Abstract
Background: Little is known about the influence of antiretroviral therapy with or without micronutrient supplementation on the micronutrient concentrations of HIV-infected lactating women in resource-constrained settings.
Objective: We examined associations of highly active antiretroviral therapy (HAART) and lipid-based nutrient supplements (LNS) with concentrations of selected micronutrients in HIV-infected Malawian women at 24 wk postpartum.
Methods: Plasma micronutrient concentrations were measured in a subsample (n = 690) of Breastfeeding, Antiretrovirals, and Nutrition (BAN) study participants who were randomly assigned at delivery to receive HAART, LNS, HAART+LNS, or no HAART/no LNS (control). HAART consisted of protease inhibitor–based triple therapy. LNS (140 g/d) met energy and micronutrient requirements of lactation. Multivariable linear regression tested the association of HAART and LNS, plus their interaction, with micronutrient concentrations, controlling for season, baseline viral load, and baseline CD4 count.
Results: We found significant HAART by LNS interactions for folate (P = 0.051), vitamin B-12 (P < 0.001), and transferrin receptors (TfRs) (P = 0.085). HAART was associated with lower folate (with LNS: −27%, P < 0.001; without LNS: −12%, P = 0.040) and higher TfR concentrations (with LNS: +14%, P = 0.004; without LNS: +28%, P < 0.001), indicating iron deficiency. LNS increased folate (with HAART: +17%, P = 0.037; without HAART: +39%, P < 0.001) and decreased TfR concentrations (with HAART only: −12%, P = 0.023). HAART was associated with lower vitamin B-12 concentrations only when LNS was present (−18%, P = 0.001), whereas LNS increased vitamin B-12 only when no HAART was present (+27%, P < 0.001). HAART, but not LNS, was associated with higher retinol-binding protein (RBP; +10%, P = 0.007). We detected no association of HAART or LNS with selenium, ferritin, or hemoglobin.
Conclusion: The association of HAART with lower folate, iron deficiency, and higher RBP plus the attenuation of LNS effects on folate and vitamin B-12 when combined with HAART has implications for the health of lactating HIV-infected women taking HAART in prevention of mother-to-child transmission programs. This trial was registered at clinicaltrials.gov as NCT00164736.
Keywords: highly active antiretroviral therapy, lipid-based nutrient supplements, micronutrient, HIV, mothers
Introduction
The WHO’s latest update on the use of antiretrovirals for the prevention of mother-to-child transmission (PMTCT)12 has precipitated a shift toward the provision of antiretrovirals to mothers rather than to infants in African countries with high HIV prevalence (1, 2). More than 1.5 million women are now eligible to receive highly active antiretroviral therapy (HAART), containing a combination of 3 drugs, either during pregnancy and breastfeeding (Option B) or starting in pregnancy and continuing for life (Option B+) (3). Although these strategies have clear benefits for preventing vertical transmission, the long-term effects of HAART on the micronutrient status of women participating in PMTCT programs are not well characterized.
Marginal micronutrient status and deficiencies are common among HIV-infected women in resource-limited countries (4–7). In patients with low CD4 counts, HAART has a beneficial impact on micronutrient status through reductions in anemia (8–10) and vitamin A (11) and selenium deficiency (12). It is not known if these positive effects of HAART on the status of some micronutrients occur in individuals with higher CD4 and/or minimal HIV disease progression, like many mothers in PMTCT programs. Some negative effects of antiretrovirals on micronutrient concentrations have been reported, particularly for vitamins D (13, 14) and B-12 (15, 16). Negative associations of antiretrovirals used for PMTCT with maternal micronutrient concentrations have implications for the health of the mother and her child, through micronutrient stores obtained in utero and micronutrients available in breast milk. In the pre-HAART era, micronutrient supplementation provided to people living with HIV improved their hematologic status (17–25). However, the association of micronutrient supplementation with plasma micronutrient concentrations in lactating women receiving HAART is unknown.
This article presents data from a selected subsample of women who participated in the Breastfeeding, Antiretrovirals, and Nutrition (BAN) study in Malawi. We examine the association of micronutrient-fortified lipid-based nutrient supplements (LNS) and protease inhibitor–based HAART regimens on maternal micronutrient concentrations. We focus on associations at 24 wk, the time at which participants had their longest exposure to study interventions.
Methods
Subjects and procedures.
From 2004 to 2009, HIV-1–infected, pregnant women were recruited into the BAN study at antenatal clinics in Lilongwe, Malawi. They received the standard of care during pregnancy from local health facilities. Mother-infant pairs were eligible for enrollment at delivery if infants had a birth weight ≥2 kg and mothers had a CD4 count ≥250 cells/mm3 (≥200 cells/mm3 until July 2006), hemoglobin ≥ 70 g/L, and no previous antiretroviral use (26).
At delivery, women were randomly assigned to the following maternal interventions: HAART, LNS, HAART+LNS, and no HAART/no LNS (control). Micronutrient biomarkers were analyzed in a subsample of stored plasma from BAN participants. We used all 24-wk specimens with a matched infant sample and available anthropometric and dietary data. Mothers were excluded if they had multiple births or their infants became HIV-positive, because we also planned to use the subsample to analyze the relation between maternal supplementation and infant growth. Multiples and HIV-positive infants have different rates of growth than do singletons and HIV-negative infants.
Nutrition and antiretroviral interventions were provided from delivery through 28 wk postpartum. Mothers were counseled to exclusively breastfeed from 0 to 24 wk and to wean their infants between 24 and 28 wk. LNS (Nutriset), containing peanut paste, nonfat milk powder, sugar, vegetable oil, and micronutrient mix, was used for the nutrition intervention. The daily LNS dose (140 g) was designed to supply the additional energy, protein, and micronutrient needs of lactation. It provided 3120 kJ (746 kcal) of energy, 20.8 g protein, and 16 vitamins and minerals. The complete nutrient content of the LNS is described elsewhere (27). Briefly, it contained 300 μg folic acid (0.6 times the RDA for lactating women), 2.6 μg cyanocobalamin (0.9 times the RDA), 75 μg sodium selenite (1.3 times the RDA), and 15 mg ferrous sulfate (1.7 times the RDA) (28–30). The LNS did not contain vitamin A due to evidence available before the start of the study that it could increase HIV transmission through breast milk (31).
The maternal antiretroviral intervention was a HAART regimen containing 3 drugs (32). All women assigned to the antiretroviral arms received lamivudine/zidovudine as a single pill (Combivir; Glaxo-SmithKline) throughout the intervention period (0–28 wk). In addition, the first 39 BAN participants randomly assigned to antiretrovirals received nevirapine as their study drug. The study switched to the second-line drug, nelfinavir (Viracept; Roche), which was given to the next 146 women, after the FDA issued a black box warning concerning the use of nevirapine in women with a CD4 count >250 cells/mm3. A further change was made to lopinavir/ritonavir (LPVr; Kaletra; Abbott) for reasons of availability, safety, and potency. Nelfinavir and LPVr are protease inhibitors, which have side effects including nausea, diarrhea, increased lipids, and lipodystrophy (33). In our micronutrient subsample, 3 women switched from nevirapine to nelfinavir, one switched from nelfinavir to LPVr, and one switched from LPVr to nelfinavir (due to reactions to LPVr). We coded these women as taking the drug used at the 24-wk visit.
Venous blood samples were collected at 24 wk postpartum. Plasma was separated from RBCs within 60 min, separated into aliquots in polypropylene storage tubes, and kept at −70°C. Mothers were asked to report their adherence to the LNS at 1, 4, 8, 12, and 21 wk. Adherence to HAART was based on pill counts at 4, 12, and 18 wk. This was calculated by using the following formula: (number of pills distributed at previous visit – number of pills returned at current visit)/(days between visits × pills prescribed per day). Adherence to the LNS regimen was obtained by questionnaire during regular study visits. Mothers were asked how much of the supplement they ate yesterday in half-packet increments, ranging from none to 2 packets (the full daily dose). A questionnaire on socioeconomic characteristics was administered to mothers during screening.
At the time when this study began, there was not yet evidence that antiretrovirals were effective at preventing HIV transmission through breast milk and it was acceptable to have study groups without drugs. In 2008, the study’s data safety and monitoring board stopped enrollment in the control arm when it became clear that use of antiretrovirals prevented transmission. Ethical approval for the study was obtained from the Malawi National Health Science Research Committee and the institutional review boards at the University of North Carolina at Chapel Hill, the US CDC, and the University of California, Davis. The trial was registered at clinicaltrials.gov (NCT00164736).
Laboratory analysis.
Plasma concentrations of most micronutrient biomarkers were measured at the USDA–Agricultural Research Service Western Human Nutrition Research Center. Vitamin B-12 and folate were analyzed by using the SimulTRAC-SNB Radioassay Kit [vitamin B-12 (57Co)/folate (12585 I); MP Biomedicals]. The analysis of retinol-binding protein (RBP) was performed with the Human Retinol BP ELISA, an immunoperoxidase assay for the determination of RBP in human sera (Immunology Consultants Laboratory). Transferrin receptors (TfRs) and inflammatory markers [C-reactive protein (CRP) and α-1-acid glycoprotein (AGP)] were measured by using a Cobas Integra 400+ analyzer (Roche Diagnostics). Ferritin concentrations were determined with the IRMA Ferritin Coat-a-Count radioimmunoassay (Siemens Health Care Diagnostics).
Selenium concentrations were analyzed at the USDA–Agricultural Research Service Grand Forks Human Nutrition Research Center by automated electrothermal atomic absorption spectrophotometry. Hemoglobin was measured in whole blood in Lilongwe by using a Beckman Coulter AcT 5-part Differential Analyzer (Beckman Coulter).
Statistical analysis.
Differences in background characteristics of participants and adherence to LNS and HAART by study group were examined by using ANOVA for continuous variables and chi-square tests for categorical variables. Natural-log transformations were used for all micronutrient outcome variables because they followed non-Gaussian distributions; outcomes were modeled as continuous variables. Multivariable linear regression was used to test associations between the LNS and HAART interventions and maternal plasma micronutrient concentrations. Adjusted geometric means for each intervention group and ratios for pairs of interventions (e.g., HAART vs. no HAART) and their 95% CIs were calculated from the models. A HAART × LNS interaction term was included in all initial models and retained if P < 0.10. For micronutrients with significant HAART × LNS interactions, exploratory analyses were conducted to examine possible differential effects of regimens containing LPVr + Combivir or nelfinavir + Combivir. In exploratory models, we estimated ratios of geometric means for pairs of groups (e.g., LPVr vs. no HAART among women receiving LNS). All models controlled for baseline CD4 count and log10 viral load as continuous variables. Season at the time of the 24-wk visit was also included in the models to control for potential differences in dietary intake and to account for the possibility that calendar time was related to the outcomes. Season was included as a binary variable denoting the presence or absence of the food-insecure period of the year (during the rainy season) based on the month and date of the woman’s study visit. Approximately 10% of the analysis sample received either sulfadoxine-pyrimethimine or cotrimoxazole (drugs with folate-inhibiting properties) during the 3 wk preceding the study visit when blood was collected. Consequently, the presence or absence of folate-inhibiting drugs was included in the folate model. To better understand the role of inflammation on the association of antiretrovirals with micronutrients, we compared multivariable linear regression models with and without markers of inflammation (measured as log CRP and log AGP and modeled as continuous variables) for biomarkers that are known to be influenced by the acute phase response (selenium, RBP, ferritin, TfR, and hemoglobin) (34).
Results
Of 709 women selected for the micronutrient subsample at 24 wk, 18 were dropped from the analysis. Nine of these stopped taking their drugs before 24 wk and 9 were taking nevirapine, a sample that was too small to produce stable estimates in regression models. There were no significant differences by study group in age, level of education, number of pregnancies, BMI, baseline viral load or CD4 count, anemia, high CRP, or high AGP (Table 1). As expected, we found significantly lower median CD4 counts and percentage of CD4 <250 cells/mm3 among women in the groups that received no HAART at 24 wk compared with those who received HAART. Characteristics of mothers in the micronutrient subsample compared with those of other BAN participants are shown in Supplemental Table 1.
TABLE 1.
Characteristics of mothers in the micronutrient analysis subsample of the BAN study1
| No LNS/no HAART(n = 237) | LNS(n = 238) | HAART(n = 104) | LNS+HAART(n = 111) | P | |
| Age, y | 26.6 ± 5.0 | 26.6 ± 5.1 | 27.3 ± 4.9 | 26.0 ± 5.2 | 0.36 |
| More than primary education, % | 34 | 39 | 37 | 35 | 0.64 |
| Number of pregnancies | 3.4 ± 1.7 | 3.3 ± 1.6 | 3.4 ± 1.4 | 3.4 ± 1.8 | 0.96 |
| BMI | |||||
| At 24 wk, kg/m2 | 22.7 ± 2.8 | 22.8 ± 3.4 | 22.3 ± 3.3 | 22.3 ± 2.6 | 0.29 |
| <18.5 kg/m2 at 24 wk, % | 4 | 4 | 7 | 6 | 0.62 |
| Viral load at baseline,2 log10 copies | 4.1 ± 0.9 | 4.1 ± 0.9 | 4.2 ± 0.9 | 4.2 ± 0.9 | 0.93 |
| CD4 | |||||
| At baseline,2 cells/μL | 428 (327, 577) | 444 (323, 600) | 460 (304, 571) | 449 (336, 580) | 0.90 |
| At 24 wk,3 cells/μL | 474 (326, 655) | 462 (337, 698) | 634 (470, 774) | 628 (434, 796) | <0.001 |
| <250 cells/mm3 at 24 wk, % | 9 | 10 | 2 | 4 | 0.03 |
| Hemoglobin at baseline,2 g/L | 109 ± 1.2 | 107 ± 1.2 | 107 ± 1.2 | 108 ± 1.1 | 0.33 |
| Anemia | |||||
| Hemoglobin <120 g/L at baseline,2 % | 54 | 58 | 55 | 52 | 0.76 |
| Hemoglobin <120 g/L at 24 wk, % | 36 | 32 | 38 | 35 | 0.75 |
| CRP >5 mg/L at 24 wk, % | 19 | 16 | 18 | 14 | 0.69 |
| AGP >1 g/L at 24 wk, % | 36 | 35 | 34 | 31 | 0.82 |
Values are means ± SDs or medians (IQRs) unless otherwise indicated; n = 690. AGP, α-1-acid glycoprotein; BAN, Breastfeeding, Antiretrovirals, and Nutrition; CRP, C-reactive protein; HAART, highly active antiretroviral therapy; LNS, lipid-based nutrient supplements.
Baseline viral load, CD4, hemoglobin, and anemia were measured during pregnancy when participants were screened.
CD4 at 24 wk: no LNS/no HAART, n = 211; LNS, n = 213; HAART, n = 92; LNS + HAART, n = 105.
Adherence to LNS and HAART was high and generally increased over time. The percentages of mothers who reported consuming the full dose (2 packets) of LNS the previous day were as follows: 1 wk, 87%; 4 wk, 89%; 8 wk, 94%; 12 wk, 94%; and 21 wk, 96%. On the basis of pill counts, mean drug adherence was 86% at 4 wk, 87% at 12 wk, and 90% at 18 wk. LNS adherence did not differ significantly between the LNS and HAART+LNS groups at any visit. Similarly, there were no differences in drug adherence by type of HAART or between the groups receiving HAART with or without LNS.
We found significant interactions of HAART and LNS for folate (P = 0.051), vitamin B-12 (P < 0.001), and TfR (P = 0.085) but not for selenium, RBP, ferritin, or hemoglobin. Folate concentrations were higher among women receiving LNS with HAART (+17%; P = 0.037) or without HAART (+39%; P < 0.001) (Table 2). HAART was associated with lower folate concentrations in women receiving LNS (−27%; P < 0.001) or no LNS (−12%; P = 0.040). Vitamin B-12 concentrations were higher among women who received LNS and no HAART than in those receiving no LNS and no HAART (+27%; P < 0.001). HAART was associated with lower vitamin B-12 concentrations in women who received LNS (−18%; P = 0.001) but not in those with no LNS. Compared with women not receiving HAART, TfR concentrations were higher in women receiving HAART with (+14%; P = 0.004) or without (+28%; P < 0.001) LNS. Among women receiving HAART, TfR concentrations were lower in women receiving LNS (−12%; P = 0.023).
TABLE 2.
Folate, vitamin B-12, and TfR concentrations at 24 wk postpartum in a sample of BAN study mothers who were untreated or given HAART with or without LNS1
| HAART | No HAART | Ratio of HAART to no HAART (95% CI) | |
| Folate, nmol/L | |||
| LNS | 19.1 (17.1, 21.1) | 26.0 (24.2, 27.8) | 0.73*** (0.65, 0.83) |
| No LNS | 16.3 (14.6, 18.0) | 18.7 (17.4, 20.0) | 0.88* (0.77, 0.99) |
| Ratio of LNS to no LNS | 1.17* (1.01, 1.35) | 1.39*** (1.26, 1.54) | |
| Vitamin B-12, pmol/L | |||
| LNS | 286 (257.4, 313.7) | 349 (325.7, 372.2) | 0.82** (0.73, 0.92) |
| No LNS | 310 (278.4, 341.1) | 276 (257.4, 294.1) | 1.12 (0.99, 1.27) |
| Ratio of LNS to no LNS | 0.92 (0.80, 1.06) | 1.27*** (1.15, 1.39) | |
| TfR, mg/L | |||
| LNS | 5.0 (4.6, 5.3) | 4.3 (4.1, 4.6) | 1.14** (1.04, 1.25) |
| No LNS | 5.6 (5.2, 6.0) | 4.4 (4.2, 4.6) | 1.28*** (1.17, 1.40) |
| Ratio of LNS to no LNS | 0.88* (0.79, 0.98) | 0.98 (0.92, 1.06) |
Values are adjusted geometric means (95% CIs) or ratios (95% CIs). Folate and vitamin B-12: no HAART, no LNS, n = 237; no HAART, LNS, n = 238; HAART, no LNS, n = 103; HAART, LNS, n = 110. TfR: no HAART, no LNS, n = 237; no HAART, LNS, n = 238; HAART, no LNS, n = 104; HAART, LNS, n = 111. Models controlled for season, baseline CD4 count, baseline viral load, and use of folate inhibitors (for folate only) and included significant HAART × LNS interactions (folate: P = 0.051; vitamin B-12: P < 0.001; TfR: P = 0.085). *,**,***Significant ratio: *P < 0.05, **P < 0.01, ***P < 0.001. BAN, Breastfeeding, Antiretrovirals, and Nutrition; HAART, highly active antiretroviral therapy; LNS, lipid-based nutrient supplements; TfR, transferrin receptor.
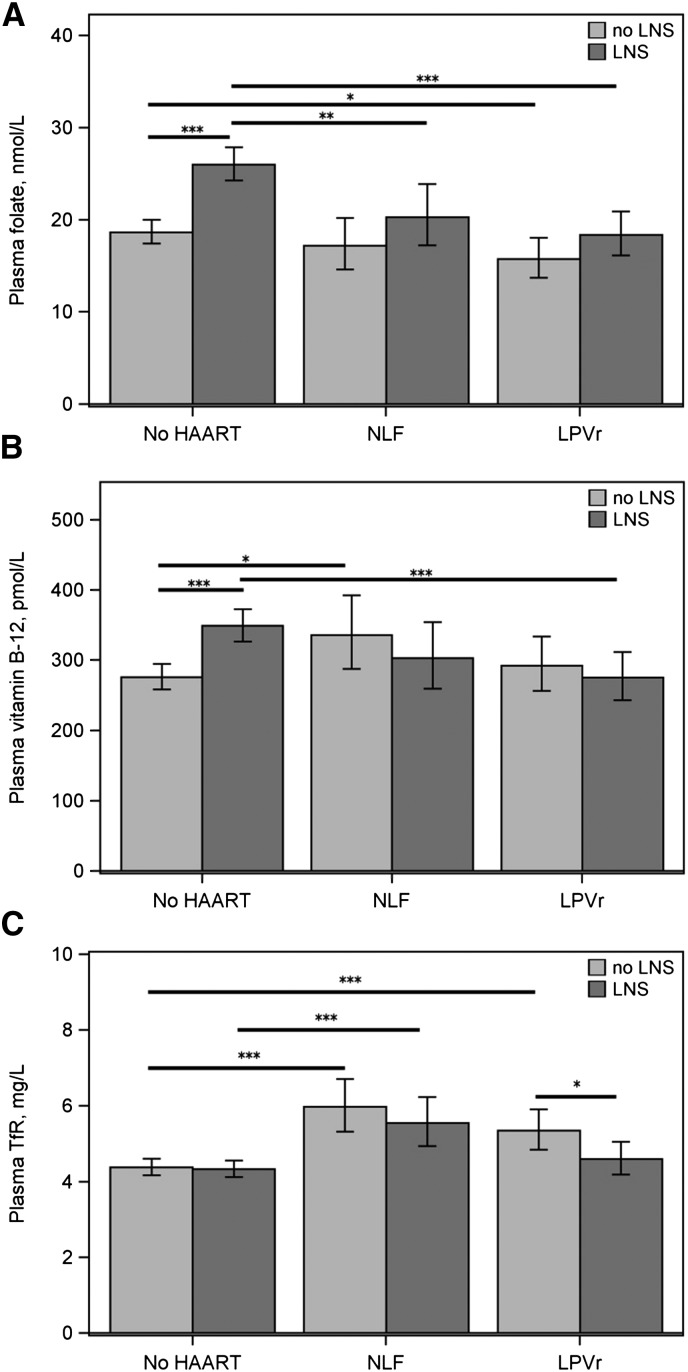
In exploratory analyses by type of drug regimen, the ratio of the geometric means indicated that women taking LPVr had lower folate concentrations if they received LNS (−35%; P < 0.001) or no LNS (−17%; P = 0.031), whereas women taking nelfinavir had lower folate only if they received LNS (−25%; P = 0.006) (Figure 1A). Women taking LPVr had lower vitamin B-12 concentrations if they received LNS (−24%; P = 0.001) but not if they did not receive LNS (Figure 1B). In contrast, women taking nelfinavir had higher vitamin B-12 concentrations if they received no LNS (+20%; P < 0.022) but not in those who received LNS. LNS increased folate and vitamin B-12 concentrations in women receiving no drugs (folate: +33%, P < 0.001; vitamin B-12: +24%, P < 0.001) but not in women receiving either LPVr or nelfinavir. Women taking LPVr had higher TfR only if they received no LNS (+20%; P < 0.001) (Figure 1C). Women taking nelfinavir had higher TfR if they received LNS (+25%; P < 0.001) or no LNS (+31%; P < 0.001). LNS was associated with lower TfR only in women receiving LPVr (−15%; P = 0.031); a similar association was not detected among women receiving nelfinavir.
FIGURE 1.
Adjusted geometric mean plasma folate (A), vitamin B-12 (B), and TfR (C) concentrations at 24 wk postpartum in a sample of BAN study mothers who were untreated or given HAART with or without LNS. Values are geometric means and 95% CIs. (A and B) No HAART, no LNS: n = 237; no HAART, LNS: n = 238; NLF, no LNS: n = 43; NLF, LNS: n = 43; LPVr, no LNS: n = 60; LPVr, LNS: n = 67. (C) No HAART, no LNS: n = 237; no HAART, LNS: n = 238; NLF, no LNS: n = 44; NLF, LNS: n = 44; LPVr, no LNS: n = 60; LPVr, LNS: n = 67. Comparisons were made between no LNS and LNS within each drug category (no HAART, NLF, and LPVr) and between NLF or LPVr and no HAART within each LNS category. *,**,***Difference between adjusted means at either end of the bar: *P < 0.05, **P < 0.01, ***P < 0.001. BAN, Breastfeeding, Antiretrovirals, and Nutrition; HAART, highly active antiretroviral therapy; LNS, lipid-based nutrient supplements; LPVr, lopinavir/ritonavir; NLF, nelfinavir; TfR, transferrin receptor.
We found no significant interactions of HAART and LNS for RBP, selenium, ferritin, or hemoglobin (Table 3). RBP concentrations were higher among women taking HAART than among those with no HAART (RBP: +10%; P = 0.007), but no associations with LNS were detected. There were no associations of either intervention with concentrations of selenium, ferritin, or hemoglobin.
TABLE 3.
Selenium, RBP, ferritin, and hemoglobin concentrations at 24 wk postpartum in a sample of BAN study mothers who were untreated or given HAART with or without LNS1
| HAART | No HAART | Ratio of HAART to no HAART | LNS | No LNS | Ratio of LNS to no LNS | |
| Selenium, μg/L | 82.0 (79.5, 84.6) | 81.7 (80.0, 83.4) | 1.00 (0.97, 1.04) | 82.9 (80.8, 84.9) | 80.7 (78.8, 82.7) | 1.03 (0.99, 1.06) |
| RBP, μmol/L | 0.98 (0.92, 1.03) | 0.89 (0.86, 0.93) | 1.10** (1.03, 1.17) | 0.92 (0.88, 0.96) | 0.92 (0.88, 0.96) | 1.00 (0.94, 1.07) |
| Ferritin, μg/L | 22.4 (19.8, 24.9) | 24.5 (22.6, 26.4) | 0.91 (0.79, 1.05) | 24.7 (22.5, 27.0) | 23.0 (20.9, 25.0) | 1.08 (0.95, 1.22) |
| Hemoglobin, g/L | 123 (121.8, 125.1) | 123 (121.5, 123.7) | 1.00 (0.99, 1.02) | 123 (121.8, 124.4) | 123 (121.2, 123.9) | 1.00 (0.99, 1.02) |
Values are adjusted geometric means (95% CIs) or ratios (95% CIs). Selenium and hemoglobin: HAART, n = 214; no HAART, n = 474; LNS, n = 348; no LNS, n = 340. RBP: HAART, n = 214; no HAART, n = 473; LNS, n = 348; no LNS, n = 339. Ferritin: HAART, n = 215; no HAART, n = 475; LNS, n = 349; no LNS, n = 341. Models controlled for season, baseline CD4 count, and baseline viral load. HAART × LNS interactions were not significant (selenium: P = 0.45; RBP: P = 0.39; ferritin: P = 0.55; hemoglobin: P = 0.66). **Significant ratio, P < 0.01. BAN, Breastfeeding, Antiretrovirals, and Nutrition; HAART, highly active antiretroviral therapy; LNS, lipid-based nutrient supplements; RBP, retinol-binding protein.
CRP, an acute phase protein, was negatively associated with RBP (P < 0.001) and hemoglobin (P < 0.001) and positively associated with ferritin (P < 0.001). AGP, a marker of chronic infection, was negatively associated with selenium (P < 0.001) and hemoglobin (P < 0.001). Including CRP and AGP in the models did not appreciably change the coefficients for selenium, RBP, ferritin, TfR, or hemoglobin (data not shown).
Discussion
This study examined the association of protease inhibitor–based HAART and LNS with maternal micronutrient concentrations after 24 wk of use. Women in this subsample had high mean CD4 counts and were assigned to HAART to test whether it prevented HIV transmission to their infants. Micronutrient concentrations and supplementation interventions have not been studied previously in women taking HAART for PMTCT. Yet, such women represent an important and growing population of HAART users due to the recent changes in recommendations to provide lifelong antiretrovirals to mothers in Option B+ PMTCT programs and to initiate HAART for all HIV-infected individuals with a CD4 count <500 cells/mm3 (1, 2).
We found that maternal folate concentrations were higher in women receiving LNS and lower in women receiving HAART. Furthermore, HAART modified the effect of LNS on folate. Intermediate folate values for the combined interventions suggest that HAART diminished the benefits of supplementation, whereas a daily dose of 0.6 times the RDA of folic acid mitigated the negative influence of the drugs. Our finding that protease inhibitor–based therapy was associated with lower folate concentrations confirms results from small cross-sectional studies in children and adults (35, 36). Lower folate concentrations among participants receiving HAART could be related to poor absorption due to drug-related changes in gut epithelial integrity (37) or drug-related diarrhea, which commonly occurs with initiation of protease inhibitors and some other antiretroviral drugs (38, 39). It is possible that HAART is related to intracellular folate metabolism. Some classes of antiretrovirals inhibit multidrug resistance–related proteins (MRPs), including MRP3, which is involved in folic acid transport out of the gut (40). Folate and homocysteine have an inverse relation in HIV-infected individuals. Several studies found an association of antiretroviral therapy or duration of therapy with hyperhomocysteinemia and low folate concentrations (35, 36, 41, 42), whereas others detected no association (43–45). Additional research is needed to confirm the relation between folate and different types and combinations of antiretrovirals and to clarify the biological mechanisms.
In our sample, LNS supplementation was associated with higher vitamin B-12 concentrations only in women not taking HAART and HAART was associated with lower vitamin B-12 concentrations only in women receiving LNS, indicating that HAART eliminated the association of LNS with vitamin B-12. This was driven by the negative association of LPVr with vitamin B-12 in women receiving LNS. Other studies also showed that vitamin B-12 concentrations can be increased through vitamin B-12 supplementation in HIV-infected children and adults, especially if they are initially deficient (16, 46, 47). Evidence from previous research on the effects of antiretrovirals on vitamin B-12 concentrations or status is mixed. One study found that zidovudine treatment was associated with lower plasma vitamin B-12 concentrations (15). A second study showed that vitamin B-12 intake was related to larger increases in serum vitamin B-12 concentrations in individuals not taking protease inhibitors than in those taking protease inhibitors (16). However, a third study found that patients receiving HAART (type not described) had higher vitamin B-12 concentrations and were less likely to be vitamin B-12 deficient than a historical cohort of patients who were not taking HAART (48). The mixed evidence for the effects of antiretrovirals on vitamin B-12 concentrations indicates a need for further research in larger samples and with adequate information on the type of drugs and adherence.
The literature on the association of HAART and vitamin A concentrations is also mixed. Participants in this study had higher RBP concentrations when they received HAART, even when controlling for concurrent markers of inflammation and baseline CD4 and viral load. Because RBP has a 1:1 relation with serum retinol (49), this finding suggests that protease inhibitor–based HAART could contribute to improved vitamin A status in populations similar to that in the BAN study, with high baseline CD4 and prevalent vitamin A deficiency. Our results agree with studies that showed that protease inhibitor–based HAART was related to higher RBP or β-carotene compared with individuals not receiving HAART (50, 51), whereas other studies found no differences in vitamin A concentrations by HAART status or type of HAART (12, 52). The variability in the literature points to the need to examine how baseline vitamin A status, initial CD4, and levels of inflammation influence changes in vitamin A concentrations before and after HAART initiation.
LNS alone was not associated with ferritin, TfR, or hemoglobin in this study. HAART was not associated with ferritin or hemoglobin but was associated with higher TfR concentrations, which indicates greater functional tissue iron deficiency in women taking these drugs. TfR can be elevated when there is iron-deficient erythropoiesis or tissue iron deficiency without anemia (53), which may be more common in people with chronic diseases and inflammation (54). The results on ferritin, TfR, and hemoglobin reported here confirm our earlier findings from a smaller, matched mother-infant subsample of BAN study participants (55).
Women in the BAN study received standard iron-folic acid tablets during pregnancy, as per Malawian guidelines, and their hemoglobin concentrations were ∼120 g/L on average both after delivery and at 24 wk postpartum. This may explain the lack of LNS effect on hemoglobin in our sample, whereas increases in hemoglobin concentrations have been documented in HIV-infected pregnant and lactating women with low initial hemoglobin concentratons who were supplemented with multiple micronutrients (17). Our findings also differ from those of several studies that showed increases in hemoglobin concentrations in HIV patients after HAART initiation (8, 9, 56–58). Some possible reasons for these differences include the short duration of HAART therapy and inclusion of zidovudine in the HAART regimen in our study. HAART has a more pronounced effect on increasing hemoglobin in patients who have taken it for >6 mo (56), whereas the use of zidovudine-containing HAART causes lower hemoglobin concentrations in some individuals (56, 58, 59).
This study had 3 main limitations. First, participants were not randomly assigned to the different drugs. They took either nelfinavir+Combivir or LPVr+Combivir on the basis of the timing of their enrollment in the study. Although calendar time could be related to our outcomes, we tried to limit possible confounding by including season in the models. Second, this analysis used a purposively selected subsample of mothers enrolled in the BAN study. We initially planned to examine effects in women with or without LNS. This resulted in a subsample with relatively small numbers of participants in the drug groups, which could limit our ability to detect differences. Choosing a subsample can result in differences in characteristics from the randomized cohort. Women in the micronutrient subsample were slightly older and the proportion with low CD4 counts or anemia was larger than in the rest of the BAN participants. However, the actual differences between the subsample and other study participants for these indicators were so small as to be clinically insignificant. In addition, we controlled for baseline CD4 count and viral load to address the possibility of selection bias. Third, with the exception of hemoglobin, we did not measure baseline micronutrient concentrations. We made this choice because micronutrient values are typically lower in pregnancy, when screening occurred, and would not allow us to look at changes related to the interventions, which were implemented postpartum. Furthermore, we would not expect to find differences in participants’ preintervention micronutrient concentrations in a randomized trial. We detected no differences between study arms in the proportion who were anemic at baseline, mean baseline hemoglobin, or any other baseline characteristic, suggesting that the groups were well balanced before initiating the study interventions.
The HAART regimens provided in this study were first-line treatment combinations at the time of study implementation and are now used as second-line regimens in PMTCT programs. On the basis of estimates of the proportion of patients who switch regimens due to treatment failure, the results of this study are applicable to ∼100,000 women in sub-Saharan Africa who are receiving second-line drugs in PMTCT programs (3, 60). Anemia, iron deficiency, and inadequate folate status are common in HIV-infected pregnant and lactating women in Africa (61–63). If confirmed in larger studies, our findings on the association of HAART with folate and TfR concentrations have implications for the health of mothers and their infants and may require supplementation or other types of interventions. Given the recent rapid expansion of PMTCT programs, further research is urgently needed to quantify the effects of second-line HAART regimens and to study the association of first-line regimens with micronutrient concentrations of women in PMTCT.
Acknowledgments
We thank all of the members of the BAN Study Team at the University of North Carolina (UNC) Chapel Hill; the CDC, Atlanta; and the UNC Project in Lilongwe including: LSA, Yusuf Ahmed, Mounir Ait-Khaled, Sandra Albrecht, Shrikant Bangdiwala, Ronald Bayer, MEB, Brian Bramson, Emily Bobrow, Nicola Boyle, Sal Butera, CSC, Charity Chavula, Maggie Chigwenembe, Maria Chikasema, Norah Chikhungu, David Chilongozi, Joseph Chimerang'ambe, Grace Chiudzu, Lenesi Chome, Anne Cole, AC, Amy Corneli, Anna Dow, Ann Duerr, Henry Eliya, Sascha Ellington, Joseph Eron, Sherry Farr, Yvonne Owens Ferguson, Susan Fiscus, VLF, Ali Fokar, Shannon Galvin, Laura Guay, Chad Heilig, Irving Hoffman, Elizabeth Hooten, Mina Hosseinipour, Michael Hudgens, Stacy Hurst, Lisa Hyde, DJJ, George Joaki (deceased), David Jones, Elizabeth Jordan-Bell, Zebrone Kacheche, Esmie Kamanga, Gift Kamanga, Coxcilly Kampani, Portia Kamthunzi, DK, Cecilia Kanyama, Angela Kashuba, Damson Kathyola, Dumbani Kayira, Peter Kazembe, Caroline C King, Rodney Knight, APK, Robert Krysiak, Jacob Kumwenda, Hana Lee, Edde Loeliger, Dustin Long, Misheck Luhanga, Victor Madhlopa, Maganizo Majawa, Alice Maida, Cheryl Marcus, Francis Martinson, Chrissie Matiki (deceased), Douglas Mayers, Isabel Mayuni, Marita McDonough, Joyce Meme, Ceppie Merry, Khama Mita, Chimwemwe Mkomawanthu, Gertrude Mndala, Ibrahim Mndala, Agnes Moses, Albans Msika, Wezi Msungama, Beatrice Mtimuni, Jane Muita, Noel Mumba, Bonface Musis, Charles Mwansambo, Gerald Mwapasa, Jacqueline Nkhoma, Megan Parker, Richard Pendame, Ellen Piwoz, Byron Raines, Zane Ramdas, John Rublein, Mairin Ryan, Ian Sanne, Christopher Sellers, Diane Shugars, Dorothy Sichali, Wendy Snowden, Alice Soko, Allison Spensley, Jean-Marc Steens, GT, Martin Tembo, Roshan Thomas, Navdeep Thoofer, Hsiao-Chuan Tien, Beth Tohill, CMvdh, Esther Waalberg, Elizabeth Widen, Jeffrey Wiener, Cathy Wilfert, Patricia Wiyo, Innocent Zgambo, and Chifundo Zimba.
We also appreciate the selenium analyses carried out in Gerald Combs’ laboratory by Laura Idso and Craig Lacher. LSA, CSC, GT, DK, APK, CMvdH, DJJ, and MEB designed and conducted the study; LHA, SS-F, and DH carried out the laboratory analyses; EJD was responsible for data management and provided statistical advice; AC helped interpret findings; NLD provided data on antiretroviral adherence; and VLF carried out the statistical analysis, drafted the manuscript, and had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AGP, α-1-acid glycoprotein; BAN, Breastfeeding, Antiretrovirals, and Nutrition; CRP, C-reactive protein; HAART, highly active antiretroviral therapy; LNS, lipid-based nutrient supplements; LPVr, lopinavir/ritonavir; MRP, multidrug resistance–related protein; PMTCT, prevention of mother-to-child transmission; RBP, retinol-binding protein; TfR, transferrin receptor.
References
- 1.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach June 2013. Geneva (Switzerland): WHO; 2013. [PubMed] [Google Scholar]
- 2.WHO. Use of antiretroviral drugs for treating pregnant women and preventing HIV infections in infants: executive summary. Geneva (Switzerland): WHO; 2012. [Google Scholar]
- 3.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2013. Geneva (Switzerland): Joint United Nations Programme on HIV/AIDS (UNAIDS); 2013. [Google Scholar]
- 4.Baum MK, Shor-Posner G, Zhang G, Lai H, Quesada JA, Campa A, Jose-Burbano M, Fletcher MA, Sauberlich H, Page JB. HIV-1 infection in women is associated with severe nutritional deficiencies. J Acquir Immune Defic Syndr Hum Retrovirol 1997;16:272–8. [DOI] [PubMed] [Google Scholar]
- 5.Tang AM, Graham NM, Semba RD, Saah AJ. Association between serum vitamin A and E levels and HIV-1 disease progression. AIDS 1997;11:613–20. [DOI] [PubMed] [Google Scholar]
- 6.Friis H. Micronutrient interventions and HIV infection: a review of current evidence. Trop Med Int Health 2006;11:1849–57. [DOI] [PubMed] [Google Scholar]
- 7.Campa A, Baum MK. Micronutrients and HIV infection. HIV Ther 2010;4:437–69. [Google Scholar]
- 8.Semba RD, Shah N, Vlahov D. Improvement of anemia among HIV-infected injection drug users receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2001;26:315–9. [DOI] [PubMed] [Google Scholar]
- 9.Semba RD, Shah N, Klein RS, Mayer KH, Schuman P, Gardner LI, Vlahov D. Highly active antiretroviral therapy associated with improved anemia among HIV-infected women. AIDS Patient Care STDS 2001;15:473–80. [DOI] [PubMed] [Google Scholar]
- 10.Moore RD, Forney D. Anemia in HIV-infected patients receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2002;29:54–7. [DOI] [PubMed] [Google Scholar]
- 11.Jones CY, Tang AM, Forrester JE, Huang J, Hendricks KM, Knox TA, Spiegelman D, Semba RD, Woods MN. Micronutrient levels and HIV disease status in HIV-infected patients on highly active antiretroviral therapy in the Nutrition for Healthy Living cohort. J Acquir Immune Defic Syndr 2006;43:475–82. [DOI] [PubMed] [Google Scholar]
- 12.Rousseau MC, Molines C, Moreau J, Delmont J. Influence of highly active antiretroviral therapy on micronutrient profiles in HIV-infected patients. Ann Nutr Metab 2000;44:212–6. [DOI] [PubMed] [Google Scholar]
- 13.Allavena C, Delpierre C, Cuzin L, Rey D, Viget N, Bernard J, Guillot P, Duvivier C, Billaud E, Raffi F. High frequency of vitamin D deficiency in HIV-infect patients: effects of HIV-related factors and antiretroviral drugs. J Antimicrob Chemother 2012;67:2222–30. [DOI] [PubMed] [Google Scholar]
- 14.Havers FP, Detrick B, Cardoso SW, Berendes S, Lama JR, Sungandhavesa P, Mwelase NH, Campbell TB, Gupta A; ACTG A5175 PEARLS and NWCS319 Study Teams. Change in vitamin D levels occurs early after antiretroviral therapy initation and depends on treatment regimen in resource-limited settings. PLoS ONE 2014;9:e95164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paltiel O, Falutz J, Veilleux M, Rosenblatt DS, Gordon K. Clinical correlates of subnormal vitamin B12 concentrations in patients infected with the human immunodeficiency virus. Am J Hematol 1995;49:318–22. [DOI] [PubMed] [Google Scholar]
- 16.Woods MN, Tang AM, Forester J, Jones C, Hendricks K, Ding B, Knox TA. Effect of dietary intake and protease inhibitors on serum vitamin B12 levels in a cohort of human immunodeficiency virus-positive patients. Clin Infect Dis 2003;37(Suppl 2):S124–31. [DOI] [PubMed] [Google Scholar]
- 17.Fawzi WW, Msamanga GI, Kupka R, Spiegelman D, Villamor E, Mugusi F, Wei R, Hunter D. Multivitamin supplementation improves hematologic status in HIV-infected women and their children in Tanzania. Am J Clin Nutr 2007;85:1335–43. [DOI] [PubMed] [Google Scholar]
- 18.Fawzi WW, Villamor E, Msamanga GI, Antelman G, Aboud S, Urassa W, Hunter D. Trial of zinc supplements in relation to pregnancy outcomes, hematologic indicators, and T cell counts among HIV-1-infected women in Tanzania. Am J Clin Nutr 2005;81:161–7. [DOI] [PubMed] [Google Scholar]
- 19.Hurwitz BE, Klaus JR, Llabre MM, Gonzalez A, Lawrence PJ, Maher KJ, Greeson JM, Baum MK, Shor-Posner G, Skyler JS, et al. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial. Arch Intern Med 2007;167:148–54. [DOI] [PubMed] [Google Scholar]
- 20.Allard JP, Aghdassi E, Chau J, Tam C, Kovacs CM, Salit IE, Walmsley SL. Effects of vitamin E and C supplementation on oxidative stress and viral load in HIV-infected subjects. AIDS 1998;12:1653–9. [DOI] [PubMed] [Google Scholar]
- 21.Austin J, Singhal N, Voigt R, Smaill F, Gill MJ, Walmsley S, Salit I, Gilmour J, Schlech WF 3rd, Choudhri S, et al. A community randomized controlled clinical trial of mixed carotenoids and micronutrient supplementation of patients with acquired immunodeficiency syndrome. Eur J Clin Nutr 2006;60:1266–76. [DOI] [PubMed] [Google Scholar]
- 22.Baeten JM, McClelland RS, Overbaugh J, Richardson BA, Emery S, Lavreys L, Mandaliya K, Bankson DD, Ndinya-Achola JO, Bwayo JJ, et al. Vitamin A supplementation and human immunodeficiency virus type 1 shedding in women: results of a randomized clinical trial. J Infect Dis 2002;185:1187–91. [DOI] [PubMed] [Google Scholar]
- 23.Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, McGrath N, Mwakagile D, Antelman G, Mbise R, Herrera G, Kapiga S, et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet 1998;351:1477–82. [DOI] [PubMed] [Google Scholar]
- 24.Jiamton S, Pepin J, Suttent R, Filteau S, Mahakkanukrauh B, Hanshaoworakul W, Chaisilwattana P, Suthipinittharm P, Shetty P, Jaffar S. A randomized trial of the impact of multiple micornutrient supplementation on mortality among HIV-infected individuals living in Bangkok. AIDS 2003;17:2461–9. [DOI] [PubMed] [Google Scholar]
- 25.Semba RD, Ricketts EP, Mehta S, Netski D, Thomas D, Kirk G, Wu AW, Vlahov D. Effect of micronutrients and iron supplementation on hemoglobin, iron status, and plasma hepatitis C and HIV RNA levels in female injection drug users: a controlled clinical trial. J Acquir Immune Defic Syndr 2007;45:298–303. [DOI] [PubMed] [Google Scholar]
- 26.van der Horst C, Chasela C, Ahmed Y, Hoffman I, Hosseinipour M, Knight R, Fiscus S, Hudgens M, Kazembe P, Bentley M, et al. Modifications of a large HIV prevention clinical trial to fit changing realities: a case study of the Breastfeeding, Antiretroviral, and Nutrition (BAN) protocol in Lilongwe, Malawi. Contemp Clin Trials 2009;30:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flax VL, Bentley ME, Chasela CS, Kayira D, Hudgens MG, Knight RJ, Soko A, Jamieson DJ, van der Horst CM, Adair LS. Use of lipid-based nutrient supplements by HIV-infected Malawian women during lactation has no effect on infant growth from 0–24 weeks. J Nutr 2012;142:1350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Institute of Medicine. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academies Press; 1998. [PubMed] [Google Scholar]
- 29.Institute of Medicine. Dietary Reference Intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 30.Institute of Medicine. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academies Press; 2001. [PubMed] [Google Scholar]
- 31.Fawzi WW, Msamanga GI, Hunter D, Renjifo B, Antelman G, Bang H, Manji K, Kapiga S, Mwakagile D, Essex M, et al. Randomized trial of vitamin supplements in relation to transmission of HIV-1 through breastfeeding and early child mortality. AIDS 2002;16:1935–44. [DOI] [PubMed] [Google Scholar]
- 32.Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, Kourtis AP, Martinson F, Tegha G, Knight RJ, Ahmed YI, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med 2010;362:2271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.AIDSMAP. Protease inhibitors (PIs). 2014 [cited 2015 Feb 6]. Available from: http://www.aidsmap.com/page/1060148.
- 34.Thurnham DI, McCabe GP. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. Geneva (Switzerland): WHO; 2012. [Google Scholar]
- 35.Vilaseca MA, Sierra C, Colome C, Artuch R, Valls C, Munos-Almagro C, Vilches M-A, Fortuny C. Hyperhomocysteinaemia and folate deficiency in human immunodeficiency virus-infected children. Eur J Clin Invest 2001;31:992–8. [DOI] [PubMed] [Google Scholar]
- 36.Coria-Ramirez E, Cisneros LN, Trevino-Perez S, Ibarra-Gonzalez I, Casillas-Rodriguez J, Majluf-Cruz A. Effect of highly active antiretroviral therapy on homocysteine plasma concentrations in HIV-1-infected patients. J Acquir Immune Defic Syndr 2010;54:477–81. [DOI] [PubMed] [Google Scholar]
- 37.Wu X, Sun L, Zha W, Studer E, Gurley E, Chen L, Wang X, Hylemon PB, Pandak WM Jr, Sanyal AJ, et al. HIV protease inhibitors induce endoplasmic reticulum stress and disrupt barrier integrity in intestinal epithelial cells. Gastroenterology 2010;138:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cahn P, Andrade-Villanueva J, Arribas JR, Gatell JM, Lama JR, Norton M, Patterson P, Madero JS, Sued O, Figueroa MI, et al. Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect Dis 2014;14:572–80. [DOI] [PubMed] [Google Scholar]
- 39.Drain PK, Kupka R, Mugusi F, Fawzi WW. Micronutrients in HIV-positive persons receiving highly active antiretroviral therapy. Am J Clin Nutr 2007;85:333–45. [DOI] [PubMed] [Google Scholar]
- 40.Weiss J, Theile D, Ketabi-Kiyanvash N, Lindenmaier H, Haefeli WE. Inhibition of MRP1/ABCC1, MRP2/ABCC2, and MRP3/ABCC3 by nucleoside, nucleotide, and non-nucleoside reverse transcriptase inhibitors. Drug Metab Dispos 2007;35:340–4. [DOI] [PubMed] [Google Scholar]
- 41.Bernasconi E, Uhr M, Magenta L, Ranno A, Telenti A. Homocysteinaemia in HIV-infected patients treated with highly active antiretroviral therapy. AIDS 2001;15:1081–2. [DOI] [PubMed] [Google Scholar]
- 42.Deminice R, Vassimon HS, Machado AA, de Paula FJA, Monteiro JP, Jordao AA. Plasma homocysteine levels in HIV-infected men with and without lipodystrophy. Nutrition 2013;29:1326–30. [DOI] [PubMed] [Google Scholar]
- 43.Uccelli MC, Torti C, Lapadula G, Labate L, Cologni G, Tirelli V, Moretti F, Costarelli S, Quiros-Roldan E, Carosi G. Influence of folate serum concentration on plasma homocysteine levels in HIV-positive patients exposed to protease inhibitors undergoing HAART. Ann Nutr Metab 2006;50:247–52. [DOI] [PubMed] [Google Scholar]
- 44.Bongiovanni M, Casana M, Pisacreta M, Tordato F, Cicconi P, Russo U, Ranieri R, Monforte A, Bini T. Predictive factors of hyperhomocysteinemia in HIV-positive patients. J Acquir Immune Defic Syndr 2007;44:117–9. [DOI] [PubMed] [Google Scholar]
- 45.Raiszadeh F, Hoover DR, Lee I, Shi A, Anastos K, Gao W, Kaplan R, Glesby MJ. Plasma homocysteine is not associated with HIV serostatus or antiretroviral therapy in women. J Acquir Immune Defic Syndr 2009;51:175–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ndeezi G, Tumwine JK, Ndugwa CM, Bolann BJ, Tylleskar T. Multiple micronutrient supplementation improves vitamin B12 and folate concentrations of HIV infected children in Uganda: a randomized controlled trial. Nutr J 2011;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Remacha AF, Cadafalch J. Cobalamin deficiency in patients infected with the human immunodeficiency virus. Semin Hematol 1999;36:75–87. [PubMed] [Google Scholar]
- 48.Remacha AF, Cadafalch J, Sarda P, Barcelo M, Fuster M. Vitamin B-12 metabolism in HIV-infected patients in the age of highly active antiretroviral therapy: role of homocysteine in assessing vitamin B-12 status. Am J Clin Nutr 2003;77:420–4. [DOI] [PubMed] [Google Scholar]
- 49.Baeten JM, Richardson BA, Bankson DD, Wener MH, Kriess JK, Lavreys L, Mandaliya K, Bwayo JJ, McClelland RS. Use of serum retinol-binding protein for prediction of vitamin A deficiency: effects of HIV-1 infection, protein malnutrition, and the acute phase response. Am J Clin Nutr 2004;79:218–25. [DOI] [PubMed] [Google Scholar]
- 50.Toma E, Devost D, Chow Lan N, Bhat PV. HIV-protease inhibitors alter retinoic acid synthesis. AIDS 2001;15:1979–84. [DOI] [PubMed] [Google Scholar]
- 51.Tang AM, Smit E, Semba RD, Shah N, Lyles CM, Li D, Vlahov D. Improved antioxidant status among HIV-infected injecting drug users on potent antiretroviral therapy. J Acquir Immune Defic Syndr 2000;23:321–6. [DOI] [PubMed] [Google Scholar]
- 52.Kaio DJ, Rondo PHC, Souza JMP, Firmino AV, Luzia LA, Segurado AA. Vitamin A and beta-carotene concentrations in adults with HIV/AIDS on highly active antiretroviral therapy. J Nutr Sci Vitaminol (Tokyo) 2013;59:496–502. [DOI] [PubMed] [Google Scholar]
- 53.Skikne BS. Serum transferrin receptor. Am J Hematol 2008;83:872–5. [DOI] [PubMed] [Google Scholar]
- 54.Noé G, Augustin J, Hausdorf S, Rich IN, Kubanek B. Serum erythropoietin and transferrin receptor levels in patients with rheumatoid arthritis. Clin Exp Rheumatol 1995;13:445–51. [PubMed] [Google Scholar]
- 55.Widen EM, Bentley ME, Chasela CS, Kayira D, Flax VL, Kourtis AP, Ellington SR, Kacheche Z, Tegha G, Jamieson DJ, et al. Antiretroviral treatment is associated with iron deficiency in HIV-infected Malawian women that is mitigated with supplementation, but is not associated with infant iron deficiency during 24 weeks of exclusive breastfeeding. J Acquir Immune Defic Syndr 2015;69:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berhane K, Karim R, Cohen MH, Masri-Lavine L, Young M, Anastos K, Augenbraun M, Watts DH, Levine AM. Impact of highly active antiretroviral therapy on anemia and relationship between anemia and survival in a large cohort of HIV-infected women: Women's Interagency HIV Study. J Acquir Immune Defic Syndr 2004;37:1245–52. [DOI] [PubMed] [Google Scholar]
- 57.Johannessen A, Naman E, Gundersen SG, Bruun JN. Antiretroviral treatment reverses HIV-associated anemia in rural Tanzania. BMC Infect Dis 2011;11:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takuva S, Maskew M, Brennan AT, Sanne I, MacPhail AP, Fox MP. Anemia among HIV-infected patients initiating antiretroviral therapy in South Africa: improvement in hemoglobin regardless of degree of immunosuppression and the initiating ART regimen. J Trop Med 2013;2013:162950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richman DD, Fischl MA, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, Leedom JM, Groopman JE, Mildvan D. The toxicity of azidothymidine AZT in the treatment of patients with AIDS and AIDS-related complex: a double-blind placebo-controlled trial. N Engl J Med 1987;317:192–7. [DOI] [PubMed] [Google Scholar]
- 60.Renaud-Thery F, Avila-Figueroa C, Stover J, Thierry S, Vitoria M, Habiyambere V, Souteyrand Y. Utilization patterns and projected demand of antiretroviral drugs in low- and middle-income countries. AIDS Res Treat 2011;2011:749041. [DOI] [PMC free article] [PubMed]
- 61.Friis H, Gomo E, Koestel P, Ndhlovu P, Nyazema N, Krarup H, Michaelsen KF. HIV and other predictors of serum folate, serum ferritin, and hemoglobin in pregnancy: a cross-sectional study in Zimbabwe. Am J Clin Nutr 2001;73:1066–73. [DOI] [PubMed] [Google Scholar]
- 62.Papathakis PC, Rollins NC, Chantry CJ, Bennish ML, Brown KH. Micronutrient status during lactation in HIV-infected and HIV-uninfected South African women during the first 6 mo after delivery. Am J Clin Nutr 2007;85:182–92. [DOI] [PubMed] [Google Scholar]
- 63.Kupka R, Msamanga GI, Mugusi F, Petraro P, Hunter DJ, Fawzi WW. Iron status is an important cause of anemia in HIV-infected Tanzanian women but is not related to accelerated HIV disease progression. J Nutr 2007;137:2317–23. [DOI] [PubMed] [Google Scholar]