Abstract
Study Design Randomized controlled trial.
Objective Despite a large number of publications of outcomes after spinal fusion surgery, there is still no consensus on the efficacy of the several different fusion methods. The aim of this study was to determine whether transforaminal lumbar interbody fusion (TLIF) results in an improved clinical outcome compared with uninstrumented posterolateral fusion (PLF) in the surgical treatment for chronic low back pain.
Methods This study included 135 patients with degenerative disk disease (n = 96) or postdiskectomy syndrome (n = 39). Inclusion criteria were at least 1 year of back pain with or without leg pain in patients aged 20 to 65 with one- or two-level disease. Exclusion criteria were sequestration of disk hernia, psychosocial instability, isthmic spondylolisthesis, drug abuse, and previous spine surgery other than diskectomy. Pain was assessed by visual analog scale (pain index). Functional disability was quantified by the disability rating index and Oswestry Disability Index. The global outcome was assessed by the patient and classified as much better, better, unchanged, or worse. The patients were randomized to conventional uninstrumented PLF (n = 67) or TLIF (n = 68). PLF was performed in a standardized fashion using autograft. TLIF was performed with pedicle titanium screw fixation and a porous tantalum interbody spacer with interbody and posterolateral autograft. The clinical outcome measurements were obtained preoperatively and at 12 and 24 months postoperatively. The 2-year follow-up rate was 98%.
Results The two treatment groups improved significantly from preoperatively to 2 years' follow-up. At final follow-up, the results in the TLIF group were significantly superior to those in the PLF group in pain index (2.0 versus 3.9, p = 0.007) and in disability rating index (22 versus 36, p = 0.003). The Oswestry Disability Index was better in the TLIF group (20 versus 28, p = 0.110, not significant). The global assessment was clearly superior in the TLIF group: 63% of patients scored “much better” in the TLIF group as compared with 48% in the PLF group (p = 0.017).
Conclusions The results of the current study support the use of TLIF rather than uninstrumented PLF in the surgical treatment of the degenerative lumbar spine. The less optimal outcome after uninstrumented PLF may be explained by the much higher reoperation rate.
Keywords: chronic low back pain, transforaminal lumbar interbody fusion, posterolateral noninstrumented fusion
Introduction
Degenerative disk disease (DDD) and postdiskectomy syndrome (PDS) may cause severe and chronic low back pain. There are still controversies about the role of surgical treatment in these disorders. Although the evidence-based medicine (EBM) situation in DDD is unclear, fusion has been shown to be superior to conservative treatment in one of three randomized controlled trials (RCTs) on DDD.1 In PDS, the EBM situation is even more unclear with no RCT at all comparing fusion to conservative treatment. Adding to the controversy, the type of fusion has not convincingly been shown to affect the clinical outcome, although the fusion rate seems to vary with the technique used. In a recent review,2 Babu et al concluded that there is level 1 data supporting that lumbar instrumentation improves fusion rates, but with no clear evidence of a consistent correlation between fusion rate and patient outcome. The rationale behind fusion is to eliminate motion in the pathologic motion segment,3 eliminating nociceptive afferent signals from disk and apophyseal joints.4 5 On theoretical grounds, interbody fusion has been suggested to result in an improved outcome compared with posterolateral fusion (PLF), because 80% of the mechanical load on the spine is anterior and the disk is probably an important pain generator. In addition, interbody fusion has an increased fusion rate, increased lordosis in short fusions, and direct or indirect foraminal decompression.6 In the 1990s, Harms and Varga introduced and popularized TLIF in treatment of degenerative disorders in the lumbar spine.9 The data to support an improved outcome with interbody fusion as compared with PLF is scarce. Høy et al recently reported no difference in the outcome between TLIF and instrumented PLF in a mixed group of patients including not only DDD but also spinal stenosis and isthmic spondylolisthesis.7 However, a previous Danish study by Videbaek et al22 showed superior data in surgery with 360-degree fusion as compared with PLF with fixation at 5 to 9 years' follow-up. TLIF has several reported advantages compared with posterolateral lumbar interbody fusion (PLIF; i.e., only unilateral approach to the spinal canal with less canal violation, less canal fibrosis, less dura- and nerve retraction, reduced incidence of dural tear, less bleeding, and shorter operative time). Stand-alone anterior interbody fusion without screw fixation is today largely abandoned because of its high pseudarthrosis rates.8 The 360-degree fusion as shown to be a method fulfilling the demands described above, but is more time-consuming as compared with TLIF.
In view of the similar outcomes observed in most RCTs comparing modern fusion techniques with traditional noninstrumented PLF, we decided to compare the simplest method, noninstrumented PLF, with TLIF, a recent addition to fusion methods in the degenerative lumbar spine.
Patients and Methods
Between 2001 and 2005, 135 patients were included in the study, 74 men and 61 women with a mean age of 44.5 years. The patient demographics and characteristics are shown in Table 1. Patients aged 20 to 65 years with at least 1-year duration of low back pain with or without leg pain were included. All patients had magnetic resonance imaging showing either DDD with or without disk hernia or a history of previous diskectomy with a clinical and radiological picture of PDS. The study was restricted to one- or two-level disease. Patients with sequestration of disk hernia, psychosocial instability, isthmic spondylolisthesis, drug abuse, and previous spine surgery other than diskectomy were excluded. In all patients, low back pain was the dominant symptom, with additional leg pain in 84% of the patients. Seventy-three percent of all included patients were on sick leave because of low back problems. Ninety-one patients (67%) were treated with one-level fusion and 44 (33%) patients with two-level fusion.
Table 1. Patients demographics and characteristics.
| TLIF (n = 68) | PLF (n = 67) | |
|---|---|---|
| Age, y (range) | 44 (25–62) | 45 (27–65) |
| Gender, M/F (%) | 53/47 | 57/43 |
| Diagnosis, n (%) | ||
| Degenerative disk disease | 46 (67) | 50 (75) |
| Postdiskectomy syndrome | 22 (33) | 17 (25) |
| Weight (mean kg) | 79 | 80 |
| Height (mean cm) | 175 | 174 |
| On sick leave (%) | 74 | 71 |
| Exercise level (%) | ||
| None | 31 | 37 |
| Once a week | 32 | 36 |
| More than once a week | 34 | 25 |
| Education (%) | ||
| Elementary only | 19 | 31 |
| College | 50 | 48 |
| University | 24 | 9 |
Abbreviations: PLF, posterolateral fusion; TLIF, transforaminal lumbar interbody fusion.
Randomization was performed by the nurse attending the outpatient clinic after inclusion of the patients into the study by the surgeon. The patient drew one of two notes indicating surgical treatment with either PLF or TLIF. The randomization resulted in a comparable number of patients in both groups: 67 patients were randomized to PLF and 68 to TLIF. The groups were comparable in sociodemographic variables as well as in the level of preoperative pain and disability. There were more two-level fusions in the TLIF group as compared with the PLF group (41% versus 24%; Table 2). In the DDD group, 50 patients were randomized to PLF and 46 patients to TLIF. In the PDS group, 17 patients were randomized to PLF and 22 patients to TLIF.
Table 2. Baseline data.
| TLIF (n = 68) | PLF (n = 67) | |
|---|---|---|
| Pain index (median) | 8.0 | 7.9 |
| DRI (median) | 56.4 | 52.5 |
| ODI (median) | 44.0 | 46.0 |
| No. of affected segments, one/two (%) | 59/41 | 76/24 |
| Levels (%) | ||
| L5–S1 | 25 | 42 |
| L4–L5 | 27 | 31 |
| L4–S1 | 32 | 22 |
| L3–L5 | 3 | 2 |
| L3–L4 | 3 | 3 |
| L2–L4 | 6 | – |
| L2–L3 | 4 | – |
Abbreviations: DRI, disability rating index; ODI, Oswestry disability index; PLF, posterolateral fusion; TLIF, transforaminal lumbar interbody fusion.
Decompression and/or diskectomy were performed if necessary in a standardized fashion. PLF was performed with decortication of the transverse processes, ala sacra, and facet joints in the PLF group as well as in the TLIF group, using iliac crest bone graft and locally gained bone. The PLF group wore a prefabricated lumbar soft corset for 6 months. TLIF was performed with the traditional technique including removal of the total facet joint unilaterally and identification and retraction of the medially passing nerve root to identify the disk.9 After distraction between the pedicle screws, thorough emptying of the disk and cleaning of the respective end plates with various instruments was performed. After placement of the ground bone graft anteriorly, a trabecular tantalum spacer (TM 300, Zimmer Spine, Minneapolis, Minnesota, United States) was positioned in the interbody space, followed by compression over the pedicle screws on each side. The spacer was placed aiming at a central transverse position using the instruments designed to turn the implant properly. The TM spacer is made of a porous material with the potential of bony ingrowth from the end plates.10 Multiaxial titanium screws and rods were used for fixation.
Clinical outcomes were measured preoperatively and at 12 and 24 months postoperatively. Pain was assessed by the visual analog scale and expressed as a pain index (PI, 0 to 10). Functional disability was assessed by a disability rating index (DRI, 0 to 100) and the Oswestry Disability Index (ODI, 0 to 100), both validated for use in degenerative lumbar spine disorders.11 12 The global outcome was assessed by the patient and classified as much better, better, unchanged, or worse.
PLF was graded according to Lenke and classified into fusion A, B, C, or D.13 The interbody fusion was defined as calcification of the anterior longitudinal ligament (sentinel sign) or central bony bridging between vertebral bodies. The fusion in the PLF group in the present study was defined as Lenke A or B, and the fusion in the TLIF group was defined as Lenke A or B (posterolaterally) or calcification of the anterior longitudinal ligament (sentinel sign) or central bony bridging between vertebrae in the absence of resorption around implants. The grading of fusion was made by an independent observer not involved with the treatment of the patients.
The Medical Ethical Committees of Huddinge University Hospital, Stockholm and Ryhov Hospital, Jönköping, Sweden approved the study.
Statistical Analysis
The statistical analysis was performed with Statistical Package for the Social Sciences, Version 18 (SPSS, Inc., Chicago, IL, United States). Quality control was performed to check for missing data and inconsistent entries. Descriptive statistics were used to characterize patients and outcomes. Data distribution was tested against normality using the Kolmogorov-Smirnov goodness-of-fit test. Because the PI, ODI, and DRI were not normally distributed, nonparametric methods were used to analyze the differences between the variables. For unpaired data, the Mann-Whitney U test was used, and for paired data, the Wilcoxon signed rank test was used. We calculated 95% confidence intervals for the median values. The chi-square test was used for the comparisons between ordered categories such as global outcome. A p value < 0.05 was considered statistically significant.
Results
The 2-year follow-up rate was 98%. At baseline the median scores for the whole group was as follows: PI 8 (range 3 to 10), DRI 55 (range 23 to 98), and ODI 46 (range 22 to 100). In longitudinal analysis, we found a significant improvement of PI, ODI, and DRI in all outcome measurements for both treatment groups of the 2-year follow-up (Figs. 1 2 3).
Fig. 1.

Median pain index (0 to 10) in patients operated on by PLF or TLIF. Vertical bars indicate 95% confidence limits. Abbreviations: ns, not significant; PLF, posterolateral fusion; TLIF, transforaminal lumbar interbody fusion.
Fig. 2.
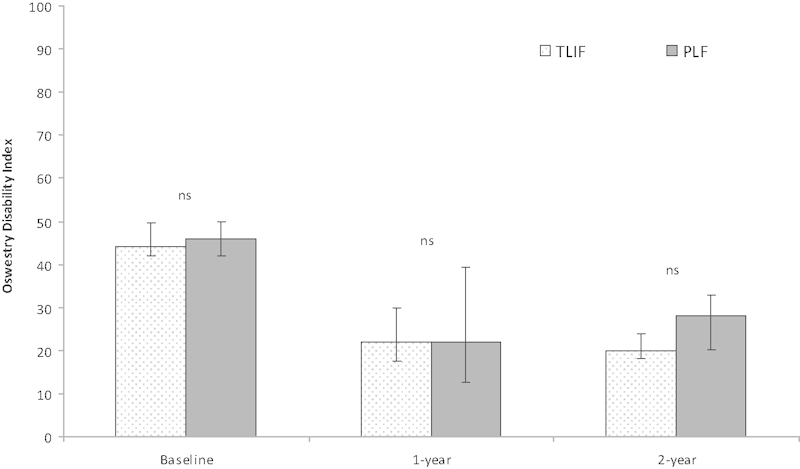
Median Oswestry Disability Index (0 to 100) in patients operated on by PLF or TLIF. Vertical bars indicate 95% confidence limits. Abbreviations: ns, not significant; PLF, posterolateral fusion; TLIF, transforaminal lumbar interbody fusion.
Fig. 3.

Median disability rating index (0 to 100) in patients operated on by PLF or TLIF. Vertical bars indicate 95% confidence limits. Abbreviations: ns, not significant; PLF, posterolateral fusion; TLIF, transforaminal lumbar interbody fusion.
When comparing the two treatment groups at 1-year follow-up, there were no significant differences in PI, ODI, or DRI. At 2-year follow-up, all three outcome measurements (PI, ODI, and DRI) were superior in the TLIF group compared with the PLF group; the difference was significant for PI and DRI, but not for ODI (Table 3). The PI in the TLIF group improved by 6 points as compared with 4 points in the PLF group (p = 0.007). The DRI in the TLIF group improved by 34.4 points as compared with 16.5 points in the PLF group (p = 0.017). However, the difference in ODI improvement between the groups was not significant; the TLIF group improved by 22.9 points compared with 18.5 points in the PLF group.
Table 3. Patient reported outcome at 2 years.
| TLIF (n = 68) | PLF (n = 67) | p Value | |
|---|---|---|---|
| Pain index (median) | 2.0 | 3.9 | 0.007 |
| DRI (median) | 22 | 36 | 0.003 |
| ODI (median) | 20 | 28 | 0.110 |
| Global assessment (%) | 0.017 | ||
| Much better | 63 | 48 | |
| Better | 28 | 21 | |
| Unchanged | 6 | 19 | |
| Worse | 3 | 12 | |
| Working | 48 | 52 | 0.617 |
Abbreviations: DRI, disability rating index; ODI, Oswestry disability index; PLF, posterolateral fusion; TLIF, transforaminal lumbar interbody fusion.
The global assessment at 2-year follow-up was superior in the TLIF group (p = 0.017) (Table 3). The percentage was much better (63%) in the TLIF group as compared with the PLF group (48%; Table 4). There were no significant differences in return to work between the groups (48 and 52% in TLIF and PLF, respectively). There were no significant differences in outcome between one-level and two-level fusions.
Table 4. Outcome according to global assessment at 2 years.
| TLIF | PLF | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | % | PI | ODI | DRI | % | PI | ODI | DRI |
| Much better | 63 | 1.0 | 20.0 | 15.8 | 48 | 0.8 | 12.0 | 16.5 |
| Better | 28 | 4.0 | 28.0 | 33.3 | 21 | 3.5 | 30.0 | 42.0 |
| Unchanged | 6 | 6.0 | 44.0 | 46.8 | 19 | 6.7 | 43.5 | 55.0 |
| Worse | 3 | 6.2 | 55.0 | 47.6 | 12 | 7.5 | 56.0 | 63.5 |
Abbreviations: DRI, disability rating index; ODI, Oswestry disability index; PI, pain index; PLF, posterolateral fusion; TLIF, transforaminal lumbar interbody fusion.
The reoperation rate was 12/67 (17.9%) in the PLF group as compared with 5/68 (7.4%) in the TLIF group (p = 0.12). The cause of reoperations in the PLF group was symptomatic pseudarthrosis. The reasons for the five reoperations in the TLIF group were as follows: novel low back pain caused by adjacent-segment degeneration in three patients, one implant removal because of local pain, and one removal of bone graft fragment from nerve root canal causing severe pain. There was no symptomatic pseudarthrosis in the TLIF group. The fusion rate was 80% in the PLF group and 87% in the TLIF group (not significant).
In total, there were eight complications (6%), four in each group (Table 5). There were two nerve root injuries with postoperative leg pain and paresthesia corresponding to the nerve root at the TLIF site. Both patients recovered spontaneously within 6 months. One patient in the TLIF group was re-operated because of severe leg pain caused by nerve root compression due to a bony fragment in the root canal. In the PLF group, there were two dural lesions, primarily sutured, with no sequelae. The L5 nerve root was cut in one patient in the PLF group with no postoperative neurologic consequence.
Table 5. Complications according to treatment.
| Treatment | n |
|---|---|
| TLIF | |
| Battered nerve root | 2 |
| Bone fragment in root canal | 1 |
| Superficial wound infection | 1 |
| PLF | |
| Dural lesion | 2 |
| Bronchial pneumonia | 1 |
| Nerve root cut off | 1 |
Abbreviations: PLF, posterolateral fusion; TLIF, transforaminal lumbar interbody fusion.
Discussion
The results of the current study showed improved clinical outcomes both in TLIF and PLF groups in a mixed-patient selection with chronic lumbar pain. The strength of the study is the randomized design, the use of multiple validated outcome measurements, including return to work and high follow-up rate. A possible drawback was the relatively limited number of patients. The power analysis, however, indicated that 98 patients were sufficient to detect a minimal clinically important difference (MCID) set at 10 points in the ODI with 90% power at significance level 0.05, a much smaller number than the actual number of patients (135) in the current study. Furthermore, although the ODI improved more in the TLIF group, the difference was not significant and far below the MCID. Similarly, the proportion of patients working at follow-up was not different between the groups.
Notwithstanding these limitations and problems in interpretation of the results, the clinical outcome of the TLIF group must be considered as clearly superior compared with the PLF group, particularly in view of the substantial difference in global assessment with 63% “much better” after TLIF, compared with 48% after PLF. In addition, in the literature generally much better rates have been reported after spinal fusion, usually 29 to 55%,1 14 compared with our results. The global assessment has the disadvantage of possible recall bias. However, in the comparison between the groups, this problem does not invalidate the main finding of a superior outcome in the TLIF group. An improved outcome with TLIF was further supported by significantly better scores in the PI and DRI. The observed difference between the groups in the PI (19 mm) was the same as the reported MCID (18 to 19 mm) by Hägg et al but lower than the MCID reported by Glassman et al (25 mm).15 16 The finding of a larger improvement in the DRI than in the ODI confirms a previous observation after fusion in adult spondylolisthesis, suggesting the DRI to be more sensitive to change than the ODI.17 It may be that the DRI is a more disease-specific questionnaire on lumbar spine functional disability compared with the ODI, which includes more general aspects such as social and sexual function as well as pain.
Although the difference in improvement between the groups were less than suggested, as seen in the MCID levels for the PI and ODI, the substantial difference in global assessment between the groups strongly suggest an improved outcome with TLIF. The concept of the MCID is controversial, and there is no consensus on either the appropriate levels or the general usefulness of the concept.18 19 20
The results in our study are somewhat in contrast to the randomized studies by Fritzell et al and by Ekman et al showing a similar outcome between PLF and PLIF.14 21 Furthermore, our study is also in contrast to the findings of Høy et al (i.e., no difference in the outcome between TLIF and instrumented PLF). An obvious possible explanation for this difference is the fact that our control group had uninstrumented PLF.7 On the other hand, Videbaek et al concluded that circumferentially fused patients had a significantly improved outcome compared with those treated by means of PLF.22 In contrast to other reports on interbody fusion using interbody cages, we used an interbody spacer made of porous tantalum with a low modulus of elasticity minimizing stress shielding and with the possibility of bony ingrowth through the implant,23 thus increasing the total possible interbody fusion surface, as compared with other devices. Whether this has affected fusion rate and ultimately clinical outcome is difficult to ascertain. Several clinical studies of TLIF have reported high fusion rates and good clinical outcomes, and with a lower rate of complications than the PLIF procedure.24 25
Complications and fusion rates for TLIF have recently been described in a meta-analysis by Wu et al,26 showing a fusion rate from 16 studies of 91% and a complication rate of 12.6%. Our fusion rate in PLF is typical of what is reported in the literature.27
In our study, we found four complications (6%) in the TLIF group related to the procedure, all with radicular pain, one of which needed reoperation. The rest recovered spontaneously from their leg pain. In the PLF group, there were only complications unrelated to the procedure. Cutting of the L5 nerve root had no consequence, probably because of multiple innervations of the leg muscles.
The randomization resulted in a skewed distribution of number of levels fused in the TLIF compared with the PLF group. This was an unfortunate event of the random allocation of patients. The effect on the outcome cannot be ascertained; on one hand, the more levels fused in the TLIF group may reflect a more severe disorder, but on the other hand, more levels fused may in fact increase the chances of a good outcome. Overall, it seems more likely that the fact that more levels were fused reflects a more severe disorder in the TLIF group, not invalidating the conclusion that TLIF seems to be a more efficient fusion technique in the degenerative spine.
Noninstrumented PLF was used as a control group because several studies have shown a similar outcome for PLFs with and without instrumentation, even at long-term follow-up.14 17 21 28 29 One can argue that the worse outcome with PLF was an effect of a much higher reoperation rate because of pseudarthrosis: 17.9% versus 0% in PLF and TLIF, respectively. This difference in the reoperation rate because of pseudarthrosis is in contrast to the fact that formal analysis of fusion rate, performed by an experienced spinal surgeon not involved with the treatment, showed no significant difference in fusion rate. However, one must be aware of the well-known difficulties to assess fusion based on standard radiographs,13 as in the present study. Furthermore, there is contradicting data in trials regarding the clinical benefits of higher fusion rates. Some authors have found that despite higher fusion rates, there is no corresponding increase in the clinical outcome,28 30 31 32 33 whereas one study reported a correlation between higher fusion rates and better clinical outcome.34
Conclusion
The results of the current study support the use of TLIF rather than PLF in the surgical treatment of chronic low back pain.
Footnotes
Disclosures Kourosh Jalalpour, none Pavel Neumann, none Christer Johansson, none Rune Hedlund, Grant: Zimmer Spine; Consulting: Depuy
References
- 1.Fritzell P Hägg O Wessberg P Nordwall A; Swedish Lumbar Spine Study Group. 2001 Volvo Award Winner in Clinical Studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group Spine (Phila Pa 1976) 200126232521–2532., discussion 2532–2534 [DOI] [PubMed] [Google Scholar]
- 2.Babu M A, Coumans J V, Carter B S. et al. A review of lumbar spinal instrumentation: evidence and controversy. J Neurol Neurosurg Psychiatry. 2011;82(9):948–951. doi: 10.1136/jnnp.2010.231860. [DOI] [PubMed] [Google Scholar]
- 3.Frymoyer J W, Selby D K. Segmental instability. Rationale for treatment. Spine (Phila Pa 1976) 1985;10(3):280–286. doi: 10.1097/00007632-198504000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Adams M A, Roughley P J. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 2006;31(18):2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 5.Edgar M A. The nerve supply of the lumbar intervertebral disc. J Bone Joint Surg Br. 2007;89(9):1135–1139. doi: 10.1302/0301-620X.89B9.18939. [DOI] [PubMed] [Google Scholar]
- 6.Christensen F B, Hansen E S, Eiskjaer S P. et al. Circumferential lumbar spinal fusion with Brantigan cage versus posterolateral fusion with titanium Cotrel-Dubousset instrumentation: a prospective, randomized clinical study of 146 patients. Spine (Phila Pa 1976) 2002;27(23):2674–2683. doi: 10.1097/00007632-200212010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Høy K, Bünger C, Niederman B. et al. Transforaminal lumbar interbody fusion (TLIF) versus posterolateral instrumented fusion (PLF) in degenerative lumbar disorders: a randomized clinical trial with 2-year follow-up. Eur Spine J. 2013;22(9):2022–2029. doi: 10.1007/s00586-013-2760-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anjarwalla N K, Morcom R K, Fraser R D. Supplementary stabilization with anterior lumbar intervertebral fusion—a radiologic review. Spine (Phila Pa 1976) 2006;31(11):1281–1287. doi: 10.1097/01.brs.0000217692.90624.ab. [DOI] [PubMed] [Google Scholar]
- 9.Harms J G, Jeszenszky D. Die posteriore, lumbale, interkorporelle Fusion in unilateraler transforaminaler Technik. Orthop Traumatol. 1998;10:90–102. doi: 10.1007/s00064-006-0112-7. [DOI] [PubMed] [Google Scholar]
- 10.Zou X, Li H, Bünger M, Egund N, Lind M, Bünger C. Bone ingrowth characteristics of porous tantalum and carbon fiber interbody devices: an experimental study in pigs. Spine J. 2004;4(1):99–105. doi: 10.1016/s1529-9430(03)00407-8. [DOI] [PubMed] [Google Scholar]
- 11.Salén B A, Spangfort E V, Nygren A L, Nordemar R. The Disability Rating Index: an instrument for the assessment of disability in clinical settings. J Clin Epidemiol. 1994;47(12):1423–1435. doi: 10.1016/0895-4356(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 12.Fairbank J C, Couper J, Davies J B, O'Brien J P. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271–273. [PubMed] [Google Scholar]
- 13.Lenke L G, Bridwell K H, Bullis D, Betz R R, Baldus C, Schoenecker P L. Results of in situ fusion for isthmic spondylolisthesis. J Spinal Disord. 1992;5(4):433–442. doi: 10.1097/00002517-199212000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Ekman P, Möller H, Tullberg T, Neumann P, Hedlund R. Posterior lumbar interbody fusion versus posterolateral fusion in adult isthmic spondylolisthesis. Spine (Phila Pa 1976) 2007;32(20):2178–2183. doi: 10.1097/BRS.0b013e31814b1bd8. [DOI] [PubMed] [Google Scholar]
- 15.Hägg O Fritzell P Nordwall A; Swedish Lumbar Spine Study Group. The clinical importance of changes in outcome scores after treatment for chronic low back pain Eur Spine J 200312112–20. [DOI] [PubMed] [Google Scholar]
- 16.Glassman S D, Copay A G, Berven S H, Polly D W, Subach B R, Carreon L Y. Defining substantial clinical benefit following lumbar spine arthrodesis. J Bone Joint Surg Am. 2008;90(9):1839–1847. doi: 10.2106/JBJS.G.01095. [DOI] [PubMed] [Google Scholar]
- 17.Ekman P, Möller H, Hedlund R. The long-term effect of posterolateral fusion in adult isthmic spondylolisthesis: a randomized controlled study. Spine J. 2005;5(1):36–44. doi: 10.1016/j.spinee.2004.05.249. [DOI] [PubMed] [Google Scholar]
- 18.Carragee E J. The rise and fall of the “minimum clinically important difference.”. Spine J. 2010;10(4):283–284. doi: 10.1016/j.spinee.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Gatchel R J, Mayer T G, Chou R. What does/should the minimum clinically important difference measure? A reconsideration of its clinical value in evaluating efficacy of lumbar fusion surgery. Clin J Pain. 2012;28(5):387–397. doi: 10.1097/AJP.0b013e3182327f20. [DOI] [PubMed] [Google Scholar]
- 20.Copay A G, Subach B R, Glassman S D, Polly D W Jr, Schuler T C. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541–546. doi: 10.1016/j.spinee.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Fritzell P Hägg O Wessberg P Nordwall A; Swedish Lumbar Spine Study Group. Chronic low back pain and fusion: a comparison of three surgical techniques: a prospective multicenter randomized study from the Swedish lumbar spine study group Spine (Phila Pa 1976) 200227111131–1141. [DOI] [PubMed] [Google Scholar]
- 22.Videbaek T S, Christensen F B, Soegaard R. et al. Circumferential fusion improves outcome in comparison with instrumented posterolateral fusion: long-term results of a randomized clinical trial. Spine (Phila Pa 1976) 2006;31(25):2875–2880. doi: 10.1097/01.brs.0000247793.99827.b7. [DOI] [PubMed] [Google Scholar]
- 23.Zou X, Li H, Teng X. et al. Pedicle screw fixation enhances anterior lumbar interbody fusion with porous tantalum cages: an experimental study in pigs. Spine (Phila Pa 1976) 2005;30(14):E392–E399. doi: 10.1097/01.brs.0000170588.80377.3f. [DOI] [PubMed] [Google Scholar]
- 24.Lowe T G, Tahernia A D, O'Brien M F, Smith D A. Unilateral transforaminal posterior lumbar interbody fusion (TLIF): indications, technique, and 2-year results. J Spinal Disord Tech. 2002;15(1):31–38. doi: 10.1097/00024720-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Humphreys S C, Hodges S D, Patwardhan A G, Eck J C, Murphy R B, Covington L A. Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine (Phila Pa 1976) 2001;26(5):567–571. doi: 10.1097/00007632-200103010-00023. [DOI] [PubMed] [Google Scholar]
- 26.Wu R H, Fraser J F, Härtl R. Minimal access versus open transforaminal lumbar interbody fusion: meta-analysis of fusion rates. Spine (Phila Pa 1976) 2010;35(26):2273–2281. doi: 10.1097/BRS.0b013e3181cd42cc. [DOI] [PubMed] [Google Scholar]
- 27.Lee C S, Hwang C J, Lee D H, Kim Y T, Lee H S. Fusion rates of instrumented lumbar spinal arthrodesis according to surgical approach: a systematic review of randomized trials. Clin Orthop Surg. 2011;3(1):39–47. doi: 10.4055/cios.2011.3.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjarke Christensen F, Stender Hansen E, Laursen M, Thomsen K, Bünger C E. Long-term functional outcome of pedicle screw instrumentation as a support for posterolateral spinal fusion: randomized clinical study with a 5-year follow-up. Spine (Phila Pa 1976) 2002;27(12):1269–1277. doi: 10.1097/00007632-200206150-00006. [DOI] [PubMed] [Google Scholar]
- 29.Andersen T, Videbaek T S, Hansen E S, Bünger C, Christensen F B. The positive effect of posterolateral lumbar spinal fusion is preserved at long-term follow-up: a RCT with 11–13 year follow-up. Eur Spine J. 2008;17(2):272–280. doi: 10.1007/s00586-007-0494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischgrund J S, Mackay M, Herkowitz H N, Brower R, Montgomery D M, Kurz L T. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine (Phila Pa 1976) 1997;22(24):2807–2812. doi: 10.1097/00007632-199712150-00003. [DOI] [PubMed] [Google Scholar]
- 31.France J C, Yaszemski M J, Lauerman W C. et al. A randomized prospective study of posterolateral lumbar fusion. Outcomes with and without pedicle screw instrumentation. Spine (Phila Pa 1976) 1999;24(6):553–560. doi: 10.1097/00007632-199903150-00010. [DOI] [PubMed] [Google Scholar]
- 32.Mardjetko S M Connolly P J Shott S Degenerative lumbar spondylolisthesis. A meta-analysis of literature 1970–1993 Spine (Phila Pa 1976) 199419(20, Suppl):2256S–2265S. [PubMed] [Google Scholar]
- 33.Fritzell P Hägg O Wessberg P Nordwall A; Swedish Lumbar Spine Study Group. Chronic low back pain and fusion: a comparison of three surgical techniques: a prospective multicenter randomized study from the Swedish lumbar spine study group Spine (Phila Pa 1976) 200227111131–1141. [DOI] [PubMed] [Google Scholar]
- 34.Zdeblick T A. A prospective, randomized study of lumbar fusion. Preliminary results. Spine (Phila Pa 1976) 1993;18(8):983–991. doi: 10.1097/00007632-199306150-00006. [DOI] [PubMed] [Google Scholar]


