Abstract
Background: In utero exposure to excessive cholesterol has been shown to increase fetal plasma cholesterol concentration and predispose adult offspring to cardiovascular disease (CVD) risk. Because lipid-lowering drugs are contraindicated during pregnancy, natural cholesterol-lowering compounds may be a safe and effective alternative to reduce CVD risk in offspring born to hypercholesterolemic mothers.
Objective: This study used the hypercholesterolemic apolipoprotein E–deficient (apoE−/−) mouse model to test the hypothesis that mothers supplemented with phytosterols during gestation and lactation would produce offspring with a more favorable lipid profile than offspring from unsupplemented mothers, despite having a genetic predisposition toward hypercholesterolemia.
Methods: Sixteen female apoE−/− mice were randomly assigned to 2 diets fed throughout the gestation and lactation periods: a cholesterol-enriched diet (CH) (0.15%) or the cholesterol-enriched diet supplemented with phytosterols (CH/PS) (2%). Serum lipids and lipoproteins were measured by enzyme assay and nuclear magnetic resonance spectroscopy, respectively, and liver cholesterol was analyzed by GC.
Results: Compared with the CH-fed dams at the end of lactation, phytosterol-supplemented dams displayed lower (P < 0.05) serum total cholesterol (−55%), non-HDL cholesterol (−56%), and LDL cholesterol (−47%), but no change (P > 0.05) in HDL cholesterol and triacylglycerol (TG) concentrations. Pups from phytosterol-fed dams demonstrated lower (P < 0.05) total cholesterol (−25%), non-HDL cholesterol (−25%), LDL cholesterol (−47%), and TGs (−41%), without any change (P > 0.05) in HDL cholesterol compared with pups from CH-fed dams. Furthermore, compared with pups from CH-fed dams, pups from phytosterol-supplemented dams displayed a lower (P < 0.05) number of total LDL particles (−34%), VLDL particles (−31%), and HDL particles (−30%).
Conclusion: Our results in apoE−/− mice suggest that even under strong genetic predisposition to hypercholesterolemia, pups born to mothers supplemented with phytosterols during gestation and lactation exhibit favorable liver and serum lipid responses compared with pups from unsupplemented mothers.
Keywords: cardiovascular disease, cholesterol, maternal programming, offspring, phytosterols
Introduction
Pregnancy is characterized by an elevation in maternal cholesterol that is essential for embryogenesis and early organ development through activation of sonic hedgehog proteins (1). However, an excessive increase in maternal serum cholesterol during pregnancy—termed maternal supraphysiological hypercholesterolemia—can manifest in women with elevated cholesterol before conception because of metabolic, genetic, or lifestyle conditions (2, 3). Excessive in utero exposure to cholesterol by direct maternal transfer in hypercholesterolemic pregnancies alters placental oxidative stress (4), modulates placental expression of cholesterol-regulatory genes (5), and produces offspring who develop premature, faster progressing arterial lesions (6, 7). Although the incidence of maternal supraphysiological hypercholesterolemia is difficult to access because of the lack of routine serum cholesterol tracking during pregnancy (8), it is likely appreciable considering the prevalence of obesity and hypercholesterolemia in women of childbearing age (9). A plasma total cholesterol concentration of >280 mg/dL has been suggested as a cutoff at which maternal hypercholesterolemia negatively alters fetal vascular reactivity and increases fetal fatty streak development (2, 7, 10).
Both animal (11, 12) and human studies (13, 14) have demonstrated the influential effect of maternal hyperlipidemia during pregnancy on lipid concentrations in offspring. Furthermore, cholesterol concentrations in newborns have been shown to be predictive of cardiovascular disease (CVD)6 risk in adulthood (15–17). Physicians treating hypercholesterolemic women who are or wish to become pregnant face a dilemma, because many cholesterol-lowering medications including statins and ezetimibe are contraindicated during pregnancy due to potential adverse effects on fetal development (18, 19). Current fertility management guidelines in women with familial hypercholesterolemia recommend discontinuing statin use 3 mo before conception and an immediate cessation of lipid-lowering medication in women who conceive while on drug therapy (19). Therefore, there is sufficient justification to explore alternative lipid-lowering strategies for use in hypercholesterolemic pregnancies that are both safe and effective for mother and offspring.
Phytosterols are plant-based bioactive compounds characterized since the early 1950s as effective cholesterol-lowering agents that work by interfering with intestinal cholesterol absorption (20). Phytosterol supplementation at ∼2 g/d has been shown to reduce serum cholesterol concentrations in the range of 8–16%, particularly in hypercholesterolemic individuals, including those with familial hypercholesterolemia (21, 22) and dyslipidemic conditions associated with metabolic syndrome (23) and diabetes (24). Although the safety of phytosterol supplementation during pregnancy has been examined in a number of preclinical studies (25–27) and a human investigations (28) without any negative effects reported, the potential application of phytosterols as a cholesterol-lowering therapy for use in hypercholesterolemic pregnancies has not been investigated. Therefore, in this study we used the hypercholesterolemic apoE–deficient (apoE−/−) mouse model to test the hypothesis that mothers supplemented with phytosterols during gestation and lactation would produce offspring with a more favorable lipid profile than offspring from unsupplemented mothers despite having a genetic predisposition toward hypercholesterolemia. The apoE −/− mouse was specifically chosen because 1) these mice exhibit hypercholesterolemia that has been shown to be developmentally programmed through altered intrauterine environmental exposure to excessive cholesterol (29, 30), and 2) phytosterol supplementation in cholesterol-fed adult apoE−/− mice is effective in protecting against hypercholesterolemia and limiting arterial lesion development (31, 32).
Methods
Mice and diets.
Sixteen mature (3-mo-old) female mice homozygous for disruption of the apoE gene (apoE−/−, strain B6.129P2-Apoetm1Unc > /J, stock no. 002052) were purchased from Jackson Laboratory. Mice were brought to the Animal Care Facility at the University at Buffalo and housed in a temperature-controlled room (20°C, 12h light/dark cycle). The mice were randomly assigned (n = 8/group) to 1 of 2 commercial unpurified diets (Teklad 2019 Harlan Laboratories, Supplemental Table 1): 1) a cholesterol-enriched diet (CH) (0.15% wt:wt, TD.140285), and 2) the cholesterol-enriched diet supplemented with phytosterols (CH/PS) (2% wt:wt; TD.140286; phytosterols sourced from Forbs Medi-Tech Corp.). In-house analysis confirmed that, as a percentage of total sterols, the phytosterol supplement was composed largely of β-sitosterol (>75%), with minor amounts of sitostanol (<15%) and campesterol (<7%). Females were mated with male apoE−/− breeders for 1 wk (1 male per 2 females) and upon confirmation of pregnancy based on daily weight gain were returned to their individual cages. After parturition, litters were randomly culled to 6 pups per dam to minimize variability in postnatal pup development influenced by litter size (33). Throughout the suckling period the dams remained on their respective diets. To prevent the pups from consuming the diet as they matured, food pellets were provided on raised caged platforms that were only accessible by the mother. At weaning (day 21), the dams and pups were anesthetized with isoflurane for blood collection. Blood was collected from food-deprived (15 h) mice by cardiac puncture and livers were excised and stored at −80°C until further processing and analyses. Mice were euthanized by exsanguination and heart removal. The mice used in this experiment were cared for in accordance with the guidelines established by the Institutional Animal Care and Use Committee. All procedures were reviewed and approved by the Animal Care Committee at the University at Buffalo (protocol no. PTE16082N).
Liver histology.
For visualization of hepatic neutral lipids, liver slices were fixed in formaldehyde, embedded in 10-μm-thick paraffin sections, and stained with 0.5% Oil Red O in propylene glycol.
Blood biochemistry.
Serum cholesterol panel (total cholesterol, HDL cholesterol, and direct LDL cholesterol) and TGs were measured by direct automated enzymatic assay, and lipoprotein particle number and size were analyzed by NMR spectroscopy (Liposcience) (34). Non-HDL cholesterol was calculated by subtracting HDL cholesterol from the total cholesterol fraction.
Serum β-sitosterol and hepatic cholesterol analysis.
Serum β-sitosterol and hepatic cholesterol were extracted and analyzed according to our previously published procedures (35, 36). Approximately 0.5 mL of serum or 0.5 g of pulverized liver was enriched with α-cholestane as internal standard and saponified in freshly prepared KOH–methanol at 100°C for 1 h. The nonsaponifiable sterol fraction was extracted with petroleum diethyl ether and dried under N2 gas. Sterol fractions were analyzed on a Shimadzu GC-17A gas chromatograph fitted with a flame ionization detector with the use of a SAC-5 capillary column (30 m × 0.25 mm × 0.25 mm, Supelco). This methodology was recently validated through the international “Survey for Sterols and Oxysterols ST1/14,” which included total cholesterol and phytosterol external reference materials from Referenzinstitut für Bioanalytik.
Statistical analyses.
Litters from each dam were considered as a single observation. Data were analyzed with a general linear model ANOVA with the use of SPSS 16 for Mac. Data are presented as means ± SEMs. Differences were considered significant at P < 0.05. Because of the cannibalism of pups by one dam after parturition, the final number of dams in each treatment was n = 8 for the CH group and n = 7 for the CH/PS group.
Results
Maternal phytosterol supplementation did not affect (P > 0.05) feed intake or the trajectory of body weight gain in dams during the gestation and lactation periods compared with the CH group (Supplemental Figure 1A and B). Similarly, litter weights during the postnatal period (Supplemental Figure 1C) or the male-to-female sex ratio (ranging from 40–60%) did not differ (P > 0.05) between litters born to CH-fed or CH/PS-fed mothers.
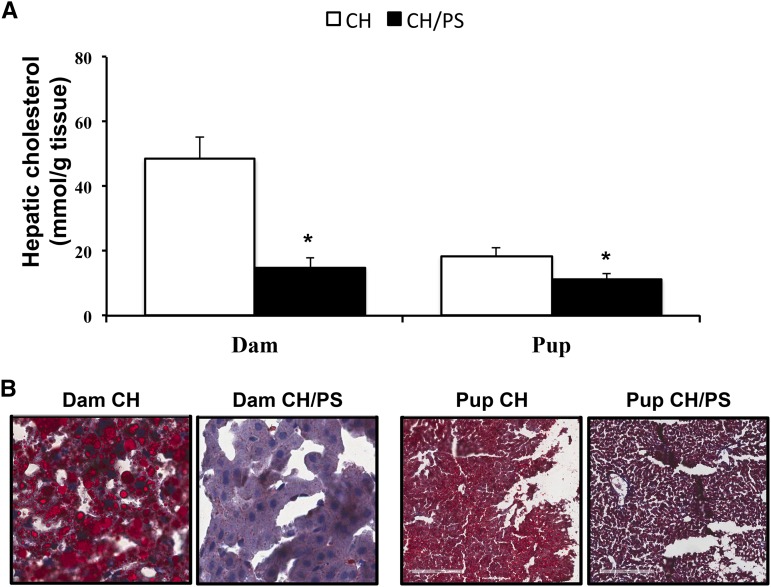
Phytosterol supplementation lowered (P < 0.05) hepatic cholesterol concentrations in both dams (−69%) and pups (−38%) compared with the CH group (Figure 1A). A lower hepatic neutral lipid content in phytosterol-supplemented dams and their pups compared with the CH group was supported by Oil Red O staining (Figure 1B).
FIGURE 1.
Effect of maternal supplementation with CH or CH/PS during gestation/lactation on hepatic cholesterol in dams and pups. Hepatic cholesterol concentration (A) and Oil Red O staining of liver slices in dams and pups (B). Data are means ± SEs, n = 8 (CH-supplemented group) and n = 7 (CH/PS supplemented group); *Difference from CH, P < 0.05. CH, cholesterol; CH/PS, cholesterol and phytosterols.
Compared with the CH-fed dams at the end of lactation, phytosterol-supplemented dams displayed lower (P < 0.05) serum total cholesterol (−55%), non-HDL cholesterol (−56%), and LDL cholesterol (−47%), but no change (P > 0.05) in HDL cholesterol and TG concentrations (Figure 2A). The total cholesterol-to-HDL cholesterol ratio was lower (−42%, P < 0.05) in phytosterol-supplemented dams than in CH-fed dams. Compared with the CH group, dams consuming the phytosterol-supplemented diet demonstrated lower (P < 0.05) total VLDL particles (−45%) resulting from reductions in the large (−62%), medium (−48%), and small (−42%) VLDL fractions (Figure 2B). HDL particle number did not differ (P > 0.05) between CH-fed and CH/PS-fed dams (Figure 2C).
FIGURE 2.
Serum lipids and lipoprotein response in dams supplemented with CH or CH/PS during gestation/lactation. Serum lipids including total cholesterol, non-HDL cholesterol, LDL cholesterol, HDL cholesterol and TGs (A); VLDL particle number (B); and HDL particle number (C). Data are means ± SEs, n = 8 (CH-supplemented group) and n = 7 (CH/PS-supplemented group); *Difference from CH, P < 0.05. CH, cholesterol; CH/PS, cholesterol and phytosterols; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; Non-HDL-C, non-HDL cholesterol; Total-C, total cholesterol.
Pups from phytosterol-fed dams demonstrated lower (P < 0.05) total cholesterol (−25%), non-HDL cholesterol (−25%), LDL cholesterol (−47%), and TGs (−41%), without any change (P > 0.05) in HDL cholesterol compared with pups from CH-fed dams (Figure 3A). The total cholesterol-to-HDL cholesterol ratio did not differ (P > 0.05) in pups from phytosterol-supplemented dams compared with pups from the CH group. Compared with pups from CH-fed dams, pups from phytosterol-supplemented dams displayed a lower (P < 0.05) number of total LDL particles (−34%) and intermediate LDL particles (−41%) without any change (P > 0.05) in large LDL particles (Figure 3B). Compared with pups from the CH group, pups born to phytosterol-supplemented dams displayed a lower (P < 0.05) total number of VLDL particles (−31%) stemming from reductions in all VLDL subclasses, including large (−39%), medium (−40%), and small (−24%) (Figure 4A). Pups from phytosterol-supplemented dams responded with a lower (P < 0.05) total HDL particle number (−30%), primarily through a reduced number of small (−37%) and medium (−45%) HDL particles, compared with pups from the CH-fed dams (Figure 4B). No difference (P > 0.05) was observed in the ratio of HDL cholesterol to HDL particle number, a measure of HDL function, between the pups from CH-fed and CH/PS-fed dams (1.80 ± 0.11 vs. 1.79 ± 0.06, respectively). Although LDL and VLDL size did not change between the pups, HDL particles were slightly larger (+3%, P < 0.05) in pups from phytosterol-fed dams than in pups from CH-fed dams (Figure 4C).
FIGURE 3.
Serum lipids and lipoprotein response in pups from dams supplemented with CH or CH/PS during gestation/lactation. Serum lipids including total cholesterol, non-HDL cholesterol, LDL cholesterol, HDL cholesterol and TGs (A); LDL particle number (B). Data are means ± SEs, n = 8 (CH-supplemented group) and n = 7 (CH/PS-supplemented group); *Difference from CH, P < 0.05. CH, cholesterol; CH/PS, cholesterol and phytosterols; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; Non-HDL-C, non-HDL cholesterol; Total-C, total cholesterol.
FIGURE 4.
Serum lipoprotein response in pups from dams supplemented with CH or CH/PS during gestation/lactation. VLDL particle number (A); HDL particle number (B); lipoprotein size, including VLDL, HDL, and LDL (C). Data are means ± SEs, n = 8 (CH-supplemented group) and n = 7 (CH/PS-supplemented group); *Difference from CH, P < 0.05. CH, cholesterol; CH/PS, cholesterol and phytosterols.
A higher (P < 0.05) serum concentration of β-sitosterol (+211%), expressed as phytosterol-to-cholesterol ratio (μmol/mmol), was observed in pups from phytosterol-fed dams (1.11 ± 0.10 μmol/mmol) compared with pups from CH-fed dams (0.35 ± 0.01 μmol/mmol), likely indicating its transfer via milk from mother to suckling pups.
Discussion
Compared with pups from CH-fed apoE−/− dams, weanling offspring from dams supplemented with phytosterols during pregnancy and lactation displayed favorable lipid responses despite a genetic predisposition to hypercholesterolemia. Specifically, these lipid responses were characterized by the following: 1) lower hepatic cholesterol concentration, 2) lower serum cholesterol (total cholesterol, non-HDL cholesterol, and LDL cholesterol) and LDL particle number, and 3) lower serum TGs with a parallel reduction in VLDL particles in the large, medium, and small subclasses. However, pups born to phytosterol-supplemented dams were also characterized by lower total HDL particles without any change in HDL cholesterol concentration compared with pups from CH-fed dams. Pups from phytosterol-supplemented mothers developed normally, without any alterations in litter birth weight, sex ratio, or neonatal growth, suggesting that maternal phytosterol supplementation is not associated with adverse health responses in offspring, at least at these whole-body indexes.
The lack of safe cholesterol-lowering strategies for use in hypercholesterolemic pregnancies has been acknowledged (37); however, the potential novel application of specific bioactive food components in ameliorating metabolic derangements programmed in offspring from hypercholesterolemic mothers has received little research attention. Napoli et al. (6) established that antioxidant intervention through maternal vitamin E supplementation was effective in reducing peroxidation and atherosclerosis in offspring of hypercholesterolemic New Zealand white rabbits. Results from our study suggest that maternal phytosterol supplementation during gestation and lactation is effective in lowering multiple CVD risk factors related to cholesterol and TG metabolism.
Although the specific mechanisms have not been examined previously, maternal phytosterol supplementation could modulate pup cholesterol metabolism through several conceivable means related to maternal–offspring cholesterol transfer. Maternal cholesterol contributes to the fetal cholesterol pool, particularly during early development, when fetal cholesterol synthetic capacity is low (38). A transplacental maternal–fetal cholesterol transport system regulates the movement of cholesterol through the placental trophoblasts and the endothelial cells (40). Although the transport system has not been completely elucidated, both cell types express a variety of cholesterol transporters, including the Niemann-Pick c1-like 1(NPC1L1), LDL receptor (LDLr), LDL-receptor-related protein 1(LRP1), VLDL receptor (VLDLr), scavenger receptor class B type 1(SCARB1), and ATP-binding cassette transporter G5 and G8 (ABCG5/G8) (40). Mechanistic studies suggest that phytosterol supplementation reduces whole-body cholesterol balance by interfering with intestinal cholesterol absorption and regulating the expression of a whole host of intracellular cholesterol regulatory and transport genes across different tissues (36, 40–42). Therefore, maternal phytosterol supplementation may have reduced offspring cholesterol balance by limiting excessive transfer of maternal cholesterol during in utero development, the result of an overall reduction in the maternal cholesterol pool and/or modulation of the expression of genes that regulate maternal-fetal cholesterol transfer. Alternatively, lowering maternal cholesterol balance through phytosterol supplementation during lactation could have also modulated the milk composition and interfered with cholesterol transfer to suckling pups.
In addition to the cholesterol-lowering response in pups from phytosterol-supplemented mothers, we also observed reductions in serum TG and VLDL particles compared with pups from unsupplemented mothers. Phytosterols are traditionally regarded as cholesterol-lowering compounds; however, recent work has explored the TG-lowering properties of phytosterols and highlighted the potential mechanisms that may underlie this response. Previous work from our laboratory (35) and others (43) suggest that at least part of the TG-lowering response to phytosterols may be related to interference with intestinal TG and/or FA absorption. Similar to maternal–fetal cholesterol transport, a placental–fetal FA transport system is recognized that involves both passive and active transport mechanisms (44). In a mechanism similar to what we proposed for cholesterol, a phytosterol-induced reduction in maternal TGs may have interfered with FA transfer to the pups during in utero development and/or postnatal lactation. However, this appears unlikely, because although VLDL particles were lower in phytosterol-supplemented dams than in CH-fed dams, no change in serum TG concentration was observed. Because phytosterols were recently demonstrated to directly reduce hepatic VLDL secretion in male C57BL/6J mice (45), it is plausible that the increased concentration of β-sitosterol in pups from phytosterol-supplemented dams caused an inhibition of hepatic VLDL secretion and an associated reduction in serum TG concentration.
Although pups from phytosterol-supplemented dams exhibited a largely favorable lipid profile, including lower serum cholesterol and LDL/VLDL particle number, compared with pups from CH-fed dams, the lower HDL particles observed in this group may be a potential cause for concern, because HDL particle number may be reflective of reverse cholesterol transport and CVD risk (46). The clinical value of blood HDL in assessing CVD risk is debated, because it is a complex lipoprotein with multiple subclasses having distinct particle sizes, lipid cargo, and specific antiatherogenic activity (47, 48). Currently, it is not clear whether the action of HDL in mediating reverse cholesterol transport is best represented by HDL cholesterol concentration, particle number, size, or some estimate of both (49). Recently, Qi et al. (50) suggested that the ratio of HDL cholesterol to HDL particle number is a better predictor of HDL function than either index alone. In a human cohort study, they reported that a high ratio of HDL cholesterol to HDL particle number reflected cholesterol-enriched HDL particles with a limited ability to promote reverse cholesterol transport. In our study, neither the HDL cholesterol-to-HDL particle number ratio nor the total cholesterol-to-HDL cholesterol ratio was different between the groups, suggesting that the reduction in HDL particle number in pups from CH/PS-fed dams may not indicate a reduction in HDL function.
In summary, we have shown that even under strong genetic predisposition to hypercholesterolemia, pups born to mothers supplemented with phytosterols during gestation and lactation exhibit favorable tissue and serum lipid responses compared with pups from unsupplemented mothers. These responses include lower hepatic cholesterol concentration, lower serum cholesterol profile (total cholesterol, LDL cholesterol, and LDL particle number), and lower serum TG and VLDL particles. The implications of lower HDL particle number in pups from phytosterol-supplemented mothers on HDL function are unknown. The specific mechanisms through which these responses occur and whether or not they modify lesion development and CVD risk into adulthood requires further investigation.
Acknowledgments
TCR designed and conducted the research, analyzed the data, and wrote the manuscript; CM, Y-TT, AI, and AR conducted the research and analyzed the data; and MSP designed the research. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ABCG5/G8, ATPbinding cassette transporter G5 and G8; apoE−/−, apoE–deficient; CVD, cardiovascular disease; CH, cholesterol-enriched diet; CH/PS, cholesterol-enriched diet supplemented with phytosterols; LDLr, LDL receptor; LRP1, LDL-receptor-related protein 1; NPC1L1, Niemann-Pick c1-like 1; SCARB1, scavenger receptor class B type 1; VLDLr, VLDL receptor.
References
- 1.Roberg-Larsen H, Strand MF, Krauss S, Wilson SR. Metabolites in vertebrate Hedgehog signaling. Biochem Biophys Res Commun 2014;446:669–74. [DOI] [PubMed] [Google Scholar]
- 2.Leiva A, de Medina CD, Salsoso R, Saez T, San Martin S, Abarzua F, Farias M, Guzman-Gutierrez E, Pardo F, Sobrevia L. Maternal hypercholesterolemia in pregnancy associates with umbilical vein endothelial dysfunction role of endothelial nitric oxide synthase and arginase II. Arterioscler Thromb Vasc Biol 2013;33:2444–53. [DOI] [PubMed] [Google Scholar]
- 3.Palinski W, Napoli C. The fetal origins of atherosclerosis: maternal hypercholesterolemia, and cholesterol-lowering or antioxidant treatment during pregnancy influence in utero programming and postnatal susceptibility to atherogenesis. FASEB J 2002;16:1348–60. [DOI] [PubMed] [Google Scholar]
- 4.Liguori A, DaposArmiento FP, Palagiano A, Balestrieri ML, Williams-Ignarro S, de Nigris F, Lerman LO, DaposAmora M, Rienzo M, Fiorito C, Ignarro LJ, Palinski W, Napoli C. Effect of gestational hypercholesterolaemia on omental vasoreactivity, placental enzyme activity and transplacental passage of normal and oxidised fatty acids. BJOG: an international journal of obstetrics and gynaecology 2007;114:1547–56. [DOI] [PubMed]
- 5.Marseille-Tremblay C, Ethier-Chiasson M, Forest JC, Giguere Y, Masse A, Mounier C, Lafond J. Impact of maternal circulating cholesterol and gestational diabetes mellitus on lipid metabolism in human term placenta. Mol Reprod Dev 2008;75:1054–62. [DOI] [PubMed] [Google Scholar]
- 6.Napoli C, Witztum JL, Calara F, de Nigris F, Palinski W. Maternal hypercholesterolemia enhances atherogenesis in normocholesterolemic rabbits, which is inhibited by antioxidant or lipid-lowering intervention during pregnancy: an experimental model of atherogenic mechanisms in human fetuses. Circ Res 2000;87:946–52. [DOI] [PubMed] [Google Scholar]
- 7.Napoli C, D'Armiento FP, Mancini FP, Postiglione A, Witztum JL, Palumbo G, Palinski W. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest 1997;100:2680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leiva A, de Medina CD, Guzmán-Gutiérrez E, Pardo F, Sobrevia L. Maternal hypercholesterolemia in gestational diabetes and the association with placental endothelial dysfunction. Placenta 2013;9:10.
- 9.Health, United States, 2013: With special feature on prescription drugs. Hyattsville (MD); 2014. [PubMed]
- 10.Napoli C, Glass CK, Witztum JL, Deutsch R, D'Armiento FP, Palinski W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet 1999;354:1234–41. [DOI] [PubMed] [Google Scholar]
- 11.Burke KT, Colvin PL, Myatt L, Graf GA, Schroeder F, Woollett LA. Transport of maternal cholesterol to the fetus is affected by maternal plasma cholesterol concentrations in the golden Syrian hamster. J Lipid Res 2009;50:1146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montoudis A, Simoneau L, Brissette L, Forest JC, Savard R, Lafond J. Impact of a cholesterol enriched diet on maternal and fetal plasma lipids and fetal deposition in pregnant rabbits. Life Sci 1999;64:2439–50. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Srinivasan SR, Bao W, Berenson GS. The magnitude of familial associations of cardiovascular risk factor variables between parents and offspring are influenced by age: the Bogalusa Heart Study. Ann Epidemiol 2001;11:522–8. [DOI] [PubMed] [Google Scholar]
- 14.Marcovecchio ML, Tossavainen PH, Heywood JJ, Dalton RN, Dunger DB. An independent effect of parental lipids on the offspring lipid levels in a cohort of adolescents with type 1 diabetes. Pediatr Diabetes 2012;13:463–9. [DOI] [PubMed] [Google Scholar]
- 15.Webber LS, Srinivasan SR, Wattigney WA, Berenson GS. Tracking of serum lipids and lipoproteins from childhood to adulthood. The Bogalusa Heart Study. Am J Epidemiol 1991;133:884–99. [DOI] [PubMed] [Google Scholar]
- 16.Porkka KV, Viikari JS, Taimela S, Dahl M, Akerblom HK. Tracking and predictiveness of serum lipid and lipoprotein measurements in childhood: a 12-year follow-up. The Cardiovascular Risk in Young Finns study. Am J Epidemiol 1994;140:1096–110. [DOI] [PubMed] [Google Scholar]
- 17.Juhola J, Magnussen CG, Viikari JS, Kahonen M, Hutri-Kahonen N, Jula A, Lehtimaki T, Akerblom HK, Pietikainen M, Laitinen T, et al. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. J Pediatr 2011;159:584–90. [DOI] [PubMed] [Google Scholar]
- 18.Rutherford JD. Maternal heterozygous familial hypercholesterolemia and its consequences for mother and child. Circulation 2011;124:1599–601. [DOI] [PubMed] [Google Scholar]
- 19.Kusters DM, Homsma SJ, Hutten BA, Twickler MT, Avis HJ, van der Post JA, Stroes ES. Dilemmas in treatment of women with familial hypercholesterolaemia during pregnancy. Neth J Med 2010;68:299–303. [PubMed] [Google Scholar]
- 20.Gylling H, Plat J, Turley S, Ginsberg HN, Ellegard L, Jessup W, Jones PJ, Lutjohann D, Maerz W, Masana L, et al. European Atherosclerosis Society Consensus Panel on P. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 2014;232:346–60. [DOI] [PubMed] [Google Scholar]
- 21.Malhotra A, Shafiq N, Arora A, Singh M, Kumar R, Malhotra S. Dietary interventions (plant sterols, stanols, omega-3 fatty acids, soy protein and dietary fibers) for familial hypercholesterolaemia. Cochrane Database Syst Rev 2014;6:CD001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moruisi KG, Oosthuizen W, Opperman AM. Phytosterols/stanols lower cholesterol concentrations in familial hypercholesterolemic subjects: a systematic review with meta-analysis. J Am Coll Nutr 2006;25:41–8. [DOI] [PubMed] [Google Scholar]
- 23.Sialvera TE, Pounis GD, Koutelidakis AE, Richter DJ, Yfanti G, Kapsokefalou M, Goumas G, Chiotinis N, Diamantopoulos E, Zampelas A. Phytosterols supplementation decreases plasma small and dense LDL levels in metabolic syndrome patients on a westernized type diet. Nutr Metab Cardiovasc Dis 2012;22:843–8. [DOI] [PubMed] [Google Scholar]
- 24.Lau VW, Journoud M, Jones PJ. Plant sterols are efficacious in lowering plasma LDL and non-HDL cholesterol in hypercholesterolemic type 2 diabetic and nondiabetic persons. Am J Clin Nutr 2005;81:1351–8. [DOI] [PubMed] [Google Scholar]
- 25.Ryökkynen A, Kayhko UR, Mustonen AM, Kukkonen JV, Nieminen P. Multigenerational exposure to phytosterols in the mouse. Reprod Toxicol 2005;19:535–40. [DOI] [PubMed] [Google Scholar]
- 26.Waalkens-Berendsen DH, Wolterbeek AP, Wijnands MV, Richold M, Hepburn PA. Safety evaluation of phytosterol esters. Part 3. Two-generation reproduction study in rats with phytosterol esters–a novel functional food. Food Chem Toxicol 1999;37:683–96. [DOI] [PubMed] [Google Scholar]
- 27.Whittaker MH, Frankos VH, Wolterbeek AP, Waalkens-Berendsen DH. Two-generation reproductive toxicity study of plant stanol esters in rats. Regul Toxicol Pharmacol 1999;29:196–204. [DOI] [PubMed] [Google Scholar]
- 28.Laitinen K, Isolauri E, Kaipiainen L, Gylling H, Miettinen TA. Plant stanol ester spreads as components of a balanced diet for pregnant and breast-feeding women: evaluation of clinical safety. Br J Nutr 2009;101:1797–804. [DOI] [PubMed] [Google Scholar]
- 29.Goharkhay N, Sbrana E, Gamble PK, Tamayo EH, Betancourt A, Villarreal K, Hankins GD, Saade GR, Longo M. Characterization of a murine model of fetal programming of atherosclerosis. American journal of obstetrics and gynecology 2007;197:416 e1–5. [DOI] [PubMed]
- 30.Alkemade FE, Gittenberger-de Groot AC, Schiel AE, VanMunsteren JC, Hogers B, van Vliet LS, Poelmann RE, Havekes LM, Willems van Dijk K, DeRuiter MC. Intrauterine exposure to maternal atherosclerotic risk factors increases the susceptibility to atherosclerosis in adult life. Arterioscler Thromb Vasc Biol 2007;27:2228–35. [DOI] [PubMed] [Google Scholar]
- 31.Moghadasian MH, Nguyen LB, Shefer S, Salen G, Batta AK, Frohlich JJ. Hepatic cholesterol and bile acid synthesis, low-density lipoprotein receptor function, and plasma and fecal sterol levels in mice: effects of apolipoprotein E deficiency and probucol or phytosterol treatment. Metabolism 2001;50:708–14. [DOI] [PubMed] [Google Scholar]
- 32.Moghadasian MH. Dietary phytosterols reduce probucol-induced atherogenesis in apo E-KO mice. Atherosclerosis 2006;188:28–34. [DOI] [PubMed] [Google Scholar]
- 33.Agnish ND, Keller KA. The rationale for culling of rodent litters. Fundamental and applied toxicology: official journal of the Society of Toxicology 1997;38:2–6. [DOI] [PubMed]
- 34.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med 2006;26:847–70. [DOI] [PubMed] [Google Scholar]
- 35.Rideout TC, Harding SV, Jones PJ. Consumption of plant sterols reduces plasma and hepatic triglycerides and modulates the expression of lipid regulatory genes and de novo lipogenesis in C57BL/6J mice. Mol Nutr Food Res 2010;54: Suppl 1:S7–13. [DOI] [PubMed] [Google Scholar]
- 36.Harding SV, Rideout TC, Jones PJ. Hepatic nuclear sterol regulatory binding element protein 2 abundance is decreased and that of ABCG5 increased in male hamsters fed plant sterols. J Nutr 2010;140:1249–54. [DOI] [PubMed] [Google Scholar]
- 37.Ito MK, McGowan MP, Moriarty PM. National Lipid Association Expert Panel on Familial H. Management of familial hypercholesterolemias in adult patients: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol 2011;5:S38–45. [DOI] [PubMed] [Google Scholar]
- 38.McConihay JA, Horn PS, Woollett LA. Effect of maternal hypercholesterolemia on fetal sterol metabolism in the Golden Syrian hamster. J Lipid Res 2001;42:1111–9. [PubMed] [Google Scholar]
- 39.Baardman ME, Kerstjens-Frederikse WS, Berger RM, Bakker MK, Hofstra RM, Plosch T. The role of maternal-fetal cholesterol transport in early fetal life: current insights. Biol Reprod 2013;88:24. [DOI] [PubMed] [Google Scholar]
- 40.Jesch ED, Seo JM, Carr TP, Lee JY. Sitosterol reduces messenger RNA and protein expression levels of Niemann-Pick C1-like 1 in FHs 74 Int cells. Nutr Res 2009;29:859–66. [DOI] [PubMed] [Google Scholar]
- 41.Calpe-Berdiel L, Escola-Gil JC, Blanco-Vaca F. New insights into the molecular actions of plant sterols and stanols in cholesterol metabolism. Atherosclerosis 2009;203:18–31. [DOI] [PubMed] [Google Scholar]
- 42.Calpe-Berdiel L, Escola-Gil JC, Blanco-Vaca F. Are LXR-regulated genes a major molecular target of plant sterols/stanols? Atherosclerosis 2007;195:210–1. [DOI] [PubMed] [Google Scholar]
- 43.Tomoyori H, Kawata Y, Higuchi T, Ichi I, Sato H, Sato M, Ikeda I, Imaizumi K. Phytosterol oxidation products are absorbed in the intestinal lymphatics in rats but do not accelerate atherosclerosis in apolipoprotein E-deficient mice. J Nutr 2004;134:1690–6. [DOI] [PubMed] [Google Scholar]
- 44.Gil-Sánchez A, Koletzko B, Larque E. Current understanding of placental fatty acid transport. Curr Opin Clin Nutr Metab Care 2012;15:265–72. [DOI] [PubMed] [Google Scholar]
- 45.Schonewille M, Brufau G, Shiri-Sverdlov R, Groen AK, Plat J. Serum TG-lowering properties of plant sterols and stanols are associated with decreased hepatic VLDL secretion. J Lipid Res 2014;55:2554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin SS, Khokhar AA, May HT, Kulkarni KR, Blaha MJ, Joshi PH, Toth PP, Muhlestein JB, Anderson JL, Knight S, et al. Lipoprotein Investigators C. HDL cholesterol subclasses, myocardial infarction, and mortality in secondary prevention: the Lipoprotein Investigators Collaborative. Eur Heart J 2015;36:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet 2014;384:618–25. [DOI] [PubMed] [Google Scholar]
- 48.Toth PP, Barter PJ, Rosenson RS, Boden WE, Chapman MJ, Cuchel M, D'Agostino RB Sr, Davidson MH, Davidson WS, Heinecke JW, et al. High-density lipoproteins: a consensus statement from the National Lipid Association. J Clin Lipidol 2013;7:484–525. [DOI] [PubMed] [Google Scholar]
- 49.Hovingh GK, Rader DJ, Hegele RA. HDL re-examined. Curr Opin Lipidol 2015;26:127–32. [DOI] [PubMed] [Google Scholar]
- 50.Qi Y, Fan J, Liu J, Wang W, Wang M, Sun J, Liu J, Xie W, Zhao F, Li Y, et al. Cholesterol-overloaded HDL particles are independently associated with progression of carotid atherosclerosis in a cardiovascular disease-free population: A community-based cohort study. J Am Coll Cardiol 2015;65:355–63. [DOI] [PubMed] [Google Scholar]