Abstract
Background: It is widely understood that the 2 primary factors affecting dietary zinc absorption in adults are the quantities of zinc and phytate in the diet. Although a similar association of absorption to dietary zinc and phytate is presumed to exist in children, to our knowledge, no large-scale examination of the relation of zinc absorption to dietary and growth factors has been conducted.
Objective: The goal was to apply an adult absorption model and related models to data from zinc absorption studies of infants and children in order to determine the nature of the relation of zinc absorption to dietary zinc and phytate, age, body size, and zinc homeostatic variables.
Methods: Data from 236 children between 8 and 50 mo of age were obtained from stable-isotope studies of zinc absorption. Statistical and mechanistic models were fit to the data using linear and nonlinear regression analysis.
Results: The effect of dietary phytate on zinc absorption when controlling for dietary zinc was very small and not statistically discernable (P = 0.29). A 500-mg/d increase in dietary phytate reduced absorbed zinc by <0.04 mg/d. Absorption was observed to vary with age, weight, and height (P < 0.0001) when controlling for dietary zinc. For example, absorption from 6 mg/d of dietary zinc increased by as much as 0.2 mg/d with a 12-mo increase in age. Absorption varied with weight and exchangeable zinc pool size (0.01 < P < 0.05) when controlling for dietary zinc and age.
Conclusions: The absence of a detectable phytate effect on zinc absorption raises caution about use of dietary phytate:zinc molar ratios to predict zinc bioavailability and does not support phytate reduction as a strategy to improve zinc status of young children. The effect of age on zinc absorption and the absence of a phytate effect should facilitate estimations of dietary zinc needs in young children.
Keywords: zinc absorption, dietary zinc, dietary phytate, infants, young children, mathematical modeling
Introduction
Although it is perhaps universally understood that the quantity of zinc absorbed from the diet increases with the quantity in the diet, the relation between absorbed zinc and dietary zinc, and how it is influenced by other dietary components, has been characterized in some detail (1–5). Of particular interest in these studies was the inhibition of zinc absorption by dietary phytate, which binds with zinc in the small intestine to form an unabsorbable complex. These analyses were conducted only on data from adults, however. In contrast, the public health concern about risk of zinc deficiency is greatest for young children living in impoverished environments who subsist on plant-based diets. Additionally, because of the lack of adequate simple biomarkers of zinc status, a great deal of attention has centered on dietary zinc intakes to evaluate the extent and severity of this problem. Interpretation of dietary data alone, however, is problematical without reliable information on the absorption of ingested zinc.
The fundamental goal of this investigation was to use several mechanistic mathematical models to investigate the relations between total daily absorbed zinc (TAZ)4 and a variety of other variables, most importantly, total daily dietary zinc (TDZ) intake, total daily dietary phytate (phytic acid) (TDPA) intake, age, and body size, to advance our understanding of and ability to predict zinc absorption in infants and young children. It was expected that the infant and child data would exhibit a saturation response of absorbed zinc to dietary zinc and an inhibition of zinc absorption by dietary phytate, both of which have been observed in adults. Furthermore, it was expected that zinc absorption would increase with subject body size and age (6), reflecting the association of absorptive capacity with increasing luminal surface area caused by, in part, lengthening of the small intestine in growing young subjects (7–9).
The modeling of the infant and child data will have potential usefulness as both predictive and descriptive tools. In the former role they may be used to predict the quantities of dietary zinc needed to ensure sufficient absorbed zinc to meet the physiologic requirements for growth in a specific dietary environment. The descriptive application may contribute to an improved understanding of the factors influencing zinc absorption.
Methods
Origin of data.
The data originated from studies of zinc absorption in 262 infants and children conducted in poor, rural communities mostly in economically developing countries (China, India, Kenya, Malawi, and Zambia) (10–16) and in a westernized, resource-adequate community in the United States (17) (Table 1). The subjects were apparently healthy, with no chronic illnesses or conditions affecting zinc absorption. With the exception of several children staying in a hospital setting (13), the subjects were all living at home. Our laboratory participated in all of the studies. In each study zinc absorption was determined by the dual-stable-isotope-tracer ratio method in which all main meals on the study day were isotopically labeled. In most studies the subjects were eating their habitual diets or study diets similar to their habitual diets on the day of the absorption study. When a study diet was used, it was administered only on the study day or for up to 6 d before the study day. Exceptions to this are 2 studies of breastfed 9-mo-old subjects who were provided complementary foods (17) or micronutrient powder (11) for addition to their complementary foods for 3 mo before the study day. TAZ, TDZ, age, body weight, body length/height, and the size of the rapidly exchanging zinc pool were measured in all of the studies (Table 2). Gender was recorded and weight-for-age z scores (WAZs) (18), height/length-for-age z scores (HAZs), and BMI were calculated. TDPA, plasma zinc concentration, and C-reactive protein (CRP) concentration were measured in most, but not all, of the studies. Only data from 236 subjects in the range of 8–50 mo of age were included in the analyses. The data were restricted to this age range because of the focus in global health on children <5 y of age. Several values (shown in parentheses) that were extreme outliers from the distributions and biologically implausible for this population were removed from the exchangeable zinc pool (EZP; 173 mg), plasma zinc (153 μg/dL), and CRP (119 and 734 mg/L) data before the calculation of summary statistics (Table 1) and analyses. They were removed on the basis of their implausibility, likely because of analytic error. Their removal eliminated concern that, being unrepresentative of the population and having high leverage in the regression analyses, their presence would have compromised the regression results.
TABLE 1.
Summary of zinc absorption studies from which modeled data were derived1
| Study | Setting | Subject | Intervention and method | Measurement |
| Krebs et al. (17) | Denver, CO | n = 42, 5- to 6-mo-old breastfed (only) infants | Randomly assigned to first CF meat, multiple-micronutrient fortified infant cereal, or iron-fortified infant cereal; zinc absorption studies at 9 mo of age | All meals (CF) and human milk of day labeled; dietary phytate measurements: TDZ, FAZ, TAZ, EZP; other: plasma zinc, CRP; anthropometry |
| Esamai et al. (11) | Rural western Kenya | n = 27, 6-mo-old, nonanemic breastfed infants; CF primarily maize and plant based | Double-blind, randomized trial of MNP, with and without iron or placebo; MNP started at 6 mo of age; zinc absorption studies at 9 mo of age | Weighed duplicate diets including MNP labeled; measurements: TDZ, FAZ, TAZ, EZP; other: serum zinc, AGP, CRP; anthropometry |
| May et al. (15) | Masika, Malawi, isolated village | n = 17, stunted 3- to 5-y-olds; habitual diets maize, rice, beans | Studied on habitual diet; repeat isotope studies after 30 d of resistant starch added to habitual diet | Weighed duplicate diets; measurements: TDZ, FAZ, TAZ, EZP; other: serum zinc; anthropometry |
| Chomba et al. (10) | Chongwe District, Zambia | n = 60, 1- to 5-y-olds; habitual diets, primarily maize | Randomly assigned to biofortified or zinc-fortified or control/local maize; studied on assigned diet × 1 d | Weighed duplicate diets; dietary phytate measurements: TDZ, FAZ, TAZ, EZP; Other: plasma zinc, AGP; anthropometry |
| Sheng et al. (16) | Xi-Chou China, Yun-nan province | n = 43, 19- to 25-mo-olds; habitual diets primarily rice, vegetables | Studied on habitual diets | Weighed duplicate diets; dietary phytate; measurements: TDZ, FAZ, TAZ, EZP; other: plasma zinc; anthropometry |
| Manary et al. (14) | Rural Malawi | n = 10; 2- to 5-y-olds; maize-based diet | Studied on habitual diets | Weighed duplicate diets; measurements: TDZ, FAZ, TAZ, EZP; other: plasma zinc, CRP; anthropometry |
| Manary et al. (13) | Blantyre, Malawi, Queen Elizabeth Central Hospital | n = 23; 3- to 13-y-olds; recovering from tuberculosis or minor injury; well children controls | Children randomly assigned to standard or phytate-reduced corn-plus-soy porridge; studied after 3–7 d on assigned porridge | Weighed duplicate diets; measurements: TDZ, FAZ, TAZ, EZP; other: plasma zinc, CRP; anthropometry |
| Kodkany et al. (12) | Kineye, Belgaum in Northern Karnataka, India | n = 40; 22- to 35-mo-olds; vegetarian diet; all subjects iron deficient | Double-blind, randomly assigned to iron and zinc biofortified or control pearl millet; studied on assigned diet × 1 d | Weighed duplicate diets; measurements: TDZ, FAZ, TAZ, EZP; other: plasma zinc, CRP; anthropometry |
AGP, α-1 acid glycoprotein; CF, complementary food; CRP, C-reactive protein; EZP, exchangeable zinc pool; FAZ, fractional absorption of zinc; MNP, micronutrient powder; TAZ, total daily absorbed zinc; TDZ, total daily dietary zinc.
TABLE 2.
Summary statistics for the absorption study data that were modeled1
| TDZ, mg/d | TDPA, mg/d | TAZ, mg/d | Age, mo | wt, kg | WAZ | Ht, cm | HAZ | BMI, kg/m2 | EZP,2mg | EZP/wt, mg/kg | PIZn,2 μg/dL | CRP,2mg/L | |
| Mean | 3.88 | 739 | 0.86 | 24.2 | 10.7 | −0.84 | 80.9 | −1.28 | 16.3 | 44.9 | 4.22 | 69.5 | 4.64 |
| Median | 3.49 | 490 | 0.74 | 24.2 | 10.6 | −0.87 | 82.0 | −1.33 | 16.2 | 42.9 | 3.93 | 68.6 | 2.20 |
| SD | 2.27 | 632 | 0.49 | 12.1 | 2.1 | 1.02 | 9.3 | 1.53 | 1.7 | 15.6 | 1.42 | 14.7 | 7.79 |
| Range | 0.52–12.4 | 31–2557 | 0.10–2.66 | 8–50 | 6.4–15.8 | −3.99–1.95 | 61.2–103.9 | −4.9–3.50 | 11.8–23.6 | 7.3–121 | 0.89–14.3 | 29.9–119.1 | 0.01–44.2 |
| Count | 236 | 174 | 236 | 235 | 236 | 235 | 225 | 224 | 225 | 226 | 226 | 204 | 147 |
There were 106 male and 128 female subjects (gender was unknown for 2 subjects). CRP, C-reactive protein concentration; EZP, exchangeable zinc pool; EZP/wt, exchangeable zinc pool/body wt; HAZ, height/length-for-age z score; Ht, body length/height; PlZn, plasma zinc concentration; TAZ, total absorbed zinc; TDPA, total dietary phytate (phytic acid); TDZ, total dietary zinc; WAZ, weight-for-age z score.
Two or less biologically implausible values were removed (see text).
Development of mechanistic models.
A published model of zinc absorption as a function of dietary zinc and phytate, the validity of which has been demonstrated in adults (1–3, 5, 19), served as the basis for several models developed for application to these data. The development of the model is described in detail elsewhere (1). The model equation is
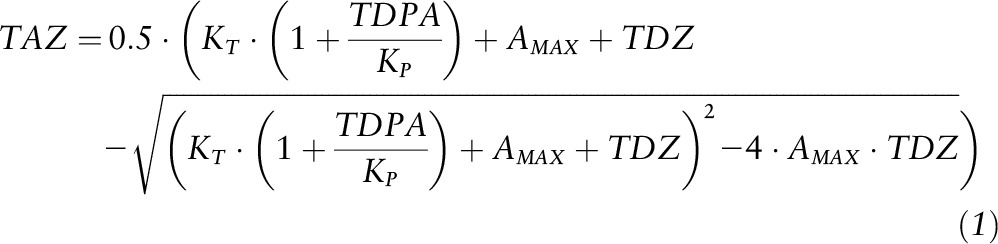 |
The model has 2 predictor variables, TDZ and TDPA, and the response variable is TAZ. All variables are expressed as mmol/d, and the model has 3 parameters: AMAX (maximal absorption, i.e., absorptive capacity), KT (zinc-transporter binding equilibrium dissociation constant), and KP (zinc-phytate binding equilibrium dissociation constant). This adult model was modified to permit absorptive capacity to vary with a variable such as age or body size. In the new versions of the model the AMAX parameter was replaced with “A*variable^E,” where “variable” is one associated with varying gut absorptive capacity, e.g., age, or a measure of body size, and A and E are new parameters. One of the new models that was most useful is expressed as
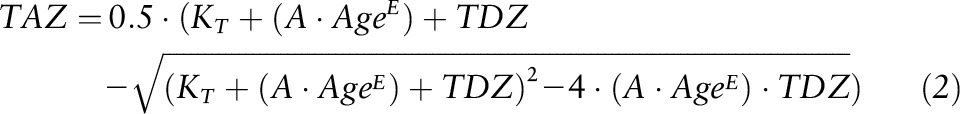 |
where age is shown as the new predictor variable, and the phytate variable and parameter have been removed. This model in turn served as the basis for several other variations created to further investigate other relations of the variables to absorbed zinc (Supplemental Equations S1–S6). For all models without TDPA as a predictor variable TDZ and TAZ were expressed as mg/d instead of mmol/d for analyses.
Data analysis and modeling.
The mechanistic models described previously were fit to the data by means of nonlinear regression analysis using the R statistical programming environment (version 3.0.2) (20) and DataFit software (version 9.0; Oakdale Engineering). Linear statistical models (with second-order polynomial terms) approximating the nonlinear models were also used to investigate the relations between variables and to derive statistical information not available from the nonlinear analyses. TAZ was the response variable in all models. The statistical significance of relations of predictor variables to TAZ in the nonlinear models was evaluated with the P values from partial F tests of the extra sum of squares distinguishing nested models with and without the predictor variable and parameter(s) being tested. Approximate CIs of parameter estimates from nonlinear models were determined using the profile likelihood method. Goodness of fit was assessed using the mean square errors of the nonlinear models and the coefficient of determination (R2) of the linear models.
The validity of the nonlinear models and the compliance with regression assumptions were evaluated using the value of parameter estimates and by examination of plots of the residuals. The existence of outliers was evaluated using residual plots and the values of standardized or studentized residuals. Data with absolute standardized residual values of >3.5 were considered extreme outliers and were removed. Assuming a normal distribution of the errors, the probability of the occurrence of such an outlier was extremely low (0.0004) and thus was judged to be an aberrance. Outliers with absolute standardized residuals <3.5 were examined and considered for removal if they were influential or questionable. When regression assumptions were not met, appropriate remedial measures were taken. For example, when the errors were observed to not have constant variance (heteroscedastic errors), nonlinear weighted least-squares regression was used to derive parameter estimates and CIs. Weights were calculated from an estimated variance function derived from the regression of the absolute or squared residuals. A significance level of α = 0.05 was used.
Because the subset of data with TDPA values were from one study conducted in a resource-adequate community in the United States and 3 studies in poor communities in other countries (China, India, and Zambia), the data were examined to determine if the United States differed from the other data. The data from the other countries were modeled without the US data, TAZ values for the US data were predicted from the model, and the mean square errors from the model and the predictions were compared. The mean square prediction error was less than the mean square error of the model, indicating that the relations of the variables were similar for all data.
Results
Throughout the analyses there was no evidence of problems with model validity or regression assumption compliance, with the exception of the frequent existence of heteroscedastic error variance. Two data with standardized residuals exceeding 3.5, attributable to unusually high TAZ values, were removed from analyses.
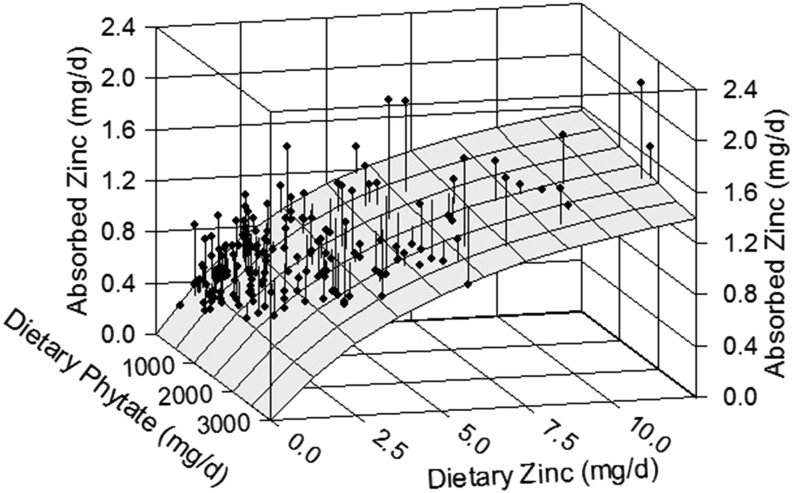
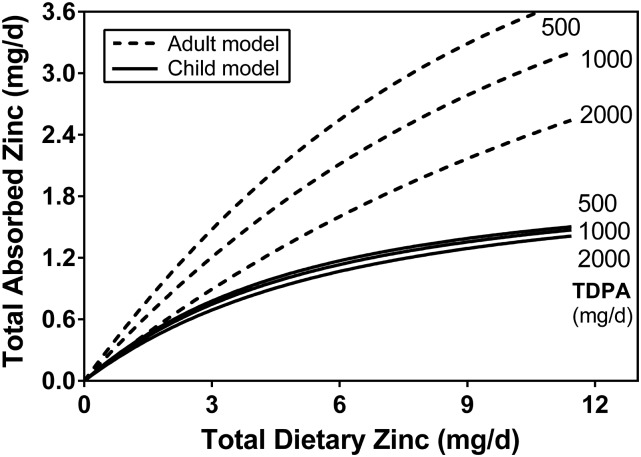
The subset of 174 data with TDPA values (10, 12, 16, 17) were initially analyzed with the adult model (Equation 1). Although an unambiguous association of TAZ to TDZ was observed (P < 0.0001), the relation of TAZ with TDPA when controlling for TDZ was not significant (P = 0.29). Plots of the data and fitted model (Figures 1 and 2) suggest the presence of a small phytate effect, but it is so small as to not have a noticeable impact on zinc absorption and too small for these data to provide statistical evidence of its existence. The coefficient of determination (R2) from the linear model of the relation of TAZ to TDZ and TDPA was 0.57, and the coefficients of partial determination were 0.42 and 0.016 for TDZ and TDPA, respectively. The nonlinear model parameter estimates and 95% CIs are listed in Table 3, along with those of the adult model for comparison. The estimated values of AMAX and KT are of the same order of magnitude between children and adults, as would be expected. In contrast, the KP value for children is much higher and has a large uncertainty. A higher KP value indicates less of a phytate effect. Figure 2 provides a graphic comparison with the adult model.
FIGURE 1.
The relation of daily absorbed zinc to dietary zinc and phytate intake in infants and young children. The surface represents the absorption response predicted by the Equation 1 model fitted to the infant/child data having TDPA values (n = 172). The vertical lines from each datum to the surface show the residual, i.e., the deviation of the point from the model’s prediction. Although an increase in absorption with increasing zinc intake is evident, there is only the suggestion of an inhibiting effect of phytate in the small downward slope of the surface with increasing phytate intake (most evident at the right side). TDPA, total daily dietary phytate (phytic acid).
FIGURE 2.
The relation of daily absorbed zinc to dietary zinc and phytate intake in infants and young children. This is a 2-dimensional version of Figure 1 showing the absorption response to dietary zinc intake for selected quantities of dietary phytate. The predicted response curves for corresponding intakes from the adult model (3) are shown for comparison of the phytate effect. The difference in the magnitude of the phytate effect is evident. TDPA, total daily dietary phytate (phytic acid).
TABLE 3.
Parameter values and CIs from fitting of the Equation 1 model to infant/child data and from the same model fit to adult data
| Infant/child |
Adult1 |
|||
| Parameter estimate | 95% CI limit | Parameter estimate | 95% CI limit | |
| AMAX | 0.0314 | 0.0234, 0.0472 | 0.0909 | 0.0794, 0.1077 |
| KT | 0.0510 | 0.0307, 0.0913 | 0.0333 | 0.0142, 0.0619 |
| KP | 8.30 | 3.572 | 0.678 | 0.290, 1.222 |
Adult parameter values reported by Hambidge et al. (3).
Upper limit could not be calculated by profile likelihood method, probably because of its magnitude.
Because this finding was unexpected, the data sets from the 4 studies with dietary phytate data were analyzed separately and in various combinations in order to detect any peculiarities in the data that might have contributed to misleading regression results. However, none of these analyses differed notably from that of all the phytate data. The KP parameter estimates from these analyses varied from 7.6 to effective infinity. P values of the KP estimates were >0.25.
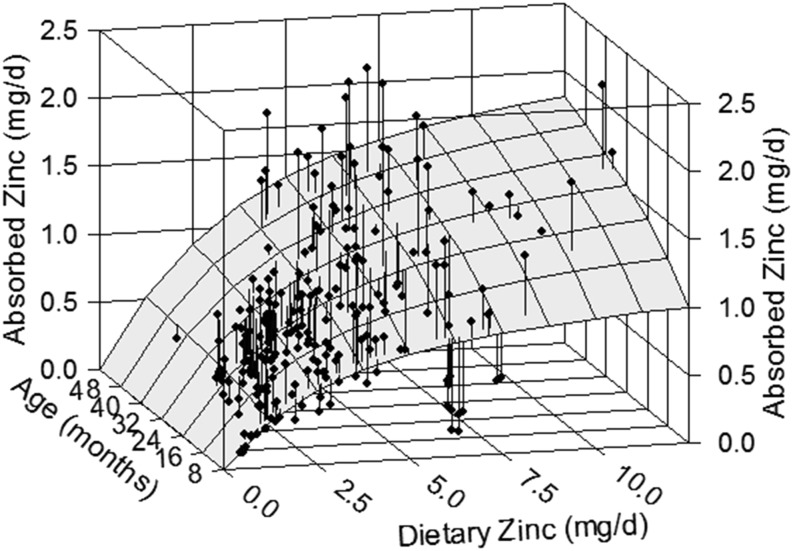
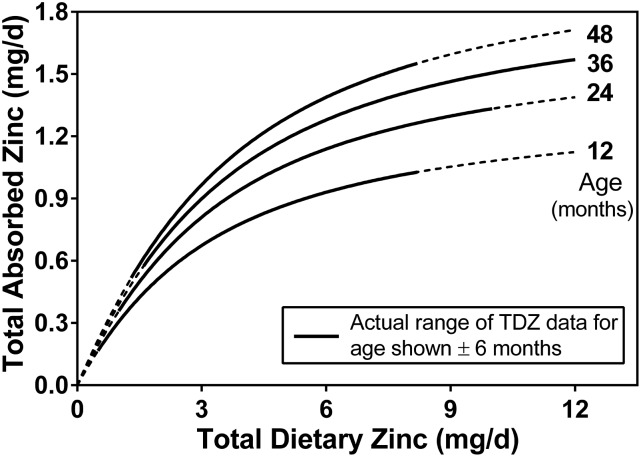
Because dietary phytate was found to not have an effect on absorbed zinc, subsequent analyses were performed on the full data set (n = 236) without using TDPA as a predictor variable. These data were modeled using the Equation 2 model with age, body weight, or height again serving as the variable influencing absorptive capacity. All 3 variables were found to have a direct relation with TAZ (P < 0.0001). Although it is reasonable to conclude that any of these relations reflect the impact of small intestine size on zinc absorption, age was viewed as a more practical predictor of TAZ because dietary and physiologic requirements are specified by age. Furthermore, the model with age demonstrated the best fit to the data of the 3 variables; weight had the next best fit. The mean square errors of the nonlinear models were 0.101 and 0.0105 and the coefficients of determination (R2) from the linear versions were 0.53 and 0.51 for age and weight models, respectively. The parameter estimates and 95% CI limits (in parentheses) from the age model were 0.633 (0.418, 0.951), 0.311 (0.211, 0.417), and 2.42 (1.61, 3.67) for A, E, and KT, respectively. Plots of the model with age show the notable increase of TAZ with age (Figures 3 and 4). The coefficients of partial determination from the linear model were 0.34 and 0.21 for TDZ and age, respectively.
FIGURE 3.
The relation of daily absorbed zinc to dietary zinc and age in infants and young children. The surface represents the absorption response predicted by the Equation 2 model fitted to all the child data (n = 233). An increase in absorption with dietary zinc or age is evident.
FIGURE 4.
The relation of daily absorbed zinc to dietary zinc and age in infants and young children. This is a 2-dimensional version of Figure 3 showing the absorption response to dietary zinc intake for selected age groups. TDZ, total daily dietary zinc.
Several more complex models derived from Equation 2 (Supplemental Equations S4 and S5) were applied to the data to investigate the possibility that when controlling for TDZ and age, there might be other variables that exhibit a relation with absorbed zinc; i.e., for a subject of a given age and having a given zinc intake is there a relation between zinc absorbed and, e.g., body weight? These models indicated that weight, WAZ, BMI, EZP, and EZP/weight all improved the fit of the model to the data (0.01 < P < 0.05), suggesting that each of the variables had a relation with TAZ when controlling for TDZ and age. In each case the relation was direct. It was surmised that all of these relations, with the exception of EZP/weight, were reflecting a common phenomenon, i.e., that there was an association of absorption with body weight or a weight-related factor among children of a particular age and zinc intake. Neither height, HAZ, plasma zinc, nor CRP were observed to have an association with TAZ when TDZ and age were controlled for.
The effects of several categorical variables (gender, malaria endemism, and environmental enteropathy endemism) were also examined using variations of the models (Supplemental Equations S2 and S3). None of these categorical variables was found to be significant in any model.
Discussion
The finding of no phytate effect or, at best, a small and not statistically discernable effect, on zinc absorption in infants and children was surprising and not amenable to ready explanation. There is a wealth of literature documenting the inhibition of zinc absorption by phytate in the diet in adults and animal models. An overview of the literature can be found in review articles (21–24). This effect of dietary phytate on zinc absorption has been widely, perhaps universally, presumed to exist in children as well, although the results of the limited number of investigations of the phenomenon in infants and young children have not been generally supportive of this view. Among the positive findings, 2- to 10-y-old Nigerian children absorbed more zinc from a single test meal of phytase-treated maize porridge than from the untreated porridge (25). Full-term infants between 2 and 6 mo of age absorbed more zinc from isotope-labeled feedings of phytase-treated vs. control soybean protein isolate-based formula (26), and low hair zinc as an indicator of chronic suboptimal zinc status was found to be associated with high dietary phytate intake in 7-y-old Guatemalan children (27).
In contrast, other studies have detected no effect of dietary phytate or phytate reduction. An early stable-isotope study of 2- to 11-mo-old infants showed no difference in zinc absorption from low-phytate and control soy-based formulas (28). No difference in linear growth velocity between 6 and 12 mo was seen in Guatemalan infants consuming control or low-phytate maize for the period (29). No difference in zinc absorption from control and phytase-treated corn-soy porridge was observed in whole-day isotope studies of 3- to 13-y-old well Malawian children, although a difference was seen in children recovering from tuberculosis (13). In another whole-day isotope absorption study, in this case of 9-y-old Guatemalan children, zinc absorption from control and low-phytate maize did not differ (30). Swedish infants exhibited no differences in serum zinc concentrations after 6 mo on randomly assigned diets of cereal porridges containing varying amounts of phytate (31). Three- to 5-y-old Malawian children showed no differences in fecal excretion of endogenous zinc after 40 d on phytate-reduced maize compared with their habitual maize diet (32). The publications of the 4 studies from which the phytate data used in this analysis originated did not report in detail on the impact of phytate on zinc absorption because this was not a focus of the studies. It is now evident that had the effect of dietary phytate been an objective of the studies, these publications would number among those reporting no effect.
Thus, it appears that the unexpected result of this analysis is actually consistent with much of the existing research on the effects of dietary phytate on zinc absorption and status in children. This analysis, nonetheless, presents a more compelling case for the negative finding than previous studies in that a larger number of data from multiple studies and having wider ranges of values were analyzed and confounding factors were controlled for by the models. This, in turn, makes the need for an explanation of this apparent difference between children and adults more pressing. Several mechanisms that could possibly explain the difference were considered. One potential mechanism thought to hold promise focuses on the role of dietary phytases. The presence of phytogenic and microbial phytases in the human diet is well established (33–35) and hydrolysis of phytate by dietary phytases in the upper gastrointestinal tract has been documented (36) with the stomach considered to be the main functional site of dietary phytase (33). This process may be more pronounced in infants and children because of the fact that gastric pH is higher in infants and children than in adults (37). Fasting pH is higher, decreasing with age, and postprandial increases in pH have been observed to peak higher (7 in neonates and 4–6 in adolescents) and last longer (2.5–4 h) than in older subjects. pH and temperature are the major factors determining enzyme activity (33) and most phytases characterized thus far are acid phytases, which exhibit maximal phytate-degrading activity in a pH range of 4–6 (33, 34). Consequently, there is a case for conditions being more favorable for the gastric hydrolysis of phytate by dietary phytases in infants and children. Finally, a noteworthy difference between the child and adult phytate-zinc absorption data used in our models is that a majority of the adult studies were conducted in the United States and Europe, whereas a majority of the studies of infants and children were performed in poor, rural areas in economically developing countries. This raises the possibility that not just age but other unrecognized contextual factors may play a role in the observed differences.
The observed effect of age and body size on zinc absorption when controlling for TDZ was consistent with expectations. Age, body weight, and length/height, all of which have been shown to have an association with small intestine length, were significant predictors of zinc absorption. The linear and nonlinear models provided evidence that age was the better predictor, because it consistently made the greater contribution to the fit of the models to the data. Relating zinc absorption to age instead of weight or height enhances the model’s practical value and has particular efficacy in the context of predictive uses of the model such as estimating dietary requirements.
The next step in the modeling of the data, investigating a possible relation of absorbed zinc to other variables when TDZ and age have been controlled for, did provide evidence of relations with weight, WAZ, BMI, EZP, and EZP/weight. All of these variables have a direct relation to weight, with the exception of EZP/weight, which obviously is inversely related to weight. It is conceivable, for a child of a given age and ingesting a given amount of dietary zinc, that zinc absorption would be further influenced by body weight or another factor related to weight. The fact that there was no relation with height or HAZ, which are strongly correlated with weight and WAZ, respectively, suggests that it is not body size but another factor related differently to weight and height that is underlying the effect. However, the size of this effect is of minor consequence. The model predicts that a kilogram increase in body size is associated with a TAZ increase of between 0.02 and 0.03 mg/d in 24-mo-old children ingesting 4 mg of zinc per day. Thus, this more-complicated model has little practical value currently, its potential usefulness to be realized only with additional data and further interpretation.
The analyses described here, using nonlinear mechanistic and linear statistical models to examine the relation of total absorbed zinc to a number of potential predictor variables for which data are available, have provided important information, notably, the absence of a phytate effect of zinc absorption. As is usually the case, it is clear that additional data are needed to better understand and characterize these important relations, and, importantly, there need to be data on additional variables that could explain more of the variation in absorbed zinc than what has been accomplished with the current measurements. For example, biomarkers specific to intestinal inflammation would be of particular interest in addition to CRP, which reflects systemic inflammation. The highly prevalent condition of environmental enteropathy has been proposed to have a negative impact on zinc absorption (11, 38, 39). From the coefficients of determination of the statistical models it was apparent that the models generally accounted for no more than 50–60% of the variance of TAZ, which is typical of modeling of individual data from stable-isotope studies of human nutrition. The R2 values from the modeling of individual data are unlikely to exceed this range until our ability to quantify the genetic and contextual variables that contribute to interindividual differences is refined.
These mechanistic models of data from isotope studies in infants and children identified the amount of dietary zinc and age as major determinants of TAZ. Importantly, and in contrast to observations in adults, dietary phytate was not significantly related to zinc absorption. These findings have implications for estimating dietary zinc requirements for young children. Specifically, the absence of a detectable phytate effect on zinc absorption raises caution about use of dietary phytate:zinc molar ratio calculations to predict zinc bioavailability. Additionally, this finding does not support phytate reduction as a strategy to improve zinc status of young children.
Acknowledgments
We thank Jamie Westcott for assistance with compiling the data. LVM created the models and performed data analyses. LVM, KMH, and NFK wrote the paper. LVM had responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CRP, C-reactive protein; EZP, exchangeable zinc pool; HAZ, height/length-for-age z score; TAZ, total daily absorbed zinc; TDPA, total daily dietary phytate (phytic acid); TDZ, total daily dietary zinc; WAZ, weight-for-age z score.
References
- 1.Miller LV, Krebs NF, Hambidge KM. A mathematical model of zinc absorption in humans as a function of dietary zinc and phytate. J Nutr 2007;137:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hambidge KM, Miller LV, Westcott JE, Krebs NF. Dietary reference intakes for zinc may require adjustment for phytate intake based upon model predictions. J Nutr 2008;138:2363–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hambidge KM, Miller LV, Westcott JE, Sheng X, Krebs NF. Zinc bioavailability and homeostasis. Am J Clin Nutr 2010;91:1478S–83S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller LV, Krebs NF, Hambidge KM. Mathematical model of zinc absorption: effects of dietary calcium, protein and iron on zinc absorption. Br J Nutr 2013;109:695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosado JL, Hambidge KM, Miller LV, Garcia OP, Westcott J, Gonzalez K, Conde J, Hotz C, Pfeiffer W, Ortiz-Monasterio I, et al. The quantity of zinc absorbed from wheat in adult women is enhanced by biofortification. J Nutr 2009;139:1920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hambidge KM, Krebs NF, Westcott JE, Miller LV. Changes in zinc absorption during development. J Pediatr 2006; 149(5 Suppl)S64–8. [DOI] [PubMed] [Google Scholar]
- 7.Siebert JR. Small-intestine length in infants and children. Am J Dis Child 1980;134:593–5. [DOI] [PubMed] [Google Scholar]
- 8.Struijs MC, Diamond IR, de Silva N, Wales PW. Establishing norms for intestinal length in children. J Pediatr Surg 2009;44:933–8. [DOI] [PubMed] [Google Scholar]
- 9.Weaver LT, Austin S, Cole TJ. Small intestinal length: a factor essential for gut adaptation. Gut 1991;32:1321–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chomba E, Westcott CM, Westcott JE, Mpabalwani EM, Krebs NF, Patinkin ZW, Palacios N, Hambidge KM. Zinc absorption from biofortified maize meets the requirements of young rural Zambian children. J Nutr 2015;145:514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esamai F, Liechty E, Ikemeri J, Westcott J, Kemp J, Culbertson D, Miller LV, Hambidge KM, Krebs NF. Zinc absorption from micronutrient powder is low but is not affected by iron in Kenyan infants. Nutrients 2014;6:5636–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kodkany BS, Bellad RM, Mahantshetti NS, Westcott JE, Krebs NF, Kemp JF, Hambidge KM. Biofortification of pearl millet with iron and zinc in a randomized controlled trial increases absorption of these minerals above physiologic requirements in young children. J Nutr 2013;143:1489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manary MJ, Hotz C, Krebs NF, Gibson RS, Westcott JE, Arnold T, Broadhead RL, Hambidge KM. Dietary phytate reduction improves zinc absorption in Malawian children recovering from tuberculosis but not in well children. J Nutr 2000;130:2959–64. [DOI] [PubMed] [Google Scholar]
- 14.Manary MJ, Hotz C, Krebs NF, Gibson RS, Westcott JE, Broadhead RL, Hambidge KM. Zinc homeostasis in Malawian children consuming a high-phytate, maize-based diet. Am J Clin Nutr 2002;75:1057–61. [DOI] [PubMed] [Google Scholar]
- 15.May T, Westcott C, Thakwalakwa C, Ordiz MI, Maleta K, Westcott J, Ryan K, Hambidge KM, Miller LV, Young G, et al. Resistant starch does not affect zinc homeostasis in rural Malawian children. J Trace Elem Med Biol 2015;30:43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheng XY, Hambidge KM, Zhu XX, Ni JX, Bailey KB, Gibson RS, Krebs NF. Major variables of zinc homeostasis in Chinese toddlers. Am J Clin Nutr 2006;84:389–94. [DOI] [PubMed] [Google Scholar]
- 17.Krebs NF, Westcott JE, Culbertson DL, Sian L, Miller LV, Hambidge KM. Comparison of complementary feeding strategies to meet zinc requirements of older breastfed infants. Am J Clin Nutr 2012;96:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO Multicentre Growth Reference Study Group. Enrollment and baseline characteristics in the WHO Multicentre Growth Reference Study. Acta Paediatr Suppl 2006;450:7–15. [DOI] [PubMed] [Google Scholar]
- 19.Hunt JR, Beiseigel JM, Johnson LK. Adaptation in human zinc absorption as influenced by dietary zinc and bioavailability. Am J Clin Nutr 2008;87:1336–45. [DOI] [PubMed] [Google Scholar]
- 20.R Core Team [Internet]. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2013 [cited 2014 Jan 1]. Available from: http://www.R-project.org/.
- 21.Lönnerdal B. Phytic acid-trace element (Zn, Cu, Mn) interactions. Int J Food Sci Nutr 2002;37:749–58. [Google Scholar]
- 22.Bel-Serrat S, Stammers AL, Warthon-Medina M, Moran VH, Iglesia-Altaba I, Hermoso M, Moreno LA, Lowe NM. Factors that affect zinc bioavailability and losses in adult and elderly populations. Nutr Rev 2014;72:334–52. [DOI] [PubMed] [Google Scholar]
- 23.Lonnerdal B. Dietary factors influencing zinc absorption. J Nutr 2000;130:(5S Suppl):1378S–83S. [DOI] [PubMed] [Google Scholar]
- 24.Wise A. Phytate and zinc bioavailability. Int J Food Sci Nutr 1995;46:53–63. [DOI] [PubMed] [Google Scholar]
- 25.Thacher TD, Aliu O, Griffin IJ, Pam SD, O'Brien KO, Imade GE, Abrams SA. Meals and dephytinization affect calcium and zinc absorption in Nigerian children with rickets. J Nutr 2009;139:926–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidsson L, Ziegler EE, Kastenmayer P, van Dael P, Barclay D. Dephytinisation of soyabean protein isolate with low native phytic acid content has limited impact on mineral and trace element absorption in healthy infants. Br J Nutr 2004;91:287–94. [DOI] [PubMed] [Google Scholar]
- 27.Cavan KR, Gibson RS, Grazioso CF, Isalgue AM, Ruz M, Solomons NW. Growth and body composition of periurban Guatemalan children in relation to zinc status: a cross-sectional study. Am J Clin Nutr 1993;57:334–43. [DOI] [PubMed] [Google Scholar]
- 28.Ziegler E, Janghorbani M, Nelson S, BB E. Effect of phytate reduction on mineral absorption from soy based infant formula. Am J Clin Nutr 1990;51:528. [Google Scholar]
- 29.Mazariegos M, Hambidge KM, Westcott JE, Solomons NW, Raboy V, Das A, Goco N, Kindem M, Wright LL, Krebs NF. Neither a zinc supplement nor phytate-reduced maize nor their combination enhance growth of 6- to 12-month-old Guatemalan infants. J Nutr 2010;140:1041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazariegos M, Hambidge KM, Krebs NF, Westcott JE, Lei S, Grunwald GK, Campos R, Barahona B, Raboy V, Solomons NW. Zinc absorption in Guatemalan schoolchildren fed normal or low-phytate maize. Am J Clin Nutr 2006;83:59–64. [DOI] [PubMed] [Google Scholar]
- 31.Lind T, Lonnerdal B, Persson LA, Stenlund H, Tennefors C, Hernell O. Effects of weaning cereals with different phytate contents on hemoglobin, iron stores, and serum zinc: a randomized intervention in infants from 6 to 12 mo of age. Am J Clin Nutr 2003;78:168–75. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy G, Hambidge KM, Manary M. A reduced phytate diet does not reduce endogenous fecal zinc in children on a habitual high-phytate diet. J Pediatr Gastroenterol Nutr 2010;51:678–9. [DOI] [PubMed] [Google Scholar]
- 33.Greiner R, Koneitzny U. Phytase for food application. Food Technol Biotechnol 2006;44:125–40. [Google Scholar]
- 34.Kumar V, Sinha AK, Makkar HPS, Becker K. Dietary roles of phytate and phytase in human nutrition: a review. Food Chem 2010;120:945–59. [Google Scholar]
- 35.Sandberg A-S, Andlid T. Phytogenic and microbial phytases in human nutrition. Int J Food Sci Technol 2002;37:823–33. [Google Scholar]
- 36.Sandberg AS, Andersson H. Effect of dietary phytase on the digestion of phytate in the stomach and small intestine of humans. J Nutr 1988;118:469–73. [DOI] [PubMed] [Google Scholar]
- 37.Nagita A, Amemoto K, Yoden A, Aoki S, Sakaguchi M, Ashida K, Mino M. Diurnal variation in intragastric pH in children with and without peptic ulcers. Pediatr Res 1996;40:528–32. [DOI] [PubMed] [Google Scholar]
- 38.Krebs NF, Miller LV, Hambidge KM. Zinc deficiency in infants and children: a review of its complex and synergistic interactions. Paediatr Int Child Health 2014;34:279–88. [DOI] [PubMed]
- 39.Lindenmayer GW, Stoltzfus RJ, Prendergast AJ. Interactions between zinc deficiency and environmental enteropathy in developing countries. Adv Nutr 2014;5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]