Abstract
Background: Short-term dietary restriction (DR) without malnutrition preconditions against surgical stress in rodents; however, the nutritional basis and underlying nutrient/energy-sensing pathways remain poorly understood.
Objectives: We investigated the relative contribution of protein restriction (PR) vs. calorie restriction (CR) to protection from renal ischemia reperfusion injury (IRI) and changes in organ-autonomous nutrient/energy-sensing pathways and hormones underlying beneficial effects.
Methods: Mice were preconditioned on experimental diets lacking total calories (0–50% CR) or protein/essential amino acids (EAAs) vs. complete diets consumed ad libitum (AL) for 1 wk before IRI. Renal outcome was assessed by serum markers and histology and integrated over a 2-dimensional protein/energy landscape by geometric framework analysis. Changes in renal nutrient/energy-sensing signal transduction and systemic hormones leptin and adiponectin were also measured. The genetic requirement for amino acid sensing via general control non-derepressible 2 (GCN2) was tested with knockout vs. control mice. The involvement of the hormone leptin was tested by injection of recombinant protein vs. vehicle during the preconditioning period.
Results: CR-mediated protection was dose dependent up to 50% with maximal 2-fold effect sizes. PR benefits were abrogated by EAA re-addition and additive with CR, with maximal benefits at any given amount of CR occurring with a protein-free diet. GCN2 was not required for functional benefits of PR. Activation and repression of nutrient/energy-sensing kinases, AMP-activated protein kinase (AMPK) and mechanistic target of rapamycin complex 1 (mTORC1), respectively, on PR reflected a state of negative energy balance, paralleled by 13% weight loss and an 87% decrease in leptin, independent of calorie intake. Recombinant leptin administration partially abrogated benefits of dietary preconditioning against renal IRI.
Conclusions: In male mice, PR and CR both contributed to the benefits of short-term DR against renal IRI independent of GCN2 but partially dependent on reduced circulating leptin and coincident with AMPK activation and mTORC1 repression.
Keywords: dietary restriction, protein restriction, calorie restriction, amino acid sensing, ischemia reperfusion injury, leptin, geometric framework, GCN2, mTOR, AMPK
Introduction
Dietary restriction (DR)10, defined as reduced food intake without malnutrition, describes various interventional approaches that consistently extend life span in most organisms tested, including yeast, fruit flies, worms, and rodents (1, 2). DR also improves metabolic fitness and increases resistance to a number of acute stressors, ranging from heat shock to oxidative stress to starvation. DR has similar benefits on metabolic fitness in humans, improving glucose homeostasis, altering circulating lipid profiles, and improving cardiovascular health (3–6).
Recent data indicate beneficial effects of short-term application of DR (in the range of days to weeks) in preclinical models (7). For example, fasting and DR can precondition against oxidative and genotoxic stress associated with endpoints that range from chemotherapy to surgical ischemia reperfusion injury (IRI) (8–11). Importantly, short-term DR can precondition against focal stroke in rats, one of the main complications of cardiovascular surgery, resulting in considerable morbidity and mortality, and for which there is currently no commonly accepted risk mitigation strategy (12).
Whether the macronutrient source of calories (carbohydrates, fat, or protein) is important in the benefits of DR and, indeed, whether calorie restriction (CR) is a better name, is still a matter of debate. In rodents, the nutritional basis of DR was most heavily studied in the context of longevity and aging-related disorders, and is dose dependent, with life span increasing with decreasing calorie intake up to near the point of starvation (13–15). Early experimental attempts to characterize the relative contribution of reduced calories vs. reduction of specific nutrients led to a range of conclusions. Some showed additive benefits of protein restriction (PR) and energy restriction (16). Others showed a negligible influence of the source of calories (protein, fat) (17). Still others showed the importance of PR in the benefits of DR (18, 19). In the fruit fly, restriction of calories from yeast (the sole source of protein) imparts greater life span extension than reduction of the same amount of calories from sucrose (20, 21). Moreover, add-back of essential amino acids (EAAs) to a restricted diet optimized for longevity abrogates life span extension (22). Together, these findings support the notion that protein sensing may play an evolutionarily conserved role in conferring the protection induced by DR.
The mechanisms underlying protein/EAA sensing and how they trigger a protective response are largely unknown. Intracellular AA-sensing pathways include the general control non-derepressible 2 (GCN2) kinase and the mechanistic target of rapamycin complex 1 (mTORC1). GCN2 senses AA insufficiency by binding to cognate uncharged transfer RNAs (tRNAs) (23). Activated GCN2 phosphorylates and inactivates the eukaryotic initiation factor 2α (eIF2α), setting off a cascade of events that result in altered translation and transcription (23). AA sufficiency, however, is sensed indirectly by mTORC1, a kinase that integrates availability of nutrients with the presence of growth factors and energy (24). In the presence of permissive AAs such as leucine (25), mTORC1 phosphorylates downstream targets, including the p70 ribosomal S6 protein kinase (S6K) and the eukaryotic initiation factor 4E-binding protein 1 (4E-BP1), with pleiotropic effects on cell growth and metabolism (26). Importantly, mTORC1 is negatively regulated by the energy-sensing protein AMP-activated protein kinase (AMPK), which is allosterically activated at low energetic cellular amounts by AMP (27). AMPK and mTORC1 activity are also coordinately regulated by fat-derived hormones (adipokines), including adiponectin and leptin, which convey information on energy availability in an endocrine fashion; AMPK is activated by adiponectin (28–30), and mTORC1 is activated by leptin (31, 32).
In mammals, the benefits of short-term DR in a preclinical model of IRI to the kidney or the liver can be achieved by a variety of dietary preconditioning regimens, including fasting, reduced food intake (8), sugar-only liquid diets (33), PR (34), or restriction of an EAA (11). In the case of tryptophan restriction, GCN2 is required for benefits against renal and hepatic IRI (11). In the liver, however, benefits of PR are maintained in mice that lack GCN2 but not in mice with constitutive mTORC1 activation on genetic ablation of the tuberous sclerosis complex (TSC) repressor complex (34). In the kidney, the relative importance of CR vs. reduced protein or EAAs and the role of associated nutrient/energy-sensing mechanisms for protection against renal IRI are not known. Here, we used diet-induced protection against renal IRI to define the contribution of protein vs. carbohydrate/fat CR in the benefits of short-term DR that lasted 1 wk, and further to define the nutrient/energy-sensing pathways involved.
Methods
Mice.
Male, 8-wk-old B6D2F1/J hybrid mice were purchased from The Jackson Laboratory. GCN2 knockout (KO) and control C57BL/6 mice were obtained as previously reported (11). Mice were maintained under standard laboratory conditions and allowed free access to water and food except as noted. Mice were killed by cervical dislocation while under isoflurane anesthesia. All experiments were performed with the approval of the Harvard Medical Area Institutional Animal Care and Use Committee.
Diets and feeding.
Semipurified diets (Research Diets) were purchased in powdered form for customization with sucrose, casein, or crystalline l-AAs (Ajinmoto North America, Inc.) (Supplemental Tables 1–3) (35) and delivered in a final 1% agar form (34) to prevent loss of food due to crumbling of the pellets, allowing for accurate measurements of daily food intake. This soft, nonpelleted form also prevented the potential of dominant mice from sequestering individual food pellets and eating more than others in the cage, thus allowing for group housing of mice and the resulting improved ability to maintain body temperature both during the preconditioning period and after the surgery. Experimental diets were provided daily at ∼1800 before the dark phase when mice consume most of their food. At the same time, weight of food remaining and individual mouse weights were recorded for calculating food intake per gram of body weight on a per cage basis each day.
Renal ischemia reperfusion model.
Bilateral renal ischemia for 30 min, followed by reperfusion for ≤3 d, was performed as described previously (11, 36). Surgery orders within each experimental group were staggered over the time it took to complete the procedures, typically between 1000 and 1500. All mice were returned to clean cages and given ad libitum (AL) access to a complete diet immediately after surgery. Urea was measured in serum collected before ischemia and daily for 3 d after reperfusion by using a modified Jung method as described previously (11, 36). Serum urea values from each of these time points were used to calculate an AUC value of renal function for each mouse, with higher serum urea AUCs representing increased renal dysfunction. Serum creatinine was measured as described previously (11, 36).
Blood and serum measurements.
Blood glucose determinations were performed on fresh blood with an Easy Check Diabetes Meter Kit (Home Aide Diagnostics) according to the manufacturer’s instructions. Serum-free AAs from a fixed volume of serum were analyzed by mass spectrometry as described previously (34). Serum leptin and adiponectin were determined with ELISA kits (R&D Systems).
Histopathology.
Kidneys were harvested 3 d after IRI and fixed in 10% buffered formalin for paraffin embedding, sectioning, and hematoxylin/eosin staining. A minimum of 3 sections, 500 μm apart on each kidney, were analyzed at a 40× magnification for signs of tubular necrosis, including tubular cell swelling, vacuolization, nuclear degradation, and disruption of the normal tubular structural characteristics. The percentage of tubular necrosis in the cortex and corticomedullar region was estimated from a minimum of 15 fields per section according to the following histopathologic grading scale: 1) 0–25% necrosis, 2) 25–50% necrosis, 3) 50–75% necrosis, and 4) 75–100% necrosis.
Immunoblotting.
Extracts from snap-frozen kidneys homogenized with an IKA T10 Ultra-Turrax were resolved by SDS-PAGE and transferred to Immobilon-P polyvinylidene difluoride membranes (Millipore). The list of antibodies and dilutions is included in Supplemental Methods. Quantitation of chemiluminescent signals on film was performed with Image J (NIH).
Leptin administration.
Recombinant mouse leptin (10 μg; R&D Systems) in saline was administered twice daily by intraperitoneal injection during the preconditioning period.
Experiment 1: Dose-dependent effects of 1 wk of CR against bilateral renal IRI.
The purpose of this experiment was to establish the dose-response relation between severity of food restriction for 1 wk and protection from renal IRI. We used an experimental design with between-subjects factor of food dose. Group-housed mice (4–5 mice/cage, 2–3 cages per group) were preconditioned for 1 wk with a complete diet (Supplemental Table 1) consumed AL or restricted up to ∼50% daily. Because food intake of AL-fed mice varied from experiment to experiment, the amount of food given to the restricted groups was based on a fixed energy intake per initial weight of mice in the cage (1.7–0.9 kJ/g) and remained constant throughout the preconditioning period. After preconditioning, all mice were subject to renal IRI. Food intake and body weight were measured daily before and after renal IRI. Blood glucose was measured immediately before renal IRI. Serum urea was measured before ischemia and daily for 3 d after reperfusion. Renal histology was assessed on day 3 after reperfusion.
Experiment 2: PR vs. carbohydrate restriction in protection from renal IRI by CR.
The purpose of this experiment was to measure the relative contribution of restriction of an equal number of calories derived specifically from protein or carbohydrate relative to a complete diet on protection from renal IRI. We used an experimental design with between-subjects factor of diet (complete, protein CR, sucrose CR). Because the magnitude of food restriction that yielded significant functional benefits in experiment 1 was on the order of 30%, we constructed the protein CR and sucrose CR diets so that >30% of calories from these macronutrients could be withheld while keeping intake of other macronutrients and micronutrients constant. The resulting complete diet contained 34% protein (casein) and 34% carbohydrate (sucrose) by weight (Supplemental Table 2). In the first arm of this experiment, group-housed mice (4–5 mice/cage, 2–3 cages per group) were preconditioned for 1 wk with the complete diet consumed AL or with restricted amounts of the protein CR or sucrose CR diets so that intake of fat and micronutrients was held constant among all groups, and that CR was specifically from an isocaloric amount of protein or sucrose, respectively. Food intake and body weight were measured daily before and after renal IRI. Blood glucose was measured immediately before renal IRI. Serum urea was measured before ischemia and daily for 3 d after reperfusion. A second arm of the experiment was performed to measure the relative contribution of restriction of protein vs. sucrose CR in the context of overall reduced calorie intake. It used a similar design as in arm 1 with 10 mice/group in cages of 5 mice each, but with a further ∼20% enforced food restriction in each diet group (complete, protein CR, sucrose CR) relative to arm 1.
Experiment 3: Role of AA composition in protection from renal IRI by PR.
The purpose of this experiment was to test whether protection from renal IRI mediated by PR was specific to restriction of total AAs or particular groups of AAs, namely EAAs or non-EAAs (NEAAs). We used an experimental design with between-subjects factor of dietary AA composition [complete, protein free (PF), NEAA only, EAA only]. In the first arm of this experiment, mice (n = 5/group) consumed AL for 1 wk before induction of renal IRI with 1 of 4 different isocaloric diets as described in Supplemental Table 3, with 18% calories from casein (complete), sucrose (PF), casein NEAA (NEAA only), or casein EAA (EAA only). Food intake and body weight were measured daily before and after renal IRI. Blood glucose was measured immediately before renal IRI. Serum urea was measured before ischemia and daily for 3 d after reperfusion. A second arm of the experiment with the same purpose, experimental design, and measures was performed with 5–10 mice/group preconditioned with the same 4 diets for 1 wk but at 50% overall CR to overcome differential intake of these diets observed on AL feeding in arm 1.
Experiment 4: Evaluation of the effects of 1 wk of combined PR and CR against renal IRI in male mice.
The purpose of this experiment was to determine whether PR and CR were additive in their ability to protect against renal IRI. We used an experimental design with between-subjects factor of food dose by using a PF diet. Group-housed mice (4–5 per cage, 2–3 cages per group) were preconditioned for 1 wk with a PF diet (Supplemental Table 1) consumed AL or restricted up to ∼40% daily, based on a fixed energy intake per initial weight of mice in the cage (1.7–1.1 kJ/g). Food intake and body weight were measured daily before and after renal IRI. Blood glucose was measured immediately before renal IRI. Serum urea was measured before ischemia and daily for 3 d after reperfusion.
Experiment 5: Molecular mechanisms of dietary preconditioning against renal IRI.
The purpose of this experiment was to investigate changes in nutrient/energy signal transduction in kidneys and systemic changes in metabolism and circulating energy metabolites and adipokine hormones as a function of PR and CR to understand underlying mechanisms of DR-mediated protection from renal IRI. A 2 × 2 study design was used in which 4 groups of mice (n = 5/group) were fed complete or PF diets (Supplemental Table 1) either AL or 50% restricted for 1 wk before harvest. Food intake and body weights were measured daily. Extracts prepared from frozen kidneys were used for immunoblotting of markers of target nutrient/energy-sensing pathways. Serum collected at harvest was used for analysis of circulating free AAs and adipokines. Serum-free AAs were divided into EAAs and NEAAs and were expressed as a percentage of the mean of the AL complete diet group.
Experiment 6: Genetic requirement for GCN2 in dietary preconditioning against renal IRI.
The purpose of this experiment was to test the genetic requirement for GCN2 in PR-mediated protection from renal IRI. We used an experimental design with between-subjects factor of protein dose (complete vs. PF diet; Supplemental Table 1). GCN2 KO mice (n = 9/group) and littermate control mice (n = 5/group) were preconditioned for 1 wk with complete or PF diets before induction of renal IRI. Serum urea was measured 1 d after reperfusion.
Experiment 7: Role of leptin in dietary preconditioning against renal IRI.
The purpose of this experiment was to test the role of reduced circulating leptin in DR-mediated protection from renal IRI. We used an experimental design with between-subjects factor of recombinant leptin treatment. Mice preconditioned for 1 wk with a PF diet (Supplemental Table 1) restricted daily by ∼40% (n = 14/group) or a complete diet (Supplemental Table 1) consumed AL (n = 10–15/group) were treated with recombinant leptin or vehicle (saline) twice daily during the preconditioning period before induction of renal IRI. Serum urea was measured 1 d after reperfusion.
Modeling.
For curve fitting, serum urea AUC data from experiments 1 and 4 were fitted to be exponentially dependent, and corresponding Pearson’s correlation was calculated with the stats package in R version 3.1.0 (R Development Core Team) (37). For geometric framework, statistical modeling of the data from experiments 1, 2, and 4 was performed with the help of thin-plate splines over the diet composition (protein vs. fat/carbohydrate calories) as described previously (20).
Statistical analysis.
Data are expressed as the mean ± SEM. Statistical analyses were performed in GraphPad Prism (GraphPad Inc.). Measures from experiments 1–4 with between-subjects designs were analyzed by 1-factor ANOVA with Dunnett’s multiple comparisons test to compare means to the corresponding complete diet control group fed on an AL basis or restricted as in the second arms of experiments 2 and 3. In experiment 1, Pearson’s correlations were calculated between food dose and preoperative weight loss, blood glucose, or change in postoperative weight and between serum urea AUC and histopathology score or change in postoperative weight. Measures from experiment 5 with a 2 × 2 design were analyzed by 2-factor ANOVA with Tukey’s multiple comparisons tests that compared all combinations of means, including main effects of dietary protein content, calorie intake, and interactions. Student’s t tests were used to compare means between diet groups within each genotype in experiment 6 and between leptin treatment groups within diet group in experiment 7. P ≤ 0.05 was considered significant.
Results
Protection against renal IRI by CR is dose dependent.
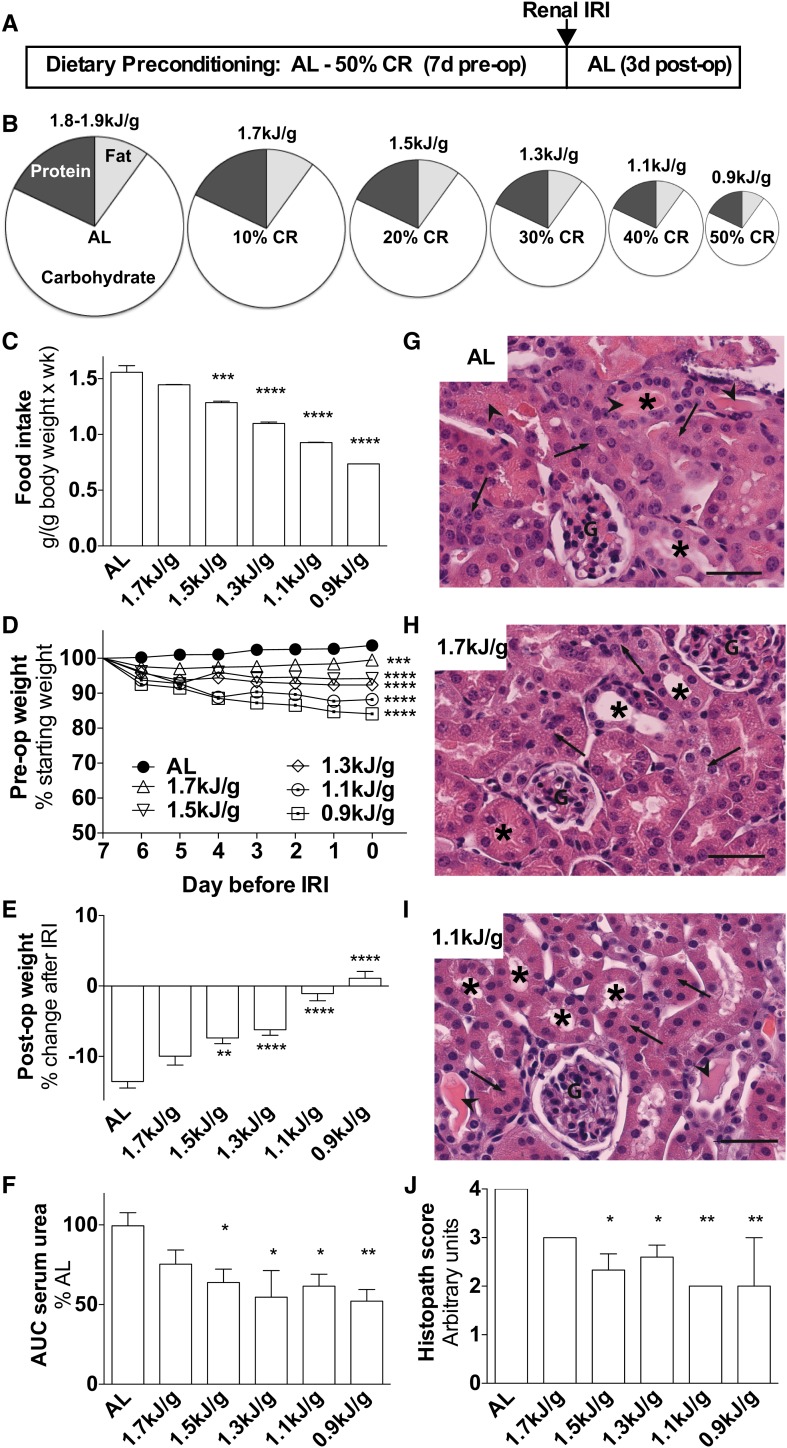
In experiment 1, mice were preconditioned over a range of restricted food amounts to establish the dose-response to 1 wk of CR ( ). Food restriction was fixed to initial body weight (Supplemental Figure 1A) and correlated significantly with changes in body weight (Figure 1D; r = 0.99, P < 0.0001) and blood glucose concentrations (Supplemental Figure 1B; r = 0.94, P = 0.0012) at the end of 1 wk of preconditioning. After surgical induction of renal IRI, all mice continued to lose body weight despite AL access to a complete diet; however, mice preconditioned at 1.5–0.9 kJ/g lost significantly less weight because of increased food intake after surgery in a dose-dependent fashion that correlated with the magnitude of food restriction before surgery (Figure 1E; r = −0.99, P = 0.0002).
FIGURE 1.
Dose-dependent effects of 1 wk of CR against bilateral renal IRI in male mice in experiment 1. (A) Schematic of preconditioning and postconditioning feeding regimens relative to the timing of surgical renal IRI induction (arrow). (B) Schematic of diets with pie slices proportional to the calorie content of the indicated macronutrient and circle areas proportional to the indicated level of daily energy intake. (C) Total food intake during the preconditioning period of the indicated groups; n = 2–3 cages/group. (D) Mouse weights during the preconditioning period are expressed as percentage of starting weight. (E) Postoperative weight on day 3 after IRI is expressed as a percentage of change after IRI. (F) Kidney function after renal IRI is expressed as a percentage of serum urea AUC of the AL group. (G–I) Representative hematoxylin and eosin–stained kidney sections from the indicated diet groups 3 d after injury. Arrows point to areas of tubular necrosis as evidenced by nuclear changes and cytoplasmic lysis or vacuolization. Arrowheads show tubular cast formation. Asterisks are positioned over tubules that show a normal structural characteristic. Scale bar = 50 μm. (J) Histopathology scores indicative of renal damage with 1 representing the least (0–25%) tubular damage and 4 representing the most (75–100%). Values are means ± SEMs; n = 8–13 mice/group. *,**,***,****Different from the AL diet group: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. AL, ad libitum; CR, calorie restriction; G, glomerulus; IRI, ischemia reperfusion injury; post-op, postoperative; pre-op, preoperative.
Serum urea AUC, a measure of renal function, was significantly improved by preconditioning at 1.5–0.9 kJ/g relative to the AL group (Figure 1F, Supplemental Figure 1C). Because functional protection also correlated with reduced weight loss over the 3-d period after IRI (r = −0.84, P < 0.04) likely because of increased intake of a complete diet (Supplemental Figure 1D), it is unlikely that reduced protein intake before injury was causative of reduced serum urea after injury. However, to confirm that differences in urea were related to actual kidney injury, we performed histopathology on kidneys on the third day after ischemia. Kidneys from mice with AL diet exhibited extensive tubular necrosis, characterized by pyknosis, karyorrhexis, and proteinaceous cast formation consistent with tubular destruction and loss of function (Figure 1G). Similar types of changes were observed in mice preconditioned with CR (Figure 1H, I) but to a significantly lesser degree, correlating with functional measures of protection (Figure 1J; r = 0.92, P < 0.01).
PR benefits are separable from carbohydrate restriction at low levels of CR.
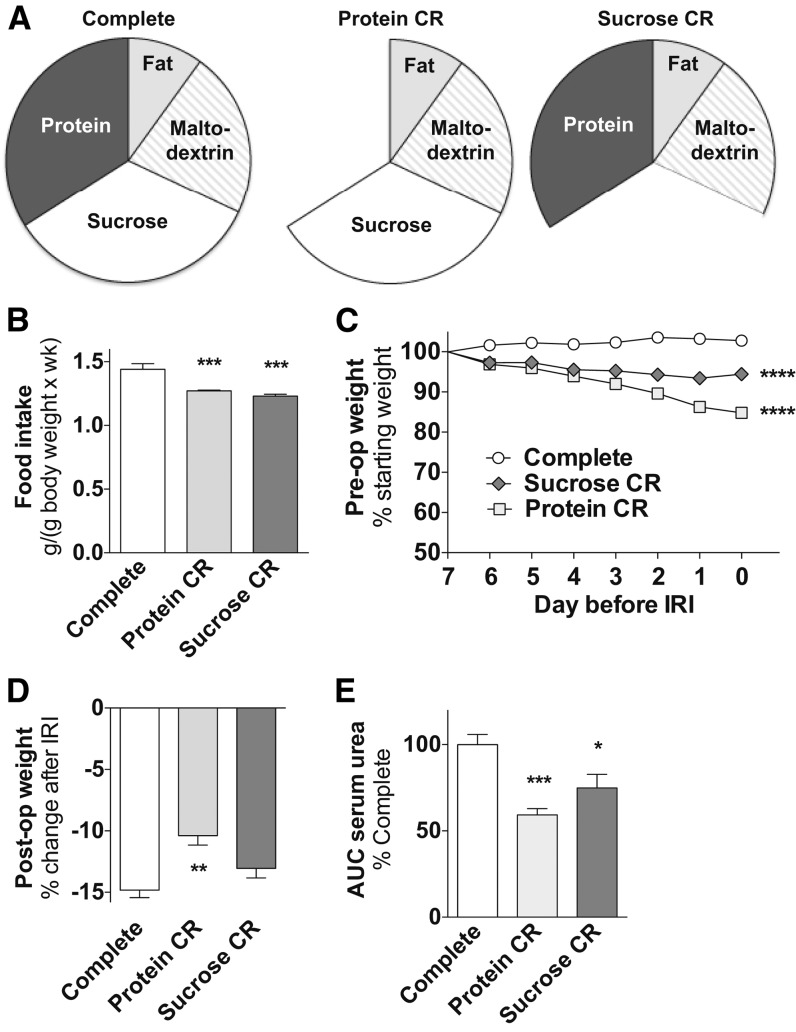
In experiment 2 we assessed the isolated contribution of PR vs. carbohydrate restriction to dietary preconditioning benefits. On the basis of the observation in experiment 1 that functional benefits within a week of CR required ∼30% restriction, we engineered a diet that contained >30% calories from protein (Supplemental Table 2) so that removing protein would reach this CR threshold. Mice given limited access (1.3 kJ/g daily; ∼20% CR) to a diet based on this complete formulation but lacking either protein (protein CR) or an isocaloric amount of sucrose (sucrose CR; Figure 2A, B) lost weight relative to the control AL complete diet group before ischemia (Figure 2C). Because mice consumed less of the complete diet than predicted, possibly because of the high-protein content, relative consumption of protein CR and sucrose CR diet resulted in ∼20% CR relative to the AL-fed complete diet group (Figure 2B). After ischemia, only the protein CR group displayed significantly reduced postoperative weight loss (Figure 2D), whereas both the protein CR and sucrose CR groups displayed significant functional benefits (Figure 2E).
FIGURE 2.
Effects of 1 wk of protein vs. carbohydrate restriction against renal IRI in male mice in experiment 2. (A) Schematic of complete or restricted diets that lack calories specifically from protein (protein CR) or sucrose (sucrose CR) with pie slices proportional to the calorie content of the indicated macronutrient. (B) Total food intake during the preconditioning period of the indicated groups, n = 2–3 cages/group. (C) Mouse weights during the preconditioning period are expressed as percentage of starting weight. (D) Postoperative weight on day 3 after IRI is expressed as a percentage change after IRI. (E) Kidney function after renal IRI is expressed as a percentage of serum urea AUC of the complete group. Values are means ± SEMs; n = 8–14 mice/group. *,**,***,****Different from complete control diet group: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. CR, calorie restriction; IRI, ischemia reperfusion injury; post-op, postoperative; pre-op, preoperative.
In a second arm of experiment 2, we assessed the effects of the complete, protein CR, and sucrose CR diets at an overall reduced energy level by restricting intake of each of these diets by an additional 20% relative to the amount eaten in the first arm (Supplemental Figure 2A). As previously observed, weight loss was greater on protein CR than on sucrose CR, despite similar calorie intake of 1.1 kJ/g (Supplemental Figure 2B). After renal IRI, we observed significant benefits on the protein CR and sucrose CR regimens for both postoperative weight change (Supplemental Figure 2C) and renal function (Supplemental Figure 2D). We conclude that restriction of calories in the form of protein or sucrose is beneficial against renal IRI at 2 different amounts of overall calorie intake.
EAAs control the benefits associated with PR.
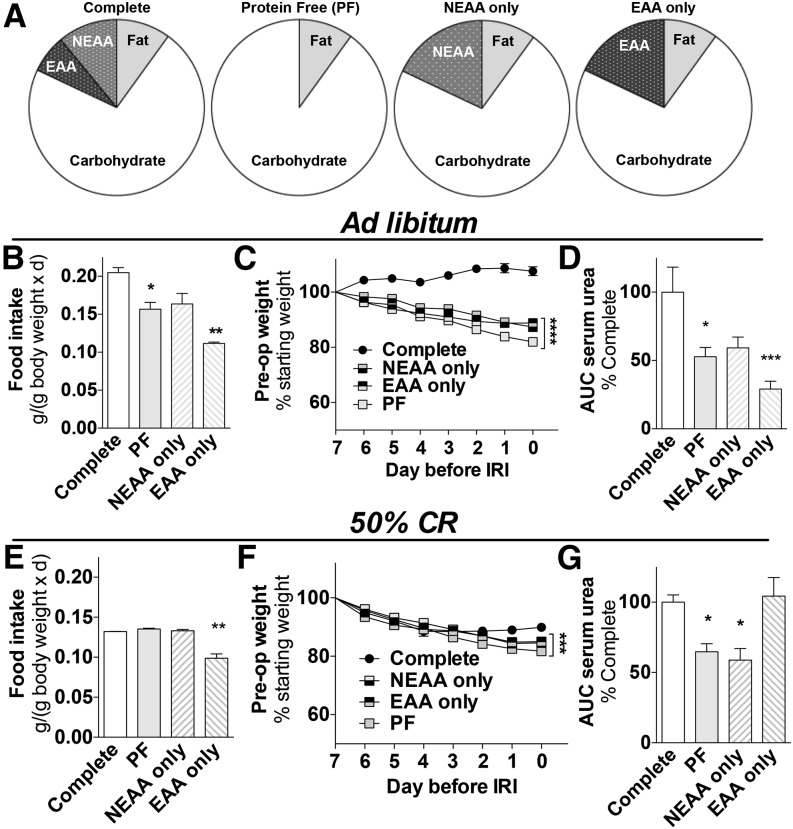
To test whether the benefits of PR were simply because of reduced calorie intake or related to signaling properties of AAs, we performed experiment 3 to test the potential role of specific AAs to PR benefits (Figure 3A). In the first arm of this experiment performed under AL feeding conditions, mice fed the PF and NEAA-only diets, both lacking EAAs, consumed ∼25% less than mice fed the complete diet, likely because of food aversion caused by lack of EAAs (38) (Figure 3B). The PF and NEAA-only groups also lost weight (Figure 3C) and displayed a similar degree of protection against renal IRI relative to the complete diet group (Figure 3D, Supplemental Figure 3A). Contrary to our hypothesis, mice fed the EAA-only diet were also protected from renal IRI (Figure 3D, Supplemental Figure 3A). However, this was likely because of severe food aversion that resulted in ∼50% CR (Figure 3B) occurring for unknown reasons, possibly related to an EAA-imbalanced diet, and obscuring our ability to test the specific role of EAAs in PR.
FIGURE 3.
EAAs control benefits of 1 wk of PR against renal IRI in male mice in experiment 3. (A) Schematic of diets in which 18% of calories are contributed by protein (complete), sucrose (PF), NEAAs (NEAA only), or EEAs (EAA only); mice were preconditioned with these diets consumed AL (B–D) or restricted daily by ∼50% (E–G). (B, E) Daily food intake is expressed relative to mouse weight; n = 2 cages/group. (C, F) Mouse weights during the preconditioning period are expressed as percentage of starting weight. (D, G) Kidney function after renal IRI is expressed as a percentage of serum urea AUC of the complete diet group. Values are means ± SEMs; n = 4–5 mice/group. *,**,***,****Different from complete control diet group: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. AL, ad libitum; CR, calorie restriction; EAA, essential amino acid; IRI, ischemia reperfusion injury; NEAA, nonessential amino acid; PF, protein free; PR, protein restriction; pre-op, preoperative.
To avoid the complications of differential food intake because of aversion, a second arm of experiment 3 was performed under conditions of enforced food restriction of ∼50%. Even at this amount of restriction, consumption of the EAA-only diet was significantly reduced relative to the other groups (Figure 3E, Supplemental Figure 3B), although weight loss was similar among groups (Figure 3F). Importantly, functional protection afforded by PR was unaffected by re-addition of NEAAs but was lost on re-addition of EAAs (Figure 3G). Together, these data are consistent with a model in which dietary EAAs control the benefits afforded by PR against renal IRI independent of their calorie or nitrogen content.
PR and energy restriction provide additive benefits against ischemic injury.
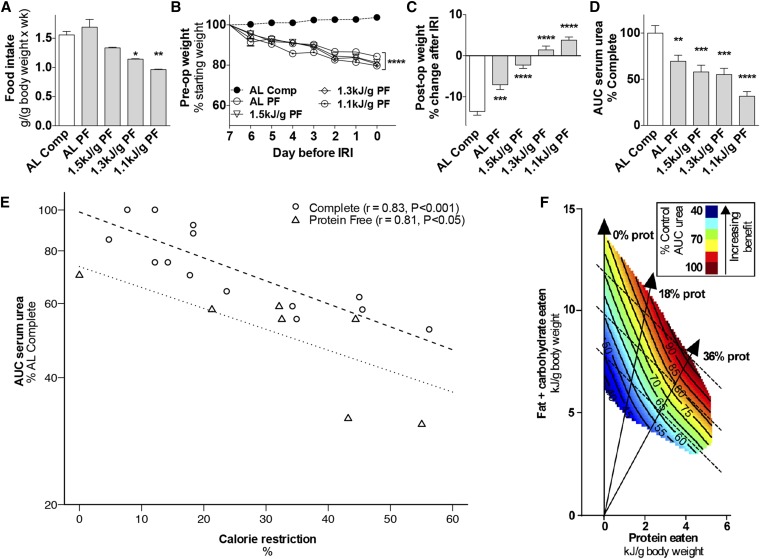
Although PR/EAA restriction improved the outcome of renal IRI under both AL and restricted feeding conditions, the relative contribution of CR and PR/EAA restriction to protection by DR remains unknown. We thus investigated if PR and CR could function additively or synergistically in protection from renal IRI in experiment 4. Mice were preconditioned for 1 wk with a PF diet (Supplemental Table 1) with either AL access or restricted up to 1.1 kJ/g daily. Despite an initial aversion, mice fed the PF diet AL ate as much food over the preconditioning period when corrected for body weight than controls fed a complete diet (Figure 4A, Supplemental Figure 4A). Weight loss and reduced blood glucose concentrations were similar among PF groups at different amounts of calorie intake (Figure 4B, Supplemental Figure 4B). Postoperative weight changes (Figure 4C) and functional protection from renal ischemic injury (Figure 4D) were significantly improved in the AL PF diet group relative to the AL complete diet group from experiment 1; both measures and postoperative food intake (Supplemental Figure 4C) were also improved on additional CR.
FIGURE 4.
Effects of 1 wk of combined protein restriction and CR against renal IRI in male mice in experiment 4. (A) Total food intake is expressed relative to body weight with the indicated diet, with PF groups at the indicated daily energy intake and the AL complete diet group from Figure 1 as a reference; n = 2–3 cages/group. (B) Mouse weights during the preconditioning period are expressed as percentage of starting weight. (C) Postoperative weight on day 3 after IRI is expressed as a percentage of change after IRI. (D) Kidney function after renal IRI is expressed as a percentage of serum urea AUC of the AL complete diet group. Values are means ± SEMs; n = 9–13 mice/group. *,**,***,****Different from complete control diet group: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (E) Exponential fit of observed AUC serum urea data calculated as a percentage of the AL complete group in the corresponding experiment relative to total energy intake and expressed as percentage of calorie restriction. Observed AUC serum urea for the complete diet groups (circles) was best fitted by the equation AUC serum urea = 99 × exp(−0.0125 × CR) with strong positive Pearson’s correlation (r = 0.83, P < 0.001) between the observed and predicted data. The observed AUC serum urea for the PF diet groups (triangles) was best fitted by the equation AUC serum urea = 73 × exp(−0.0114 × CR), also with a strong Pearson’s correlation (r = 0.81, P = 0.029). (F) Geometric framework-based model of the nutritional basis of short-term dietary restriction. Axes represent daily energy intake of protein (x) or carbohydrates and fat (y). Colors indicate functional outcome of renal IRI as a percentage of dysfunction in mice that consumed a complete diet AL, with dark red indicating maximal dysfunction and dark blue maximal protection. Each dotted line represents a continuum of isocaloric diets that differ in macronutrient ratios; solid vertical lines represent diets with 0%, 18%, or 36% calories from protein as indicated. AL, ad libitum; Comp, complete diet; CR, calorie restriction; IRI, ischemia reperfusion injury; PF, protein free; post-op, postoperative; pre-op, preoperative; prot, protein.
To assess the possible additive effects of PR and CR, AUC serum urea data from mice fed complete or PF diets at various amounts of total calorie intake from experiments 1 and 4 were fitted to exponential functions (Figure 4E). The correlation between observed and predicted data were strong for both complete (r = 0.83, P < 0.001) and PF diets (r = 0.81, P = 0.029). Although both curves decayed at a similar rate, the intercept for the PF diet was ∼25% lower than for the complete diet. This lower intercept indicates that PR contributes to functional protection independently of CR and supports an additive effect between the two.
The combined effects of macronutrient ratios (protein to fats/carbohydrates) and calorie intake during 1 wk of preconditioning on functional protection from renal IRI were modeled with the geometric framework (GF) (39) (Figure 4F). This approach allows visualization of the functional outcome (serum urea AUC) at different macronutrient ratios and different amounts of overall calorie intake. According to the GF, protection increased with reduced protein intake at each amount of total calorie intake (Figure 4F dotted lines). Protection also increased with decreasing calorie intake independent of protein intake. Thus, although protection could be achieved with any given diet by reduction of total calorie intake below AL amounts, optimal protection at any given amount of calorie intake was achieved at the lowest protein to carbohydrate/fat ratio (Figure 4F).
Modulation of AMPK and mTORC1 but not GCN2 in the kidney on dietary PR/CR.
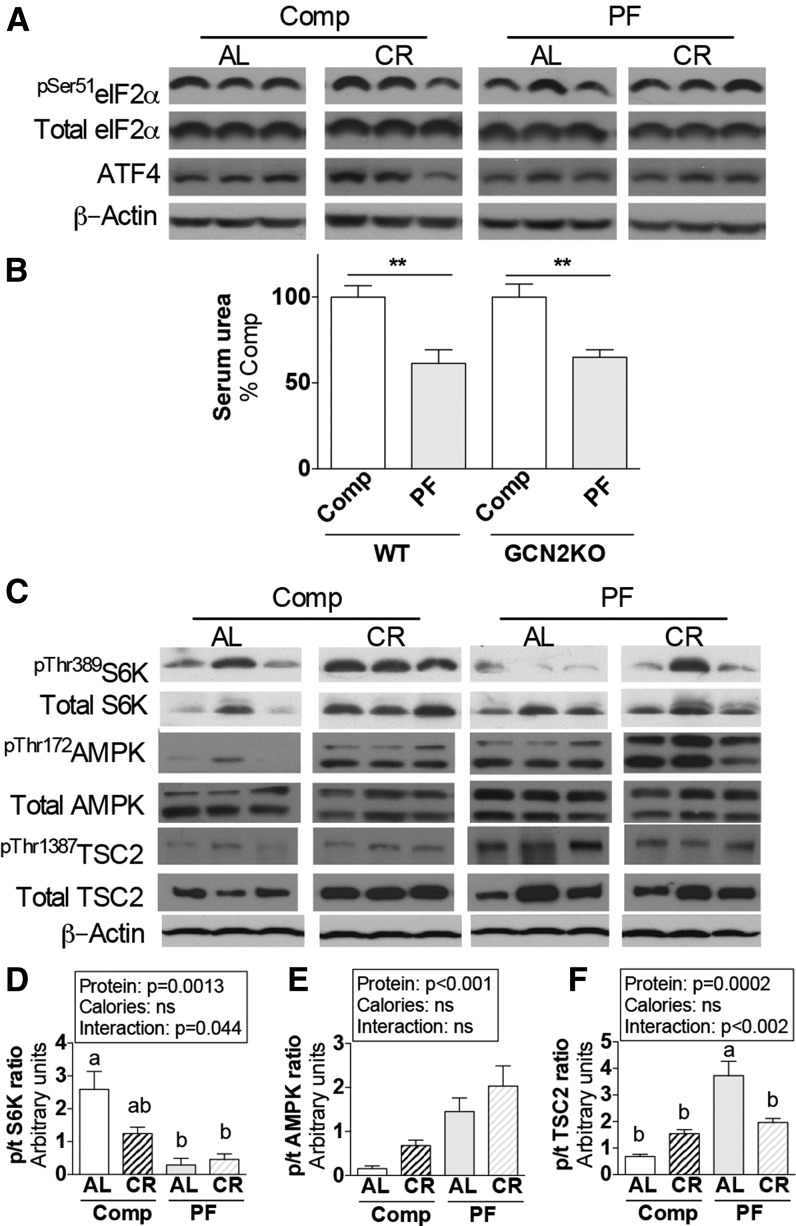
To understand the potential role of evolutionarily conserved nutrient- and energy-sensing signal transduction pathways in the benefits of short-term PR and CR, experiment 5 was undertaken to assess the activation status of GCN2, mTORC1, and AMPK in kidneys. One week of dietary PR and/or CR did not change markers of GCN2 activation in the kidney, including phosphorylation of its target, eIF2α (Figure 5A). Consistent with this finding, benefits of a PF diet against renal ischemic injury were intact in GCN2 KO mice in experiment 6 (Figure 5B).
FIGURE 5.
Protein- and energy-sensing signal transduction in kidney extracts from male mice after 1 wk of protein and/or CR in experiment 5. (A) Immunoblots of markers of GCN2 activation in kidney extracts from mice fed the indicated Comp or PF diet consumed AL or restricted ∼50% (CR). (B) Serum urea on day 1 after renal IRI of WT and GCN2 KO mice preconditioned with the indicated diet 1 wk before surgery in experiment 6; n = 5–9 mice/group. **Different from complete control within genotype, P < 0.01. (C) Immunoblots of mTORC1 target S6K, AMPK, and AMPK target TSC2 in kidney extracts from mice fed the indicated diets. (D–F) Quantitation of p/t ratios of S6K pThr389 (D), AMPK pThr172 (E), TSC2 pThr1387 (F); n = 3–5 mice/group. Main effects of dietary protein (protein = Comp vs. PF), food restriction (calories = AL vs. CR), and interactions are indicated above each graph with P values; ns, P > 0.05. Means without a common letter differ, P < 0.05. Values are means ± SEMs. AL, ad libitum; AMPK, AMP-activated protein kinase; ATF4, activating transcription factor 4; Comp, complete diet; CR, calorie restriction; eIF2α, eukaryotic initiation factor 2α GCN2, general control non-derepressible 2; IRI, ischemia reperfusion injury; KO, knockout; PF, protein-free diet; p/t, phospho/total; S6K, p70 ribosomal S6 protein kinase; TSC2, tuberous sclerosis complex 2; WT, wild-type.
To assess mTORC1 activation status in the kidney, we monitored phosphorylation of its direct target S6K. Although 50% restriction of a complete diet reduced phosphorylation of S6K at Thr389, the PF diet did as well independent of overall calorie intake (Figure 5C, D). To distinguish between the role of nutrient and energy restriction in mTORC1 inhibition, we assessed the activation status of the energy-sensing kinase AMPK. Phosphorylation of AMPK Thr172, indicative of activation, was significantly increased in the kidney on combined PR and CR (Figure 5C, E). Similarly, phosphorylation of the mTORC1 repressor tuberous sclerosis complex 2 (TSC2) at the AMPK site Thr1387 increased with a PF diet (Figure 5C, F). Among these 3 markers of nutrient/energy status in the kidney, more than one-half of the variability was because of PR (58%, P = 0.0013 for pS6K; 58%, P = 0.0006 for pAMPK; 50%, P = 0.0002 for pTSC2), whereas CR did not contribute significantly to variability. The percentage of the variability due to the interaction between PR and CR was significant only for pS6K (14%; P = 0.044) and pTSC1 (29%; P = 0.0018).
PR alters energy metabolism and hormonal status independent of calorie intake.
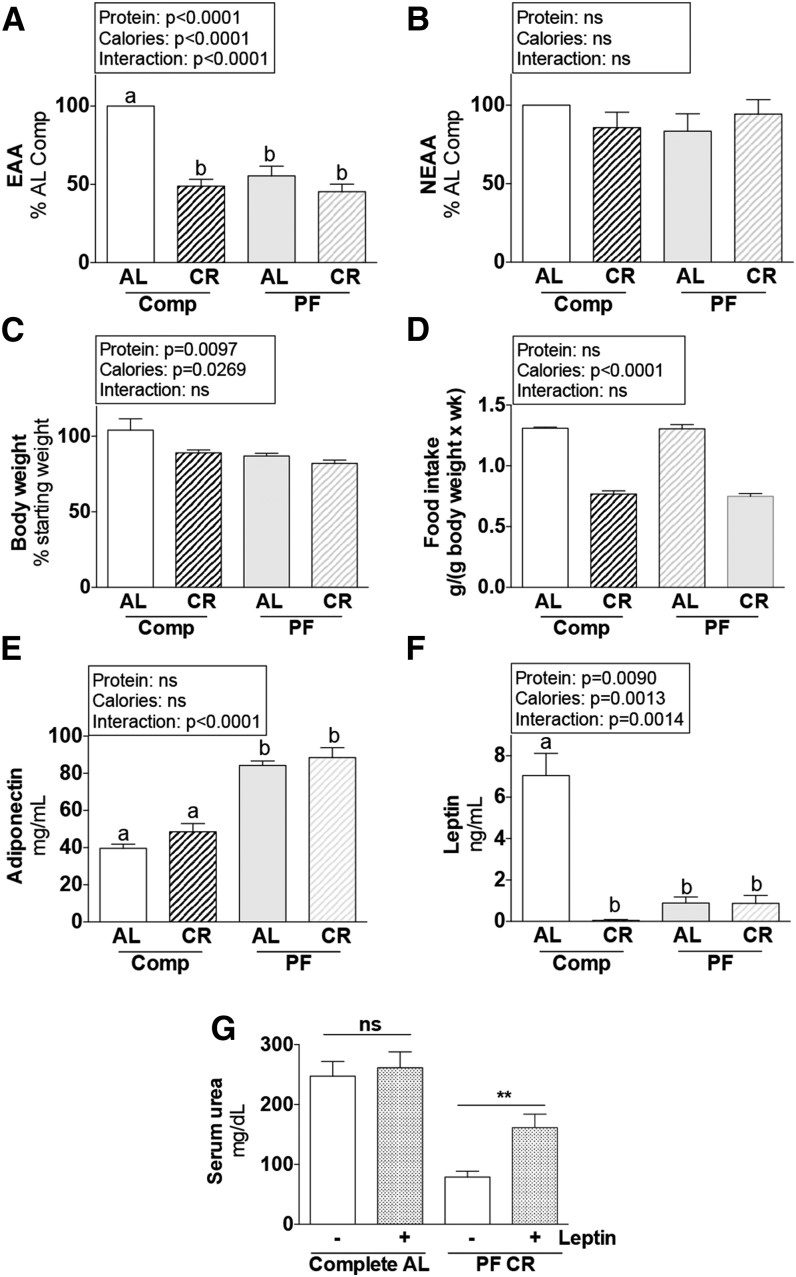
To explain the appearance of an energy-deprived state in the kidney on PR independent of calorie intake, we looked at circulating concentrations of nutrient/energy substrates and adipokine hormones in vivo in serum from mice in experiment 5. PR resulted in a significant reduction in circulating free EAAs that was not further reduced by CR (Figure 6A), whereas NEAAs were not significantly affected by diet (Figure 6B). Body weight was significantly reduced (Figure 6C) independent of calorie intake of the PF diet (Figure 6D), and circulating adipokines were significantly altered, namely increased adiponectin (Figure 6E) and reduced leptin (Figure 6F) with a PF diet, independent of CR.
FIGURE 6.
Metabolic and serum variables of male mice after 1 wk of protein and/or CR in experiment 5. (A, B) Combined means of individual serum EAAs (A) and NEAAs (B) calculated as a percentage of the AL complete diet group, after 1 wk fed the indicated diet. (C) Body weight is expressed as a percentage of the starting value. (D) Total food intake during the preconditioning period of the indicated groups; n = 2 cages/group. (E, F) Serum concentrations of adiponectin (E) and leptin (F) after 1 wk fed the indicated diet. Main effects of dietary protein (protein = complete vs. PF), food restriction (calories = AL vs. CR), and interactions are indicated above each graph with P values; ns, P > 0.05. Means without a common letter differ, P < 0.05; n = 5 mice/group. (G) Serum urea on day 1 after renal IRI of mice preconditioned with the indicated diet 1 wk before surgery and treated with leptin or vehicle in experiment 7 as indicated. **Different from vehicle control within diet, P < 0.01; n = 10–15 mice/group. Values are means ± SEMs. AL, ad libitum; Comp, complete diet; CR, ∼50% calorie restriction; EAA, essential amino acids; IRI, ischemia reperfusion injury; KO, knockout; NEAA, nonessential amino acids; PF, protein-free diet; WT, wild-type.
Finally, in experiment 7 we tested the functional relevance of systemic changes in circulating leptin, the adipokine that demonstrated the greatest percentage of change on dietary preconditioning. Recombinant leptin supplementation during the preconditioning period significantly reduced the benefits of dietary preconditioning against renal IRI (Figure 6G).
Discussion
The primary goal of this study was to elucidate the nutritional basis of short-term dietary preconditioning against surgical stress in a preclinical model of renal IRI. For daily restriction of a complete diet for 1 wk, protection increased with increasing food restriction, reaching significance by ∼30% CR and increasing up to at least 50% CR (Figure 1F, J; Supplemental Figure 4D). This is similar to longevity benefits of long-term CR, which are dose dependent up to nearly the point of starvation of ∼60% CR (13). Although these studies were not designed to determine the maximal achievable protection against renal IRI or the minimal duration of CR required for protection, they do point to significant effects in a potentially clinically relevant time frame. Previously, we demonstrated that 3 d of food deprivation (100% CR) and 2–4 wk of 30% CR induced similar functional benefits against renal IRI (8). Future studies will be required to determine whether longer time periods at any given amount of CR, or shorter periods at a higher amount of restriction, increase functional protection and, more importantly, if such effects translate to humans.
We also found that select removal of protein yielded important benefits against renal IRI that were separable from removal of an isocaloric amount of sucrose at 2 different overall amounts of CR (∼20%, Figure 2E; ∼40%, Supplemental Figure 2D), with functional benefits converging at higher amounts of overall CR, possibly because they approached a maximal effect size of up to 2-fold typical of hormetic mechanisms (40). In the context of a diet that lacked protein, note that the reduction in serum urea over the 3-d period after surgery was related to preservation of kidney function and not an artifact of lack of nitrogen in the diet during the preconditioning period. In support of this conclusion, intake of a complete diet (and thus nitrogen) after surgery was increased in proportion to functional protection (Figure 4C).
Having established the ability of PR to contribute to dietary preconditioning, we next found that EAAs can influence dietary preconditioning independent of their caloric content, and that EAA removal underlies the benefits of CR attributable to PR. Unexpectedly, the experimental design was complicated by an extreme aversion to the EAA-only diet that prevented direct comparisons of functional protection with isocaloric PF or NEAA-only diet (Figure 3B; Supplemental Figure 3). Although aversion is expected from diets that lack ≥1 EAAs, it can also be caused by imbalances in dietary AAs (41), although underlying mechanisms remain unknown. Nonetheless, by restricting access to each of these diets, we were able to normalize calorie intake among groups and show that functional benefits observed with the PF diet were maintained with the NEAA-only diet but were lost on the EAA-only diet (Figure 3G).
Interestingly, although weight loss was consistently observed in association with diets that offered resistance to renal IRI, the magnitude of weight loss did not always correlate with the magnitude of protection. For example, mice fed the EAA-only diet lost a similar amount of weight as the other diet-restricted groups (Figure 3F) but did not gain the benefits observed in the PF or NEAA-only diet group (Figure 3G). Two distinct mechanisms appeared to underlie weight loss in this study. The first mechanism involved reduced food intake, either as a result of enforced food restriction (CR) or aversion to food intake due to EAA deficiency or imbalance. The second mechanism was likely because of protein deficiency itself, which resulted in steady weight loss independent of total calorie intake. Indeed, compensatory food intake after initial aversion did not stem weight loss as observed here (Figure 4A, B; Supplemental Figure 4A) and in previous studies (11). Curiously, although diets that lacked EAAs cause food aversion and subsequent weight loss at least in part on activation of GCN2 and phosphorylation of eIF2α in the hypothalamus (42), protein-rich diets are also associated with weight loss due to reduced food intake but likely via a fundamentally different mechanism that involves satiety induction on mTORC1 activation by increased AAs such as leucine in the hypothalamus (43).
Finally, we observed that, although PR contributed to protection from renal IRI without CR, further restriction of energy led to increasing benefits (Figure 4D). Modeling of the relation between functional protection and calorie intake in mice preconditioned with complete vs. PF diets revealed an additive effect between PR and CR on dietary preconditioning against renal IRI (Figure 4E).
Our collective functional results on the nutritional basis of preconditioning are summarized with the GF (Figure 4F), which allows for visualization of the magnitude of any chosen biological outcome as a function of intake of different amounts of food at different ratios of macronutrients (39, 44). Recently, the GF was used to map the nutritional landscape of rodent longevity, revealing the importance of dietary protein intake in mammalian health span and life span (45). Here, the GF indicated that dietary preconditioning could be achieved with diets that differed widely in protein content (0–36% calories) by reducing intake of those diets, as indicated by solid lines in Figure 4F, with benefits increasing toward the origin. At the same time, the optimal preconditioning diet at any given amount of calorie intake (dashed lines in Figure 4F) was the diet that contained the least protein, as indicated by benefits increasing from right to left along any given dashed line. A limitation of this study is the focus on only 1 organ, the kidney. Although dietary preconditioning against IRI works in multiple tissues, including the liver (8, 11) and brain (12), future studies are required to determine whether the nutritional basis of preconditioning against IRI in these organs is the same as in the kidney.
The second goal of this study was to elucidate nutrient/energy-signaling molecules underlying functional protection. On the basis of our identification of roles for both EAA restriction independent of reduced calorie intake and overall energy restriction, we predicted involvement of AA sensing via GCN2 and/or mTORC1 pathways in response to EAAs and the energy sensor AMPK and mTORC1 in response to carbohydrate/fat CR.
Contrary to our prediction, AA sensing by GCN2 in the kidney itself did not appear to play a direct role in protection, and GCN2 was not required for functional benefits of PR (Figure 5A, B). This stands in contrast to the requirement for GCN2 in protection mediated by restriction of the single EAA tryptophan (11). GCN2 can in principle sense the reduction of any individual AA due to accumulation of the corresponding uncharged tRNA; however, GCN2 was not activated in the kidney by protein deprivation, despite a significant reduction in all of the EAAs in circulation (Figure 6A). This failure to activate GCN2 is not because of constitutive repression of this pathway in the kidney, because GCN2 is capable of being activated in this organ on systemic treatment with the prolyl tRNA synthetase inhibitor halofuginone (11). Instead, maintenance of AA concentrations in the kidney despite protein deprivation, for example, by increased renal reabsorption of free AAs released from muscle stores, could potentially explain the failure to activate GCN2 (46). Taken together, these data indicate that, although GCN2 is required under certain circumstances for preconditioning benefits on individual AA restriction, it is redundant when total dietary protein is restricted, similar to recent findings on dietary preconditioning against ischemic injury in the liver (34).
Because mTORC1 activity responds to AA availability, specifically leucine, it is tempting to speculate that the mTORC1 signal transduction pathway, whose activity was reduced on PR (Figure 5C, D), is a functionally relevant AA-sensing pathway in the kidney on PR. However, although free EAA concentrations (including leucine) were reduced in serum, reduced leucine in the kidney would also be expected to activate GCN2, which was not observed. As an alternate hypothesis, reduced mTORC1 activity on PR could be influenced by availability of growth factors or energy, both of which are required, such as leucine, to activate mTORC1. In support of this hypothesis, PR resulted in a significant reduction in circulating glucose (Supplemental Figure 4B), reduced body weight (Figure 6C), increased adiponectin (Figure 6E), and reduced leptin (Figure 6F). Consistent with a state of negative energy balance despite normal intake of calories in the form of fat and carbohydrate, PR also resulted in activation of AMPK (Figure 5C, E) and increased phosphorylation of AMPK target TSC2 (Figure 5C, F), a repressor of mTORC1 activity.
Previously, we showed that reduced mTORC1 activity is critical to PR-mediated protection from IRI in the liver by using hepatocyte-specific ablation of the mTORC1 repressor TSC1 (34) and that this protection depends on endogenous hydrogen sulfide production (47). Although the role of hydrogen sulfide in protection against renal IRI is currently not known, this latter study revealed a key difference between dietary preconditioning against IRI in the liver vs. the kidney, namely that PR in the absence of CR has a large beneficial impact that is not further improved by CR as it was here in the renal system (47). This is important because it demonstrates that different organs may react differently to the same dietary preconditioning regimen.
We also previously found that reduced mTORC1 activity on CR protects against a maladaptive proinflammatory response to infection in a rodent malaria model and that this protection is abrogated by recombinant leptin treatment (48). Here, we found that leptin administration during the preconditioning period abrogated full protection by combined PR/CR. Although leptin was substantially reduced on PR and CR, a limitation of this study is that we did not measure the relative contribution of reduced leptin to protection by PR in the absence of CR. A further limitation of this study is that we do not know the cellular or molecular targets of leptin action relevant to protection from ischemic injury. It is possible that leptin activates mTORC1 in the kidney and in immune cells, both of which could contribute to loss of protection. Future studies are required to decipher the relative importance of AMPK activation and/or mTORC1 downregulation in the benefits of dietary preconditioning. However, the finding that AMPK activation by the allosteric activator 5-aminoimidazole-4-carboxamide ribonucleotide protects against ischemic injury in rats when given at high doses before injury (49) provides proof of principle of the potential role of this energy-sensing pathway in protection by either PR or CR.
One direct translation of this preclinical model of dietary preconditioning against renal IRI would be in the context of abdominal aortic aneurysm surgery, in which the blood flow to the kidneys is temporarily occluded and postoperative renal dysfunction is a major clinical problem. Currently, no such preoperative prophylactic strategies exist. Because dietary preconditioning also prevents IRI in preclinical models of hepatic ischemia (8, 11) and stroke (12), we have also proposed its use more generally in vascular surgical procedures to precondition against the risk of complications, including heart attack and stroke, that occur with varying frequency, depending on the procedure and other risk factors associated with the patient (50). We predict that maximal compliance, and thus clinical feasibility, of dietary preconditioning will result from minimally restrictive food intake. Thus, the finding here that PR can contribute to protection independent of calorie intake has important implications for designing clinically feasible preconditioning regimens. Future studies will be required to test the safety, compliance rates, and efficacy of such regimens in the clinic.
Acknowledgments
We thank Will Mair and Chih-Hao Lee for critical reading of the manuscript. LTR, JHT-V, PM, CKO, BSK, and JRM designed the experiments; LTR, JHT-V, PM, EH, CH, DV, HZ, and JRM performed the experiments; YG performed the statistical analyses; SJS performed the geometric framework analysis; LTR, JHT-V, PM, CKO, BSK, and JRM analyzed the data; JHT-V and JRM wrote the paper; and JRM had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AA, amino acid; AL, ad libitum; AMPK, AMP-activated protein kinase; CR, calorie restriction; DR, dietary restriction; EAA, essential amino acid; eIF2α, eukaryotic initiation factor 2α GCN2, general control non-derepressible 2; GF, geometric framework; IRI, ischemia reperfusion injury; KO, knockout; mTORC1, mechanistic target of rapamycin complex 1; NEAA, nonessential amino acid; PF, protein free; PR, protein restriction; S6K, p70 ribosomal S6 protein kinase; tRNA, transfer RNA; TSC2, tuberous sclerosis complex 2; 4E-BP1, factor 4E-binding protein 1.
References
- 1.Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med 2011;32:159–221. [DOI] [PubMed] [Google Scholar]
- 2.Masoro EJ. Subfield history: caloric restriction, slowing aging, and extending life. Sci Aging Knowledge Environ 2003;2003:RE2. [DOI] [PubMed]
- 3.Stein PK, Soare A, Meyer TE, Cangemi R, Holloszy JO, Fontana L. Caloric restriction may reverse age-related autonomic decline in humans. Aging Cell 2012;11:644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy JC, McDaniel JL, Mora K, Villareal DT, Fontana L, Weiss EP. Preferential reductions in intermuscular and visceral adipose tissue with exercise-induced weight loss compared with calorie restriction. J Appl Physiol 2012;112:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 2006;295:1539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walford RL, Harris SB, Gunion MW. The calorically restricted low-fat nutrient-dense diet in Biosphere 2 significantly lowers blood glucose, total leukocyte count, cholesterol, and blood pressure in humans. Proc Natl Acad Sci USA 1992;89:11533–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson LT, Mitchell JR. Benefits of short-term dietary restriction in mammals. Exp Gerontol 2013;48:1043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell JR, Verweij M, Brand K, van de Ven M, Goemaere N, van den Engel S, Chu T, Forrer F, Muller C, de Jong M, et al. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell 2010;9:40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci USA 2008;105:8215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S, Merlino A, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Science Transl Med 2012;4:124ra127. [DOI] [PMC free article] [PubMed]
- 11. Peng W, Robertson L, Gallinetti J, Mejia P, Vose S, Charlip A, Chu T, Mitchell JR. Surgical stress resistance induced by single amino acid deprivation requires Gcn2 in mice. Sci Transl Med 2012;4:118ra111. [DOI] [PMC free article] [PubMed]
- 12.Varendi K, Airavaara M, Anttila J, Vose S, Planken A, Saarma M, Mitchell JR, Andressoo JO. Short-term preoperative dietary restriction is neuroprotective in a rat focal stroke model. PLoS One 2014;9:e93911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of ageing in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr 1986;116:641–54. [DOI] [PubMed] [Google Scholar]
- 14.McCay CM, Crowel MF, Maynard LA. The effect of retarded growth upon the length of the life span and upon the ultimate body size. J Nutr 1935;10:63–79. [PubMed] [Google Scholar]
- 15.Merry BJ. Molecular mechanisms linking calorie restriction and longevity. Int J Biochem Cell Biol 2002;34:1340–54. [DOI] [PubMed] [Google Scholar]
- 16.Ross MH. Length of life and nutrition in the rat. J Nutr 1961;75:197–210. [DOI] [PubMed] [Google Scholar]
- 17.Masoro EJ. Assessment of nutritional components in prolongation of life and health by diet. Proc Soc Exp Biol Med 1990;193:31–4. [DOI] [PubMed] [Google Scholar]
- 18.Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol 1985;40:657–70. [DOI] [PubMed] [Google Scholar]
- 19.Maeda H, Gleiser CA, Masoro EJ, Murata I, McMahan CA, Yu BP. Nutritional influences on aging of Fischer 344 rats: II. Pathology. J Gerontol 1985;40:671–88. [DOI] [PubMed] [Google Scholar]
- 20.Raubenheimer D, Simpson SJ, Tait AH. Match and mismatch: conservation physiology, nutritional ecology and the timescales of biological adaptation. Philos Trans R Soc Lond B Biol Sci 2012;367:1628–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol 2005;3:e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 2009;462:1061–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallinetti J, Harputlugi E, Mitchell JR. Amino acid sensing and translational control in dietary restriction-mediated longevity and stress resistance: contrasting roles of signal transducing kinases Gcn2 and mTOR. Biochem J 2013;449:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tokunaga C, Yoshino K, Yonezawa K. mTOR integrates amino acid- and energy-sensing pathways. Biochem Biophys Res Commun 2004;313:443–6. [DOI] [PubMed] [Google Scholar]
- 25.Dodd KM, Tee AR. Leucine and mTORC1: a complex relationship. Am J Physiol Endocrinol Metab 2012;302:E1329–42. [DOI] [PubMed] [Google Scholar]
- 26.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 2009;10:307–18. [DOI] [PubMed] [Google Scholar]
- 27.Inoki K, Kim J, Guan KL. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol 2012;52:381–400. [DOI] [PubMed] [Google Scholar]
- 28.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002;8:1288–95. [DOI] [PubMed] [Google Scholar]
- 29.Hattori Y, Nakano Y, Hattori S, Tomizawa A, Inukai K, Kasai K. High molecular weight adiponectin activates AMPK and suppresses cytokine-induced NF-kappaB activation in vascular endothelial cells. FEBS Lett 2008;582:1719–24. [DOI] [PubMed] [Google Scholar]
- 30.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003;423:762–9. [DOI] [PubMed] [Google Scholar]
- 31.Maya-Monteiro CM, Bozza PT. Leptin and mTOR: partners in metabolism and inflammation. Cell Cycle 2008;7:1713–7. [DOI] [PubMed] [Google Scholar]
- 32.Finlay D, Cantrell DA. Metabolism, migration and memory in cytotoxic T cells. Nat Rev Immunol 2011;11:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verweij M, van de Ven M, Mitchell JR, van den Engel S, Hoeijmakers JH, Ijzermans JN, de Bruin RW. Glucose supplementation does not interfere with fasting-induced protection against renal ischemia/reperfusion injury in mice. Transplantation 2011;92:752–8. [DOI] [PubMed] [Google Scholar]
- 34.Harputlugil E, Hine C, Vargas D, Robertson L, Manning BD, Mitchell JR. The TSC complex is required for the benefits of dietary protein restriction on stress resistance in vivo. Cell Reports 2014;8:1160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 36.Mauro CR, Tao M, Yu P, Trevino-Villerreal JH, Longchamp A, Kristal BS, Ozaki CK, Mitchell JR. Preoperative dietary restriction reduces intimal hyperplasia and protects from ischemia-reperfusion injury. J Vasc Surg 2014. Aug 8 (Epub ahead of print; DOI: 10.1016/j.jvs.2014.07.004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. R Core Team. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2013.
- 38.Gietzen DW, Hao S, Anthony TG. Mechanisms of food intake repression in indispensable amino acid deficiency. Annu Rev Nutr 2007;27:63–78. [DOI] [PubMed] [Google Scholar]
- 39.Simpson SJ, Raubenheimer D. Geometric analysis of macronutrient selection in the rat. Appetite 1997;28:201–13. [DOI] [PubMed] [Google Scholar]
- 40.Calabrese EJ. Hormesis: toxicological foundations and role in aging research. Exp Gerontol 2013;48:99–102. [DOI] [PubMed] [Google Scholar]
- 41.Harper AE, Benevenga NJ, Wohlhueter RM. Effects of ingestion of disproportionate amounts of amino acids. Physiol Rev 1970;50:428–558. [DOI] [PubMed] [Google Scholar]
- 42.Maurin AC, Benani A, Lorsignol A, Brenachot X, Parry L, Carraro V, Guissard C, Averous J, Jousse C, Bruhat A, et al. Hypothalamic eIF2alpha signaling regulates food intake. Cell Reports 2014;6:438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science 2006;312:927–30. [DOI] [PubMed] [Google Scholar]
- 44.Piper MD, Partridge L, Raubenheimer D, Simpson SJ. Dietary restriction and aging: a unifying perspective. Cell Metab 2011;14:154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 2014;19:418–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van de Poll MC, Soeters PB, Deutz NE, Fearon KC, Dejong CH. Renal metabolism of amino acids: its role in interorgan amino acid exchange. Am J Clin Nutr 2004;79:185–97. [DOI] [PubMed] [Google Scholar]
- 47.Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee BC, Brace L, Longchamp A, Trevino-Villarreal JH, Mejia P, Ozaki CK, et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 2015;160:132–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mejia P, Treviño-Villarreal JH, Hine C, Harputlugil E, Lang S, Calay E, Rogers R, Wirth D, Duraisingh MT, Mitchell JR. Dietary restriction protects against experimental cerebral malaria via leptin modulation and T cell mTORC1 suppression. Nat Comm 2015;6:6050 [DOI] [PMC free article] [PubMed]
- 49.Lempiäinen J, Finckenberg P, Levijoki J, Mervaala E. AMPK activator AICAR ameliorates ischaemia reperfusion injury in the rat kidney. Br J Pharmacol 2012;166:1905–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell JR, Beckman JA, Nguyen LL, Ozaki CK. Reducing elective vascular surgery perioperative risk with brief preoperative dietary restriction. Surgery 2013;153:594–8. [DOI] [PMC free article] [PubMed] [Google Scholar]