Abstract
Study Design Case report.
Objective Although May-Thurner syndrome or iliac vein compression syndrome is covered in the vascular literature, it remains absent from the orthopedic and neurosurgery literature and has not been previously reported to occur in concordance with spine surgery. We review the salient points of disease presentation, diagnosis, and treatment.
Methods A 33-year-old woman was followed postoperatively via clinical and radiographic findings. Her presentation, operative treatment, postoperative extensive deep venous thrombosis (DVT) formation, and management are described.
Results We present a unique case of a healthy 33-year-old woman who developed an extensive left iliac vein DVT after anterior lumbar spine fusion. Although she had multiple risk factors for thrombosis, the size of the thrombus was atypical. A subsequent venogram showed compression of the left common iliac vein by the right common iliac artery, consistent with May-Thurner syndrome.
Conclusions May-Thurner syndrome or iliac vein compression syndrome is a rare diagnosis that is absent from the spine literature. The condition can predispose patients to extensive iliac vein DVT. The contributing anatomy and subsequent clot often require catheter-directed thrombolysis and stenting to achieve a favorable outcome.
Keywords: May-Thurner, anatomy, thrombosis, DVT, lumbar spine surgery, ALIF
Case Report
A 33-year-old woman presented to her primary care physician with a chief complaint of low back pain and radiating right leg pain. These complaints had progressively worsened over the course of 1 year. Magnetic resonance imaging (MRI) of the lumbar spine revealed degenerative disk disease at L5–S1 with bilateral foraminal stenosis, greater on the right side than the left. Her primary care physician attempted to treat her with nonoperative measures including physical therapy, anti-inflammatory medications, and two epidural steroid injections, which gave her only transient relief of her symptoms.
Her primary care physician referred her to the spine clinic; she had been unable to work for 3 months secondary to worsening lower back and right leg pain in the L5 distribution. She had a 30 pack-year history of smoking but quit more than 1 year prior to presentation. She was taking oral contraceptives at the time of presentation. Her body mass index at presentation was 25. Upon examination, the patient walked with an antalgic gait, holding her lower back due to pain. She had subjectively decreased sensation in the right L5 distribution. She had normal symmetric reflexes and no weakness. She had a positive straight leg raise bilaterally, both causing right leg pain. Her exam was otherwise unremarkable.
There was no evidence of fracture or instability (Fig. 1). The MRI showed loss of disk height at L5–S1 with degenerative changes across the disk space. No significant disk herniation or narrowing of the canal was noted. There was compression at the L5 nerve roots secondary to foraminal narrowing (Fig. 2).
Fig. 1.
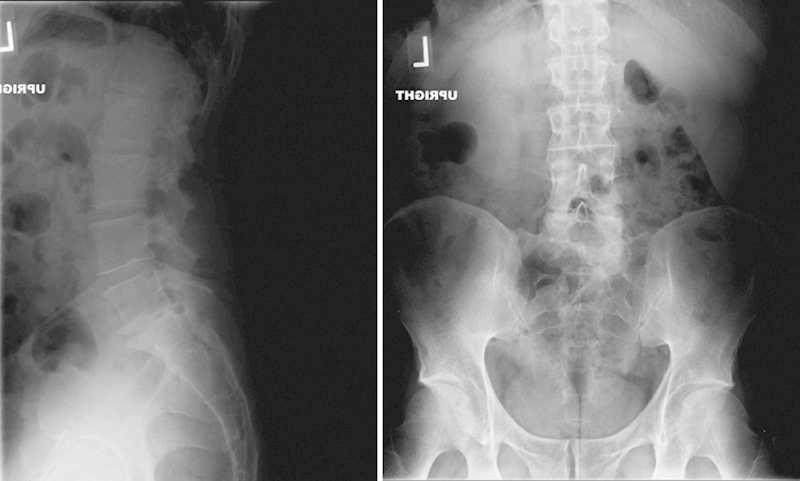
Preoperative anteroposterior and lateral radiographs.
Fig. 2.
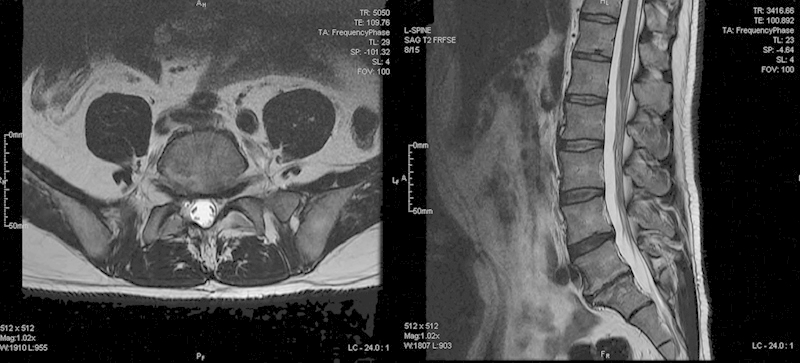
Preoperative axial and sagittal magnetic resonance imaging.
The patient had exhausted conservative measures and elected to proceed with an uncomplicated L5–S1 anterior lumbar interbody fusion using a lordotic polyetheretherketone cage, which was 16 mm in height with 10 degrees of lordosis (Fig. 3). InFuse Bone Graft recombinant human bone morphogenic protein 2 (rhBMP-2) (Medtronic Sofamor Danek, Memphis, Tennessee, United States) 5.6 mL was applied to a collagen sponge and inserted into the cage. There was no excess retraction of the vasculature; the bifurcation above L5–S1 left ample space to prepare the disk space and insert the implant. The procedure was completed without vascular injury. The patient did well postoperatively and was discharged within 5 days of the procedure. In accordance with our standard protocol, only mechanical deep venous thrombosis (DVT) prophylaxis was used in the hospital, and no chemoprophylaxis was used postoperatively.
Fig. 3.
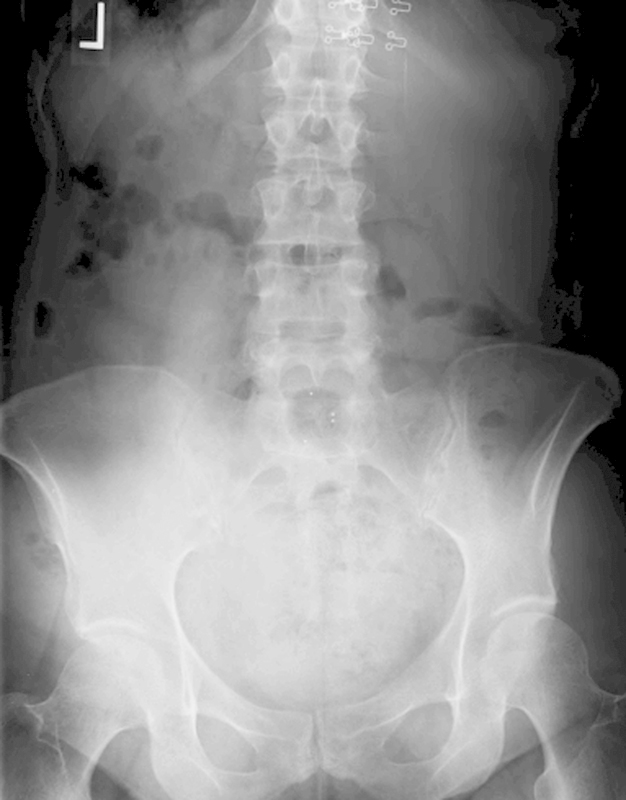
Postoperative anteroposterior radiograph.
At her first postoperative clinic visit 8 days after surgery, she complained of new-onset left leg pain and swelling throughout the left lower extremity. Her left leg was subjectively larger than the contralateral side with tenderness to palpation over the groin. She denied chest pain or shortness of breath. A duplex ultrasound preformed at that time revealed an acute DVT with extensive propagation throughout the left proximal common femoral vein, femoral vein, and profunda femoris. The patient was admitted to the hospital and placed on a heparin drip.
Although this patient certainly had risk factors for DVT formation (postoperative status, anterior spine surgery, previous smoker, and oral contraceptive use), due to the extreme size of the thrombus, the vascular surgery and hematology departments were consulted and a hypercoagulable workup was done, which was negative for a genetic predisposition for DVT. The role of rhBMP-2 in contributing to pelvic thrombus formation is thus far unclear. An abdominal computed tomography (CT) scan was obtained with contrast showing a large thrombus anterior to the L5 vertebral body (Fig. 4). A venogram was performed and found subsequent thrombolysis from a popliteal access point. An extensive thrombus was found in the left common iliac vein and the adjacent inferior vena cava. The anatomic compression of the left femoral vein between the right iliac artery and the L5 vertebral body was noted and the diagnosis of May-Thurner syndrome was made. Tissue plasminogen activator was used as a thrombolytic agent. Serial venograms were performed 24 and 48 hours after the initial thrombolysis and showed resolution of flow in the left common iliac vein (Fig. 5). During the third venogram, stents were placed in the left common iliac vein and external iliac veins and the sheath was removed. The patient continued heparin therapy until she was transitioned to coumadin, which was continued for 6 months post-thrombolysis.
Fig. 4.
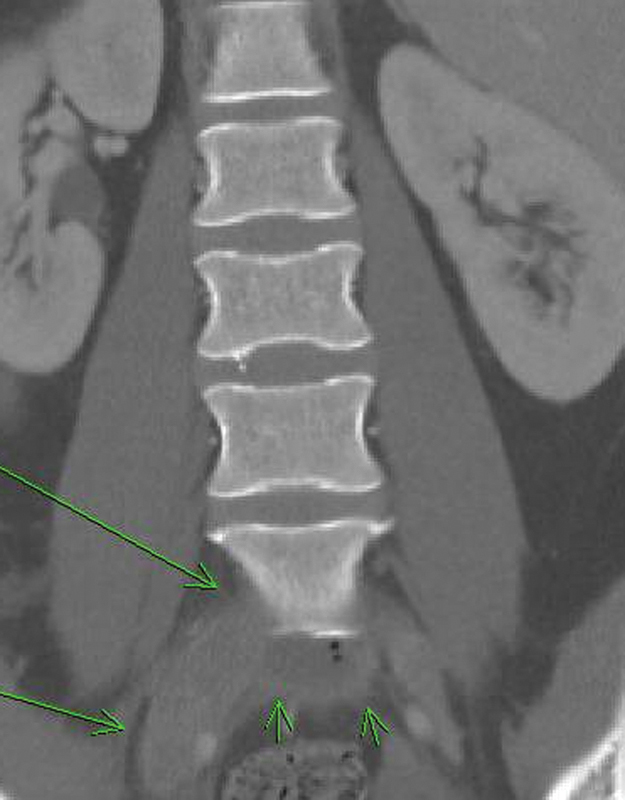
Abdomen/pelvis coronal computed tomography. Long arrows show the thrombosed left common iliac vein. Short arrows show the interbody device placed at L5-S1.
Fig. 5.
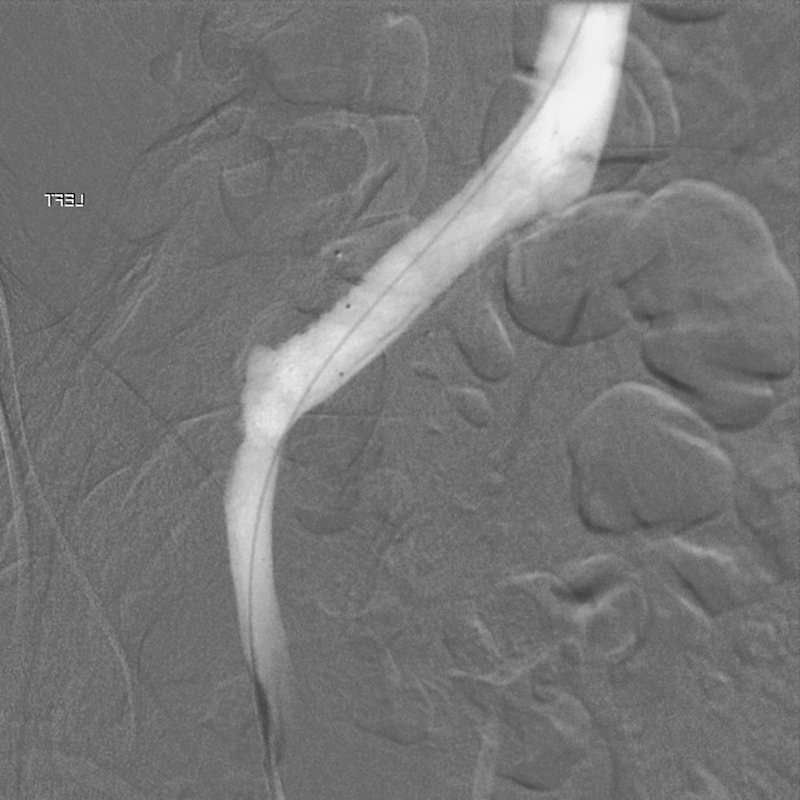
Post-thrombectomy venogram.
She has since recovered fully with no subsequent thrombotic events. She was able to complete her rehabilitation for her L5–S1 anterior lumbar interbody fusion and achieved resolution of her right leg pain and lower back pain. She was able to return to work 3 months postoperatively.
Discussion
DVT is a known complication after anterior spine surgery. This patient had clear risk factors for postoperative thrombosis including anterior approach, 30 pack-year history of smoking, and oral contraceptive usage. It is clear that her thrombosis was multifactorial in nature. However, the extent of her thrombosis raised concerns about other possible factors such as genetic, hematologic, and anatomic contributions. The diagnosis of iliac vein compression syndrome (IVCS) made by the interventional vascular team was one unfamiliar to our spine surgery department and warranted further investigation and education.
Etiology
May-Thurner syndrome or IVCS is an anatomic variant that results in compression of the left iliac vein by the right iliac artery. In 1851, Virchow described a similar compression phenomenon.1 However, it was not until 1957 that May and Thurner described a syndrome in which compression between the L5 vertebral body and the right iliac artery caused compression of the left iliac vein. They postulated that in response to repetitive trauma to the vein at the site of bony contact, thickening of the vascular endothelium leads to a “spur” of intimal proliferation in the vein wall and increased compression and obstruction of the vein over time.2 This cycle ends with an increased predisposition to form DVT in the left lower extremity. Fig. 6 illustrates the anatomic compression of the left iliac vein by the crossing right iliac artery and the resultant lateral, double lumen, and webbing spur patterns. In detailed dissections of 430 cadavers and examinations of the vascular histopathology, May and Thurner found compression of the left iliac vein between the right iliac artery and the L5 vertebral body in 22% of their specimens. Spurs were categorized as lateral with two spurs encroaching toward the lumen, central with a single septation across the lumen, or partial obliteration corresponding to webbing across the lumen.2
Fig. 6.
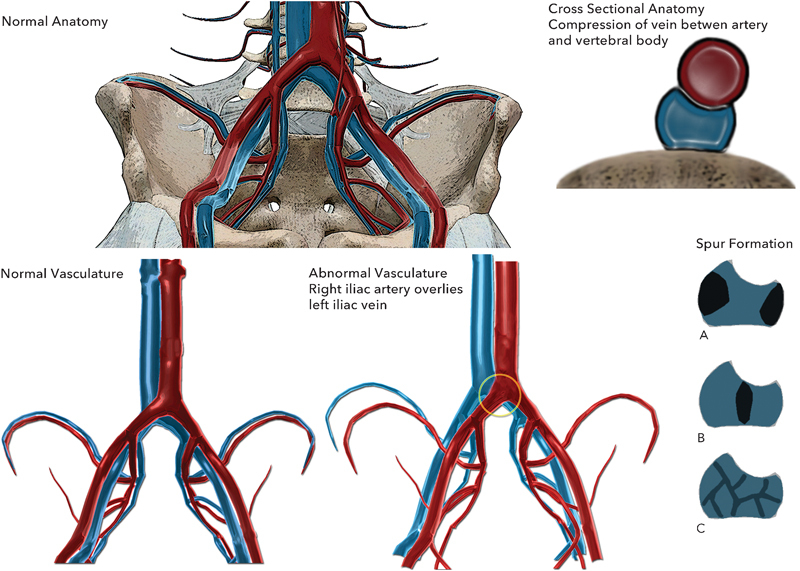
Iliac vein compression syndrome.
Since its original naming, the anatomic compression has been known by multiple titles: iliocaval compression syndrome, iliac vein compression syndrome, compression syndrome.2 Cockett and Thomas again described this anatomy in 1965 and went on to name the syndrome Cockett syndrome.3 They found that the left side was more commonly affected than the right, and women were most often affected during their second to fourth decades of life. They described an acute phase of swelling often found postoperatively or during pregnancy as well as a chronic phase that results in chronic lower-extremity venous stasis.3
Originally thought to be a rare condition only found on cadaveric dissections, IVCS has since become easier to diagnose with the development of directed venography. The incidence of IVCS in left-sided lower-extremity DVT is estimated to be between 18 and 49%.4 It is likely that many cases of IVCS-related DVT go undiagnosed. Commonly, a workup is initiated only when an extensive pelvic DVT is found. Although the standard and most common configuration of IVCS is compression of the left iliac vein by the right iliac artery, multiple anatomic variants causing venous obstruction in this region are also possible. Left iliac arterial compression of the left iliac vein, right-sided iliac vein compression from the right-sided artery, and iliac vein compression from the right-sided iliac artery have all been previously described in the literature.2 5 6 7 This syndrome has never been previously described in association with anterior spine surgery.
Presentation
IVCS is usually found during workup of an extensive, acute, left lower-extremity DVT involving the left iliac vein. Patients often present with left lower-extremity pain and swelling. Young women between their second and fourth decades of life are thought to be most at risk especially during pregnancy or after periods of immobilization such as the postoperative period.3 In addition to the acute phase of symptoms, patient can present with chronic IVCS and resultant changes consistent with chronic venous stasis. Mild swelling, chronic leg pain, loss of hair, hyperpigmentation, venous varicosities, telangiectasias, or even leg ulcerations may occur in the left leg.8 9 A thorough history should be taken for each patient being considered. Family history of DVT and hypercoagulability should be ascertained. Periods of immobilization or inactivity secondary to injury or lengthy travel should be noted. Often patients may describe low-level symptoms of venous claudication or inability to tolerate venous compression stockings.
Kim and Orron described stages of IVCS in 1992: stage I, anatomic abnormalities that remain asymptomatic; stage II, development of a venous spur pattern within the lumen; stage III, development of a deep thrombosis within the iliac vein.10
Diagnosis
IVCS is often a diagnosis of exclusion. Other causes such as trauma, malignancy, radiation, previous catheterization, or iatrogenic injury should be carefully considered.11 A hypercoagulable workup should be initiated in patients suspected of having IVCS. For patients presenting with acute left-sided painful swollen leg, venous duplex ultrasound is the first line of diagnostic modalities. Although the compression of the iliac veins cannot be adequately assessed on duplex ultrasound, identification of the acute iliac vein DVT is critical to the diagnosis.12 Advanced imaging for those patients suspected of IVCS include venography, abdominal CT, CT venography, intravascular ultrasound, and magnetic resonance venography.
Abdominal CT scan with contrast is useful to rule out extrinsic causes of compression; however, given the size of the venous spurs in IVCS, slices smaller than the standard 10 mm should be used. Secondary to chronic fibrotic changes in the setting of an acute large thrombus, some venous spurs may not be visualized.13 Although the noninvasive studies listed here have been able to diagnose IVCS in some patients, the gold standard has been femoral access contrast venography with the option for catheter-directed therapy at the time of diagnosis.8 14 15 16
Although once treated by open venous bypass grafting, successful treatment can generally be achieved by intravascular means. Since 1995 when Berger et al reported use of an endovascular stent to relieve compression of the left iliac vein, multiple techniques have been described.17 Local thrombolytic therapy avoids the complications associated with systemic high-dose anticoagulation. Routine anticoagulation is preferred by most vascular surgeons for up to 6 months postoperatively. O'Sullivan et al reported 79 and 85% symptomatic relief at 1 year after endovascular thrombolysis, angioplasty, and stent placement in a series of 39 patients.15 Long-term 5-year stent patency has been reported to be as high as 80% by some authors.18 19 Generally, intravascular chemical or mechanical thrombolysis with subsequent stenting to prevent repeat compression and obstruction of the vein results in resolution of symptoms and prevents further obstruction.
Conclusions
Orthopedic surgery is a field commonly complicated by DVT formation. This case report illustrates a patient who sustained an extensive DVT as a result of anterior lumbar spine surgery. A multifactorial etiology led to her clot formation with one anatomic component in particular predisposing her to extensive pelvic thrombosis. Although seldom reported, IVCS is an important anatomical consideration leading to the development of left-sided extensive clot formation in the iliac vein. It is important to recognize this as a potential contributing factor in those patients who present postoperatively with an extensive left-sided iliac vein DVT. The diagnosis of IVCS-influenced DVT should be in the differential for nonradicular postoperative pain with extensive clot formation. After obtaining a duplex ultrasound with confirmation of an iliac vein DVT, a venogram can both diagnose IVCS and facilitate endovascular treatment. Potential interventions include thrombolysis, angioplasty, and stenting, which generally restore patency and prevent recurrence. In a patient known to have IVCS, posterior or lateral approaches should be considered as this anatomic variant may increase the risk of clot formation in already thrombosis-prone anterior surgical approaches. Preoperative recognition of this problem is extremely rare. By familiarizing ourselves with the natural history of IVCS, we hope to optimize diagnosis and treatment of this condition when recognized postoperatively.
We have presented a case of extensive DVT formation in a 33-year-old woman after anterior lumbar spine fusion who had multiple risk factors for DVT. We feel that IVCS anatomically contributed to the large size of her DVT, and successful diagnosis and endovascular treatment of this condition led to a favorable outcome.
Footnotes
Disclosures Deepak Reddy, none Mark M. Mikhael, none Gary S. Shapiro, none Tony Farrell, none
References
- 1.Virchow R. Ueber die Erweiterung kleinerer Gefäfse. Virchows Arch. 1851;3:427–462. [Google Scholar]
- 2.May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8(5):419–427. doi: 10.1177/000331975700800505. [DOI] [PubMed] [Google Scholar]
- 3.Cockett F B, Thomas M L. The iliac compression syndrome. Br J Surg. 1965;52(10):816–821. doi: 10.1002/bjs.1800521028. [DOI] [PubMed] [Google Scholar]
- 4.Kasirajan K, Gray B, Ouriel K. Percutaneous AngioJet thrombectomy in the management of extensive deep venous thrombosis. J Vasc Interv Radiol. 2001;12(2):179–185. doi: 10.1016/s1051-0443(07)61823-5. [DOI] [PubMed] [Google Scholar]
- 5.Burke R M, Rayan S S, Kasirajan K, Chaikof E L, Milner R. Unusual case of right-sided May-Thurner syndrome and review of its management. Vascular. 2006;14(1):47–50. doi: 10.2310/6670.2006.00012. [DOI] [PubMed] [Google Scholar]
- 6.Dheer S, Joseph A E, Drooz A. Retroperitoneal hematoma caused by a ruptured pelvic varix in a patient with iliac vein compression syndrome. J Vasc Interv Radiol. 2003;14(3):387–390. doi: 10.1097/01.rvi.0000058411.01661.2b. [DOI] [PubMed] [Google Scholar]
- 7.Fretz V, Binkert C A. Compression of the inferior vena cava by the right iliac artery: a rare variant of May-Thurner syndrome. Cardiovasc Intervent Radiol. 2010;33(5):1060–1063. doi: 10.1007/s00270-009-9671-y. [DOI] [PubMed] [Google Scholar]
- 8.Baron H C, Shams J, Wayne M. Iliac vein compression syndrome: a new method of treatment. Am Surg. 2000;66(7):653–655. [PubMed] [Google Scholar]
- 9.Doenz F. [Iliac vein compression syndrome, the “May Thurner Syndrome”] Praxis (Bern 1994) 2006;95(12):460–463. doi: 10.1024/0369-8394.95.12.460. [DOI] [PubMed] [Google Scholar]
- 10.Kim D Orron D E, eds. Peripheral Vascular Imaging and Intervention St. Louis, MO: Mosby; 1992 [Google Scholar]
- 11.Shebel N D Whalen C C Diagnosis and management of iliac vein compression syndrome J Vasc Nurs 200523110–17., quiz 18–19 [DOI] [PubMed] [Google Scholar]
- 12.Mousa A Y. New York, NY: Springer; 2014. May-Thurner Syndrome. Endovascular Interventions; pp. 1133–1148. [Google Scholar]
- 13.Kibbe M R, Ujiki M, Goodwin A L, Eskandari M, Yao J, Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39(5):937–943. doi: 10.1016/j.jvs.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 14.Chung J W, Yoon C J, Jung S I. et al. Acute iliofemoral deep vein thrombosis: evaluation of underlying anatomic abnormalities by spiral CT venography. J Vasc Interv Radiol. 2004;15(3):249–256. doi: 10.1097/01.rvi.0000109402.52762.8d. [DOI] [PubMed] [Google Scholar]
- 15.O'Sullivan G J, Semba C P, Bittner C A. et al. Endovascular management of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2000;11(7):823–836. doi: 10.1016/s1051-0443(07)61796-5. [DOI] [PubMed] [Google Scholar]
- 16.Oguzkurt L, Tercan F, Pourbagher M A, Kizilkilic O, Turkoz R, Boyvat F. Computed tomography findings in 10 cases of iliac vein compression (May-Thurner) syndrome. Eur J Radiol. 2005;55(3):421–425. doi: 10.1016/j.ejrad.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Berger A, Jaffe J W, York T N. Iliac compression syndrome treated with stent placement. J Vasc Surg. 1995;21(3):510–514. doi: 10.1016/s0741-5214(95)70295-4. [DOI] [PubMed] [Google Scholar]
- 18.Hood D B, Alexander J Q. Endovascular management of iliofemoral venous occlusive disease. Surg Clin North Am. 2004;84(5):1381–1396, viii. doi: 10.1016/j.suc.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Kölbel T, Lindh M, Åkesson M, Wassèlius J, Gottsäter A, Ivancev K. Chronic iliac vein occlusion: midterm results of endovascular recanalization. J Endovasc Ther. 2009;16(4):483–491. doi: 10.1583/09-2719.1. [DOI] [PubMed] [Google Scholar]


