Abstract
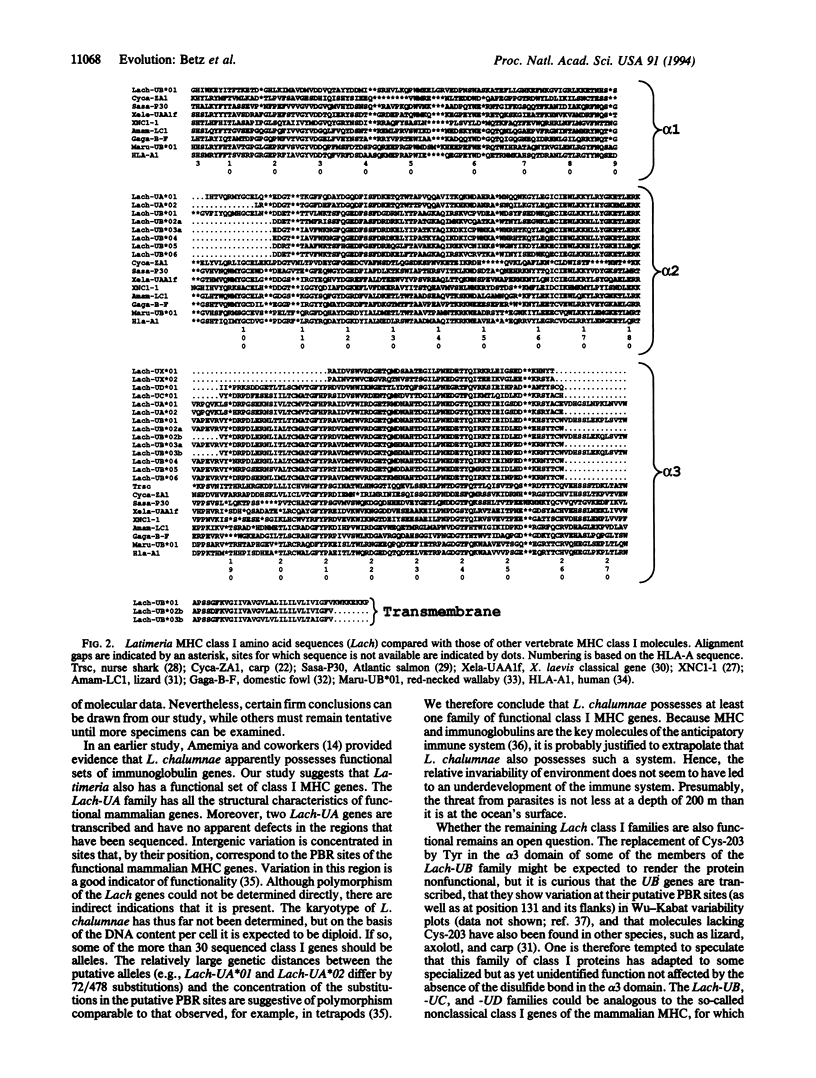
The coelacanth fish Latimeria chalumnae is the sole surviving species of a phylogenetic lineage that was founded more than 400 million years ago and that has changed morphologically very little since that time. Little is known about the molecular evolution of this "living fossil," considered by some taxonomists to be the closest living relative of tetrapods. Here we describe the isolation and characterization of L. chalumnae major histocompatibility complex (MHC) class I genes. The exon-intron organization of these genes is the same as that of their mammalian counterparts. The genes fall into four families, which we designate Lach-UA through Lach-UD. There are multiple loci in all of the families. Genes of the first two families are transcribed. The Lach-UA family bears the characteristics of functional, polymorphic class I genes; the other three families may be represented by nonclassical genes. All the Lach loci arose by duplication from an ancestral gene after the foundation of the coelacanth lineage. Intergenic variation is highest at positions corresponding to the mammalian peptide-binding region. The closest relatives of the Lach genes among the MHC genes sequenced thus far are those of the amphibian Xenopus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amemiya C. T., Ohta Y., Litman R. T., Rast J. P., Haire R. N., Litman G. W. VH gene organization in a relict species, the coelacanth Latimeria chalumnae: evolutionary implications. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6661–6665. doi: 10.1073/pnas.90.14.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly J. M., Hansen T. H., Ingold A. L., Potter T. A. Recognition by CD8 on cytotoxic T lymphocytes is ablated by several substitutions in the class I alpha 3 domain: CD8 and the T-cell receptor recognize the same class I molecule. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2137–2141. doi: 10.1073/pnas.87.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis P. D., Jackson A. P., Parham P. Molecular cloning of bovine class I MHC cDNA. J Immunol. 1988 Jul 15;141(2):642–651. [PubMed] [Google Scholar]
- Flajnik M. F., Kasahara M., Shum B. P., Salter-Cid L., Taylor E., Du Pasquier L. A novel type of class I gene organization in vertebrates: a large family of non-MHC-linked class I genes is expressed at the RNA level in the amphibian Xenopus. EMBO J. 1993 Nov;12(11):4385–4396. doi: 10.1002/j.1460-2075.1993.tb06123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorr T., Kleinschmidt T., Fricke H. Close tetrapod relationships of the coelacanth Latimeria indicated by haemoglobin sequences. Nature. 1991 May 30;351(6325):394–397. doi: 10.1038/351394a0. [DOI] [PubMed] [Google Scholar]
- Grimholt U., Hordvik I., Fosse V. M., Olsaker I., Endresen C., Lie O. Molecular cloning of major histocompatibility complex class I cDNAs from Atlantic salmon (Salmo salar). Immunogenetics. 1993;37(6):469–473. doi: 10.1007/BF00222473. [DOI] [PubMed] [Google Scholar]
- Grossberger D., Parham P. Reptilian class I major histocompatibility complex genes reveal conserved elements in class I structure. Immunogenetics. 1992;36(3):166–174. doi: 10.1007/BF00661093. [DOI] [PubMed] [Google Scholar]
- Guillemot F., Billault A., Pourquié O., Béhar G., Chaussé A. M., Zoorob R., Kreibich G., Auffray C. A molecular map of the chicken major histocompatibility complex: the class II beta genes are closely linked to the class I genes and the nucleolar organizer. EMBO J. 1988 Sep;7(9):2775–2785. doi: 10.1002/j.1460-2075.1988.tb03132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Nakanishi T., Kurosawa Y. Identification of a shark sequence resembling the major histocompatibility complex class I alpha 3 domain. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2209–2212. doi: 10.1073/pnas.89.6.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges S. B., Hass C. A., Maxson L. R. Relations of fish and tetrapods. Nature. 1993 Jun 10;363(6429):501–502. doi: 10.1038/363501b0. [DOI] [PubMed] [Google Scholar]
- Horton R. M., Hildebrand W. H., Martinko J. M., Pease L. R. Structural analysis of H-2Kf and H-2Kfm1 by using H-2K locus-specific sequences. J Immunol. 1990 Sep 15;145(6):1782–1787. [PubMed] [Google Scholar]
- Jauregui-Adell J., Pechere J. F. Parvalbumins from coelacanth muscle. III. Amino acid sequence of the major component. Biochim Biophys Acta. 1978 Sep 26;536(1):275–282. doi: 10.1016/0005-2795(78)90074-0. [DOI] [PubMed] [Google Scholar]
- Klein J. Are invertebrates capable of anticipatory immune responses? Scand J Immunol. 1989 May;29(5):499–505. doi: 10.1111/j.1365-3083.1989.tb01152.x. [DOI] [PubMed] [Google Scholar]
- Klein J., Bontrop R. E., Dawkins R. L., Erlich H. A., Gyllensten U. B., Heise E. R., Jones P. P., Parham P., Wakeland E. K., Watkins D. I. Nomenclature for the major histocompatibility complexes of different species: a proposal. Immunogenetics. 1990;31(4):217–219. doi: 10.1007/BF00204890. [DOI] [PubMed] [Google Scholar]
- Klein J., O'hUigin C. The conundrum of nonclassical major histocompatibility complex genes. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6251–6252. doi: 10.1073/pnas.91.14.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J., Satta Y., O'hUigin C., Takahata N. The molecular descent of the major histocompatibility complex. Annu Rev Immunol. 1993;11:269–295. doi: 10.1146/annurev.iy.11.040193.001413. [DOI] [PubMed] [Google Scholar]
- Kolb E., Harris J. I., Bridgen J. Triose phosphate isomerase from the coelacanth. An approach to the rapid determination of an amino acid sequence with small amounts of material. Biochem J. 1974 Feb;137(2):185–197. doi: 10.1042/bj1370185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden D. R., Gorga J. C., Strominger J. L., Wiley D. C. The structure of HLA-B27 reveals nonamer self-peptides bound in an extended conformation. Nature. 1991 Sep 26;353(6342):321–325. doi: 10.1038/353321a0. [DOI] [PubMed] [Google Scholar]
- Marshall C., Schultze H. P. Relative importance of molecular, neontological, and paleontological data in understanding the biology of the vertebrate invasion of land. J Mol Evol. 1992 Aug;35(2):93–101. doi: 10.1007/BF00183220. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Fremont D. H., Peterson P. A., Wilson I. A. Emerging principles for the recognition of peptide antigens by MHC class I molecules. Science. 1992 Aug 14;257(5072):927–934. doi: 10.1126/science.1323878. [DOI] [PubMed] [Google Scholar]
- Mayer W. E., Williams N. S., O'hUigin C., Vincek V., Zaleska-Rutczynska Z., Klein J. Class I major histocompatibility complex genes of the red-necked Wallaby, Macropus rufogriseus. Mol Phylogenet Evol. 1993 Mar;2(1):23–30. doi: 10.1006/mpev.1993.1004. [DOI] [PubMed] [Google Scholar]
- Meyer A., Wilson A. C. Origin of tetrapods inferred from their mitochondrial DNA affiliation to lungfish. J Mol Evol. 1990 Nov;31(5):359–364. doi: 10.1007/BF02106050. [DOI] [PubMed] [Google Scholar]
- Okamura K., Nakanishi T., Kurosawa Y., Hashimoto K. Expansion of genes that encode MHC class I molecules in cyprinid fishes. J Immunol. 1993 Jul 1;151(1):188–200. [PubMed] [Google Scholar]
- Ono H., Klein D., Vincek V., Figueroa F., O'hUigin C., Tichy H., Klein J. Major histocompatibility complex class II genes of zebrafish. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11886–11890. doi: 10.1073/pnas.89.24.11886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P., Lawlor D. A., Lomen C. E., Ennis P. D. Diversity and diversification of HLA-A,B,C alleles. J Immunol. 1989 Jun 1;142(11):3937–3950. [PubMed] [Google Scholar]
- Pechere J. F., Rochat H., Ferraz C. Parvalbumins from coelacanth muscle. II. Amino acid sequence of the two less acidic components. Biochim Biophys Acta. 1978 Sep 26;536(1):269–274. doi: 10.1016/0005-2795(78)90073-9. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
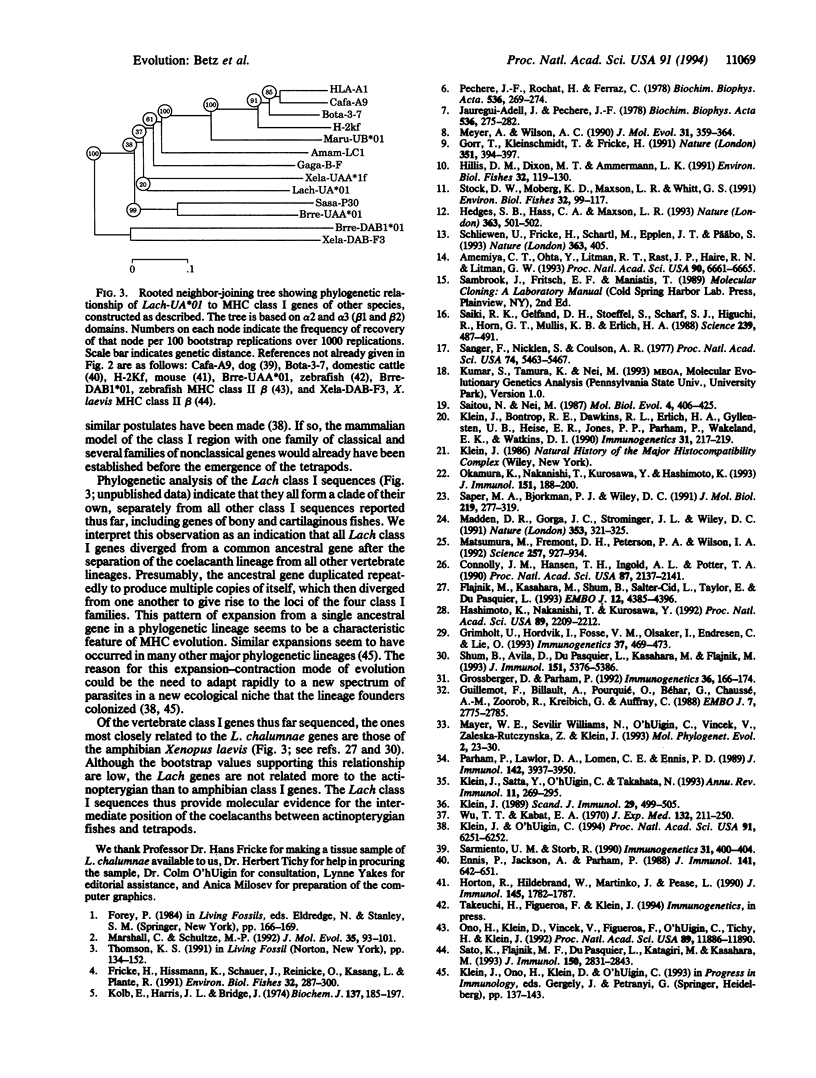
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper M. A., Bjorkman P. J., Wiley D. C. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6 A resolution. J Mol Biol. 1991 May 20;219(2):277–319. doi: 10.1016/0022-2836(91)90567-p. [DOI] [PubMed] [Google Scholar]
- Sarmiento U. M., Storb R. Nucleotide sequence of a dog class I cDNA clone. Immunogenetics. 1990;31(5-6):400–404. doi: 10.1007/BF02115019. [DOI] [PubMed] [Google Scholar]
- Sato K., Flajnik M. F., Du Pasquier L., Katagiri M., Kasahara M. Evolution of the MHC: isolation of class II beta-chain cDNA clones from the amphibian Xenopus laevis. J Immunol. 1993 Apr 1;150(7):2831–2843. [PubMed] [Google Scholar]
- Shum B. P., Avila D., Du Pasquier L., Kasahara M., Flajnik M. F. Isolation of a classical MHC class I cDNA from an amphibian. Evidence for only one class I locus in the Xenopus MHC. J Immunol. 1993 Nov 15;151(10):5376–5386. [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970 Aug 1;132(2):211–250. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]



