Abstract
Study Design Retrospective case series.
Objective Ankylosing spondylitis (AS) and diffuse idiopathic skeletal hyperostosis (DISH) are two related diseases that significantly increase the risk of unstable spinal fractures from seemingly trivial trauma. Given the older age and higher surgical risk profile of most of these patients, minimally invasive (MIS) approaches to the treatment of such fractures may reduce operative risk and physiologic stress.
Methods Eleven consecutive patients with hyperextension thoracolumbar injuries and a diagnosis of AS or DISH admitted to a single level I trauma center between June 2009 and June 2014 were retrospectively reviewed. All patients were treated with MIS stabilization. In addition, the patients were administered the Oswestry Disability Index and EuroQol-5D surveys to evaluate patient-reported outcomes regarding disability and health-related quality of life, respectively.
Results Of the 11 patients, 10 were alive at the time of review. The mean follow-up time was 28 months. The average age was 77 years old with a mean body mass index of 34. All patients had severe systemic disease, American Society of Anesthesiologists grade III, with multiple medical comorbidities. Seven segments on average were included in the operative construct. There were no instrumentation failures or nonunions requiring revision surgery. The average postoperative Oswestry disability index was 21.5% (range: 0 to 34%), corresponding to low to moderate disability, and the average EuroQol-5D utility score was 0.77 (range: 0.60 to 1), a similar average postoperative utility value to those published in the literature on elective surgery for degenerative lumbar conditions.
Conclusions MIS stabilization, when used on patients with good preoperative neurologic status, can successfully manage spinal fractures in patients with AS and DISH and preserve a favorable postoperative quality of life with limited disability.
Keywords: minimally invasive, MIS, AS, DISH, ankylosing, spine trauma, ODI, EQ-5D
Introduction
Ankylosing diseases of the spine include two major entities: ankylosing spondylitis (AS) and diffuse idiopathic skeletal hyperostosis (DISH). These diseases have unique pathophysiologies but similar clinical presentations later in the disease course.1 AS is a seronegative spondyloarthropathy, an inflammatory condition with negative laboratory markers common to autoimmune conditions (e.g., anti-nuclear antibody, serum rheumatoid factor). However, there is a genetic predisposition, with 90% of individuals with AS being positive for the HLA-B27 allele.2 The disease causes bony replacement of ligaments, fusion of joints of the axial spine including the intervertebral disks and facet joints, and subsequent osteoporosis. AS generally starts at the sacroiliac (SI) joints and progresses in a rostral direction. DISH, on the other hand, is a noninflammatory condition without a clear pathognomonic mechanism or genetic predisposition, but it is correlated with advanced age, diabetes mellitus, hypertension, and obesity.3 DISH also leads to the progressive ossification of longitudinal ligaments and most commonly affects the thoracic spine, followed by the lumbar and cervical segments. However, unlike AS, the SI and zygapophysial joints are spared.
The prevalence of AS is 0.1 to 1.4% in the general population and as high as 5 to 6% in HLA-B27 (+) individuals.4 5 The prevalence of DISH varies considerably based on age and the population studied. For example, a Finnish study concluded that the prevalence in the general population above age 40 was 3.8% for men and 2.6% in women, and in those over 70 years, the prevalence was 10.1 and 6.8%, respectively. In an American study on patients over 50 years, the prevalence was found to be 25% for men and 15% for women.6 The diagnosis of AS is based on the modified New York Criteria, which include radiographic and clinical criteria.5 AS generally begins in the second or third decade, but because the disease is progressive, it may not become clinically apparent until later in life. The diagnosis of DISH is based strictly on radiographs, with anterolateral bridging ossifications seen on at least four contiguous vertebrae without involvement of the SI or zygapophysial joints.7
The restricted motion and overall kyphotic spinal alignment of patients with AS and DISH increases the risk of hyperextension injuries from seemingly trivial trauma, such as falls from standing.8 In fact, patients with AS have four times the rate of spinal fractures compared with the general public, and although the fracture risk for patients with DISH has not been reliably quantified, it is hypothesized that the same mechanisms are at play.9 10 Previous studies have shown that these fractures most commonly occur in the cervical spine, followed by thoracic and lumbar segments.8 11 Because the spinal column becomes progressively fused, the fracture pattern is often similar to that of a long bone fracture, with lever arms above and below the fracture.11 The substantial lever arms can be quite unstable and place the patient at high risk for a neurologic injury, particularly if the cervical segment is involved. In addition, there is a high incidence of missed fractures and epidural hematomas in these patients, leading to higher rates of spinal cord injury.
Treatment for ankylosed-spine fractures may be operative or nonoperative, depending on patient age, medical comorbidities, fracture pattern, and neurologic status. Based on larger patient series, the average age of patients with an ankylosed spine-related fracture ranges from 59 to 72 years, and they tend to have increased medical comorbidities compared with the overall patient population with thoracolumbar spinal trauma.8 11 12 Conservative treatment generally consists of bracing and bed rest. However, previous studies have demonstrated a trend for higher complication rates in nonoperative patients, and one series showed a mortality rate of 51% in the nonoperative group.11 It should be noted that most patients who receive nonoperative treatment do so because of high surgical risk, and most morbidity is due to the development of pulmonary complications.11 13 14
Due to the torque associated with the lever arms, in conjunction with poor, osteoporotic bone quality, surgical treatment often requires multiple points of pedicle screw fixation above and below the affected level to appropriately stabilize the spinal column and heal the fracture. Most series on surgery for ankylosed-spine fractures were based on traditional open procedures with pedicle screw placement and creation of a fusion bed. Although percutaneous pedicle screw placement has been criticized for a lack of data regarding rates of pseudarthrosis and hardware failure, a recently developed algorithm for the treatment of traumatic thoracolumbar injuries proposed reserving the use of percutaneous nonfusion techniques to patients with AS/DISH.15 Given the high risk profile of most patients with AS/DISH, our approach has likewise been to treat these patients with minimally invasive (MIS) stabilization procedures to reduce blood loss, physiologic stress, and perioperative morbidity. We hypothesize that MIS treatment in this patient population leads to favorable disability profiles and health-related quality-of-life (HRQoL) measures.
Methods
We retrospectively identified consecutive patients with hyperextension thoracolumbar injuries admitted to a single level I trauma center between June 2009 and June 2014. All patients were diagnosed with either AS or DISH and an acute spine fracture based on computed tomography (CT) scans, and magnetic resonance imaging scans were obtained if there was any question to the diagnosis of fracture or concern for involvement of the neural structures (Fig. 1). Patients were subsequently treated with MIS stabilization. Data were collected by an Institutional Review Board (IRB)-approved protocol at the University of Pennsylvania. In addition to data from the hospitalization, outpatient follow-up data including radiographs were collected at the latest point of follow-up available. Patients were then contacted and administered the Oswestry Disability Index (ODI) and EuroQol-5D (EQ-5D) surveys via mail to assess postoperative disability and HRQoL, respectively.
Fig. 1.
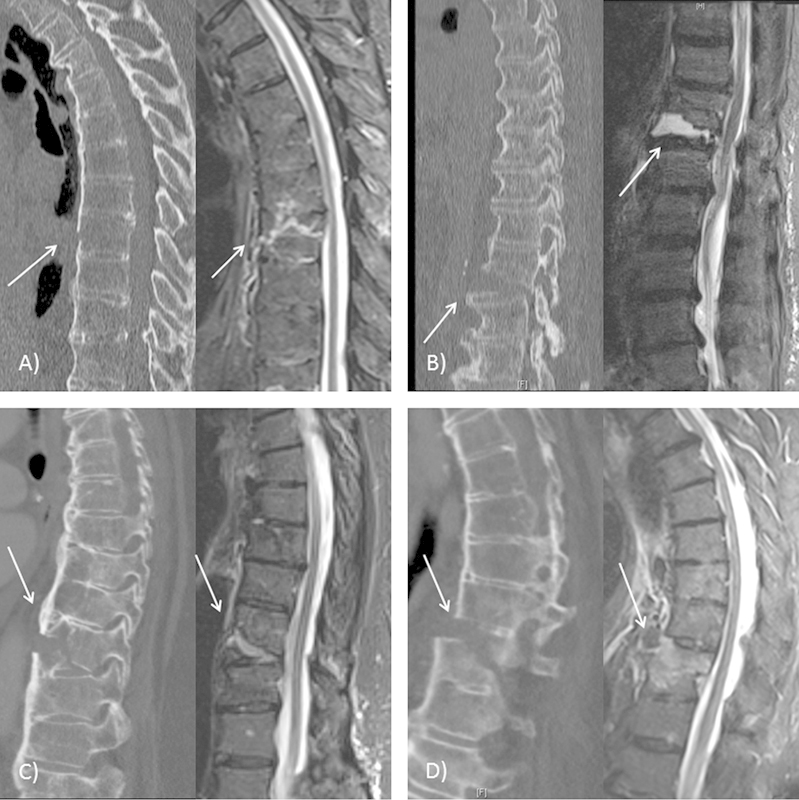
Preoperative computed tomography and magnetic resonance imaging scans demonstrating acute vertebral fractures (arrows) for patients 5 (A), 6 (B), 11 (C), and 10 (D).
Surgical Technique
All surgeries were performed under general anesthesia by the senior author. Because of the general kyphotic spinal alignment, all patients were positioned on a radiolucent Wilson frame on the flat Jackson operating table rather than in the prone modular Jackson table (Mizuho OSI, Union City, California, United States), which increases thoracolumbar extension. A midline skin incision was made, and the inferiormost spinous process was exposed to anchor the fiducial array for the StealthStation navigation system (Medtronic, Minneapolis, Minnesota, United States). An O-arm intraoperative CT scan was obtained to be used with the StealthStation. Two planning scans were often necessary to incorporate all of the necessary levels. The midline skin incision was extended as needed, and the entry points were planned using the navigation probe. Small, paraspinal fascial incisions were made, and the tip of a navigated drill guide was used to bluntly dissect down to bone. Using the navigated drill guide, pilot holes were made, followed by a navigated tap and navigated screwdriver to place the pedicle screws with screw extensions (VIPER MIS Spine System, Depuy-Synthes, Raynham, Massachusetts, United States). Rods were subsequently cut to size, contoured, and passed under the muscle and fascia through the screw heads and screw extensions. The rods were secured to the screws with locking caps and the screw extensions removed. Intraoperatively, postinstrumentation CT scans were obtained to verify appropriate position of the instrumentation. The small fascial incisions were closed and the skin incision closed in layers. Patients 1 and 2 received orthotic braces to be worn for 6 weeks postoperatively, although no subsequent patient was assigned a brace.
Results
There were 11 patients in the study, 9 with AS and 2 with DISH. Patient-reported outcome data were available for 7 of the 11 patients. Patient 6, whose fracture occurred subsequent to positioning for a brain tumor operation, was admitted approximately 2 months postoperatively for a cardiopulmonary event and died in the hospital. Patient 5 returned to living in a nursing home due to advanced dementia and thus could not complete the functional outcomes surveys. Patients 4 and 10 declined to participate in the outcomes surveys, but both live independently and report doing functionally well. All other patients returned to living independently.
Details on patient demographics, comorbidities, and surgical metrics are presented in Table 1. The average follow-up time was 28 months (range: 5 to 58). The average age at surgery was 77 (range: 52 to 88), and 45% were male (5/11). All patients had an American Society of Anesthesiologists (ASA) grade of III, representing severe systemic disease. All fractures occurred in the thoracic, thoracolumbar junction, or lumbar spine, with an average of seven segments included in the operative construct. Of the 11 patients, 5 (45%) suffered a low impact injury, with the most common mechanism being a fall from standing. All patients had an American Spinal Injury Association (ASIA) impairment scale of ASIA-E (neurologically intact), except for patient 3, who was ASIA-D due to weakness associated with concomitant jumped cervical facets. Average body mass index (BMI) was 34 (range: 20.4 to 44.5), with 73% (8/11) being obese (BMI ≥ 30). Average operative time was 227 minutes (range: 79 to 449), and the average blood loss was 251 mL (range: 25 to 900 mL). The average postoperative length of stay was 14.4 days (range: 4 to 60 days), and 36% of patients (4/11) required wound revision for superficial breakdown, of which two (patients 7 and 11) grew organisms from surgical cultures. No infection was deep to the muscle fascia or involved instrumentation. There were no instrumentation failures or nonunions requiring revision surgery. One patient (patient 3) had her iliac wing screws removed approximately 2 years after the initial surgery due to a bothersome prominence.
Table 1. Patient demographics and case details.
| Patient | Gender | Age (years) | ASA grade | Diagnosis | Mechanism | BMI | Fracture level | Segments in construct | Op time (min) | EBL | Postoperative LOS (d) | Follow-up (mo) | Wound breakdown/infection |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 88 | 3 | AS | Fall from standing (low impact) | 30.3 | T11 | 7 | 309 | 50 | 14 | 58 | N |
| 2 | M | 82 | 3 | AS | Ped struck (high impact) | 30.9 | T10 | 8 | 246 | 350 | 22 | 57 | N |
| 3 | F | 65 | 3 | AS | MVC (high impact) | 20.4 | L5 | 6 | 449 | 25 | 7 | 40 | Y |
| 4 | F | 52 | 3 | AS | MVC (high impact) | 38.1 | T7 | 7 | 334 | 900 | 4 | 38 | N |
| 5 | M | 86 | 3 | AS | Fall from scooter (low impact) | 25.1 | T8 | 7 | 79 | 75 | 5 | 36 | N |
| 6 | M | 82 | 3 | AS | OR positioning (low impact) | 25.9 | T12–L1 | 6 | 114 | 215 | 60 | N/A | N |
| 7 | M | 77 | 3 | AS | Fall from ladder (high impact) | 37.7 | T7 | 6 | 81 | 200 | 8 | 22 | Y |
| 8 | M | 80 | 3 | AS | Ped struck (high impact) | 39.2 | T9–10 | 6 | 213 | 100 | 16 | 12 | N |
| 9 | F | 77 | 3 | AS | Fall down stairs (high impact) | 39.1 | T7, T12 | 10 | 292 | 500 | 10 | 10 | N |
| 10 | F | 80 | 3 | DISH | Fall from standing (low impact) | 44.5 | T11 | 7 | 213 | 250 | 8 | 5 | Y |
| 11 | F | 79 | 3 | DISH | Fall from standing (low impact) | 43.1 | T10 | 7 | 166 | 100 | 4 | 5 | Y |
Abbreviations: AS, ankylosing spondylitis; ASA, American Society of Anesthesiologists; BMI, body mass index; DISH, diffuse idiopathic skeletal hyperostosis; EBL, estimated blood loss; LOS, length of stay; MVC, motor vehicle collision; N, no; N/A, not available; Op, operation; OR, operating room; Ped, pedestrian; Y, yes.
The results of patient-reported outcome data are presented in Table 2. The average ODI was 21.5% (range: 0 to 34%), corresponding to low to moderate disability (defined as 21 to 40%). The average EQ-5D utility score was 0.77 (range: 0.60 to 1). The range of EQ-5D utility scores in the U.S. population ranges from −0.109 to 1, where 1 represents perfect health, 0 represents death, and negative numbers are deemed to be a state worse than death per the sample cohort. We only report EQ-5D values for patients who were alive with self-reported EQ-5D surveys.
Table 2. Summary of patient details, surgical metrics, and patient-reported outcomes.
| Parameter | Average value (range) or % |
|---|---|
| Age at surgery | 77 (52–88) |
| Male | 45% |
| ASA grade | 3 |
| Low impact mechanism | 45% |
| BMI | 34 (20.4–44.5) |
| Number of segments incorporated | 7 (6–10) |
| Operative time (min) | 227 (79–449) |
| Blood loss (mL) | 251 (25–900) |
| Postoperative LOS (d) | 14.4 (4–60) |
| Postoperative ODI | 21.5% (0–34%) |
| Postoperative EQ-5D Utility Score | 0.77 (0.60–1.0) |
| Follow-up time (mo) | 28 (5–58) |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; EQ-5D, EuroQol-5D; LOS, length of stay; ODI, Oswestry Disability Index.
Discussion
Spine fractures in patients with ankylosing spinal disorders are often devastating events because most of the patients tend to be older with multiple medical comorbidities. In our series, the average combined patient age of 77 years was in line with average ages published in the literature, which range from 68 to 81.5 in combined AS and DISH cohorts.8 11 12 13 14 16 17 Moreover, all patients in our series had at least one major medical comorbidity and an ASA grade of III, representing severe systemic disease, including reversible conditions such as multiorgan injury from polytrauma. The acquired global kyphosis in this patient population frequently causes decreased pulmonary compliance, as well. These factors cumulatively place patients at a higher operative risk than typical patients with spinal trauma.
The rates of neurologic injury at presentation in ankylosed spine fractures tend to be high. One series found 58% of all patients presented with neurologic deficits, and another larger meta-analysis found that 67% of patients with AS and 40% with DISH presented with deficits.8 11 In particular, a Finnish study found patients with AS have greater than 11 times the risk of a spinal cord injury compared with the general population.18 Additionally, these patients have high rates of delayed diagnosis and thus an increased risk of secondary injury. Missed fractures may be particularly common when relying on plain radiographs. For example, in one series of 20 patients with AS, the initial plain films were negative in 60% (12/20).19 Caron et al quantified the danger of missed diagnoses; 81% of patients with a delayed diagnosis experienced neurologic deterioration during the delay.11
It should be noted that these large series include cervical spine fractures, which not only are the most common fractured segment in ankylosing spinal diseases, but also carry the highest rates of neurologic deficits. In our series, all but one patient was neurologically intact. Patient 3 had concomitant jumped cervical facets resulting in a spinal cord injury attributable to the cervical spinal cord. There was one delayed diagnosis: patient 6 was originally admitted for meningioma surgery and the fracture likely occurred during operative positioning. He had persistent back pain that led to neuroimaging several days later, identifying a spine fracture, but the patient did not have deficits attributable to the fracture.
Given the poor functional status and high operative risk of most patients with AS and DISH, most series on ankylosed spine fractures demonstrate high rates of nonoperative treatment, ranging from 33 to 46% among the combined cohorts.8 11 14 Although we could not find any series that demonstrated a statistically significant difference in morbidity and mortality between operative and nonoperative cohorts, in general most major complications and deaths among nonoperative patients were related to the development of a pulmonary complication, presumably due to a combination of prolonged stasis, decreased pulmonary compliance from a kyphotic habitus, and bracing. When the operative risk is acceptable, we believe that early definitive surgical treatment of the fracture allows patients to mobilize earlier, preventing some of those adverse events from occurring.
Although a variety of surgical approaches have been used to treat these fractures, including anterior, posterior, and combined approaches, the most common approach based on the published series appears to be posterior instrumentation three levels above and three levels below the fracture. We took that approach in all of our patients (Fig. 2), although we used percutaneous instrumentation rather than a traditional open exposure. Percutaneous instrumentation has become increasingly popular in the treatment of thoracolumbar trauma,20 21 22 23 24 25 and two recent series have been published on the specific use of MIS in the treatment of spine fractures in ankylosing spinal disorders.16 17 However, the two series took divergent views on the number of levels to include in the construct; one group sought to instrument the fewest number of levels (average of 1.8 levels), and the other primarily instrumented three levels above and below the fracture.
Fig. 2.
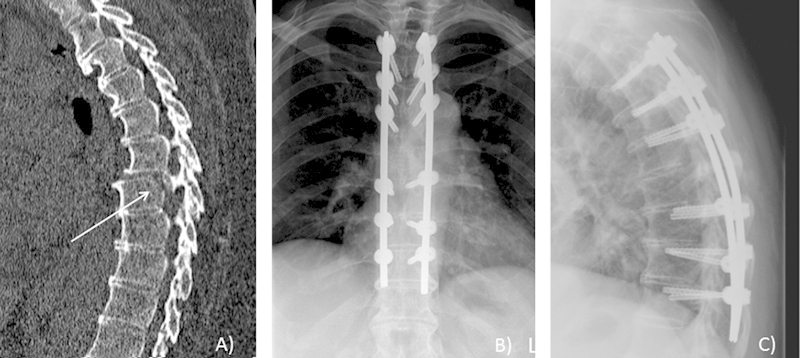
Patient 4's sagittal computed tomography scan demonstrating an unstable T7 fracture with listhesis (A). Postoperative anteroposterior (B) and lateral (C) X-rays of the operative construct demonstrating reduction of the fracture.
Positioning patients with ankylosed spines can be very precarious, as demonstrated by patient 6, who suffered an iatrogenic fracture. Because these patients develop a global kyphosis with limited mobility, placing them in a prone position that increases lordosis may exacerbate their fracture, cause a new fracture, or cause neurologic injury due to the unstable lever arms flanking the fracture. We used neurophysiologic monitoring in all cases and obtained both pre- and postpositioning signals.
Although other series on percutaneous instrumentation for ankylosed spine fractures have used fluoroscopy as the primary imaging modality, we used CT guidance for the screw placement and intraoperative CT to confirm the appropriate screw position prior to completing the surgery. As Yeoh et al have described, the fused joints and osteoporotic bone quality in ankylosing spinal disorders make X-rays very difficult to interpret.17 CT overcomes this challenge with increased detail that facilitates more accurate screw trajectories. Additionally, the poor bone quality increases the risk of the screw loosening and hardware migration. Ensuring good screw–rod interface can help minimize the risk of hardware failure, thus it is critical to prebend the rods to the exact contour of the screw extensions about the skin and avoid using persuader-type devices to fit and lock the rods in place. We did not need to reposition or revise instrumentation in any of our cases.
We identified one large study specific to AS/DISH fractures that reported mean operative times of 264 minutes for patients with AS, 203 minutes for patients with DISH, and 220 minutes for control patients, along with mean blood loss of 960 mL, 788 mL, and 613 mL for each respective cohort.14 That study included fractures throughout all spinal segments and included a combination of long-segment and short-segment fusions, as well as a small number of MIS fixations. Although our mean operative time is similar, the mean estimated blood loss in our series was considerably less. Given the small number of cases in this series, we believe that operative times will continue to decline as we move further along the learning curve.
Although none of the patients in our series required late revision of the instrumentation, 4/11 patients did require reoperation for wound breakdown. Patients 7, 10, and 11 were all morbidly obese (BMI 37.7, 44.5, 43.1, respectively) with active diabetes. Patient 3 developed a postoperative urinary tract infection and had the longest operative time of the series (449 minutes). It should also be noted that only patients 7 and 11 had grown organisms from cultures obtained during the operation (methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus, respectively), although all four patients were treated with antibiotics. Although none of the infections were deep to the fascia or required removal of instrumentation, all patients required hospitalization and a second surgical procedure. Obesity was the most common risk factor for wound breakdown. We had converted to a single midline skin incision as opposed to bilateral, paramedian incisions out of concern for devitalizing the skin in the midline between the parallel incisions. However, this method also increases the amount of the suprafascial dead space. In the obese patients, we placed a temporary subcutaneous drain to reduce the dead space and prevent seroma formation, but we will consider utilizing smaller parallel incisions if there are no pre-existing skin issues.
Outcomes in spine surgery have largely been reported as assessment of radiologic parameters (e.g., healed fracture, kyphotic angulation, etc.) or neurologic status (e.g., ASIA scale), although increasing attention has been given to patient-reported outcome measures in the field. In this series, we tracked the patient-reported health outcome measures using one disease-specific survey and one HRQoL survey, the ODI and EQ-5D, respectively. To our knowledge, one other series reported ODI results, and we are the first to report EQ-5D values. Yeoh et al performed MIS long-segment stabilization on 10 patients with AS and DISH, and they reported an average postoperative ODI of 16%, compared with a value of 21.5% in our series.17 Our average EQ-5D utility of 0.77 suggests that the patients have a reasonably high quality of life, although the precise improvement could not be determined due to a lack of preoperative values. Because of the underlying diagnoses of AS or DISH, in addition to old age, these patients will naturally have a certain baseline level of disability and diminished HRQoL had the fractures not even occurred. For comparison, the SPORT trial reported similar 1- and 2-year postoperative utility values in patients undergoing surgery for lumbar stenosis and degenerative spondylolisthesis.26 The results from our series suggest that patients undergoing percutaneous dorsal instrumentation for ankylosed spine fractures tend to have limited disability and high levels of health utility, despite old age, obesity, and multiple medical comorbidities in the average patient.
For our patients, we only obtain imaging through 3 months postoperatively unless the patient has specific signs or symptoms to suggest neurologic deterioration or a hardware malfunction, as determined by IRB restrictions. This approach precludes the ability to assess the long-term radiographic outcomes, such as fracture healing and deformity correction. Although we have an average follow-up time of 28 months, hardware-related complications can be encountered several years after the surgery, thus the duration of postoperative imaging is determined by the discretion of the treating surgeon.
Limitations in this study include its retrospective nature, small number of patients, and lack of preoperative patient-reported outcome measures. Moving forward, we plan to establish a prospective registry of patients treated with MIS stabilization. Unfortunately, because most of these patients present with acute trauma, obtaining preoperative patient-reported outcome measures will be exceedingly difficult, if not impossible, to reliably obtain.
Conclusion
AS and DISH are two ankylosing spinal disorders that significantly increase the risk of spinal fractures. Given the old age and multiple medical comorbidities associated with the average patient, surgery in this patient population is high risk, with high rates of postoperative morbidity and mortality. MIS techniques in spine surgery are becoming increasingly common, both in routine practice and in trauma settings. Based on our results, MIS stabilization, when used on patients with good preoperative neurologic status, can be successfully used to manage spinal fractures in the patients with AS and DISH and can preserve a good postoperative quality-of-life with limited disability.
Footnotes
Disclosures Nikhil R. Nayak, none Jared M. Pisapia, none Kalil G. Abdullah, none James M. Schuster, none
References
- 1.Olivieri I, D'Angelo S, Cutro M S. et al. Diffuse idiopathic skeletal hyperostosis may give the typical postural abnormalities of advanced ankylosing spondylitis. Rheumatology (Oxford) 2007;46(11):1709–1711. doi: 10.1093/rheumatology/kem227. [DOI] [PubMed] [Google Scholar]
- 2.Brewerton D A, Hart F D, Nicholls A, Caffrey M, James D C, Sturrock R D. Ankylosing spondylitis and HL-A 27. Lancet. 1973;1(7809):904–907. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- 3.Mader R, Verlaan J J, Buskila D. Diffuse idiopathic skeletal hyperostosis: clinical features and pathogenic mechanisms. Nat Rev Rheumatol. 2013;9(12):741–750. doi: 10.1038/nrrheum.2013.165. [DOI] [PubMed] [Google Scholar]
- 4.Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369(9570):1379–1390. doi: 10.1016/S0140-6736(07)60635-7. [DOI] [PubMed] [Google Scholar]
- 5.van der Linden S, Valkenburg H A, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4):361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 6.Weinfeld R M, Olson P N, Maki D D, Griffiths H J. The prevalence of diffuse idiopathic skeletal hyperostosis (DISH) in two large American Midwest metropolitan hospital populations. Skeletal Radiol. 1997;26(4):222–225. doi: 10.1007/s002560050225. [DOI] [PubMed] [Google Scholar]
- 7.Resnick D, Niwayama G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH) Radiology. 1976;119(3):559–568. doi: 10.1148/119.3.559. [DOI] [PubMed] [Google Scholar]
- 8.Westerveld L A, Verlaan J J, Oner F C. Spinal fractures in patients with ankylosing spinal disorders: a systematic review of the literature on treatment, neurological status and complications. Eur Spine J. 2009;18(2):145–156. doi: 10.1007/s00586-008-0764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkelstein J A, Chapman J R, Mirza S. Occult vertebral fractures in ankylosing spondylitis. Spinal Cord. 1999;37(6):444–447. doi: 10.1038/sj.sc.3100837. [DOI] [PubMed] [Google Scholar]
- 10.Paley D, Schwartz M, Cooper P, Harris W R, Levine A M. Fractures of the spine in diffuse idiopathic skeletal hyperostosis. Clin Orthop Relat Res. 1991;(267):22–32. [PubMed] [Google Scholar]
- 11.Caron T, Bransford R, Nguyen Q, Agel J, Chapman J, Bellabarba C. Spine fractures in patients with ankylosing spinal disorders. Spine (Phila Pa 1976) 2010;35(11):E458–E464. doi: 10.1097/BRS.0b013e3181cc764f. [DOI] [PubMed] [Google Scholar]
- 12.Whang P G, Goldberg G, Lawrence J P. et al. The management of spinal injuries in patients with ankylosing spondylitis or diffuse idiopathic skeletal hyperostosis: a comparison of treatment methods and clinical outcomes. J Spinal Disord Tech. 2009;22(2):77–85. doi: 10.1097/BSD.0b013e3181679bcb. [DOI] [PubMed] [Google Scholar]
- 13.Mathews M, Bolesta M J. Treatment of spinal fractures in ankylosing spondylitis. Orthopedics. 2013;36(9):e1203–e1208. doi: 10.3928/01477447-20130821-25. [DOI] [PubMed] [Google Scholar]
- 14.Westerveld L A, van Bemmel J C, Dhert W J, Oner F C, Verlaan J J. Clinical outcome after traumatic spinal fractures in patients with ankylosing spinal disorders compared with control patients. Spine J. 2014;14(5):729–740. doi: 10.1016/j.spinee.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 15.Dhall S S, Wadhwa R, Wang M Y, Tien-Smith A, Mummaneni P V. Traumatic thoracolumbar spinal injury: an algorithm for minimally invasive surgical management. Neurosurg Focus. 2014;37(1):E9. doi: 10.3171/2014.5.FOCUS14108. [DOI] [PubMed] [Google Scholar]
- 16.Krüger A, Frink M, Oberkircher L, El-Zayat B F, Ruchholtz S, Lechler P. Percutaneous dorsal instrumentation for thoracolumbar extension-distraction fractures in patients with ankylosing spinal disorders: a case series. Spine J. 2014 doi: 10.1016/j.spinee.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Yeoh D, Moffatt T, Karmani S. Good outcomes of percutaneous fixation of spinal fractures in ankylosing spinal disorders. Injury. 2014;45(10):1534–1538. doi: 10.1016/j.injury.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Alaranta H, Luoto S, Konttinen Y T. Traumatic spinal cord injury as a complication to ankylosing spondylitis. An extended report. Clin Exp Rheumatol. 2002;20(1):66–68. [PubMed] [Google Scholar]
- 19.Sapkas G, Kateros K, Papadakis S A. et al. Surgical outcome after spinal fractures in patients with ankylosing spondylitis. BMC Musculoskelet Disord. 2009;10:96. doi: 10.1186/1471-2474-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grossbach A J, Dahdaleh N S, Abel T J, Woods G D, Dlouhy B J, Hitchon P W. Flexion-distraction injuries of the thoracolumbar spine: open fusion versus percutaneous pedicle screw fixation. Neurosurg Focus. 2013;35(2):E2. doi: 10.3171/2013.6.FOCUS13176. [DOI] [PubMed] [Google Scholar]
- 21.Jiang X Z, Tian W, Liu B. et al. Comparison of a paraspinal approach with a percutaneous approach in the treatment of thoracolumbar burst fractures with posterior ligamentous complex injury: a prospective randomized controlled trial. J Int Med Res. 2012;40(4):1343–1356. doi: 10.1177/147323001204000413. [DOI] [PubMed] [Google Scholar]
- 22.Lee J K Jang J W Kim T W Kim T S Kim S H Moon S J Percutaneous short-segment pedicle screw placement without fusion in the treatment of thoracolumbar burst fractures: is it effective? Comparative study with open short-segment pedicle screw fixation with posterolateral fusion Acta Neurochir (Wien) 2013155122305–2312., discussion 2312 [DOI] [PubMed] [Google Scholar]
- 23.Ni W F, Huang Y X, Chi Y L. et al. Percutaneous pedicle screw fixation for neurologic intact thoracolumbar burst fractures. J Spinal Disord Tech. 2010;23(8):530–537. doi: 10.1097/BSD.0b013e3181c72d4c. [DOI] [PubMed] [Google Scholar]
- 24.Vanek P, Bradac O, Konopkova R, de Lacy P, Lacman J, Benes V. Treatment of thoracolumbar trauma by short-segment percutaneous transpedicular screw instrumentation: prospective comparative study with a minimum 2-year follow-up. J Neurosurg Spine. 2014;20(2):150–156. doi: 10.3171/2013.11.SPINE13479. [DOI] [PubMed] [Google Scholar]
- 25.Wild M H, Glees M, Plieschnegger C, Wenda K. Five-year follow-up examination after purely minimally invasive posterior stabilization of thoracolumbar fractures: a comparison of minimally invasive percutaneously and conventionally open treated patients. Arch Orthop Trauma Surg. 2007;127(5):335–343. doi: 10.1007/s00402-006-0264-9. [DOI] [PubMed] [Google Scholar]
- 26.Tosteson A N, Skinner J S, Tosteson T D. et al. The cost effectiveness of surgical versus nonoperative treatment for lumbar disc herniation over two years: evidence from the Spine Patient Outcomes Research Trial (SPORT) Spine (Phila Pa 1976) 2008;33(19):2108–2115. doi: 10.1097/brs.0b013e318182e390. [DOI] [PMC free article] [PubMed] [Google Scholar]


