Abstract
Background: Preterm birth is one of the leading causes of neonatal morbidity in the United States. Despite decades of research, the etiology is largely unknown.
Objective: The purpose of our study was to examine the association between maternal dietary patterns during pregnancy and preterm birth.
Methods: This prospective cohort study used data from the PIN (Pregnancy, Infection, and Nutrition) study (n = 3143). Dietary intake was assessed at 26–29 wk of gestation by using a food-frequency questionnaire, and patterns were derived by using factor analysis and the Dietary Approaches to Stop Hypertension (DASH) diet. Associations between dietary patterns and preterm birth were assessed by logistic regression.
Results: Four dietary patterns were identified from the factor analysis characterized by high intakes of the following: 1) fruits, vegetables, low-fat dairy, high-fiber and fortified cereals, nonfried chicken and fish, and wheat bread; 2) beans, corn, French fries, hamburgers or cheeseburgers, white potatoes, fried chicken, mixed dishes, and ice cream; 3) collard greens, coleslaw or cabbage, red and processed meats, cornbread or hushpuppies, whole milk, and vitamin C–rich drinks; and 4) shellfish, pizza, salty snacks, and refined grains. Increased odds of preterm birth were found for a diet characterized by a high consumption of collard greens, coleslaw or cabbage, red meats, fried chicken and fish, processed meats, cornbread or hushpuppies, eggs or egg biscuits, gravy, whole milk, and vitamin C–rich drinks such as Kool-Aid (Kraft Foods) and Hi-C (Minute Maid Co.) (adjusted OR for quartile 4 vs. quartile 1: 1.55; 95% CI: 1.07, 2.24). Greater adherence to the DASH diet was associated with decreased odds of preterm birth compared with women in the lowest quartile (adjusted OR for quartile 4 vs. quartile 1: 0.59; 95% CI: 0.40, 0.85).
Conclusions: Diet quality during pregnancy is associated with preterm birth; thus, preconceptional and early prenatal dietary counseling promoting healthy dietary intake could improve pregnancy outcomes.
Keywords: maternal diet, preterm birth, dietary patterns, factor analysis, Dietary Approaches to Stop Hypertension
Introduction
Preterm birth is one of the leading causes of neonatal morbidity, accounting for nearly 35% of all neonatal deaths in the United States (1). Despite decades of research, the incidence of preterm birth remains close to 11% and the etiology is largely unknown (2). With regard to diet, maternal nutrition during pregnancy has an important role in providing the necessary nutrients for fetal growth (3); however, the relation between maternal diet and preterm birth is not well established.
The use of dietary patterns, a measure of overall diet, has become widespread in nutrition research as an alternative approach to studying individual components of the diet. Unlike single-food and -nutrient studies, dietary patterns research examines the influence of foods eaten in combination and allows for interactions between nutrients (4, 5). To examine the association between maternal dietary patterns and preterm birth, studies have used both hypothesis-oriented dietary patterns based on predefined guidelines and empirically derived dietary patterns using statistical methods. Studies that used hypothesis-oriented methods, such as the Mediterranean-style diet and Diet Quality Index, in relation to preterm birth found no clear evidence of an association (6–8). The Mediterranean-style diet is based on dietary habits of the Mediterranean region (9, 10). A randomized controlled trial conducted in Norway among nonsmoking white women showed that women randomly assigned to a diet similar to the Dietary Approaches to Stop Hypertension (DASH)7 diet, as opposed to continuing their usual diet, had a reduced risk of preterm birth (11). The DASH diet is an alternative to the Mediterranean-style diet and is more applicable to women living in the southern United States.
Results from prospective studies examining empirically derived dietary patterns, before and during pregnancy, have varied in relation to preterm birth (12–14). Empirically derived dietary patterns are population-specific and influenced by social, cultural, and environmental factors (15–17). Despite this, identical names are often used across studies to identify dietary patterns, regardless of the different underlying food components, making it difficult to compare results across studies. To our knowledge, no studies to date have compared the results from both hypothesis-oriented and empirically derived methods of dietary patterns in relation to preterm birth in a pregnant US population. The primary objective of our study was to examine the association between maternal dietary patterns during pregnancy and preterm birth using both factor analysis and the DASH diet.
Methods
Study design and population.
This study used data from the Pregnancy, Infection, and Nutrition (PIN) Study, a prospective cohort study in central North Carolina designed to investigate the etiology of and risk factors for preterm birth (18). The first and second cohorts (PIN 1 and PIN 2) of pregnant women were recruited at 24–29 wk of gestation from prenatal clinics at the Wake County Human Services Department, Wake Medical Center, Wake Area Health Education Center, and University of North Carolina (UNC) hospitals from August 1995 to June 2000. A third cohort of pregnant women (PIN 3) was recruited to participate beginning in January 2001 to June 2005 from prenatal clinics at UNC hospitals at <20 wk of gestation. All participants gave written informed consent at the time of recruitment.
Data were collected via telephone interviews and self-administered questionnaires, including an FFQ to collect dietary information. Sociodemographic information and health behaviors were collected via telephone interviews. Smoking status (smoker vs. nonsmoker) during the first 6 mo of pregnancy was collected from self-administered questionnaires. Prepregnancy BMI was based on height measured at the first prenatal clinic visit and self-reported prepregnancy weight. Missing prepregnancy weight was imputed on the basis of the first prenatal care visit (19). A total of 5038 pregnant women, accounting for 5169 pregnancies, were enrolled into the PIN study. Only pregnancies with complete dietary information were included in this study (n = 4227). Because women could have >1 pregnancy during the PIN study period, we randomly selected 1 pregnancy per woman to include in this analysis, resulting in a total sample of 3941. Study protocols and procedures were reviewed and approved in accordance with the ethical standards of the Institutional Review Board of the UNC School of Medicine and Wake Medical Center.
Assessment of preterm birth.
Preterm birth was the outcome of interest in this study, defined as birth before 37 completed weeks of gestation. Gestational age was estimated by combining last menstrual period with ultrasound data; 14 women with missing gestational age data were excluded. When both measures were available and there was agreement within 14 d, the last menstrual period was used to assign gestational age; otherwise, the ultrasound data were used. Preterm birth subtype was also examined in our study. Spontaneous or medically indicated preterm births were determined by 1 of 3 obstetricians. Spontaneous preterm births were preceded by either preterm labor or premature rupture of the membranes resulting in delivery, whereas medically indicated preterm births were defined as induction of labor or cesarean delivery without labor before 37 wk of gestation (20).
Ascertainment of dietary intake.
Dietary intake was assessed by using a self-administered Block FFQ collected at 26–29 wk of gestation, which was modified to incorporate local foods and to reflect the previous 3-mo period (21). This tool measured frequency of intakes and portion sizes by using a serving-size visual, allowing calculation of total consumption. Dietsys 4.0 software (National Cancer Institute) was used to analyze daily intakes from the 109-food-item FFQ used in PIN 1, with nutrient values based on data from the USDA’s 1994–1996 Continuing Survey of Food Intakes by Individuals for women aged 19–44 y and updated folate values for fortified foods from the USDA’s 1998 nutrient database. PIN 2 and PIN 3 used a 119-food-item FFQ, which was analyzed by using Dietsys+Plus version 5.6 with an updated food-composition table based on nutrient values from the third NHANES (NHANES III) and the USDA’s 1998 nutrient database. Because PIN 1 used a different FFQ than PIN 2 and PIN 3, only the food items common to both questionnaires were included in this analysis (95 food items). Food items that were rarely consumed (<10% consumption) were also excluded from the analyses (n = 10). Low-fat milk, including 1% and 2% milk, was combined into 1 group due to very low counts. A total of 84 food items were included in the analyses. Because the food items had nonnormal distributions due to a high frequency of nonconsumers, food variables were dichotomized as consumer vs. nonconsumer, which has been previously shown to be an appropriate alternative (22). We excluded women with daily energy intakes below the 2.5th or above the 97.5th percentiles (<1148 and >5748 kcal, respectively), which could be a result of misreporting, eating disorders, or measurement errors (n = 164).
Statistical analysis.
To empirically derive dietary patterns, factor analysis with principal factors was used with weighted least-squares estimation and orthogonal (Varimax) rotation. The number of retained factors was based on the scree plot of eigenvalues and the interpretability of factor loadings. For each retained dietary pattern, a factor score was computed for each woman by summing the food items consumed weighted by their factor loadings. The amount of variance explained by each dietary pattern was calculated by summing the squared factor loadings on that factor divided by the number of observed variables (95 food items). Factor loadings ≥0.30 (absolute value) were used to indicate the meaningful influence of the food item on the overall dietary pattern. To facilitate comparisons of our factor analysis results with future studies, we did not provide names for our patterns but instead described the main food components.
The DASH eating pattern was used as the hypothesis-oriented pattern (23). A previously developed scoring method was used to determine DASH adherence, in which participants received points on the basis of their quintile of intake for each of the 8 components (24). Briefly, high intakes of fruits, vegetables, nuts and legumes, low-fat dairy, and whole grains were given 1 point for each quintile ranking (e.g., lowest quintile = 1 point, highest quintile = 5 points). Sodium, red and processed meats, and sweetened beverage intakes were reverse-scored, in which the lowest quintile of intake received 5 points and the highest quintile of intake was assigned 1 point. The 8 food component scores were summed to derive a total DASH score for each participant, which could range from 8 to 40.
We further excluded women with missing data for federal poverty level (n = 393), prepregnancy BMI (n = 148), smoking status (n = 70), parity (n = 8), and educational level (n = 1), resulting in a total of 3143 participants in the analyses. Dietary pattern scores (factor and DASH) were divided into quartiles for analyses, with the highest quartile representing greater dietary pattern adherence. We compared factor and DASH scores according to baseline sociodemographic characteristics for each dietary pattern using ANOVA. Nutrient intakes for women with factor and DASH scores in quartiles 1 and 4 were compared by using ANOVA. Descriptive statistics were expressed as means ± SDs unless otherwise specified.
Logistic regression was used to examine the association between dietary pattern quartiles and preterm birth, with estimates presented as ORs and 95% CIs. Quartile 1 was the referent category in all regression analyses. Using multinomial logistic regression, we further investigated the association between dietary patterns and preterm birth subtype, which was treated as a 3-level variable (term birth, spontaneous preterm birth, and medically indicated preterm birth), in which ORs and 95% CIs were presented. We performed 2 separate regression analyses, one that included all 4 dietary patterns in the same model and a second with only the DASH diet in the model. All regression analyses were adjusted for maternal age (continuous), race (black vs. nonblack), maternal prepregnancy BMI (continuous), educational level (less than high school, high school, and more than high school), marital status (married vs. unmarried), parity (nulliparous vs. parous), and family income based on the 1996 federal poverty level (<185%, 185–350%, >350%), smoking status in the first 6 mo of pregnancy (smoker vs. nonsmoker), and energy intake (continuous) on the basis of a priori knowledge and directed acyclic graphs (25). We examined potential effect measure modification of the association by maternal race using stratified regression analyses. Furthermore, we performed sensitivity analyses to examine the impact of extreme energy intake (±5th percentile) on our study results. In addition, women in the PIN 1 and 2 cohorts were recruited from sites that were different than those for women in the PIN 3 cohort; therefore, we assessed the sensitivity of our results when the PIN 3 cohort was excluded. All reported P values were two-tailed, and P < 0.05 was considered significant. Exploratory factor analysis was performed by using STATA version 13 (StataCorp). All other analyses were conducted in SAS version 9.3 (SAS Institute).
Results
The exploratory factor analysis identified 4 distinct dietary patterns (Supplemental Table 1). Factor 1 was characterized by high factor loadings for fruits, tomatoes, broccoli, spinach, carrots, green salads, sweet potatoes, low-fat milk, yogurt, high-fiber and highly fortified cereals, nonfried chicken and fish, and wheat bread. Factor 2 included high factor loadings for beans, corn, French fries, hamburgers or cheeseburgers, white potatoes, fried chicken, spaghetti dishes, cheese dishes such as macaroni and cheese, cornbread or hushpuppies, processed meats, biscuits, and ice cream. Factor 3 had high factor loadings for foods such as collard greens, coleslaw or cabbage, red meats, fried chicken and fish, processed meats, cornbread or hushpuppies, eggs or egg biscuits, gravy, whole milk, and vitamin C–rich drinks such as Kool-Aid and Hi-C. Factor 4 was characterized by high factor loadings for shellfish, pizza, salty snacks, candies, pancakes, tacos or burritos, and cakes and cookies. These 4 patterns explained 52.9% of the variation in food items consumed. DASH scores ranged from 9 to 38 in our study population, with a mean of 24.3 ± 4.9. The percentile cutoffs corresponding to each variable were as follows: factor 1(25th:−0.57; 50th: −0.03; 75th: 0.56), factor 2 (25th: −0.67; 50th: −0.07; 75th: 0.56), factor 3 (25th: −0.60; 50th: −0.13; 75th: 0.44), factor 4 (25th: −0.58; 50th: −0.06; 75th: 0.56), and DASH (25th: 21; 50th: 24; 75th: 28).
Maternal characteristics according to dietary pattern scores are shown in Table 1. On average, women with greater adherence to factor 1, factor 4, and the DASH pattern were older, nonblack, had a higher educational level, and a higher income (P < 0.01). Women with greater adherence to factors 2 and 3 were more likely to be younger, black, unmarried, and smokers and have lower educational and income levels (P < 0.01). In addition, women with greater adherence to factors 2 and 3 were, on average, more likely to be obese (P < 0.01).
TABLE 1.
Factor and DASH adherence scores according to select maternal characteristics in 3143 pregnant women in the PIN study, 1996–20051
| n | Factor 1 | Factor 2 | Factor 3 | Factor 4 | DASH | |
| Age, y | ||||||
| <25 | 1056 | −0.30 ± 0.66 | 0.30 ± 0.79 | 0.08 ± 0.78 | −0.09 ± 0.77 | 22 ± 4 |
| 25–29 | 884 | 0.06 ± 0.79 | −0.09 ± 0.82 | −0.10 ± 0.08 | 0.07 ± 0.86 | 25 ± 5 |
| 30–34 | 814 | 0.28 ± 0.78 | −0.29 ± 0.79 | −0.14 ± 0.77 | 0.09 ± 0.87 | 26 ± 5 |
| ≥35 | 389 | 0.42 ± 0.75 | −0.36 ± 0.80 | −0.16 ± 0.79 | 0.12 ± 0.84 | 27 ± 5 |
| P | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Race | ||||||
| Nonblack | 2256 | 0.15 ± 0.79 | −0.15 ± 0.84 | −0.29 ± 0.68 | 0.14 ± 0.84 | 25 ± 5 |
| Black | 887 | −0.24 ± 0.72 | 0.24 ± 0.78 | 0.54 ± 0.74 | −0.26 ± 0.74 | 22 ± 5 |
| P | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Education, y | ||||||
| Less than high school | 389 | −0.41 ± 0.64 | 0.36 ± 0.73 | 0.16 ± 0.73 | −0.21 ± 0.74 | 21 ± 4 |
| High school | 713 | −0.25 ± 0.68 | 0.26 ± 0.82 | 0.15 ± 0.80 | −0.09 ± 0.80 | 22 ± 4 |
| More than high school | 2041 | 0.22 ± 0.79 | −0.23 ± 0.81 | −0.18 ± 0.78 | 0.11 ± 0.85 | 26 ± 5 |
| P | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Federal poverty level | ||||||
| <185% | 1295 | −0.21 ± 0.71 | 0.29 ± 0.77 | 0.14 ± 0.78 | −0.20 ± 0.75 | 23 ± 5 |
| 185–350% | 634 | 0.01 ± 0.79 | 0.00 ± 0.85 | 0.01 ± 0.86 | 0.07 ± 0.87 | 24 ± 5 |
| >350% | 1214 | 0.32 ± 0.78 | −0.42 ± 0.75 | −0.31 ± 0.70 | 0.24 ± 0.84 | 26 ± 4 |
| P | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Prepregnancy BMI, kg/m2 | ||||||
| <18.5 | 152 | 0.01 ± 0.79 | 0.19 ± 0.88 | 0.01 ± 0.73 | 0.03 ± 0.84 | 24 ± 5 |
| 18.5–24.9 | 1707 | 0.17 ± 0.81 | −0.15 ± 0.83 | −0.17 ± 0.77 | 0.07 ± 0.84 | 25 ± 5 |
| 25.0–29.9 | 607 | −0.08 ± 0.74 | 0.01 ± 0.83 | 0.02 ± 0.81 | 0.03 ± 0.82 | 24 ± 5 |
| ≥30.0 | 677 | −0.18 ± 0.72 | 0.13 ± 0.84 | 0.13 ± 0.81 | −0.08 ± 0.84 | 23 ± 5 |
| P | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Marital status | ||||||
| Married | 2005 | 0.20 ± 0.79 | −0.22 ± 0.81 | −0.22 ± 0.75 | 0.11 ± 0.85 | 25 ± 5 |
| Unmarried | 1138 | −0.24 ± 0.70 | 0.27 ± 0.80 | 0.22 ± 0.79 | −0.13 ± 0.79 | 22 ± 5 |
| P | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Parity | ||||||
| Nulliparous | 1593 | 0.10 ± 0.79 | −0.10 ± 0.83 | −0.15 ± 0.76 | 0.04 ± 0.82 | 25 ± 5 |
| Parous | 1550 | −0.02 ± 0.79 | 0.02 ± 0.85 | 0.03 ± 0.81 | 0.01 ± 0.85 | 24 ± 5 |
| P | <0.01 | <0.01 | < 0.01 | 0.43 | <0.01 | |
| Smoking status | ||||||
| Nonsmoker | 2537 | 0.11 ± 0.79 | −0.12 ± 0.83 | −0.09 ± 0.79 | 0.04 ± 0.84 | 25 ± 5 |
| Smoker | 606 | −0.25 ± 0.69 | 0.28 ± 0.80 | 0.05 ± 0.80 | −0.01 ± 0.80 | 22 ± 5 |
| P | <0.01 | <0.01 | < 0.01 | 0.20 | <0.01 |
Values are means ± SDs unless otherwise indicated. Higher scores indicate greater dietary pattern adherence. Mean factor and adherence scores were not adjusted for confounders. Factor 1 includes high factor loadings for fruits, tomatoes, broccoli, spinach, carrots, green salads, sweet potatoes, low-fat milk, yogurt, high-fiber and highly fortified cereals, nonfried chicken and fish, and wheat bread. Factor 2 includes high factor loadings for beans, corn, French fries, hamburgers or cheeseburgers, white potatoes, fried chicken, spaghetti dishes, cheese dishes such as macaroni and cheese, cornbread or hushpuppies, processed meats, biscuits, and ice cream. Factor 3 includes high factor loadings for collard greens, coleslaw or cabbage, red meats, fried chicken and fish, processed meats, cornbread or hushpuppies, eggs or egg biscuits, gravy, whole milk, and vitamin C–rich drinks. Factor 4 includes high factor loadings for shellfish, pizza, salty snacks, candies, pancakes, tacos or burritos, and cakes or cookies. P values <0.05 were considered significant. DASH, Dietary Approaches to Stop Hypertension; PIN, Pregnancy, Infection, and Nutrition.
Nutrient intakes according to factor and DASH score quartiles (quartile 1 compared with quartile 4) are presented in Table 2. On average, women with scores in the highest quartile of factors 1–4 reported higher energy intake and had higher intakes of sodium, folate, and vitamin C (P < 0.05). Women with better adherence to the DASH diet (quartile 4) had lower energy, saturated fat, and sodium intakes but higher intakes of fiber, folate, and vitamins C and A (P < 0.05).
TABLE 2.
Nutrient intake according to factor and DASH score quartile: PIN study, 1996–20051
| Factor 1 |
Factor 2 |
Factor 3 |
Factor 4 |
DASH |
||||||
| Q1 (n = 786) | Q4 (n = 785) | Q1 (n = 786) | Q4 (n = 785) | Q1 (n = 785) | Q4 (n = 785) | Q1 (n = 786) | Q4 (n = 785) | Q1 (n = 714) | Q4 (n = 837) | |
| Energy intake, kcal/d | 2310 ± 980 | 2610 ± 940* | 1870 ± 560 | 3240 ± 1080* | 2230 ± 820 | 2860 ± 1110* | 2130 ± 830 | 2700 ± 960* | 2720 ± 1080 | 2250 ± 750* |
| Saturated fat, g/d | 31 ± 16 | 30 ± 14* | 21 ± 8 | 43 ± 17* | 28 ± 13 | 36 ± 17* | 25 ± 13 | 35 ± 15* | 38 ± 18 | 24 ± 10* |
| Sodium, mg/d | 2680 ± 1260 | 3240 ± 1210* | 2340 ± 860 | 3790 ± 1360* | 2840 ± 1080 | 3310 ± 1410* | 2370 ± 1010 | 3460 ± 1200* | 3250 ± 1370 | 2700 ± 970* |
| Fiber, g/d | 15 ± 7 | 29 ± 11* | 17 ± 8 | 27 ± 12* | 21 ± 9 | 23 ± 12* | 22 ± 11 | 22 ± 9* | 17 ± 9 | 26 ± 10* |
| Folate, mg/d | 364 ± 162 | 619 ± 244* | 396 ± 175 | 598 ± 242* | 469 ± 206 | 529 ± 250* | 472 ± 235 | 505 ± 202* | 424 ± 199 | 551 ± 225* |
| Vitamin C, mg/d | 174 ± 132 | 270 ± 148* | 208 ± 112 | 233 ± 170* | 174 ± 107 | 288 ± 179* | 211 ± 146 | 235 ± 137* | 185 ± 131 | 246 ± 129* |
| Vitamin A, μg REs/d | 954 ± 534 | 2360 ± 1240* | 1360 ± 860 | 1870 ± 1180* | 1490 ± 840 | 1780 ± 1150* | 1600 ± 1100 | 1600 ± 989 | 1160 ± 732 | 2060 ± 1100* |
Values are means ± SDs; n = 3143. *Different from Q1, P < 0.05. Factor 1 includes high factor loadings for fruits, tomatoes, broccoli, spinach, carrots, green salads, sweet potatoes, low-fat milk, yogurt, high-fiber and highly fortified cereals, nonfried chicken and fish, and wheat bread. Factor 2 includes high factor loadings for beans, corn, French fries, hamburgers or cheeseburgers, white potatoes, fried chicken, spaghetti dishes, cheese dishes such as macaroni and cheese, cornbread or hushpuppies, processed meats, biscuits, and ice cream. Factor 3 includes high factor loadings for collard greens, coleslaw or cabbage, red meats, fried chicken and fish, processed meats, cornbread or hushpuppies, eggs or egg biscuits, gravy, whole milk, and vitamin C–rich drinks. Factor 4 includes high factor loadings for shellfish, pizza, salty snacks, candies, pancakes, tacos or burritos, and cakes or cookies. DASH, Dietary Approaches to Stop Hypertension; PIN, Pregnancy, Infection, and Nutrition; Q, quartile; RE, retinol equivalent.
Nearly 12% (n = 364) of the women delivered preterm, of which 175 were categorized as spontaneous and 167 as medically indicated preterm deliveries. Table 3 reports the associations between dietary patterns and preterm birth, adjusting for maternal age, race, maternal prepregnancy BMI status, educational level, household income, parity, marital status, smoking status, and energy intake. We found that a diet characterized by high consumption of beans, corn, French fries, hamburgers or cheeseburgers, white potatoes, fried chicken, spaghetti dishes, cheese dishes such as macaroni and cheese, cornbread or hushpuppies, processed meats, biscuits, and ice cream (factor 2) was associated with increased odds of preterm birth (adjusted OR: 1.53; 95% CI: 1.02, 2.30). Similarly, the odds of preterm birth were 1.55 times higher for women in the highest quartile of factor 3, compared with the lowest quartile, after adjusting for potential confounding factors (adjusted OR: 1.55; 95% CI: 1.07, 2.24). In contrast, greater adherence to the DASH diet (quartile 4) was associated with decreased odds of preterm birth compared with women in the lowest quartile (adjusted OR: 0.59; 95% CI: 0.40, 0.85). We did not find an association between factors 1, 2, and 4 and preterm birth. Associations were similar after excluding women with daily energy intakes at the ±5th percentile cutoff (1240.7 and 4464.7 kcal; data not shown). The odds of preterm birth for factor 3 increased as related to preterm birth when women from the PIN 3 cohort were excluded (adjusted OR: 1.94; 95% CI: 1.23, 3.06; data not shown). In the stratified analysis, we did not find any differences in the association between dietary patterns and preterm birth by race. Although the association between factor 3 and preterm birth appeared to be greater among black women, the CIs for both groups overlapped, indicating no difference in the effect estimates (data not shown).
TABLE 3.
Associations between dietary patterns and preterm birth: PIN study, 1996–20051
| Q1 | Q2 | Q3 | Q4 | |
| Factor 12 | ||||
| PTBs/pregnancies, n/n | 92/786 | 105/786 | 92/786 | 75/785 |
| Crude | 1.00 | 1.20 (0.88, 1.63) | 1.06 (0.77, 1.44) | 0.88 (0.63, 1.22) |
| Adjusted | 1.00 | 1.20 (0.88, 1.64) | 1.07 (0.77, 1.49) | 0.87 (0.60, 1.27) |
| Factor 23 | ||||
| PTBs/pregnancies, n/n | 80/786 | 77/785 | 99/787 | 108/785 |
| Crude | 1.00 | 0.98 (0.70, 1.37) | 1.29 (0.94, 1.78) | 1.41 (1.03, 1.93) |
| Adjusted | 1.00 | 1.01 (0.72, 1.42) | 1.37 (0.96 1.96) | 1.53 (1.02, 2.30) |
| Factor 34 | ||||
| PTBs/pregnancies, n/n | 73/785 | 87/786 | 92/787 | 112/785 |
| Crude | 1.00 | 1.24 (0.89, 1.73) | 1.33 (0.96, 1.86) | 1.62 (1.18, 2.23) |
| Adjusted | 1.00 | 1.25 (0.89, 1.74) | 1.32 (0.94, 1.86) | 1.55 (1.07, 2.24) |
| Factor 45 | ||||
| PTBs/pregnancies, n/n | 93/786 | 84/786 | 97/786 | 90/785 |
| Crude | 1.00 | 0.91 (0.66, 1.25) | 1.12 (0.82, 1.53) | 1.08 (0.79, 1.48) |
| Adjusted | 1.00 | 0.94 (0.68, 1.31) | 1.18 (0.85, 1.64) | 1.13 (0.79, 1.63) |
| DASH6 | ||||
| PTBs/pregnancies, n/n | 101/714 | 73/658 | 118/934 | 72/837 |
| Crude | 1.00 | 0.83 (0.61, 1.12) | 0.90 (0.69, 1.19) | 0.55 (0.39, 0.78) |
| Adjusted | 1.00 | 0.85 (0.63, 1.16) | 0.96 (0.72, 1.29) | 0.59 (0.40, 0.85) |
Values are ORs (95% CIs) unless otherwise indicated; n = 3143. Regression analysis for factors 1–4 includes all factors in the same model and adjusted for maternal age, race, maternal prepregnancy BMI status, educational level, household income, parity, marital status, smoking status, and energy intakes. DASH, Dietary Approaches to Stop Hypertension; PIN, Pregnancy, Infection, and Nutrition; PTB, preterm birth; Q, quartile.
Factor 1 includes high factor loadings for fruits, tomatoes, broccoli, spinach, carrots, green salads, sweet potatoes, low-fat milk, yogurt, high-fiber and highly fortified cereals, nonfried chicken and fish, and wheat bread. Quartile medians (range): Q1 = −0.85 (−1.83, −0.56), Q2 = −0.29 (−0.56, −0.03), Q3 = 0.2 (−0.03, 0.56), and Q4 = 1.0 (0.56, 2.85).
Factor 2 includes high factor loadings for beans, corn, French fries, hamburgers or cheeseburgers, white potatoes, fried chicken, spaghetti dishes, cheese dishes such as macaroni and cheese, cornbread or hushpuppies, processed meats, biscuits, and ice cream. Quartile medians (range): Q1 = −1.00 (−2.63, −0.67), Q2 = −0.37 (−0.67, −0.07), Q3 = 0.23 (−0.07, 0.57), and Q4 = 1.00 (0.57, 2.67).
Factor 3 includes high factor loadings for collard greens, coleslaw or cabbage, red meats, fried chicken and fish, processed meats, cornbread or hushpuppies, eggs or egg biscuits, gravy, whole milk, and vitamin C–rich drinks. Quartile median (range): Q1 = −0.94 (−2.20, −0.60), Q2 = −0.35 (−0.60, −0.13), Q3 = 0.14 (−0.13, 0.44), and Q4 = 0.90 (0.45, 2.92).
Factor 4 includes high factor loadings for shellfish, pizza, salty snacks, candies, pancakes, tacos or burritos, and cakes or cookies. Quartile median (range): Q1 = −0.91 (−2.30, −0.57), Q2 = −0.31 (−0.58, −0.06), Q3 = 0.23 (−0.06, 0.56), and Q4 = 1.05 (0.56, 3.18).
Quartile median (range): Q1 = 18 (9, 20), Q2 = 22 (21, 23), Q3 = 25 (24, 27), and Q4 = 30 (28, 38).
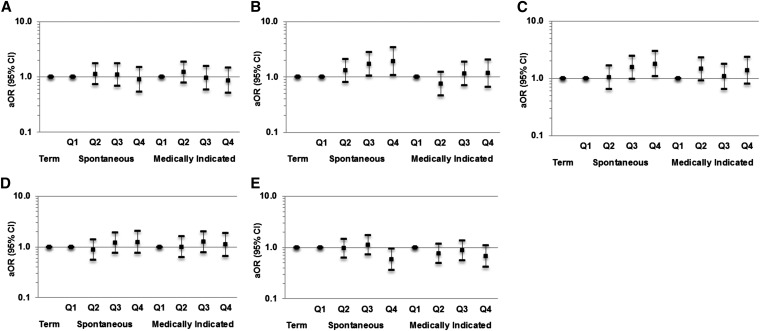
We further investigated the association between dietary pattern and preterm birth subtype (Figure 1). In the multinomial logistic regression, women with factor scores in the highest quartile of factors 2 and 3 were nearly 2 times as likely to have a spontaneous preterm birth than were women with scores in the lowest quartile [adjusted ORs (95% CI) for factors 2 and 3: 1.92 (1.08, 3.41) and 1.78 (1.07, 2.96), respectively]. We also found some indication of a decrease in the odds of spontaneous preterm birth for women in the highest quartile of the DASH diet after adjusting for potential confounders (adjusted OR: 0.58; 95% CI: 0.36, 0.95).
FIGURE 1.
Multinomial logistic regression analysis of the association between maternal dietary patterns and spontaneous and medically indicated preterm birth. (A) Factor 1, (B) factor 2, (C) factor 3, (D) factor 4, and (E) DASH diet in the PIN study, 1995–2005 (n = 3120). Multinomial logistic regression models adjusted for maternal age, race, maternal prepregnancy BMI status, educational level, household income, parity, marital status, smoking status, and energy intake. Values are aORs (95% CIs). Regression analysis for factors 1–4 includes all factors in the same model. Factor 1 includes high factor loadings for fruits, tomatoes, broccoli, spinach, carrots, green salads, sweet potatoes, low-fat milk, yogurt, high-fiber and highly fortified cereals, nonfried chicken and fish, and wheat bread. Factor 2 includes high factor loadings for beans, corn, French fries, hamburgers or cheeseburgers, white potatoes, fried chicken, spaghetti dishes, cheese dishes such as macaroni and cheese, cornbread or hushpuppies, processed meats, biscuits, and ice cream. Factor 3 includes high factor loadings for collard greens, coleslaw or cabbage, red meats, fried chicken and fish, processed meats, cornbread or hushpuppies, eggs or egg biscuits, gravy, whole milk, and vitamin C–rich drinks. Factor 4 includes high factor loadings for shellfish, pizza, salty snacks, candies, pancakes, tacos or burritos, and cakes or cookies. aOR, adjusted OR; DASH, Dietary Approaches to Stop Hypertension; PIN, Pregnancy, Infection, and Nutrition; Q, quartile.
Discussion
We identified 4 dietary patterns from factor analysis, of which adherence to a pattern characterized by high intakes of beans, corn, French fries, hamburgers or cheeseburgers, white potatoes, fried chicken, spaghetti dishes, cheese dishes such as macaroni and cheese, cornbread or hushpuppies, processed meats, biscuits, and ice cream (factor 2) or high consumption of collard greens, coleslaw or cabbage, red and processed meats, fried chicken, fried fish, cornbread or hushpuppies, eggs or egg biscuits, gravy, whole milk, and vitamin C–rich drinks (factor 3) was associated with increase odds of preterm birth. This association was mainly related to spontaneous preterm deliveries. Furthermore, better adherence to the DASH diet, a diet that is consistent with the Dietary Guidelines for Americans, was associated with a decrease in the odds of preterm birth.
Numerous mechanisms could possibly explain the findings of our study. Women in our study with factor scores in the highest quartile of factors 2 and 3, dietary patterns high in refined grains, processed meats, and sweetened beverages, had significantly higher intakes of saturated fat than did women in the lowest quartile, which is known to be a proinflammatory marker (26). We hypothesize that following a dietary pattern that promotes inflammation may increase the possibility of preterm birth by limiting the transfer of nutrients for adequate fetal growth. Furthermore, we postulate that women with greater adherence to the DASH diet, which was associated with higher amounts of folate, fiber, and vitamin A from foods such as fruits and vegetables, reduces the risk of preterm birth by reducing inflammation and promoting fetal growth. Although we have identified some nutrients and foods that could explain a biological mechanism for preterm birth, it is important to keep in mind that it is often not possible to separate the effects of individual nutrients and foods but rather it is the composition of the overall diet and the grouping of foods in certain amounts that may explain the synergistic and cumulative effects on health and disease (27).
To our knowledge, this is the first study to examine the relation between adherence to the DASH diet and preterm birth. We found that greater adherence to the DASH diet during pregnancy lowered the odds of preterm birth. The DASH diet, which is just one of several dietary indexes, has been shown to be effective in improving metabolic profiles in pregnant women (28), which could be part of the mechanistic explanation. The Mediterranean-style diet, like the DASH diet, also encourages high consumption of fruits, vegetables, legumes, and grains; moderate consumption of fish and dairy products; and low meat intake (8, 9). Despite the common aspects of these dietary patterns, previous studies examining a Mediterranean-style diet produced inconsistent findings in relation to preterm birth. In a prospective cohort study in pregnant Danish women, a decrease in the risk of early preterm birth (defined as birth before 35 wk of gestation) was found to be related to meeting all of the criteria for the Mediterranean-style diet during pregnancy (OR: 0.28; 95% CI: 0.11, 0.76); however, no association was found with birth before 37 wk of gestation (8). In a separate study in a cohort of pregnant Norwegian women, no association was found between the Mediterranean-style diet and preterm birth (6). In a retrospective study in women from a French Caribbean Mother-Child Cohort Study (TIMOUN), adherence to a Mediterranean-style diet was associated with a lower risk of preterm birth only in overweight and obese women (OR: 0.70; 95% CI: 0.60, 0.90) (29). With the use of a Mediterranean Diet Score and the Diet Quality Index, no association with preterm birth was found for either of the indexes in the year before pregnancy in the National Birth Defects Prevention Study (7). The conflicting study results may be a consequence of regional variations in the Mediterranean diet due to social, cultural, and geographical differences leading to varying definitions of the Mediterranean diet across studies (30, 31). Also, the timing of diet assessment (during pregnancy vs. before pregnancy) and data collection (during pregnancy vs. after birth) also differed, which may have contributed to the inconsistent findings.
Similar to a previously published study that used factor analysis based only on our PIN 3 cohort, we identified 3 dietary patterns that followed a prudent, Western, and southern diet (32). A slightly different fourth dietary pattern was identified in our study, which consisted of high intakes of shellfish, pizza, salty snacks, candies, pancakes, tacos or burritos, and cakes and cookies. The emergence of this fourth pattern is likely due to differences in sociodemographic characteristics found between the PIN 1 and 2 cohorts and the PIN 3 cohort. The PIN 1 and 2 cohorts contributed a higher proportion of black women and women with lower educational and income levels to the total sample than the PIN 3 cohort. For instance, black women from the PIN 1 and 2 cohorts represented 23% of the total sample, whereas black women from the PIN 3 cohort represented only 5% of the total sample. The differences in maternal characteristics between study cohorts could also explain the change in the association between factor 3 and preterm birth observed in the sensitivity analysis when the PIN 3 cohort was excluded from regression analyses.
An increase in the odds of preterm birth was found to be related to a dietary pattern consisting of high intakes of beans, corn, French fries, hamburgers or cheeseburgers, white potatoes, fried chicken, spaghetti dishes, cheese dishes such as macaroni and cheese, cornbread or hushpuppies, processed meats, biscuits, and ice cream and a dietary pattern characterized by high consumption of collard greens, coleslaw/cabbage, red and processed meats, fried chicken, fried fish, cornbread or hushpuppies, eggs or egg biscuits, gravy, whole milk, and vitamin C–rich drinks. Likewise, Rasmussen et al. (13) reported higher odds of preterm birth among women following a similar dietary pattern called the Western diet. In a study in women participating in the Norwegian Mother and Child Cohort Study, Englund-Ögge et al. (12) found a lower risk of preterm birth for women following a prudent or traditional dietary pattern. The prudent and traditional dietary patterns had characteristics similar to the DASH diet; the former consisted of high intakes of vegetables, salad, onions/leeks/garlic, fruits and berries, nuts, vegetable oils, whole-grain cereals, poultry, whole grains, and fiber-rich breads, but low intakes of processed meats, white bread, and pizza/tacos, whereas the latter was characteristic of a high consumption of boiled potatoes, fish products, gravy, lean fish, margarine, rice pudding, low-fat milk, and cooked vegetables.
Several points should be considered when interpreting the results of our study. The critical timing for the effect of maternal diet on preterm birth remains unknown. In this study, we ascertained diet at 26–29 wk of gestation. It may be that preconception diet is also important, as shown previously by Grieger et al. (14); however, previous research suggests that there are minimal changes in dietary patterns during pregnancy from preconception (33, 34). FFQs are valid and reliable instruments to assess dietary patterns over a period of time (35). Despite women being asked to report dietary intakes over the previous 3 mo, it is possible that the most recent intakes could influence reports. In addition, FFQs also limit the number and types of foods assessed, which may influence the creation of patterns or scores. Because our study was observational, conclusions about causality cannot be drawn. However, a previous randomized trial in a small sample of Norwegian pregnant women found that a diet such as the DASH diet aimed at increasing the intakes of fruits, vegetables, legumes, whole grains, fish, low-fat meats, dairy products, and oils and reduced the rate of preterm birth by 90% compared with the control group (11). We understand that complete-covariate analysis is just one of many methods to account for missing data. We believe that a complete-covariate analysis is appropriate in this study because we observed not more than 10% of missing data for any of the covariates (federal poverty level: 10%; BMI: 4%; smoking status during pregnancy: 2%; parity and education: <1%). Therefore, it is unlikely that our results are sensitive to the missing data. A large proportion of our study population included white women of high socioeconomic status, which may limit the generalizability of our findings. We were able to adjust for several potential confounding factors; however, we are not able to dismiss the possible influence of unmeasured confounding on our results. Maternal race/ethnicity is an important covariate in this study. Due to limited sample sizes, we were unable to separate Hispanics and other racial/ethnic groups from the nonblack category. We explored the sensitivity of our results to classifying white women separately from Hispanic or other racial/ethnic groups by performing regression analyses including only women classified as either non-Hispanic black or non-Hispanic white. We did not observe any significant differences in the results. Last, although black women accounted for 28% of our study population, we recognize that the distribution within each factor and DASH quartile may not be sufficient to completely eliminate residual confounding.
In this prospective cohort study of dietary patterns during the second trimester and preterm birth, we were able to show that diet quality during pregnancy was associated with preterm birth. Specifically, greater adherence to a healthy dietary pattern, such as the DASH diet, reduced the odds of preterm birth, whereas greater adherence to a dietary pattern of poorer quality, such as factors 2 and 3, increased the odds of preterm birth. The findings highlight the importance of dietary counseling during pregnancy as it relates to preterm birth. Diet quality is an important modifiable risk factor that could be a useful tool in dietary interventions and strategies aimed to improve birth outcomes.
Acknowledgments
CLM conceptualized the study, analyzed and interpreted the data, and wrote the manuscript; DS-A assisted with data analysis and interpretation of the results; and AMS-R was the co-investigator of the PIN studies, was responsible for the acquisition of the data, and assisted with the design, analysis, and interpretation of the results for this manuscript. All authors provided intellectual input into the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: DASH, Dietary Approaches to Stop Hypertension; PIN, Pregnancy, Infection, and Nutrition; UNC, University of North Carolina.
References
- 1.Matthews TJ, MacDorman MF. Infant mortality statistics from the 2010 period linked birth/infant death data set. Natl Vital Stat Rep 2014;63:1–20. [PubMed] [Google Scholar]
- 2.Hamilton BE, Martin JA, Osterman MJ, Curtin SC. Births: preliminary data for 2013. Natl Vital Stat Rep 632014. [Google Scholar]
- 3.Blumfield ML, Hure AJ, MacDonald-Wicks LK, Smith R, Simpson SJ, Giles WB, Raubenheimer D, Collins CE. Dietary balance during pregnancy is associated with fetal adiposity and fat distribution. Am J Clin Nutr 2012;96:1032–41. [DOI] [PubMed] [Google Scholar]
- 4.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- 5.Newby PK, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev 2004;62:177–203. [DOI] [PubMed] [Google Scholar]
- 6.Haugen M, Meltzer HM, Brantsaeter AL, Mikkelsen T, Osterdal ML, Alexander J, Olsen SF, Bakketeig L. Mediterranean-type diet and risk of preterm birth among women in the Norwegian Mother and Child Cohort Study (MoBa): a prospective cohort study. Acta Obstet Gynecol Scand 2008;87:319–24. [DOI] [PubMed] [Google Scholar]
- 7.Carmichael SL, Yang W, Shaw GM; National Birth Defects Prevention Study. Maternal dietary nutrient intake and risk of preterm delivery. Am J Perinatol 2013;30:579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikkelsen TB, Osterdal ML, Knudsen VK, Haugen M, Meltzer HM, Bakketeig L, Olsen SF. Association between a Mediterranean-type diet and risk of preterm birth among Danish women: a prospective cohort study. Acta Obstet Gynecol Scand 2008;87:325–30. [DOI] [PubMed] [Google Scholar]
- 9.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr 1995;61(Suppl):1402S–6S. [DOI] [PubMed] [Google Scholar]
- 10.Trichopoulou A, Lagiou P. Healthy traditional Mediterranean diet: an expression of culture, history, and lifestyle. Nutr Rev 1997;55:383–9. [DOI] [PubMed] [Google Scholar]
- 11.Khoury J, Henriksen T, Christophersen B, Tonstad S. Effect of a cholesterol-lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: a randomized clinical trial. Am J Obstet Gynecol 2005;193:1292–301. [DOI] [PubMed] [Google Scholar]
- 12.Englund-Ögge L, Brantsaeter AL, Sengpiel V, Haugen M, Birgisdottir BE, Myhre R, Meltzer HM, Jacobsson B. Maternal dietary patterns and preterm delivery: results from large prospective cohort study. BMJ 2014;348:g1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasmussen MA, Maslova E, Halldorsson TI, Olsen SF. Characterization of dietary patterns in the Danish national birth cohort in relation to preterm birth. PLoS ONE 2014;9:e93644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grieger JA, Grzeskowiak LE, Clifton VL. Preconception dietary patterns in human pregnancies are associated with preterm delivery. J Nutr 2014;144:1075–80. [DOI] [PubMed] [Google Scholar]
- 15.Balder HF, Virtanen M, Brants HA, Krogh V, Dixon LB, Tan F, Mannisto S, Bellocco R, Pietinen P, Wolk A, et al. Common and country-specific dietary patterns in four European cohort studies. J Nutr 2003;133:4246–51. [DOI] [PubMed] [Google Scholar]
- 16.Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc 2009;109:1004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Northstone K, Emmett PM, Rogers I. Dietary patterns in pregnancy and associations with nutrient intakes. Br J Nutr 2008;99:406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savitz DA, Dole N, Williams J, Thorp JM, McDonald T, Carter AC, Eucker B. Determinants of participation in an epidemiological study of preterm delivery. Paediatr Perinat Epidemiol 1999;13:114–25. [DOI] [PubMed] [Google Scholar]
- 19.Mehta UJ, Siega-Riz AM, Herring AH. Effect of body image on pregnancy weight gain. Matern Child Health J 2011;15:324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vahratian A, Siega-Riz AM, Savitz DA, Thorp JM Jr. Multivitamin use and the risk of preterm birth. Am J Epidemiol 2004;160:886–92. [DOI] [PubMed] [Google Scholar]
- 21.Siega-Riz AM, Bodnar LM, Savitz DA. What are pregnant women eating? Nutrient and food group differences by race. Am J Obstet Gynecol 2002;186:480–6. [DOI] [PubMed] [Google Scholar]
- 22.Smith AD, Emmett PM, Newby PK, Northstone K. Dietary patterns obtained through principal components analysis: the effect of input variable quantification. Br J Nutr 2013;109:1881–91. [DOI] [PubMed] [Google Scholar]
- 23.Sacks FM, Obarzanek E, Windhauser MM, Svetkey LP, Vollmer WM, McCullough M, Karanja N, Lin PH, Steele P, Proschan MA, et al. Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH): a multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol 1995;5:108–18. [DOI] [PubMed] [Google Scholar]
- 24.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–20. [DOI] [PubMed] [Google Scholar]
- 25.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37–48. [PubMed] [Google Scholar]
- 26.Santos S, Oliveira A, Lopes C. Systematic review of saturated fatty acids on inflammation and circulating levels of adipokines. Nutr Res 2013;33:687–95. [DOI] [PubMed] [Google Scholar]
- 27.Dietary Guidelines Advisory Committee. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010, to the Secretary of Agriculture and the Secretary of Health and Human Services. Washington (DC): USDA, Agricultural Research Service; 2010. [cited 2015 Jan 23]. Available from: http://www.cnpp.usda.gov/DGAs2010-DGACReport.htm.
- 28.Asemi Z, Tabassi Z, Samimi M, Fahiminejad T, Esmaillzadeh A. Favourable effects of the Dietary Approaches to Stop Hypertension diet on glucose tolerance and lipid profiles in gestational diabetes: a randomised clinical trial. Br J Nutr 2013;109:2024–30. [DOI] [PubMed] [Google Scholar]
- 29.Saunders L, Guldner L, Costet N, Kadhel P, Rouget F, Monfort C, Thome JP, Multigner L, Cordier S. Effect of a Mediterranean diet during pregnancy on fetal growth and preterm delivery: results from a French Caribbean Mother-Child Cohort Study (TIMOUN). Paediatr Perinat Epidemiol 2014;28:235–44. [DOI] [PubMed] [Google Scholar]
- 30.Martínez-González MA, Holgado B, Gibney M, Kearney J, Martinez JA. Definitions of healthy eating in Spain as compared to other European member states. Eur J Epidemiol 2000;16:557–64. [DOI] [PubMed] [Google Scholar]
- 31.Noah A, Truswell AS. There are many Mediterranean diets. Asia Pac J Clin Nutr 2001;10:2–9. [DOI] [PubMed] [Google Scholar]
- 32.Sotres-Alvarez D, Herring AH, Siega-Riz AM. Latent class analysis is useful to classify pregnant women into dietary patterns. J Nutr 2010;140:2253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM. Women's dietary patterns change little from before to during pregnancy. J Nutr 2009;139:1956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cucó G, Fernandez-Ballart J, Sala J, Viladrich C, Iranzo R, Vila J, Arija V. Dietary patterns and associated lifestyles in preconception, pregnancy and postpartum. Eur J Clin Nutr 2006;60:364–71. [DOI] [PubMed] [Google Scholar]
- 35.Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 1999;69:243–9. [DOI] [PubMed] [Google Scholar]