales (re-experiencing, avoidance, and hyperarousal) to evaluate whether specific traumatic stress symptoms drive the association between TSS and neutophil recovery. Re-experiencing symptoms were unrelated to initial neutrophil status (B = 0.02 SE = 0.05, p = 0.69) but were significantly associated with a shallower slope of neutrophil recovery (B = −0.05 SE = 0.03, p = 0.04). Similarly, hyperarousal symptoms were unrelated to initial neutrophil status (B = 0.01 SE = 0.06, p = 0.85) but were significantly associated with a shallower slope of neutrophil recovery (B = −0.06 SE = 0.03, p = 0.04). By contrast, avoidance symptoms were not related to initial neutrophil status (B = 0.01 SE = 0.05, p = 0.89) or slope of neutrophil recovery (B = −0.04 SE = 0.03, p = 0.16). Notably, depression and the interaction of depression with time were not significant predictors of neutrophil count in any of the models. A diagnosis of multiple myeloma was the only other significant predictor of neutrophil counts.
Discussion
To our knowledge, the current study is the first to document an independent relationship between TSS and rate of neutrophil recovery following autologous SCT. Participants undergoing autologous SCT reported significant levels of distress during the week following transplant, with approximately one in six experiencing clinically significant TSS. Consistent with our primary hypothesis, TSS measured 7 days after transplant were associated with a slower rate of recovery in the neutrophil cell line 9 to 30 days after transplant. This relationship between TSS at day 7 and subsequent neutrophil recovery held after accounting for TSS prior to transplant, depression, distress related to physical symptoms, and potential medical confounds including diagnosis, risk status, and chemotherapeutic regimens. Adjustment for distress related to cancer symptoms at day 7 provides evidence that the association between TSS and the slope of neutrophil engraftment is not explained by uncomfortable symptoms at the low point of treatment. Moreover, adjustment for preexisting TSS prior to SCT suggests that TSS during the active phase of treatment may be the more potent predictor of neutrophil recovery. To illustrate, individuals with TSS falling one SD above the mean had rates of neutrophil recovery that were approximately half compared with individuals with TSS falling one SD below the mean.
Our findings extend the recent work of McGregor and colleagues [17] and Knight and colleagues [38]. Whereas McGregor documented an association between a composite measure of psychological distress and white blood cell recovery and Knight documented an association between anxiety and neutrophil status, our study more specifically isolated a relationship between TSS and neutrophil recovery. Exploratory analysis revealed that symptoms of re-experiencing and hyperarousal significantly predicted slower neutrophil recovery. In contrast, avoidance symptoms were not significantly related to neutrophil recovery, lending further support to the hypothesis that it is the unique aspects of reliving trauma and hyperarousal that compromise neutrophil status following SCT. This finding is consistent with the hypothesis that symptoms associated with SNS arousal predict slower immune recovery. The nonsignificant relationship between avoidance and neutrophil recovery could be attributed to several possibilities including an inability to avoid trauma reminders while at the hospital.
The results of this analysis warrant further investigation of the biochemical mechanisms by which TSS impact neutrophil recovery and related medical outcomes in SCT patients. Moreover, future studies should examine the specific forms of psychological distress and symptoms that are most closely linked to immune recovery. For instance, plasma norepinephrine levels were found to be higher among war veterans with PTSD alone compared with veterans with PTSD and depression [39]. The unique symptoms of re-experiencing and hyperasoual are avenues for further exploration as these symptoms are particularly associated elevated SNS activity and hypersecretion of catecholamines [21]. Researchers should explore the possibility of measuring norepinephrine and other lymphoid organ neurotransmitters to determine whether alterations in these chemicals mediate the relationship between TSS and impaired neutrophil reconstitution.
Strengths and limitations
Strengths of the current study include the longitudinal assessment of neutrophil recovery over a 21-day period, and the use of validated measures of TSS and depression symptoms. In total, 492 neutrophil measurements were analyzed with advanced hierarchical growth modeling techniques, and potential confounders including preexisting TSS, depression symptoms, disease type, medical risk, and distress related to acute symptoms were addressed in the final model. The limited period of the study limits interpretations of whether our measure captures PTSD or acute stress reactions, and more research is needed to clarify whether the time course of TSS relates to more distal transplant outcomes such as cardiovascular complications, diabetes, cancer recurrence, and mortality. It is also unclear how these results may generalize to other oncology samples and whether the longitudinal relationship between TSS and subsequent neutrophil recovery is causal in nature or related to confounding variables unmeasured in the current analysis. Finally, although neutrophil recovery is the primary benchmark for evaluating the acute effectiveness of SCT, it is just one indicator of immune reconstitution and other markers such as total white blood cell count, natural killer cells, monocytes, and inflammatory markers should be considered in future research [8,16].
Implications
Results of the current study have important implications for psycho-oncology services for patients undergoing SCT. Although limited, much of psycho-oncology literature has emphasized the role of depression in transplant recovery and survival. More research is needed to determine the specific aspects of the SCT process that may exacerbate or evoke new symptoms of TSS. Our results suggest a more targeted investigation of prevention and intervention efforts aimed at the reduction of traumatic stress utilizing principles of trauma informed care [12]. Traumatic stress may be a fairly normative response to SCT; however, these symptoms, and intrusive re-experiencing and hyperarasoual in particular, may interfere with neutrophil recovery and overall health. Although evidence-based treatments are available for the treatment of traumatic stress, these treatments are often contraindicated during the initial period following SCT because cognitive resources required to engage in traditional psychotherapy may be limited by medical procedures, medication, and fatigue. A promising alternative strategy would instead activate and preserve coping resources in the individual, family, and medical team [12] as part of a comprehensive multi-level intervention [40]. Such an intervention should focus on the regulation of acute TSS directly in the medical context of the SCT unit.
Acknowledgments
This research was supported in part by the Charles and Margaret Roberts Fund. Additional support was provided by the Rush Center for Urban Health Equity (NIH-NHLBI 1P50HL105189). Alyson Zalta receives grant support from NIH (K23 MH103394).
The authors would like to thank Catalina Vechiu and Samantha Chesney for their contributions in data collection.
Footnotes
Conflict of Interest
All authors declare that there are no conflicts of interest.
References
- 1.Stanton AL. Psychosocial concerns and interventions for cancer survivors. J Clin Oncol. 2006;24(32):5132–5137. doi: 10.1200/JCO.2006.06.8775. [DOI] [PubMed] [Google Scholar]
- 2.Varela VS, Ng A, Mauch P, Recklitis CJ. Posttraumatic stress disorder (PTSD) in survivors of Hodgkin’s lymphoma: prevalence of PTSD and partial PTSD compared with sibling controls. Psycho-Oncology. 2013;22(2):434–440. doi: 10.1002/pon.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DuHamel KN, Mosher CE, Winkel G, et al. Randomized clinical trial of telephone-administered cognitive–behavioral therapy to reduce post-traumatic stress disorder and distress symptoms after hematopoietic stem-cell transplantation. J Clin Oncol. 2010;28(23):3754–3761. doi: 10.1200/JCO.2009.26.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vose JM. Hematopoietic stem cell transplantation. In: Goldman L, Schafer AI, editors. Goldman’s Cecil Medicine. Saunders Elsevier; Philadelphia: 2011. Chap 181. [Google Scholar]
- 5.Fife BL, Huster GA, Cornetta KG, Kennedy VN, Akard LP, Broun ER. Longitudinal study of adaptation to the stress of bone marrow transplantation. J Clin Oncol. 2000;18(7):1539–1549. doi: 10.1200/JCO.2000.18.7.1539. [DOI] [PubMed] [Google Scholar]
- 6.Molassiotis A, Van den Akker OBA, Milligan DW, Goldman JM. Symptom distress, coping style and biological variables as predictors of survival after bone marrow transplantation. J Psychosom Res. 1997;42:275–285. doi: 10.1016/S0022-3999(96)00298-X. [DOI] [PubMed] [Google Scholar]
- 7.Hoodin F, Uberti JP, Lynch TJ, Steele P, Ratanatharathorn V. Do negative or positive emotions differentially impact mortality after adult stem cell transplant? Bone Marrow Transplant. 2006;38(4):255–264. doi: 10.1038/sj.bmt.1705419. [DOI] [PubMed] [Google Scholar]
- 8.Costanzo ES, Juckett MB, Coe CL. Biobehavioral influences on recovery following hematopoietic stem cell transplantation. Brain Behav Immun. 2013;30(Suppl):S68–S74. doi: 10.1016/j.bbi.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel SD. Psychosocial considerations in hematopoietic stem cell transplantation: implications for patient quality of life and posttransplant survival. Community Oncol. 2008;5:407–411. doi: 10.1016/S1548-5315(11)70475-2. [DOI] [Google Scholar]
- 10.Jantunen E, Itälä M, Lehtinen T, et al. Early treatment-related mortality in adult autologous stem cell transplant recipients: a nationwide survey of 1482 transplanted patients. Eur J Haematol. 2006;76(3):245–250. doi: 10.1111/j.1600-0609.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- 11.Anderson KO, Giralt SA, Mendoza TR, et al. Symptom burden in patients undergoing autologous stem-cell transplantation. Bone Marrow Transplant. 2007;39(12):759–766. doi: 10.1038/sj.bmt.1705664. [DOI] [PubMed] [Google Scholar]
- 12.Hobfoll SE, Stevens N, Gerhart JI, et al. Traumatic stress, health, and strategies for multilevel bio-psycho-social interventions. In: Moore K, Kaniasty K, Buchwald P, editors. Stress and Anxiety. Logos Verlag; Berlin, Germany: 2013. [Google Scholar]
- 13.Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med. 2011;208(3):421–428. doi: 10.1084/jem.20110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellinger DL, Millar BA, Perez S, et al. Innervation of lymphoid organs: clinical implications. Clin Neurosci Res. 2006;6(1–2):3–33. doi: 10.1016/j.cnr.2006.04.003. [DOI] [Google Scholar]
- 15.Récalde A, Richart A, Guérin C, et al. Sympathetic nervous system regulates bone marrowderived cell egress through endothelial nitric oxide synthase activation: role in postischemic tissue remodeling. Arterioscler Thromb Vasc Biol. 2012;32:643–665. doi: 10.1161/ATVBAHA.111.244392. [DOI] [PubMed] [Google Scholar]
- 16.Coe CL. All roads lead to psychoneuroimmunology. In: Suls JM, Davidson KW, Kaplan RM, editors. Handbook of Health Psychology and Behavioral Medicine. The Guilford Press; New York: 2010. pp. 182–199. [Google Scholar]
- 17.McGregor BA, Syrjala KL, Dolan ED, Langer SL, Redman M. The effect of pre-transplant distress on immune reconstitution among adult autologous hematopoietic cell transplantation patients. Brain Behav Immun. 2013;30(Suppl):S142–S148. doi: 10.1016/j.bbi.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill JM, Saligan L, Woods S, Page G. PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care. 2009;45(4):262–277. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- 19.Kawamura N, Kim Y, Asukai N. Suppression of cellular immunity in men with a past history of posttraumatic stress disorder. Am J Psychiatr. 2001;158(3):484–486. doi: 10.1176/appi.ajp.158.3.484. [DOI] [PubMed] [Google Scholar]
- 20.Bowirrat A, Chen TJ, Blum K, et al. Neuro-psychopharmacogenetics and neurological antecedents of posttraumatic stress disorder: unlocking the mysteries of resilience and vulnerability. Curr Neuropharmacol. 2010;8(4):335–358. doi: 10.2174/157015910793358123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris MC, Rao U. Psychobiology of PTSD in the acute aftermath of trauma: integrating research on coping, HPA function and sympathetic nervous system activity. Asian J Psychiatr. 2012;6(1):3–21. doi: 10.1016/j.ajp.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heath NM, Chesney SA, Gerhart JI, et al. Interpersonal violence, PTSD, and inflammation: potential psychogenic pathways to higher C-reactive protein levels. Cytokine. 2013;63:172–178. doi: 10.1016/j.cyto.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ironson G, Wynings C, Schneiderman N, et al. Posttraumatic stress symptoms, intrusive thoughts, loss, and immune function after Hurricane Andrew. Psychosom Med. 1997;59(2):128–141. doi: 10.1097/00006842-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res. 2002;53(4):873–876. doi: 10.1016/S0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 25.Taylor-Clift A, Morris BH, Rottenberg J, Kovacs M. Emotion-modulated startle in anxiety disorders is blunted by co-morbid depressive episodes. Psychol Med. 2011;41(1):129–139. doi: 10.1017/s003329171000036x. [DOI] [PubMed] [Google Scholar]
- 26.Yoon KL, Joormann J. Stress reactivity in social anxiety disorder with and without comorbid depression. J Abnorm Psychol. 2012;121(1):250–255. doi: 10.1037/a0025079. [DOI] [PubMed] [Google Scholar]
- 27.Weiss DS, Marmar CR. The Impact of Event Scale—Revised. In: Wilson JP, Keane TM, editors. Assessing Psychological Trauma and PTSD. Guilford Press; New York: 1997. pp. 399–411. [Google Scholar]
- 28.Beck JG, Grant DM, Read JP, et al. The Impact of Event Scale—Revised: psychometric properties in a sample of motor vehicle accident survivors. J Anxiety Disord. 2008;22:187–198. doi: 10.1016/j.janxdis.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 30.Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, Van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27(2):363–370. doi: 10.1017/S0033291796004382. [DOI] [PubMed] [Google Scholar]
- 31.Syrjala KL, Langer SL, Abrams JR, et al. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA. 2004;291(19):2335–2343. doi: 10.1001/jama.291.19.2335. [DOI] [PubMed] [Google Scholar]
- 32.Wile MJ, Homer LD, Gaehler S, Phillips S, Millan J. Manual differential cell counts help predict bacterial infection: a multivariate analysis. Am J Clin Pathol. 2001;115(5):644–649. doi: 10.1309/j905-ckyw-4g7p-kuk8. [DOI] [PubMed] [Google Scholar]
- 33.IBM Corp. IBM SPSS Statistics for Windows, Version 20.0. IBM Corp; Armonk, NY: 2011. [Google Scholar]
- 34.Little RJA. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc. 1998;83:1198–1202. doi: 10.2307/2290157. [DOI] [Google Scholar]
- 35.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford University Press; USA: 2003. [Google Scholar]
- 36.Creamer M, Bell R, Failla S. Psychometric properties of the Impact of Event Scale—Revised. Behav Res Ther. 2003;41:1489–1496. doi: 10.1016/j.brat.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/S0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 38.Knight JM, Moynihan JA, Lyness JM. Peritransplant psychosocial factors and neutrophil recovery following hematopoietic stem cell transplantation. PLoS. 2014;9(6):1–6. doi: 10.1371/journal.pone.0099778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yehuda R, Siever LJ, Teicher MH, et al. Plasma norepinephrine and 3-methoxy-4-hydroxyphenylglycol concentrations and severity of depression in combat posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 1998;44(1):56–63. doi: 10.1016/S0006-3223(98)80007-3. [DOI] [PubMed] [Google Scholar]
- 40.Ellis RA. Filling the prevention gap: multifactor, multi-system, multi-level intervention. J Prim Prev. 1998;19:57–71. doi: 10.1023/A:1022617425365. [DOI] [Google Scholar]


