Abstract
Background: Olive oil polyphenols have shown protective effects on cardiovascular risk factors. Their consumption decreased oxidative stress biomarkers and improved some features of the lipid profile. However, their effects on LDL concentrations in plasma and LDL atherogenicity have not yet been elucidated.
Objective: Our objective was to assess whether the consumption of olive oil polyphenols could decrease LDL concentrations [measured as apolipoprotein B-100 (apo B-100) concentrations and the total number of LDL particles] and atherogenicity (the number of small LDL particles and LDL oxidizability) in humans.
Methods: The study was a randomized, cross-over controlled trial in 25 healthy European men, aged 20–59 y, in the context of the EUROLIVE (Effect of Olive Oil Consumption on Oxidative Damage in European Populations) study. Volunteers ingested 25 mL/d raw low-polyphenol-content olive oil (LPCOO; 366 mg/kg) or high-polyphenol-content olive oil (HPCOO; 2.7 mg/kg) for 3 wk. Interventions were preceded by 2-wk washout periods. Effects of olive oil polyphenols on plasma LDL concentrations and atherogenicity were determined in the sample of 25 men. Effects on lipoprotein lipase (LPL) gene expression were assessed in another sample of 18 men from the EUROLIVE study.
Results: Plasma apo B-100 concentrations and the number of total and small LDL particles decreased (mean ± SD: by 5.94% ± 16.6%, 11.9% ± 12.0%, and 15.3% ± 35.1%, respectively) from baseline after the HPCOO intervention. These changes differed significantly from those after the LPCOO intervention, which resulted in significant increases of 6.39% ± 16.6%, 4.73% ± 22.0%, and 13.6% ± 36.4% from baseline (P < 0.03). LDL oxidation lag time increased by 5.0% ± 10.3% from baseline after the HPCOO intervention, which was significantly different only relative to preintervention values (P = 0.038). LPL gene expression tended to increase by 26% from baseline after the HPCOO intervention (P = 0.08) and did not change after the LPCOO intervention.
Conclusion: The consumption of olive oil polyphenols decreased plasma LDL concentrations and LDL atherogenicity in healthy young men. This trial was registered at www.controlled-trials.com as ISRCTN09220811.
Keywords: olive oil polyphenols, randomized clinical trial, low-density lipoproteins, apolipoprotein B-100, LDL particle number, small LDL particles, LDL oxidation, lipoprotein lipase, healthy individuals
Introduction
Virgin olive oil consumption protects against the development of cardiovascular diseases (1) due to its content of MUFAs (2) and polyphenols (3). To assess the beneficial properties of olive oil polyphenols on lipid profile and oxidation, the Effect of Olive Oil Consumption on Oxidative Damage in European Populations (EUROLIVE)18 Study was conducted. This project showed that the consumption of polyphenol-rich olive oil was beneficial for the oxidative status of LDLs. Olive oil polyphenols dose-dependently decreased circulating concentrations of oxidized LDLs, C18 hydroxy-FAs, and uninduced conjugated dienes (4). In a previous study, our group also observed that olive oil polyphenols induced changes in LDL composition, increasing the LDL content of oleic acid, vitamin E, and olive oil phenolic compounds (5).
However, the effects of olive oil polyphenols on LDL concentrations, LDL proatherogenic properties, such as LDL size, and the expression of some key genes related to LDL concentrations, such as lipoprotein lipase (LPL), have not been determined in vivo in humans. Thus, our objective was to determine whether the consumption of a polyphenol-rich olive oil would improve all of these properties.
Methods
Study participants
Our study population consisted of a subsample of the EUROLIVE study, a parallel, crossover, randomized controlled trial conducted in 180 healthy men, aged 20–59 y, from 6 European cities. The purpose of the study was to determine the effects of olive oil polyphenols on lipid profile and oxidative stress biomarkers. Local institutional ethics committees approved the protocol of the study, the details of which have been previously described (4). In all cases, written informed consent was provided by the participants before joining the trial. The protocol of the EUROLIVE study is registered as ISRCTN09220811 (www.controlled-trials.com).
We studied the effects of olive oil polyphenols on lipid profile, apo B-100 concentrations, LDL particle distribution, and LDL oxidizability ex vivo in a random subsample of 25 EUROLIVE volunteers from 3 centers (9 from Potsdam, Germany; 9 from Kuopio, Finland; and 7 from Barcelona, Spain). We assessed the effects of polyphenols on the expression of LPL in another random subsample of 18 volunteers of the EUROLIVE study, 8 of whom were also present in the first subsample of individuals. Blood samples were taken from fasting participants before and after dietary interventions with high-polyphenol-content olive oil (HPCOO; a natural virgin olive oil with 366 mg/kg polyphenols) and low-polyphenol-content olive oil (LPCOO; a refined olive oil with 2.7 mg/kg polyphenols). Polyphenols become degraded during the refinement process and thus the refined olive oil had a lower phenolic content. The composition of both olive oils was identical, except for their polyphenol content (4). Samples were stored at −80°C until the present experiments commenced. No thaw-freeze cycles were applied to the samples before the present work.
As shown in the crossover design (Supplemental Figure 1), volunteers were assigned to 3-wk intervention periods in which they ingested 25 mL/d raw olive oil distributed among meals. Participants were instructed to replace other dietary fats with olive oil. Intervention periods were preceded by a 2-wk washout period, during which olive oil, olives, and antioxidant-rich foods were avoided. A 2-wk washout period was sufficient to eliminate olive oil polyphenols between interventions, considering the half-life of the sum of the main olive oil phenolic compounds (8 h) (6). The washout period was also sufficient if the half-life of the LDL particle is considered (3 d) (7). A more detailed description of the diet of the participants was previously published (4, 8).
Study measurements
Dietary adherence, physical activity, and oxidative status of volunteers.
Dietary adherence was determined through 24-h urinary excretion of tyrosol and hydroxytyrosol, which are the 2 main phenolic compounds in olive oil and are considered as biomarkers of olive oil consumption. These compounds were determined by GC and MS, as previously described (6). Participants’ diet was controlled through a 3-d dietary record. This dietary control was performed at the beginning of the study and after each dietary intervention. Volunteers were asked to maintain their usual diet during the whole study. The physical activity level of participants was also measured and was calculated at the beginning and the end of the study by using the validated Minnesota Leisure Time Physical Activity Questionnaire (4). The oxidative status of the volunteers was also assessed by means of the determination of different oxidative biomarkers (oxidized LDLs, C18 hydroxy-FAs, and reduced ascorbic acid and dehydroascorbic acid), as previously reported (4).
Lipid profile and apo B-100 determination.
We performed lipid profile and apo B-100 analyses with the use of an ABX Pentra 400 autoanalyzer (Horiba Diagnostics). TGs and total cholesterol were measured by using enzymatic methods (ABX Pentra Triglycerides CP and ABX Pentra Cholesterol CP, respectively; Horiba Diagnostics). HDL cholesterol was determined by the Accelerator Selective Detergent method (ABX Pentra HDL Direct CP; Horiba Diagnostics), and apo B-100 concentrations were measured by immunoturbidimetry (ABX Pentra Apo B; Horiba Diagnostics). Interassay CVs of the previous determinations were as follows: 1.48% for TGs, 1.54% for total cholesterol, 3.34% for HDL cholesterol, and 1.95% for apoB. We also calculated LDL-cholesterol concentrations by the Friedewald formula whenever TGs were <300 mg/dL.
LDL particle analyses.
We determined LDL-cholesterol concentrations (directly measured) and the number of total LDL particles, total VLDL particles, small LDL particles, large LDL particles, small VLDL particles, and medium+large VLDL particles by NMR spectroscopy. Values were calculated from the measured amplitudes of the NMR signals of the lipid methyl groups in the samples (9). These analyses were performed by using a Vantera Clinical Analyzer (LipoScience) and could only be performed in the available samples of 20 of the volunteers. This technique showed an interassay CV of 5.30% for the determination of total LDL particle number for the range of low values (typical in healthy individuals), under the most unfavorable conditions.
Analyses of LDL resistance to oxidation.
Before analysis, LDLs were isolated from K2-EDTA plasma of the volunteers by density gradient ultracentrifugation (10) and stored at −80°C in 2.5% sucrose until the analyses. In the isolated LDL samples, we determined LDL resistance to oxidation by measuring the accumulation of Cu2+-induced conjugated dienes in the lipoprotein. First, we dialyzed the isolated LDLs with PBS to discard possible contaminants. We then incubated the dialyzed LDLs (at a final concentration of 10 mg/dL LDL cholesterol) in the presence of CuSO4 to induce the oxidation reaction (at a final concentration of 5 μM CuSO4) at 37°C for 4 h. During incubation, absorbance at 234 nm was determined every 3 min by using an INFINITE M200 reader (Tecan Group Ltd.).
Consecutive measurements of absorbance defined the LDL oxidation kinetic curves. For each of these curves, we calculated the following: 1) lag time (the time when maximal oxidation started, in minutes) and 2) oxidation rate [the slope of the kinetic curve at peak velocity, expressed as the increase in the concentration (mM) of conjugated dienes per minute and milligrams of LDL cholesterol], as previously described (5). All determinations were performed in duplicate. We used an LDL pool from healthy volunteers as an interassay control. The interassay CVs of the previous determinations were as follows: 2.89% for lag time and 4.77% for oxidation rate.
LPL gene expression analyses.
First, we isolated total RNA from peripheral blood mononuclear cells by means of a liquid-liquid method. We then checked RNA purity and integrity and converted RNA to cDNA. Afterward, LPL gene expression was quantified by using a real-time PCR in TaqMan Low Density microfluidic cards (Applied Biosystems, Life Technologies). Four replicates of each RNA sample were used in the experiments. Data were analyzed by using the Sequence Detection System software (SDS 2.1.; Applied Biosystems, Life Technologies), following the manufacturer’s instructions. LPL gene expression was finally calculated by the relative quantification method (using the 2−ΔΔCt formula). This technique presents an interassay CV of 0.98%, calculated in the control pool for the housekeeping gene (GAPDH).
Sample size calculation
Biochemical determinations.
A sample size of 25 individuals allowed ≥80% power to detect significant differences of 4 mg/dL apo B-100 concentrations between both olive oil interventions, considering a 2-sided type I error of 0.05. Calculations were made by using previous data on our group, considering the SD of apo B-100 concentrations in healthy volunteers.
LPL gene expression.
A sample size of 18 individuals allowed ≥80% power to detect significant differences of 0.5 units of log2 ratio relative quantification in the expression of a reference gene [human interferon γ (IFNG)] between both olive oil interventions, considering a 2-sided type I error of 0.05. Calculations were made by using previous data on our group, considering the SD of IFNG gene expression in healthy volunteers (8).
Statistical analyses
We confirmed the normal distribution of the continuous variables by normal probability plots and the Shapiro-Wilk test. To take into consideration interindividual variability in the variables studied, we investigated possible differences in baseline values between the 2 interventions by a paired t test, and we expressed the differences between baseline and postintervention as percentage changes. These percentage changes were calculated as follows: (postintervention value – baseline value)/baseline value × 100.
We evaluated the effect of olive oil interventions compared with baseline, and the differences between treatments, in a mixed linear model. We considered the interaction between treatment (LPCOO or HPCOO) and the pre-post intervention differences as the term of interest and included the following variables as adjustment variables: study period, age, and country of origin of the volunteers. Moreover, taking into consideration the fact that we performed repeated measurements in the study due to the study design (crossover), we used “individual” as a factor of random effect in the model. We checked the period-by-treatment interactions to discard possible carry-over effects. We tested the relations between variables through Pearson’s correlation analyses.
In all cases, we considered significant any P value <0.05. All of the previous analyses were performed with R software, version 3.0.2 (R Foundation for Statistical Computing) and with SPSS software, version 18.0 (IBM Corporation). Mixed models were adjusted by using the lme4 package in R (11).
Results
General characteristics of the participants.
Supplemental Figure 1 shows the design of the study. No significant differences in baseline values were found between our subsamples and the total EUROLIVE study population (Table 1). Dietary patterns and energy expenditure in leisure-time physical activity also did not change. As previously reported (4), participants’ compliance was correct, because urinary excretions of tyrosol and hydroxytyrosol were 9-fold and 18-fold higher, respectively, after the HPCOO intervention relative to baseline. These increases were significantly greater than those after the LPCOO intervention, which were 0.3-fold and 0.7-fold higher, respectively, and were significant (P < 0.001 in both cases). The lipid profile of the volunteers (TGs, total cholesterol, HDL cholesterol, and LDL cholesterol calculated by the Friedewald formula) did not differ between the intervention periods (data not shown).
TABLE 1.
Baseline characteristics and plasma lipid profile of participants in the 2 subsamples of the study compared with the whole EUROLIVE study population1
| Subsample |
|||
| Biochemical analyses (n = 25) | Gene expression (n = 18) | EUROLIVE study population (n = 180) | |
| Age, y | 32.3 ± 11.2 | 36.9 ± 12.3 | 33.2 ± 11.0 |
| Weight, kg | 78.2 ± 10.9 | 78.1 ± 10.9 | 76.4 ± 10.5 |
| Height, m | 1.79 ± 0.08 | 1.79 ± 0.08 | 1.79 ± 0.07 |
| Total cholesterol, mmol/L | 4.5 ± 1.2 | 4.8 ± 0.9 | 4.5 ± 1.1 |
| LDL cholesterol, mmol/L | 2.6 ± 1.0 | 2.8 ± 0.9 | 2.5 ± 0.9 |
| HDL cholesterol, mmol/L | 1.3 ± 0.3 | 1.4 ± 0.3 | 1.4 ± 0.3 |
| TGs,2 mmol/L | 1.1 (0.8, 1.4) | 1.2 (0.9, 1.5) | 1.1 (0.8, 1.5) |
Values are means ± SDs unless otherwise indicated. EUROLIVE, Effect of Olive Oil Consumption on Oxidative Damage in European Populations.
Values are medians (first quartile, third quartile).
The consumption of olive oil polyphenols improved the oxidative status of the volunteers. We observed significant decreases in concentrations of oxidized LDL and C18 hydroxy-FAs, equivalent to those previously reported (4). The ratio between the reduced and the oxidized forms of ascorbic acid increased significantly after the HPCOO intervention when compared with baseline values (P = 0.018). Data for these and other variables are shown in Supplemental Table 1.
Olive oil polyphenols decreased LDL concentrations.
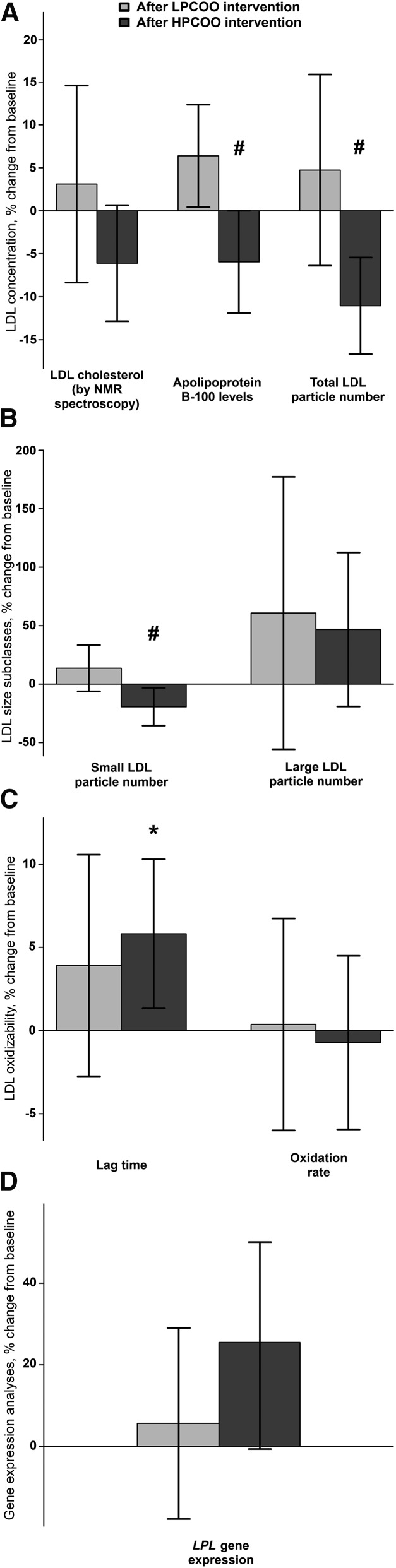
We directly determined LDL concentrations by 3 different approaches: directly measured LDL cholesterol, apo B-100 concentrations, and the number of total LDL particles. As shown in Figure 1A, after the HPCOO intervention, apo B-100 concentrations and the number of total LDL particles decreased by 5.9% ± 16.6% and 11.9% ± 12.0%, respectively, relative to baseline values. These variables increased by 6.4% ± 16.6% and 4.7% ± 22.0%, respectively, after the LPCOO intervention. Both decreases in apo B-100 concentrations and the number of total LDL particles after the HPCOO intervention were significant compared with the LPCOO intervention (P = 0.004 and P = 0.013, respectively).
FIGURE 1.
Percent changes from baseline of directly measured LDL concentrations (determined as LDL cholesterol, apo B-100 concentrations and total LDL particle number) (A), LDL size distribution (B), LDL oxidizability (C), and LPL gene expression (D) in healthy European men, aged 20–59 y, after 3-wk consumption of LPCOO or HPCOO. Values are means ± SEMs; n = 25 (for panels A, B, and C) and n = 18 (for panel D). *Different from baseline, P < 0.05. #Different from LPCOO intervention, P < 0.05. HPCOO, high-polyphenol-content olive oil; LPCOO, low-polyphenol-content olive oil; LPL, lipoprotein lipase.
Olive oil polyphenols decreased the number of small LDL particles.
As shown in Figure 1B, the number of small LDL particles decreased by 15.3% ± 35.1% after the HPCOO intervention relative to baseline values. However, after the LPCOO intervention, this value increased by 13.6% ± 36.4% relative to baseline. The decrease in the number of small LDL particles after the HPCOO intervention was significant when compared with the LPCOO intervention (P = 0.029). No significant changes in the number of large LDL particles were found after either intervention. High adherence to the consumption of olive oil polyphenols (reflected as an increase in urinary tyrosol excretion) and improvements in oxidative status after the HPCOO intervention (reflected as an increase in the reduced/oxidized ascorbic acid ratio) correlated with greater decreases in small LDL particle numbers (P = 0.042, r = −0.53, and P = 0.005, r = −0.66, respectively).
Olive oil polyphenols increased the resistance of LDLs to oxidation.
As shown in Figure 1C, the LDL oxidation lag time increased by 5.01% ± 10.3% after the HPCOO intervention and by 3.17% ± 19.1% after the LPCOO intervention. After the HPCOO intervention, lag-time values were significantly higher only compared with baseline (P = 0.038). Changes in lag time did not differ between interventions. LDL oxidation rate did not change significantly after either intervention.
Changes in LPL gene expression.
LPL gene expression tended to increase by 26%, relative to baseline, after the HPCOO intervention (P = 0.08) (Figure 1D). It did not change significantly after the LPCOO intervention. Changes in this variable did not differ between the interventions.
Discussion
The present work shows that 3-wk consumption of olive oil polyphenols decreased LDL concentrations and LDL atherogenicity in vivo and reveals, to date, some of the most considerable decreases in the number of total and small LDL particles that have been reported in humans due to dietary bioactive compounds.
LDL-cholesterol concentrations are directly and strongly associated with coronary artery disease risk (12). This association justifies their determination in most epidemiologic and interventional cardiovascular studies. Although the direct quantification of LDL cholesterol is possible, in several studies LDL-cholesterol concentrations were calculated by using indirect equations such as the Friedewald formula (an approximation based on TGs and total and HDL cholesterol) (13). These formulas may underestimate LDL-cholesterol concentrations compared with direct measurements, particularly in nonpathologic ranges of TG values. Thus, direct and more precise determinations of apo B-100 concentrations or the total number of LDL particles are recommended (13). Moreover, apo B-100 concentrations and the total number of LDL particles are more accurate than LDL cholesterol to quantify cardiovascular risk in high-risk patients (e.g., individuals who have suffered premature coronary events or with metabolic syndrome) (14) and both are directly related to a greater incidence of cardiovascular events (15, 16).
In this context, we directly assessed the effects of olive oil polyphenols on LDL concentrations. The consumption of olive oil polyphenols was significantly associated with a decrease in apo B-100 concentrations and total number of LDL particles (5.9% and 11.9%, respectively). Similar decreases have been reported after other antioxidant-rich dietary approaches. Concentrations of apo B-100 decreased after consumption of a hazelnut-enriched diet (17) and concentrated red grape juice (18). The number of LDL particles also decreased after long-term consumption of a high-fiber oat cereal (19).
The decrease in LDL concentrations may be explained through an improvement in the systemic oxidative status or by an increase in the gene expression of LPL, as observed in our study. Three different mechanisms may be involved in this hypothesis. First, oxidative stress states are associated with increased LDL concentrations, especially due to an increased number of small LDL particles (20). An improved oxidative status due to the consumption of olive oil polyphenols may counteract increases in LDL concentrations by decreasing the number of small LDL particles, as we reported. Second, increases in the expression of LPL may help the organism to decrease concentrations of TG-rich lipoproteins (e.g., LDLs), because LPL is the main enzyme involved in the removal of TGs from the blood and presents some LDL receptor activity (21). Finally, improvements in general oxidative status have been associated with a better activity of LPL (22, 23).
LDLs are more atherogenic when they are small and dense because 1) LPL does not recognize them properly, 2) they easily traverse the endothelial barrier, and 3) they are easily oxidized in the subendothelial space (24). They are thus associated with early atherosclerosis and high cardiovascular risk (25, 26) and were directly related to a greater incidence of cardiovascular events in some studies (16). In our data, the number of small LDL particles decreased by 15.3% after the consumption of olive oil polyphenols. This decrease was greater when there was a higher adherence to the HPCOO intervention.
The decrease in the number of small LDL particles may be explained by an improvement in oxidative status. As we previously commented, a better oxidative status may result in a lower production of small LDLs, because the number of small LDL particles increases when the levels of oxidative stress are higher (20). This hypothesis concurs with the significant correlation between the decrease in the number of small LDL particles and the increase in the ratio between reduced and oxidized ascorbic acid in our data. In addition, similar effects were also observed after other antioxidant-rich dietary interventions, such as a Mediterranean diet supplemented with nuts (27) and the consumption of a polyphenol-rich supplement made from freeze-dried strawberries (28).
LDL oxidation is considered to be a trigger for the biochemical processes that take place in the subendothelial space and leads to the formation of an atherosclerotic plaque (29). In particular, LDL resistance to oxidation ex vivo predicts artery dysfunction, even when adjusted for other cardiovascular risk factors (30). In our study, olive oil polyphenols increased LDL resistance to oxidation. Increases in LDL antioxidant defenses after the consumption of olive oil polyphenols explain this beneficial effect (5). Our results confirm the decrease in this LDL atherogenic trait after consumption of virgin olive oil (31) and after consumption of an antioxidant-rich vegetarian diet (32).
One of the strengths of the present study is its crossover design, which reduced interference from confounding variables. We administered real-life doses of a food that cannot be consumed in great quantities. Thus, some of the changes observed were modest. However, the LDL-related traits that we describe in this work help to explain residual cardiovascular risk (33) and are directly related to a greater incidence of cardiovascular diseases (15, 16). Therefore, even modest decreases in these variables may be protective against the development of cardiovascular events. A possible limitation of our work is that we performed systemic and gene expression analyses in 2 different subsamples of individuals. However, both subgroups did not present significantly different baseline characteristics and were representative of the whole EUROLIVE population. Other limitations of the study are that the amount of polyphenols equivalent to that provided by the HPCOO intervention could have come from other food types or that synergistic effects between olive oil polyphenols and other olive oil components on LDL biology have not yet been identified.
In conclusion, the consumption of olive oil polyphenols decreased LDL concentrations directly measured as concentrations of apo B-100 and the total number of LDL particles. The consumption of olive oil polyphenols also decreased LDL atherogenicity, as reflected in the smaller number of small LDL particles and enhanced LDL resistance to oxidation. An improved oxidative status and an increased gene expression of LPL may contribute to explain these changes. These data support previous evidence indicating that olive oil polyphenols can contribute highly to the control of cardiovascular risk.
Acknowledgments
We thank Stephanie Lonsdale for her help in the editing of the English text. ÁH, M-IC, and M Fitó designed the research; ÁH, ATR, M Farràs, SF-C, HS, MF-M, DM-A, MS, RS, M Farré, RdlT, M-CL-S, KN, and H-JFZ conducted the research; ÁH and IS analyzed the data and performed the statistical analyses; ÁH and M Fitó wrote the manuscript; and M Fitó had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: EUROLIVE, Effect of Olive Oil Consumption on Oxidative Damage in European Populations; HPCOO, high-polyphenol content olive oil; IFNG, interferon γ LPCOO, low-polyphenol content olive oil; LPL, lipoprotein lipase.
References
- 1.Martín-Peláez S, Covas MI, Fitó M, Kušar A, Pravst I. Health effects of olive oil polyphenols: recent advances and possibilities for the use of health claims. Mol Nutr Food Res 2013;57:760–71. [DOI] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration. Press release P04-100, November 1, 2004 [cited 2015 Mar 24]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2004/ucm108368.htm.
- 3.EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on the substantiation of health claims related to polyphenols in olive oil and protection of LDL particles from oxidative damage. EFSA J 2011;9 [cited 2015 Mar 24]. Available from: http://www.efsa.europa.eu/en/efsajournal/pub/2033.htm.
- 4.Covas MI, Nyyssönen K, Poulsen HE, Kaikkonen J, Zunft HF, Kiesewetter H, Gaddi A, de la Torre R, Mursu J, Bäumler H, et al. The effect of polyphenols in olive oil on heart disease risk factors: a randomized trial. Ann Intern Med 2006;145:333–41. [DOI] [PubMed] [Google Scholar]
- 5.Gimeno E, Fitó M, Lamuela-Raventós RM, Castellote AI, Covas M, Farré M, de La Torre-Boronat MC, López-Sabater MC. Effect of ingestion of virgin olive oil on human low-density lipoprotein composition. Eur J Clin Nutr 2002;56:114–20. [DOI] [PubMed] [Google Scholar]
- 6.Miró-Casas E, Farré Albaladejo M, Covas MI, Rodriguez JO, Menoyo Colomer E, Lamuela Raventós RM, de la Torre R. Capillary gas chromatography-mass spectrometry quantitative determination of hydroxytyrosol and tyrosol in human urine after olive oil intake. Anal Biochem 2001;294:63–72. [DOI] [PubMed] [Google Scholar]
- 7.Langer T, Strober W, Levy RI. The metabolism of low density lipoprotein in familial type II hyperlipoproteinemia. J Clin Invest 1972;51:1528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castañer O, Covas MI, Khymenets O, Nyyssonen K, Konstantinidou V, Zunft HF, de la Torre R, Muñoz-Aguayo D, Vila J, Fitó M. Protection of LDL from oxidation by olive oil polyphenols is associated with a downregulation of CD40-ligand expression and its downstream products in vivo in humans. Am J Clin Nutr 2012;95:1238–44. [DOI] [PubMed] [Google Scholar]
- 9.Tsai MY, Steffen BT, Guan W, McClelland RL, Warnick R, McConnell J, Hoefner DM, Remaley AT. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2014;34:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman MJ, Goldstein S, Lagrange D, Laplaud PM. A density gradient ultracentrifugal procedure for the isolation of the major lipoprotein classes from human serum. J Lipid Res 1981;22:339–58. [PubMed] [Google Scholar]
- 11.Bates D, Maechler M, Bolker BM, Walker S. lme4: Linear mixed-effects models using Eigen and S4. J Stat Softw [Internet]. c2014 [cited 2015 Mar 24]. Available from: http://arxiv.org/abs/1406.5823.
- 12.Castelli WP, Anderson K, Wilson PWF, Levy D. Lipids and risk of coronary heart disease: the Framingham Study. Ann Epidemiol 1992;2:23–8. [DOI] [PubMed] [Google Scholar]
- 13.Sniderman AD, Blank D, Zakarian R, Bergeron J, Frohlich J. Triglycerides and small dense LDL: the twin Achilles heels of the Friedewald formula. Clin Biochem 2003;36:499–504. [DOI] [PubMed] [Google Scholar]
- 14.Davidson MH. Low-density lipoprotein cholesterol, non-high-density lipoprotein, apolipoprotein, or low-density lipoprotein particle: what should clinicians measure? J Am Coll Cardiol 2012;60:2616–7. [DOI] [PubMed] [Google Scholar]
- 15.Benn M, Nordestgaard BG, Jensen GB, Tybjaerg-Hansen A. Improving prediction of ischemic cardiovascular disease in the general population using apolipoprotein B: the Copenhagen City Heart Study. Arterioscler Thromb Vasc Biol 2007;27:661–70. [DOI] [PubMed] [Google Scholar]
- 16.Ip S, Lichtenstein AH, Chung M, Lau J, Balk EM. Systematic review: association of low-density lipoprotein subfractions with cardiovascular outcomes. Ann Intern Med 2009;150:474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mercanligil SM, Arslan P, Alasalvar C, Okut E, Akgül E, Pinar A, Geyik PO, Tokgözoğlu L, Shahidi F. Effects of hazelnut-enriched diet on plasma cholesterol and lipoprotein profiles in hypercholesterolemic adult men. Eur J Clin Nutr 2007;61:212–20. [DOI] [PubMed] [Google Scholar]
- 18.Castilla P, Echarri R, Dávalos A, Cerrato F, Ortega H, Teruel JL, Lucas MF, Gómez-Coronado D, Ortuño J, Lasunción MA. Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am J Clin Nutr 2006;84:252–62. [DOI] [PubMed] [Google Scholar]
- 19.Davy BM, Davy KP, Ho RC, Beske SD, Davrath LR, Melby CL. High-fiber oat cereal compared with wheat cereal consumption favorably alters LDL-cholesterol subclass and particle numbers in middle-aged and older men. Am J Clin Nutr 2002;76:351–8. [DOI] [PubMed] [Google Scholar]
- 20.Kotani K, Tsuzaki K, Taniguchi N, Sakane N. LDL particle size and reactive oxygen metabolites in dyslipidemic patients. Int J Prev Med. 2012;3:160–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Otarod JK, Goldberg IJ. Lipoprotein lipase and its role in regulation of plasma lipoproteins and cardiac risk. Curr Atheroscler Rep 2004;6:335–42. [DOI] [PubMed] [Google Scholar]
- 22.Yang R, Le G, Li A, Zheng J, Shi Y. Effect of antioxidant capacity on blood lipid metabolism and lipoprotein lipase activity of rats fed a high-fat diet. Nutrition 2006;22:1185–91. [DOI] [PubMed] [Google Scholar]
- 23.Kalmár T, Seres I, Balogh Z, Káplár M, Winkler G, Paragh G. Correlation between the activities of lipoprotein lipase and paraoxonase in type 2 diabetes mellitus. Diabetes Metab 2005;31:574–80. [DOI] [PubMed] [Google Scholar]
- 24.Borst JW, Visser NV, Kouptsova O, Visser AJ. Oxidation of unsaturated phospholipids in membrane bilayer mixtures is accompanied by membrane fluidity changes. Biochim Biophys Acta 2000;1487:61–73. [DOI] [PubMed] [Google Scholar]
- 25.Kathiresan S, Otvos JD, Sullivan LM, Keyes MJ, Schaefer EJ, Wilson PWF, D’Agostino RB, Vasan RS, Robins SJ. Increased small low-density lipoprotein particle number: a prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation 2006;113:20–9. [DOI] [PubMed] [Google Scholar]
- 26.Gentile M, Panico S, Mattiello A, Ubaldi S, Iannuzzo G, De Michele M, Iannuzzi A, Rubba P. Association between small dense LDL and early atherosclerosis in a sample of menopausal women. Clin Chim Acta 2013;426:1–5. [DOI] [PubMed] [Google Scholar]
- 27.Damasceno NRT, Sala-Vila A, Cofán M, Pérez-Heras AM, Fitó M, Ruiz-Gutiérrez V, Martínez-González M-Á, Corella D, Arós F, Estruch R, et al. Mediterranean diet supplemented with nuts reduces waist circumference and shifts lipoprotein subfractions to a less atherogenic pattern in subjects at high cardiovascular risk. Atherosclerosis 2013;230:347–53. [DOI] [PubMed] [Google Scholar]
- 28.Basu A, Betts NM, Nguyen A, Newman ED, Fu D, Lyons TJ. Freeze-dried strawberries lower serum cholesterol and lipid peroxidation in adults with abdominal adiposity and elevated serum lipids. J Nutr 2014;144:830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999;340:115–26. [DOI] [PubMed] [Google Scholar]
- 30.Hendrickson A, McKinstry LA, Lewis JK, Lum J, Louie A, Schellenberg GD, Hatsukami TS, Chait A, Jarvik GP. Ex vivo measures of LDL oxidative susceptibility predict carotid artery disease. Atherosclerosis 2005;179:147–53. [DOI] [PubMed] [Google Scholar]
- 31.Marrugat J, Covas MI, Fitó M, Schröder H, Miró-Casas E, Gimeno E, López-Sabater MC, de la Torre R, Farré M. Effects of differing phenolic content in dietary olive oils on lipids and LDL oxidation—a randomized controlled trial. Eur J Nutr 2004;43:140–7. [DOI] [PubMed] [Google Scholar]
- 32.Lu SC, Wu WH, Lee CA, Chou HF, Lee HR, Huang PC. LDL of Taiwanese vegetarians are less oxidizable than those of omnivores. J Nutr 2000;130:1591–6. [DOI] [PubMed] [Google Scholar]
- 33.Malave H, Castro M, Burkle J, Voros S, Dayspring T, Honigberg R, Pourfarzib R. Evaluation of low-density lipoprotein particle number distribution in patients with type 2 diabetes mellitus with low-density lipoprotein cholesterol <50 mg/dl and non-high-density lipoprotein cholesterol <80 mg/dl. Am J Cardiol 2012;110:662–5. [DOI] [PubMed] [Google Scholar]