Abstract
Background: Human milk is the gold standard of nutrition for infants, providing both protective and essential nutrients. Although much is known about milk from mothers giving birth to term infants, less is known about milk from mothers giving birth to premature infants. In addition, little is known about the composition and diversity of small molecules in these milks and how they change over the first month of lactation.
Objective: The objective was to understand how milk metabolites vary over the first month of lactation in mothers giving birth to term and preterm infants.
Methods: 1H nuclear magnetic resonance (NMR) metabolomics was used to characterize metabolites that were present in micromolar to molar concentrations in colostrum (day 0–5 postpartum), transition milk (day 14), and mature milk (day 28) from mothers who delivered term (n = 15) and preterm (n = 13) infants. Principal components analysis, linear mixed-effects models (LMMs), and linear models (LMs) were used to explore the relation between infant maturity and the postpartum day of collection of milk samples.
Results: By using a standard NMR metabolite library, 69 metabolites were identified in the milks, including 15 sugars, 23 amino acids and derivatives, 11 energy-related metabolites, 10 fatty acid–associated metabolites, 3 nucleotides and derivatives, 2 vitamins, and 5 bacteria-associated metabolites. Many metabolite concentrations followed a similar progression over time in both term and preterm milks, with more biological variation in metabolite concentrations in preterm milk. However, although lacto-N-neotetraose (LMM, P = 4.0 × 10−5) and lysine (LM, P = 1.5 × 10−4) significantly decreased in concentration in term milk over time, they did not significantly change in preterm milk.
Conclusion: Overall, the metabolic profile of human milk is dynamic throughout the first month of lactation, with more variability in preterm than in term milk and subtle differences in some metabolite concentrations. This trial was registered at clinicaltrials.gov as NCT01841268.
Keywords: metabolomics, lactation, human milk, term, preterm, human milk oligosaccharides
Introduction
Human milk is recommended for infants because it provides a number of nutritional, immunologic, gastrointestinal, and neurodevelopmental benefits (1). Proper nutrition is particularly important for premature infants (gestational age <37 wk). Unfortunately, for women giving birth to premature infants, it can be difficult to establish and sustain a milk supply, because mothers are separated from their infants and premature infants <34 wk of gestation have not developed the skill to suck and swallow. Moreover, preterm milk has been shown to differ from term milk, with preterm milk initially higher in protein, fat, and free amino acids and lower in calcium (2, 3). Despite the body of literature describing the difference in composition between preterm and term milks, few studies have focused on lower molecular weight compounds; and of these, most studies focused on characterizing lactose and oligosaccharide content. For instance, lactose, the most abundant milk sugar, has been reported to be lower (4, 5) or not significantly different (6, 7) in preterm milk compared with term milk. Total oligosaccharide and free, oligosaccharide-bound, and protein-bound sialic acid concentrations were similarly reported to be higher in preterm milk during the first month of lactation (4, 8, 9). Several studies also found greater variation in the concentration of human milk oligosaccharides (HMOs)8 in preterm milk than in term milk (9, 10).
It is well known that human milk decreases the incidence of necrotizing enterocolitis (11, 12), late-onset sepsis (13), retinopathy of prematurity (14, 15), metabolic syndrome later in life (16), and rehospitalizations (17) and improves neurodevelopment (17–19). Thus, for mothers unable to provide adequate milk volume, current recommendations are to provide heat-treated donor human milk (1). Because donor human milk is mature milk provided by women who deliver at term, and there are differences between term and preterm milk, a complete understanding of the differences between milk from women delivering term and preterm infants over time, particularly in the first month postpartum, has clinical relevance.
In an effort to increase the body of knowledge regarding the composition of small molecules in human milk, both as it changes over lactation and how it is affected by infant maturity at birth, we used targeted 1H NMR metabolomics to analyze colostrum (milk expressed within the first week postpartum), transition milk (14 d postpartum), and mature milk (28 d postpartum) from mothers of 15 term and 13 preterm infants.
Methods
Participants.
This was a prospective observational study in mothers of term infants from the UC (University of California) Davis Foods for Health Institute Lactation Study and mothers of preterm infants of varying gestational ages whose children had been admitted to the neonatal intensive care unit at University of California Davis Children’s Hospital in Sacramento, California. Informed consent was obtained before participation and milk collection procedures were performed in accordance with the ethical standards of the responsible committee on human experimentation. Breast milk was collected at enrollment (0–5 d) and at 14 and 28 d postpartum by using a modified published method (20) involving milk collection from 1 breast using a Harmony Manual Breast pump (Medela, Inc.) as previously described (21). Although mothers of term infants collected milk between 2 and 4 h after feeding, for mothers of preterm infants milk was collected at the time of expression for their infants and was not specifically associated with infant feeding. Term milk was stored in subjects’ kitchen freezers (typically −18°C) and preterm milk was stored in the hospital freezer at −20°C until sample pick-up within 2 wk after collection and subsequently transported to the laboratory on dry ice and stored at −80°C until processing. Detailed medical, diet, and exposure histories were obtained at enrollment and at 2 and 4 wk of age. This study was registered at clinicaltrials.gov (NCT01841268).
Sample preparation.
Milk aliquots for NMR analysis (0.4–1.0 mL) were removed from storage at −80°C, defrosted, and prepared for NMR analysis as previously described (21). Only those metabolites that exist free in solution were quantified and reported herein.
Identification and quantification of metabolites.
1H NMR was used to identify and quantify metabolites in human milk because of the ease of sample preparation, its large dynamic range (can simultaneously measure multiple metabolite concentrations in the micromolar to molar range), and its ability to provide absolute quantitation (22). 1H NMR spectra were acquired on a Bruker Avance 600-MHz NMR spectrometer equipped with a SampleJet autosampler with the use of a NOESY-presaturation pulse sequence (noesypr) at 25°C as previously described (21, 23). All spectra were processed and metabolites identified and quantified (24) by using a combination of the 600-MHz library from the NMR Suite version 7.7 Profiler (Chenomx) and an in-house library of metabolites analytically prepared as pure compounds and NMR spectra acquired under identical conditions as previously described (21). For the majority of compounds, the accuracy of the reported concentrations was within 10% of the actual concentration (23), with a mean ± SD precision in measurement of 6.1% ± 2.3% (21). For an annotated NMR spectrum of human milk, the reader is referred to reference 25.
Statistical analysis.
Statistical analysis was performed by using a combination of Simca version 13 (Umetrics), Prism version 6.0 (GraphPad Software), and R software version 3.1.0. Means ± SDs and percentage of the CV (%CV), which was calculated as SD/mean × 100, are reported for each metabolite. All concentrations were log10-transformed before principal components analysis (PCA) or analyzed by using linear mixed-effects models (LMMs) and linear models (LMs) as described below.
PCA was used to explore the relation between the postpartum day of milk collection and infant maturity (term or preterm) at birth on the milk metabolome. To further investigate whether milk metabolites were significantly affected by infant maturity while taking into consideration the postpartum day of milk collection, either an LMM or an LM was used on the basis of which model had the lowest Akaike information criterion value (best fit) for each metabolite. The lmer function (R package “lmerTest”) was used for the LMM with postpartum lactation day and infant maturity at birth as interacting fixed effects and milk donor as a random effect, with the restricted maximum likelihood estimation set to false. The resulting P values were adjusted for multiple comparisons by the false discovery rate. In cases in which an LM was appropriate, postpartum lactation day and infant maturity at birth were modeled as interacting variables and the resulting P values were also adjusted by the false discovery rate. To test the significance of secretor status when modeling carbohydrates, secretor status [defined as a sample containing 2′-fucosyllactose (2′-FL) above the detection threshold of NMR] was included as a fixed effect in the LMM and LM, and if it was determined to be significant (P < 0.05) it was included in the model for a given sugar. Likewise, secretor status was modeled as interacting with postpartum lactation day and infant maturity at birth only if the interaction was determined to be significant. To determine which metabolites in milk significantly changed over time, the same procedure was repeated, but infant maturity was removed from the models and the term and preterm milks were treated independently. The term and preterm milk metabolite means at each lactation time point were compared by using two-tailed t tests assuming unequal variance. Significant differences were defined as P < 0.05 for all statistical tests.
Results
Self-reported participant demographic and pregnancy data are shown in Supplemental Table 1, and infant birth characteristics are shown in Supplemental Table 2. Metadata were not collected from one of the mothers who delivered a preterm infant. Most of the mothers who delivered preterm infants were aged <25 y, whereas the majority of mothers with term infants were aged >25 y. Although the concentrations of several metabolites were affected by maternal age (Supplemental Table 3), it is unknown whether these results are biologically important or merely an artifact of the distribution of ages in mothers giving birth to term or preterm infants and the small number of mothers in each arbitrarily assigned age group.
In total, 69 metabolites at 3 postpartum time points were identified in the NMR spectra of milk from mothers giving birth to term (≥37 wk gestation) and preterm (<37 wk gestation) infants. We previously reported on 65 metabolites at day 90 postpartum in women delivering term infants (21). Here, an additional 5 metabolites, including 3′-galactosyllactose (3′-GSL), 2-hydroxybutyrate, methionine, acetoin, and dimethyl sulfone, are reported. Except for dimethyl sulfone, these metabolites were in high concentrations in colostrum, decreasing over time in term milk, which may explain why they were not quantified in our previous study (their concentrations were below the detection limit of the NMR at day 90). In contrast, dimethyl sulfone started at a low concentration in colostrum and increased in concentration over the first month of lactation. Cytidine and niacinamide were observed near the detection limit in our previous study but were not detected here.
In total, 15 sugars (including monosaccharides, disaccharides, and oligosaccharides), 23 amino acids and derivatives, 11 energy-related metabolites, 10 FA-associated metabolites, 3 nucleotides and derivatives, 2 vitamins, and 5 metabolites related to microbial metabolism were measured (Tables 1–5). In terms of HMOs, the neutral oligosaccharides—2′-FL, 3-fucosyllactose (3-FL), 3′-GSL, lactodifucotetraose, lacto-N-fucopentaose (LNFP) I, LNFP II, LNFP III, lacto-N-neotetraose (LNnT), and lacto-N-tetraose—and the charged oligosaccharides—3′-sialyllactose (3′-SL) and 6′-sialyllactose (6′-SL)—were identified and quantified in all milks. Secretor status of the mothers was determined by the ability to detect 2′-FL (secretor) or not (nonsecretor) in milk by using NMR spectroscopy (21, 26). In total, 10 of 15 mothers giving birth to term infants and 10 of 13 mothers giving birth to preterm infants were classified as secretors, which is consistent with previous estimates of predominantly Caucasian populations (27).
TABLE 1.
Mean term and preterm human milk carbohydrate concentrations measured over the first month of lactation by 1H NMR spectroscopy1
| Day 0–5 |
Day 14 |
Day 28 |
|||||
| Sugars and infant maturity at birth | P2 | Concentration, mmol/L | Biological CV, % | Concentration, mmol/L | Biological CV, % | Concentration, mmol/L | Biological CV, % |
| 2′-FL | |||||||
| T | <0.01 | 5.43 ± 4.55 | 84 | 4.22 ± 2.90 | 69 | 3.59 ± 2.83 | 79 |
| PT | 0.10 | 4.96 ± 4.70 | 95 | 3.40 ± 3.02 | 89 | 2.32 ± 2.54 | 109 |
| 3-FL | |||||||
| T | <0.0001 | 0.91 ± 1.05 | 115 | 1.19 ± 1.16 | 98 | 1.57 ± 1.34 | 85 |
| PT | 0.15 | 0.99 ± 0.77 | 78 | 1.21 ± 0.68 | 56 | 1.98 ± 1.39 | 70 |
| 3′-GSL | |||||||
| T | 0.02 | 0.83 ± 0.26 | 31 | 0.67 ± 0.30 | 45 | 0.51 ± 0.15 | 30 |
| PT | 0.37 | 1.00 ± 0.29 | 29 | 0.93 ± 0.33 | 36 | 0.82 ± 0.40 | 49 |
| 3′-SL | |||||||
| T | <0.0001 | 0.36 ± 0.10 | 28 | 0.26 ± 0.06 | 24 | 0.23 ± 0.05 | 20 |
| PT | 0.10 | 0.36 ± 0.11 | 30 | 0.29 ± 0.05 | 19 | 0.28 ± 0.08 | 30 |
| 6′-SL | |||||||
| T | <0.001 | 0.82 ± 0.24 | 29 | 0.88 ± 0.22 | 24 | 0.58 ± 0.17 | 29 |
| PT | 0.61 | 0.86 ± 0.46 | 54 | 1.14 ± 0.44 | 38 | 1.04 ± 0.66 | 63 |
| Fucose | |||||||
| T | <0.001 | 0.23 ± 0.18 | 78 | 0.14 ± 0.12 | 81 | 0.12 ± 0.10 | 86 |
| PT | 0.10 | 0.30 ± 0.27 | 89 | 0.15 ± 0.13 | 84 | 0.11 ± 0.08 | 71 |
| Galactose | |||||||
| T | 0.24 | 0.01 ± 0.04 | 49 | 0.07 ± 0.04 | 56 | 0.07 ± 0.04 | 57 |
| PT | 0.85 | 0.06 ± 0.04 | 65 | 0.06 ± 0.02 | 40 | 0.07 ± 0.05 | 70 |
| Glucose | |||||||
| T | <0.01 | 0.91 ± 0.37 | 41 | 1.10 ± 0.50 | 45 | 1.54 ± 0.54 | 35 |
| PT | 0.15 | 0.60 ± 0.52 | 87 | 0.90 ± 0.38 | 42 | 1.03 ± 0.67 | 65 |
| LDFT | |||||||
| T | 0.39 | 0.25 ± 0.24 | 95 | 0.28 ± 0.29 | 105 | 0.22 ± 0.26 | 118 |
| PT | 0.98 | 0.52 ± 0.60 | 115 | 0.32 ± 0.33 | 104 | 0.31 ± 0.38 | 120 |
| LNFP I | |||||||
| T | 0.08 | 1.65 ± 1.35 | 82 | 1.01 ± 0.86 | 85 | 0.64 ± 0.60 | 93 |
| PT | 0.60 | 1.08 ± 1.03 | 96 | 0.80 ± 1.00 | 126 | 0.30 ± 0.39 | 134 |
| LNFP II | |||||||
| T | 0.41 | 0.47 ± 0.54 | 115 | 0.42 ± 0.45 | 106 | 0.43 ± 0.41 | 95 |
| PT | 0.02 | 0.40 ± 0.34 | 84 | 0.52 ± 0.30 | 57 | 0.79 ± 0.54 | 69 |
| LNFP III | |||||||
| T | <0.0001 | 0.42 ± 0.22 | 53 | 0.29 ± 0.13 | 45 | 0.26 ± 0.09 | 34 |
| PT | 0.86 | 0.28 ± 0.12 | 41 | 0.24 ± 0.05 | 20 | 0.27 ± 0.10 | 38 |
| LNnT | |||||||
| T | <0.0001 | 0.36 ± 0.16 | 45 | 0.21 ± 0.10 | 47 | 0.16 ± 0.10 | 61 |
| PT | 0.83 | 0.23 ± 0.16 | 68 | 0.17 ± 0.09 | 52 | 0.20 ± 0.08 | 41 |
| LNT | |||||||
| T | 0.80 | 1.49 ± 1.39 | 93 | 1.23 ± 0.88 | 71 | 1.06 ± 0.68 | 64 |
| PT | 0.17 | 0.96 ± 1.03 | 107 | 1.37 ± 0.94 | 68 | 1.81 ± 1.46 | 80 |
| Lactose | |||||||
| T | <0.001 | 160 ± 12.8 | 8 | 170 ± 9.95 | 6 | 180 ± 14.6 | 8 |
| PT | 0.04 | 150 ± 16.5 | 11 | 170 ± 6.69 | 4 | 170 ± 12.6 | 8 |
| Total HMOs measured | |||||||
| T | <0.0001 | 13.0 ± 3.91 | 30 | 10.7 ± 2.14 | 20 | 9.25 ± 1.91 | 21 |
| PT | 0.90 | 11.6 ± 4.81 | 41 | 10.4 ± 2.96 | 29 | 10.1 ± 1.71 | 17 |
| Total neutral HMOs measured | |||||||
| T | <0.0001 | 11.8 ± 4.00 | 34 | 9.53 ± 2.22 | 23 | 8.44 ± 1.95 | 23 |
| PT | 0.90 | 10.4 ± 4.84 | 46 | 8.95 ± 2.83 | 32 | 8.79 ± 1.38 | 16 |
| Total charged HMOs measured | |||||||
| T | <0.0001 | 1.18 ± 0.23 | 20 | 1.14 ± 0.23 | 20 | 0.81 ± 0.19 | 24 |
| PT | 0.86 | 1.22 ± 0.48 | 39 | 1.43 ± 0.46 | 32 | 1.33 ± 0.71 | 53 |
Values are means ± SDs unless otherwise indicated; day 0–5, n = 15 term and n = 10 preterm; day 14, n = 14 term and n = 10 preterm; and day 28, n = 15 term and n = 6 preterm. Mean concentrations include both secretors and nonsecretors. The biological percentage CV (%CV) was calculated as SD/mean × 100. HMO, human milk oligosaccharide; LDFT, lactodifucotetraose; LNFP, lacto-N-fucopentaose; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetraose; PT, preterm; T, term; 2′-FL, 2′-fucosyllactose; 3-FL, 3-fucosyllactose; 3′-GSL, 3′-galactosyllactose; 3′-SL, 3′-sialyllactose; 6′-SL, 6′-sialyllactose.
P values are based on linear mixed-effects or linear models, which tested whether term or preterm milk metabolite concentrations significantly changed over the first month of lactation.
TABLE 5.
Mean term and preterm human milk nucleotide and derivatives, vitamin, and bacteria-associated metabolite concentrations measured over the first month of lactation by 1H NMR spectroscopy1
| Day 0–5 |
Day 14 |
Day 28 |
|||||
| Other metabolites and infant maturity at birth | P2 | Concentration, μmol/L | Biological CV, % | Concentration, μmol/L | Biological CV, % | Concentration, μmol/L | Biological CV, % |
| Nucleotides and derivatives | |||||||
| AMP | |||||||
| T | 0.01 | 2.1 ± 2.9 | 136 | 4.4 ± 4.1 | 93 | 5.4 ± 3.9 | 72 |
| PT | 0.30 | ADL | 250 | 1.6 ± 1.5* | 96 | 2.8 ± 3.6 | 126 |
| Hypoxanthine | |||||||
| T | 0.48 | 3.5 ± 1.5 | 42 | 2.7 ± 1.3 | 47 | 2.9 ± 0.9 | 30 |
| PT | 0.29 | 2.2 ± 0.7* | 33 | 3.0 ± 1.4 | 48 | 3.1 ± 1.4 | 45 |
| Uridine | |||||||
| T | 0.07 | 6.4 ± 6.3 | 98 | 3.3 ± 1.7 | 51 | 3.6 ± 1.4 | 40 |
| PT | 0.85 | 8.0 ± 7.8 | 98 | 5.7 ± 4.6 | 81 | 7.1 ± 4.2 | 60 |
| Vitamins | |||||||
| Ascorbate | |||||||
| T | 0.92 | 191 ± 99.5 | 52 | 162 ± 60.8 | 38 | 180 ± 59.7 | 33 |
| PT | 0.95 | 174 ± 116 | 67 | 196 ± 117 | 60 | 139 ± 65.4 | 47 |
| Pantothenate | |||||||
| T | 0.12 | 12.2 ± 7.8 | 64 | 16.4 ± 9.4 | 58 | 15.3 ± 5.2 | 34 |
| PT | 0.20 | 7.5 ± 4.1 | 54 | 12.3 ± 7.0 | 57 | 13.7 ± 8.7 | 63 |
| Bacteria-associated metabolites | |||||||
| Acetoin | |||||||
| T | 0.62 | ADL | 82 | ADL | 103 | 0.6 ± 0.5 | 85 |
| PT | 0.04 | ADL | 60 | ADL | 134 | 2.8 ± 3.5 | 126 |
| Dimethyl sulfone | |||||||
| T | <0.0001 | 4.7 ± 2.1 | 44 | 6.9 ± 3.2 | 46 | 10.1 ± 6.2 | 62 |
| PT | 0.95 | 3.8 ± 1.7 | 44 | 3.6 ± 1.7** | 48 | 3.9 ± 1.6** | 41 |
| Formate | |||||||
| T | 0.11 | 17.1 ± 11.5 | 67 | 11.9 ± 4.4 | 37 | 11.5 ± 6.0 | 52 |
| PT | 0.82 | 12.0 ± 5.3 | 45 | 9.1 ± 4.0 | 45 | 11.4 ± 6.5 | 57 |
| Hippurate | |||||||
| T | <0.01 | 32.0 ± 37.8 | 118 | 12.3 ± 10.7 | 88 | 9.7 ± 7.5 | 78 |
| PT | 0.20 | 21.0 ± 28.1 | 134 | 8.8 ± 6.2 | 70 | 10.8 ± 14.3 | 132 |
| Methanol | |||||||
| T | 0.05 | 23.7 ± 12.7 | 53 | 12.9 ± 4.9 | 38 | 14.7 ± 8.1 | 55 |
| PT | 0.20 | 12.7 ± 9.3* | 73 | 12.5 ± 7.4 | 59 | 10.8 ± 6.8 | 63 |
Values are means ± SDs unless otherwise indicated; day 0–5, n = 15 term and n = 10 preterm; day 14, n = 14 term and n = 10 preterm; and day 28, n = 15 term and n = 6 preterm. Mean concentrations include both secretors and nonsecretors. *,**Significant differences between mean concentrations of term and preterm milk as determined by two-tailed t test: *P < 0.05, **P < 0.001. The biological percentage CV (%CV) was calculated as SD/mean × 100. ADL, at the detection limit; PT, preterm; T, term.
P values are based on linear mixed-effects or linear models, which tested whether term or preterm milk metabolite concentrations significantly changed over the first month of lactation.
Term and preterm milk metabolites change over the course of lactation.
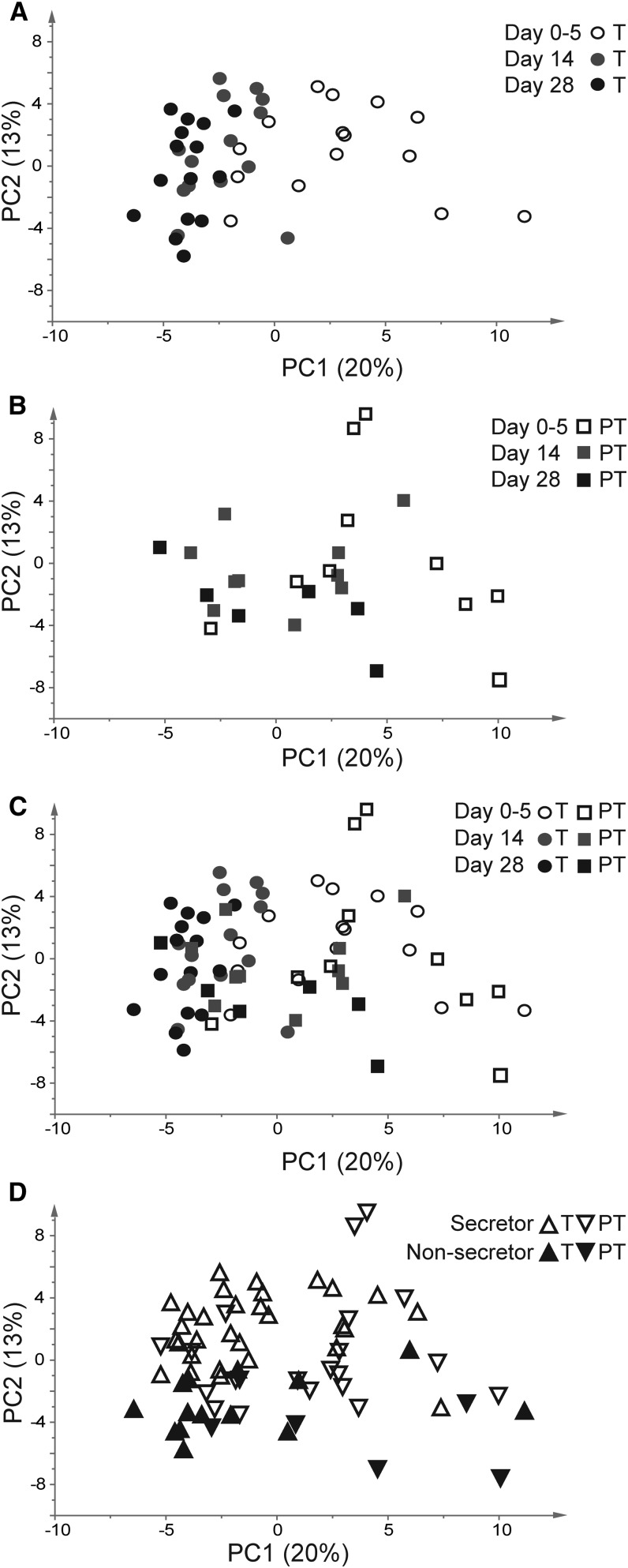
To examine correlations in milk metabolites between term and preterm milk, PCA was performed (Figure 1). A clear progression along principal component 1 (which explains 20% of the variance) was observed from the right-hand side of the plot to the left-hand side of the plot and could be explained by the day of collection. Samples of colostrum (collected between 0 and 5 d postpartum) were clustered primarily on the right side of the plot, whereas samples collected at 28 d postpartum were clustered on the left side of the plot (Figure 1A). The larger distribution of the first time point likely reflects the fact that this sample was not collected on a specific day, whereas the other samples were taken precisely on postpartum days 14 and 28. Preterm milk followed the same trend as term milk but with more variation (Figure 1B). Although PCA revealed no significant difference in clustering between milk from mothers giving birth to term and mothers giving birth to preterm infants, preterm milk samples clustered less at all time points (Figure 1C). The second principal component, principal component 2 (which explains 13% of the variance), revealed differences in secretor status, with secretors in the lower half of the plot and nonsecretors in the upper half of the plot (Figure 1D).
FIGURE 1.
PCA of term and preterm milks during the first month of lactation. (A) Term milk only. (B) Preterm milk only. (C) Original PCA of all milk samples. (D) PCA colored on the basis of secretor status. PC, principal component; PCA, principal components analysis; PT, preterm; T, term.
Changes in milk carbohydrate concentrations over time.
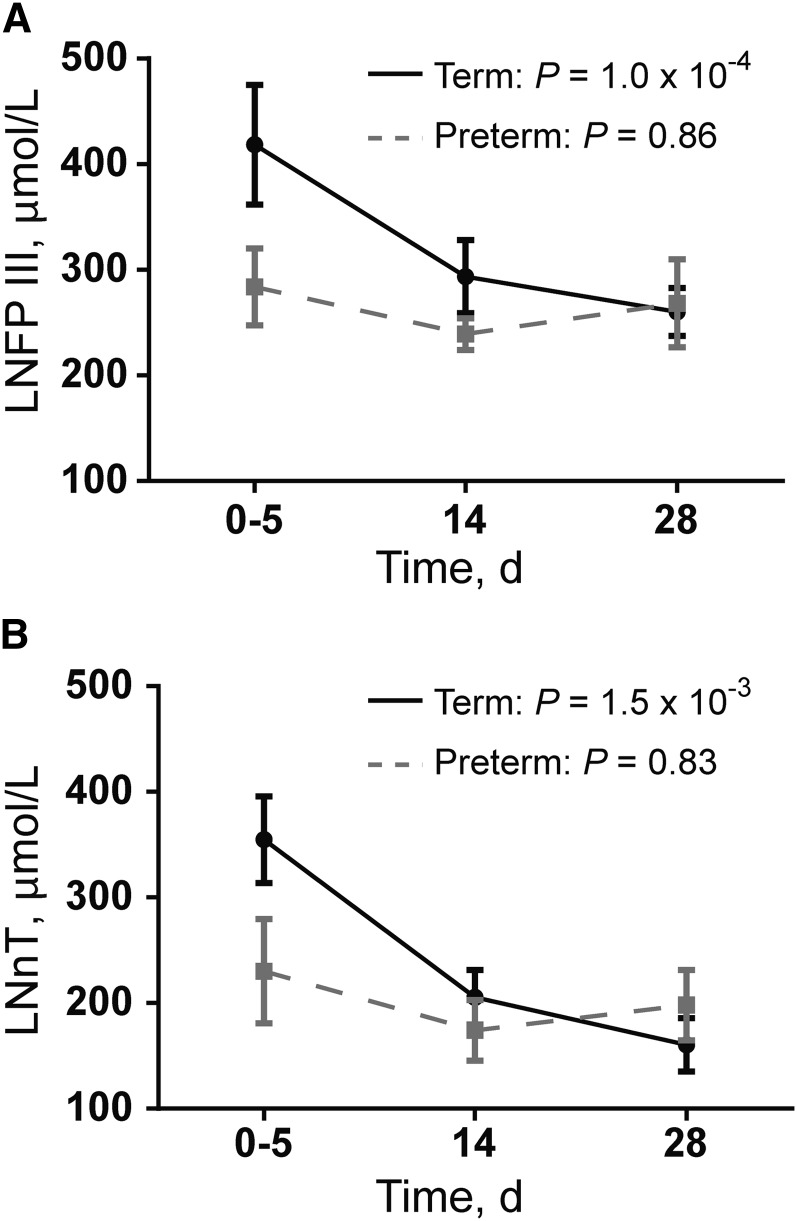
In term milk, clear trends in carbohydrate concentration over time were observed, with some sugars increasing (lactose, 3-FL, and glucose) and others decreasing (2′-FL, 3′-GSL, 3′-SL, 6′-SL, LNFP III, fucose, as well as total (n = 11), neutral (n = 9), and charged (n = 2) oligosaccharides) in concentration over time (Table 1). In preterm milk, most sugars did not significantly change over time (Table 1), except for lactose, which increased. For example, LNFP III significantly decreased over time in term milk (LMM, P = 1.0 × 10−4) but did not significantly change in preterm milk (LM, P = 0.86) (Figure 2A). LNnT in milk was significantly affected by infant maturity at birth (LMM, P = 3.5 × 10−2), postpartum day of lactation (LMM, P = 1.5 × 10−3), and secretor status (LMM, P = 2.5 × 10−5); however, none of the LMM interaction terms were significant (Figure 2B).
FIGURE 2.
Human milk oligosaccharides that significantly differ in concentration between term and preterm milk. Values are means ± SEMs. (A) LNFP III. P values represent the significance of postpartum day of lactation on LNFP III concentration by using an LMM (term) or an LM (preterm). (B) LNnT. P values represent the significance of postpartum day of lactation on LNnT concentration by using an LMM (term) or an LM (preterm). For term milk, the LNnT concentration was also influenced by maturity of the infant at birth (LMM, P = 3.5 × 10−2) and secretor status (LMM, P = 2.5 × 10−5), but none of the interaction terms were significant. For both LNFP III and LNnT: milk collected on postpartum day 0–5, n = 15 term and n = 10 preterm; milk collected on postpartum day 14, n = 14 term and n = 10 preterm; and milk collected on postpartum day 28, n = 15 term and n = 6 preterm. LM, linear model; LMM, linear mixed-effects model; LNFP, lacto-N-fucopentaose; LNnT, lacto-N-neotetraose.
The composition of preterm milk has been reported to be more variable than term milk (10). To assess whether milk carbohydrate concentrations were consistent with this trend, we calculated the %CV for all of the sugars including the total (sum of the 11 measured oligosaccharides), neutral (sum of the 9 neutral oligosaccharides), and charged (sum of the 2 neutral oligosaccharides) oligosaccharides (Table 1). All of the individual sugars in this study were highly variable in concentration in term and preterm milk, with the exception of lactose, which had a %CV between 4% and 11% in both at each time point. For term and preterm milk, the %CV of the combined total oligosaccharide concentration decreased as lactation progressed (colostrum: 30% in term, 41% in preterm; transition milk: 20% in term, 29% in preterm; mature milk: 21% in term, 17% in preterm). The combined neutral oligosaccharide concentrations, which constituted 9 of the 11 total measured oligosaccharides, followed the same trend. The charged oligosaccharides (represented by 3′-SL and 6′-SL) varied considerably in preterm milk at all time points (colostrum: 20% in term, 39% in preterm; transition milk: 20% in term, 32% in preterm; mature milk: 24% in term, 53% in preterm). Indeed, overall, the %CV of milk carbohydrates was higher in preterm milk.
Changes in milk amino acid and derivative concentrations over time.
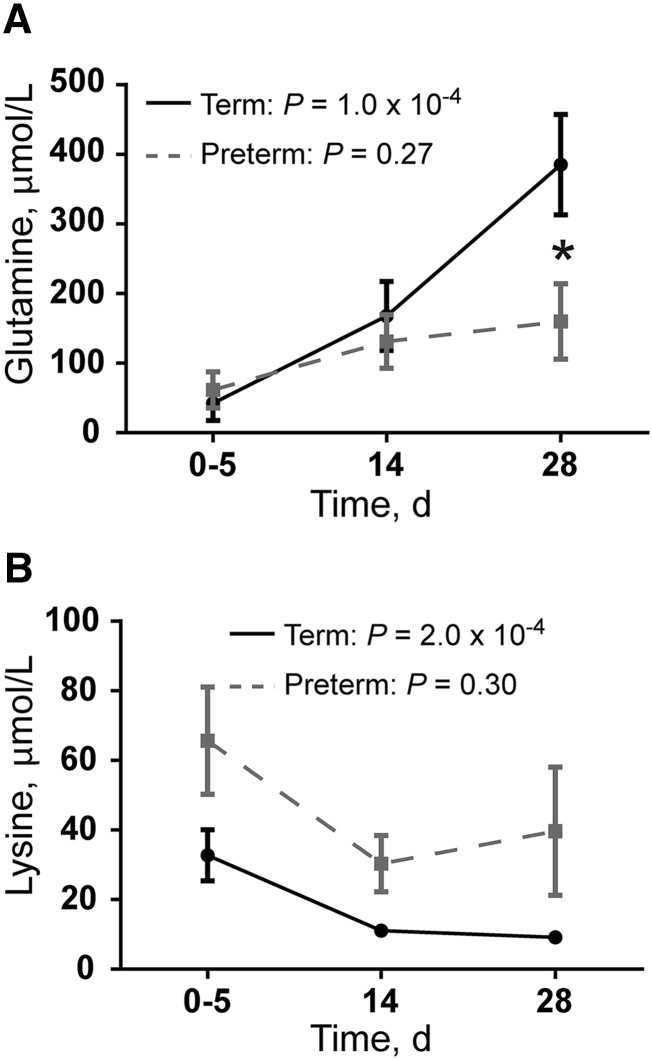
Many of the amino acids and their derivatives changed in concentration during the first month of lactation (Table 2). In term milk, 2-aminobutyrate, alanine, carnitine, glutamate, glutamine, histidine, urea, and valine increased over time. In contrast, most preterm milk amino acids and derivatives did not significantly increase over time (Table 2), except for alanine. Glutamine concentrations in colostrum were similar between term and preterm samples, but by 4 wk the concentration in term milk was significantly higher than in preterm milk (P = 2.2 × 10−2) (Figure 3A). Acetylcarnitine, betaine, isoleucine, lysine, and taurine all decreased in term milk, and in all cases these metabolites did not significantly change in preterm milk (Table 2). Lysine was the only amino acid that was significantly affected by infant maturity at birth (LM: infant maturity P = 6.7 × 10−3, postpartum day of lactation P = 1.0 × 10−4) (Figure 3B). However, the interaction between postpartum day of lactation and infant maturity at birth was not significant (LM, P = 0.97).
TABLE 2.
Mean term and preterm human milk amino acid and derivative concentrations measured over the first month of lactation by 1H NMR spectroscopy1
| Day 0–5 |
Day 14 |
Day 28 |
|||||
| Amino acids and derivatives and infant maturity at birth | P2 | Concentration, mmol/L | Biological CV, % | Concentration, mmol/L | Biological CV, % | Concentration, mmol/L | Biological CV, % |
| 2-Aminobutyrate | |||||||
| T | <0.0001 | 0.005 ± 0.004 | 84 | 0.009 ± 0.005 | 62 | 0.011 ± 0.005 | 47 |
| PT | 0.22 | 0.005 ± 0.004 | 80 | 0.007 ± 0.004 | 55 | 0.008 ± 0.004 | 53 |
| 2-Hydroxybutyrate | |||||||
| T | 0.14 | 0.006 ± 0.003 | 45 | 0.004 ± 0.001 | 33 | 0.004 ± 0.001 | 35 |
| PT | 0.88 | 0.006 ± 0.004 | 57 | 0.007 ± 0.004* | 54 | 0.006 ± 0.002 | 34 |
| Acetylcarnitine | |||||||
| T | <0.0001 | 0.033 ± 0.010 | 30 | 0.022 ± 0.009 | 40 | 0.015 ± 0.005 | 32 |
| PT | 0.37 | 0.041 ± 0.017 | 41 | 0.024 ± 0.010 | 41 | 0.035 ± 0.022 | 62 |
| Alanine | |||||||
| T | 0.01 | 0.135 ± 0.056 | 41 | 0.171 ± 0.081 | 47 | 0.213 ± 0.074 | 35 |
| PT | 0.02 | 0.137 ± 0.123 | 90 | 0.206 ± 0.092 | 45 | 0.271 ± 0.109 | 40 |
| Asparagine | |||||||
| T | 0.42 | 0.012 ± 0.007 | 54 | 0.012 ± 0.007 | 59 | 0.014 ± 0.007 | 49 |
| PT | 0.92 | 0.017 ± 0.015 | 85 | 0.011 ± 0.005 | 48 | 0.016 ± 0.008 | 48 |
| Aspartate | |||||||
| T | 0.10 | 0.049 ± 0.014 | 27 | 0.032 ± 0.016 | 51 | 0.040 ± 0.028 | 71 |
| PT | 0.10 | 0.044 ± 0.026 | 57 | 0.049 ± 0.018* | 37 | 0.078 ± 0.038 | 49 |
| Betaine | |||||||
| T | <0.0001 | 0.051 ± 0.011 | 22 | 0.030 ± 0.006 | 21 | 0.031 ± 0.006 | 18 |
| PT | 0.27 | 0.050 ± 0.024 | 47 | 0.037 ± 0.010 | 28 | 0.033 ± 0.005 | 15 |
| Carnitine | |||||||
| T | <0.001 | 0.011 ± 0.006 | 54 | 0.016 ± 0.010 | 61 | 0.022 ± 0.009 | 43 |
| PT | 0.85 | 0.017 ± 0.012 | 68 | 0.019 ± 0.011 | 60 | 0.017 ± 0.009 | 50 |
| Glutamate | |||||||
| T | <0.001 | 0.747 ± 0.288 | 39 | 0.929 ± 0.346 | 37 | 1.30 ± 0.421 | 32 |
| PT | 0.22 | 0.878 ± 0.674 | 77 | 1.16 ± 0.364 | 31 | 1.34 ± 0.345 | 26 |
| Glutamine | |||||||
| T | <0.0001 | 0.042 ± 0.095 | 224 | 0.168 ± 0.186 | 111 | 0.385 ± 0.279 | 72 |
| PT | 0.27 | 0.061 ± 0.083 | 134 | 0.131 ± 0.121 | 92 | 0.160 ± 0.133* | 83 |
| Histidine | |||||||
| T | 0.03 | 0.018 ± 0.006 | 33 | 0.021 ± 0.008 | 40 | 0.025 ± 0.008 | 33 |
| PT | 0.16 | 0.020 ± 0.013 | 67 | 0.021 ± 0.008 | 36 | 0.028 ± 0.005 | 19 |
| Isoleucine | |||||||
| T | 0.03 | 0.012 ± 0.012 | 104 | 0.008 ± 0.005 | 65 | 0.006 ± 0.004 | 61 |
| PT | 0.72 | 0.020 ± 0.019 | 96 | 0.011 ± 0.005 | 48 | 0.016 ± 0.010 | 59 |
| Leucine | |||||||
| T | 0.56 | 0.031 ± 0.041 | 131 | 0.020 ± 0.011 | 53 | 0.021 ± 0.009 | 42 |
| PT | 0.93 | 0.052 ± 0.060 | 115 | 0.023 ± 0.009 | 38 | 0.035 ± 0.016 | 45 |
| Lysine | |||||||
| T | <0.001 | 0.033 ± 0.028 | 87 | 0.011 ± 0.005 | 48 | 0.009 ± 0.002 | 25 |
| PT | 0.30 | 0.066 ± 0.049 | 74 | 0.030 ± 0.026* | 85 | 0.040 ± 0.045 | 114 |
| Methionine | |||||||
| T | 0.12 | 0.009 ± 0.006 | 66 | 0.007 ± 0.003 | 40 | 0.006 ± 0.002 | 29 |
| PT | 0.76 | 0.014 ± 0.011 | 81 | 0.007 ± 0.004 | 52 | 0.010 ± 0.005 | 44 |
| Phenylalanine | |||||||
| T | 0.29 | 0.010 ± 0.005 | 49 | 0.010 ± 0.004 | 37 | 0.012 ± 0.005 | 42 |
| PT | 0.23 | 0.013 ± 0.010 | 75 | 0.011 ± 0.003 | 27 | 0.017 ± 0.005 | 32 |
| Proline | |||||||
| T | 0.24 | 0.062 ± 0.078 | 125 | 0.026 ± 0.016 | 62 | 0.025 ± 0.009 | 35 |
| PT | 0.45 | 0.069 ± 0.073 | 106 | 0.026 ± 0.011 | 41 | 0.035 ± 0.025 | 73 |
| Taurine | |||||||
| T | <0.001 | 0.348 ± 0.155 | 45 | 0.259 ± 0.064 | 25 | 0.188 ± 0.062 | 33 |
| PT | 0.42 | 0.323 ± 0.103 | 32 | 0.286 ± 0.073 | 25 | 0.261 ± 0.109 | 42 |
| Threonine | |||||||
| T | 0.27 | 0.065 ± 0.024 | 37 | 0.065 ± 0.036 | 55 | 0.083 ± 0.042 | 51 |
| PT | 0.61 | 0.081 ± 0.050 | 61 | 0.083 ± 0.039 | 47 | 0.093 ± 0.036 | 39 |
| Tryptophan | |||||||
| T | 0.88 | 0.003 ± 0.006 | 168 | 0.002 ± 0.002 | 86 | 0.003 ± 0.002 | 67 |
| PT | 0.27 | 0.010 ± 0.014 | 132 | 0.002 ± 0.002 | 100 | 0.003 ± 0.004 | 127 |
| Tyrosine | |||||||
| T | 0.34 | 0.016 ± 0.012 | 78 | 0.012 ± 0.006 | 48 | 0.012 ± 0.005 | 38 |
| PT | 0.98 | 0.029 ± 0.022 | 75 | 0.019 ± 0.011 | 56 | 0.028 ± 0.018 | 63 |
| Urea | |||||||
| T | 0.02 | 2.73 ± 0.965 | 35 | 3.46 ± 0.987 | 29 | 3.61 ± 0.912 | 25 |
| PT | 0.37 | 2.90 ± 0.838 | 29 | 3.56 ± 1.33 | 37 | 3.72 ± 1.25 | 34 |
| Valine | |||||||
| T | 0.01 | 0.032 ± 0.022 | 67 | 0.032 ± 0.010 | 31 | 0.043 ± 0.013 | 31 |
| PT | 0.85 | 0.062 ± 0.048 | 77 | 0.043 ± 0.018 | 41 | 0.060 ± 0.024 | 40 |
Values are means ± SDs unless otherwise indicated; day 0–5, n = 15 term and n = 10 preterm; day 14, n = 14 term and n = 10 preterm; and day 28, n = 15 term and n = 6 preterm. Mean concentrations include both secretors and nonsecretors. *Significant difference between mean concentrations of term and preterm milk as determined by two-tailed t test, P < 0.05. The biological percentage CV (%CV) was calculated as SD/mean × 100. PT, preterm; T, term.
P values are based on linear mixed-effects or linear models, which tested whether term or preterm milk metabolite concentrations significantly changed over the first month of lactation.
FIGURE 3.
Human milk amino acids that differ significantly in concentration between term and preterm milk. Values are means ± SEMs. (A) Glutamine. P values represent the significance of postpartum day of lactation on glutamine concentration by using an LMM (term) or an LM (preterm). (B) Lysine. P values represent the significance of postpartum day of lactation on lysine concentration by using an LMM (term) or an LM (preterm). Lysine concentration is also influenced by the maturity of the infant (P = 6.7 × 10−3) *Significant difference in mean concentration between term and preterm milk at a specific time point, P < 0.05. For both glutamine and lysine: milk collected on postpartum day 0–5, n = 15 term and n = 10 preterm; milk collected on postpartum day 14, n = 14 term and n = 10 preterm; and milk collected on postpartum day 28, n = 15 term and n = 6 preterm. LM, linear model; LMM, linear mixed-effects model.
All of the amino acids and derivatives exhibited high biological variability with the exception of betaine, where the %CV remained at <25% at all time points in term milk. In preterm milk, this metabolite had high biological variability in colostrum (47%) but by 28 d postpartum became less variable (28%CV in transition milk and 15%CV in mature milk).
Changes in other milk metabolite concentrations over time.
FAs and associated metabolites changed in concentration over time in term and preterm milk, with more significant changes in term milk and high degrees of biological variability overall (Table 3). Energy-related molecules were stable over time and similar between term and preterm milk except for 2-oxoglutarate (P = 3.8 × 10−2), acetone (P = 1.3 × 10−2), lactate (P = 8.6 × 10−3), and pyruvate (P = 3.4 × 10−2), which were higher in preterm milk at day 28, and creatinine, which was higher in term milk at day 14 (P = 6.0 × 10−3) and day 28 (P = 1.4 × 10−2) (Table 4). Human milk nucleotides were similar between term and preterm milk, except for hypoxanthine, which was higher in term milk at day 0–5 (P = 1.1 × 10−2) and AMP, which was higher in term milk at day 14 (P = 3.0 × 10−2). Finally, the microbial associated metabolite concentrations were similar in the milks, with the exception of dimethyl sulfone, which was significantly lower in preterm milk at day 14 (P = 6.4 × 10−3) and day 28 (P = 2.3 × 10−3) (Table 5). Methanol was also significantly lower in preterm milk at day 0–5 (P = 2.7 × 10−2).
TABLE 3.
Mean term and preterm human milk FA-associated metabolite concentrations measured over the first month of lactation by 1H NMR spectroscopy1
| Day 0–5 |
Day 14 |
Day 28 |
|||||
| FA-associated metabolites and infant maturity at birth | P2 | Concentration, mmol/L | Biological CV, % | Concentration, mmol/L | Biological CV, % | Concentration, mmol/L | Biological CV, % |
| Acetate | |||||||
| T | 0.12 | 0.019 ± 0.010 | 49 | 0.021 ± 0.014 | 69 | 0.015 ± 0.010 | 67 |
| PT | 0.24 | 0.016 ± 0.004 | 23 | 0.015 ± 0.009 | 65 | 0.035 ± 0.022 | 62 |
| Azelate | |||||||
| T | <0.0001 | 0.018 ± 0.017 | 97 | 0.065 ± 0.052 | 80 | 0.071 ± 0.064 | 90 |
| PT | 0.04 | 0.008 ± 0.010 | 125 | 0.033 ± 0.043 | 132 | 0.059 ± 0.044 | 74 |
| Butyrate | |||||||
| T | <0.0001 | 0.010 ± 0.011 | 110 | 0.022 ± 0.018 | 83 | 0.046 ± 0.039 | 84 |
| PT | 0.04 | 0.006 ± 0.006 | 97 | 0.016 ± 0.013 | 85 | 0.056 ± 0.059 | 105 |
| Caprate | |||||||
| T | <0.001 | 0.007 ± 0.004 | 60 | 0.019 ± 0.010 | 50 | 0.026 ± 0.021 | 81 |
| PT | 0.18 | 0.008 ± 0.007 | 83 | 0.025 ± 0.025 | 100 | 0.033 ± 0.026 | 79 |
| Caprylate | |||||||
| T | <0.0001 | 0.023 ± 0.017 | 74 | 0.057 ± 0.032 | 56 | 0.100 ± 0.081 | 82 |
| PT | 0.04 | 0.024 ± 0.036 | 151 | 0.077 ± 0.084 | 110 | 0.212 ± 0.203 | 96 |
| Choline | |||||||
| T | <0.01 | 0.106 ± 0.061 | 57 | 0.079 ± 0.043 | 55 | 0.070 ± 0.028 | 41 |
| PT | 0.93 | 0.141 ± 0.084 | 60 | 0.105 ± 0.067 | 64 | 0.138 ± 0.103 | 75 |
| Ethanolamine | |||||||
| T | <0.01 | 0.035 ± 0.013 | 38 | 0.070 ± 0.017 | 24 | 0.057 ± 0.016 | 27 |
| PT | 0.27 | 0.047 ± 0.017 | 37 | 0.058 ± 0.025 | 43 | 0.071 ± 0.024 | 34 |
| Glycero-3-phosphocholine | |||||||
| T | <0.001 | 0.210 ± 0.193 | 92 | 0.587 ± 0.158 | 27 | 0.512 ± 0.164 | 32 |
| PT | 0.04 | 0.149 ± 0.137 | 92 | 0.522 ± 0.332 | 63 | 0.506 ± 0.217 | 43 |
| myo-Inositol | |||||||
| T | <0.0001 | 1.59 ± 0.413 | 26 | 0.916 ± 0.267 | 29 | 0.972 ± 0.227 | 23 |
| PT | 0.24 | 1.42 ± 0.789 | 56 | 1.01 ± 0.319 | 32 | 0.800 ± 0.195 | 24 |
| Phosphocholine | |||||||
| T | 0.14 | 0.626 ± 0.337 | 54 | 0.697 ± 0.128 | 18 | 0.714 ± 0.129 | 18 |
| PT | 0.22 | 0.432 ± 0.203 | 47 | 0.705 ± 0.188 | 27 | 0.682 ± 0.371 | 54 |
Values are means ± SDs unless otherwise indicated; day 0–5, n = 15 term and n = 10 preterm; day 14, n = 14 term and n = 10 preterm; and day 28, n = 15 term and n = 6 preterm. Mean concentrations include both secretors and nonsecretors. The biological percentage CV (%CV) was calculated as SD/mean × 100. PT, preterm; T, term.
P values are based on linear mixed-effects or linear models, which tested whether term or preterm milk metabolite concentrations significantly changed over the first month of lactation.
TABLE 4.
Mean term and preterm human milk energy metabolite concentrations measured over the first month of lactation by 1H NMR spectroscopy1
| Day 0–5 |
Day 14 |
Day 28 |
|||||
| Energy metabolites and infant maturity at birth | P2 | Concentration, mmol/L | Biological CV, % | Concentration, mmol/L | Biological CV, % | Concentration, mmol/L | Biological CV, % |
| 2-Oxoglutarate | |||||||
| T | <0.001 | 0.042 ± 0.014 | 35 | 0.035 ± 0.012 | 33 | 0.028 ± 0.008 | 30 |
| PT | 0.44 | 0.046 ± 0.013 | 29 | 0.049 ± 0.022 | 45 | 0.067 ± 0.035* | 52 |
| Acetone | |||||||
| T | 0.03 | 0.005 ± 0.003 | 61 | 0.006 ± 0.003 | 49 | 0.007 ± 0.004 | 63 |
| PT | 0.92 | 0.004 ± 0.002 | 44 | 0.004 ± 0.002 | 40 | 0.004 ± 0.001* | 14 |
| cis-Aconitate | |||||||
| T | 0.09 | 0.003 ± 0.001 | 48 | 0.003 ± 0.001 | 39 | 0.003 ± 0.002 | 50 |
| PT | 0.85 | 0.005 ± 0.004 | 87 | 0.003 ± 0.001 | 39 | 0.003 ± 0.001 | 27 |
| Citrate | |||||||
| T | 0.90 | 3.74 ± 1.57 | 42 | 3.58 ± 0.985 | 28 | 3.21 ± 0.825 | 26 |
| PT | 0.96 | 3.07 ± 1.17 | 38 | 3.42 ± 0.993 | 29 | 2.86 ± 0.463 | 16 |
| Creatine | |||||||
| T | <0.001 | 0.079 ± 0.032 | 41 | 0.056 ± 0.011 | 19 | 0.048 ± 0.015 | 31 |
| PT | 0.06 | 0.095 ± 0.038 | 40 | 0.063 ± 0.025 | 39 | 0.062 ± 0.034 | 55 |
| Creatine phosphate | |||||||
| T | 0.02 | 0.029 ± 0.018 | 61 | 0.036 ± 0.012 | 33 | 0.043 ± 0.014 | 33 |
| PT | 0.36 | 0.021 ± 0.012 | 56 | 0.029 ± 0.008 | 29 | 0.029 ± 0.014 | 50 |
| Creatinine | |||||||
| T | 0.14 | 0.041 ± 0.009 | 22 | 0.047 ± 0.006 | 13 | 0.045 ± 0.007 | 14 |
| PT | 0.85 | 0.035 ± 0.009 | 25 | 0.037 ± 0.009* | 24 | 0.036 ± 0.008* | 22 |
| Fumarate | |||||||
| T | <0.0001 | 0.006 ± 0.003 | 43 | 0.003 ± 0.002 | 62 | 0.003 ± 0.002 | 69 |
| PT | 0.83 | 0.005 ± 0.003 | 55 | 0.006 ± 0.004 | 72 | 0.006 ± 0.004 | 77 |
| Lactate | |||||||
| T | <0.001 | 0.280 ± 0.237 | 84 | 0.104 ± 0.072 | 69 | 0.071 ± 0.038 | 53 |
| PT | 0.85 | 0.271 ± 0.193 | 71 | 0.156 ± 0.123 | 79 | 0.200 ± 0.078** | 39 |
| Pyruvate | |||||||
| T | <0.001 | 0.012 ± 0.009 | 79 | 0.004 ± 0.004 | 88 | 0.003 ± 0.003 | 87 |
| PT | 0.66 | 0.018 ± 0.021 | 117 | 0.008 ± 0.009 | 110 | 0.012 ± 0.007* | 61 |
| Succinate | |||||||
| T | 0.93 | 0.012 ± 0.004 | 29 | 0.010 ± 0.002 | 23 | 0.013 ± 0.004 | 30 |
| PT | 0.45 | 0.013 ± 0.007 | 51 | 0.012 ± 0.004 | 32 | 0.016 ± 0.004 | 25 |
Values are means ± SDs unless otherwise indicated; day 0–5, n = 15 term and n = 10 preterm; day 14, n = 14 term and n = 10 preterm; and day 28, n = 15 term and n = 6 preterm. Mean concentrations include both secretors and nonsecretors. *,**Significant differences between mean concentrations of term and preterm milk as determined by two-tailed t test: *P < 0.05, **P < 0.001. The biological percentage CV (%CV) was calculated as SD/mean × 100. PT, preterm; T, term.
P values are based on linear mixed-effects or linear models, which tested whether term or preterm milk metabolite concentrations significantly changed over the first month of lactation.
Discussion
The chemical makeup of human milk changes during the course of lactation but particularly in the first month postpartum when breast tissue matures. Colostrum is the first milk produced by the mother and is rich in immunologic compounds and growth factors (28–30). As the mammary epithelial cells begin to form tight junctions, lactose and citrate increase in concentration (31), whereas total protein and sodium decrease (30) and transition milk is produced. This progression varies among women but usually occurs within a few days postpartum. By 4–6 wk, milk is considered fully mature and its composition does not fluctuate as much as in the first month postpartum (32). Consistent with other findings (4, 32, 33), we observed that lactose concentrations increased in both term and preterm milk over the first month of lactation, with low biological %CV. Indeed, lactose is an important osmolyte that is highly regulated (34), and the small %CV observed in this study and our previous study (21) supports this observation. No significant difference in the lactose concentration between term and preterm milk was observed in this study, which is also consistent with previous findings (6, 7).
In addition to providing nutrients for the growing infant, human milk also supplies prebiotics in the form of HMOs. These structurally diverse compounds are a conspicuously abundant component of human milk. Indeed, HMOs are the third most abundant component in human milk after lactose and fat (35) and act as prebiotics for commensal bacteria. HMOs contain a common lactose core decorated with combinations of glucose, galactose, N-acetylglucosamine, fucose, and sialic acid (36, 37). A group of HMOs that contain α-1,2 fucosyl linkages, which include 2′-FL, are produced through the action of the fucosyltransferase 2 (FUT2) gene. Mutations in both FUT2 alleles render an individual unable to create these linkages, and these individuals are referred to as “nonsecretors.” Those individuals who are heterozygotes or who lack any of the common FUT2 mutations readily create these linkages in surface and secreted molecules and are named “secretors” (38). Because the infant gut lacks glycosidases to catabolize HMOs, these molecules are indigestible and survive intact through the upper digestive tract (39) and thus are able to serve as a food source for specific commensal bacteria in the distal small bowel and colon (40–42), and potentially protect the infant from enteric pathogens by acting as decoys (43).
The concentrations of many of the HMOs measured in this study were dynamic during the first 28 d postpartum in both term and preterm milk. Given the small number of nonsecretors in this study and because only 1 preterm milk sample was collected at day 28 from a nonsecretor, meaningful results regarding differences in term and preterm milk on the basis of secretor status could not be drawn. Nonetheless, most of the HMOs in term milk decreased over time, consistent with findings in other studies (33, 44–50). 3-FL was the only HMO to increase during the first 28 d of lactation in both milks and was previously reported to increase in term milk over time (48–50). LNFP III and LNnT were less abundant in preterm colostrum than in term colostrum, but by day 28, they approached similar mean concentrations in term and preterm milks. The core N-acetylglucosamine of the tetrasaccharide LNnT may be fucosylated through an α-1,3 linkage to form LNFP III (48), which may explain the similar trend of these oligosaccharides over time. LNnT was previously shown to decrease in term milk over the first 3 mo of lactation (47, 48), and although it is not one of the most abundant HMOs, it is consumed by bifidobacteria (42). The total measured HMOs in this study were highly consistent in term and preterm mature milks, which is in agreement with our previous study in which we observed the conservation of total HMO concentration in term milk at day 90 postpartum (21). Taken together, these results suggest that oligosaccharide production is highly regulated within the mammary gland. Interestingly, total charged oligosaccharide concentrations, which were dominated by 6′-SL, did not vary greatly throughout the first month of lactation in term milk, because the %CV ranged from 20% to 24%. However, in preterm milk, these oligosaccharides had higher biological variability, particularly in mature milk (53%CV), suggesting that sialylation may not be as tightly regulated at 1 mo postpartum in mothers who deliver preterm infants.
Free amino acids comprise 8–22% of nonprotein nitrogen and 5–10% of total amino acids in human milk (51–53). They are an important nitrogen source for the growing infant and are more readily absorbed than protein-derived amino acids (54). In a systematic review of the literature, Zhang et al. (55) found that many of the free amino acids in term human milk decrease in concentration during the first 2 mo of lactation, whereas glutamine and glutamate increase. We determined that in addition to glutamine and glutamate, histidine and valine also increased significantly in term milk, as did alanine in both term and preterm milk over the first month of lactation. Interestingly, glutamine concentrations were similar in term and preterm colostrum but became significantly higher in term milk at 1 mo. Glutamine was previously reported to be higher in term transitional milk (55). This amino acid provides fuel for rapidly dividing cells such as enterocytes and immune cells and promotes the differentiation and proliferation of intestinal epithelial cells in combination with nucleotides. A recent meta-analysis of 11 clinical trials of glutamine supplementation in premature infants did not show significant benefits in reduction in mortality or morbidity or improvement in neurodevelopmental outcome; however, enteral administration of glutamine did reduce invasive infections and shorten the time to full enteral feedings (56). Unbound lysine, an essential amino acid, was found to be higher in preterm milk at all time points. Zhang et al. (55) also found free lysine to be higher in transitional preterm milk. Lysine is necessary for protein synthesis and its deficiency has been found to cause weight loss in neonates (57).
Premature infants have a higher risk of neonatal death, infection, and neurodevelopmental delays than do term infants. Human milk improves the health outcome for preterm infants (1); however, most studies to date have only compared mother’s own preterm milk with formula. The increased use of donor human milk (generally heat-treated mature term milk) for premature infants underscores the need to consider differences between term and preterm milk and how milk changes over time. Here, 69 metabolites were described in term and preterm human milk over the first 28 d of lactation with the use of 1H NMR spectroscopy. We found that the term and preterm human milk metabolomes are dynamic throughout the first month of lactation, with subtle differences in the concentrations of a few carbohydrates and amino acids and more biological variation in preterm human milk. Future studies aimed at understanding the consequences of these differences will be valuable.
Acknowledgments
We thank Xuan He for help with the statistical analysis. JTS, MAU, JBG, and CMS designed the study; ARS, JTS, and ELC conducted the research; ARS analyzed the data; ARS, JTS, and CMS wrote the manuscript; and CMS had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: FUT2, fucosyltransferase 2; HMO, human milk oligosaccharide; LM, linear model; LMM, linear mixed-effects model; LNFP, lacto-N-fucopentaose; LNnT, lacto-N-neotetraose; PCA, principal components analysis; 2′-FL, 2′-fucosyllactose; 3-FL, 3-fucosyllactose; 3′-GSL, 3′-galactosyllactose; 3′-SL, 3′-sialyllactose; 6′-SL, 6′-sialyllactose.
References
- 1.Eidelman AI, Schanler RJ, Johnston M, Landers S, Noble L, Szucs K, Viehmann L, Feldman-Winter L, Lawrence R, Kim S, et al. Breastfeeding and the use of human milk. Pediatrics 2012;129:e827–41. [DOI] [PubMed] [Google Scholar]
- 2.Underwood MA. Human milk for the premature infant. Pediatr Clin North Am 2013;60:189–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson GH. The effect of prematurity on milk composition and its physiological basis. Fed Proc 1984;43:2438–42. [PubMed] [Google Scholar]
- 4.Gabrielli O, Zampini L, Galeazzi T, Padella L, Santoro L, Peila C, Giuliani F, Bertino E, Fabris C, Coppa GV. Preterm milk oligosaccharides during the first month of lactation. Pediatrics 2011;128(6):e1520–31. [DOI] [PubMed]
- 5.Gross SJ, Geller J, Tomarelli RM. Composition of breast milk from mothers of preterm infants. Pediatrics 1981;68:490–3. [PubMed] [Google Scholar]
- 6.Saarela T, Kokkonen J, Koivisto M. Macronutrient and energy contents of human milk fractions during the first six months of lactation. Acta Paediatr 2005;94:1176–81. [DOI] [PubMed] [Google Scholar]
- 7.Lemons JA, Moye L, Hall D, Simmons M. Differences in the composition of preterm and term human milk during early lactation. Pediatr Res 1982;16:113–7. [DOI] [PubMed] [Google Scholar]
- 8.Coppa GV, Pierani P, Zampini L, Gabrielli O, Carlucci A, Catassi C, Giorgi PL. Lactose, oligosaccharide and monosaccharide content of milk from mothers delivering preterm newborns over the first month of lactation. Minerva Pediatr 1997;49:471–5. [PubMed] [Google Scholar]
- 9.Wang B, Brand-Miller J, McVeagh P, Petocz P. Concentration and distribution of sialic acid in human milk and infant formulas. Am J Clin Nutr 2001;74:510–5. [DOI] [PubMed] [Google Scholar]
- 10.De Leoz ML, Gaerlan SC, Strum JS, Dimapasoc LM, Mirmiran M, Tancredi DJ, Smilowitz JT, Kalanetra KM, Mills DA, German JB, et al. Lacto-N-tetraose, fucosylation, and secretor status are highly variable in human milk oligosaccharides from women delivering preterm. J Proteome Res 2012;11:4662–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet 1990;336:1519–23. [DOI] [PubMed] [Google Scholar]
- 12.Quigley MA, Henderson G, Anthony MY, McGuire W. Formula milk versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev 2007;4:CD002971. [DOI] [PubMed] [Google Scholar]
- 13.Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics 1999;103:1150–7. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto T, Shirai M, Kokubo M, Takahashi S, Kajino M, Takase M, Sakata H, Oki J. Human milk reduces the risk of retinal detachment in extremely low-birthweight infants. Pediatr Int 2007;49:894–7. [DOI] [PubMed] [Google Scholar]
- 15.Maayan-Metzger A, Avivi S, Schushan-Eisen I, Kuint J. Human milk versus formula feeding among preterm infants: short-term outcomes. Am J Perinatol 2012;29:121–6. [DOI] [PubMed] [Google Scholar]
- 16.Singhal A, Cole TJ, Lucas A. Early nutrition in preterm infants and later blood pressure: two cohorts after randomised trials. Lancet 2001;357:413–9. [DOI] [PubMed] [Google Scholar]
- 17.Vohr BR, Poindexter BB, Dusick AM, McKinley LT, Wright LL, Langer JC, Poole WK, Network NNR. Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics 2006;118:e115–23. [DOI] [PubMed] [Google Scholar]
- 18.Isaacs EB, Fischl BR, Quinn BT, Chong WK, Gadian DG, Lucas A. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr Res 2010;67:357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vohr BR, Poindexter BB, Dusick AM, McKinley LT, Higgins RD, Langer JC, Poole WK; for the National Institute of Child Health and Human Development National Research Network. Persistent beneficial effects of breast milk ingested in the neonatal intensive care unit on outcomes of extremely low birth weight infants at 30 months of age. Pediatrics 2007;120:e953–9. [DOI] [PubMed] [Google Scholar]
- 20.Ferris AM, Jensen RG. Lipids in human milk: a review. 1: Sampling, determination, and content. J Pediatr Gastroenterol Nutr 1984;3:108–22. [DOI] [PubMed] [Google Scholar]
- 21.Smilowitz JT, O'Sullivan A, Barile D, German JB, Lonnerdal B, Slupsky CM. The human milk metabolome reveals diverse oligosaccharide profiles. J Nutr 2013;143:1709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slupsky CM. Nuclear magnetic resonance-based analysis of urine for the rapid etiological diagnosis of pneumonia. Expert Opin Med Diagn 2011;5:63–73. [DOI] [PubMed] [Google Scholar]
- 23.Slupsky CM, Rankin KN, Wagner J, Fu H, Chang D, Weljie AM, Saude EJ, Lix B, Adamko DJ, Shah S, et al. Investigations of the effects of gender, diurnal variation, and age in human urinary metabolomic profiles. Anal Chem 2007;79:6995–7004. [DOI] [PubMed] [Google Scholar]
- 24.Weljie AM, Newton J, Mercier P, Carlson E, Slupsky CM. Targeted profiling: quantitative analysis of 1H NMR metabolomics data. Anal Chem 2006;78:4430–42. [DOI] [PubMed] [Google Scholar]
- 25.O'Sullivan A, He X, McNiven EM, Hinde K, Haggarty NW, Lonnerdal B, Slupsky CM. Metabolomic phenotyping validates the infant rhesus monkey as a model of human infant metabolism. J Pediatr Gastroenterol Nutr 2013;56:355–63. [DOI] [PubMed] [Google Scholar]
- 26.Totten SM, Zivkovic AM, Wu S, Ngyuen U, Freeman SL, Ruhaak LR, Darboe MK, German JB, Prentice AM, Lebrilla CB. Comprehensive profiles of human milk oligosaccharides yield highly sensitive and specific markers for determining secretor status in lactating mothers. J Proteome Res 2012;11:6124–33. [DOI] [PubMed] [Google Scholar]
- 27.Taylor-Cousar JL, Zariwala MA, Burch LH, Pace RG, Drumm ML, Calloway H, Fan HY, Weston BW, Wright FA, Knowles MR, et al. Histo-blood group gene polymorphisms as potential genetic modifiers of infection and cystic fibrosis lung disease severity. PLoS ONE 2009;4:e4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castellote C, Casillas R, Ramirez-Santana C, Perez-Cano FJ, Castell M, Moretones MG, Lopez-Sabater MC, Franch A. Premature delivery influences the immunological composition of colostrum and transitional and mature human milk. J Nutr 2011;141:1181–7. [DOI] [PubMed] [Google Scholar]
- 29.Pang WW, Hartmann PE. Initiation of human lactation: secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia 2007;12:211–21. [DOI] [PubMed] [Google Scholar]
- 30.Kulski JK, Hartmann PE. Changes in human milk composition during the initiation of lactation. Aust J Exp Biol Med Sci 1981;59:101–14. [DOI] [PubMed] [Google Scholar]
- 31.Kent JC, Arthur PG, Retallack RW, Hartmann PE. Calcium, phosphate and citrate in human milk at initiation of lactation. J Dairy Res 1992;59:161–7. [DOI] [PubMed] [Google Scholar]
- 32.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 2013;60:49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coppa GV, Gabrielli O, Pierani P, Catassi C, Carlucci A, Giorgi PL. Changes in carbohydrate composition in human milk over 4 months of lactation. Pediatrics 1993;91:637–41. [PubMed] [Google Scholar]
- 34.Neville MC. The physiological basis of milk secretion. Ann N Y Acad Sci 1990;586:1–11. [DOI] [PubMed] [Google Scholar]
- 35.Newburg DS, Neubauer SH. Carbohydrates in milks: analysis, quantities, and significance. In: Jensen RG, editor. Handbook of milk composition. San Diego (CA): Academic Press; 1995. p. 273–349. [Google Scholar]
- 36.Wu S, Grimm R, German JB, Lebrilla CB. Annotation and structural analysis of sialylated human milk oligosaccharides. J Proteome Res 2011;10:856–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu S, Tao N, German JB, Grimm R, Lebrilla CB. Development of an annotated library of neutral human milk oligosaccharides. J Proteome Res 2010;9:4138–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viverge D, Grimmonprez L, Cassanas G, Bardet L, Solere M. Discriminant carbohydrate components of human milk according to donor secretor types. J Pediatr Gastroenterol Nutr 1990;11:365–70. [DOI] [PubMed] [Google Scholar]
- 39.Chaturvedi P, Warren CD, Buescher CR, Pickering LK, Newburg DS. Survival of human milk oligosaccharides in the intestine of infants. Adv Exp Med Biol 2001;501:315–23. [DOI] [PubMed] [Google Scholar]
- 40.LoCascio RG, Desai P, Sela DA, Weimer B, Mills DA. Broad conservation of milk utilization genes in Bifidobacterium longum subsp. infantis as revealed by comparative genomic hybridization. Appl Environ Microbiol 2010;76:7373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turroni F, Bottacini F, Foroni E, Mulder I, Kim JH, Zomer A, Sanchez B, Bidossi A, Ferrarini A, Giubellini V, et al. Genome analysis of Bifidobacterium bifidum PRL2010 reveals metabolic pathways for host-derived glycan foraging. Proc Natl Acad Sci USA 2010;107:19514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruiz-Moyano S, Totten SM, Garrido DA, Smilowitz JT, German JB, Lebrilla CB, Mills DA. Variation in consumption of human milk oligosaccharides by infant gut-associated strains of Bifidobacterium breve. Appl Environ Microbiol 2013;79:6040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr 2005;25:37–58. [DOI] [PubMed] [Google Scholar]
- 44.Montreuil J, Mullet S. [Study of the variations of the glucide constituents of human milk during lactation.] Bull Soc Chim Biol (Paris) 1960;42:365–77 (in French). [PubMed] [Google Scholar]
- 45.Viverge D, Grimmonprez L, Cassanas G, Bardet L, Bonnet H, Solere M. Variations of lactose and oligosaccharides in milk from women of blood types secretor A or H, secretor Lewis, and secretor H/nonsecretor Lewis during the course of lactation. Ann Nutr Metab 1985;29:1–11. [DOI] [PubMed] [Google Scholar]
- 46.Thurl S, Munzert M, Henker J, Boehm G, Muller-Werner B, Jelinek J, Stahl B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr 2010;104:1261–71. [DOI] [PubMed] [Google Scholar]
- 47.Coppa GV, Pierani P, Zampini L, Carloni I, Carlucci A, Gabrielli O. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr Suppl 1999;88:89–94. [DOI] [PubMed] [Google Scholar]
- 48.Erney RM, Malone WT, Skelding MB, Marcon AA, Kleman-Leyer KM, O'Ryan ML, Ruiz-Palacios G, Hilty MD, Pickering LK, Prieto PA. Variability of human milk neutral oligosaccharides in a diverse population. J Pediatr Gastroenterol Nutr 2000;30:181–92. [DOI] [PubMed] [Google Scholar]
- 49.Chaturvedi P, Warren CD, Altaye M, Morrow AL, Ruiz-Palacios G, Pickering LK, Newburg DS. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology 2001;11:365–72. [DOI] [PubMed] [Google Scholar]
- 50.Sumiyoshi W, Urashima T, Nakamura T, Arai I, Saito T, Tsumura N, Wang B, Brand-Miller J, Watanabe Y, Kimura K. Determination of each neutral oligosaccharide in the milk of Japanese women during the course of lactation. Br J Nutr 2003;89:61–9. [DOI] [PubMed] [Google Scholar]
- 51.Agostoni C, Carratu B, Boniglia C, Lammardo AM, Riva E, Sanzini E. Free glutamine and glutamic acid increase in human milk through a three-month lactation period. J Pediatr Gastroenterol Nutr 2000;31:508–12. [DOI] [PubMed] [Google Scholar]
- 52.Atkinson SA, Schnurr C, Donovan SM, Lönnerdal B. The non-protein nitrogen components of human milk: biochemistry and potential functional role. In: Atkinson SA, Lönnerdal B, editors. Protein and non-protein nitrogen in human milk. Boca Raton, FL: CRC Press; 1989. p. 117–33. [Google Scholar]
- 53.Svanberg U, Gebre-Medhin M, Ljungqvist B, Olsson M. Breast milk composition in Ethiopian and Swedish mothers. III. Amino acids and other nitrogenous substances. Am J Clin Nutr 1977;30:499–507. [DOI] [PubMed] [Google Scholar]
- 54.Schanler RJ, Garza C. Plasma amino acid differences in very low birth weight infants fed either human milk or whey-dominant cow milk formula. Pediatr Res 1987;21:301–5. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Z, Adelman AS, Rai D, Boettcher J, Lonnerdal B. Amino acid profiles in term and preterm human milk through lactation: a systematic review. Nutrients 2013;5:4800–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moe-Byrne T, Wagner JV, McGuire W. Glutamine supplementation to prevent morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2012;3:CD001457. [DOI] [PubMed] [Google Scholar]
- 57.Snyderman SE, Norton PM, Fowler DI, Holt LE Jr. The essential amino acid requirements of infants: lysine. AMA J Dis Child 1959;97:175–85. [DOI] [PubMed] [Google Scholar]