Abstract
Background: Previous trials of prenatal iron supplementation had limited measures of maternal or neonatal iron status.
Objective: The purpose was to assess effects of prenatal iron-folate supplementation on maternal and neonatal iron status.
Methods: Enrollment occurred June 2009 through December 2011 in Hebei, China. Women with uncomplicated singleton pregnancies at ≤20 wk gestation, aged ≥18 y, and with hemoglobin ≥100 g/L were randomly assigned 1:1 to receive daily iron (300 mg ferrous sulfate) or placebo + 0.40 mg folate from enrollment to birth. Iron status was assessed in maternal venous blood (at enrollment and at or near term) and cord blood. Primary outcomes were as follows: 1) maternal iron deficiency (ID) defined in 2 ways as serum ferritin (SF) <15 μg/L and body iron (BI) <0 mg/kg; 2) maternal ID anemia [ID + anemia (IDA); hemoglobin <110 g/L]; and 3) neonatal ID (cord blood ferritin <75 μg/L or zinc protoporphyrin/heme >118 μmol/mol).
Results: A total of 2371 women were randomly assigned, with outcomes for 1632 women or neonates (809 placebo/folate, 823 iron/folate; 1579 mother-newborn pairs, 37 mothers, 16 neonates). Most infants (97%) were born at term. At or near term, maternal hemoglobin was significantly higher (+5.56 g/L) for iron vs. placebo groups. Anemia risk was reduced (RR: 0.53; 95% CI: 0.43, 0.66), as were risks of ID (RR: 0.74; 95% CI: 0.69, 0.79 by SF; RR: 0.65; 95% CI: 0.59, 0.71 by BI) and IDA (RR: 0.49; 95% CI: 0.38, 0.62 by SF; RR: 0.51; 95% CI: 0.40, 0.65 by BI). Most women still had ID (66.8% by SF, 54.7% by BI). Adverse effects, all minor, were similar by group. There were no differences in cord blood iron measures; >45% of neonates in each group had ID. However, dose-response analyses showed higher cord SF with more maternal iron capsules reported being consumed (β per 10 capsules = 2.60, P < 0.05).
Conclusions: Prenatal iron supplementation reduced anemia, ID, and IDA in pregnant women in rural China, but most women and >45% of neonates had ID, regardless of supplementation. This trial was registered at clinicaltrials.gov as NCT02221752.
Keywords: pregnant women, neonates, iron supplementation, iron deficiency, iron deficiency anemia, randomized clinical trial
Introduction
Reducing maternal anemia and preventing iron deficiency (ID)8 are global health priorities (1). Public health efforts have reduced maternal anemia in many settings, but 38% of pregnant women worldwide are still anemic, mostly due to ID (1), potentially affecting millions of women and their infants. The problem is not restricted to limited-resource countries. In the United States, for instance, 30–34% of pregnant women are anemic (2) or iron-deficient (3). Severe maternal anemia/ID can contribute to adverse pregnancy outcomes (4) and affect newborn iron status (5). ID during infancy is associated with poorer cognitive, motor, and social-emotional development (6).
This randomized clinical trial was designed to assess effects of prenatal iron-folate supplementation on maternal and neonatal iron status, with a secondary goal of examining effects on maternal anemia and perinatal outcomes. The study was conducted in China, where prenatal micronutrient supplements other than folate before conception were not routinely recommended at the time. The study is pertinent to 3 recent meta-analyses/systematic reviews of routine prenatal iron supplementation (7–9) and 2 large micronutrient supplementation trials in China that included iron-folate supplementation groups (10–12). Virtually all of the studies provided data on maternal hemoglobin and anemia, but few had additional measures of maternal iron status. A recent report from one of the China trials is a notable exception (12). The only trial in a systematic review (9) to report cord blood iron measures found no effect of maternal iron supplementation but observed differences later in infancy (13).
With more comprehensive measures for both pregnant women and neonates than in previous studies, we predicted that iron supplementation would improve maternal and fetal-neonatal iron status and reduce ID and ID anemia (IDA). We also expected that prenatal iron supplementation would increase maternal hemoglobin, reduce maternal anemia, and improve perinatal outcomes.
Methods
Study setting and design.
The study, conducted in rural Sanhe County, Hebei Province, China, involved collaboration between Peking University First Hospital and 3 local hospitals [Sanhe Maternity and Child Health Care Center (MCHC), Sanhe General County Hospital, and Sanhe Hospital of Traditional Chinese Medicine]. The study initially involved only Sanhe MCHC, but interim analyses of drop-out rates led us to appreciate that, according to local custom, women often did not give birth at the same place they received prenatal care. Women in this area had the choice of delivering at >2 dozen local hospitals and clinics, which might differ in convenience, closeness to home, or cost. It was not possible to collect cord blood for study participants in all these locations, but we made arrangements to do so at 2 other major hospitals during the second half of the study. Eligible pregnant women were enrolled from June 2009 through December 2011 and randomly assigned in a 1:1 ratio to iron and folate or placebo and folate. The study and the assessments of maternal and neonatal iron status were approved by ethics committees of the Peking University First Hospital and the University of Michigan.
Participants.
Women were recruited at their first prenatal visit at Sanhe MCHC. Women were eligible if they had an uncomplicated singleton pregnancy, a first Sanhe MCHC visit at ≤20 wk gestation, and plans to give birth at a participating hospital. Exclusion criteria were age <18 y, not living in Sanhe, not mentally competent, having a chronic health problem, hemoglobin <100 g/L, or having taken medicinal iron for any duration. A total of 2371 women were recruited.
Enrollment and informed consent.
Staff from Sanhe MCHC explained the project to expecting mothers and obtained signed informed consent for those agreeing to participate.
Randomization and masking.
Women received 2 supplements: iron/placebo and folate. Taiyuan Satellite Pharmaceutical Co., Ltd. prepared equal quantities of iron or placebo capsules and folate capsules and assigned 4-digit package numbers beginning with 0001 according to a random-number chart prepared by a statistician who was not part of the study. Study participants, personnel, and investigators were unaware of supplement group. The code was not broken until the study and primary analyses were completed.
Intervention.
Project personnel provided participants with medication packs from consecutive package numbers on the basis of date of first prenatal MCHC visit. Supplement packs, differing in appearance only in the number, consisted of capsules with iron [300 mg ferrous sulfate (60 mg elemental iron)] or placebo (starch, dextrin, sucrose, and magnesium stearate) and capsules with 0.40 mg folate. Participants were instructed to take one of each kind of supplement daily from enrollment to delivery and to return to the clinic for more supplements when they ran out. Women received 100 capsules of each supplement upon enrollment and after ∼3 mo, typically at 26–32 wk gestation. Project personnel asked about the number of capsules consumed at this follow-up visit and again at or near term. We estimated the percentage of adherence by iron or placebo capsules consumed divided by days from enrollment to the assessment at or near term. Physicians were free to use clinical judgment in treating anemic women. Local clinical practice was to prescribe medicinal iron therapy (ferrous sulfate, ferrous succinate, or ferric ammonium citrate) and/or to make dietary recommendations. Women were free to obtain over-the-counter iron-containing supplements on their own, regardless of group assignment or whether or not they were anemic.
Study outcomes.
Primary outcomes were iron status of pregnant women at or near term and of neonates at birth, almost all of whom were born at term (37–41 wk). Other outcomes were maternal anemia and infant gestational age and birth weight.
Gestational age was based on last menstrual period. Maternal blood samples were obtained by venipuncture for research and/or clinical purposes at enrollment and generally at 26–32 wk, at or near term, and 1–3 d after delivery. Cord blood samples were obtained for research purposes by sterile needle puncture immediately after cord clamping. Cord clamping generally occurred within 60 s of delivery, with the infant ∼20 cm below the perineum for vaginal births and on the mother’s abdomen for cesarean deliveries. A complete blood count, including hemoglobin and mean corpuscular volume (MCV), was performed by using a Sysmex KX-21N Auto Hematology Analyzer for clinical purposes at each time point at the Sanhe MCHC and at the research time points at Peking University First Hospital (enrollment, at or near term, and cord blood). In Sanhe, whole blood for zinc protoporphyrin/heme (ZPP/H) was stored at 4°C and protected from light; serum for other iron measures was stored at −20°C. Samples were transferred weekly in cooled and chilled transfer boxes, respectively, to Peking University First Hospital where ZPP/H was analyzed by hematofluorometer (Aviv Biomedical), serum ferritin (SF) and serum transferrin receptor (sTfR) by chemiluminescent immunoassay (Beckman Coulter Access 2 Immunoassay System), and serum C-reactive protein (CRP) by rate nephelometry (Hitachi 7600). Both laboratories maintained standard quality-control procedures.
We defined maternal anemia per WHO guidelines as hemoglobin <110 g/L (1, 2) and ID in the following 2 ways: SF <15 μg/L (14) and body iron (BI) <0 mg/kg (15). IDA was defined as anemia plus ID. We considered fetal-neonatal ID as cord blood SF <75 μg/L or as ZPP/H >118 μmol/mol. The SF cutoff has been used in studies of prenatal ID neurodevelopmental effects (16–18). The ZPP/H cutoff represents the US 90th percentile (19). Birth weight and length were measured by obstetrical staff.
Sample size.
The targeted sample of 1200/group (total n = 2400) was adequate to detect a reduction in maternal IDA of ≥4.5% (RR: 0.78) and a difference in birth weight of ≥45 g, with 80% power and α = 0.05. Although we did not reach the targeted sample number because of budgetary constraints, there was little reduction in power. The actual numbers of women with outcome data were 802 in the placebo group and 814 in the iron-supplemented group. With this sample size we had 80% power to detect a ≥5.4% reduction in maternal IDA (RR: 0.73). With the observed sample size of 792 neonates in the placebo group and 803 in the iron-supplemented group, there was 80% power to detect a difference in birth weight of ≥55 g and, in general, small differences (effect size of ≥0.14) in any continuous outcome, such as cord blood measures of iron status. The study was not powered to detect differences in uncommon outcomes (e.g., low birth weight, fetal loss).
Statistical analysis.
Primary analyses were based on intention-to-treat. Chi-square tests were used to test for group differences in maternal anemia, ID, and IDA, fetal-neonatal ID, and other categorical variables. We used two-sample t tests to test for group differences in maternal and cord blood iron measures, infant gestational age and birth weight, and other continuous variables. In addition, we used multiple regression or logistic regression to adjust for potential confounding factors, such as baseline iron measures, duration of supplementation, gestational age at birth, etc. We used a similar approach in secondary analyses within the iron-supplemented group to model dose-response relations between the number of iron capsules reported being consumed and maternal and neonatal iron status outcomes among women in the iron-folate group who had complete data on adherence. Descriptive data are expressed as means ± SDs. Results for group comparisons of outcomes are expressed as means (95% CIs) for continuous variables and RRs (95% CIs) for categorical ones. Significance was set at P < 0.05.
Results
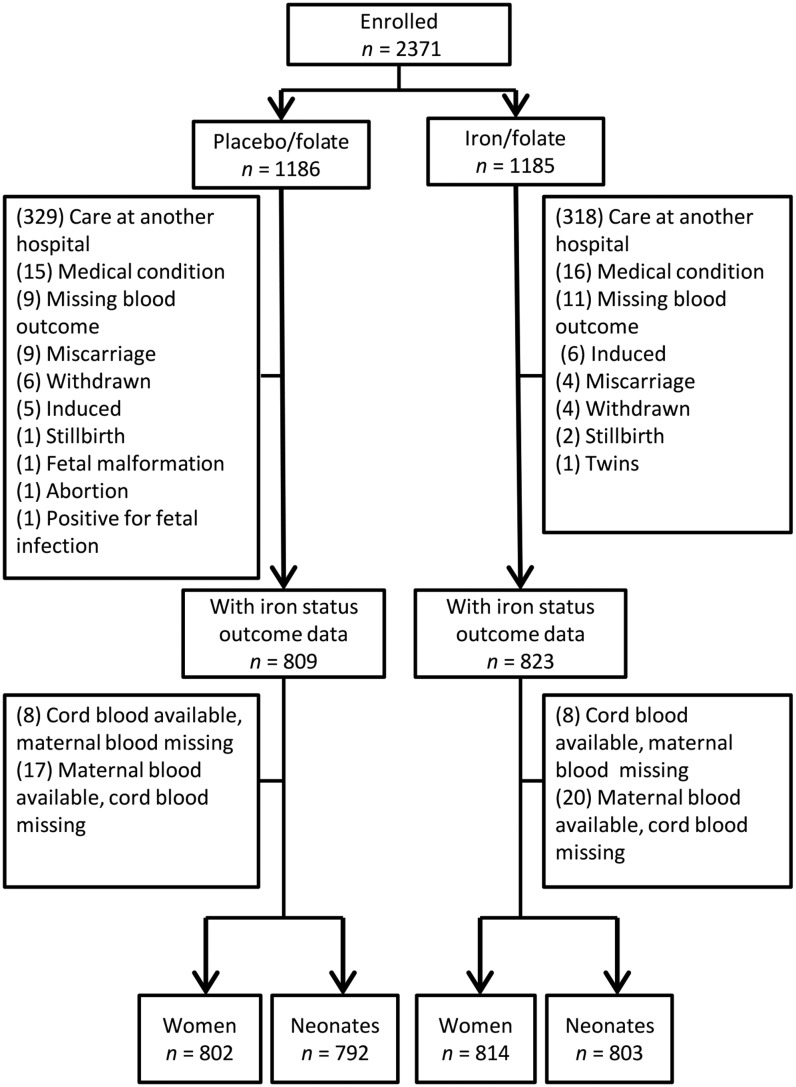
The 2371 pregnant women, enrolled at a mean gestational age of 15.9 ± 1.9 wk, were randomly assigned in approximately equal numbers to receive placebo and folate or iron and folate (Figure 1). Attrition affected the groups similarly both in proportion [31.8% (377 of 1186) in the placebo/folate group and 30.5% (362 of 1185) in the iron/folate group] and reason. Most attrition [87.5% (647 of 739)] was due to delivery at a nonparticipating hospital/health center. There was little attrition due to pregnancy complications (similar by group), which were generally referred to a tertiary-level general hospital, or for other reasons (Figure 1).
FIGURE 1.
Flowchart of participants in a randomized clinical trial among women in China, 2009–2011, by placebo/folate and iron/folate groups. One woman in the placebo/folate group with hemoglobin of 98 g/L, who was enrolled by mistake, provided outcome data and was retained in the study. Two women in the iron/folate group who were 17 y of age were also enrolled by mistake. They provided outcome data and were retained in the study.
Outcome data were available for 1632 women or neonates (1579 mother-newborn pairs, 37 mothers only, and 16 neonates only). Maternal iron status outcome data were obtained at or near term, generally within 1 wk of birth (mean gestational age: 39.4 ± 1.2 wk); 97% of the infants were born at term (37–41 wk).
Iron status at enrollment was similar for those with and without outcome data. For those with outcome data, maternal and family characteristics at enrollment were similar by supplement group (809 placebo/folate, 823 iron/folate) (Table 1), as was iron status (Table 2). Information on adherence was available for most women for the first 3-mo period of supplementation (95.8%; 1564 of 1632) but for less than half in the second 3-mo period (46.4%; 757 of 1632). Incomplete information on adherence was more likely for women who gave birth at a collaborating hospital other than Sanhe MCHC. Women with incomplete adherence data had more education and higher family incomes but were enrolled a half week later in gestation than those with complete data and had poorer iron status at the study conclusion (data not shown). On the basis of women with complete data, adherence was similar by group and generally good: the mean percentage of capsules reported being consumed was 83.7% (median = 90.1%) for the placebo group and 85.1% (median = 91.3%) for the iron-supplemented group. Weight gain in pregnancy was ∼18 kg in both groups but averaged 0.6 kg more in the placebo group than in the iron-supplemented group (Table 2).
TABLE 1.
Maternal and family characteristics at enrollment of pregnant women randomly assigned to receive placebo/folate or iron/folate supplements1
| Placebo/folate | Iron/folate | P2 | |
| n | 809 | 823 | |
| Maternal age, y | 24.5 ± 3.6 | 24.7 ± 3.6 | 0.20 |
| Education (middle school or less), n/total n (%) | 516/782 (66.0) | 538/797 (67.5) | 0.52 |
| Net family income ≤50,000 yuan/y, n/total n (%) | 402/756 (53.2) | 422/754 (56.0) | 0.28 |
| Primiparous, n/total n (%) | 605/776 (78.0) | 622/791 (78.6) | 0.75 |
| Gestational age at enrollment, wk | 15.8 ± 1.9 | 15.9 ± 1.9 | 0.97 |
| Prepregnancy weight, kg | 57.0 ± 9.3 | 57.0 ± 9.3 | 0.94 |
| Height, cm | 161 ± 4 | 161 ± 5 | 0.95 |
| Prepregnancy BMI, kg/m2 | 21.9 ± 3.3 | 21.9 ± 3.5 | 0.90 |
Values are means ± SDs for continuous variables and n/total n (%) for categorical variables. Numbers (n) vary slightly due to missing data. Information is based on women with hematology outcome data.
Obtained by using t tests for continuous variables and chi-square tests for categorical variables.
TABLE 2.
Supplement intake, pregnancy weight gain, and hematology upon enrollment and at or near term in women randomly assigned to receive placebo/folate or iron/folate supplements in pregnancy1
| Placebo/folate | Iron/folate | P | |
| At enrollment, n | 1186 | 1185 | |
| At or near term, n | 802 | 814 | |
| Time from enrollment to delivery, d | 167 (166, 168) | 167 (166, 168) | 0.94 |
| Capsules reported consumed,2 n | 138 (135, 142) | 139 (136, 142) | 0.67 |
| Weight gain in pregnancy, kg | 18.2 (17.8, 18.6) | 17.6 (17.2, 18.0) | 0.043 |
| Hemoglobin, g/L | |||
| Enrollment | 123 (122, 123) | 123 (122, 123) | 0.82 |
| At or near term | 117 (116, 117) | 122 (121, 123) | <0.001 |
| Mean corpuscular volume, fL | |||
| Enrollment | 85.2 (85.0, 85.4) | 85.1 (84.9, 85.3) | 0.60 |
| At or near term | 85.2 (84.9, 85.5) | 87.3 (86.9, 87.6) | <0.001 |
| ZPP/H,4 μmol/mol heme | |||
| Enrollment | 50.1 (48.8, 51.4) | 51.7 (50.4, 53.0) | 0.09 |
| At or near term | 81.7 (79.1, 84.3) | 65.8 (63.8, 68.0) | <0.001 |
| SF,4 μg/L | |||
| Enrollment | 30.7 (29.4, 32.1) | 30.7 (29.3, 32.1) | 0.98 |
| At or near term | 11.1 (10.7, 11.6) | 15.3 (14.6, 16.1) | <0.001 |
| sTfR,4 nmol/L | |||
| Enrollment | 14.7 (14.5, 14.9) | 14.8 (14.5, 15.0) | 0.67 |
| At or near term | 28.5 (27.8, 29.2) | 23.8 (23.2, 24.4) | <0.001 |
| Log(sTfR:SF) ratio | |||
| Enrollment | −0.74 (−0.79, −0.68) | −0.73 (−0.79, −0.68) | 0.88 |
| At or near term | 0.94 (0.89, 1.00) | 0.44 (0.37, 0.51) | <0.001 |
| BI, mg/kg | |||
| Enrollment | 5.32 (5.12, 5.51) | 5.30 (5.10, 5.49) | 0.89 |
| At or near term | −0.72 (−0.93, −0.52) | 1.09 (0.85, 1.33) | <0.001 |
| Platelets, 109/L | |||
| Enrollment | 227 (224, 230) | 230 (227, 233) | 0.27 |
| At or near term | 219 (215, 223) | 208 (204, 211) | <0.001 |
| CRP,4 mg/L | |||
| Enrollment | 2.06 (1.93, 2.20) | 1.99 (1.86, 2.13) | 0.45 |
| At or near term | 4.13 (3.85, 4.43) | 4.05 (3.80, 4.33) | 0.71 |
| Anemia (hemoglobin <110 g/L), n/total n (%) | |||
| Enrollment | 104/1180 (8.81) | 88/1179 (7.46) | 0.23 |
| At or near term | 201/802 (25.1) | 109/814 (13.4) | <0.001 |
| Hemoglobin <100 g/L at or near term, n/total n (%) | 29/802 (3.6) | 18/814 (2.2) | 0.09 |
| Iron deficiency,5 n/total n (%) | |||
| SF <15 μg/L | |||
| Enrollment | 224/1180 (19.0) | 223/1179 (18.9) | 0.97 |
| At or near term | 618/802 (77.1) | 462/815 (56.8) | <0.001 |
| BI6 <0 mg/kg | |||
| Enrollment | 94/1180 (8.0) | 87/1179 (7.4) | 0.59 |
| At or near term | 533/802 (66.5) | 351/814 (43.1) | <0.001 |
| Iron deficiency anemia,5 n/total n (%) | |||
| By SF | |||
| Enrollment | 29/1180 (2.5) | 25/1179 (2.1) | 0.58 |
| At or near term | 174/802 (21.7) | 86/814 (10.6) | <0.001 |
| By BI6 | |||
| Enrollment | 18/1180 (1.5) | 22/1157 (1.9) | 0.52 |
| At or near term | 159/802 (19.8) | 82/814 (10.1) | <0.001 |
Values are means (95% CIs) for continuous variables and n/total n (%) for categorical variables unless otherwise indicated. Numbers (n) vary slightly due to missing data. BI, body iron; CRP, C-reactive protein; SF, serum ferritin; sTfR, serum transferrin receptor; ZPP/H, zinc protoporphyrin/heme.
Values are based on women with complete adherence data and iron status outcomes (n = 360 and 380 in the placebo/folate and iron/folate groups, respectively).
Obtained by multiple regression with gestational age as a significant confounding factor.
Values are geometric means (95% CIs); t tests were conducted on log-transformed SF, sTfR, ZPP/H, and CRP.
Iron deficiency and iron deficiency anemia results are presented by 2 different criteria for iron deficiency: SF <15 μg/L and BI <0 mg/kg.
BI was calculated by using SF and sTfR according to the formula in Cook et al. (15): BI (mg/kg) = −[log10(sTfR × 1000/ferritin) – 2.8229]/0.1207. This formula used an sTfR assay described in Flowers et al. (20). To convert Beckman Coulter sTfR concentrations for use in the formula, we built on published data for Flowers, Ramco, and Beckman Coulter sTfRs. As reported in Pfeiffer et al. (21), the Ramco assay was similar to Flowers et al. Ramco and Beckman Coulter assays were part of a WHO study that used a standard reference reagent for sTfR (22). The Ramco assay yielded sTfR concentrations 4.3 times higher than those of Beckman Coulter, so the Flowers sTfR equivalent was calculated by the following formula: Flowers sTfR = 4.3 × Beckman Coulter sTfR.
Maternal iron status.
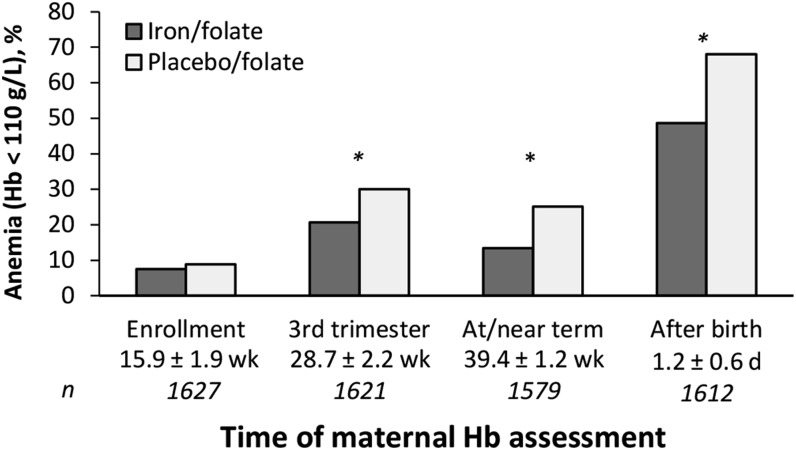
At enrollment, ∼8% of women were mildly anemic (hemoglobin: 100–109 g/L) (Table 2). At or near term, maternal hemoglobin averaged 5.56 g/L higher in the iron-supplemented group than in the placebo group (effect size = 0.50), and the prevalence of anemia was lower (13.4% vs. 25.1%; RR: 0.53; 95% CI: 0.43, 0.66). Compared with placebo, iron supplementation reduced the risk of ID and IDA at or near term (RR: 0.74; 95% CI: 0.69, 0.79 for ID defined as SF <15 μg/L; RR: 0.65; 95% CI: 0.59, 0.71 for ID defined as BI <0 mg/kg; RR: 0.49; 95% CI: 0.38, 0.62 for IDA by low SF; RR: 0.51; 95% CI: 0.40, 0.65 for IDA by low BI). However, the majority of women still had ID at or near term (66.8% by low SF, 54.7% by low BI). There was little IDA at the beginning of the trial (2.5% by low SF, 1.5% by low BI). Without iron supplementation, there was a prevalence of IDA of 21.7% at or near term by low SF and of 19.7% by low BI; corresponding IDA proportions with iron supplementation were 10.6% by low SF and 10.1% by low BI. All hematologic and biochemical measures of maternal iron status—MCV, ZPP/H, SF, sTfR, sTfR:SF ratio, and BI—improved significantly at or near term with iron supplementation. Effect sizes ranged from 0.44 for MCV to 0.57 for sTfR:SF ratio and BI. There was also a significant reduction in platelets in the iron-supplemented group (effect size = 0.20). Despite improved iron status in the iron/folate group, ID was widespread regardless of supplementation or criterion: more than half of the women who received supplemental iron and three-quarters of those who did not had SF <15 μg/L. The impact of iron supplementation on anemia reduction (Figure 2) was apparent by the clinical visit at 26–32 wk (RR: 0.69; 95% CI: 0.58, 0.82). Postpartum (90% of samples on day 1), anemia was reduced by 19.4% (RR: 0.71; 95% CI: 0.66, 0.78). The results were similar with and without adjustment for confounding factors. Covariate analyses using multiple or logistic regression included parity, gestational age, duration of supplementation, and CRP at or near term. For postpartum anemia, number of days after birth was an additional confounding factor.
FIGURE 2.
Anemia prevalence at enrollment, during the early third trimester, at or near term, and ∼1 d after birth in women randomly assigned to receive iron/folate or placebo/folate supplements during pregnancy. Time of hemoglobin assessment is expressed as means ± SDs for weeks of gestation and for days after birth. *Iron/folate different from placebo/folate, P < 0.01. Hb, hemoglobin.
Fetal-neonatal iron status and other outcomes.
There were no significant group differences in cord blood measures of fetal-neonatal iron status (Table 3). Regardless of maternal iron supplementation, >45% of neonates had cord SF <75 μg/L or ZPP/H >118 μmol/mol. The groups were similar in other fetal-neonatal outcomes, such as gestational age and birth weight and length. The results were similar with and without adjustment for confounding factors (parity, initial pregnancy iron measures, gestational age, duration of supplementation, and CRP).
TABLE 3.
Birth outcomes and cord blood iron status of neonates born to women who were randomly assigned to receive placebo/folate or iron/folate supplements in pregnancy1
| Placebo/folate, (n = 792) | Iron/folate(n =803) | P | |
| Infant outcomes | |||
| Gestational age, wk | 39.7 (39.6, 39.7) | 39.6 (39.6, 39.7) | 0.76 |
| Birth weight, g | 3368 (3341, 3395) | 3355 (3330, 3383) | 0.55 |
| Birth length, cm | 49.7 (49.6, 49.8) | 49.7 (49.6, 49.8) | 0.65 |
| Cesarean delivery,2 n/total n (%) | 527/799 (66.0) | 571/815 (70.1) | 0.08 |
| Male, n/total n (%) | 422/812 (52.0) | 433/820 (52.8) | 0.74 |
| Cord blood iron status | |||
| Hemoglobin, g/L | 152 (151, 153) | 153 (152, 154) | 0.14 |
| Mean corpuscular volume, fL | 102 (102, 103) | 102 (102, 103) | 0.98 |
| ZPP/H,3 μmol/mol heme | 99.6 (97.2, 102.1) | 97.0 (94.7, 99.3) | 0.13 |
| SF,3 μg/L | 103 (97.9, 108) | 105 (101, 110) | 0.43 |
| sTfR,3 nmol/L | 30.9 (30.2, 31.6) | 30.5 (29.8, 31.2) | 0.44 |
| Log(sTfR:SF) ratio | −1.18 (-1.24, -1.13) | −1.21 (-1.27, -1.16) | 0.44 |
| Iron deficiency (SF <75 μg/L or ZPP/H >118 μmol/mol), n/total n (%) | 352/782 (45.0) | 371/789 (47.0) | 0.42 |
Values are means (95% CIs) for continuous variables and n/total n (%) for categorical variables unless otherwise indicated. Numbers (n) vary slightly due to missing data. SF, serum ferritin; sTfR, serum transferrin receptor; ZPP/H, zinc protoporphyrin/heme.
Per usual clinical practice in China, birth by cesarean delivery was common.
Values are geometric means (95% CIs); t tests were conducted on log-transformed SF, sTfR, and ZPP/H.
Although the study was not powered to detect significant differences in uncommon serious adverse birth outcomes (i.e., miscarriage, still birth, prematurity, or major congenital malformation), there were almost twice as many serious adverse outcomes in the placebo group (15 of 831; 1.81%) than in the iron-supplemented group (8 of 840; 0.95%) (χ2 = 2.24, P = 0.13; Figure 1). More than two-thirds of the women in each group reported minor adverse symptoms such as nausea, vomiting, diarrhea, or constipation (68.2% vs. 68.4% in placebo and iron-supplemented groups, respectively).
Analyses of adherence and dose-response relations.
We conducted further analyses to understand the reasons for missing data on adherence and use of medicinal iron or iron-containing supplements outside the study. As noted above, data were complete for the first 3-mo period of study participation but decreased to approximately half for the second period. In analyzing the rate of missing adherence data over time, we noted an increase corresponding to the time that 2 additional local hospitals were included. The rate continued to increase gradually thereafter. Women with iron status outcome data but who were missing second-batch adherence data also had poorer iron status than those with complete adherence data (data not shown).
We analyzed dose-response relations, controlling for significant confounding factors, between number of capsules reportedly consumed and outcome in the 380 women in the iron-folate group who had complete data on adherence. Even though adherence was generally high (>85% of possible capsules were reported to have been consumed), there was variation in the number of doses reported being consumed (mean: 139 ± 30; range: 28–192). Potential confounding factors were maternal and family factors that might influence both the number of capsules reportedly consumed and iron status outcomes such as maternal age, parity, and education; family income; and duration of study participation. There were significant relations between the number of capsules reportedly consumed and maternal and neonatal iron measures (Table 4). The results uniformly indicated that the more capsules reportedly consumed, the better the maternal iron status. There was also a significant relation with cord blood SF (more capsules reported being consumed, higher SF) and a suggestive trend with cord blood ZPP/H (more capsules reported being consumed, lower ZPP/H).
TABLE 4.
Relations between iron capsules reported being consumed (per 10 capsules) and maternal and neonatal iron status outcome measures among women in the iron/folate group with complete adherence data1
| β (SE) | P | |
| Maternal iron measures at or near term | ||
| Hemoglobin, g/L | 0.727 (0.188) | <0.001 |
| Mean corpuscular volume, fL | 0.204 (0.074) | 0.006 |
| ZPP/H,2 μmol/mol heme | −0.042 (0.008) | <0.001 |
| SF,23 μg/L | 0.051 (0.012) | <0.001 |
| sTfR,2 nmol/L | −0.023 (0.006) | <0.001 |
| BI, mg/kg | 0.272 (0.062) | <0.001 |
| Cord blood iron measures | ||
| Hemoglobin, g/L | −0.089 (0.265) | 0.738 |
| ZPP/H,2 μmol/mol heme | −0.011 (0.006) | 0.065 |
| SF,3 μg/L | 2.60 (1.26) | 0.040 |
n = 380. The β and P values are based on multiple linear regression controlling for days in the study and significant confounding factors. Maternal ZPP/H, SF, and sTfR and cord ZPP/H were log-transformed to normalize the distribution. BI, body iron; SF, serum ferritin; sTfR, serum transferrin receptor; ZPP/H, zinc protoporphyrin/heme.
β Values (per 10 capsules) are based on log transformation of the indicated iron status outcome measures.
For SF outcomes, values >370 μg/L were excluded because they may indicate infection or inflammation. Six cord blood SF values were excluded for this reason; no maternal values were > 370 μg/L.
Discussion
We found that iron supplementation in pregnancy increased maternal hemoglobin at or near term by 5.56 g/L and reduced the risk of anemia, ID, and IDA. The improvement in maternal iron status did not occur at the expense of fetal iron status. Fetal-neonatal iron status in the placebo group was similar to that in the iron-supplemented group, but maternal iron status was considerably worse. There were no differences in birth weight or other infant outcomes with iron supplementation.
Our findings on reduced risk of maternal ID/IDA can be compared with recent meta-analyses of randomized trials of prenatal iron supplementation (7–9). Few studies assessed iron status, but most that did so defined ID as low SF. The reduced RR of ID we observed (RR: 0.74) is within the range of the most recent systematic review/meta-analysis (RR: 0.59; 95% CI: 0.44, 0.79; 8 trials) (7). Our ID results are similar to those of a large trial, which was also conducted in Hebei Province (10), and jointly supported by the US CDC, in which iron status was assessed in 834 of ∼19,000 participating women (12). Although we obtained iron measures at or near term, whereas that study did so at 28–32 wk, and SF cutoffs differed [<15 vs. <12 μg/L (12)], mean SF at the study conclusion was almost identical in the folate groups in that trial and in ours (11.3 vs. 11.1 μg/L) and was similar in the iron-folate groups (16.7 vs. 15.3 μg/L). Depending on ID criterion (low SF or BI <0 mg/kg), 59.6–69.9% of women receiving folate in that trial had ID, as did 34.3–53.6% of those receiving iron-folate (12); our corresponding numbers were slightly higher (66.5–77.0% and 42.8–56.8%, respectively).
With regard to IDA, the reduced risk we observed (RR: 0.49) is similar to that in the meta-analyses (mean RRs: 0.33–0.44; 5–7 trials) (7–9) but contrasts with no reduction in the other Hebei study. Because mean SF and ID prevalence postsupplementation were quite similar in both Hebei clinical trials, differing results for IDA likely hinge on differences in hemoglobin and anemia. Our results for hemoglobin increase and anemia reduction are consistent with the meta-analyses. Iron supplementation increased maternal hemoglobin at or near term by 4.59 g/L in 1 review (7) and 8.88 g/L in another (9). In contrast, hemoglobin increased only 0.4 g/L in the other Hebei trial (10). Although the risk was reduced, anemia was uncommon, only ∼6.5%, with surprisingly little change from enrollment despite the emergence of ID in the majority of women. Direct comparisons between our study and that trial are limited by important differences in methodology, but the marked difference in anemia prevalence warrants further investigation.
Improvements in maternal hemoglobin and reductions in anemia, ID, and IDA in our study and the meta-analyses can be clinically meaningful. Shifting the hemoglobin curve to the right should help reduce the number of pregnant women with severe anemia and associated serious complications (1). Better iron status and less anemia may also contribute to maternal well-being in other ways, such as work performance, household activities, and parenting (23, 24)
The reduction in platelets we observed with iron supplementation is unlikely to be clinically important, because counts were in the normal range. An impact of prenatal iron supplements on platelets was not mentioned in the systematic reviews or meta-analyses of randomized clinical trials. However, reactive thrombocytosis has been reported with IDA; thrombocytopenia is also seen with severe IDA (25–27).
Despite improved maternal iron status with iron supplementation in our study, most women had marginal-to-poor iron status at or near term. This was also observed in our previous study in southeastern China (5) and in the other Hebei trial (12). These findings point out the challenge of meeting iron needs of pregnant women in China and elsewhere. Dietary factors and pregnancy physiology (28) are relevant, but the combination of relatively small or thin mothers and large newborns may be important. Mean birth weight in our sample was similar to the US mean (29), but mean prepregnancy weight was 16.4 kg less than nonpregnant US women in their 20s (30), BMI was considerably lower (in kg/m2; 21.9 vs. 27.5) (30), and pregnancy weight gain was ∼2 kg above the upper limit recommended in the United States (31). When thinner or smaller women and larger or heavier women give birth to infants of similar size, thinner or smaller women are likely to be at higher risk of ID. They enter pregnancy with lower blood volume and body mass, and hence, a lower total absolute amount of iron in their bodies than larger women. Because the iron needs of the placenta and fetus do not depend on maternal size, a thinner or smaller woman has to transfer proportionally more of the iron in her body to the placenta and fetus than a larger woman for a similarly sized fetus. Our results suggest that lower maternal BMI is a risk factor for poor iron status, despite iron supplementation, especially if combined with high pregnancy weight gain and good fetal growth.
The lack of significant group differences in neonatal iron measures does not appear to be due to limited power. The sample size was adequate to detect small effect sizes (d = 0.14) in continuous newborn outcomes, including cord blood iron measures. Thus, any missed differences in iron status would be very small and not clinically meaningful.
Improvements in maternal iron status with prenatal iron supplementation without group differences in fetal-neonatal iron status underscore the complexities of iron regulation between mother, placenta, and fetus. Previous cohort studies reported little association between maternal and neonatal iron status unless mothers are markedly anemic or have severe ID (5). These findings indicate that the fetal blood compartment is buffered until maternal iron status is very compromised. Maternal iron status has been shown to influence iron transfer to the fetus late in pregnancy by using stable isotopes (32), but fetus and placenta also play a role. Placental iron transport is upregulated as fetal iron status becomes compromised (33, 34), resulting in more iron transfer to the fetus. This is at the expense of the mother if external sources of iron are inadequate to meet iron needs. These processes may help explain a lack of group differences in cord blood iron status with prenatal iron supplementation despite substantial differences in maternal iron status.
The findings do not mean, however, that infants receive no benefit from maternal iron supplementation. Although effects were small, the more iron capsules reportedly consumed, the higher the cord blood SF (P = 0.04) and the lower the cord blood ZPP/H (suggestive trend, P = 0.07). The analyses controlled for factors that might influence both the number of capsules reportedly consumed and outcome, including duration of study participation, but causality cannot be inferred. Nonetheless, the implication is that maternal iron supplementation may affect neonatal iron status if “enough” iron is consumed. More research is needed to determine how much iron is enough under conditions of such high iron needs. It is also important to emphasize that iron to the fetus is a concern not only for blood but also for the brain (35). In fact, a large randomized clinical trial in Nepal found that children of iron-supplemented mothers had higher neurocognitive test scores at 7–9 y (36) than those whose mothers did not receive iron. Thus, there could be positive fetal effects of prenatal iron supplementation, even if there is little or no demonstrable improvement in cord blood measures of iron status.
We did not observe an increase in birth weight with iron supplementation, in contrast to meta-analysis findings. As noted above, power was adequate to detect a ≥55-g difference in birth weight, but the actual group difference in birth weight was trivial, 13.4 g. This small difference, which would not be clinically meaningful regardless of sample size, and the power calculation show that the lack of significance was not due to limited statistical power. There was also no birth weight difference in the other Hebei trial (10) and only in the poorest women in another China study (11). It is unclear why some studies found increased birth weight and/or reduced low birth weight with iron supplementation but others did not. An important factor may be differences in sample birth weight characteristics. In both Hebei trials, there was little room for improvement, because infants were generally healthy, mean birth weights were >3200 g, and low birth weight was rare. Iron supplementation may have little impact on birth weight in similarly healthy populations.
Attrition was higher than anticipated, because we had not appreciated that women often gave birth at a local health care facility other than where they received initial prenatal care. That a considerable proportion of study participants (∼30%) chose to give birth at a nonparticipating hospital is evidence that they understood the voluntary nature of their study involvement. Although drop-out rates were similar across study groups and the randomized design was not compromised, the high drop-out rate is a limitation of the study.
Reliance on maternal self-report regarding adherence and use of medicinal iron or over-the-counter iron-containing supplements is another study limitation, as is incomplete data for these measures for the second 3-mo supplementation period. “Missingness” appears to be due in part to birth at a participating hospital other than Sanhe MCHC. However, poorer iron status in women with missing adherence data suggests that some “missingness” was due to poorer adherence. This could make our dose-response analyses underestimate the true impact of consuming more iron capsules. Analysis of primary outcomes by intention-to-treat is also likely to provide a conservative estimate of the effects of prenatal iron supplementation.
In addition, our study was not powered to detect differences in serious adverse outcomes, which were infrequent. Despite a more comprehensive panel of iron measures in both mothers and neonates than in other studies, we did not obtain some sensitive iron measures, such as reticulocyte hemoglobin and reticulocyte ZPP/H (37) or hepcidin.
In sum, our study showed that prenatal iron supplementation improved maternal hemoglobin and iron status and reduced anemia, ID, and IDA. However, our results emphasize that “improved” does not mean “problem solved.” The majority of women and >45% of the neonates had indications of ID, regardless of supplementation. These results point to challenges in preventing ID and IDA among pregnant women and neonates in at-risk populations.
Acknowledgments
We appreciate the guidance of the Independent Data Monitoring Committee for the NIH grant. Members included: Sean Lynch, Zuguo Mei, Christine McLaren, and Zu-Pei Chen.
GZ, ZZ, TT, ML, and BL contributed to the study conception and design; GZ, GX, MZ, YJ, ZZ, TT, and ML conducted the research; BR and NK performed statistical analysis; GZ, MZ, YJ, KMC, and BL wrote the manuscript; GZ and BL had primary responsibility for final content; and MKG contributed to interpretation of the findings. All authors critically revised the manuscript and read and approved the final manuscript.
Footnotes
Abbreviations used: BI, body iron; CRP, C-reactive protein; ID, iron deficiency; IDA, iron deficiency anemia; MCHC, Maternity and Child Health Care Center; MCV, mean corpuscular volume; SF, serum ferritin; sTfR, serum transferrin receptor; ZPP/H, zinc protoporphyrin/heme.
References
- 1.Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, Pena-Rosas JP, Bhutta ZA, Ezzati M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health 2013;1:e16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalenius K, Brindley P, Smith B, Reinold C, Grummer-Strawn L. Pregnancy Nutrition Surveillance 2010 Report. Atlanta (GA): US Department of Health and Human Services, CDC; 2012. [Google Scholar]
- 3.Mei Z, Cogswell ME, Looker AC, Pfeiffer CM, Cusick SE, Lacher DA, Grummer-Strawn LM. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Am J Clin Nutr 2011;93:1312–20. [DOI] [PubMed] [Google Scholar]
- 4.Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr 2000;71(5, Suppl):1280S–4S. [DOI] [PubMed] [Google Scholar]
- 5.Shao J, Lou J, Rao R, Georgieff MK, Kaciroti N, Felt BT, Zhao ZY, Lozoff B. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J Nutr 2012;142:2004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lozoff B. Iron deficiency and child development. Food Nutr Bull 2007;28:S560–71. [DOI] [PubMed] [Google Scholar]
- 7.Haider BA, Olofin I, Wang M, Spiegelman D, Ezzati M, Fawzi WW. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. BMJ 2013;346:f3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imdad A, Bhutta ZA. Routine iron/folate supplementation during pregnancy: effect on maternal anaemia and birth outcomes. Paediatr Perinat Epidemiol 2012;26(Suppl 1):168–77. [DOI] [PubMed] [Google Scholar]
- 9.Pena-Rosas JP, De-Regil LM, Dowswell T, Viteri FE. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev 2012. Issue 12. Art. No.: CD004736. DOI: 10.1002/14651858.CD004736.pub4. [DOI] [PMC free article] [PubMed]
- 10.Liu JM, Mei Z, Ye R, Serdula MK, Ren A, Cogswell ME. Micronutrient supplementation and pregnancy outcomes: double-blind randomized controlled trial in China. JAMA Intern Med 2013;173:276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng L, Yan H, Cheng Y, Dibley MJ. Modifying effects of wealth on the response to nutrient supplementation in pregnancy on birth weight, duration of gestation and perinatal mortality in rural western China: double-blind cluster randomized controlled trial. Int J Epidemiol 2011;40:350–62. [DOI] [PubMed] [Google Scholar]
- 12.Mei Z, Serdula MK, Liu JM, Flores-Ayala RC, Wang L, Ye R, Grummer-Strawn LM. Iron-containing micronutrient supplementation of Chinese women with no or mild anemia during pregnancy improved iron status but did not affect perinatal anemia. J Nutr 2014;144:943–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preziosi P, Prual A, Galan P, Daouda H, Boureima H, Hercberg S. Effect of iron supplementation on the iron status of pregnant women: consequences for newborns. Am J Clin Nutr 1997;66:1178–82. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Vitamin and Mineral Nutrition Information System. Geneva (Switzerland): World Health Organization; 2011. (WHO/NMH/NHD/MNM/11.2) [cited 2014 Jul 14.] Available from: http://www.who.int/vmnis/indicators/serum_ferritin.pdf.
- 15.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood 2003;101:3359–64. [DOI] [PubMed] [Google Scholar]
- 16.Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, et al. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr 2002;140:165–70. [DOI] [PubMed] [Google Scholar]
- 17.Amin SB, Orlando M, Eddins A, MacDonald M, Monczynski C, Wang H. In utero iron status and auditory neural maturation in premature infants as evaluated by auditory brainstem response. J Pediatr 2010;156:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armony-Sivan R, Eidelman AI, Lanir A, Sredni D, Yehuda S. Iron status and neurobehavioral development of premature infants. J Perinatol 2004;24:757–62. [DOI] [PubMed] [Google Scholar]
- 19.McLimore HM, Phillips AK, Blohowiak SE, Pham DQ, Coe CL, Fischer B, et al. Impact of multiple risk factors on newborn iron status. J Pediatr Hematol Oncol 2013;35:473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flowers CH, Skikne BS, Covell AM, Cook JD. The clinical measurement of serum transferrin receptor. J Lab Clin Med 1989;114:368–77. [PubMed] [Google Scholar]
- 21.Pfeiffer CM, Cook JD, Mei Z, Cogswell ME, Looker AC, Lacher DA. Evaluation of an automated soluble transferrin receptor (sTfR) assay on the Roche Hitachi analyzer and its comparison to two ELISA assays. Clin Chim Acta 2007;382:112–6. [DOI] [PubMed] [Google Scholar]
- 22.Thorpe SJ, Heath A, Sharp G, Cook J, Ellis R, Worwood M. A WHO reference reagent for the serum transferrin receptor (sTfR): international collaborative study to evaluate a recombinant soluble transferrin receptor preparation. Clin Chem Lab Med 2010;48:815–20. [DOI] [PubMed] [Google Scholar]
- 23.Haas J, Brownlie T. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr 2001;131(Suppl):676S–90S. [DOI] [PubMed]
- 24.Li R, Chen X, Yan H, Deurenberg P, Garby L, Hautvast JGAJ. Functional consequences of iron supplementation in iron-deficient female cotton workers in Beijing, China. Am J Clin Nutr 1994;59:908–13. [DOI] [PubMed] [Google Scholar]
- 25.Kadikoylu G, Yavasoglu I, Bolaman Z, Senturk T. Platelet parameters in women with iron deficiency anemia. J Natl Med Assoc 2006;98:398–402. [PMC free article] [PubMed] [Google Scholar]
- 26.Morris VK, Spraker HL, Howard SC, Ware RE, Reiss UM. Severe thrombocytopenia with iron deficiency anemia. Pediatr Hematol Oncol 2010;27:413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuku I, Kaya S, Yologlu R, Gokdeniz R, Baydin A. Platelet counts in adults with iron deficiency anemia. Platelets 2009;20:401–5. [DOI] [PubMed] [Google Scholar]
- 28.Pena-Rosas JP, Viteri FE. Effects of routine oral iron supplementation with or without folic acid for women during pregnancy. Cochrane Database Syst Rev 2006;3:CD004736. [DOI] [PubMed] [Google Scholar]
- 29.Donahue SM, Kleinman KP, Gillman MW, Oken E. Trends in birth weight and gestational length among singleton term births in the United States, 1990–2005. Obstet Gynecol 2010;115:357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fryar CD, Gu Q, Ogden CL. Anthropometric reference data for children and adults: United States, 2007#x20131010. 2012. National Center for Health Statistics. Vital Health Stat 11(252). [PubMed]
- 31.Institute of Medicine. Weight gain during pregnancy: reexamining the guidelines. Washington (DC): National Academies Press; 2009. [PubMed] [Google Scholar]
- 32.O'Brien KO, Zavaleta N, Abrams SA, Caulfield LE. Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy. Am J Clin Nutr 2003;77:924–30. [DOI] [PubMed] [Google Scholar]
- 33.McArdle HJ, Lang C, Hayes H, Gambling L. Role of the placenta in regulation of fetal iron status. Nutr Rev 2011;69:S17–22. [DOI] [PubMed] [Google Scholar]
- 34.Bradley J, Leibold EA, Harris ZL, Wobken JD, Clarke S, Zumbrennen KB, Eisenstein RS, Georgieff MK. Influence of gestational age and fetal iron status on IRP activity and iron transporter protein expression in third-trimester human placenta. Am J Physiol Regul Integr Comp Physiol 2004;287:R894–901. [DOI] [PubMed] [Google Scholar]
- 35.Wachs TD, Georgieff M, Cusick S, McEwen BS. Issues in the timing of integrated early interventions: contributions from nutrition, neuroscience, and psychological research. Ann N Y Acad Sci 2014;1308:89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christian P, Murray-Kolb L, Khatry SK, Katz J, Schaefer BA, Cole PM, LeClerq SC, Tielsch J. Prenatal micronutrient supplementation and intellectual and motor function in early school-aged children in Nepal. JAMA 2010;304:2716–23. [DOI] [PubMed] [Google Scholar]
- 37.Phillips AK, Roy SC, Lundberg R, Guilbert TW, Auger AP, Blohowiak SE, Coe CL, Kling PJ. Neonatal iron status is impaired by maternal obesity and excessive weight gain during pregnancy. J Perinatol 2014;34:513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]